Abstract
Benzo[a]pyrene (B[a]P), a highly carcinogenic polycyclic aromatic hydrocarbon primarily formed during incomplete organic matter combustion, undergoes a series of hepatic metabolic reactions once absorbed into the body. B[a]P contributes to liver damage, ranging from molecular DNA damage to the onset and progression of various diseases, including cancer. Specifically, B[a]P induces oxidative stress via reactive oxygen species generation within cells. Consequently, more research has focused on exploring the underlying mechanisms of B[a]P-induced oxidative stress and potential strategies to counter its hepatic toxicity. Flavonoids, natural compounds abundant in plants and renowned for their antioxidant properties, possess the ability to neutralize the adverse effects of free radicals effectively. Although extensive research has investigated the antioxidant effects of flavonoids, limited research has delved into their potential in regulating B[a]P metabolism to alleviate oxidative stress. This review aims to consolidate current knowledge on B[a]P-induced liver oxidative stress and examines the role of flavonoids in mitigating its toxicity.
1. Introduction
Benzo[a]pyrene (B[a]P) is a prevalent environmental pollutant present in tobacco smoke, vehicle emissions, and industrial processes, posing serious health risks to human health and the environment [1]. Upon absorption, B[a]P primarily undergoes hepatic metabolic transformations through the actions of enzymes, such as CYP1A1 and CYP1B1, to form B[a]P-7,8-epoxide [2]. Further metabolic processing involving epoxide hydrolase leads to the formation of B[a]P-7,8-dihydrodiol, which is subsequently converted by CYP1 enzymes into B[a]P-7,8-diol-9,10-epoxide (BPDE), a highly reactive intermediate [3].
BPDE, identified as a potent carcinogen, induces DNA damage by forming covalent adducts upon interaction with DNA molecules, thereby leading to mutations and initiating tumorigenesis [1]. The liver, central in B[a]P metabolism, is particularly vulnerable to its toxic effects [4]. Liver tissues have exhibited BPDE-DNA adducts, which are associated with liver cancer development [5]. Additionally, BPDE can induce oxidative stress, leading to an increase in reactive oxygen species (ROS) production [6]. ROS can further damage cellular components, including DNA, proteins, and lipids, contributing to cellular dysfunction and inflammation [7]. This oxidative stress cascade can exacerbate the toxicity of B[a]P and its metabolites, resulting in a spectrum of adverse health effects [8].
Minimizing human exposure to B[a]P and controlling environmental pollution are imperative due to the severe health implications associated with its exposure. Understanding the metabolic and toxic impacts of B[a]P is pivotal in developing preventive and therapeutic measures aimed at safeguarding human liver health. The liver is an important organ that is involved in various metabolic activities. Chronic hepatic damage leads to conditions such as cirrhosis, fibrosis, fatty liver, and even hepatocellular carcinoma, raising serious epidemiological concerns [9]. Despite remarkable progress in modern medicine, the lack of suitable and effective hepatoprotective drugs remains a persistent concern. The withdrawal of numerous drugs from the market due to drug-induced liver injury (DILI) has prompted research into alternative treatment approaches [10]. Currently, the use of naturally derived phytochemicals to treat a variety of diseases is becoming increasingly accepted. Among these bioactive compounds, flavonoids are well-known for their natural origin. Ongoing research into flavonoids has revealed promising health benefits, particularly their reported positive effects on human health [11]. Considering the hepatic metabolism of xenobiotic flavonoids, a comprehensive review of their specific impacts on liver health is essential. Especially, the single or synergistic effect of flavonoids against B[a]P-induced toxicity and drug metabolism have been studied. By exploring their mechanisms of action and interactions within the liver, this review aims to understand the role of flavonoids in promoting liver health and countering B[a]P-induced oxidative stress. In Section 2, we primarily aimed to communicate about oxidative damage during B[a]P metabolism and the subsequent liver damage. Then, in Section 3, the focus shifts to exploring flavonoids as a potential solution to mitigate this damage.
