Comparison of the Regenerative Metabolic Efficiency of Lipid Extracts from Microalgae Nannochloropsis oceanica and Chlorococcum amblystomatis on Fibroblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Cell Culture Treatment
2.1.1. Microalgaes
2.1.2. Microalgae Lipid Extracts
2.1.3. Phospholipid and Glycolipid Quantification
2.1.4. Lipid Profiles of Nannochloropsis oceanica and Chlorococcum amblystomatis Extracts Analysis
2.1.5. Cell Culture
2.2. Methods
2.2.1. Determination of ROS Generation
2.2.2. Antioxidant Enzyme Activity and Low-Molecular Antioxidant Level Estimation
2.2.3. Protein Level
2.2.4. Lipid Peroxidation Product (4-HNE) Determination
2.2.5. Adducts of 4-HNE—Protein Determination
2.2.6. Protein Carbonyl Group (CBO) Determination
2.2.7. Determination of the Activity of Enzyme-Metabolizing Phospholipids and Free PUFAs
2.2.8. Phospholipid and Free Fatty Acid Determination
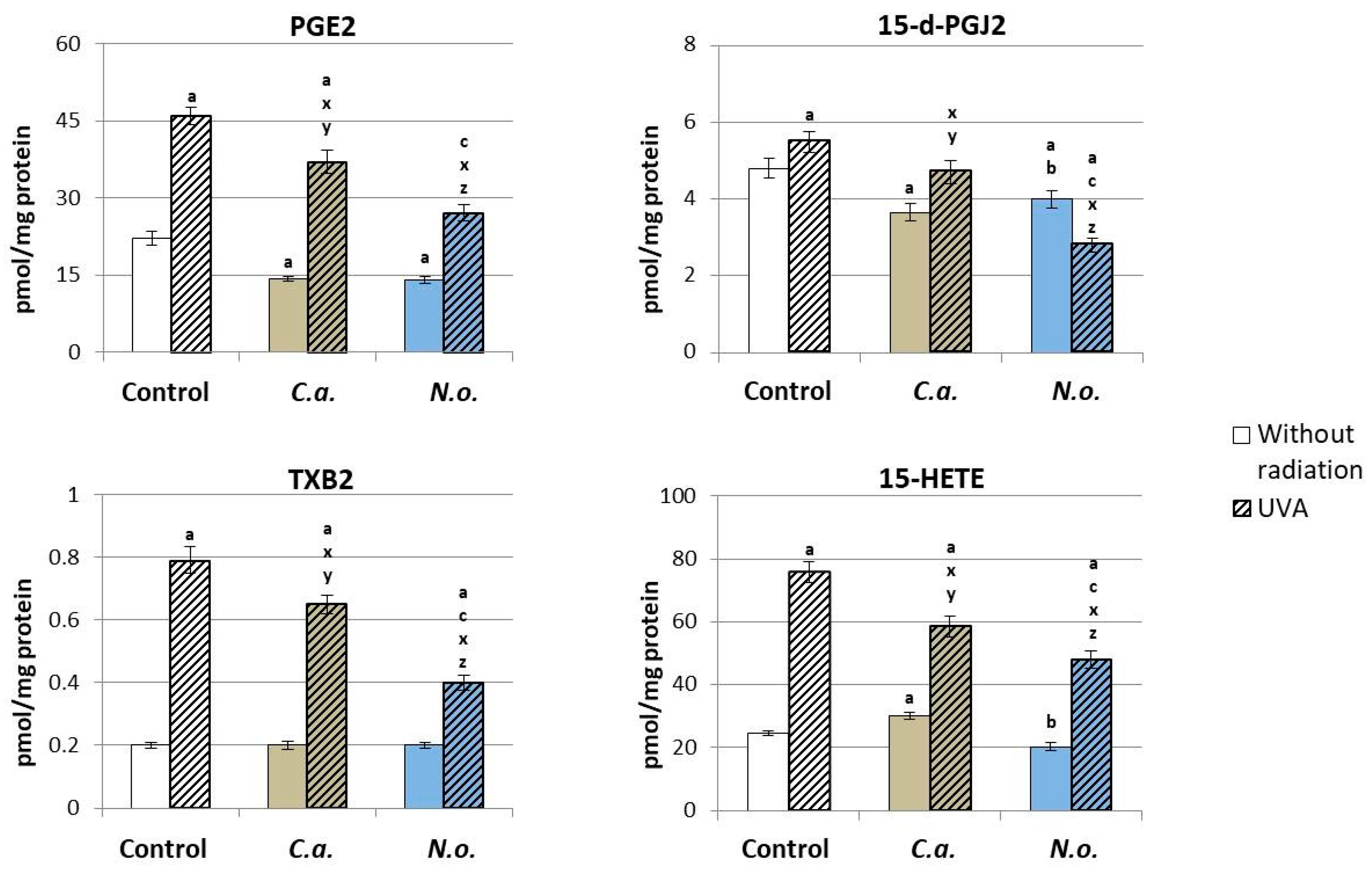
2.2.9. Determination of the Level of Eicosanoids
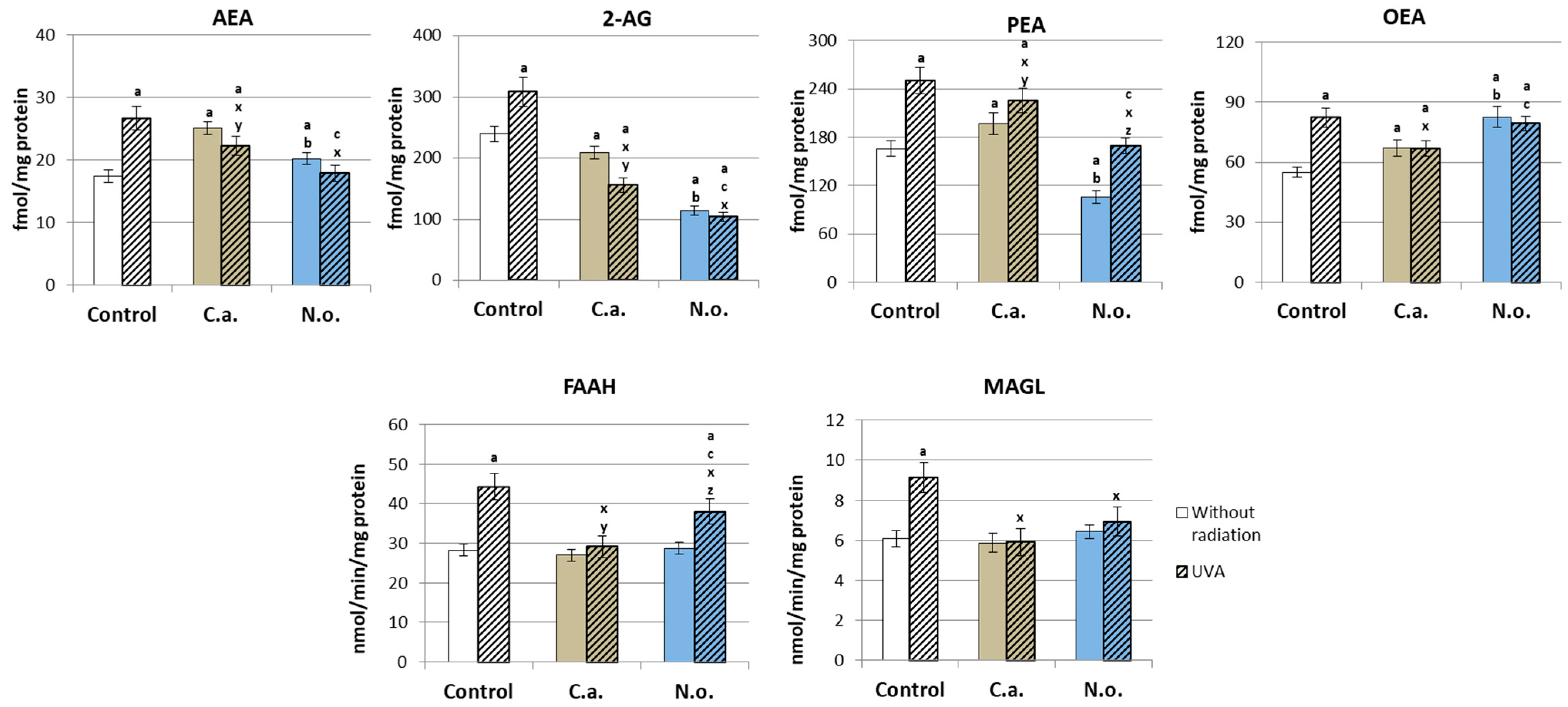
2.2.10. Determination of the Level of Endocannabinoids and Its Degrading Enzymes
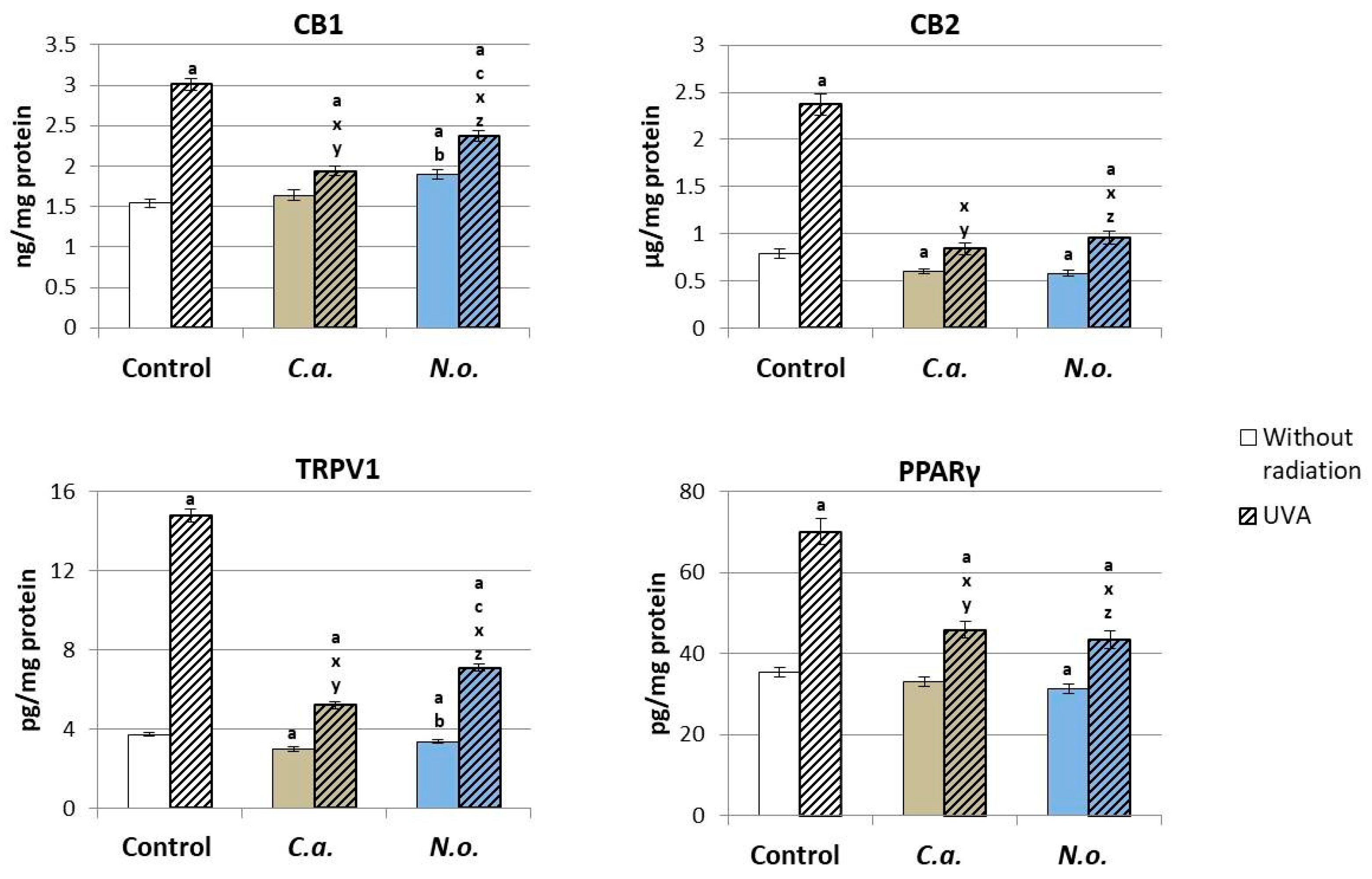
2.2.11. Determination of Expression of Membrane Receptors
2.2.12. Statistical Analysis
3. Results
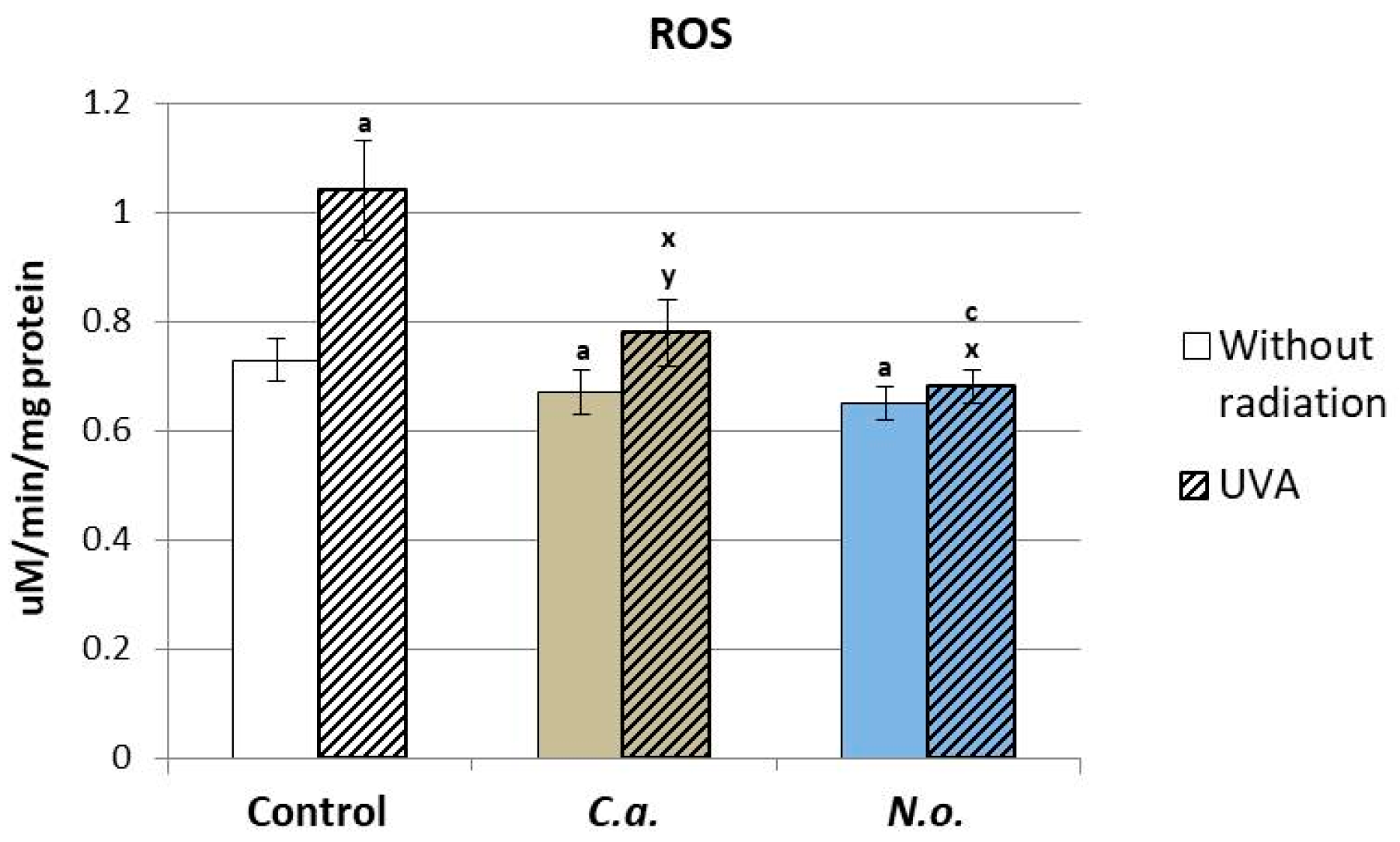
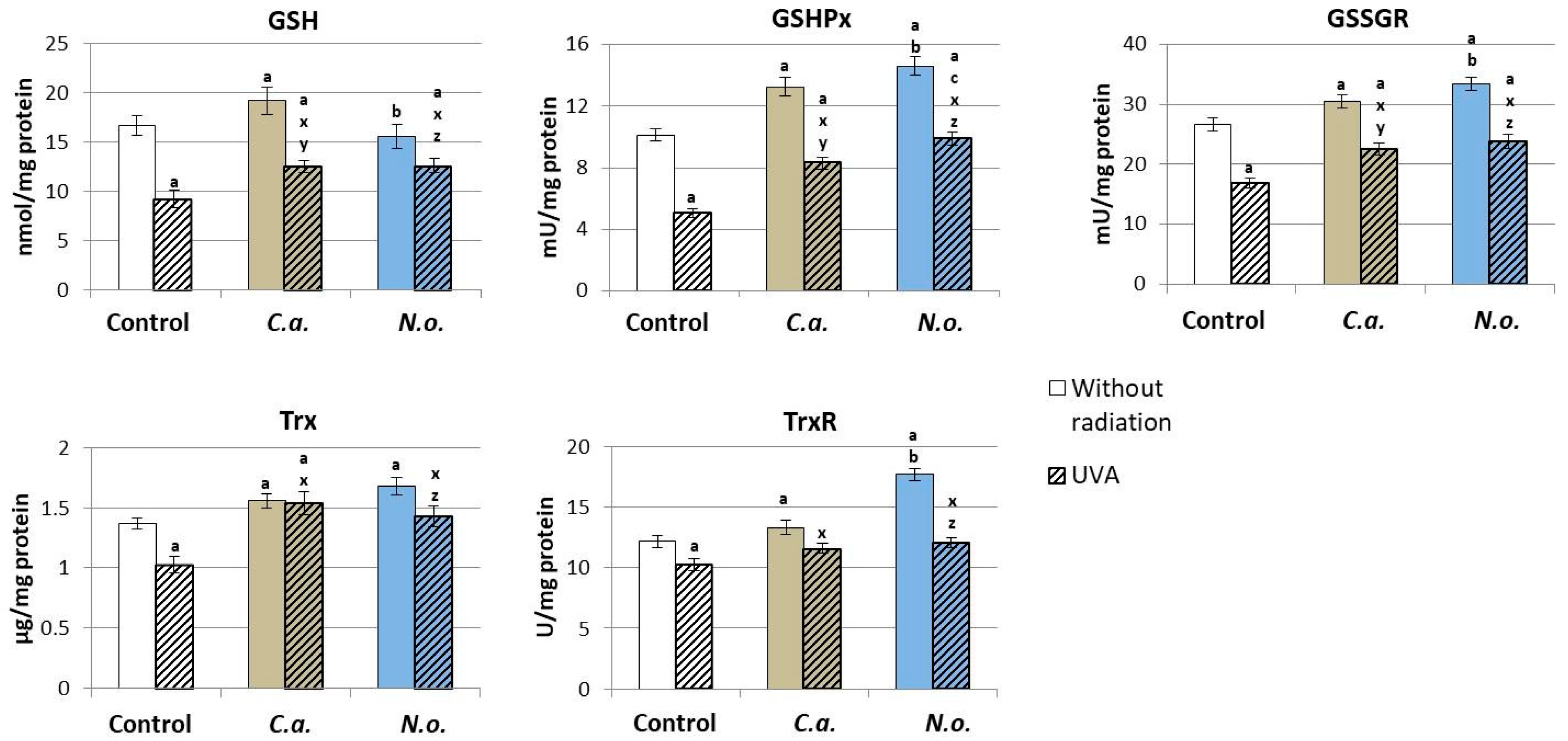
3.1. The Influence of Lipid Extracts from the Microalgae Chlorococcum amblystomatis and Nannochloropsis oceanica on Redox Balance Disturbance Caused by UVA Irradiation of Fibroblasts
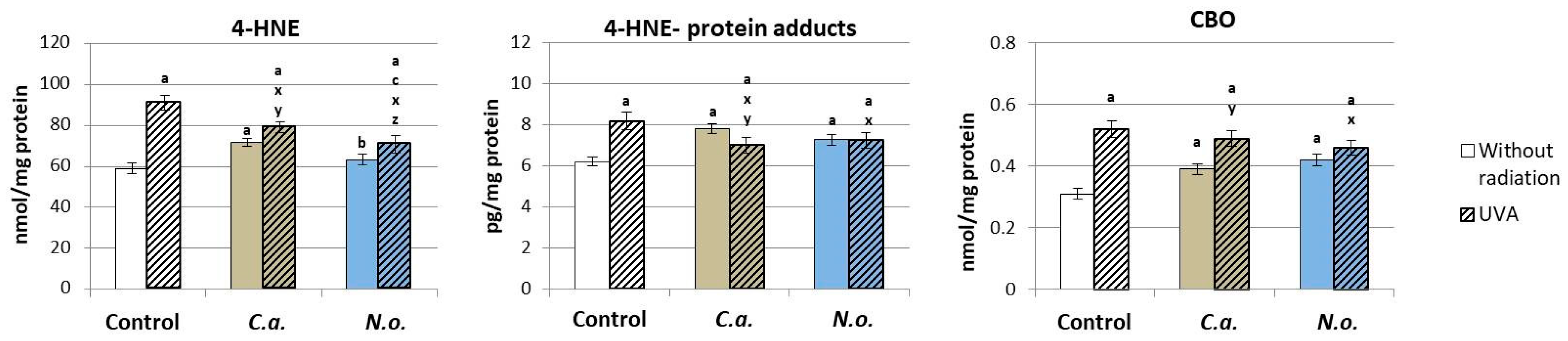
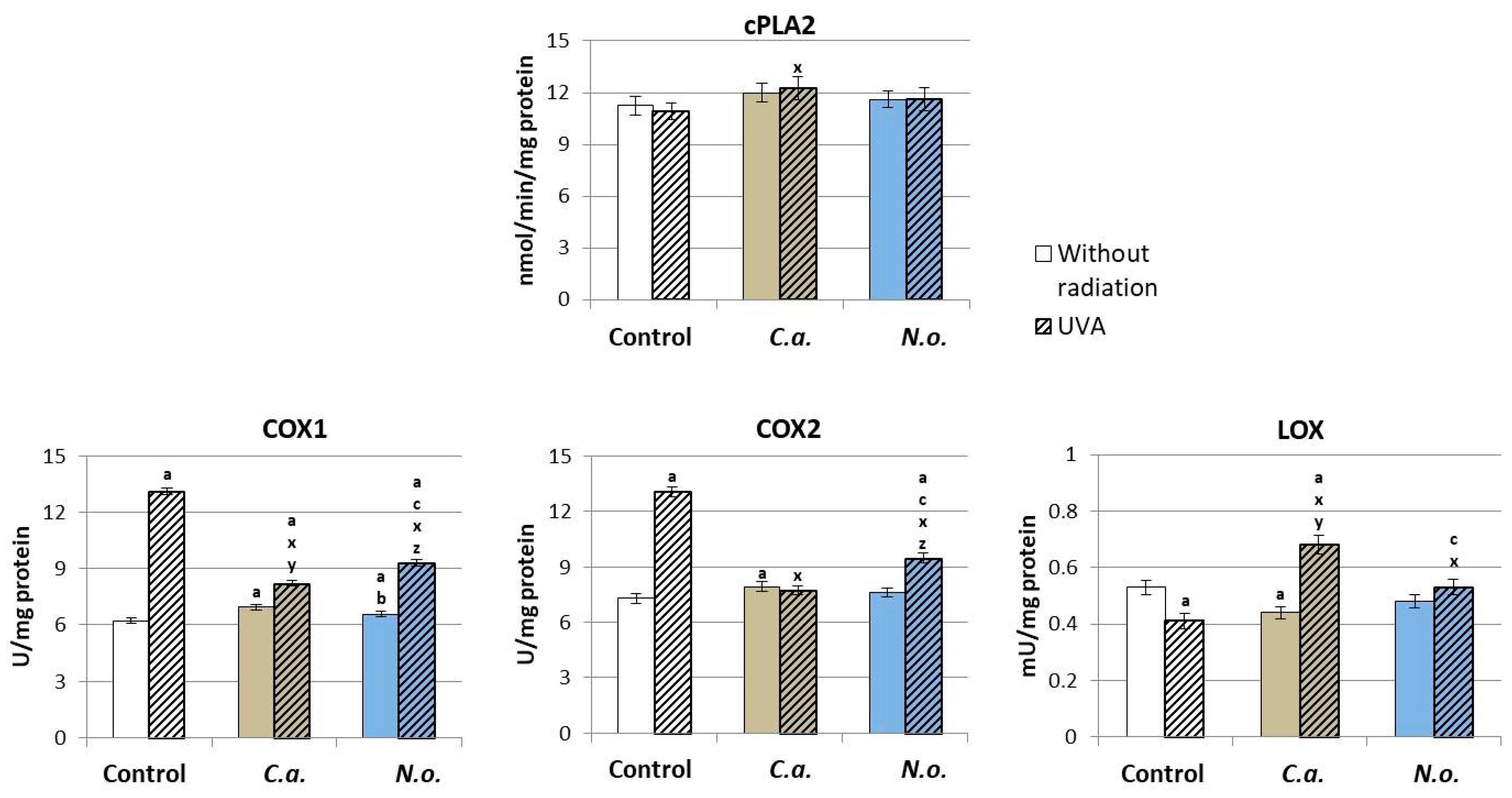
3.2. The Influence of Lipid Extracts from the Microalgae Chloroccocum amblystomatis and Nannochloropsis oceanica on Lipid Peroxidation Caused by UVA Irradiation of Fibroblasts
4. Discussion
4.1. Protective Effect of Marine and Freshwater Microalgae on Skin Fibroblasts
4.2. Regenerative Effect of the Microalgae Nannochloropsis oceanica and Chlorococcum amblystomatis on the Metabolism of UVA-Irradiated Fibroblasts
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dąbrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The Relationship between Skin Function, Barrier Properties, and Body-Dependent Factors. Skin Res. Technol. 2018, 24, 165–174. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Plonka, P.M.; Szaflarski, J.P.; Paus, R. How UV Light Touches the Brain and Endocrine System Through Skin, and Why. Endocrinology 2018, 159, 1992–2007. [Google Scholar] [CrossRef] [PubMed]
- Vangipuram, R.; Feldman, S. Ultraviolet Phototherapy for Cutaneous Diseases: A Concise Review. Oral Dis. 2016, 22, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Bernerd, F.; Passeron, T.; Castiel, I.; Marionnet, C. The Damaging Effects of Long UVA (UVA1) Rays: A Major Challenge to Preserve Skin Health and Integrity. Int. J. Mol. Sci. 2022, 23, 8243. [Google Scholar] [CrossRef]
- Nisar, M.F.; Parsons, K.S.G.; Bian, C.X.; Zhong, J.L. UVA Irradiation Induced Heme Oxygenase-1: A Novel Phototherapy for Morphea. Photochem. Photobiol. 2015, 91, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative Stress in the Skin: Impact and Related Protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Chaiprasongsuk, A.; Lohakul, J.; Soontrapa, K.; Sampattavanich, S.; Akarasereenont, P.; Panich, U. Activation of Nrf2 Reduces UVA-Mediated MMP-1 Upregulation via MAPK/AP-1 Signaling Cascades: The Photoprotective Effects of Sulforaphane and Hispidulin. J. Pharmacol. Exp. Ther. 2017, 360, 388–398. [Google Scholar] [CrossRef]
- Priya Dharshini, L.C.; Vishnupriya, S.; Sakthivel, K.M.; Rasmi, R.R. Oxidative Stress Responsive Transcription Factors in Cellular Signalling Transduction Mechanisms. Cell Signal. 2020, 72, 109670. [Google Scholar] [CrossRef]
- Panov, A.V.; Dikalov, S.I. Cardiolipin, Perhydroxyl Radicals, and Lipid Peroxidation in Mitochondrial Dysfunctions and Aging. Oxidative Med. Cell. Longev. 2020, 2020, e1323028. [Google Scholar] [CrossRef]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, e5080843. [Google Scholar] [CrossRef]
