Alterations in Mitochondrial Oxidative Phosphorylation System: Relationship of Complex V and Cardiac Dysfunction in Human Heart Failure
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Samples Collection
2.2. RNA Extraction and Integrity
2.3. mRNA-Seq Analysis
2.4. ncRNA-Seq Analysis
2.5. Western Blot
2.6. Tissue Processing for Electron Microscopy
2.7. Statistical Methods
3. Results
3.1. Clinical Characteristics of Patients
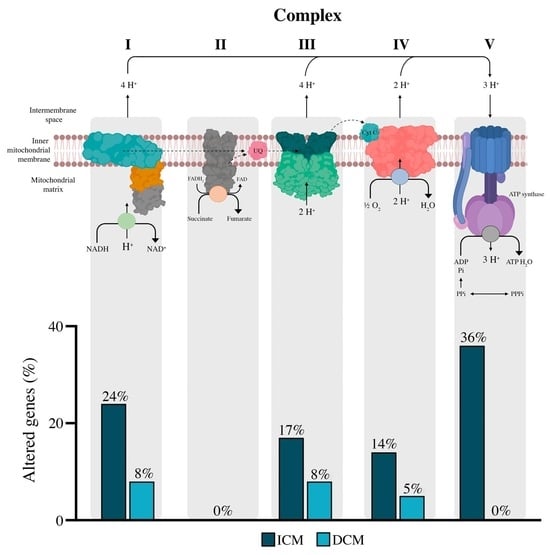
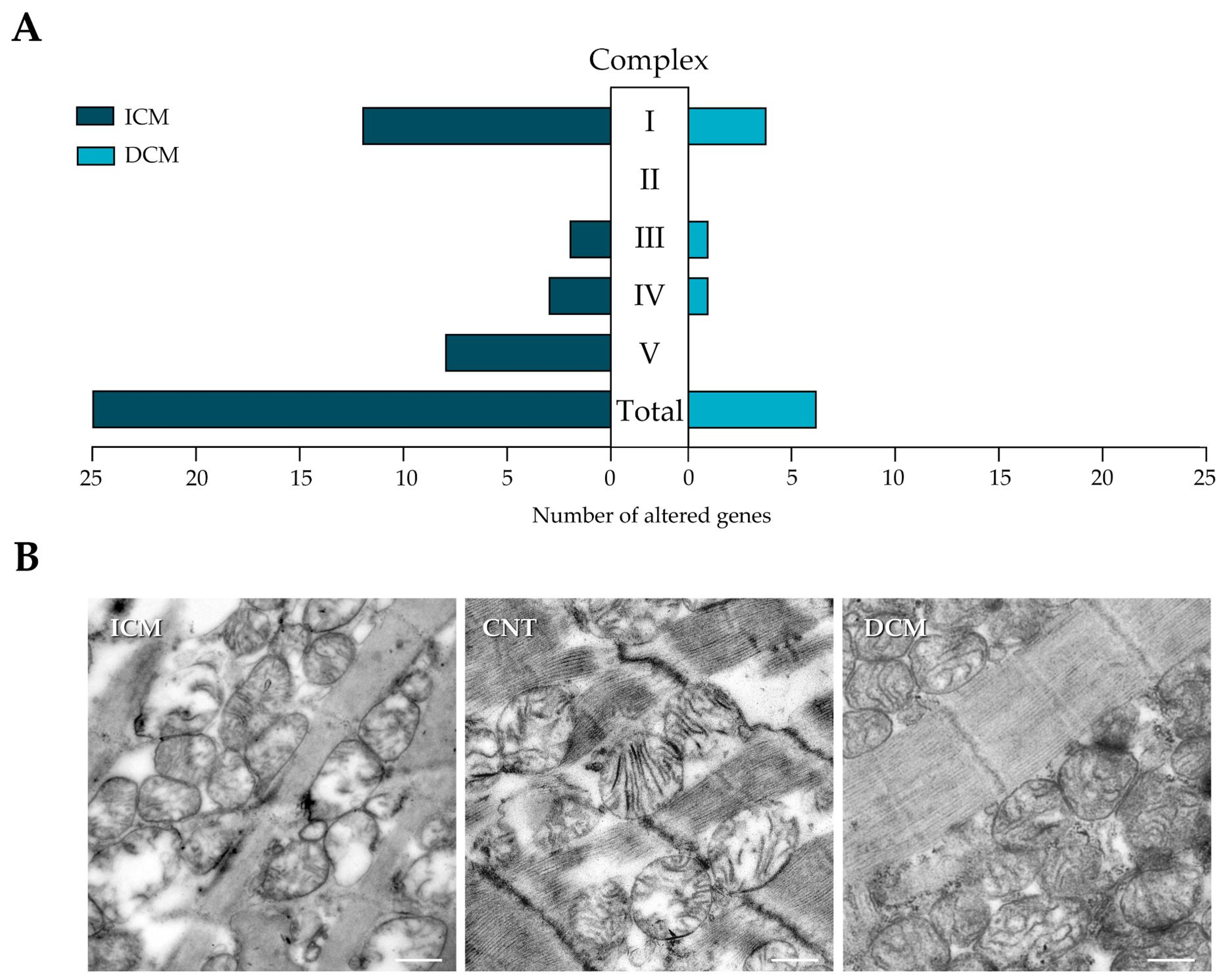
3.2. mRNA Expression of Oxidative Phosphorylation Complexes and Mitochondrial Morphology in Heart Failure Patients
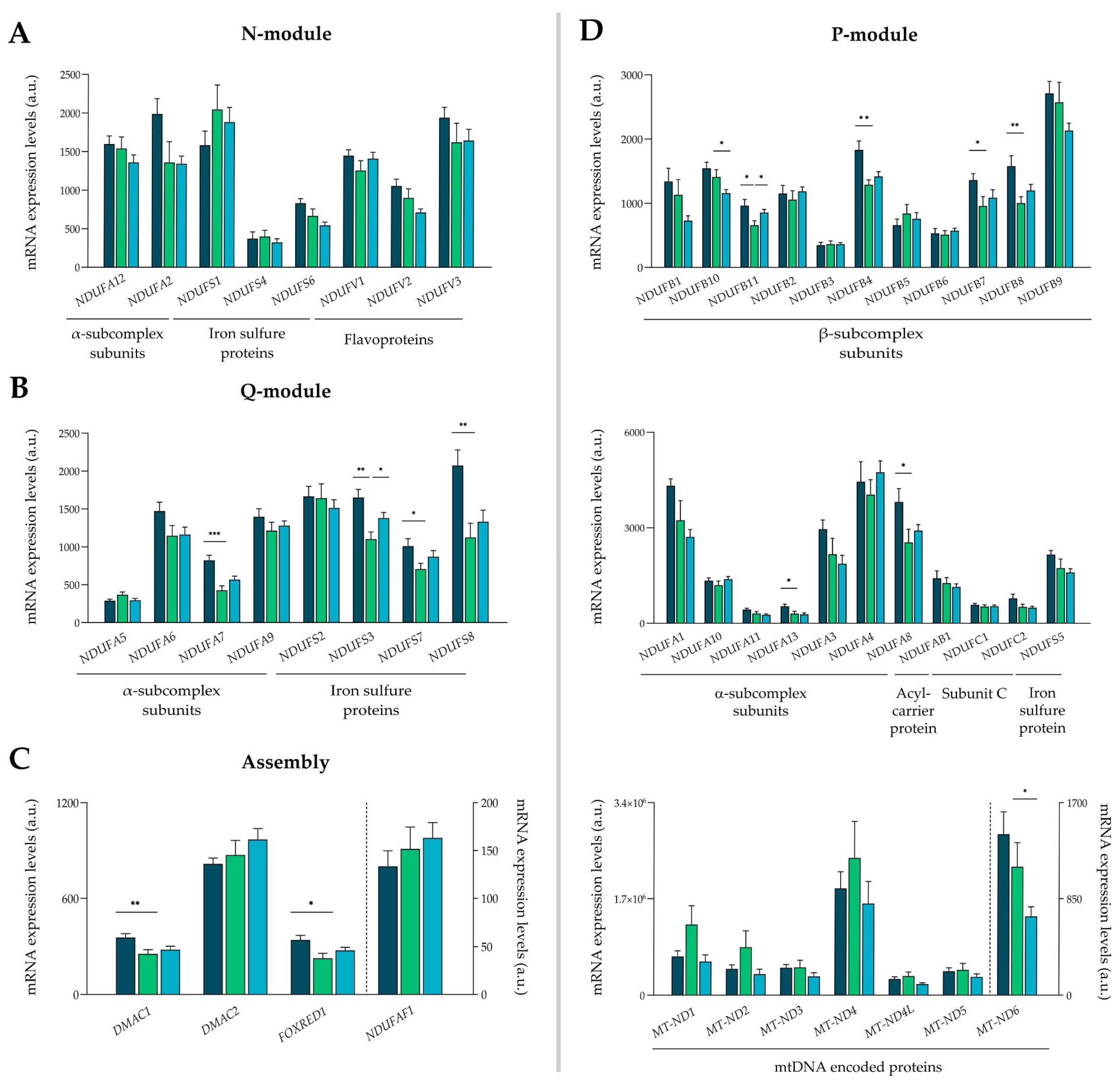
3.3. Complex I
3.4. Complex II, III, and IV
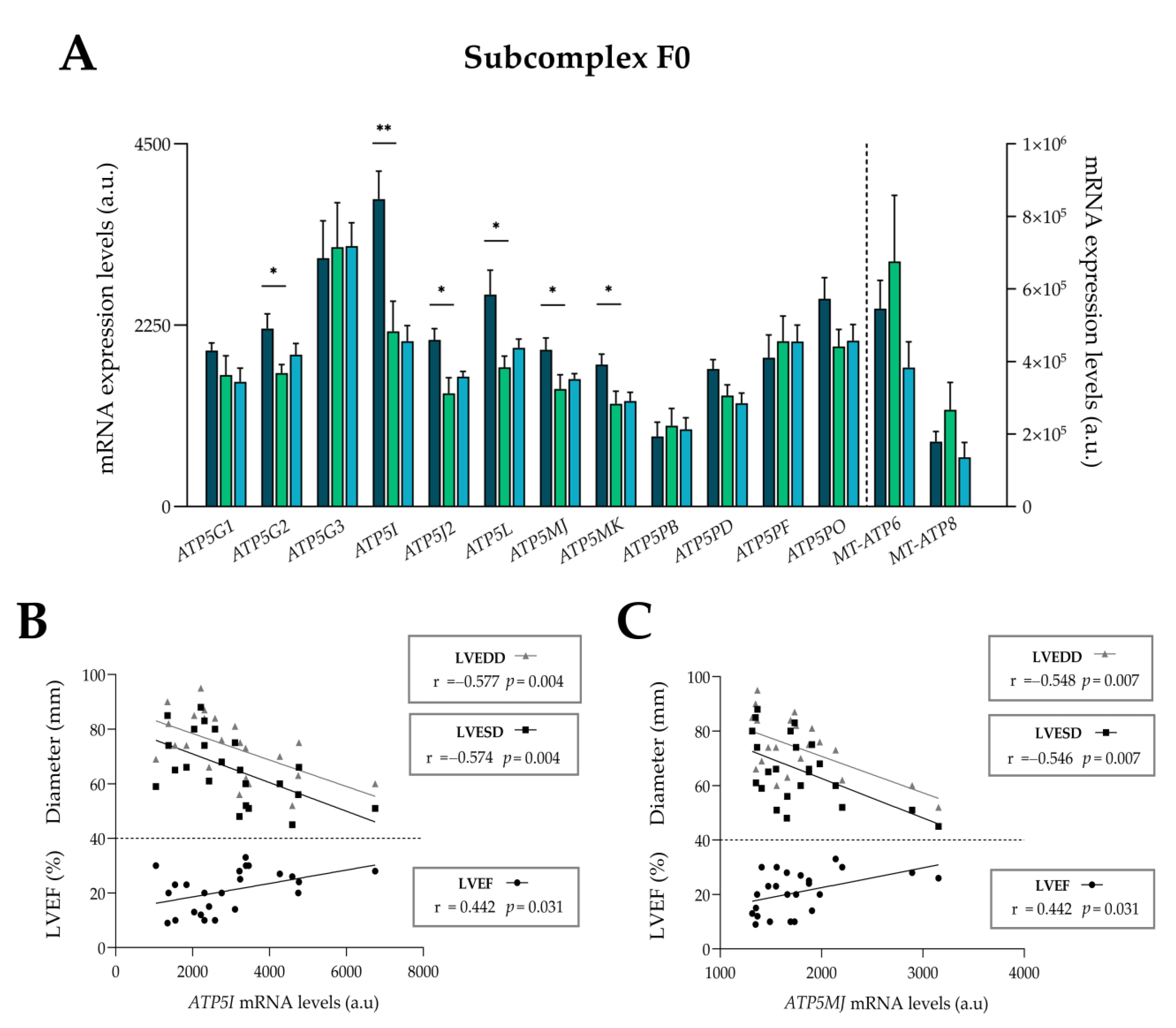
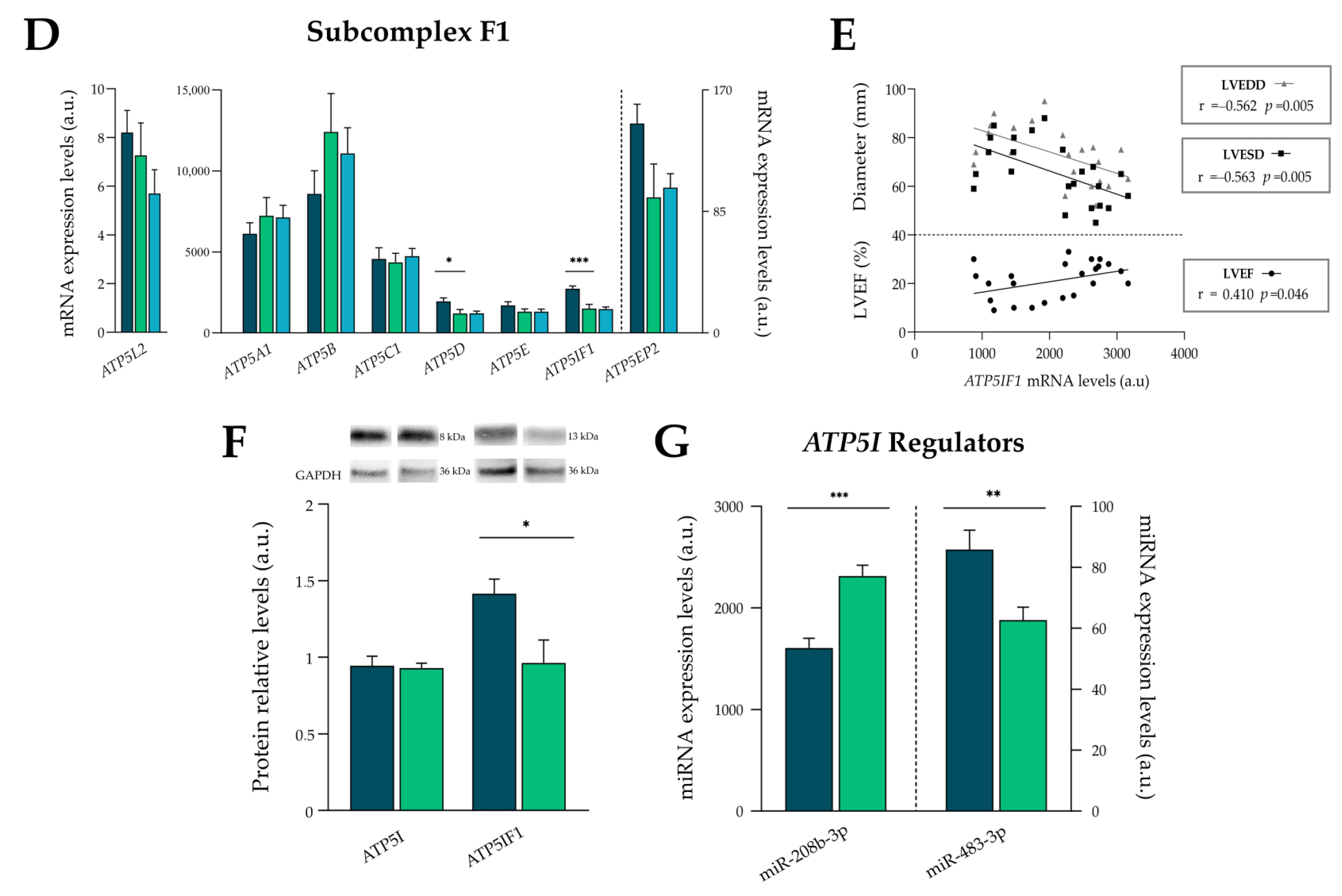
3.5. Complex V
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Roger, V.L. Epidemiology of Heart Failure: A Contemporary Perspective. Circ. Res. 2021, 128, 1421–1434. [Google Scholar] [CrossRef]
- Baldasseroni, S.; Opasich, C.; Gorini, M.; Lucci, D.; Marchionni, N.; Marini, M.; Campana, C.; Perini, G.; Deorsola, A.; Masotti, G.; et al. Italian Network on Congestive Heart Failure Investigators. Left bundle-branch block is associated with increased 1-year sudden and total mortality rate in 5517 outpatients with congestive heart failure: A report from the Italian network on congestive heart failure. Am. Heart J. 2002, 143, 398–405. [Google Scholar]
- Herrer, I.; Roselló-Lletí, E.; Rivera, M.; Molina Navarro, M.M.; Tarazón, E.; Ortega, A.; Martínez-Dolz, L.; Triviño, J.C.; Lago, F.; González-Juanatey, J.R.; et al. RNA-sequencing analysis reveals new alterations in cardiomyocyte cytoskeletal genes in patients with heart failure. Lab. Investig. 2014, 94, 645–653. [Google Scholar] [CrossRef]