2. B[a]P-Induced Oxidative Liver Damage
2.1. Oxidative Damage Induced by B[a]P in the Liver
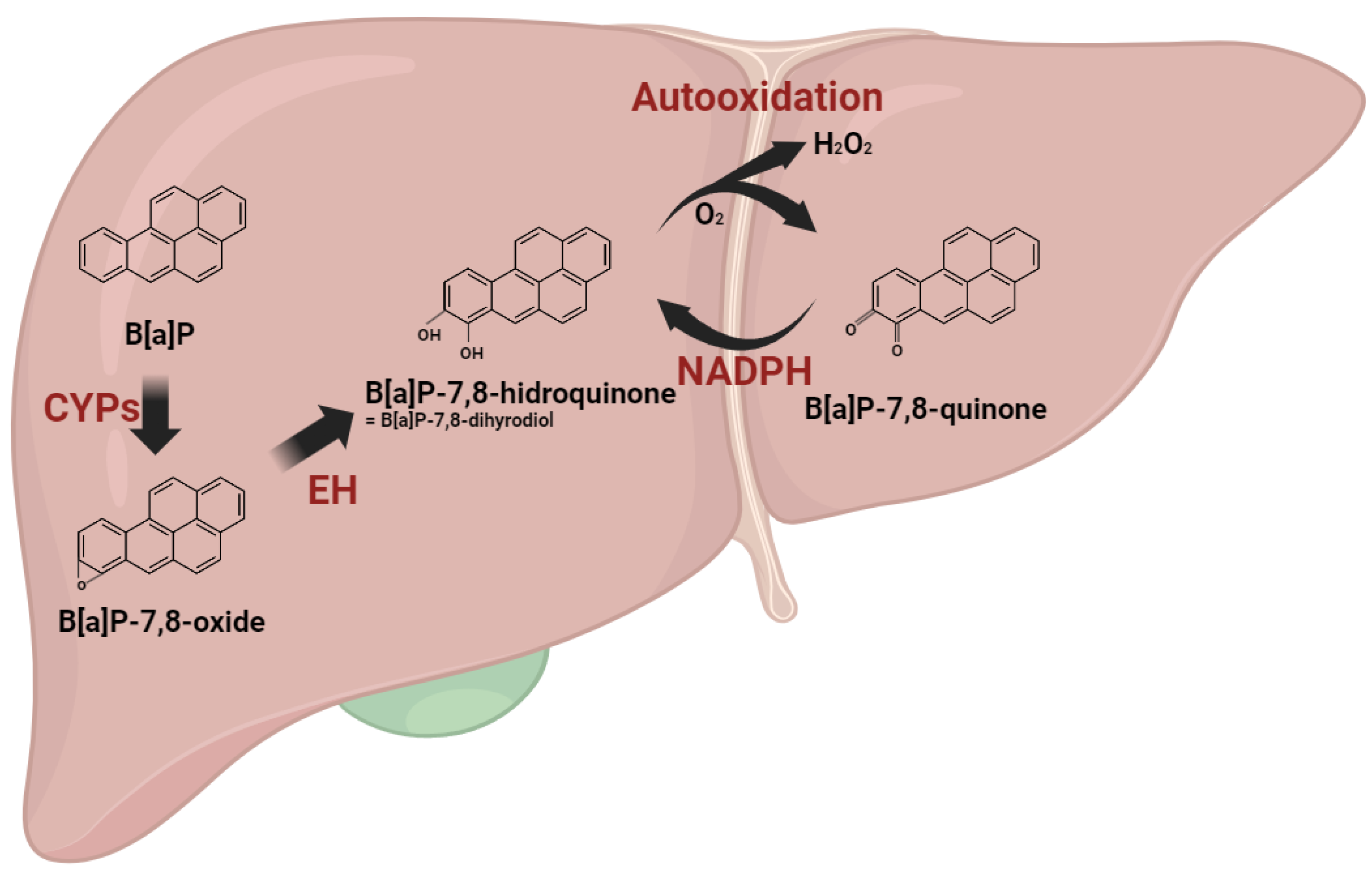
Polycyclic aromatic hydrocarbons (PAHs) undergo metabolic transformation via xenobiotic-metabolizing enzymes upon body absorption. Among these, B[a]P, a PAH, engages in a carcinogenic activation process through three distinct pathways: (1) Radical-cation; (2) Diol epoxide; (3) Quinone pathways [3,12]. In the radical-cation pathway, hydroxylated metabolites, including B[a]P-quinones, such as B[a]P-1,6-dione and B[a]P-6,12-dione, are produced. These B[a]P-quinones, highly reactive due to their chemical structure, undergo enzymatic reduction by NAD(P)H quinone dehydrogenase 1 (NQO1), forming hydroquinone equivalents. Generated from B[a]P-quinones, hydroquinones swiftly undergo two autooxidation cycles, yielding radicals while regenerating quinones. Consequently, these futile cycles result in the combination of molecular oxygen (O2) molecules with B[a]P metabolites, generating superoxide anion radicals (O2−) and hydrogen peroxide (H2O2) [13]. The metabolic processing of B[a]P inevitably leads to the production of ROS (Figure 1).
