Evaluation of the Shelf life of Ready-to-Eat Fresh Bamboo Sprouts (Phyllostachys edulis) Packaged in a Modified Atmosphere or Vacuum: A Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials
2.3. Sample Preparation and Experimental Design
- -
- polypropylene (PP) heat-sealed trays sealed with a polyamide/polyethylene (PA/PE) film. Inside MAP1, there was the following mixture of gases: 2% O2, 5% CO2, 93% N2.
- -
- polypropylene (PP) heat-sealed trays sealed with a polyamide/polyethylene (PA/PE) film. The following gas mixture was inserted in this group 3% O2, 7% CO2, 90% N2 (MAP2).
- -
- vacuum packed in food-grade multi-layer polythene (PE) bags measuring 350 × 180 × 150 mm (OLPACK 510, Interprise Breussel S.A., Belgium).
2.4. Physical Quality Parameter Analysis
2.5. Evolution of Color Parameters during Storage
2.6. Firmness Analysis
2.7. Headspace Composition
2.8. Microbiological Analysis
2.9. Evaluation of Organic Acids during Storage
2.10. Extraction Procedure
2.11. Total Phenol, Flavonoid, and Carotenoid Contents and UHPLC Analysis of Fresh Sprout
Bamboo Sprouts Bioactive Compounds Evolution during Storage
2.12. Bamboo Sprouts Antioxidant Activity
2.13. Statistical Analysis
3. Results and Discussion
3.1. Evolution of Fresh Bamboo Sprouts Quality Parameters during Storage
3.2. Effect of Storage on Packaged Bamboo Sprouts CIELab Parameters
3.3. Microbiological Determination
3.4. Analysis of Selected Organic Acids
3.5. Evolution of Bioactive Compounds (TPC, TFC, and TCC) in Bamboo Sprouts during Storage
3.6. Degradation Kinetics of TPC, TFC, and TCC during Storage
3.7. UHPLC Analysis of Fresh Bamboo Sprouts
3.8. Investigation on the Evolution of Bamboo Sprouts Antioxidant Activity during Storage
3.9. Principal Component Analysis (PCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Acharya, B.; Behera, A.; Sahu, P.K.; Dilnawaz, F.; Behera, S.; Chowdhury, B.; Mishra, D.P. Bamboo shoots: An exploration into its culinary heritage in India and its nutraceutical potential. J. Ethn. Food 2023, 10, 2–15. [Google Scholar] [CrossRef]
- Choudhury, D.; Sahu, J.K.; Sharma, G.D. Value addition to bamboo shoots: A review. J. Food Sci. Technol. 2012, 49, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Satya, S.; Bal, L.M.; Singhal, P.; Naik, S.N. Bamboo shoot processing: Food quality and safety aspect (a review). Trends Food Sci. Technol. 2010, 21, 181–189. [Google Scholar] [CrossRef]
- Rawat, K.; Kumari, A.; Kumar, S.; Kumar, R.; Gehlot, R. Traditional fermented products of India. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1873–1883. [Google Scholar] [CrossRef]
- Chongtham, N.; Bisht, M.S.; Haorongbam, S. Nutritional properties of bamboo shoots: Potential and prospects for utilization as a health food. Compr. Rev. Food Sci. 2011, 10, 153–168. [Google Scholar] [CrossRef]
- Kumar, P.S.; Kumari, U.; Devi, M.; Choudhary, V.K.; Sangeetha, A. Bamboo shoot as a source of nutraceuticals and bioactive compounds: A review. Indian J. Nat. Prod. Resour. 2017, 8, 32–46. [Google Scholar] [CrossRef]
- Bajwa, H.K.; Santosh, O.; Nirmala, C. Bamboo shoot for food and nutritional security. J. Pharm. Phytochem. 2021, 10, 24–30. [Google Scholar]
- Nongdam, P.; Tikendra, L. The Nutritional Facts of Bamboo Shoots and Their Usage as Important Traditional Foods of Northeast India. Int. Sch. Res. Notices. 2014, 2014, 679073. [Google Scholar] [CrossRef]
- Chongtham, N.; Bisht, M.S. Bamboo Shoot: Superfood for Nutrition, Health, and Medicine; CRC Press: Boca Raton, FL, USA, 2020; pp. 1–201. [Google Scholar]
