Abstract
Ozone-based chemiluminescence detection (CLD) has been widely applied for determining nitric oxide (•NO) and its derived species in many different fields, such as environmental monitoring and biomedical research. In humans and animals, CLD has been applied to determine exhaled •NO and •NO metabolites in plasma and tissues. The main advantages of CLD are high sensitivity and selectivity for quantitative analysis in a wide dynamic range. Combining CLD with analytical separation techniques like chromatography allows for the analytes to be quantified with less disturbance from matrix components or impurities. Sampling techniques like microdialysis and flow injection analysis may be coupled to CLD with the possibility of real-time monitoring of •NO. However, details and precautions in experimental practice need to be addressed and clarified to avoid wrong estimations. Therefore, using CLD as a detection tool requires a deep understanding of the sample preparation procedure and chemical reactions used for liberating •NO from its derived species. In this review, we discuss the advantages and pitfalls of CLD for determining •NO species, list the different applications and combinations with other analytical techniques, and provide general practical notes for sample preparation. These guidelines are designed to assist researchers in comprehending CLD data and in selecting the most appropriate method for measuring •NO species.
1. Introduction
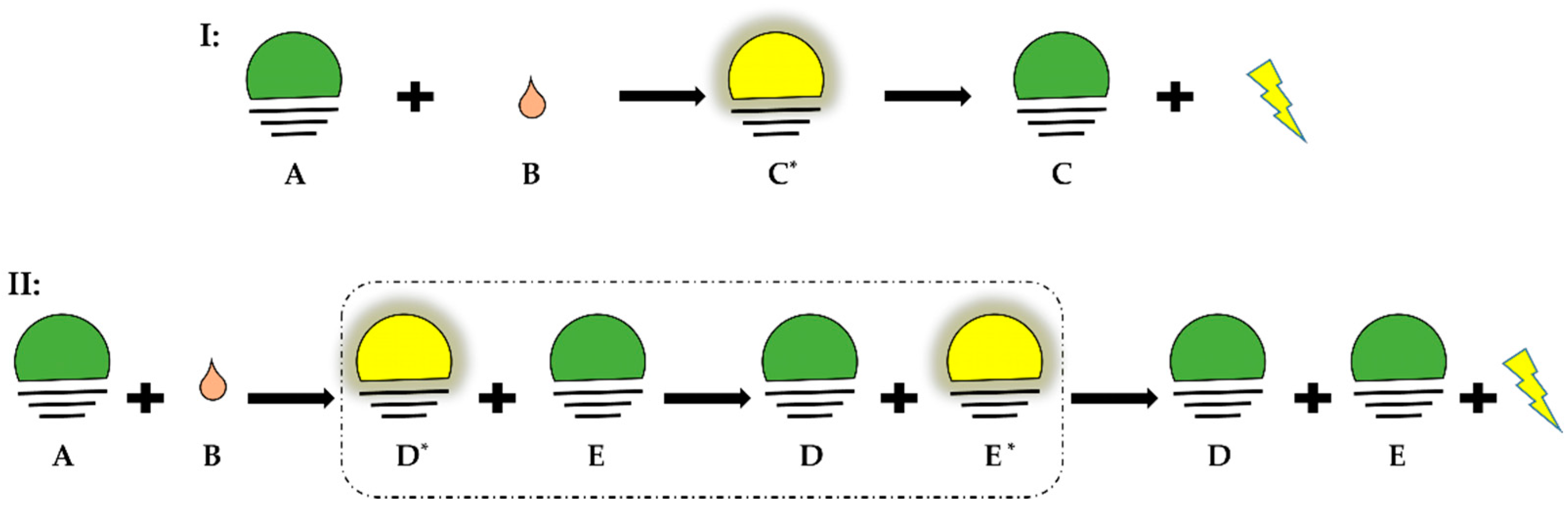
Chemiluminescence is defined as the emission of light as the result of a chemical reaction. The reactants or intermediates are chemically activated via oxidation into an electronically excited state and then can release light by two distinct mechanisms defined as direct and indirect chemiluminescence (Figure 1). In direct chemiluminescence, the chemiluminescent molecules (A) are oxidized to unstable excited intermediates (C*) that then return to the ground state (C) by releasing energy in the form of photons. In indirect chemiluminescence, the excited intermediates (D*), instead of directly decaying, transfer energy through an optical process to the surrounding fluorophores (E), which then become excited (E*) and release energy by light emission. This phenomenon is called chemiluminescence resonance energy transfer of light.
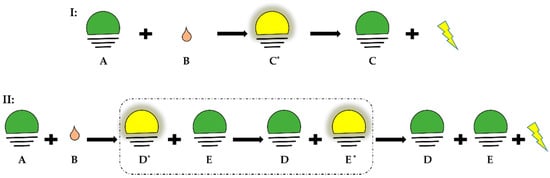
Figure 1.
Direct and indirect chemiluminescence. Green: basal state; yellow: excited state. (I) Direct chemiluminescence, A: chemiluminescent molecule, B: oxidant, C*: excited state of intermediate, C: ground state of intermediate. (II) Indirect chemiluminescence, D*: excited state of intermediate, D: ground state of intermediate, E: ground state of fluorophore, E*: excited state of fluorophore.
Luminol was one of the first chemiluminescent molecules applied for the detection of oxidants and free radicals using direct chemiluminescence [1,2]. Luminol emits blue light with a wavelength of 425 nm after being oxidized [1,2,3,4]. Luminol chemiluminescence was applied for the detection of oxidants such as superoxide, hydrogen peroxide, and peroxynitrite [1,5,6]. However, the use of luminol chemiluminescence has strong limitations and is only semiquantitative. The main reason is that the oxidation of luminol is an autocatalytic process, which yields unspecific signal potentiation. Moreover, the oxidation yield is enhanced by the presence of bicarbonate–carbon dioxide (through the formation of ONOOCO2− that decomposes into CO3•−). The oxidation can be inhibited by free thiols and other antioxidants [7]. Therefore, luminol-dependent chemiluminescence assay is now only applied to analyze overall oxidant formation under specific and controlled conditions [1,8].
Ozone-based chemiluminescence detection (CLD) for nitric oxide (•NO) species is well recognized as a highly sensitive and specific approach for quantifying gaseous •NO. The detector quantifies the light produced by the reaction of •NO with ozone in the gas phase [9]. The reaction produces excited nitrogen dioxide (NO2*) (Reaction (1)), which then emits photons when it returns to the ground state (Reaction (2)). The emitted light is then amplified with a photomultiplier tube and detected.
•NO + O3 → NO2* + O2
NO2* → NO2 + hv
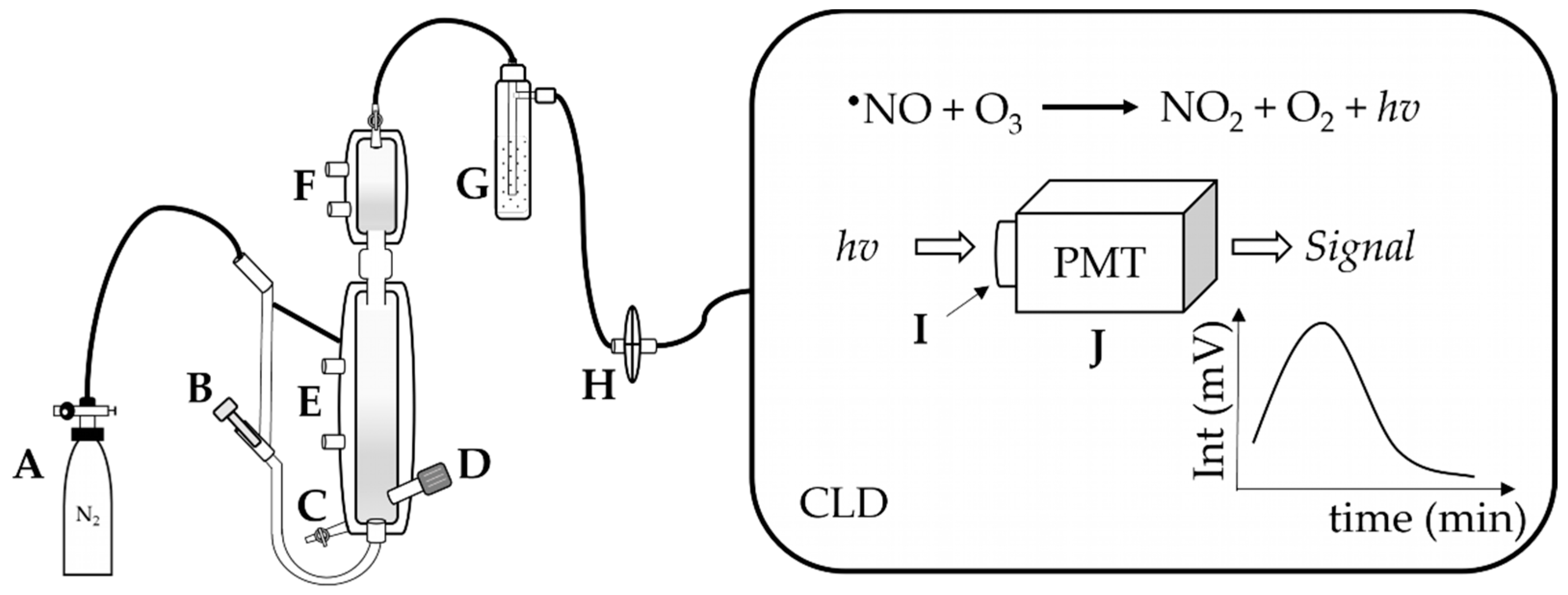
Typically, •NO is generated in a reaction chamber purged with an inert carrier gas (N2 or argon), which carries the generated •NO along the tubing connecting the chamber to the CLD. The chamber contains a reductive or oxidative solution prepared for the reaction under acidic or neutral conditions and temperated with defined temperature conditions (e.g., 60 °C). The settings of the reaction chamber depend on the type of analyte and the type of •NO derivatives that one may want to quantify. After leaving the reaction chamber, the carrier gas together with •NO is then purged into a “NaOH trap”, consisting of a solution of NaOH (1 N), which prevents high-temperature acid vapor from entering and damaging the chemiluminescence detector (Figure 2) [10,11].
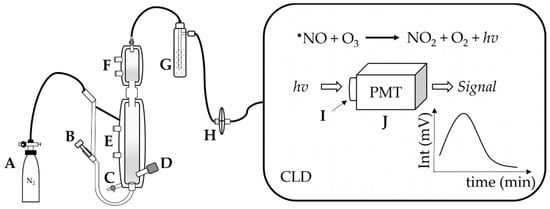
Figure 2.
Apparatus for ozone-based chemiluminescence. A: supply gas, N2, B: supply gas fine control for purging, C: waste outlet, D: injection port of sample, E: heating circulation, F: cooling circulation, G: NaOH trap, H: gas filter; in the CLD: the emission hv is collected by the I: optical filter and then converted by the J: photomultiplier tube (PMT) to amplify the signal in mV.
2. NO and Its Biologically Relevant Derivatives
•NO is a moderately reactive uncharged free radical, which attracted a lot of attention in the late 1980s for its biological properties. It was found to be the endothelium-derived relaxing factor (EDRF), which uncovered how endothelial cells are involved in the vasodilatory effect of smooth muscle cells in the vasculature [11,12,13]. The nature of EDRF as •NO was indeed revealed using CLD [11,14]. The Nobel Prize in Physiology or Medicine for 1998 was awarded jointly to Robert F. Furchgott, Louis J. Ignarro, and Ferid Murad for their discoveries concerning “nitric oxide as a signaling molecule in the cardiovascular system”.