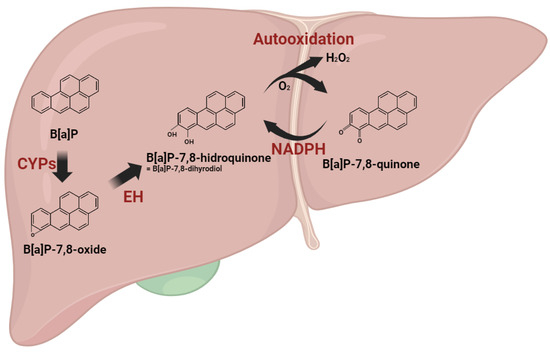
Figure 1.
Reactive oxygen species generation during benzo[a]pyrene metabolism. B[a]P undergoes metabolic transformations initiated by cytochrome P450 enzymes (CYPs), leading to the formation of B[a]P-oxide, which is further converted into B[a]P-hydroquinone. Within the radical cation pathway, hydroxylated metabolites, including B[a]P-quinones, are generated. These B[a]P-quinones undergo enzymatic reduction facilitated by NAD(P)H quinone dehydrogenase 1 (NQO1), resulting in the formation of hydroquinone equivalents. Generated from B[a]P-quinones, hydroquinones swiftly undergo two autooxidation cycles, yielding radicals while regenerating quinones. The metabolic processing of B[a]P inevitably leads to the production of ROS.
ROS function as critical signaling molecules that determine cell fate; however, their excessive accumulation can lead to irreversible cellular damage or death [14]. ROS is a highly reactive molecule that attacks DNA [7]. Particularly, guanine, with the lowest ionization potential among nucleic acid components, becomes a preferred target for one-electron oxidizers, either directly or indirectly [15]. Consequently, the predominant manifestation of oxidative DNA damage is 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxo-dG), generated through the oxidation of the reducing purine radical. Furthermore, the side-oxidation of 8-oxo-dG by highly oxidizing oxyl radicals may lead to the synthesis of oxazolone and imidazolone nucleosides [16]. B[a]P induces ROS release, promoting genotoxicity by generating 8-oxo-dG [17]. In mice orally administered 2 mg/kg of B[a]P for 55 days, a significant increase in 8-oxo-dG levels occurred in the liver, stomach, colon, and kidney by more than a 2–3 fold change. Therefore, 8-oxo-dG is a common form of oxidative DNA damage and serves as a prominent marker of oxidative DNA damage within the DNA molecule [18]. This type of DNA damage can cause mutations in the genetic material, DNA by influencing DNA replication and DNA repair systems, potentially leading to the accumulation of DNA damage and mutations [19]. These mutations can disrupt basic cellular functions such as cell cycle control, cell division, DNA replication, and DNA repair, often associated with cancer development. Specifically, 8-oxo-dG is an important indicator of DNA damage, with numerous studies reporting its relevance to cancer [20,21,22]. The increased presence of 8-oxo-dG correlates with cancer initiation and progression, potentially enhancing carcinogenicity [23]. This accumulation of DNA damage and mutations, with 8-oxo-dG as a key contributor, can significantly contribute to cancer development, positioning it as a crucial biological marker associated with cancer initiation and progression.