- Kleinhenz, V.; Gosbee, M.; Elsmore, S.; Lyall, T.W.; Blackburn, K.; Harrower, K.; Midmore, D.J. Storage methods for extending shelf life of fresh, edible bamboo shoots [Bambusa oldhamii (Munro)]. Postharvest Biol. Technol. 2000, 19, 253–271. [Google Scholar] [CrossRef]
- Nirmala, C.; Bisht, M.S.; Laishram, M. Bioactive compounds in bamboo shoots: Health benefits and prospects for developing functional foods. Int. J. Food Sci. Nutr. 2014, 49, 1425–1431. [Google Scholar] [CrossRef]
- Zeng, F.; Luo, Z.; Xie, J.; Feng, S. Gamma Radiation Control Quality and Lignification of Bamboo Shoots (Phyllostachys Praecox f. prevernalis.) Stored at Low Temperature. Postharvest Biol. Technol. 2015, 102, 17–24. [Google Scholar] [CrossRef]
- Chen, H.; Ling, J.; Wu, F.; Zhang, L.; Sun, Z.; Yang, H. Effect of hypobaric storage on flesh lignification, active oxygen metabolism and related enzyme activities in bamboo shoots. LWT-Food Sci. Technol. 2013, 51, 190–195. [Google Scholar] [CrossRef]
- Oliveira, M.; Abadias, M.; Usall, J.; Torres, R.; Teixidó, N.; Viñas, I. Application of modified atmosphere packaging as a safety approach to fresh-cut fruits and vegetables—A review. Trends Food Sci. Technol. 2015, 46, 13–26. [Google Scholar] [CrossRef]
- Fang, Y.; Wakisaka, M. A Review on the modified atmosphere preservation of fruits and vegetables with cutting-edge technologies. Agriculture 2021, 11, 992. [Google Scholar] [CrossRef]
- Ahvenainen, R. New approaches in improving the shelf life of minimally processed fruit and vegetables. Trends Food Sci. Technol. 1996, 7, 179–187. [Google Scholar] [CrossRef]
- Gorny, J.R. A summary of CA and MA requirements and recommendation for fresh-cut (minimally processed) fruits and vegetables. In Fresh-Cut Fruits and Vegetables and MAP; Gorny, J.R., Ed.; International Society for Horticultural Science: Davis, CA, USA, 1997; Volume 5, pp. 30–67. [Google Scholar]
- Varoquaux, P.; Mazollier, J.; Albagnac, G. The influence of raw material characteristics on the storage life of fresh-cut butterhead lettuce. Postharvest Biol. Technol. 1996, 9, 127–139. [Google Scholar] [CrossRef]
- Perdue, R. Vacuum Packaging. In Encyclopaedia of Packaging Technology, 3rd ed.; Yam, K.L., Ed.; John Wiley & Sons, Inc., Publication: Hoboken, NJ, USA, 2010; pp. 1–1366. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Arlington, TX, USA, 2005. [Google Scholar]
- Sicari, V.; Romeo, R.; Leporini, M.; Pellicanò, T.M.; Tundis, R.; Loizzo, M.R. Comparison of traditional hot water and vacuum assisted blanching methods on the physico-chemical quality parameters and antioxidant activity of zucchini (Cucurbita pepo L.) slices. Food Meas. 2022, 16, 281–294. [Google Scholar] [CrossRef]
- Mokrzycki, W.; Tatol, M. Color diference Delta E: A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- De Bruno, A.; Gattuso, A.; Ritorto, D.; Piscopo, A.; Poiana, M. Effect of edible coating enriched with natural antioxidant extract and bergamot essential oil on the shelf life of strawberries. Foods 2023, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, A.; Zappia, A.; Princi, M.P.; De Bruno, A.; Araniti, F.; Lupini, A.; Abenavoli, M.R.; Poiana, M. Quality of shredded carrots minimally processed by different dipping solutions. J. Food Sci. Technol. 2019, 56, 2584–2593. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, C.; Sun, J.; Xin, M.; Yi, P.; He, X.; Sheng, J.; Zhou, Z.; Ling, D.; Zheng, F.; et al. Synergistic effects of ultraviolet light irradiation and high-oxygen modified atmosphere packaging on physiological quality, microbial growth and lignification metabolism of fresh-cut carrots. Post. Biol. Technol. 2021, 173, 111365. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Zappia, C.; Capocasale, M.; Poiana, M.; Sidari, R.; Di Donna, L.; Bartella, L.; Sindona, G.; Corradini, G.; Giudici, P.; et al. Vinegar production to valorise Citrus bergamia by-products. Eur. Food Res. Technol. 2019, 245, 667–675. [Google Scholar] [CrossRef]