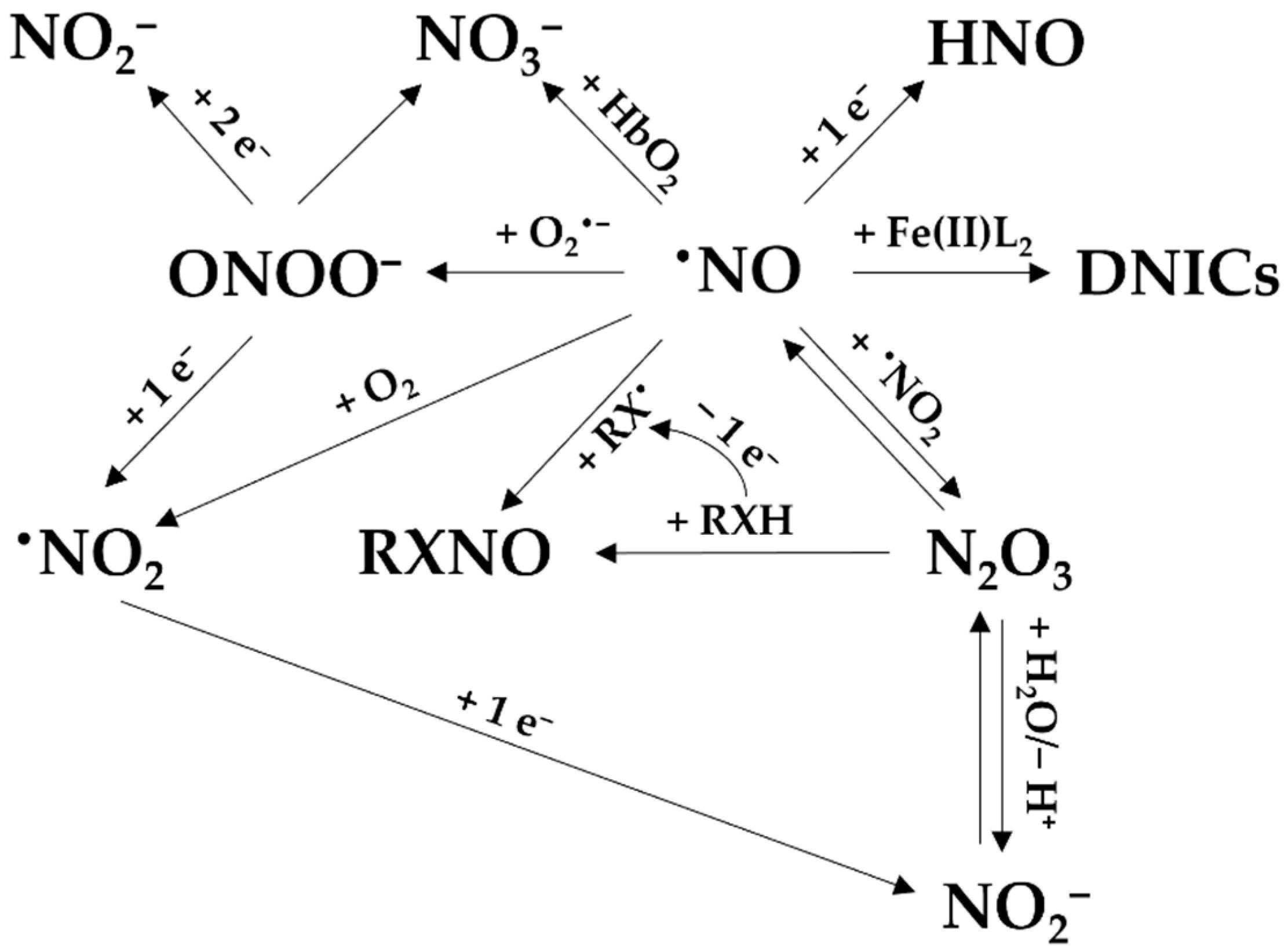
By reacting with other radicals and molecules produced in a biological environment, •NO leads to the formation of reactive nitrogen species. The reactive nitrogen species are in turn involved in the oxidation or nitrosation of biomolecules [15,16]. Other downstream species include dinitrogen trioxide (N2O3), nitrosothiols and nitrosamines (often abbreviated as RSNO and RNNO, respectively), intracellular dinitrosyl iron complexes (DNICs), nitroxyl (HNO), •NO2, peroxynitrite (ONOO−), nitrite, and nitrate (Figure 3) [8]. Together with reactive oxygen and sulfur species, they are part of the reactive species interactome [17].
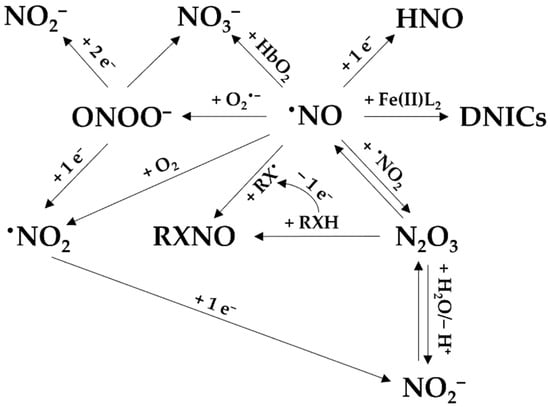
Figure 3.
The reactions of •NO to form its derived species. Nitric oxide (•NO) can react with O2•− to form peroxynitrite (ONOO−), which then can yield nitrate (NO3−) or be reduced to NO2− and •NO2. NO3− can be also derived from the reaction between •NO and oxyhemoglobin (HbO2). Nitrosyl (HNO) can be formed by reduction of •NO. •NO can bind to iron (Fe(II)) to form dinitrosyl iron complexes (DNICs). •NO can also react with nitrogen dioxide (•NO2), with N2O3 as intermediate, to generate nitrite (NO2−) and nitrosated product (RXNO).
N2O3, as an intermediate in the autoxidation of •NO, is derived from the reaction of •NO with •NO2 (Reactions (3) and (4)). N2O3 can be then hydrolyzed to two molecules of nitrite or rapidly nitrosate thiols and amines, leading to the formation of RSNO, RNNO, and nitrite (Reactions (5) and (6)) [16,18,19,20,21].
2•NO + O2 → 2•NO2
•NO + •NO2 ⇌ N2O3
N2O3 + H2O → 2HNO2 ⇌ 2NO2− + 2H+
N2O3 + R2NH → R2NNO + NO2− + H+
Instead of direct nitrosation of thiols by N2O3, RSNO was also proposed to be formed in the reaction of RS− with •NO2 to generate RS•, which then reacts with •NO to obtain RSNO (Reactions (7) and (8)) [22].
RS− + •NO2 → RS• + NO2−
RS• + •NO → RSNO
In addition, •NO can target also Fe-heme and bind to iron from or partially from the chelatable iron pool together with ligands such as glutathione to form DNICs [23].
NO− is formed by the one-electron reduction of •NO, which is found only in its protonated form, HNO [8]. HNO could also react with oxygen leading to the formation of ONOO−. However, this reaction has low relevance under biological conditions due to a relatively slow reaction rate (Reaction (9)) [24,25].
NO− + O2 → OONO−
ONOO− is formed mainly by the reaction between •NO and O2•− (Reaction (10)). ONOO− can undergo one- or two-electron reduction yielding •NO2 and NO2−, respectively [26,27]. ONOO− can be also isomerized to NO3− in the presence of metmyoglobin or methemoglobin as a catalyst (Reaction (11)) [28,29].
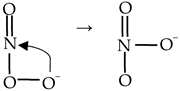
•NO + O2•− → OONO−

Nitrate can be derived by the reaction between •NO and oxyhemoglobin (Reaction (12)) [30]. Under certain conditions such as in the presence of oral bacteria or the enzyme xanthine oxidoreductase, nitrate can be reduced back to nitrite. Nitrite is an important source of •NO in the body [31,32,33,34,35,36].
Nitrite can be reduced to •NO by proteins such as deoxyhemoglobin, deoxymyoglobin, and other globins under hypoxic conditions. These mechanisms contribute to many biological processes such as vasodilation, neurotransmission, immune response, and other physiological signaling pathways (Reaction (13)) [37,38].
•NO + oxyHb → NO3− + metHb
NO2− + deoxyHb → •NO + metHb
•NO was also described to react with deoxyhemoglobin to form nitrosylhemoglobin, where •NO forms a complex with the iron heme. Nitrosylhemoglobin was described as a transporter of •NO bioactivity, as an intermediate for the formation of s-nitrosohemoglobin, and as an intermediate formed during nitrite reduction into •NO by deoxyhemoglobin [37,39,40,41].
3. Measurement of •NO Metabolites Using Chemiluminescence
In the reaction chamber, the •NO metabolites need to be converted back to •NO by reduction (nitrate, nitrite, RSNO, RNNO) or oxidation reactions (NO-heme or DNIC).
3.1. Reduction of Nitrite, RSNO, and RNNO with Tri-Iodide, Cys/CuCl, and Hydroquinone/Quinone
The most widely used reductive solution applied in CLD is tri-iodide. The tri-iodide-based reductive reagent consists of potassium iodide (KI) and iodine (I2) in glacial acetic acid. Under acidic conditions, nitrite can be protonated to form nitrous acid with acid dissociation constant at a logarithmic scale (pKa) of 3.4. Nitrous acid is unstable at low pH, for example, in glacial acetic acid, it goes through disproportionation to form •NO or by-product N2O3, which interferes with the detection by variation in the background signal. The KI/I2 mixture is very widely used for reducing nitrite to •NO in biological samples (Reaction (14)) [10,42]. In addition to nitrite, the tri-iodide assay can also be applied for the detection of other biological •NO metabolites such as nitrosated (S- or N-nitroso) products (RXNO) (Reactions (15)–(17)).
I− + NO2− + 2H+ → •NO + 1/2 I2 + H2O
I2 + I− → I3−
I3− + 2RSNO → 3I− + RS-SR + 2NO+
2NO+ + 2I− → 2NO + I2
Sample preparation for the detection of S-nitrosated products (RSNO) requires a pre-treatment of the samples with a stabilization solution containing N-ethylmaleimide (NEM) that prevents artificial S-nitrosation of free thiols. Treatments with NEM have some drawbacks as NEM is often contaminated by nitrite, and the contamination may vary from one lot number to another. It is therefore necessary to check contamination before adding NEM to samples.
In addition, samples need to be treated with sulfanilamide prior detection. This treatment serves to eliminate nitrite by converting it into an undetectable diazonium cation. This procedure ensures that the detection process specifically captures signals related to RSNO and residual species during the tri-iodide assay.
For the detection of RNNO, samples need to be pre-treated with HgCl2. This process converts RSNO into NO+ and leads to the subsequent formation of NO2−. Then, the addition of sulfanilamide will remove the formed NO2−, thus revealing the “remnants” or the RNNOs. Consequently, the combined pre-treatment with HgCl2 and sulfanilamide offers an alternative approach to differentiate RSNO and RNNO from other species [43].
The measurement of RSNO can be also carried out using cysteine/CuCl as a reducing matrix (1 mM L-cysteine/0.1 mM CuCl) with a sensitivity of 10 pmol for the RSNO standards or using a hydroquinone/quinone reducing matrix (0.1 M hydroquinone/0.01 M quinone) with quantitative detection down to 10 nM in PBS buffer or rat plasma (see also Table 1) [44,45].
3.2. Measurement of Nitrate with Vanadium Chloride
In comparison to nitrite and other species, nitrate is present at a much higher concentration in biological specimens and makes up the major part of total •NO species. To be reduced, it requires a stronger reductive reagent as compared with tri-iodide or an enzymatic reaction catalyzed by a bacterial nitrate reductase. Chemical reduction of nitrate is carried out with vanadium chloride (VCl3) prepared in hydrochloric acid (HCl) in a final concentration of 0.1 mol/L of VCl3 in 2 mol/L HCl (Reaction (18)) [46,47]. It is worth mentioning that the VCl3/HCl reagent will not just reduce nitrate but also all the other metabolites able to be reduced using the tri-iodide method. To obtain the accurate nitrate level, therefore, the results from the tri-iodide assay need to be subtracted from the observed signals in the VCl3/HCl method.
2NO3− + 3V3+ + 2H2O → 2•NO + 3VO2+ + 4H+
3.3. Measurement of NO-Heme with Ferricyanide
Gladwin et al. proposed an oxidation method based on one-electron oxidation of NO-heme with ferricyanide for the determination of NO-heme, with a sensitivity down to 5 nM in human blood after removing the background signal of nitrite (Reaction (19)) [48]. This method was further modified by Bryan et al., who used 0.05 M instead of 0.2 M ferricyanide in PBS at pH 7.5 to determine NO-heme in rat tissues such as the brain, heart, liver, etc., as well as in the blood components, plasma, and erythrocytes [20].
NO-heme (Fe2+) + Ferricyanide (Fe3+) → medHb (Fe3+) + Ferrocyanide (Fe2+) + •NO
Some common reductive and oxidative solutions for the detection of •NO metabolites are listed in Table 1.

Table 1.
Reductive and oxidative reaction solution for NO metabolites.
Table 1.
Reductive and oxidative reaction solution for NO metabolites.
| Reaction Solution | Conditions | Target NO Metabolites | References |
|---|---|---|---|
| Iodine/iodide | 60 mM I−/6 to 20 mM I2/1M HCl, RT | NO2−, RNNO, RSNO; RNNO, RSNO with the addition of sulfanilamide and HgCl2 | [44] |
| 56 mM I−/2 mM I2, 4 mM CuCl, CH3COOH, 68 °C | [49] | ||
| 45 mM I−/10 mM I2, CH3COOH, 60 °C | [10] | ||
| Cysteine/CuCl | 1 mM L-cysteine/0.1 mM CuCl | RSNO | [45] |
| Hydroquinone/quinone | 0.1 M hydroquinone/0.01 M quinone | RSNO | [44] |
| VCl3/H+ | 0.1 M vanadium(III) in 2 M HCl | NO3−, NO2−, RNNO, RSNO | [50] |
| Ferricyanide | 0.2 M ferricyanide in PBS, pH 7.5 | NO-heme | [48] |
| 0.05 M ferricyanide in PBS, pH 7.5 | [20] |
4. Multi-Level Analytical Approaches for a Comprehensive Analysis of •NO Metabolites
4.1. Chemiluminescence Coupled with Chromatography or Mass Spectrometry (MS)
Chemiluminescence compared with other optical techniques such as fluorescence detection, needs no external light source, has a low background signal and, therefore, has a high signal-to-noise ratio and thus is highly sensitive [51,52]. A chemiluminescence nitrogen detector was developed in 1970 based on the reaction of •NO with ozone for continuously monitoring gaseous pollutants in the air [9].