In addition to DNA damage, B[a]P-induced ROS triggers various cellular dysfunctions, particularly impacting mitochondrial DNA, which constitutes approximately 1% of total cellular DNA and is believed to be highly susceptible to oxidative stress [24]. Persistent damage to mitochondrial DNA eventually leads to mutations within the mitochondrial genome, exacerbating mitochondrial dysfunction and contributing to the onset and progression of diverse diseases. Moreover, studies reveal a dose-dependent increase in mitochondrial depletion following B[a]P exposure, particularly impacting the liver more significantly than other organs [25]. This emphasizes the significant influence of B[a]P on mitochondrial dysfunction and its potential implications for overall physiological pathogenesis. Mitochondrial dysfunctions disrupt the electron flow in the electron transport chain (ETC), causing electron leakage onto O2, thereby elevating ROS production within damaged mitochondria. Altered ROS levels linked to mitochondrial activity result from disruptions in mitochondrial metabolic processes, such as NADH accumulation in dysfunctional mitochondria. Additionally, damaged mitochondria indirectly elevate ROS production from the endoplasmic reticulum surface [26]. These damaged mitochondria can prematurely release electrons, particularly from Complex I (NADH:ubiquinone oxidoreductase) or Complex II (succinate dehydrogenase) within the ETC, reacting with O2 to produce superoxide radicals (O2•−)—a primary source of ROS [27]. Moreover, damaged mitochondria exhibit impairments in electron carriers and ETC proteins, rendering them more susceptible to electron leakage and subsequent ROS generation [28]. The ROS produced within damaged mitochondria then contribute to a “vicious cycle” by causing additional damage to mitochondrial components, such as lipids, proteins, and nucleic acids (including mtDNA). This cycle of mitochondrial dysfunction and ROS production culminates in further cellular damage and impairments in cellular function.
The response to B[a]P-induced ROS damage varies across different organs [29]. Acute B[a]P exposure significantly decreased body weight across various doses. Previous research indicates variations in oxidative DNA damage among organ tissues, with a prevalence sequence as follows: liver > lung > kidney > brain > stomach [29]. Moreover, B[a]P induces relatively higher oxidative stress in the liver and lungs but exhibits less oxidative stress in the stomach and brain. The liver is one of the essential organs in xenobiotic metabolism and is predominantly involved in B[a]P metabolism. Chronic liver diseases consistently exhibit increased oxidative stress, regardless of their origin. ROS plays a pivotal role in the initiation and progression of numerous liver disorders. Additionally, oxidative stress influences liver fibrogenesis [30].
Exploring the interactions between B[a]P and ROS yields valuable insights into its potential carcinogenicity, cellular dysfunctions, and diverse organ impacts, particularly the liver. Understanding these mechanisms is essential for formulating effective strategies to alleviate the adverse effects of B[a]P and improve B[a]P-induced liver diseases.