- Tundis, R.; Augimeri, G.; Vivacqua, A.; Romeo, R.; Sicari, V.; Bonofiglio, D.; Loizzo, M.R. Anti-inflammatory and antioxidant effects of leaves and sheath from bamboo (Phyllostacys edulis J. Houz). Antioxidants 2023, 12, 1239. [Google Scholar] [CrossRef]
- Carvalho Tavares, I.M.; Rocchetti Sumere, B.; Gómez-Alonso, S.; Gomes, E.; Hermosín-Gutiérrez, I.; Da-Silva, R.; Silva Lago-Vanzela, E. Storage stability of the phenolic compounds, color and antioxidant activity of jambolan juice powder obtained by foam mat drying. Food Res. Int. 2020, 128, 108750. [Google Scholar] [CrossRef]
- Caleb, O.J.; Mahajan, P.V.; Al-Said, F.A.; Opara, U.L. Modified atmosphere packaging technology of fresh and fresh-cut produce and the microbial consequences—A review. Food Bioproc Technol. 2013, 6, 303–329. [Google Scholar] [CrossRef] [PubMed]
- Reche, J.; García-Pastor, M.E.; Valero, D.; Hernández, F.; Almansa, M.S.; Legua, P.; Amorós, A. Effect of modified atmosphere packaging on the physiological and functional characteristics of Spanish jujube (Ziziphus jujuba Mill.) cv ‘Phoenix’ during cold storage. Sci. Hortic. 2019, 258, 108743. [Google Scholar] [CrossRef]
- Moradinezhad, F.; Khayyat, M.; Ranjbari, F.; Maraki, Z. Physiological and quality responses of Shishe-Kab pomegranates to short-term high CO2 treatment and modified atmosphere packaging. Int. J. Fruit Sci. 2018, 18, 287–299. [Google Scholar] [CrossRef]
- Li, X.; Xing, X.; Shui, Y.; Cao, X.; Xu, R.; Xu, Q.; Bi, X.; Liu, X. Quality of bamboo shoots during storage as affected by high hydrostatic pressure processing. Int. J. Food Prop. 2021, 24, 656–676. [Google Scholar] [CrossRef]
- Xu, X.L.; Yan, B.F. Accumulation of lignin and involvement of enzymes in bamboo shoot during storage. Eur. Food Res. Technol. 2008, 226, 635–640. [Google Scholar] [CrossRef]
- Pongprasert, N.; Wongs-Aree, C.; Srilaong, V.; Kanlayanarat, S. Alleviation of browning and lignification in minimally processed sweet bamboo (Dendrocalamus asper) shoots by packaging. N. Z. J. Crop Hort. Sci. 2007, 35, 253–257. [Google Scholar] [CrossRef]
- Changchai, S.; Sumrit, T.; Kitpot, T.; Inrirai, P. Impact of storage condition on postharvest preservation of fresh Bambusa sp. Shoot. Food Res. 2020, 4, 57–63. [Google Scholar] [CrossRef]
- Song, L.; Chen, H.; Gao, H.; Fang, X.; Yuan, Y.; Yang, Q.; Jiang, Y. Combined modified atmosphere packaging and low temperature storage delay lignification and improve the defence response of minimally processed water bamboo shoot. Chem. Cen. J. 2013, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Nugrahedi, P.Y.; Lusiani, M.; Persijin, S.T. Effect of Modified Atmosphere Packing on Selected Phenomena Affecting Quality of Fresh, Edible Bamboo Shoots. Indones. Food Nutr. Prog. 2004, 11, 20–24. [Google Scholar]
- Luo, Z.S.; Xu, X.L.; Cai, Z.Z.; Yan, B.F. Effects of Ethylene and 1-methylcyclopropene (1-MCP) on lignification of postharvest bamboo shoot. Food Chem. 2007, 105, 521–527. [Google Scholar] [CrossRef]