- Tarazón, E.; Roselló-Lletí, E.; Ortega, A.; Molina-Navarro, M.M.; Sánchez-Lázaro, I.; Lago, F.; González-Juanatey, J.R.; Rivera, M.; Portolés, M. Differential gene expression of C-type natriuretic peptide and its related molecules in dilated and ischemic cardiomyopathy. A new option for the management of heart failure. Int. J. Cardiol. 2014, 174, e84–e86. [Google Scholar] [CrossRef] [PubMed]
- Tarazón, E.; Roselló-Lletí, E.; Rivera, M.; Ortega, A.; Molina-Navarro, M.M.; Triviño, J.C.; Lago, F.; González-Juanatey, J.R.; Orosa, P.; Montero, J.A.; et al. RNA sequencing analysis and atrial natriuretic peptide production in patients with dilated and ischemic cardiomyopathy. PLoS ONE 2014, 9, e90157. [Google Scholar] [CrossRef] [PubMed]
- Cortés, R.; Roselló-Lletí, E.; Rivera, M.; Martínez-Dolz, L.; Salvador, A.; Azorín, I.; Portolés, M. Influence of heart failure on nucleocytoplasmic transport in human cardiomyocytes. Cardiovasc. Res. 2010, 85, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Roselló-Lletí, E.; Alonso, J.; Cortés, R.; Almenar, L.; Martínez-Dolz, L.; Sánchez-Lázaro, I.; Lago, F.; Azorín, I.; Juanatey, J.R.; Portolés, M.; et al. Cardiac protein changes in ischaemic and dilated cardiomyopathy: A proteomic study of human left ventricular tissue. J. Cell Mol. Med. 2012, 16, 2471–2486. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, Z.; Zhang, W.; Liu, X. Mitochondrial dysfunction and mitochondrial therapies in heart failure. Pharmacol. Res. 2022, 175, 106038. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Investig. 2018, 128, 3716–3726. [Google Scholar] [CrossRef] [PubMed]