Coupling CLD with analytical separative techniques like gas chromatography (GC) or high-performance liquid chromatography (HPLC) allows for the combination of such a highly sensitive detection technique with powerful analytical separation methods. The selective elution of analytes from the chromatography column can help separate the target analytes from unwanted interferants or impurities in the sample matrix, which could improve the accuracy and the sensitivity of CLD even further [53,54].
4.1.1. Gas Chromatography
A chemiluminescence detector for •NO species coupled with GC was applied for the detection of atmospheric •NO, ammonia, amines, and some nitrogen-containing compounds [9,55,56]. To establish a GC-CLD system, particular attention is required for many critical aspects concerning both GC and CLD. Regarding GC, key considerations include the sample injection (split/splitless, which determine the amount of sample entering the GC system), the column (including type of stationary phase, film thickness, column inner diameter, and length of the column), and the GC-CLD interface (involving a transfer line through which analytes travel from GC to the CLD). These elements are essential components for the proper functioning of the system [56].
GC is an analytical technique for the quantitative analysis of volatile compounds. The volatility of the analytes is important for assuring their volatilization and ability to enter the gaseous mobile phase in a way that they can be transported for further separation through the column. For samples with liquid matrices (like wastewater), the injected samples need to be introduced at a proper flow rate for more complete combustion at the injection port of GC, which eliminates the risk of samples with high water content reaching the chemiluminescence detector [56].
To enhance the peak capacity and improve the resolution, CLD was coupled to two-dimensional gas chromatography (GCxGC) with orthogonality for separation between two applied GC columns. This coupling was used for the quantitative analysis of nitrogen-containing compounds in microalgae-based bio-oils, food samples, and urban aerosol samples [57,58,59].
In GC-CLD, GC introduces the chance for further separation in the GC column; however, it also brings the requirement for the volatility of the samples. The drawbacks of this technique are particularly notable when dealing with complex samples, especially biological tissues or cells. The development of sample preparation methods and instrumental parameters might be time-consuming and costly, which requires more careful consideration and more prudent handling. To the best of our knowledge, GCxGC-CLD was never applied to biological specimens, probably because of the complexity of the sample matrix.
4.1.2. Liquid Chromatography
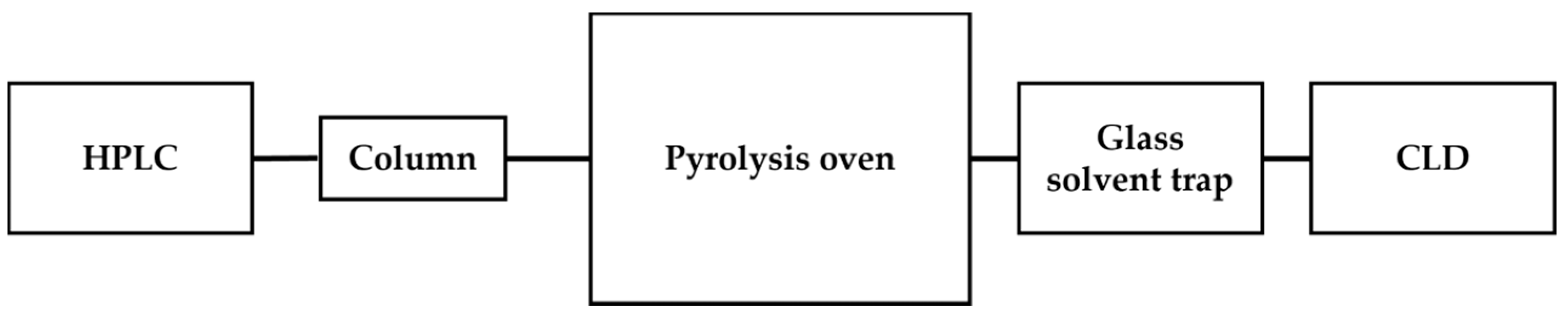
Attempts also have been made to couple CLD with HPLC for detecting •NO species. Using an UPLC system allows for the direct analysis of non-volatile samples in liquid matrices without vaporization [53,60]. HPLC-CLD was applied for the analysis of nitrated polycyclic aromatic hydrocarbons, which are environmental combustion pollutants. In the literature, there is an example of a HPLC-CLD system made by connecting the outlet of an HPLC column to a quartz tubing placed in a pyrolysis oven by using a stainless-steel tubing. The pyrolysis oven is heated at 900 °C and converts the liquid matrices, along with samples, into the gaseous state (Figure 4). It was found that such conversion into the gaseous state before reaching the chemiluminescence detector significantly impacts the sensitivity, which becomes worse with increasing aqueous content in the mobile phase [61].

Figure 4.
Schematic representation of HPLC-CLD with a pyrolysis oven, as described in Ref. [61]. The eluents from an HPLC column were transformed into the gaseous state within the pyrolysis oven. This system showed some sensitivity issue in the presence of water in aqueous matrixes.
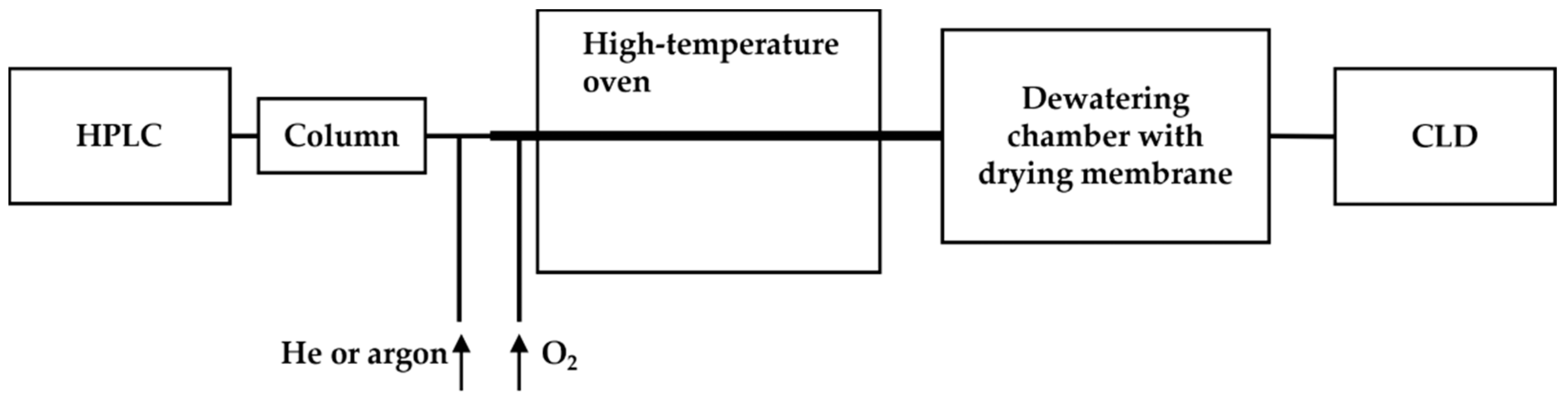
To overcome this, a dewatering chamber equipped with a drying membrane was added to the system (as shown in Figure 5). The eluents from the HPLC column were mixed with inert gas (helium or argon) along with oxygen to facilitate the oxidation of all nitrogen-containing compounds to •NO in a high-temperature oven (approximately 1000 °C). The resulting •NO and gases were then transported through a membrane in the dewatering chamber to remove the aqueous content before reacting with ozone in the reaction chamber for chemiluminescence [62]. The modified HPLC-CLD system equipped with a Hamilton PRP-X200 ion chromatography column was validated for the detection limit of ammonium nitrogen down to 5 ng in wastewater and also the capability of profiling nitrate of 80 ng and nitrite of 160 ng in a nitrite–nitrate mixture [62].
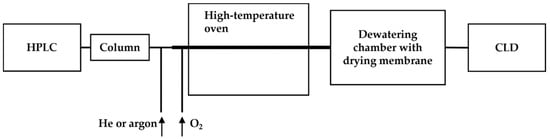
Figure 5.
Schematic representation of the HPLC-CLD coupled with a high-temperature oven and a dewatering chamber, as described in Ref. [62]. The dewatering chamber, positioned post the high-temperature oven, serves to remove the aqueous content in the sample flow, thereby enhancing the sensitivity of CLD detection.
The application of HPLC-CLD for the nitrogen-specific detection of commercially synthesized peptides, including crude peptide mixtures, further demonstrated its superiority over simultaneous ultraviolet detection in peptide profiling [53,63]. On the other hand, an HPLC-CLD system was also applied for quantifying nucleosides based on their nitrogen content [60]. Another example of HPLC-CLD system involving a CLD-10A chemiluminescence detector (Shimadzu, Kyoto, Japan) was used for determining hydroxyl-1-nitropyrenes (OHNPs) and metabolites of 1-nitropyrene, including 3-OHNP, 6-OHNP, and 8-OHNP formed by cytochrome P450s [64].
4.1.3. Mass Spectrometry
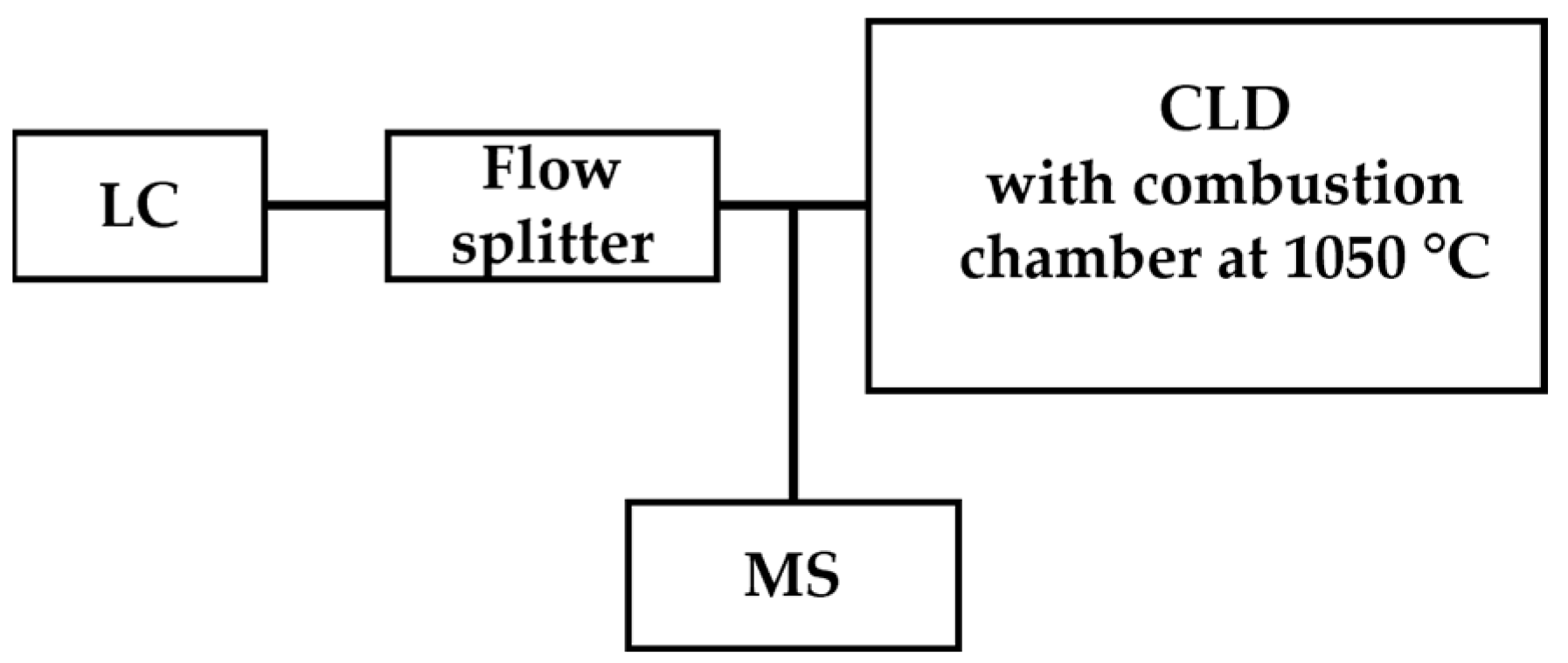
Mass spectrometry is a powerful detection technique based on the determination of the mass-to-charge ratio (m/z) of target ions and has been applied for the qualitative analysis and quantitation of •NO-derived species such as nitrite and nitrate by coupling with chromatography as GC-MS or LC-MS [65,66]. Instead of sole MS detection, CLD was coupled for online quantitative analysis to LC-MS or GC-MS. For the sample class with a known formula, quantitation by CLD eliminates the need for preparing primary standards for each individual analyte within the known class with an unknown concentration, which allows high-throughput analysis. Hence, the combination of LC-MS/CLD shows the advantage of CLD for quantitation in high-throughput analysis and also brings the power of MS for specific demands of qualification. An example of this application is the analysis of 24 selected nitrogen-containing compounds such as C10H10N2, C11H9N3O2, etc. [67]. The scheme of LC-MS/CLD is shown in Figure 6.
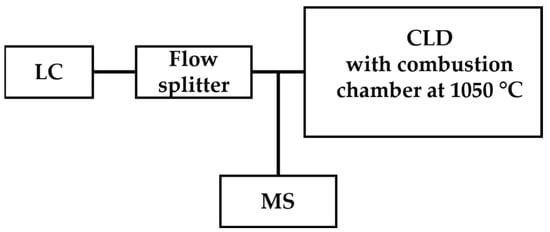
Figure 6.