- Beaudry, R.M. Effect of O2 and CO2 partial pressure on selected phenomena affecting fruit and vegetable quality. Postharvest Biol. Technol. 1999, 15, 293–303. [Google Scholar] [CrossRef]
- Shen, Q.; Kong, F.; Wang, Q. Effect of modified atmosphere packing on the browning and lignification of bamboo shoots. J. Food Eng. 2006, 77, 348–354. [Google Scholar] [CrossRef]
- Liu, R.; Wang, H.; Yang, H.; Zhang, H.; Chen, J.; Gao, H.; Chen, H. Effect of ozone treatment on lignification and postharvest quality of water bamboo shoots. eFood 2022, 3, e24. [Google Scholar] [CrossRef]
- Chen, C.; Jing, L.; Guiguang, C.; Yaping, L.; Yongxin, Y.; Dongjie, C.; Xuejiao, W.; Jianxin, C. Flavor changes and microbial evolution in fermentation liquid of sour bamboo shoots. J. Food Compost. Anal. 2023, 120, 105273. [Google Scholar] [CrossRef]
- Badwaik, L.S.; Borah, P.K.; Borah, K.; Das, A.J.; Deka, S.C.; Sharma, H.K. Influence of fermentation on nutritional compositions, antioxidant activity, total phenolic and microbial load of bamboo shoot. Food Sci. Technol. Res. 2014, 20, 255–262. [Google Scholar] [CrossRef]
- Badwaik, L.S.; Borah, P.K.; Deka, S.C. Production and purification of anti-bacterial biometabolite from wild-type Lactobacillus, isolated from fermented bamboo shoot: Future suggestions and a proposed system for secondary metabolite onsite recovery during continuous fermentation. Appl. Biochem. Biotechnol. 2015, 175, 1915–1925. [Google Scholar] [CrossRef]
- Saini, N.; Nirmala, C.; Bisht, M.S. Bamboo resource of Himachal Pradesh (India) and potential of shoots in socio-economic development of the state. In Proceedings of the 10th World Bamboo Congress: Community and Economic Development, Damyang, Republic of Korea, 17–22 September 2015. [Google Scholar]
- Lu, S.M.; Xu, Y.G. Physiological and biochemical changes of fresh-cut bamboo shoot (Phyllostachys heterocycla var pubescens) during cold storage. J. Sci. Food Agric. 2004, 84, 772–776. [Google Scholar] [CrossRef]
- Bhardwaj, D.R.; Rana, S.; Kumar, D.; Sharma, P. Nutritive value of tender shoots of different bamboo species in relation to harvesting height in mid-hills of north-western Himalayas. Appl. Food Res. 2023, 3, 100244. [Google Scholar] [CrossRef]
- Pandey, A.K.; Ojha, V. Standardization of harvesting age of bamboo shoots with respect to nutritional and anti-nutritional components. J. For. Res. 2013, 24, 83–90. [Google Scholar] [CrossRef]
- Kozukue, E.; Kozukue, N.; Tsuchida, H. Identification and changes of phenolic compounds in bamboo [Gramineae] shoots during storage at 20 degrees centigrade. J. Jpn. Soc. Hortic. Sci. 1998, 67, 805–811. [Google Scholar] [CrossRef][Green Version]
- Yang, H.; Zhou, C.; Wu, F.; Cheng, J. Effect of nitric oxide on browning and lignification of peeled bamboo shoots. Postharvest Biol. Technol. 2010, 57, 72–76. [Google Scholar] [CrossRef]
- Rana, S.; Siddiqui, S.; Gandhi, K. Effect of individual vacuum and modified atmosphere packaging on shelf life of guava. Int. J. Chem. Stud. 2018, 6, 966–972. [Google Scholar]
- Razak, N.A.; Hamid, N.A.; Shaari, A.R. Effect of vacuum and non-vacuum packaging on total phenolic content of encapsulated orthosiphon stamineus spray-dried powder during storage. Solid State Phenom. 2018, 280, 330–334. [Google Scholar] [CrossRef]