- Signes, A.; Fernandez-Vizarra, E. Assembly of mammalian oxidative phosphorylation complexes I-V and supercomplexes. Essays Biochem. 2018, 62, 255–270. [Google Scholar] [PubMed]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Brown, D.A.; Perry, J.B.; Allen, M.E.; Sabbah, H.N.; Stauffer, B.L.; Shaikh, S.R.; Cleland, J.G.; Colucci, W.S.; Butler, J.; Voors, A.A.; et al. Expert consensus document: Mitochondrial function as a therapeutic target in heart failure. Nat. Rev. Cardiol. 2017, 14, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Liu, J.; Zhang, B.; Liang, C.; Hua, J.; Meng, Q.; Wei, M.; Wang, W.; Yu, X.; Xu, J. Mitochondria-Related Transcriptome Characterization Associated with the Immune Microenvironment, Therapeutic Response and Survival Prediction in Pancreatic Cancer. Int. J. Mol. Sci. 2023, 24, 3270. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Shen, H. Common methods in mitochondrial research (Review). Int. J. Mol. Med. 2022, 50, 126. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Macrae, D.J. The Council for International Organizations and Medical Sciences (CIOMS) guidelines on ethics of clinical trials. Proc. Am. Thorac. Soc. 2007, 4, 176–179. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Roselló-Lletí, E.; Tarazón, E.; Ortega, A.; Gil-Cayuela, C.; Carnicer, R.; Lago, F.; González-Juanatey, J.R.; Portolés, M.; Rivera, M. Protein Inhibitor of NOS1 Plays a Central Role in the Regulation of NOS1 Activity in Human Dilated Hearts. Sci. Rep. 2016, 6, 30902. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Neubauer, S. The failing heart-an engine out of fuel. N. Engl. J. Med. 2007, 356, 1140–1151. [Google Scholar] [CrossRef]