Schematic representation of LC-MS/CLD, as described in Ref. [67], featuring a flow splitter positioned after LC. The flow splitter divides eluents from LC into two streams, with one stream directed toward CLD for quantitative analysis and the other directed toward MS for qualitative analysis.
The main pitfall of LC-MS/CLD techniques is the tailing of the peak, possibly due to clogging of the splitter and the nebulizer. This issue can be overcome with the adjustment of flow rate, tubing length, and replacement with a variable flow splitter [67]. LC-MS/CLD was applied for the identification and quantitation of in vivo metabolites in complex biological matrixes such as bile, urine, and plasma [68].
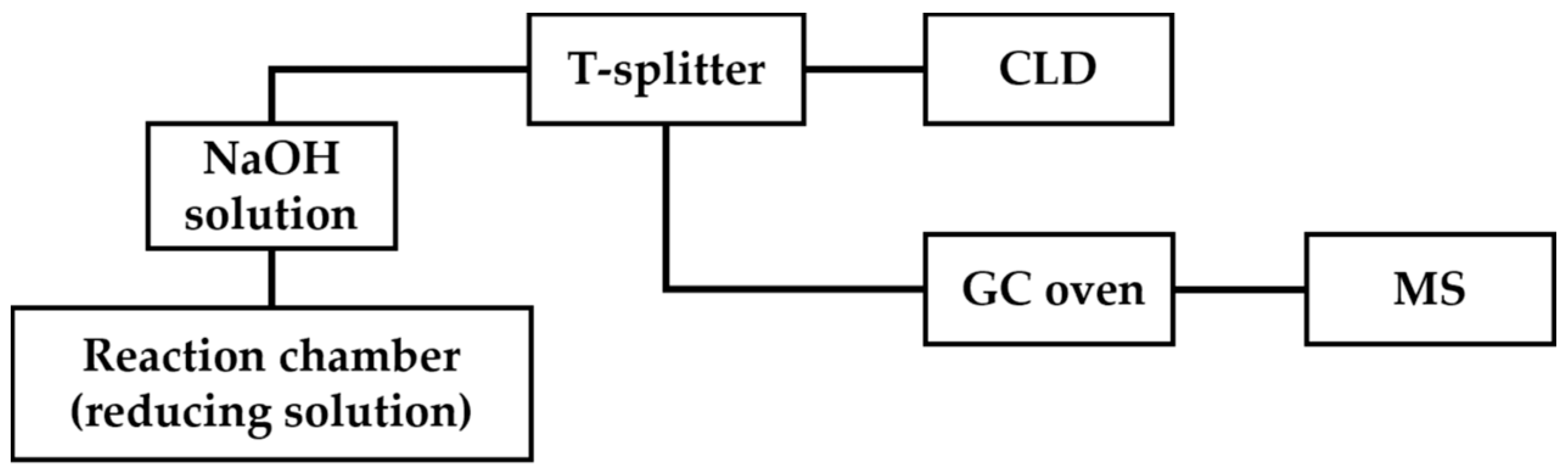
In GC-MS/CLD, a T-splitter is placed after the trapping chamber containing a NaOH solution, which splits the •NO generated by the chemiluminescence reaction into a chemiluminescence detector and mass spectrometer (Figure 7) [69]. With GC-MS/CLD, total •NO can be quantified routinely with CLD; furthermore, the 14NO and isotope-labeled 15NO can be differentiated according to m/z with MS. An example application of this technique is the measurement of nitrite reductase activity in a macrophage cell line lysate. J774.2 macrophage cell lysate was treated with 15NO3−, which was then reduced to 15NO2− by nitrate reductase activity. Both the original 14NO2− and produced 15NO2− were reduced with chemiluminescence assay to 14NO and 15NO, respectively, followed by MS analysis to distinguish them. In this case, GC-MS/CLD held the detection limit of •NO-related products around 10 nM in 100 μL of the sample, which was demonstrated to be beneficial for the study of nitrate reductase activity or related enzymatic pathways [69].
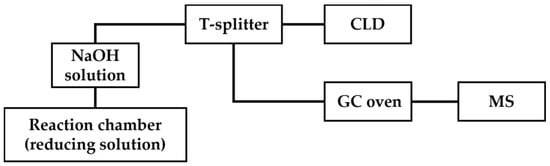
Figure 7.
Schematic representation of GC-MS/CLD, as described in Ref. [69], featuring a T-splitter positioned after the NaOH solution chamber. The T-splitter divides the eluents into two streams, with one stream directed toward CLD for quantitative analysis and the other directed toward GC oven followed by MS detection.
4.2. Coupled with Microdialysis
Microdialysis is a technique used for sampling soluble molecules from the extracellular fluid through a semipermeable membrane. It is based on the principle of osmosis: molecules diffuse through a semipermeable membrane according to the concentration from an area of high concentration to an area of low concentration. It was found that by increasing the surface area of the dialysis membrane, the collection of samples from the interstitial space of the brain and other tissues became feasible [70]. A special microdialysis probe with a three-layer membrane was designed to collect •NO in vivo from the blood or brain tissues of rats and rabbits, which was then combined with luminol-based chemiluminescence. This system was proposed for the real-time monitoring of changes in •NO concentration in vivo, which allows to trace variations in •NO metabolism in different physiological states, such as changes in body temperature. Microdialisis was also used to evaluate the impact of •NO donors on the concentration of •NO in blood and tissues [71].
4.3. Coupled with Flow Injection Analysis
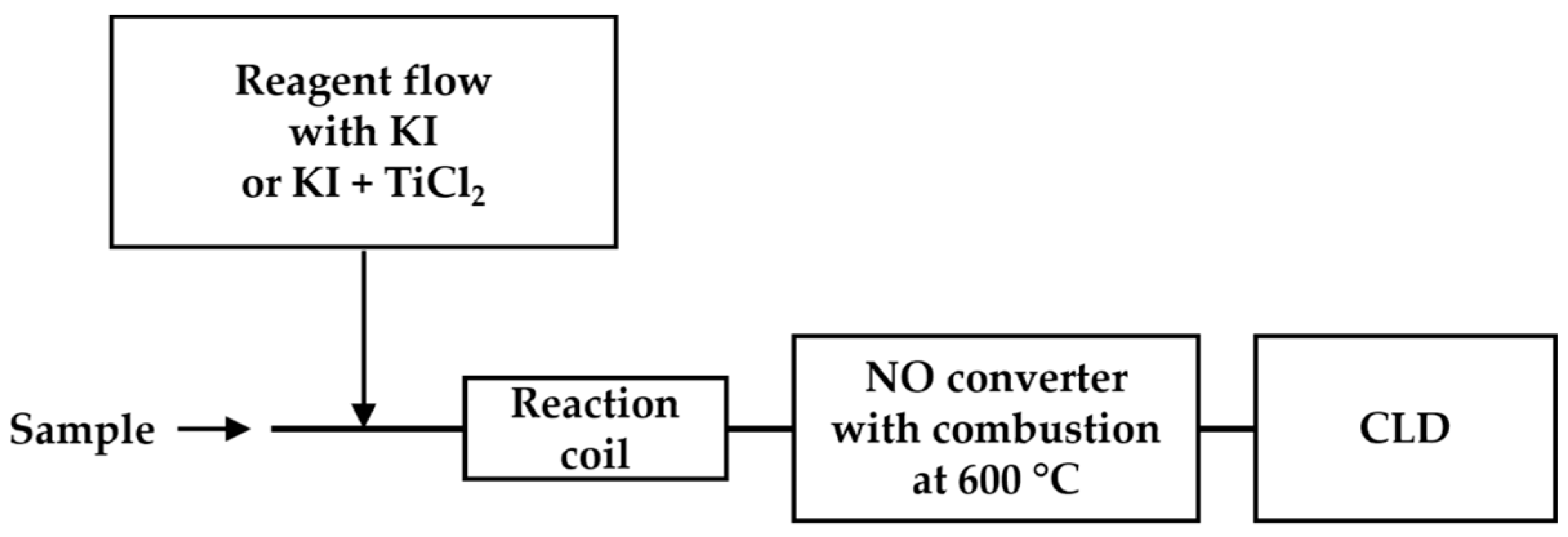
Flow injection analysis (FIA) involves the injection of samples into a continuous carrier flow (a liquid stream), which then is mixed with other reagent flows for the reaction before reaching the detector [72,73,74]. Aqueous ammonia, nitrite, and nitrate were quantified using a flow injection system equipped with a chemiluminescence detector (Figure 8) [73,74]. In this system, an aqueous sample stream is continuously mixed with various reducing reagents (KI for the detection of nitrite only and titanium chloride for the detection of total nitrate and nitrite). Moreover, high-temperature combustion is applied to remove aqueous content. The detection limits of 10 nM for nitrite and nitrate were demonstrated in a performance study with standards.
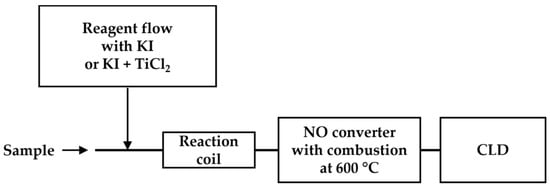
Figure 8.
Schematic representation of FIA-CLD, as described in Ref. [73]. An aqueous sample is mixed with KI or the mixture (KI + TiCl2) before reaching the reaction coil. After high-temperature combustion in the •NO converter, the •NO products are detected using CLD.
Another FIA/chemiluminescence system was constructed for the monitoring of peroxynitrite in biological samples. This system is based on the collection of samples applying a dialysis membrane and the detection of peroxynitrite using luminol chemiluminescence [75]. With this setup, the detection limits were 10 and 100 pM for the calibrations with and without a membrane, respectively, which was proposed to be useful for in vivo monitoring of peroxynitrite as combined with microdialysis.
5. Advantages and Disadvantages of Chemiluminescence for Detecting •NO Species in Biological Specimens
5.1. Advantages
Chemiluminescence is currently the most used technique for the quantification of •NO in biological specimens [76] because of its high sensitivity, which allows for the detection of pM concentration of •NO [77]; its selectivity over a wide dynamic linear range usually from 0.5 ppb to 5600 ppm •NO [76]; its ability in the real-time monitoring of •NO [78]; and its commercial availability with relatively affordable price.
As mentioned above, ozone-based chemiluminescence can be used not only for the detection of •NO but also for nitrite, nitrate, S-nitrosothiols, nitrosyl–metal complex, and N-nitrosamines in biological samples [10,40,44,79,80,81,82,83].
5.2. Pitfalls
As mentioned above, an ozone-based chemiluminescence detector can detect the concentration of nitrite in the pM range. Therefore, it is important to avoid contamination coming from the water used for the preparation of reagents, standards, samples, and cleaning procedures. Milli-Q water was demonstrated to contain the lowest level of nitrite among the other sources of water and must be used for this application [84].
Contamination is not the only reason why sample preparation is a critical step. In fact, since •NO has a short half-life and nitrite in the blood rapidly reacts with oxyhemoglobin to form nitrate, it is essential that the sample preparation is carried out in conditions that allow for the preservation of endogenous •NO metabolites. This requires working quickly during both sample collecting and processing [85]. Moreover, different machines have different sensitivities. This variability arises from the fact that the reproducibility and sensitivity of the measurements are influenced by the ozone gas stream and the carrier gas flow rate, which are difficult to keep stable [86]. Therefore, for comparing data among experimental groups, the measurements need to be performed with the same machine [84].
Moreover, chemiluminescence methods require very specific equipment, time-consuming detection procedures, and frequent equipment maintenance, as well as a deep knowledge and understanding of the reactions that occur during measurement and data analysis [76,77]. Manual injection and manual data analysis (peak integration) are time-consuming and require experienced scientists.
However, if the proper precautions are taken to avoid contamination, interference in measurement, and errors in data analysis, ozone-based chemiluminescence can be considered one of the best techniques for the determination of •NO metabolites, mainly due to its high sensitivity, high selectivity, and the possibility of coupling with other analytical techniques.
6. Alternatives for the Detection of •NO Species in Biological Samples
CLD is one of the most widely used techniques for the determination of •NO and •NO derivatives, but many other methods are also currently in use. In this section, we give a quick overview of the alternatives that can be used, which are also summarized in Table 2.
6.1. Electrochemical Sensors (Electrodes)
An electrochemical sensor detects the electric current that is produced from the chemical reactions occurring at the electrode. The electrochemical detection of •NO is based on the ability of •NO to be oxidized or reduced. However, the oxidation of •NO is mainly used in commercial electrodes [87,88]. There are also electrochemical sensors like the ion-selective electrode (ISE) that can be used specifically for nitrate detection (NO3−-ISE) [89].
6.2. Fluorescence
Fluorescent probes allow for the indirect detection of •NO through its reaction with fluorophore, resulting in the formation of a fluorescent molecule. The most used fluorescent agents are 2,3-diaminonaphthalene (DAN) and diaminofluorescein (DAF). DANs react with nitrite under acidic conditions to produce fluorescent deprotonated naphthotriazol [90]. DAFs can be oxidized in the presence of •NO, forming a fluorescent derivative triazolofluorescein [91,92]. The specificity and applicability of DANs and DAFs are limited by a number of issues, including the formation of secondary fluorescent products, the autocatalytic effects of the reactions, and the dependency of the reactions on the presence of other radicals and antioxidants.