- Catalá, A. Lipid Peroxidation of Membrane Phospholipids Generates Hydroxy-Alkenals and Oxidized Phospholipids Active in Physiological and/or Pathological Conditions. Chem. Phys. Lipids 2009, 157, 1–11. [Google Scholar] [CrossRef]
- Gęgotek, A.; Jastrząb, A.; Dobrzyńska, M.; Biernacki, M.; Skrzydlewska, E. Exogenous Antioxidants Impact on UV-Induced Changes in Membrane Phospholipids and the Effectiveness of the Endocannabinoid System in Human Skin Cells. Antioxidants 2021, 10, 1260. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Frisardi, V.; Panza, F.; Seripa, D.; Farooqui, T.; Farooqui, A.A. Glycerophospholipids and Glycerophospholipid-Derived Lipid Mediators: A Complex Meshwork in Alzheimer’s Disease Pathology. Prog. Lipid Res. 2011, 50, 313–330. [Google Scholar] [CrossRef] [PubMed]
- Jarc, E.; Petan, T. A Twist of FATe: Lipid Droplets and Inflammatory Lipid Mediators. Biochimie 2020, 169, 69–87. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Biological Effect of Protein Modifications by Lipid Peroxidation Products. Chem. Phys. Lipids 2019, 221, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, F.; Mijouin, L.; Pichon, C. Skin Immune Landscape: Inside and Outside the Organism. Mediat. Inflamm. 2017, 2017, e5095293. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Łuczaj, W.; Skrzydlewska, E. Effects of Natural Antioxidants on Phospholipid and Ceramide Profiles of 3D-Cultured Skin Fibroblasts Exposed to UVA or UVB Radiation. Antioxidants 2021, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Kwiecien, K.; Zegar, A.; Jung, J.; Brzoza, P.; Kwitniewski, M.; Godlewska, U.; Grygier, B.; Kwiecinska, P.; Morytko, A.; Cichy, J. Architecture of Antimicrobial Skin Defense. Cytokine Growth Factor. Rev. 2019, 49, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Szachowicz-Petelska, B.; Łuczaj, W.; Wroński, A.; Jastrząb, A.; Dobrzyńska, I. The Differential Effect of Cannabidiol on the Composition and Physicochemical Properties of Keratinocyte and Fibroblast Membranes from Psoriatic Patients and Healthy People. Membranes 2021, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- da Silva Vaz, B.; Moreira, J.B.; de Morais, M.G.; Costa, J.A.V. Microalgae as a New Source of Bioactive Compounds in Food Supplements. Curr. Opin. Food Sci. 2016, 7, 73–77. [Google Scholar] [CrossRef]
- Conde, T.A.; Neves, B.F.; Couto, D.; Melo, T.; Neves, B.; Costa, M.; Silva, J.; Domingues, P.; Domingues, M.R. Microalgae as Sustainable Bio-Factories of Healthy Lipids: Evaluating Fatty Acid Content and Antioxidant Activity. Mar. Drugs 2021, 19, 357. [Google Scholar] [CrossRef]
- Ravindran, B.; Gupta, S.K.; Cho, W.-M.; Kim, J.K.; Lee, S.R.; Jeong, K.-H.; Lee, D.J.; Choi, H.-C. Microalgae Potential and Multiple Roles—Current Progress and Future Prospects—An Overview. Sustainability 2016, 8, 1215. [Google Scholar] [CrossRef]
- Conde, T.A.; Couto, D.; Melo, T.; Costa, M.; Silva, J.; Domingues, M.R.; Domingues, P. Polar Lipidomic Profile Shows Chlorococcum Amblystomatis as a Promising Source of Value-Added Lipids. Sci. Rep. 2021, 11, 4355. [Google Scholar] [CrossRef]
- Correia, N.; Pereira, H.; Silva, J.T.; Santos, T.; Soares, M.; Sousa, C.B.; Schüler, L.M.; Costa, M.; Varela, J.; Pereira, L.; et al. Isolation, Identification and Biotechnological Applications of a Novel, Robust, Free-Living Chlorococcum (Oophila) amblystomatis Strain Isolated from a Local Pond. Appl. Sci. 2020, 10, 3040. [Google Scholar] [CrossRef]
- Astudillo, A.M.; Meana, C.; Bermúdez, M.A.; Pérez-Encabo, A.; Balboa, M.A.; Balsinde, J. Release of Anti-Inflammatory Palmitoleic Acid and Its Positional Isomers by Mouse Peritoneal Macrophages. Biomedicines 2020, 8, 480. [Google Scholar] [CrossRef]
- Bermúdez, M.A.; Pereira, L.; Fraile, C.; Valerio, L.; Balboa, M.A.; Balsinde, J. Roles of Palmitoleic Acid and Its Positional Isomers, Hypogeic and Sapienic Acids, in Inflammation, Metabolic Diseases and Cancer. Cells 2022, 11, 2146. [Google Scholar] [CrossRef]