2.2. Metabolism and Excretion of B[a]P and Their Hepatic Consequences
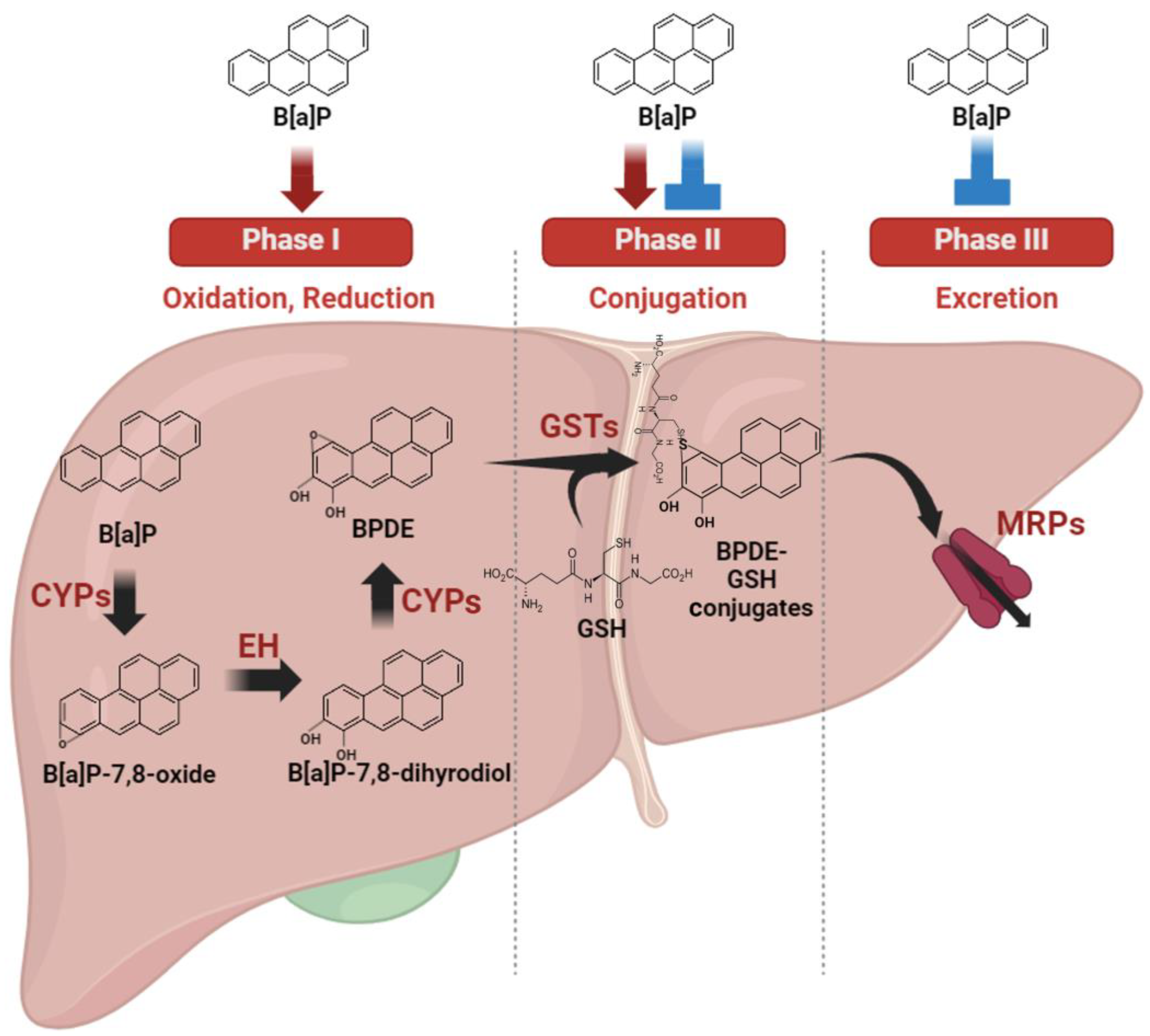
The liver exhibits heightened sensitivity to B[a]P compared with other organs due to its central role as the primary site for drug metabolism (Figure 2). As the primary organ responsible for xenobiotic drug metabolism, the liver significantly contributes to minimizing drug toxicity. This organ undertakes a multi-phase process in xenobiotic metabolism. Phase I involves cytochrome P450 oxidases catalyzing the oxidation or reduction in xenobiotic materials. In phase II, these modified xenobiotics are conjugated with other polar compounds by specific phase II enzymes. Finally, phase III involves the excretion of conjugated xenobiotic materials from cells through efflux transporters, effectively eliminating them from the cells [31].
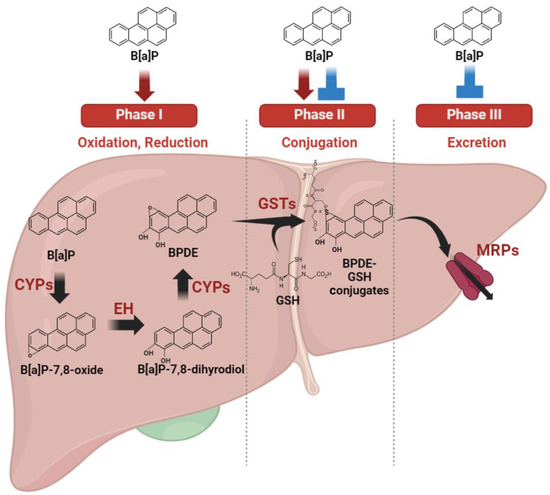
Figure 2.