- Bajwa, H.K.; Santosh, O.; Chongtham, N. Bioactive Compounds in Bamboo Shoot. In Bioactive Compounds in Underutilized Vegetables and Legumes; Reference Series in, Phytochemistry; Murthy, H.N., Paek, K.Y., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Park, E.J.; Jhon, D.Y. The antioxidant, angiotensin converting enzyme inhibition activity, and phenolic compounds of bamboo shoot extracts. LWT 2010, 43, 655–659. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Deng, Z.; Li, H.; Yu, Y.; Zhang, B. Comparison of free, conjugated, and insoluble-bound phenolics and their antioxidant activities in oven-drying and freeze-drying bamboo (Phyllostachys edulis) shoot tips. J. Food Sci. 2021, 86, 4223–4243. [Google Scholar] [CrossRef]
- Sonam, K.S.; Guleria, S. Synergistic antioxidant activity of natural products. Ann. Pharmacol. Pharm. 2017, 2, 1086–1092. [Google Scholar]
- Tanongkankit, Y.; Chiewchan, N.; Devahastin, S. Evolution of antioxidants in dietary fiber powder produced from white cabbage outer leaves: Effects of blanching and drying methods. Food Sci. Technol. 2015, 52, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Rawat, K.; Sharma, V.; Saini, N.; Nirmala, C.; Bisht, M.S. Impact of different boiling and soaking treatments on the release and retention of antinutrients and nutrients from the edible shoots of three bamboo species. Am. J. Food Sci. Nutr. Res. 2016, 3, 31–41. [Google Scholar]
- Bajwa, H.K.; Nirmala, C.; Koul, A.; Bisht, M.S. Changes in organoleptic, physicochemical and nutritional qualities of shoots of an edible bamboo Dendrocalamus hamiltonii (Roxb.) Nees during processing. J. Food Process. Pres. 2016, 4, 1309–1317. [Google Scholar] [CrossRef]
- Santosh, O.; Bajwa, H.K.; Bisht, M.S.; Chongtham, N. Antioxidant activity and sensory evaluation of crispy salted snacks fortified with bamboo shoot rich in bioactive compounds. Appl. Food Res. 2021, 1, 100018–100027. [Google Scholar] [CrossRef]
- Iwansyah, A.C.; Kumalasari, R.; Darmajana, D.A.; Ratnawati, L. Antioxidant properties and toxicity of selected bamboo shoots “iwung” extract: A comparative study. IOP Conf. Ser. Earth Environ. Sci. 2019, 251, 012017. [Google Scholar] [CrossRef]
- Milani, G.; Curci, F.; Cavalluzzi, M.M.; Crupi, P.; Pisano, I.; Lentini, G.; Clodoveo, M.L.; Franchini, C.; Corbo, F. Optimization of microwave-assisted extraction of antioxidants from bamboo shoots of Phyllostachys pubescens. Molecules 2020, 25, 215. [Google Scholar] [CrossRef]
- D’Agostino, M.F.; Sanz, J.; Martínez-Castro, I.; Giuffré, A.M.; Sicari, V.; Soria, A.C. Statistical analysis for improving data precision in the spme GC-MS analysis of blackberry (Rubus ulmifolius) volatiles. Talanta 2014, 125, 249–256. [Google Scholar] [CrossRef]
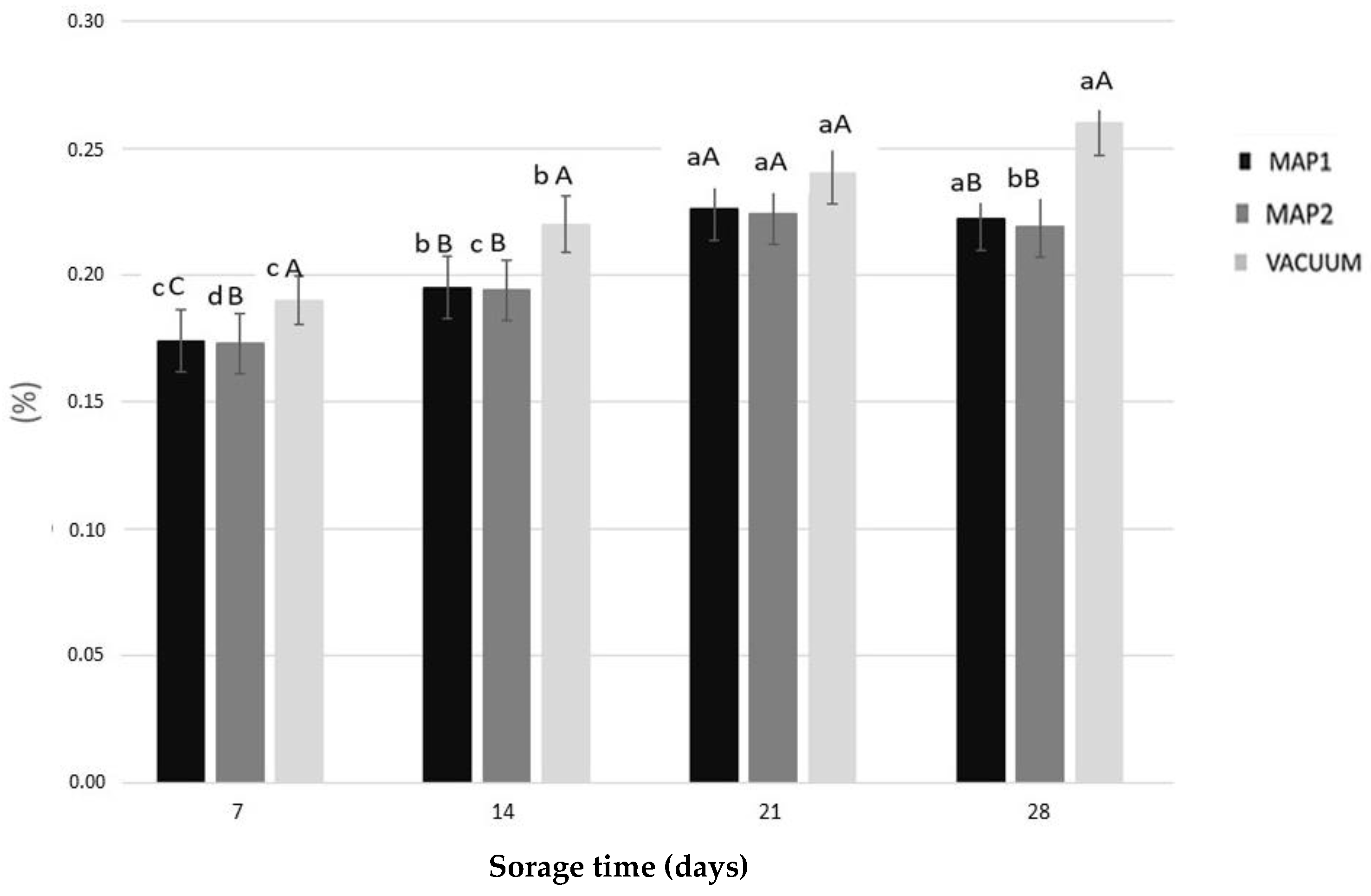
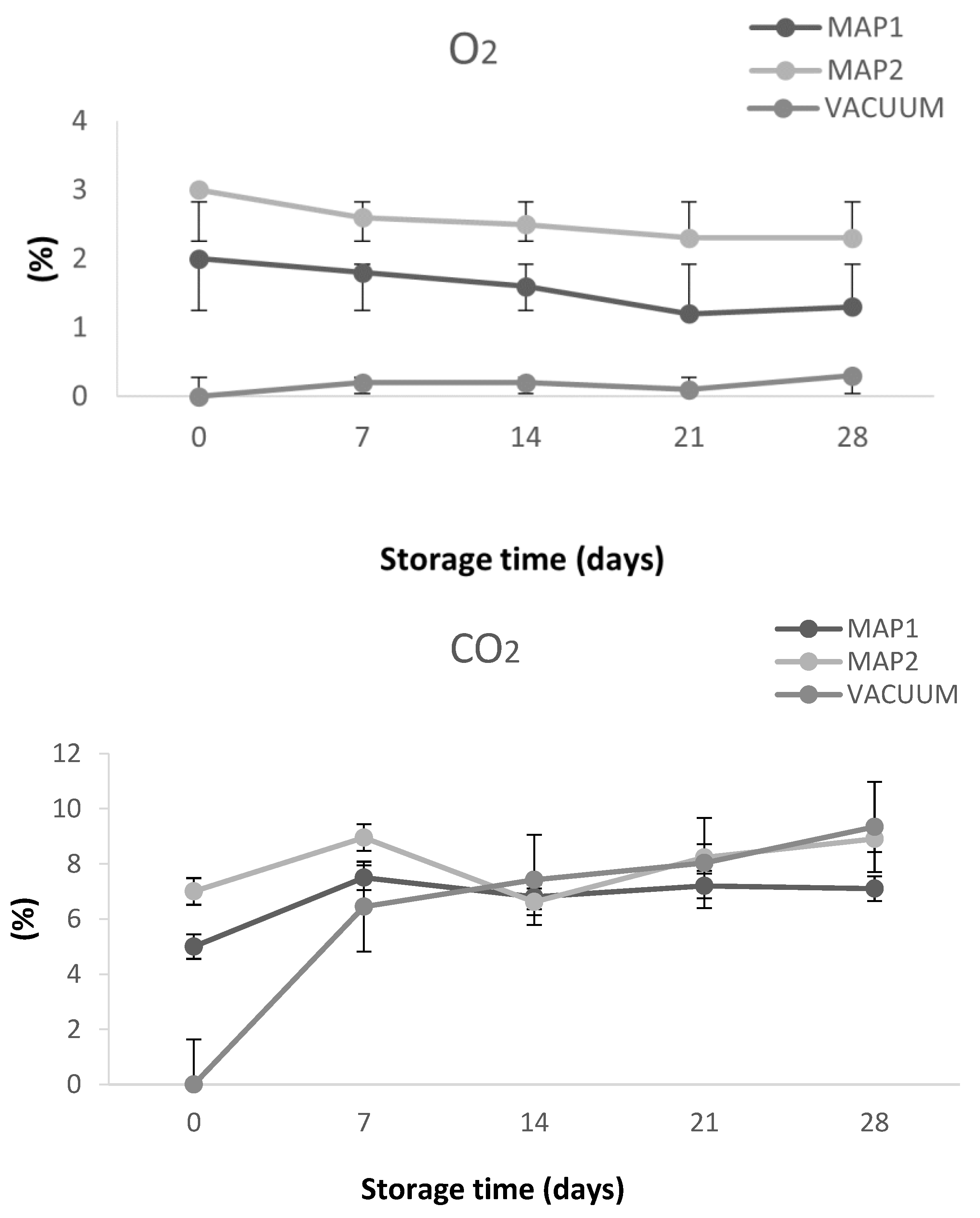
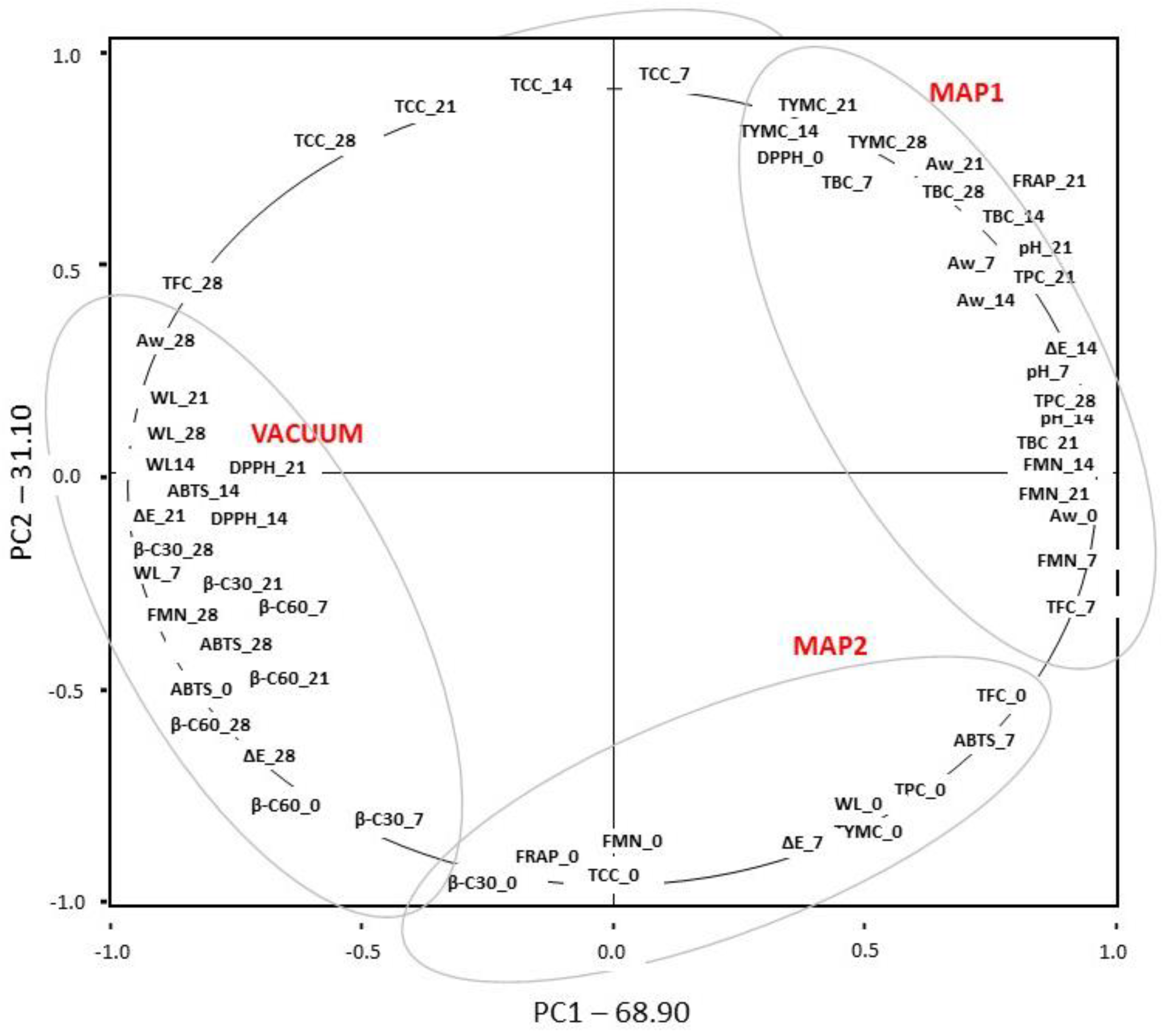
| Storage Time (Days) | MAP1 | MAP2 | VACUUM | |
|---|---|---|---|---|
| Apical section | Sign. | |||
| 0 | 8.68 ± 0.87 aA | 8.68 ± 0.87 aA | 8.68 ± 0.87 aA | ns |
| 7 | 9.79 ± 1.44 bA | 9.62 ± 1.67 bA | 8.89 ± 0.67 bB | ** |
| 14 | 10.16 ± 2.43 cA | 10.16 ± 1.33 cA | 9.22 ± 0.89 cB | ** |
| 21 | 10.82 ± 0.88 dA | 10.8 ± 1.08 bA | 9.60 ± 0.78 dB | ** |
| 28 | 16.72 ± 1.22 eA | 16.79 ± 1.78 eA | 16.72 ± 1.43 eA | ** |
| Sign. | ** | ** | ** | |
| Basal section | Sign. | |||
| 0 | 8.53 ± 0.56 aA | 8.53 ± 0.56 aA | 8.53 ± 0.56 eA | ns |
| 7 | 10.71 ± 1.67 bA | 10.71 ± 2.02 bA | 8.71 ± 1.78 bB | ** |
| 14 | 11.99 ± 0.99 bA | 11.99 ± 1.41 bA | 8.98 ± 1.45 cB | ** |
| 21 | 12.19 ± 2.31 cA | 12.09 ± 1.78 cA | 10.14 ± 1.69 dB | ** |
| 28 | 29.03 ± 1.56 dA | 29.03 ± 2.67 dA | 16.43 ± 0.435 dB | ** |
| Sign. | ** | ** | ** | |
| Storage Time (Days) | MAP1 | MAP2 | VACUUM | Sign. |
|---|---|---|---|---|
| Apical section | ||||