- Correia, N.; Pereira, H.; Schulze, P.S.C.; Costa, M.M.; Santo, G.E.; Guerra, I.; Trovão, M.; Barros, A.; Cardoso, H.; Silva, J.L.; et al. Heterotrophic and Photoautotrophic Media Optimization Using Response Surface Methodology for the Novel Microalga Chlorococcum Amblystomatis. Appl. Sci. 2023, 13, 2089. [Google Scholar] [CrossRef]
- Martins, T.; Barros, A.N.; Rosa, E.; Antunes, L. Enhancing Health Benefits through Chlorophylls and Chlorophyll-Rich Agro-Food: A Comprehensive Review. Molecules 2023, 28, 5344. [Google Scholar] [CrossRef]
- Bazinet, R.P.; Metherel, A.H.; Chen, C.T.; Shaikh, S.R.; Nadjar, A.; Joffre, C.; Layé, S. Brain Eicosapentaenoic Acid Metabolism as a Lead for Novel Therapeutics in Major Depression. Brain Behav. Immun. 2020, 85, 21–28. [Google Scholar] [CrossRef]
- Kim, N.; Kang, M.S.; Nam, M.; Kim, S.A.; Hwang, G.-S.; Kim, H.S. Eicosapentaenoic Acid (EPA) Modulates Glucose Metabolism by Targeting AMP-Activated Protein Kinase (AMPK) Pathway. Int. J. Mol. Sci. 2019, 20, 4751. [Google Scholar] [CrossRef]
- Mitra, M.; Mishra, S. A Biorefinery from Nannochloropsis Spp. Utilizing Wastewater Resources. In Application of Microalgae in Wastewater Treatment: Volume 2: Biorefinery Approaches of Wastewater Treatment; Gupta, S.K., Bux, F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 123–145. ISBN 978-3-030-13909-4. [Google Scholar]
- Couto, D.; Conde, T.A.; Melo, T.; Neves, B.; Costa, M.; Cunha, P.; Guerra, I.; Correia, N.; Silva, J.T.; Pereira, H.; et al. Effects of Outdoor and Indoor Cultivation on the Polar Lipid Composition and Antioxidant Activity of Nannochloropsis oceanica and Nannochloropsis Limnetica: A Lipidomics Perspective. Algal Res. 2022, 64, 102718. [Google Scholar] [CrossRef]
- Dutta, S.; Ray, S.; Nagarajan, K. Glutamic Acid as Anticancer Agent: An Overview. Saudi Pharm. J. 2013, 21, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Melville, G.W.; Siegler, J.C.; Marshall, P.W.M. The Effects of D-Aspartic Acid Supplementation in Resistance-Trained Men over a Three Month Training Period: A Randomised Controlled Trial. PLoS ONE 2017, 12, e0182630. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, N.; Melchior, D.; Obled, C. Modifications of Protein and Amino Acid Metabolism during Inflammation and Immune System Activation. Livest. Prod. Sci. 2004, 87, 37–45. [Google Scholar] [CrossRef]
- Barros, A.; Pereira, H.; Campos, J.; Marques, A.; Varela, J.; Silva, J. Heterotrophy as a Tool to Overcome the Long and Costly Autotrophic Scale-up Process for Large Scale Production of Microalgae. Sci. Rep. 2019, 9, 13935. [Google Scholar] [CrossRef]
- Stasiewicz, A.; Conde, T.; Gęgotek, A.; Domingues, M.R.; Domingues, P.; Skrzydlewska, E. Prevention of UVB Induced Metabolic Changes in Epidermal Cells by Lipid Extract from Microalgae Nannochloropsis oceanica. Int. J. Mol. Sci. 2023, 24, 11302. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, E.M.; Lewis, D.H. Spectrophotometric Determination of Phosphate Esters in the Presence and Absence of Orthophosphate. Anal. Biochem. 1970, 36, 159–167. [Google Scholar] [CrossRef]
- Weinman, E.O.; Chaikoff, I.L.; Entenman, C.; Dauben, W.G. Turnover rates of phosphate and fatty acid moieties of plasma phospholipides. J. Biol. Chem. 1950, 187, 643–649. [Google Scholar] [CrossRef]
- Koch, A.K.; Käppeli, O.; Fiechter, A.; Reiser, J. Hydrocarbon Assimilation and Biosurfactant Production in Pseudomonas Aeruginosa Mutants. J. Bacteriol. 1991, 173, 4212–4219. [Google Scholar] [CrossRef]
- Bell, B.M.; Daniels, D.G.H.; Fearn, T.; Stewart, B.A. Lipid Compositions, Baking Qualities and Other Characteristics of Wheat Varieties Grown in the U.K. J. Cereal Sci. 1987, 5, 277–286. [Google Scholar] [CrossRef]