- Signorile, A.; De Rasmo, D. Mitochondrial Complex I, a Possible Sensible Site of cAMP Pathway in Aging. Antioxidants 2023, 12, 221. [Google Scholar] [CrossRef]
- Jonckheere, A.I.; Smeitink, J.A.; Rodenburg, R.J. Mitochondrial ATP synthase: Architecture, function and pathology. J. Inherit. Metab. Dis. 2012, 35, 211–225. [Google Scholar] [CrossRef]
- Ramaccini, D.; Montoya-Uribe, V.; Aan, F.J.; Modesti, L.; Potes, Y.; Wieckowski, M.R.; Krga, I.; Glibetić, M.; Pinton, P.; Giorgi, C.; et al. Mitochondrial Function and Dysfunction in Dilated Cardiomyopathy. Front. Cell Dev. Biol. 2021, 8, 624216. [Google Scholar] [CrossRef]
- Tahrir, F.G.; Langford, D.; Amini, S.; Mohseni Ahooyi, T.; Khalili, K. Mitochondrial quality control in cardiac cells: Mechanisms and role in cardiac cell injury and disease. J. Cell Physiol. 2019, 234, 8122–8133. [Google Scholar] [CrossRef]
- Sabbah, H.N. Targeting the Mitochondria in Heart Failure: A Translational Perspective. JACC Basic Transl. Sci. 2020, 5, 88–106. [Google Scholar] [CrossRef]
- Ait-Aissa, K.; Blaszak, S.C.; Beutner, G.; Tsaih, S.W.; Morgan, G.; Santos, J.H.; Flister, M.J.; Joyce, D.L.; Camara, A.K.S.; Gutterman, D.D.; et al. Mitochondrial Oxidative Phosphorylation defect in the Heart of Subjects with Coronary Artery Disease. Sci. Rep. 2019, 9, 7623. [Google Scholar] [CrossRef] [PubMed]
- Chaanine, A.H.; Joyce, L.D.; Stulak, J.M.; Maltais, S.; Joyce, D.L.; Dearani, J.A.; Klaus, K.; Nair, K.S.; Hajjar, R.J.; Redfield, M.M. Mitochondrial Morphology, Dynamics, and Function in Human Pressure Overload or Ischemic Heart Disease With Preserved or Reduced Ejection Fraction. Circ. Heart Fail. 2019, 12, e005131. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.A.B.S.; Araújo, T.T.; Dionizio, A.; Trevizol, J.S.; Pereira, F.S.; Iano, F.G.; Faria Ximenes, V.; Buzalaf, M.A.R. Increase of complex I and reduction of complex II mitochondrial activity are possible adaptive effects provoked by fluoride exposure. Heliyon 2021, 7, e06028. [Google Scholar] [CrossRef] [PubMed]
- Van Houten, B.; Woshner, V.; Santos, J.H. Role of mitochondrial DNA in toxic responses to oxidative stress. DNA Repair 2006, 5, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Tsuchiya, M.; Bartlett, J.D. Fluoride induces endoplasmic reticulum stress and inhibits protein synthesis and secretion. Environ. Health Perspect. 2008, 116, 1142–1146. [Google Scholar] [CrossRef]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar]
- Dröse, S.; Brandt, U. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv. Exp. Med. Biol. 2012, 748, 145–169. [Google Scholar] [PubMed]
- Bleier, L.; Dröse, S. Superoxide generation by complex III: From mechanistic rationales to functional consequences. Biochim. Biophys. Acta 2013, 1827, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Xia, Y.; Chen, Z.; Chen, A.; Wu, Y.; Jia, J.; Sun, A.; Zou, Y.; Qian, J.; Ge, J. Cardiac Proteome Profiling in Ischemic and Dilated Cardiomyopathy Mouse Models. Front. Physiol. 2019, 10, 750. [Google Scholar] [CrossRef]
- Visapää, I.; Fellman, V.; Vesa, J.; Dasvarma, A.; Hutton, J.L.; Kumar, V.; Payne, G.S.; Makarow, M.; Van Coster, R.; Taylor, R.W.; et al. GRACILE syndrome, a lethal metabolic disorder with iron overload, is caused by a point mutation in BCS1L. Am. J. Hum. Genet. 2002, 71, 863–876. [Google Scholar] [CrossRef]