Metabolic regulation and autoregulation of benzo[a]pyrene metabolism. B[a]P undergoes a sequence of metabolic transformations catalyzed primarily, called phase I, by cytochrome P450 oxidases (CYPs) enzymes, such as CYP1A1 and CYP1B1. Phase II enzymes play a pivotal role in xenobiotic drug metabolism by converting endogenous substances and foreign compounds into more easily excretable forms. Phase III enzymes, including a diverse group of membrane transporters such as the multidrug resistance protein family (MRP), aid in the elimination of conjugated metabolites from cells. B[a]P is a substrate metabolized by these phase enzymes, and concurrently, it has the ability to regulate the expression or activity of phase enzymes.
B[a]P undergoes a sequence of metabolic transformations catalyzed primarily by CYP1 enzymes, such as CYP1A1 and CYP1B1. Initially, B[a]P is metabolized into B[a]P-7,8-epoxide, which is subsequently modified via hydrolysis by epoxide hydrolase, resulting in the formation of B[a]P-7,8-dihydrodiol. Further enzymatic action by CYP1 converts B[a]P-7,8-dihydrodiol into BPDE, a highly reactive compound [32]. BPDE, a major metabolite, forms covalent bonds with DNA by intercalating and reacting with nucleophilic guanine bases, binding specifically to guanine at the N2 position with multiple alignments within the helix [33]. This process results in the formation of bulky guanine adducts [34]. Specifically, the (K)-7S,8R,9R,10S+anti-B(a)PDE enantiomer (10S) DNA adduct positions within the minor groove, oriented towards the 5′ end of the helix, affecting neighboring bases around the guanine adduct [35]. Consequently, these BPDE DNA adducts induce distortions in DNA structure, leading to molecular modifications [36]. Such genetic alterations can impede proper DNA repair mechanisms. Failure in repair processes may result in the transmission of these DNA lesions to daughter cells during cell division, resulting in the accumulation of DNA damage and potentially contributing to cancer development [37]. Clearly, B[a]P metabolites, particularly BPDE, play a crucial role in inducing genetic damage, which, if left unrepaired, can lead to cancer progression. The crucial involvement of CYP1 enzymes and epoxide hydrolase in B[a]P metabolism to form BPDE cannot be overstated. CYP1 enzymes and epoxide hydrolase are regulated by a common transcription factor, the aryl hydrocarbon receptor (AhR) [38]. B[a]P, functioning as an AhR ligand, activates the receptor, subsequently inducing the expression of downstream genes such as CYP1 enzymes and epoxide hydrolase [39]. In essence, B[a]P, by activating AhR, elevates the expression levels of CYP1 enzymes and epoxide hydrolase, thereby increasing the production of carcinogenic BPDE molecules. This mechanism underscores the significance of AhR signaling in B[a]P-induced carcinogenesis and the potential for liver cancer development.