- Gęgotek, A.; Bielawska, K.; Biernacki, M.; Zaręba, I.; Surażyński, A.; Skrzydlewska, E. Comparison of Protective Effect of Ascorbic Acid on Redox and Endocannabinoid Systems Interactions in in Vitro Cultured Human Skin Fibroblasts Exposed to UV Radiation and Hydrogen Peroxide. Arch. Dermatol. Res. 2017, 309, 285–303. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, G.; Timbrell, J.A. In Vitro Cytotoxicity Assays: Comparison of LDH, Neutral Red, MTT and Protein Assay in Hepatoma Cell Lines Following Exposure to Cadmium Chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Nouroozi, R.V.; Noroozi, M.V.; Ahmadizadeh, M. Determination of Protein Concentration Using Bradford Microplate Protein Quantification Assay. Int. Electron. J. Med. 2015, 4, 11–17. [Google Scholar] [CrossRef]
- Dikalov, S.; Griendling, K.K.; Harrison, D.G. Measurement of Reactive Oxygen Species in Cardiovascular Studies. Hypertension 2007, 49, 717–727. [Google Scholar] [CrossRef]
- Geller, B.L.; Winge, D.R. A Method for Distinguishing Cu,Zn- and Mn-Containing Superoxide Dismutases. Anal. Biochem. 1983, 128, 86–92. [Google Scholar] [CrossRef]
- Sykes, J.A.; McCormack, F.X., Jr.; O’Brien, T.J. A Preliminary Study of the Superoxide Dismutase Content of Some Human Tumors1. Cancer Res. 1978, 38, 2759–2762. [Google Scholar]
- Aebi, H. Catalase in Vitro. In Methods in Enzymology; Oxygen Radicals in Biological Systems; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the Quantitative and Qualitative Characterization of Erythrocyte Glutathione Peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Mize, C.E.; Langdon, R.G. Hepatic Glutathione Reductase: I. Purification and general kinetic properties. J. Biol. Chem. 1962, 237, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Maeso, N.; García-Martínez, D.; Rupérez, F.J.; Cifuentes, A.; Barbas, C. Capillary Electrophoresis of Glutathione to Monitor Oxidative Stress and Response to Antioxidant Treatments in an Animal Model. J. Chromatogr. B Biomed. Appl. 2005, 822, 61–69. [Google Scholar] [CrossRef]
- Holmgren, A.; Lu, J. Thioredoxin and Thioredoxin Reductase: Current Research with Special Reference to Human Disease. Biochem. Biophys. Res. Commun. 2010, 396, 120–124. [Google Scholar] [CrossRef]
- Butler, J.E. Enzyme-Linked Immunosorbent Assay. J. Immunoass. 2000, 21, 165–209. [Google Scholar] [CrossRef]
- Tsikas, D.; Rothmann, S.; Schneider, J.Y.; Gutzki, F.-M.; Beckmann, B.; Frölich, J.C. Simultaneous GC-MS/MS Measurement of Malondialdehyde and 4-Hydroxy-2-Nonenal in Human Plasma: Effects of Long-Term L-Arginine Administration. Anal. Biochem. 2017, 524, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Žarković, N.; Jastrząb, A.; Jarocka-Karpowicz, I.; Orehovec, B.; Baršić, B.; Tarle, M.; Kmet, M.; Lukšić, I.; Łuczaj, W.; Skrzydlewska, E. The Impact of Severe COVID-19 on Plasma Antioxidants. Molecules 2022, 27, 5323. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Morgan, P.E.; Davies, M.J. Quantification of Protein Modification by Oxidants. Free Radic. Biol. Med. 2009, 46, 965–988. [Google Scholar] [CrossRef]
- Ling, H.; Jia, X.; Zhang, Y.; Gapter, L.A.; Lim, Y.; Agarwal, R.; Ng, K. Pachymic Acid Inhibits Cell Growth and Modulates Arachidonic Acid Metabolism in Nonsmall Cell Lung Cancer A549 Cells. Mol. Carcinog. 2010, 49, 271–282. [Google Scholar] [CrossRef]
- Petrovic, N.; Murray, M. Using N,N,N’,N’-Tetramethyl-p-Phenylenediamine (TMPD) to Assay Cyclooxygenase Activity In Vitro. In Advanced Protocols in Oxidative Stress II; Armstrong, D., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; pp. 129–140. ISBN 978-1-60761-411-1. [Google Scholar]
- Christie, W. Preparation of Ester Derivatives of Fatty Acids for Chromatographic Analysis. In Advances in Lipid Methodology–Two; Oily Press: Dundee, UK, 1993; pp. 69–111. [Google Scholar]
- Watkins, B.A.; Kim, J.; Kenny, A.; Pedersen, T.L.; Pappan, K.L.; Newman, J.W. Circulating Levels of Endocannabinoids and Oxylipins Altered by Dietary Lipids in Older Women Are Likely Associated with Previously Identified Gene Targets. Biochim. Biophys. Acta 2016, 1861, 1693–1704. [Google Scholar] [CrossRef]
- Luque-Córdoba, D.; Calderón-Santiago, M.; Luque de Castro, M.D.; Priego-Capote, F. Study of Sample Preparation for Determination of Endocannabinoids and Analogous Compounds in Human Serum by LC–MS/MS in MRM Mode. Talanta 2018, 185, 602–610. [Google Scholar] [CrossRef]