- Incognito, C.; Hedley, J.; Posadas, K.T.; Wang, X.; Desai, M. Pathogenic BCS1L Mutation Resulting in Hypertrophic Cardiomyopathy: A Unique Presentation of Nuclear Mitochondrial Disease. Tex. Heart Inst. J. 2023, 50, e217730. [Google Scholar] [CrossRef] [PubMed]
- Al-Owain, M.; Colak, D.; Albakheet, A.; Al-Younes, B.; Al-Humaidi, Z.; Al-Sayed, M.; Al-Hindi, H.; Al-Sugair, A.; Al-Muhaideb, A.; Rahbeeni, Z.; et al. Clinical and biochemical features associated with BCS1L mutation. J. Inherit. Metab. Dis. 2013, 36, 813–820. [Google Scholar] [CrossRef]
- Vogt, S.; Ramzan, R.; Cybulski, P.; Rhiel, A.; Weber, P.; Ruppert, V.; Irqsusi, M.; Rohrbach, S.; Niemann, B.; Mirow, N.; et al. The ratio of cytochrome c oxidase subunit 4 isoform 4I1 and 4I2 mRNA is changed in permanent atrial fibrillation. ESC Heart Fail. 2023. [Google Scholar] [CrossRef]
- Lazarou, M.; Smith, S.M.; Thorburn, D.R.; Ryan, M.T.; McKenzie, M. Assembly of nuclear DNA-encoded subunits into mitochondrial complex IV, and their preferential integration into supercomplex forms in patient mitochondria. FEBS J. 2009, 276, 6701–6713. [Google Scholar] [CrossRef]
- Maurer, S.F.; Fromme, T.; Grossman, L.I.; Hüttemann, M.; Klingenspor, M. The brown and brite adipocyte marker Cox7a1 is not required for non-shivering thermogenesis in mice. Sci. Rep. 2015, 5, 17704. [Google Scholar] [CrossRef]
- Roselló-Lletí, E.; Carnicer, R.; Tarazón, E.; Ortega, A.; Gil-Cayuela, C.; Lago, F.; González-Juanatey, J.R.; Portolés, M.; Rivera, M. Human Ischemic Cardiomyopathy Shows Cardiac Nos1 Translocation and its Increased Levels are Related to Left Ventricular Performance. Sci. Rep. 2016, 6, 24060. [Google Scholar] [CrossRef]