6.3. Electron Paramagnetic Resonance (EPR)
EPR is a specific method used to indirectly detect •NO formation in cells or tissues using spin traps like Hb, nitronylnitroxides, and the iron–dithiocarbamate complex [93,94,95]. Moreover, •NO-derived produced in biological systems like nitrosylhemoglobin (HbNO) and DINCs can be specifically detected with EPR.
6.4. Membrane Inlet Mass Spectrometry
Membrane inlet mass spectrometry was used to measure •NO in aqueous solution with a detection limit of 10 nM and linear response of 50 μM. This technique uses a semipermeable membrane that allows for the introduction of selectively small molecules like •NO into the MS [96].
6.5. UV-Visible Spectrophotometry for •NO Determination
Spectrometric detection of •NO is based on the change in the absorption spectrum when oxyhemoglobin is oxidized to methemoglobin in the presence of •NO in aqueous solution [97]. This method was applied to determine •NO formation in endothelial cells. It has the advantage of being highly specific for •NO. However, its applicability is limited to enzymatic reactions producing •NO in the absence of background or interference.
6.6. Griess Assay
The Griess assay is a colorimetric method used for the determination of nitrite. This method is based on the formation of a red-violet azo compound (λmax ≈ 540 nm) by the reaction of nitrite with the amino group of sulfanilic acid to form a diazonium cation, which then couples to α-naphthylamine. For the determination of nitrate, a reduction from nitrate to nitrite is needed before conducting the assay [98].
An automated system for the analysis of nitrite and nitrate was successfully established in 1982 [99]. Later, it was commercialized and developed as ENO-10, ENO-20, and the ENO-30 NOx analyzer (Eicom Corporation) [100,101]. Such a system was constructed to obtain high sensitivity by coupling HPLC with the diazotization reaction method (Griess reaction). In brief, nitrite and nitrate in the samples are firstly separated on the reversed-phase HPLC column, followed by flowing through a copper-plated cadmium reduction column, where nitrate is reduced to nitrite. The resolved eluents then react with the Griess reagent to form the azo dye compounds, which are detected with a spectrophotometer [99,101].
An FIA/HPLC system was also applied for the reproducible determination of plasma nitrite levels based on the Griess reaction. This method may enable high-throughput measurements in plasma, e.g., clinical studies with a detection limit of 10 nM [102].

Table 2.
Overview of the main techniques applied for the determination of •NO and •NO derivatives. The limit of detection, limit of quantitation, and dynamic range are highly dependent on the matrix and need to be determined for each experimental setting.
Table 2.
Overview of the main techniques applied for the determination of •NO and •NO derivatives. The limit of detection, limit of quantitation, and dynamic range are highly dependent on the matrix and need to be determined for each experimental setting.
| Method | Common Applications | Applications | Analytical Parameters | References |
|---|---|---|---|---|
| Electrochemical sensors | Direct on-line •NO detection with electrooxidation or electroreduction | Real-time •NO quantification in biological systems; •NO detection in tissues and cells | Sensitivity: 3.5–106 pA per 1 µM change in •NO concentration | [87,88] |
| Fluorescence | Indirect detection of •NO with the formation of a fluorescent molecule | Detection of •NO in cells and tissues | Limit of detection: 3–5 nM; Sensitivity: matrix-dependent | [90,91,92] |
| Electron paramagnetic resonance | Indirect detection of •NO; direct detection of HbNO and dinitrosyl iron complex | •NO and HbNO detection in cells and tissues | Limit of detection: 500 nM | [93,94,95] |
| Mass spectrometry | Detection of •NO with multiple ion detection | •NO detection in aqueous solution | Limit of detection: 10 nM | [96] |
| UV-visible spectrophotometry | Indirect detection of •NO with oxyhemoglobin oxidation | •NO formation in cells and tissues | Limit of detection: 0.2 nmol/min | [97,103] |
| Griess assay | Determination of nitrite and nitrate with the formation of an azo dye in acidic condition | Determination of nitrite levels in biological systems | Limit of detection: 1–2 µM | [98,104] |
7. Practical Considerations about Choosing CLD as Detection Method
Many techniques are capable of detecting •NO species in biological samples. However, it is necessary to choose the technique depending on the specific needs of the experiments. For example, as the main players in the nitrate–nitrite-•NO/nitrosothiols biological pathway, nitrite and nitrate can be measured using the Griess assay (with the diazotiation of nitrite, where nitrate requires reduction to nitrite first) or using ozone-based CLD of •NO [105,106,107,108]. The Griess assay with the colorimetric determination of the diazotization product is simple, fast, and affordable (chemicals and UV-vis spectrophotometer are relatively low costs as compared with other instruments). As mentioned before in Section 5, ozone-based chemiluminescence depends on the availability of a •NO analyzer for the direct measurement of gaseous •NO or those derived from the reduction of nitrite and nitrate. The detection procedures using ozone-based chemiluminescence also require more experience to avoid contamination and to carry out the reactions, but it is superior in sensitivity.
As reported by many researchers, the Griess assay has a limit of detection of around 1 μM in plasma [108,109,110,111]. In contrast, ozone-based chemiluminescence is able to quantify nitrite and other NO metabolites at the nM range in biological matrixes, which is much more sensitive compared with the Griess assay [43]. Moreover, direct quantification allows for an ozone-based chemiluminescence analyzer to measure even exhaled •NO [112,113].
The choice of the technique needs to be based on the detection range and the sample type. For certain samples, such as wastewater or soil, with a rather complex matrix, a coupled technique such as HPLC-CLD or GC-CLD can be a solution instead of relying on CLD alone. As mentioned above, such a coupling system with chromatography provides additional separation before CLD, which reduces the matrix effect and the chance of under- or over-estimation. Furthermore, MS can bring highly accurate qualitative analysis and even the possibility of isotopic tracing with 15N-labeled substrate for the study of enzymatic pathways and metabolomics.
As with all semiquantitative and quantitative analytical techniques, calibrants should be prepared in a matrix as close as possible to the samples, which means the interference from other compounds in the sample matrix also needs to be included in the calibrants. The classes of target analytes (nitrite, nitrate, etc.) and their estimated levels in the samples need to be confirmed to define the calibration range. The detected concentrations of samples need to be located in the linear range of the calibration; otherwise, the samples need to be diluted.
Chemiluminescence depends on the reaction of •NO with ozone. However, some species such as ethylenic hydrocarbons, sulfur compounds, and carbonyls can react with ozone and produce chemiluminescence signals, which may lead to interference for accurate detection [114]. Specifically, some substituted ethylenes can react with ozone and produce strong chemiluminescence signals at higher or lower pressure in the analyzer. Sulfur compounds, such as hydrogen sulfide or dimethyl sulfide, can produce intensive signals once they react with ozone. Carbonyls can be introduced from experimental treatments, such as treatment with CO to block oxy/deoxyhemoglobin, which interferes with detection by reacting with ozone. The disturbance of interferants like ethylenic hydrocarbons and sulfur to the •NO/ozone reaction can be eliminated by increasing the detection wavelength to >600 nm (440 to 470 nm for ethylenic hydrocarbons, <400 nm for sulfur). However, even if some of the interference can be reduced or overcome with methods such as adjusting the wavelength, adjusting the pressure of the analyzer, or filtering, artificial contamination needs to be avoided or carefully evaluated before starting.
Some critical points for the technical procedures of ozone-based chemiluminescence in biological samples have been summarized in Table 3.

Table 3.
Technical notes for performing ozone-based chemiluminescence in biological samples.
8. Successful Application in Different Fields
CLD has been applied to determine •NO and its metabolites in different fields of research. The analysis of nitrogen species NOx in the air for the monitoring of air pollution as well as the composition of exhausted air from motors and cars was one of the first applications of CLD for determining •NO species and is still used today [115,116,117]. Another application is the determination of the concentration of NO3− (derived from fertilizer) and other •NO species in lakes, oceans, rivers, and drinking water [118,119,120].
The analysis of •NO species in the gas phase is not only used for environmental monitoring but also for biomedical and clinical research. Already in 1991, it was shown that endogenous •NO is present in the exhaled air of different animal species and humans and can be measured with CLD [121]. Follow-up studies found differences in exhaled •NO in asthmatic patients compared with healthy individuals [122]. CLD was also applied to detect •NO released by vasoactive drugs in exhaled air from pigs [123]. In this interesting pioneering study, the authors concluded that the measurement of exhaled •NO could be a possible indicator of pulmonary endothelial dysfunction or hypertension. Measurement of exhaled •NO is still used as a reproducible noninvasive indicator of inflammation of the airways [124,125].
The measurement of •NO metabolites in plasma, urine, or tissues was also used to investigate the role of endogenous •NO production or the effect of intake of nitrate in the body [126,127,128,129].
This is based on the principle that •NO produced in the body is immediately oxidized into nitrite, nitrate, and nitroso compounds that can be measured as •NO metabolites [130,131]. Nitrite concentration in plasma was proposed as an indicator of endothelial dysfunction and endothelial NO synthase (eNOS)-derived NO bioavailability [102,132]. Global eNOS knock-out (KO) mice show decreased circulating nitrite and nitrate metabolites [130,131,133,134]. Recently, we found that mice lacking eNOS in endothelial cells show a decrease in circulating nitrite and nitrate in plasma. However, restoring eNOS expression only in endothelial cells of global eNOS KO mice does not lead to the recovery of nitrite levels in the plasma. This indicates that the levels of nitrite in plasma are not only dependent on eNOS activity in the endothelium. Another finding of this cited study was that also eNOS expressed in RBCs contributes to the circulating nitrite levels [39,106].
In addition, the activity of nNOS and iNOS may also contribute to overall •NO metabolites [131]. Recently, it was reported that COVID-19 patients show a decrease in •NO and sulfide metabolites in plasma [135].
Due to the biological variability in circulating •NO, nitrate, and nitrite levels, the measurements of these metabolites could not be used as clinical biomarkers until now. However, measuring these metabolites with CLD is still considered an important approach to understanding the systemic •NO pathway in health and disease.
Regarding nitrate and nitrite levels in the circulation, it is important to note that a main factor in modulating those levels is the dietary intake of nitrate. There are multiple studies using CLD for analyzing nitrate and nitrite levels in food and drinking water [136], which is also important due to the fact that a high intake of nitrate has been considered harmful and cancerogenic due to the conversion of nitrate into nitrite by bacteria and further metabolism into nitrosamine [137,138]. Furthermore, sodium nitrate and potassium nitrate as well as sodium nitrite and potassium nitrite are used in the food industry, especially for the preservation of meat as additives [139]. Therefore, it was proposed to control nitrate and nitrite levels as a precaution to avoid potential health risks [140]. On the other hand, it has been shown that the supplementation of nitrate lowers blood pressure in humans, is cardioprotective, and shows the importance of dietary nitrate intake [128,129,141].
•NO signaling is known to play an important role in plant biology and is involved in multiple processes [142]. Similar to animals and humans, plants can use NADH and nitrite to generate •NO but do not express a NO synthase. The measurement of these molecules is not only important for better insight into the signaling of plants but can also provide information about the accumulation of nitrate in the leaves and vegetables of plants due to the use of fertilizers.
In conclusion, the detection of •NO with CLD is a widely used technique in multiple fields and is continuously improved and adapted for new possible usage.
9. Summary and Conclusions
The observation that •NO reacts with ozone, leading to the emission of light, was first described and used to quantify •NO by amplifying the signal in a photomultiplier tube. Chemiluminescence-based •NO detection allowed researchers to confirm the nature of EDRF as •NO and afterward to investigate •NO and its derived species in biological samples in a specific, precise, and direct way.
Its high sensitivity and wide dynamic range make CLD an indispensable tool for quantitative analysis. To increase the applicability and specificity of the detection of nitrite, nitrate, or other metabolites, many scientists have combined the advantages of CLD with various analytical separative approaches, such as chromatography, MS, microdialysis, and FIA. These multilevel approaches enabled researchers to obtain both quantitative and qualitative profiles including the isotope tracing of 14NO and 15NO for enzymatic activity or in vivo monitoring of important •NO-derived species such as nitrite and peroxynitrite.
Chemiluminescence-based •NO detection has been applied in various fields, including the investigation of environmental pollution related to inorganic nitrates, as well as biomedical and clinical research on endogenous •NO metabolites as indicators of endothelial dysfunction. It is important to note that ozone-based CLD is a highly sensitive method for the detection of •NO species, but it needs to be operated under highly controlled conditions. Indeed, the system requires meticulous calibration, and both sample preparation and analysis must be conducted with careful consideration of sample composition and target analytes. Factors such as the presence of contaminants and molecules that could potentially interfere with the reactions in the chamber or the detector also need attention. Additionally, it is crucial to account for aspects like sample collection and storage, as well as technical issues such as system leakage, baseline fluctuations, contamination from surrounding air, and the exhaustion of ozone in the chamber.