- Siegmund, S.V.; Seki, E.; Osawa, Y.; Uchinami, H.; Cravatt, B.F.; Schwabe, R.F. Fatty Acid Amide Hydrolase Determines Anandamide-Induced Cell Death in the Liver. J. Biol. Chem. 2006, 281, 10431–10438. [Google Scholar] [CrossRef]
- Ulloa, N.M.; Deutsch, D.G. Assessment of a Spectrophotometric Assay for Monoacylglycerol Lipase Activity. AAPS J. 2010, 12, 197–201. [Google Scholar] [CrossRef]
- Hnasko, R.; Lin, A.; McGarvey, J.A.; Stanker, L.H. A Rapid Method to Improve Protein Detection by Indirect ELISA. Biochem. Biophys. Res. Commun. 2011, 410, 726–731. [Google Scholar] [CrossRef]
- Ávila-Román, J.; García-Gil, S.; Rodríguez-Luna, A.; Motilva, V.; Talero, E. Anti-Inflammatory and Anticancer Effects of Microalgal Carotenoids. Mar. Drugs 2021, 19, 531. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, A.R.J.; Guilherme-Fernandes, J.; Valente, I.M.; Almeida, A.; Lima, S.A.C.; Fonseca, A.J.M.; Maia, M.R.G. Nutritional Composition and Untargeted Metabolomics Reveal the Potential of Tetradesmus Obliquus, Chlorella Vulgaris and Nannochloropsis oceanica as Valuable Nutrient Sources for Dogs. Animals 2022, 12, 2643. [Google Scholar] [CrossRef]
- Conde, T.; Couto, D.; Melo, T.; Moreira, A.S.P.; Ferreira, P.; Costa, M.; Silva, J.; Neves, B.; Domingues, P.; Domingues, M.R. Production of a Food Grade Extract of Chlorococcum Amblystomatis Rich in Omega-3 Lipids Using Ethanol Assisted with Ultrasound and Deep Characterization by Lipidomics. J. Appl. Phycol. 2022, 34, 3011–3024. [Google Scholar] [CrossRef]
- Couto, D.; Conde, T.A.; Melo, T.; Neves, B.; Costa, M.; Silva, J.; Domingues, R.; Domingues, P. The Chemodiversity of Polar Lipidomes of Microalgae from Different Taxa. Algal Res. 2023, 70, 103006. [Google Scholar] [CrossRef]
- Figueiredo, A.R.P.; da Costa, E.; Silva, J.; Domingues, M.R.; Domingues, P. The Effects of Different Extraction Methods of Lipids from Nannochloropsis oceanica on the Contents of Omega-3 Fatty Acids. Algal Res. 2019, 41, 101556. [Google Scholar] [CrossRef]
- Melo, T.; Figueiredo, A.R.P.; da Costa, E.; Couto, D.; Silva, J.; Domingues, M.R.; Domingues, P. Ethanol Extraction of Polar Lipids from Nannochloropsis oceanica for Food, Feed, and Biotechnology Applications Evaluated Using Lipidomic Approaches. Mar. Drugs 2021, 19, 593. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Skrzydlewska, E. The Role of Transcription Factor Nrf2 in Skin Cells Metabolism. Arch. Dermatol. Res. 2015, 307, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Superoxide Anion Chemistry—Its Role at the Core of the Innate Immunity. Int. J. Mol. Sci. 2023, 24, 1841. [Google Scholar] [CrossRef] [PubMed]
- Jastrząb, A.; Skrzydlewska, E. Thioredoxin-Dependent System. Application of Inhibitors. J. Enzym. Inhib. Med. Chem. 2021, 36, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Cichoński, J.; Chrzanowski, G. Microalgae as a Source of Valuable Phenolic Compounds and Carotenoids. Molecules 2022, 27, 8852. [Google Scholar] [CrossRef] [PubMed]
- Khavari, F.; Asadi, F.; Nouri, F.; Taheri, M.; Mohammadi, F.; Mohammadi, M.; Habibi, P.; Asghari, B. Chapter 10—Marine Antioxidants from Microalgae. In Marine Antioxidants; Kim, S.-K., Shin, K.-H., Venkatesan, J., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 141–160. ISBN 978-0-323-95086-2. [Google Scholar]
- Zhuang, D.; He, N.; Khoo, K.S.; Ng, E.-P.; Chew, K.W.; Ling, T.C. Application Progress of Bioactive Compounds in Microalgae on Pharmaceutical and Cosmetics. Chemosphere 2022, 291, 132932. [Google Scholar] [CrossRef] [PubMed]
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef] [PubMed]
- Raederstorff, D.; Wyss, A.; Calder, P.C.; Weber, P.; Eggersdorfer, M. Vitamin E Function and Requirements in Relation to PUFA. Br. J. Nutr. 2015, 114, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Ritu, J.R.; Ambati, R.R.; Ravishankar, G.A.; Shahjahan, M.; Khan, S. Utilization of Astaxanthin from Microalgae and Carotenoid Rich Algal Biomass as a Feed Supplement in Aquaculture and Poultry Industry: An Overview. J. Appl. Phycol. 2023, 35, 145–171. [Google Scholar] [CrossRef]