Phase II enzymes play a pivotal role in xenobiotic drug metabolism by converting endogenous substances and foreign compounds into more easily excretable forms. This involves processes such as conjugation with glutathione, glucuronidation, or glycine, contributing to the metabolic deactivation of pharmacologically active substances. Glutathione S-transferases (GST), part of the phase II enzyme group, catalyze the conjugation of reduced glutathione (GSH) with xenobiotic substrates, a crucial step in detoxification [40]. The conjugation of BPDE with GSH represents a pivotal mechanism in cellular detoxification [41]. Another essential superfamily within phase II metabolic enzymes, uridine diphosphate glucuronosyltransferases (UGTs), facilitate the transfer of glucuronosyl groups from uridine 5′-diphosphate-glucuronic acid to substrates containing alcohols, amines, or carboxylic acids as functional groups [42]. Notably, key UGT isoforms such as UGT1A3 and 2B7 have been found to inhibit B[a]P-7,8 catechol. Consequently, decreased phase II enzyme metabolism capacity can lead to the accumulation of xenobiotic material toxicity [43]. Hence, maintaining the activity and expression of phase II enzymes is critical in mitigating the toxic effects of B[a]P. However, research suggests that B[a]P may disrupt the normal regulation of phase II enzymes. For instance, in mice treated with B[a]P, GST activity showed no significant changes in the liver compared with the non-treated group, contrary to the expected activation of GST by typical phase II activation pathways [44]. Typically, phase II enzymes are effectively activated to detoxify xenobiotics formed by phase I enzymes. This discrepancy indicates that the increased BPDE produced in the activated phase I step might pose challenges for proper detoxification. Several studies reveal varying activity and expression levels of GST depending on specific enzyme types and organ types, suggesting diverse susceptibility to B[a]P. Notably, in the lungs, B[a]P significantly induces total GST expression, although they are not activated by B[a]P in the lung [44]. Recent findings revealed the capacity of B[a]P to enhance the activity of specific GST enzymes such as GSTA2, M1, and P1 while concurrently reducing GSTA4 activity [45]. This highlights the crucial need for precise regulation of phase II enzymes inhibited by benzo[a]pyrene to effectively mitigate its toxic impact.