In conclusion, chemiluminescence-based •NO determination is a very useful and versatile tool for the quantitative analysis of •NO and its metabolites in various samples and can be applied in many fields. The power of CLD for the detection of •NO species can be further enhanced when coupled with other analytical separative techniques, delivering a more vivid picture of the status of •NO metabolism in various samples. These techniques, coupled with the cell-specific genetic manipulation of NOS enzyme and/or pharmacological/dietetic intervention, will continue to reveal how •NO metabolism is regulated in vivo.
Author Contributions
Conceptualization, J.L. and M.M.C.-K.; writing—original draft preparation, J.L.; writing—review and editing, J.L., A.L., S.K.H. and M.M.C.-K.; visualization, J.L.; funding acquisition, J.L. and M.M.C.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by research grants from the Research Commission, Medical Faculty of the Heinrich Heine University Duesseldorf (to J.L.); from the German Research Council (DFG) (to M.M.C.-K. and Dr. Johannes Stegbauer), grant number 263779315; and from DFG (to M.M.C.-K.), grant number 521638178.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are indebted to Zhixin Li for her editorial help.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wardman, P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: Progress, pitfalls, and prospects. Free Radic. Biol. Med. 2007, 43, 995–1022. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Darley-Usmar, V.; Davies, K.J.; Dennery, P.A.; Forman, H.J.; Grisham, M.B.; Mann, G.E.; Moore, K.; Roberts, L.J., 2nd; Ischiropoulos, H. Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free Radic. Biol. Med. 2012, 52, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, H.O. Über die Chemiluminescenz des Aminophthalsäurehydrazids. Z. Für Phys. Chem. 1928, 136U, 321–330. [Google Scholar] [CrossRef]
- Whitehead, T.P.; Thorpe, G.H.G.; Carter, T.J.N.; Groucutt, C.; Kricka, L.J. Enhanced luminescence procedure for sensitive determination of peroxidase-labelled conjugates in immunoassay. Nature 1983, 305, 158–159. [Google Scholar] [CrossRef]
- Radi, R.; Cosgrove, T.P.; Beckman, J.S.; Freeman, B.A. Peroxynitrite-induced luminol chemiluminescence. Biochem. J. 1993, 290 Pt 1, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Kooy, N.W.; Royall, J.A. Agonist-induced peroxynitrite production from endothelial cells. Arch. Biochem. Biophys. 1994, 310, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Radi, R.; Peluffo, G.; Alvarez, M.N.; Naviliat, M.; Cayota, A. Unraveling peroxynitrite formation in biological systems. Free Radic. Biol. Med. 2001, 30, 463–488. [Google Scholar] [CrossRef] [PubMed]
- Moller, M.N.; Rios, N.; Trujillo, M.; Radi, R.; Denicola, A.; Alvarez, B. Detection and quantification of nitric oxide-derived oxidants in biological systems. J. Biol. Chem. 2019, 294, 14776–14802. [Google Scholar] [CrossRef] [PubMed]
- Fontijn, A.; Sabadell, A.J.; Ronco, R.J. Homogeneous chemiluminescent measurement of nitric oxide with ozone. Implications for continuous selective monitoring of gaseous air pollutants. Anal. Chem. 1970, 42, 575–579. [Google Scholar] [CrossRef]
- Feelisch, M.; Rassaf, T.; Mnaimneh, S.; Singh, N.; Bryan, N.S.; Jourd’Heuil, D.; Kelm, M. Concomitant S-, N-, and heme-nitros(yl)ation in biological tissues and fluids: Implications for the fate of NO in vivo. FASEB J. 2002, 16, 1775–1785. [Google Scholar] [CrossRef]
- Palmer, R.M.; Ferrige, A.G.; Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L.J.; Buga, G.M.; Wood, K.S.; Byrns, R.E.; Chaudhuri, G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. USA 1987, 84, 9265–9269. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Cherry, P.D.; Zawadzki, J.V.; Jothianandan, D. Endothelial cells as mediators of vasodilation of arteries. J. Cardiovasc. Pharmacol. 1984, 6 (Suppl. 2), S336–S343. [Google Scholar] [CrossRef]
- Kelm, M.; Feelisch, M.; Spahr, R.; Piper, H.M.; Noack, E.; Schrader, J. Quantitative and kinetic characterization of nitric oxide and EDRF released from cultured endothelial cells. Biochem. Biophys. Res. Commun. 1988, 154, 236–244. [Google Scholar] [CrossRef]
- Doyle, M.P.; Hoekstra, J.W. Oxidation of nitrogen oxides by bound dioxygen in hemoproteins. J. Inorg. Biochem. 1981, 14, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.S.; Tannenbaum, S.R.; Deen, W.M. Kinetics of N-Nitrosation in Oxygenated Nitric Oxide Solutions at Physiological pH: Role of Nitrous Anhydride and Effects of Phosphate and Chloride. J. Am. Chem. Soc. 1995, 117, 3933–3939. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Koning, A.; Kuhnle, G.G.C.; Nagy, P.; Bianco, C.L.; Pasch, A.; Wink, D.A.; Fukuto, J.M.; Jackson, A.A.; van Goor, H.; et al. The Reactive Species Interactome: Evolutionary Emergence, Biological Significance, and Opportunities for Redox Metabolomics and Personalized Medicine. Antioxid. Redox Signal. 2017, 27, 684–712. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Czapski, G. Kinetics of Nitric Oxide Autoxidation in Aqueous Solution in the Absence and Presence of Various Reductants. The Nature of the Oxidizing Intermediates. J. Am. Chem. Soc. 1995, 117, 12078–12084. [Google Scholar] [CrossRef]
- Goldstein, S.; Czapski, G. Mechanism of the Nitrosation of Thiols and Amines by Oxygenated •NO Solutions: the Nature of the Nitrosating Intermediates. J. Am. Chem. Soc. 1996, 118, 3419–3425. [Google Scholar] [CrossRef]
- Bryan, N.S.; Rassaf, T.; Maloney, R.E.; Rodriguez, C.M.; Saijo, F.; Rodriguez, J.R.; Feelisch, M. Cellular targets and mechanisms of nitros(yl)ation: An insight into their nature and kinetics in vivo. Proc. Natl. Acad. Sci. USA 2004, 101, 4308–4313. [Google Scholar] [CrossRef]
- Moller, M.N.; Li, Q.; Vitturi, D.A.; Robinson, J.M.; Lancaster, J.R., Jr.; Denicola, A. Membrane “lens” effect: Focusing the formation of reactive nitrogen oxides from the *NO/O2 reaction. Chem. Res. Toxicol. 2007, 20, 709–714. [Google Scholar] [CrossRef]
- Jourd’heuil, D.; Jourd’heuil, F.L.; Feelisch, M. Oxidation and nitrosation of thiols at low micromolar exposure to nitric oxide. Evidence for a free radical mechanism. J. Biol. Chem. 2003, 278, 15720–15726. [Google Scholar] [CrossRef]
- Toledo, J.C., Jr.; Bosworth, C.A.; Hennon, S.W.; Mahtani, H.A.; Bergonia, H.A.; Lancaster, J.R., Jr. Nitric oxide-induced conversion of cellular chelatable iron into macromolecule-bound paramagnetic dinitrosyliron complexes. J. Biol. Chem. 2008, 283, 28926–28933. [Google Scholar] [CrossRef] [PubMed]
- Bartberger, M.D.; Liu, W.; Ford, E.; Miranda, K.M.; Switzer, C.; Fukuto, J.M.; Farmer, P.J.; Wink, D.A.; Houk, K.N. The reduction potential of nitric oxide (NO) and its importance to NO biochemistry. Proc. Natl. Acad. Sci. USA 2002, 99, 10958–10963. [Google Scholar] [CrossRef] [PubMed]
- Smulik, R.; Debski, D.; Zielonka, J.; Michalowski, B.; Adamus, J.; Marcinek, A.; Kalyanaraman, B.; Sikora, A. Nitroxyl (HNO) reacts with molecular oxygen and forms peroxynitrite at physiological pH. Biological Implications. J. Biol. Chem. 2014, 289, 35570–35581. [Google Scholar] [CrossRef]
- Blough, N.V.; Zafiriou, O.C. Reaction of superoxide with nitric oxide to form peroxonitrite in alkaline aqueous solution. Inorg. Chem. 1985, 24, 3502–3504. [Google Scholar] [CrossRef]
- Ferrer-Sueta, G.; Campolo, N.; Trujillo, M.; Bartesaghi, S.; Carballal, S.; Romero, N.; Alvarez, B.; Radi, R. Biochemistry of Peroxynitrite and Protein Tyrosine Nitration. Chem. Rev. 2018, 118, 1338–1408. [Google Scholar] [CrossRef]
- Herold, S.; Shivashankar, K. Metmyoglobin and methemoglobin catalyze the isomerization of peroxynitrite to nitrate. Biochemistry 2003, 42, 14036–14046. [Google Scholar] [CrossRef]
- Romero, N.; Radi, R.; Linares, E.; Augusto, O.; Detweiler, C.D.; Mason, R.P.; Denicola, A. Reaction of human hemoglobin with peroxynitrite. Isomerization to nitrate and secondary formation of protein radicals. J. Biol. Chem. 2003, 278, 44049–44057. [Google Scholar] [CrossRef]
- Joshi, M.S.; Ferguson, T.B., Jr.; Han, T.H.; Hyduke, D.R.; Liao, J.C.; Rassaf, T.; Bryan, N.; Feelisch, M.; Lancaster, J.R., Jr. Nitric oxide is consumed, rather than conserved, by reaction with oxyhemoglobin under physiological conditions. Proc. Natl. Acad. Sci. USA 2002, 99, 10341–10346. [Google Scholar] [CrossRef] [PubMed]
- Rosier, B.T.; Buetas, E.; Moya-Gonzalvez, E.M.; Artacho, A.; Mira, A. Nitrate as a potential prebiotic for the oral microbiome. Sci. Rep. 2020, 10, 12895. [Google Scholar] [CrossRef]
- Millar, T.M.; Stevens, C.R.; Benjamin, N.; Eisenthal, R.; Harrison, R.; Blake, D.R. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998, 427, 225–228. [Google Scholar] [CrossRef]
- Li, H.; Cui, H.; Liu, X.; Zweier, J.L. Xanthine oxidase catalyzes anaerobic transformation of organic nitrates to nitric oxide and nitrosothiols: Characterization of this mechanism and the link between organic nitrate and guanylyl cyclase activation. J. Biol. Chem. 2005, 280, 16594–16600. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Haydar, S.M.; Pearl, V.; Lundberg, J.O.; Weitzberg, E.; Ahluwalia, A. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic. Biol. Med. 2013, 55, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef]
- Jansson, E.A.; Huang, L.; Malkey, R.; Govoni, M.; Nihlen, C.; Olsson, A.; Stensdotter, M.; Petersson, J.; Holm, L.; Weitzberg, E.; et al. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat. Chem. Biol. 2008, 4, 411–417. [Google Scholar] [CrossRef]
- Keller, T.C.S.t.; Lechauve, C.; Keller, A.S.; Brooks, S.; Weiss, M.J.; Columbus, L.; Ackerman, H.; Cortese-Krott, M.M.; Isakson, B.E. The role of globins in cardiovascular physiology. Physiol. Rev. 2022, 102, 859–892. [Google Scholar] [CrossRef]
- Cosby, K.; Partovi, K.S.; Crawford, J.H.; Patel, R.P.; Reiter, C.D.; Martyr, S.; Yang, B.K.; Waclawiw, M.A.; Zalos, G.; Xu, X.; et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003, 9, 1498–1505. [Google Scholar] [CrossRef]
- LoBue, A.; Heuser, S.K.; Lindemann, M.; Li, J.; Rahman, M.; Kelm, M.; Stegbauer, J.; Cortese-Krott, M.M. Red blood cell endothelial nitric oxide synthase: A major player in regulating cardiovascular health. Br. J. Pharmacol. 2023. [Google Scholar] [CrossRef]
- Jia, L.; Bonaventura, C.; Bonaventura, J.; Stamler, J.S. S-nitrosohaemoglobin: A dynamic activity of blood involved in vascular control. Nature 1996, 380, 221–226. [Google Scholar] [CrossRef]
- Gladwin, M.T.; Schechter, A.N.; Shelhamer, J.H.; Pannell, L.K.; Conway, D.A.; Hrinczenko, B.W.; Nichols, J.S.; Pease-Fye, M.E.; Noguchi, C.T.; Rodgers, G.P.; et al. Inhaled nitric oxide augments nitric oxide transport on sickle cell hemoglobin without affecting oxygen affinity. J. Clin. Investig. 1999, 104, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bryan, N.S.; MacArthur, P.H.; Rodriguez, J.; Gladwin, M.T.; Feelisch, M. Measurement of nitric oxide levels in the red cell: Validation of tri-iodide-based chemiluminescence with acid-sulfanilamide pretreatment. J. Biol. Chem. 2006, 281, 26994–27002. [Google Scholar] [CrossRef]
- MacArthur, P.H.; Shiva, S.; Gladwin, M.T. Measurement of circulating nitrite and S-nitrosothiols by reductive chemiluminescence. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 851, 93–105. [Google Scholar] [CrossRef]
- Samouilov, A.; Zweier, J.L. Development of chemiluminescence-based methods for specific quantitation of nitrosylated thiols. Anal. Biochem. 1998, 258, 322–330. [Google Scholar] [CrossRef]