- Kanwugu, O.N.; Glukhareva, T.V. Activation of Nrf2 Pathway as a Protective Mechanism against Oxidative Stress-Induced Diseases: Potential of Astaxanthin. Arch. Biochem. Biophys. 2023, 741, 109601. [Google Scholar] [CrossRef]
- Moratilla-Rivera, I.; Sánchez, M.; Valdés-González, J.A.; Gómez-Serranillos, M.P. Natural Products as Modulators of Nrf2 Signaling Pathway in Neuroprotection. Int. J. Mol. Sci. 2023, 24, 3748. [Google Scholar] [CrossRef]
- du Preez, R.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Nannochloropsis oceanica as a Microalgal Food Intervention in Diet-Induced Metabolic Syndrome in Rats. Nutrients 2021, 13, 3991. [Google Scholar] [CrossRef]
- Bisogno, T.; Maccarrone, M. Endocannabinoid Signaling and Its Regulation by Nutrients. BioFactors 2014, 40, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Biernacki, M.; Conde, T.; Stasiewicz, A.; Surażyński, A.; Domingues, M.R.; Domingues, P.; Skrzydlewska, E. Restorative Effect of Microalgae Nannochloropsis oceanica Lipid Extract on Phospholipid Metabolism in Keratinocytes Exposed to UVB Radiation. Int. J. Mol. Sci. 2023, 24, 14323. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A.; Lewandowska-Polak, A.; Moskwa, S.; Kowalski, M.L.; Grzegorczyk, J.Ł. IgE-Mediated 15-Hydroxyeicosatetraenoic Acid (15-HETE) Generation by Peripheral Blood Leukocytes: Its Association with Basophil Activation. Adv. Dermatol. Allergol. 2015, 32, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Jiang, L.; Wang, Y.; Yao, B.; Yang, S.; Zhang, B.; Zhang, M.-Z. 12/15 Lipoxygenase Regulation of Colorectal Tumorigenesis Is Determined by the Relative Tumor Levels of Its Metabolite 12-HETE and 13-HODE in Animal Models. Oncotarget 2014, 6, 2879–2888. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.G.; Chávez, C.L.; Zhang, C.; Sowden, M.; Yan, C.; Berk, B.C. The Lipid Peroxidation Product 4-Hydroxynonenal Inhibits NLRP3 Inflammasome Activation and Macrophage Pyroptosis. Cell Death Differ. 2022, 29, 1790–1803. [Google Scholar] [CrossRef] [PubMed]
- Milkovic, L.; Zarkovic, N.; Marusic, Z.; Zarkovic, K.; Jaganjac, M. The 4-Hydroxynonenal–Protein Adducts and Their Biological Relevance: Are Some Proteins Preferred Targets? Antioxidants 2023, 12, 856. [Google Scholar] [CrossRef] [PubMed]
- Jaganjac, M.; Milkovic, L.; Gegotek, A.; Cindric, M.; Zarkovic, K.; Skrzydlewska, E.; Zarkovic, N. The Relevance of Pathophysiological Alterations in Redox Signaling of 4-Hydroxynonenal for Pharmacological Therapies of Major Stress-Associated Diseases. Free Radic. Biol. Med. 2020, 157, 128–153. [Google Scholar] [CrossRef] [PubMed]
- Dias, I.H.K.; Milic, I.; Heiss, C.; Ademowo, O.S.; Polidori, M.C.; Devitt, A.; Griffiths, H.R. Inflammation, Lipid (Per)Oxidation, and Redox Regulation. Antioxid. Redox Signal. 2020, 33, 166–190. [Google Scholar] [CrossRef]
- Ivanov, I.V.; Mappes, T.; Schaupp, P.; Lappe, C.; Wahl, S. Ultraviolet Radiation Oxidative Stress Affects Eye Health. J. Biophotonics 2018, 11, e201700377. [Google Scholar] [CrossRef]
- Raone, B.; Patrizi, A.; Gurioli, C.; Gazzola, A.; Ravaioli, G.M. Cutaneous Carcinogenic Risk Evaluation in 375 Patients Treated with Narrowband-UVB Phototherapy. Photodermatol. Photoimmunol. Photomed. 2018, 34, 302–306. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, Oxidative Stress and Autophagy in Skin Aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Wang, F.; Huang, L.; Gao, B.; Zhang, C. Optimum Production Conditions, Purification, Identification, and Antioxidant Activity of Violaxanthin from Microalga Eustigmatos cf. polyphem (Eustigmatophyceae). Mar. Drugs 2018, 16, 190. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Xie, B.; Ma, S.; Zhu, X.; Fan, G.; Pan, S. Evaluation of Antioxidant Activities of Principal Carotenoids Available in Water Spinach (Ipomoea aquatica). J. Food Compost. Anal. 2011, 24, 288–297. [Google Scholar] [CrossRef]