| 0 | 59.22 ± 3.89 aA | 59.22 ± 2.78 aA | 20.62 ± 2.08 bB | ** |
| 7 | 52.29 ± 4.76 cA | 51.39 ± 4.02 cB | 20.63 ± 2.03 abC | ** |
| 14 | 51.02 ± 3.66 dA | 51.01 ± 3.61 dA | 20.62 ± 2.89 bB | ** |
| 21 | 47.31 ± 3.67 eA | 47.12 ± 4.09 eB | 20.63 ± 2.99 bC | ** |
| 28 | 53.82 ± 5.01 bA | 52.84 ± 4.67 bB | 20.65 ± 3.09 aC | ** |
| Sign. | ** | ** | * | |
| Basal section | Sign. | |||
| 0 | 59.72 ± 4.87 aA | 59.72 ± 4.74 aA | 20.65 ± 2.78 aB | ** |
| 7 | 42.12 ± 3.81 eA | 41.12 ± 4.04 eB | 20.66 ± 1.98 aC | ** |
| 14 | 54.82 ± 2.89 bA | 53.82 ± 4.06 bB | 20.64 ± 2.84 aC | ** |
| 21 | 50.12 ± 2.88 cA | 49.12 ± 4.88 cB | 20.67 ± 2.77 aC | ** |
| 28 | 48.22 ± 3.66 dA | 46.22 ± 4.07 dB | 20.65 ± 3.89 aC | ** |
| Sign. | ** | ** | ns | |
| Storage Time (Days) | MAP1 | MAP2 | VACUUM | |
|---|---|---|---|---|
| Apical section | Sign. | |||
| 0 | 19.43 ± 1.21 aA | 19.43 ± 1.12 aA | 1.59 ± 0.02 aB | ** |
| 7 | 2.45 ± 0.03 dA | 2.36 ± 0.06 cB | 1.17 ± 0.01 cC | ** |
| 14 | 2.32 ± 0.02 eA | 2.25 ± 0.04 cB | 1.38 ± 0.01 bC | ** |
| 21 | 2.76 ± 0.06 cA | 2.72 ± 0.03 bA | 1.17 ± 0.01 cB | ** |
| 28 | 7.75 ± 0.11 bA | 2.70 ± 0.03 bB | 1.59 ± 0.01 aC | ** |
| Sign. | ** | ** | ** | |
| Basal section | Sign. | |||
| 0 | 19.98 ± 2.12 aA | 19.98 ± 1.97 aA | 1.17 ± 0.02 bB | ** |
| 7 | 2.1 ± 0.03 eA | 2.10 ± 0.03 eA | 1.17 ± 0.01 bB | ** |
| 14 | 3.81 ± 0.07 bA | 3.67 ± 0.05 bB | 1.59 ± 0.04 aC | ** |
| 21 | 3.05 ± 0.05 cA | 3.05 ± 0.04 cA | 1.18 ± 0.02 bB | ** |
| 28 | 2.61 ± 0.07 dA | 2.58 ± 0.04 dA | 1.58 ± 0.02 aB | ** |
| Sign. | ** | ** | ** | |
| Storage Time (Days) | MAP1 | MAP2 | VACUUM | Sign. |
|---|---|---|---|---|
| Basal section | ||||
| 0 | 43.52 ± 4.98 aA | 43.52 ± 4.76 aA | 11.76 ± 1.78 aB | ** |
| 7 | 11.76 ± 1.67 dA | 11.77 ± 1.54 dA | 11.77 ± 1.45 aA | ns |
| 14 | 29.41 ± 2.78 bA | 29.18 ± 3.03 bB | 11.76 ± 3.44 aC | ** |
| 21 | 17.65 ± 1.04 cA | 17.65 ± 2.33 cA | 11.77 ± 2.62 aB | ** |
| 28 | 17.64 ± 1.38 cA | 11.77 ± 1.44 dB | 11.76 ± 0.37 aB | ** |
| Sign. | ** | ** | ns | |
| Apical section | Sign. | |||
| 0 | 42.36 ± 3.44 aA | 42.36 ± 3.83 aA | 11.76 ± 1.43 aB | ** |
| 7 | 11.77 ± 1.23 cA | 11.78 ± 1.32 cA | 5.88 ± 0.57 aB | ** |
| 14 | 11.76 ± 0.93 cA | 11.79 ± 0.89 cA | 11.76 ± 0.49 aA | ns |
| 21 | 11.65 ± 0.54 bA | 11.65 ± 0.67 bA | 11.76 ± 0.64 aB | ** |
| 28 | 11.77 ± 0.87 cA | 11.77 ± 0.97 cA | 11.76 ± 0.85 aA | ns |
| Sign. | ** | ** | ns | |
| TBC | MAP1 | MAP2 | VACUUM | Sign. |
|---|---|---|---|---|
| 0 | 4.77 ± 0.20 aC | 4.77 ± 0.20 aD | 4.47 ± 0.20 bD | ** |
| 7 | 4.30 ± 0.29 aD | 3.90 ± 0.29 bE | 3.86 ± 0.29 bE | ** |
| 14 | 6.80 ± 0.09 aB | 6.40 ± 0.05 bC | 6.10 ± 0.05 cC | ** |
| 21 | 6.96 ± 0.08 aB | 6.86 ± 0.04 bB | 6.26 ± 0.04 cB | ** |
| 28 | 9.60 ± 0.56 aA | 9.00 ± 0.46 bA | 8.65 ± 0.46 cA | ** |
| Sign. | ** | ** | ** | |
| TYMC | MAP1 | MAP2 | VACUUM | |
| 0 | nd | nd | nd | ns |
| 7 | 1.81 ± 0.28 aD | 1.71 ± 0.28 bD | 1.70 ± 0.28 bD | ** |
| 14 | 3.00 ± 0.02 aC | 2.20 ± 0.02 bC | 2.18 ± 0.02 bC | ** |
| 21 | 3.86 ± 0.41 aB | 3.16 ± 0.41 bB | 3.10 ± 0.41 bB | ** |
| 28 | 4.90 ± 0.06 aA | 3.90 ± 0.06 bA | 3.80 ± 0.06 bA | ** |
| Sign. | ** | ** | ** |
| Ossalic Acid | Tartaric Acid | Malic Acid | Citric Acid | Succinic Acid | Fumaric Acid | |
|---|---|---|---|---|---|---|
| MAP1 | ||||||
| 0 | 323.84 ± 0.20 a | 145.14 ± 0.35 b | 79.6 ± 0.31 a | 307.09 ± 0.26 a | 133.76 ± 1.00 a | 0.6 ± 0.00 e |
| 7 | 273.04 ± 0.27 b | 224.96 ± 0.31 a | nd | 76.33 ± 0.46 c | nd | 1.26 ± 0.00 c |
| 14 | 115.82 ± 0.22 d | nd | nd | 36.04 ± 1.22 c | nd | 0.65 ± 0.00 d |