- Fang, K.; Ragsdale, N.V.; Carey, R.M.; MacDonald, T.; Gaston, B. Reductive assays for S-nitrosothiols: Implications for measurements in biological systems. Biochem. Biophys. Res. Commun. 1998, 252, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Smarason, A.K.; Allman, K.G.; Young, D.; Redman, C.W. Elevated levels of serum nitrate, a stable end product of nitric oxide, in women with pre-eclampsia. Br. J. Obstet. Gynaecol. 1997, 104, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Abu-Alghayth, M.; Vanhatalo, A.; Wylie, L.J.; McDonagh, S.T.; Thompson, C.; Kadach, S.; Kerr, P.; Smallwood, M.J.; Jones, A.M.; Winyard, P.G. S-nitrosothiols, and other products of nitrate metabolism, are increased in multiple human blood compartments following ingestion of beetroot juice. Redox Biol. 2021, 43, 101974. [Google Scholar] [CrossRef] [PubMed]
- Gladwin, M.T.; Wang, X.; Reiter, C.D.; Yang, B.K.; Vivas, E.X.; Bonaventura, C.; Schechter, A.N. S-Nitrosohemoglobin is unstable in the reductive erythrocyte environment and lacks O2/NO-linked allosteric function. J. Biol. Chem. 2002, 277, 27818–27828. [Google Scholar] [CrossRef]
- Marley, R.; Feelisch, M.; Holt, S.; Moore, K. A chemiluminescense-based assay for S-nitrosoalbumin and other plasma S-nitrosothiols. Free Radic. Res. 2000, 32, 1–9. [Google Scholar] [CrossRef]
- Ewing, J.F.; Janero, D.R. Specific S-nitrosothiol (thionitrite) quantification as solution nitrite after vanadium(III) reduction and ozone-chemiluminescent detection. Free Radic. Biol. Med. 1998, 25, 621–628. [Google Scholar] [CrossRef]
- Yan, Y.; Shi, P.; Song, W.; Bi, S. Chemiluminescence and Bioluminescence Imaging for Biosensing and Therapy: In Vitro and In Vivo Perspectives. Theranostics 2019, 9, 4047–4065. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhou, W.; Wu, R.; Guan, W.; Ye, N. Recent Advances in Nanomaterial-Based Chemiluminescence Probes for Biosensing and Imaging of Reactive Oxygen Species. Nanomaterials 2023, 13, 1726. [Google Scholar] [CrossRef] [PubMed]
- Fujinari, E.M.; Manes, J.D. Nitrogen-specific detection of peptides in liquid chromatography with a chemiluminescent nitrogen detector. J. Chromatogr. A 1994, 676, 113–120. [Google Scholar] [CrossRef]
- Brannegan, D.; Ashraf-Khorassani, M.; Taylor, L.T. High-performance liquid chromatography coupled with chemiluminescence nitrogen detection for the study of ethoxyquin antioxidant and related organic bases. J. Chromatogr. Sci. 2001, 39, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Kashihira, N.; Makino, K.; Kirita, K.; Watanabe, Y. Chemiluminescent nitrogen detector-gas chromatography and its application to measurement of atmospheric ammonia and amines. J. Chromatogr. A 1982, 239, 617–624. [Google Scholar] [CrossRef]
- Fujinari, E.M. Gas chromatography-chemiluminescent nitrogen detection: GC-CLND. In Developments in Food Science; Wetzel, D.L.B., Charalambous, G., Eds.; Elsevier: Amsterdam, The Netherlands, 1998; Volume 39, pp. 385–424. [Google Scholar]
- Toraman, H.E.; Franz, K.; Ronsse, F.; Van Geem, K.M.; Marin, G.B. Quantitative analysis of nitrogen containing compounds in microalgae based bio-oils using comprehensive two-dimensional gas-chromatography coupled to nitrogen chemiluminescence detector and time of flight mass spectrometer. J. Chromatogr. A 2016, 1460, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Kocak, D.; Ozel, M.Z.; Gogus, F.; Hamilton, J.F.; Lewis, A.C. Determination of volatile nitrosamines in grilled lamb and vegetables using comprehensive gas chromatography—Nitrogen chemiluminescence detection. Food Chem. 2012, 135, 2215–2220. [Google Scholar] [CrossRef] [PubMed]
- Ozel, M.Z.; Hamilton, J.F.; Lewis, A.C. New sensitive and quantitative analysis method for organic nitrogen compounds in urban aerosol samples. Environ. Sci. Technol. 2011, 45, 1497–1505. [Google Scholar] [CrossRef]
- Fujinari, E.M.; Damon Manes, J. Nitrogen-specific liquid chromatography detection of nucleotides and nucleosides by HPLC-CLND. In Developments in Food Science; Charalambous, G., Ed.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 37, pp. 379–396. [Google Scholar]
- Robbat, A.; Corso, N.P.; Liu, T.Y. Evaluation of a nitrosyl-specific gas-phase chemiluminescence detector with high-performance liquid chromatography. Anal. Chem. 1988, 60, 173–174. [Google Scholar] [CrossRef]
- Fujinari, E.M.; Courthaudon, L.O. Nitrogen-specific liquid chromatography detector based on chemiluminescence: Application to the analysis of ammonium nitrogen in waste water. J. Chromatogr. A 1992, 592, 209–214. [Google Scholar] [CrossRef]
- Fujinari, E.M.; Manes, J.D.; Bizanek, R. Peptide content determination of crude synthetic peptides by reversed-phase liquid chromatography and nitrogen-specific detection with a chemiluminescent nitrogen detector. J. Chromatogr. A 1996, 743, 85–89. [Google Scholar] [CrossRef]
- Hayakawa, K.; Lu, C.; Mizukami, S.; Toriba, A.; Tang, N. Determination of 1-nitropyrene metabolites by high-performance liquid chromatography with chemiluminescence detection. J. Chromatogr. A 2006, 1107, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Simultaneous derivatization and quantification of the nitric oxide metabolites nitrite and nitrate in biological fluids by gas chromatography/mass spectrometry. Anal. Chem. 2000, 72, 4064–4072. [Google Scholar] [CrossRef]
- Shin, S.; Fung, H.L. Evaluation of an LC-MS/MS assay for 15N-nitrite for cellular studies of L-arginine action. J. Pharm. Biomed. Anal 2011, 56, 1127–1131. [Google Scholar] [CrossRef]
- Corens, D.; Carpentier, M.; Schroven, M.; Meerpoel, L. Liquid chromatography–mass spectrometry with chemiluminescent nitrogen detection for on-line quantitative analysis of compound collections: Advantages and limitations. J. Chromatogr. A 2004, 1056, 67–75. [Google Scholar] [CrossRef]
- Taylor, E.W.; Jia, W.; Bush, M.; Dollinger, G.D. Accelerating the drug optimization process: Identification, structure elucidation, and quantification of in vivo metabolites using stable isotopes with LC/MSn and the chemiluminescent nitrogen detector. Anal. Chem. 2002, 74, 3232–3238. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, J.; Tran, T.; Turner, N.; Piazza, A.; Mills, L.; Slack, R.; Hauser, S.; Alexander, J.S.; Grisham, M.B.; Feelisch, M.; et al. Isotope tracing enhancement of chemiluminescence assays for nitric oxide research. Biol. Chem. 2009, 390, 181–189. [Google Scholar] [CrossRef]
- Delgado, J.M.; DeFeudis, F.V.; Roth, R.H.; Ryugo, D.K.; Mitruka, B.M. Dialytrode for long term intracerebral perfusion in awake monkeys. Arch. Int. Pharmacodyn. Ther. 1972, 198, 9–21. [Google Scholar] [PubMed]
- Yao, D.; Vlessidis, A.G.; Evmiridis, N.P.; Evangelou, A.; Karkabounas, S.; Tsampalas, S. Luminol chemiluminescense reaction: A new method for monitoring nitric oxide in vivo. Anal. Chim. Acta 2002, 458, 281–289. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Pyszynska, M. Flow-Injection Methods in Water Analysis-Recent Developments. Molecules 2022, 27, 1410. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Fukuda, S.; Hosoi, Y.; Mukai, H. Rapid flow injection analysis method for successive determination of ammonia, nitrite, and nitrate in water by gas-phase chemiluminescence. Anal. Chim. Acta 1997, 349, 11–16. [Google Scholar] [CrossRef]
- Hendgen-Cotta, U.; Grau, M.; Rassaf, T.; Gharini, P.; Kelm, M.; Kleinbongard, P. Reductive gas-phase chemiluminescence and flow injection analysis for measurement of the nitric oxide pool in biological matrices. Methods Enzymol. 2008, 441, 295–315. [Google Scholar] [CrossRef]
- Dai, K.; Vlessidis, A.G.; Evmiridis, N.P. Dialysis membrane sampler for on-line flow injection analysis/chemiluminescence-detection of peroxynitrite in biological samples. Talanta 2003, 59, 55–65. [Google Scholar] [CrossRef]
- Gill, A.; Zajda, J.; Meyerhoff, M.E. Comparison of electrochemical nitric oxide detection methods with chemiluminescence for measuring nitrite concentration in food samples. Anal. Chim. Acta 2019, 1077, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.N.; Green, J.R.; Mutus, B. Fluorescein isothiocyanate, a platform for the selective and sensitive detection of S-Nitrosothiols and hydrogen sulfide. Talanta 2022, 237, 122981. [Google Scholar] [CrossRef]
- Yao, D.; Vlessidis, A.G.; Evmiridis, N.P. Determination of nitric oxide in biological samples. Microchim. Acta 2004, 147, 1–20. [Google Scholar] [CrossRef]
- Garside, C. A chemiluminescent technique for the determination of nanomolar concentrations of nitrate and nitrite in seawater. Mar. Chem. 1982, 11, 159–167. [Google Scholar] [CrossRef]
- Kallakunta, V.M.; Slama-Schwok, A.; Mutus, B. Protein disulfide isomerase may facilitate the efflux of nitrite derived S-nitrosothiols from red blood cells. Redox Biol. 2013, 1, 373–380. [Google Scholar] [CrossRef]
- Doctor, A.; Platt, R.; Sheram, M.L.; Eischeid, A.; McMahon, T.; Maxey, T.; Doherty, J.; Axelrod, M.; Kline, J.; Gurka, M.; et al. Hemoglobin conformation couples erythrocyte S-nitrosothiol content to O2 gradients. Proc. Natl. Acad. Sci. USA 2005, 102, 5709–5714. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J.S.; Jaraki, O.; Osborne, J.; Simon, D.I.; Keaney, J.; Vita, J.; Singel, D.; Valeri, C.R.; Loscalzo, J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc. Natl. Acad. Sci. USA 1992, 89, 7674–7677. [Google Scholar] [CrossRef] [PubMed]
- Gladwin, M.T.; Shelhamer, J.H.; Schechter, A.N.; Pease-Fye, M.E.; Waclawiw, M.A.; Panza, J.A.; Ognibene, F.P.; Cannon, R.O., 3rd. Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proc. Natl. Acad. Sci. USA 2000, 97, 11482–11487. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.K.; Vivas, E.X.; Reiter, C.D.; Gladwin, M.T. Methodologies for the sensitive and specific measurement of S-nitrosothiols, iron-nitrosyls, and nitrite in biological samples. Free Radic. Res. 2003, 37, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Piknova, B.; Park, J.W.; Cassel, K.S.; Gilliard, C.N.; Schechter, A.N. Measuring Nitrite and Nitrate, Metabolites in the Nitric Oxide Pathway, in Biological Materials using the Chemiluminescence Method. J. Vis. Exp. 2016, 118, e54879. [Google Scholar] [CrossRef]
- Bateman, R.M.; Ellis, C.G.; Freeman, D.J. Optimization of nitric oxide chemiluminescence operating conditions for measurement of plasma nitrite and nitrate. Clin. Chem. 2002, 48, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.D.; Schoenfisch, M.H. Electrochemical Nitric Oxide Sensors: Principles of Design and Characterization. Chem. Rev. 2019, 119, 11551–11575. [Google Scholar] [CrossRef] [PubMed]
- Shibuki, K. An electrochemical microprobe for detecting nitric oxide release in brain tissue. Neurosci. Res. 1990, 9, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, B.; Yin, T.; Qin, W. Alternative coulometric signal readout based on a solid-contact ion-selective electrode for detection of nitrate. Anal. Chim. Acta 2020, 1129, 136–142. [Google Scholar] [CrossRef]
- Wiersma, J.H. 2,3-Diaminonaphthalene as a spectrophotometric and fluorometric reagent for the determination of nitrite ion. Anal. Lett. 1970, 3, 123–132. [Google Scholar] [CrossRef]
- Kojima, H.; Sakurai, K.; Kikuchi, K.; Kawahara, S.; Kirino, Y.; Nagoshi, H.; Hirata, Y.; Nagano, T. Development of a fluorescent indicator for nitric oxide based on the fluorescein chromophore. Chem. Pharm. Bull. 1998, 46, 373–375. [Google Scholar] [CrossRef]