- Lee, J.-J.; An, S.; Kim, K.B.; Heo, J.; Cho, D.-H.; Oh, H.-M.; Kim, H.-S.; Bae, S. Extract of Ettlia Sp. YC001 Exerts Photoprotective Effects against UVB Irradiation in Normal Human Dermal Fibroblasts. J. Microbiol. Biotechnol. 2016, 26, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Atalay Ekiner, S.; Gęgotek, A.; Skrzydlewska, E. The Molecular Activity of Cannabidiol in the Regulation of Nrf2 System Interacting with NF-ΚB Pathway under Oxidative Stress. Redox Biol. 2022, 57, 102489. [Google Scholar] [CrossRef] [PubMed]
- Ryšavá, A.; Vostálová, J.; Rajnochová Svobodová, A. Effect of Ultraviolet Radiation on the Nrf2 Signaling Pathway in Skin Cells. Int. J. Radiat. Biol. 2021, 97, 1383–1403. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.P.; Rodrigo, M.J.; Zacarias, L. Dietary Carotenoid Roles in Redox Homeostasis and Human Health. J. Agric. Food Chem. 2018, 66, 5733–5740. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Ferruzzi, M.G. Update on the Bioavailability and Chemopreventative Mechanisms of Dietary Chlorophyll Derivatives. Nutr. Res. 2020, 81, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Al-Kandari, N.; Fadel, F.; Al-Saleh, F.; Khashab, F.; Al-Maghrebi, M. The Thioredoxin System Is Regulated by the ASK-1/JNK/P38/Survivin Pathway During Germ Cell Apoptosis. Molecules 2019, 24, 3333. [Google Scholar] [CrossRef]
- Hasan, A.A.; Kalinina, E.; Tatarskiy, V.; Shtil, A. The Thioredoxin System of Mammalian Cells and Its Modulators. Biomedicines 2022, 10, 1757. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, M.; Podolski-Renić, A.; Krasavin, M.; Pešić, M. The Role of the Thioredoxin Detoxification System in Cancer Progression and Resistance. Front. Mol. Biosci. 2022, 9, 883297. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Zou, L.; Peana, M.; Chasapis, C.T.; Hangan, T.; Lu, J.; Maes, M. The Role of the Thioredoxin System in Brain Diseases. Antioxidants 2022, 11, 2161. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, C.; Chakraborty, S.; Sengupta, R. NO News: S-(de)Nitrosylation of Cathepsins and Their Relationship with Cancer. Anal. Biochem. 2022, 655, 114872. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.G.; Zhu, J.-H.; Cheng, W.-H.; Bao, Y.; Ho, Y.-S.; Reddi, A.R.; Holmgren, A.; Arnér, E.S.J. Paradoxical Roles of Antioxidant Enzymes: Basic Mechanisms and Health Implications. Physiol. Rev. 2016, 96, 307–364. [Google Scholar] [CrossRef]
- Schwenk, R.W.; Holloway, G.P.; Luiken, J.J.F.P.; Bonen, A.; Glatz, J.F.C. Fatty Acid Transport across the Cell Membrane: Regulation by Fatty Acid Transporters. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 149–154. [Google Scholar] [CrossRef]
- Ventura, R.; Martínez-Ruiz, I.; Hernández-Alvarez, M.I. Phospholipid Membrane Transport and Associated Diseases. Biomedicines 2022, 10, 1201. [Google Scholar] [CrossRef]
- Paterson, S.; Gómez-Cortés, P.; de la Fuente, M.A.; Hernández-Ledesma, B. Bioactivity and Digestibility of Microalgae Tetraselmis Sp. and Nannochloropsis Sp. as Basis of Their Potential as Novel Functional Foods. Nutrients 2023, 15, 477. [Google Scholar] [CrossRef]
- Hayward, S.; Cilliers, T.; Swart, P. Lipoxygenases: From Isolation to Application. Compr. Rev. Food Sci. Food Saf. 2017, 16, 199–211. [Google Scholar] [CrossRef]
- Sá, M.; Ferrer-Ledo, N.; Wijffels, R.; Crespo, J.G.; Barbosa, M.; Galinha, C.F. Monitoring of Eicosapentaenoic Acid (EPA) Production in the Microalgae Nannochloropsis oceanica. Algal Res. 2020, 45, 101766. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Rhodes, L.E.; Al-Aasswad, N.M.I.; Massey, K.A.; Nicolaou, A. Impact of EPA Ingestion on COX- and LOX-Mediated Eicosanoid Synthesis in Skin with and without a pro-Inflammatory UVR Challenge--Report of a Randomised Controlled Study in Humans. Mol. Nutr. Food Res. 2014, 58, 580–590. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Kim, H. Lutein as a Modulator of Oxidative Stress-Mediated Inflammatory Diseases. Antioxidants 2021, 10, 1448. [Google Scholar] [CrossRef] [PubMed]