- Pagotto, S.; Colorito, M.L.; Nicotra, A.; Apuzzo, T.; Tinari, N.; Protasi, F.; Stassi, G.; Visone, R.; Di Franco, S.; Veronese, A. A perspective analysis: microRNAs, glucose metabolism, and drug resistance in colon cancer stem cells. Cancer Gene Ther. 2022, 29, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cheng, Y.; Xu, H.; Huang, H.; Tang, S.; Song, F.; Zhou, J. TRESK Regulates Gm11874 to Induce Apoptosis of Spinal Cord Neurons via ATP5i Mediated Oxidative Stress and DNA Damage. Neurochem. Res. 2021, 46, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Schwemmlein, J.; Maack, C.; Bertero, E. Mitochondria as Therapeutic Targets in Heart Failure. Curr. Heart Fail. Rep. 2022, 19, 27–37. [Google Scholar] [CrossRef] [PubMed]

| mRNA-Seq | ncRNA-Seq | Western Blot | ||
|---|---|---|---|---|
| ICM (n = 13) | DCM (n = 13) | ICM (n = 22) | ICM (n = 25) | |
| Age (years) | 54 ± 8 | 51 ± 11 | 55 ± 8 | 55 ± 8 |
| Gender male (%) | 100 | 92 | 100 | 100 |
| NYHA class | III–IV | III–IV | III–IV | III–IV |
| BMI (kg/m2) | 27 ± 4 | 27 ± 5 | 26 ± 3 | 27 ± 4 |
| Hemoglobin (mg/dL) | 14 ± 3 | 13 ± 3 | 14 ± 2 | 13 ± 3 |
| Hematocrit (%) | 41 ± 6 | 39 ± 7 | 41 ± 6 | 39 ± 7 |
| Total cholesterol (mg/dL) | 162 ± 41 | 147 ± 37 | 175 ± 45 | 192 ± 46 |
| Prior smoking (%) | 92 * | 50 | 81 | 81 |
| Prior hypertension (%) | 33 | 17 | 40 | 48 |
| Prior diabetes mellitus (%) | 42 | 17 | 45 | 57 |
| LVEF (%) | 25 ± 5 * | 17 ± 8 | 24 ± 6 | 23 ± 7 |
| LVESD (mm) | 57 ± 8 *** | 74 ± 10 | 53 ± 8 | 54 ± 8 |
| LVEDD (mm) | 65 ± 8 *** | 81 ± 8 | 62 ± 9 | 64 ± 9 |
| Left ventricle mass index (g/m2) | 139 ± 26 *** | 245 ± 64 | 133 ± 30 | 136 ± 30 |
| Left ventricle mass (g) | 263 ± 53 *** | 466 ± 105 | 246 ± 54 | 262 ± 61 |