| 21 | 242.99 ± 0.09 e | 14.94 ± 0.21 d | nd | 93.6 ± 0.27 b | nd | 1.66 ± 0.00 b |
| 28 | 246.04 ± 0.11 c | 53.93 ± 0.10 c | nd | 72.01 ± 0.27 c | nd | 1.92 ± 0.00 a |
| Sign. | ** | ** | ** | ** | ** | ** |
| MAP2 | ||||||
| 0 | 323.84 ± 0.20 a | 145.14 ± 0.35 b | 79.6 ± 0.31 a | 307.09 ± 0.26 a | 133.76 ± 1.00 a | 0.6 ± 0.00 e |
| 7 | 268.04 ± 0.27 b | 212.96 ± 0.31 a | nd | 75.33 ± 0.46 c | nd | 1.23 ± 0.00 c |
| 14 | 120.82 ± 0.22 d | nd | nd | 32.04 ± 1.22 c | nd | 0.63 ± 0.00 d |
| 21 | 222.89 ± 0.09 e | 12.34 ± 0.21 d | nd | 85.36 ± 0.27 b | nd | 1.61 ± 0.00 b |
| 28 | 236.44 ± 0.11 c | 49.83 ± 0.10 c | nd | 70.11 ± 0.27 c | nd | 1.90 ± 0.00 a |
| Sign. | ** | ** | ** | ** | ** | ** |
| VACUUM | ||||||
| 0 | 321.52 ± 0.20 a | 144.14 ± 0.35 b | 79.6 ± 0.31 a | 306.19 ± 0.26 a | 133.76 ± 1.00 a | 0.6 ± 0.00 e |
| 7 | 266.04 ± 0.27 b | 209.96 ± 0.31 a | nd | 75.03 ± 0.46 c | nd | 1.02 ± 0.00 c |
| 14 | 118.12 ± 0.22 d | nd | nd | 31.84 ± 1.22 c | nd | 0.61 ± 0.00 d |
| 21 | 202.88 ± 0.09 e | 12.04 ± 0.21 d | nd | 84.96 ± 0.27 b | nd | 1.58 ± 0.00 b |
| 28 | 234.41 ± 0.11 c | 48.83 ± 0.10 c | nd | 68.21 ± 0.27 c | nd | 1.86 ± 0.00 b |
| Sign. | ** | ** | ** | ** | ** | ** |
| Total Phenol Content (TPC) | ||||
|---|---|---|---|---|
| Storage Time (Days) | MAP 1 | MAP 2 | VACUUM | Sign |
| 0 | 85.42 ± 4.1 aA | 85.42 ± 4.1 aA | 85.42 ± 4.1 aA | ns |
| 7 | 66.34 ± 5.3 bA | 66.30 ± 5.5 bA | 67.11 ± 5.8 bA | ** |
| 14 | 66.87 ± 4.4 bA | 66.80 ± 4.8 bA | 66.94 ± 4.7 bA | ** |
| 21 | 52.08 ± 5.2 cA | 52.00 ± 5.5 cA | 54.53 ± 5.0 CA | ** |
| 28 | 43.30 ± 5.0 dA | 40.21 ± 5.6 dB | 45.24 ± 5.3 dC | ** |
| Sign. | ** | ** | ** | |
| Total flavonoid content (TFC) | ||||
| Storage time (days) | MAP 1 | MAP 2 | VACUUM | Sign. |
| 0 | 46.97 ± 4.6 aA | 46.97 ± 4.6 aA | 46.97 ± 4.6 aA | ns |
| 7 | 36.74 ± 4.3 bA | 38.88 ± 4.4 bB | 31.32 ± 3.4 bC | ** |
| 14 | 36.30 ± 3.3 bA | 35.14 ± 3.7 cB | 26.75 ± 4.2 cC | ** |
| 21 | 28.60 ± 4.1 cA | 27.50 ± 4.0 dB | 25.87 ± 4.0 dC | ** |
| 28 | 20.31 ± 5.5 dA | 18.88 ± 3.9 eA | 23.07 ± 4.1 eB | * |
| Sign. | ** | ** | ** | |
| Total carotenoid content (TCC) | ||||
| Storage time (days) | MAP 1 | MAP 2 | VACUUM | Sign. |
| 0 | 29.80 ± 4.2 aA | 29.80 ± 4.2 aA | 29.80 ± 4.2 aB | ns |
| 7 | 25.65 ± 4.1 bA | 23.46 ± 4.0 bB | 24.52 ± 3.8 cC | ** |
| 14 | 23.38 ± 3.8 bA | 21.22 ± 3.7 cB | 22.92 ± 3.4 cC | ** |
| 21 | 23.20 ± 3.7 bA | 21.38 ± 3.6 cA | 23.44 ± 3.2 dB | ** |
| 28 | 18.20 ± 1.5 dA | 16.60 ± 3.2 dA | 18.86 ± 2.7 bB | ** |
| Sign. | ** | ** | ** | |
| Bioactives | Packaging Systems | Reaction Order | K-Value | Equation | R2 | Half-Life (t1/2) (Days) |
|---|---|---|---|---|---|---|
| TPC | ||||||
| MAP1 | 0 | 1.4071 | y = −1.4071x + 82.502 R2 = 0.9366 | 0.9447 | 0.4927 | |
| MAP2 | 0 | 1.4960 | y = −1.496x + 83.09 R2 = 0.9415 | 0.9415 | 0.4634 | |
| VACUUM | 0 | 1.3270 | y = −1.327x + 82.436 R2 = 0.9403 | 0.9403 | 0.5223 | |
| TFC | ||||||
| MAP1 | 0 | 0.8780 | y = −0.878x + 46.076 R2 = 0.9506 | 0.9506 | 0.7894 | |
| MAP2 | 0 | 0.9651 | y = −0.9651x + 46.986 R2 = 0.9862 | 0.9862 | 0.7182 | |
| VACUUM | 0 | 0.7607 | y = −0.7607x + 41.446 R2 = 0.7829 | 0.7829 | 0.9112 | |
| TCC | ||||||
| MAP1 | 0 | 2.5650 | Y = −2.565x + 31.741 R2 = 0.9264 | 0.9264 | 0.2702 | |
| MAP2 | 0 | 2.8480 | y = −2.848x + 31.036 R2 = 0.88825 | 0.8825 | 0.2433 | |
| VACUUM | 0 | 2.2960 | y = −2.296x + 3.4515 R2 = 0.8535 | 0.8535 | 0.3019 |
| DPPH Test (IC50, µg/mL) | ||||
|---|---|---|---|---|
| Storage Time (Days) | MAP 1 | MAP 2 | VACUUM | Sign. |