- Padmanabha, S.; Vallikannan, B. Fatty Acids Modulate the Efficacy of Lutein in Cataract Prevention: Assessment of Oxidative and Inflammatory Parameters in Rats. Biochem. Biophys. Res. Commun. 2018, 500, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, H.L.; Li, Y.; Hannon, K.; Watkins, B.A. Eicosapentaenoic Acid Decreases Expression of Anandamide Synthesis Enzyme and Cannabinoid Receptor 2 in Osteoblast-like Cells. J. Nutr. Biochem. 2011, 22, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Omega-6 and Omega-3 Fatty Acids: Endocannabinoids, Genetics and Obesity. OCL 2020, 27, 7. [Google Scholar] [CrossRef]
- Di Marzo, V.; Bisogno, T.; De Petrocellis, L. The Biosynthesis, Fate and Pharmacological Properties of Endocannabinoids. In Cannabinoids; Pertwee, R.G., Ed.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2005; pp. 147–185. ISBN 978-3-540-26573-3. [Google Scholar]
- Piscitelli, F.; Di Marzo, V. Cannabinoids: A Class of Unique Natural Products with Unique Pharmacology. Rend. Lincei. Sci. Fis. Nat. 2021, 32, 5–15. [Google Scholar] [CrossRef]
- Aghazadeh Tabrizi, M.; Baraldi, P.G.; Borea, P.A.; Varani, K. Medicinal Chemistry, Pharmacology, and Potential Therapeutic Benefits of Cannabinoid CB2 Receptor Agonists. Chem. Rev. 2016, 116, 519–560. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.J. The Endocannabinoid System–Current Implications for Drug Development. J. Intern. Med. 2021, 290, 2–26. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Endocannabinoids and Their Pharmacological Actions. In Endocannabinoids; Handbook of Experimental Pharmacology Series; Pertwee, R.G., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–37. ISBN 978-3-319-20825-1. [Google Scholar]
- Gallelli, C.A.; Calcagnini, S.; Romano, A.; Koczwara, J.B.; de Ceglia, M.; Dante, D.; Villani, R.; Giudetti, A.M.; Cassano, T.; Gaetani, S. Modulation of the Oxidative Stress and Lipid Peroxidation by Endocannabinoids and Their Lipid Analogues. Antioxidants 2018, 7, 93. [Google Scholar] [CrossRef]
- Lipina, C.; Hundal, H.S. Modulation of Cellular Redox Homeostasis by the Endocannabinoid System. Open Biol. 2016, 6, 150276. [Google Scholar] [CrossRef]
- Muzio, G.; Barrera, G.; Pizzimenti, S. Peroxisome Proliferator-Activated Receptors (PPARs) and Oxidative Stress in Physiological Conditions and in Cancer. Antioxidants 2021, 10, 1734. [Google Scholar] [CrossRef] [PubMed]
- Subramoniam, A.; Asha, V.V.; Nair, S.A.; Sasidharan, S.P.; Sureshkumar, P.K.; Rajendran, K.N.; Karunagaran, D.; Ramalingam, K. Chlorophyll Revisited: Anti-Inflammatory Activities of Chlorophyll a and Inhibition of Expression of TNF-α Gene by the Same. Inflammation 2012, 35, 959–966. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasiewicz, A.; Conde, T.; Domingues, M.d.R.; Domingues, P.; Biernacki, M.; Skrzydlewska, E. Comparison of the Regenerative Metabolic Efficiency of Lipid Extracts from Microalgae Nannochloropsis oceanica and Chlorococcum amblystomatis on Fibroblasts. Antioxidants 2024, 13, 276. https://doi.org/10.3390/antiox13030276
Stasiewicz A, Conde T, Domingues MdR, Domingues P, Biernacki M, Skrzydlewska E. Comparison of the Regenerative Metabolic Efficiency of Lipid Extracts from Microalgae Nannochloropsis oceanica and Chlorococcum amblystomatis on Fibroblasts. Antioxidants. 2024; 13(3):276. https://doi.org/10.3390/antiox13030276
Chicago/Turabian StyleStasiewicz, Anna, Tiago Conde, Maria do Rosario Domingues, Pedro Domingues, Michał Biernacki, and Elżbieta Skrzydlewska. 2024. "Comparison of the Regenerative Metabolic Efficiency of Lipid Extracts from Microalgae Nannochloropsis oceanica and Chlorococcum amblystomatis on Fibroblasts" Antioxidants 13, no. 3: 276. https://doi.org/10.3390/antiox13030276
APA StyleStasiewicz, A., Conde, T., Domingues, M. d. R., Domingues, P., Biernacki, M., & Skrzydlewska, E. (2024). Comparison of the Regenerative Metabolic Efficiency of Lipid Extracts from Microalgae Nannochloropsis oceanica and Chlorococcum amblystomatis on Fibroblasts. Antioxidants, 13(3), 276. https://doi.org/10.3390/antiox13030276