| Duration of disease (months) | 45 ± 40 | 75 ± 68 | 36 ± 35 | 74 ± 67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giménez-Escamilla, I.; Benedicto, C.; Pérez-Carrillo, L.; Delgado-Arija, M.; González-Torrent, I.; Vilchez, R.; Martínez-Dolz, L.; Portolés, M.; Tarazón, E.; Roselló-Lletí, E. Alterations in Mitochondrial Oxidative Phosphorylation System: Relationship of Complex V and Cardiac Dysfunction in Human Heart Failure. Antioxidants 2024, 13, 285. https://doi.org/10.3390/antiox13030285
Giménez-Escamilla I, Benedicto C, Pérez-Carrillo L, Delgado-Arija M, González-Torrent I, Vilchez R, Martínez-Dolz L, Portolés M, Tarazón E, Roselló-Lletí E. Alterations in Mitochondrial Oxidative Phosphorylation System: Relationship of Complex V and Cardiac Dysfunction in Human Heart Failure. Antioxidants. 2024; 13(3):285. https://doi.org/10.3390/antiox13030285
Chicago/Turabian StyleGiménez-Escamilla, Isaac, Carlota Benedicto, Lorena Pérez-Carrillo, Marta Delgado-Arija, Irene González-Torrent, Roger Vilchez, Luis Martínez-Dolz, Manuel Portolés, Estefanía Tarazón, and Esther Roselló-Lletí. 2024. "Alterations in Mitochondrial Oxidative Phosphorylation System: Relationship of Complex V and Cardiac Dysfunction in Human Heart Failure" Antioxidants 13, no. 3: 285. https://doi.org/10.3390/antiox13030285
APA StyleGiménez-Escamilla, I., Benedicto, C., Pérez-Carrillo, L., Delgado-Arija, M., González-Torrent, I., Vilchez, R., Martínez-Dolz, L., Portolés, M., Tarazón, E., & Roselló-Lletí, E. (2024). Alterations in Mitochondrial Oxidative Phosphorylation System: Relationship of Complex V and Cardiac Dysfunction in Human Heart Failure. Antioxidants, 13(3), 285. https://doi.org/10.3390/antiox13030285