| 0 | 24.77 ± 3.14 aA | 24.77 ± 3.14 aA | 24.77 ± 3.14 aA | ns |
| 7 | 39.92 ± 0.48 eC | 39.02 ± 2.03 cB | 44.41 ± 2.03 bA | ** |
| 14 | 37.44 ± 0.27 dC | 38.00 ± 1.56 cB | 47.10 ± 1.56 cA | ** |
| 21 | 33.26 ± 1.04 cC | 34.98 ± 3.37 bB | 69.63 ± 3.37 dA | ** |
| 28 | 31.97 ± 0.27 bC | 32.74 ± 3.67 bB | 71.12 ± 3.67 eA | ** |
| Sign. | ** | ** | ** | |
| ABTS test (IC50, µg/mL) | ||||
| Storage time (days) | MAP 1 | MAP 2 | VACUUM | Sign. |
| 0 | 0.78 ± 0.04 aA | 0.78 ± 0.04 aA | 0.78 ± 0.04 aA | ns |
| 7 | 0.82 ± 0.48 bA | 0.89 ± 0.05 bA | 0.80 ± 0.53 aA | * |
| 14 | 0.96 ± 0.27 bC | 1.09 ± 0.56 cB | 1.84 ± 0.56 bA | ** |
| 21 | 1.29 ± 0.44 cB | 1.34 ± 0.37 dB | 2.10 ± 3.37 A | ** |
| 28 | 2.27 ± 0.47 dC | 3.56 ± 0.67 deB | 4.41 ± 3.67 cdA | ** |
| Sign. | ** | ** | ** | |
| β-Carotene bleaching test (30 min incubation) (IC50, µg/mL) | ||||
| Storage time (days) | MAP 1 | MAP 2 | VACUUM | Sign. |
| 0 | 7.72 ± 1.85 aA | 7.72 ± 1.85 aA | 7.72 ± 1.85 aA | ns |
| 7 | 6.30 ± 0.71 aC | 8.83 ± 0.97 aB | 9.34 ± 0.97 aA | ** |
| 14 | 6.35 ± 0.48 bC | 9.27 ± 2.03 cB | 12.32 ± 2.02 cA | ** |
| 21 | 9.31 ± 0.37 bC | 11.28 ± 1.56 cB | 14.35 ± 1.53 cA | ** |
| 28 | 11.35 ± 0.89 cC | 13.27 ± 3.27 bB | 17.36 ± 3.34 bA | ** |
| Sign. | ** | ** | ** | |
| β-Carotene bleaching test (60 min incubation) (IC50, µg/mL) | ||||
| Storage time (days) | MAP 1 | MAP 2 | VACUUM | Sign. |
| 0 | 10.54 ± 1.97 aA | 10.54 ± 1.97 aA | 10.54 ± 1.97 aA | ns |
| 7 | 7.32 ± 0.71 aC | 9.34 ± 0.97 aB | 11.35 ± 0.97 aA | ** |
| 14 | 9.27 ± 0.48 bC | 11.31 ± 2.03 cB | 13.32 ± 2.02 cA | ** |
| 21 | 11.24 ± 0.37 bC | 14.28 ± 1.56 cB | 16.20 ± 1.53 cA | ** |
| 28 | 14.29 ± 1.00 cC | 16.89 ± 3.27 bB | 19.31 ± 3.34 bA | ** |
| Sign. | ** | ** | ** | |
| FRAP test (FRAP value, μM Fe (II)/g) | ||||
| Storage time (days) | MAP 1 | MAP 2 | VACUUM | Sign. |
| 0 | 14.26 ± 2.75 aA | 14.26 ± 2.75 aA | 14.26 ± 2.75 aA | ns |
| 7 | 0.52 ± 0.48 bA | 0.30 ± 0.03 bB | 0.08 ± 0.03 cC | ** |
| 14 | 0.49 ± 0.27 cA | 0.26 ± 0.06 bA | 0.09 ± 0.06 cB | * |
| 21 | 0.47 ± 0.04 cA | 0.18 ± 0.37 cB | 0.10 ± 0.37 bC | ** |
| 28 | 0.44 ± 0.27 cA | 0.14 ± 0.67 cB | 0.11 ± 0.67 bB | ** |
| Sign. | ** | ** | ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sicari, V.; Tundis, R.; Romeo, R.; Reitano, A.; Belsito, E.L.; Leggio, A.; Loizzo, M.R. Evaluation of the Shelf life of Ready-to-Eat Fresh Bamboo Sprouts (Phyllostachys edulis) Packaged in a Modified Atmosphere or Vacuum: A Comparative Study. Antioxidants 2024, 13, 185. https://doi.org/10.3390/antiox13020185
Sicari V, Tundis R, Romeo R, Reitano A, Belsito EL, Leggio A, Loizzo MR. Evaluation of the Shelf life of Ready-to-Eat Fresh Bamboo Sprouts (Phyllostachys edulis) Packaged in a Modified Atmosphere or Vacuum: A Comparative Study. Antioxidants. 2024; 13(2):185. https://doi.org/10.3390/antiox13020185
Chicago/Turabian StyleSicari, Vincenzo, Rosa Tundis, Rosa Romeo, Antonella Reitano, Emilia Lucia Belsito, Antonella Leggio, and Monica Rosa Loizzo. 2024. "Evaluation of the Shelf life of Ready-to-Eat Fresh Bamboo Sprouts (Phyllostachys edulis) Packaged in a Modified Atmosphere or Vacuum: A Comparative Study" Antioxidants 13, no. 2: 185. https://doi.org/10.3390/antiox13020185
APA StyleSicari, V., Tundis, R., Romeo, R., Reitano, A., Belsito, E. L., Leggio, A., & Loizzo, M. R. (2024). Evaluation of the Shelf life of Ready-to-Eat Fresh Bamboo Sprouts (Phyllostachys edulis) Packaged in a Modified Atmosphere or Vacuum: A Comparative Study. Antioxidants, 13(2), 185. https://doi.org/10.3390/antiox13020185