- Li, J.; LoBue, A.; Heuser, S.K.; Leo, F.; Cortese-Krott, M.M. Using diaminofluoresceins (DAFs) in nitric oxide research. Nitric Oxide 2021, 115, 44–54. [Google Scholar] [CrossRef]
- Kleschyov, A.L.; Mollnau, H.; Oelze, M.; Meinertz, T.; Huang, Y.; Harrison, D.G.; Munzel, T. Spin trapping of vascular nitric oxide using colloid Fe(II)-diethyldithiocarbamate. Biochem. Biophys. Res. Commun. 2000, 275, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Kalyanaraman, B.; Hyde, J.S. Trapping of nitric oxide by nitronyl nitroxides: An electron spin resonance investigation. Biochem. Biophys. Res. Commun. 1993, 192, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Piknova, B.; Gladwin, M.T.; Schechter, A.N.; Hogg, N. Electron paramagnetic resonance analysis of nitrosylhemoglobin in humans during NO inhalation. J. Biol. Chem. 2005, 280, 40583–40588. [Google Scholar] [CrossRef]
- Goodwin, J.M.; Chrestensen, C.A.; Moomaw, E.W. Detection of Nitric Oxide by Membrane Inlet Mass Spectrometry. Methods Mol. Biol. 2018, 1747, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Feelisch, M.; Noack, E.A. Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase. Eur. J. Pharmacol. 1987, 139, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Griess, P. Bemerkungen zu der Abhandlung der HH. Weselsky und Benedikt “Ueber einige Azoverbindungen”. Eur. J. Inorg. Chem. 1879, 12, 426–428. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Tanaka, S.; Akaike, T.; Fang, J.; Beppu, T.; Ogawa, M.; Tamura, F.; Miyamoto, Y.; Maeda, H. Antiapoptotic effect of haem oxygenase-1 induced by nitric oxide in experimental solid tumour. Br. J. Cancer 2003, 88, 902–909. [Google Scholar] [CrossRef]
- Bryan, N.S.; Grisham, M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic. Biol. Med. 2007, 43, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Kleinbongard, P.; Dejam, A.; Lauer, T.; Jax, T.; Kerber, S.; Gharini, P.; Balzer, J.; Zotz, R.B.; Scharf, R.E.; Willers, R.; et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic. Biol. Med. 2006, 40, 295–302. [Google Scholar] [CrossRef]
- Kelm, M.; Schrader, J. Control of coronary vascular tone by nitric oxide. Circ. Res. 1990, 66, 1561–1575. [Google Scholar] [CrossRef]
- Guevara, I.; Iwanejko, J.; Dembinska-Kiec, A.; Pankiewicz, J.; Wanat, A.; Anna, P.; Golabek, I.; Bartus, S.; Malczewska-Malec, M.; Szczudlik, A. Determination of nitrite/nitrate in human biological material by the simple Griess reaction. Clin. Chim. Acta 1998, 274, 177–188. [Google Scholar] [CrossRef]
- Nagababu, E.; Rifkind, J.M. Measurement of plasma nitrite by chemiluminescence. Methods Mol. Biol. 2010, 610, 41–49. [Google Scholar] [CrossRef]
- Leo, F.; Suvorava, T.; Heuser, S.K.; Li, J.; LoBue, A.; Barbarino, F.; Piragine, E.; Schneckmann, R.; Hutzler, B.; Good, M.E.; et al. Red Blood Cell and Endothelial eNOS Independently Regulate Circulating Nitric Oxide Metabolites and Blood Pressure. Circulation 2021, 144, 870–889. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Rassaf, T.; Dejam, A.; Kerber, S.; Kelm, M. Griess method for nitrite measurement of aqueous and protein-containing samples. Methods Enzymol. 2002, 359, 158–168. [Google Scholar] [CrossRef]
- Giustarini, D.; Rossi, R.; Milzani, A.; Dalle-Donne, I. Nitrite and nitrate measurement by Griess reagent in human plasma: Evaluation of interferences and standardization. Methods Enzymol. 2008, 440, 361–380. [Google Scholar] [CrossRef]
- Tsikas, D. Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: Appraisal of the Griess reaction in the L-arginine/nitric oxide area of research. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 851, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.A.; Storm, W.L.; Coneski, P.N.; Schoenfisch, M.H. Inaccuracies of nitric oxide measurement methods in biological media. Anal. Chem. 2013, 85, 1957–1963. [Google Scholar] [CrossRef] [PubMed]
- Brizzolari, A.; Dei Cas, M.; Cialoni, D.; Marroni, A.; Morano, C.; Samaja, M.; Paroni, R.; Rubino, F.M. High-Throughput Griess Assay of Nitrite and Nitrate in Plasma and Red Blood Cells for Human Physiology Studies under Extreme Conditions. Molecules 2021, 26, 4569. [Google Scholar] [CrossRef] [PubMed]
- Robbins, R.A.; Floreani, A.A.; Von Essen, S.G.; Sisson, J.H.; Hill, G.E.; Rubinstein, I.; Townley, R.G. Measurement of exhaled nitric oxide by three different techniques. Am. J. Respir. Crit. Care Med. 1996, 153, 1631–1635. [Google Scholar] [CrossRef]
- Binding, N.; Muller, W.; Czeschinski, P.A.; Witting, U. NO chemiluminescence in exhaled air: Interference of compounds from endogenous or exogenous sources. Eur. Respir. J. 2000, 16, 499–503. [Google Scholar] [CrossRef]
- Bates, J.N. Nitric oxide measurement by chemiluminescence detection. Neuroprotocols 1992, 1, 141–149. [Google Scholar] [CrossRef]
- Kuik, F.; Kerschbaumer, A.; Lauer, A.; Lupascu, A.; von Schneidemesser, E.; Butler, T.M. Top–down quantification of NOx emissions from traffic in an urban area using a high-resolution regional atmospheric chemistry model. Atmos. Chem. Phys. 2018, 18, 8203–8225. [Google Scholar] [CrossRef]
- Suarez-Bertoa, R.; Astorga, C. Impact of cold temperature on Euro 6 passenger car emissions. Environ. Pollut. 2018, 234, 318–329. [Google Scholar] [CrossRef]
- Joseph, D.W.; Spicer, C.W. Chemiluminescence method for atmospheric monitoring of nitric acid and nitrogen oxides. Anal. Chem. 1978, 50, 1400–1403. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, K.-K.; Yang, G.-P.; Li, P.-F.; Liu, C.-Y.; Ingeniero, R.C.O.; Bange, H.W. Continuous Chemiluminescence Measurements of Dissolved Nitric Oxide (NO) and Nitrogen Dioxide (NO2) in the Ocean Surface Layer of the East China Sea. Environ. Sci. Technol. 2021, 55, 3668–3675. [Google Scholar] [CrossRef]
- Kanda, Y.; Taira, M. Flow-Injection Analysis Method for the Determination of Nitrite and Nitrate in Natural Water Samples Using a Chemiluminescence NOx Monitor. Anal. Sci. 2003, 19, 695–699. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bijay, S.; Craswell, E. Fertilizers and nitrate pollution of surface and ground water: An increasingly pervasive global problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar] [CrossRef]
- Gustafsson, L.E.; Leone, A.M.; Persson, M.G.; Wiklund, N.P.; Moncada, S. Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem. Biophys. Res. Commun. 1991, 181, 852–857. [Google Scholar] [CrossRef]
- Alving, K.; Weitzberg, E.; Lundberg, J.M. Increased amount of nitric oxide in exhaled air of asthmatics. Eur. Respir. J. 1993, 6, 1368–1370. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, R.E.; Tornberg, D.C.; Settergren, G.; Liska, J.; Angdin, M.; Lundberg, J.O.; Weitzberg, E. Endogenous nitric oxide release by vasoactive drugs monitored in exhaled air. Am. J. Respir. Crit. Care Med. 2003, 168, 114–120. [Google Scholar] [CrossRef]
- Berry, M.; Hargadon, B.; Morgan, A.; Shelley, M.; Richter, J.; Shaw, D.; Green, R.H.; Brightling, C.; Wardlaw, A.J.; Pavord, I.D. Alveolar nitric oxide in adults with asthma: Evidence of distal lung inflammation in refractory asthma. Eur. Respir. J. 2005, 25, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Khatri, S.B.; Tejwani, V. Measuring exhaled nitric oxide when diagnosing and managing asthma. Cleve. Clin. J. Med. 2023, 90, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, A.; Mitchell, K.; Blackwell, J.R.; Vanhatalo, A.; Jones, A.M. High-nitrate vegetable diet increases plasma nitrate and nitrite concentrations and reduces blood pressure in healthy women. Public. Health Nutr. 2015, 18, 2669–2678. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.D.; Marsh, A.P.; Dove, R.W.; Beavers, D.; Presley, T.; Helms, C.; Bechtold, E.; King, S.B.; Kim-Shapiro, D. Plasma nitrate and nitrite are increased by a high-nitrate supplement but not by high-nitrate foods in older adults. Nutr. Res. 2012, 32, 160–168. [Google Scholar] [CrossRef]
- Kapil, V.; Milsom, A.B.; Okorie, M.; Maleki-Toyserkani, S.; Akram, F.; Rehman, F.; Arghandawi, S.; Pearl, V.; Benjamin, N.; Loukogeorgakis, S.; et al. Inorganic nitrate supplementation lowers blood pressure in humans: Role for nitrite-derived NO. Hypertension 2010, 56, 274–281. [Google Scholar] [CrossRef]
- Larsen, F.J.; Ekblom, B.; Sahlin, K.; Lundberg, J.O.; Weitzberg, E. Effects of dietary nitrate on blood pressure in healthy volunteers. N. Engl. J. Med. 2006, 355, 2792–2793. [Google Scholar] [CrossRef] [PubMed]
- Kleinbongard, P.; Dejam, A.; Lauer, T.; Rassaf, T.; Schindler, A.; Picker, O.; Scheeren, T.; Godecke, A.; Schrader, J.; Schulz, R.; et al. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic. Biol. Med. 2003, 35, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Milsom, A.B.; Fernandez, B.O.; Garcia-Saura, M.F.; Rodriguez, J.; Feelisch, M. Contributions of nitric oxide synthases, dietary nitrite/nitrate, and other sources to the formation of NO signaling products. Antioxid. Redox Signal. 2012, 17, 422–432. [Google Scholar] [CrossRef]
- Lauer, T.; Preik, M.; Rassaf, T.; Strauer, B.E.; Deussen, A.; Feelisch, M.; Kelm, M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc. Natl. Acad. Sci. USA 2001, 98, 12814–12819. [Google Scholar] [CrossRef]
- Wood, K.C.; Cortese-Krott, M.M.; Kovacic, J.C.; Noguchi, A.; Liu, V.B.; Wang, X.; Raghavachari, N.; Boehm, M.; Kato, G.J.; Kelm, M.; et al. Circulating blood endothelial nitric oxide synthase contributes to the regulation of systemic blood pressure and nitrite homeostasis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Piknova, B.; Huang, P.L.; Noguchi, C.T.; Schechter, A.N. Effect of blood nitrite and nitrate levels on murine platelet function. PLoS ONE 2013, 8, e55699. [Google Scholar] [CrossRef] [PubMed]
- Dominic, P.; Ahmad, J.; Bhandari, R.; Pardue, S.; Solorzano, J.; Jaisingh, K.; Watts, M.; Bailey, S.R.; Orr, A.W.; Kevil, C.G.; et al. Decreased availability of nitric oxide and hydrogen sulfide is a hallmark of COVID-19. Redox Biol. 2021, 43, 101982. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Bhatoee, M.; Singh, P.; Kaladhar, V.C.; Yadav, N.; Paul, D.; Loake, G.J.; Gupta, K.J. Detection of Nitric Oxide from Chickpea Using DAF Fluorescence and Chemiluminescence Methods. Curr. Protoc. 2022, 2, e420. [Google Scholar] [CrossRef] [PubMed]
- Picetti, R.; Deeney, M.; Pastorino, S.; Miller, M.R.; Shah, A.; Leon, D.A.; Dangour, A.D.; Green, R. Nitrate and nitrite contamination in drinking water and cancer risk: A systematic review with meta-analysis. Environ. Res. 2022, 210, 112988. [Google Scholar] [CrossRef]
- Tannenbaum, S.R.; Correa, P. Nitrate and gastric cancer risks. Nature 1985, 317, 675–676. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, T.; Di Taranto, A.; Berardi, G.; Vita, V.; Iammarino, M. Nitrate as food additives: Reactivity, occurrence, and regulation. In Nitrate Handbook; CRC Press: Boca Raton, FL, USA, 2022; pp. 281–300. [Google Scholar]
- Ziarati, P.; Zahedi, M.; Shirkhan, F.; Mostafidi, M. Potential health risks and concerns of high levels of nitrite and nitrate in food sources. SF Pharma J. 2018, 1, 2. [Google Scholar]
- Kapil, V.; Khambata, R.S.; Robertson, A.; Caulfield, M.J.; Ahluwalia, A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: A randomized, phase 2, double-blind, placebo-controlled study. Hypertension 2015, 65, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Tiwari, S.; Singh, V.P.; Prasad, S.M. Nitric oxide in plants: An ancient molecule with new tasks. Plant Growth Regul. 2020, 90, 1–13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).