Phase III enzymes, including a diverse group of membrane transporters such as the multidrug resistance protein family [16], aid in the elimination of conjugated metabolites from cells. These proteins, belonging to the ATP-binding cassette (ABC) transporters, facilitate ATP-dependent transport of various hydrophobic anions [46]. Consequently, ABC transporters play a critical role in expelling phase II metabolites out of cells, enabling further metabolism or excretion to occur [47]. The complex detoxification response of ABC transporters to B[a]P exposure warrants further investigation due to limited available information. Nevertheless, some studies suggest their involvement in B[a]P excretion. For instance, in various species, ABC transporters have been identified as responsible for eliminating B[a]P metabolites [48,49]. Furthermore, B[a]P has been shown to reduce the expression levels of ABCA12 [50] and decrease the protein expression level of ABCC1 [51], suggesting a potential hindrance in the proper removal of detoxified B[a]P metabolites from cells due to reduced transporter expression.
In summary, exploring the regulation of genes associated with the xenobiotic metabolism B[a]P will provide valuable insights into the susceptibility of the liver to B[a]P.
3. Hepatoprotective Potential of Flavonoids against B[a]P-Induced Liver Damage
3.1. Classification of Flavonoids
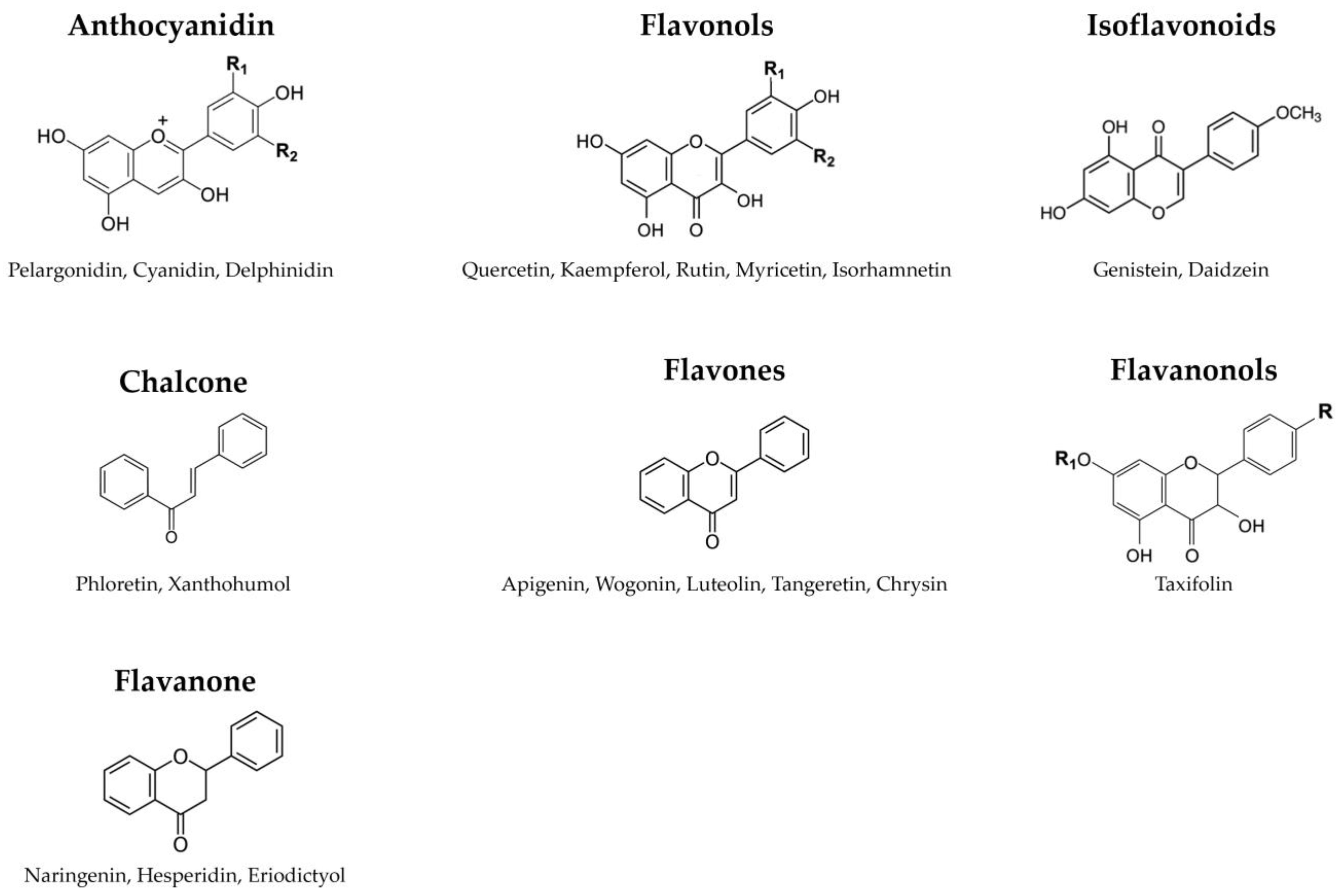
Flavonoids, a subgroup of polyphenols, represent plant-derived compounds renowned for their antioxidant properties [52]. These low-molecular-weight compounds are secondary metabolites found in plants [53], playing significant roles by contributing to color, aroma, and important responses to biological and non-biological environmental factors, such as their growth and defense against pathogens and UV radiation [54,55]. Consequently, flavonoids are ubiquitous across plant species. Chemically, flavonoids feature a 15-carbon backbone structure composed of two benzene rings linked by a heterocyclic pyran ring [56]. Hence, they are denoted as C6-C3-C6 compounds (Figure 3).
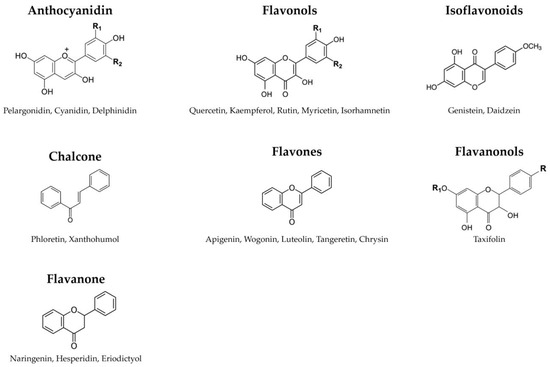
Figure 3.
The classification of flavonoids. Flavonoids feature a 15-carbon backbone structure composed of two benzene rings linked by a heterocyclic pyran ring.
Flavonoids, distinguished by their diverse chemical structures and properties, are categorized into several groups based on their chemical structure, oxidation degree, and unsaturation in the linking chain (C3). These groups encompass anthocyanidins, chalcones, flavonols, flavanones, flavanonols, flavones, and isoflavonoids. Anthocyanidins are a group of colorful pigments in plants responsible for vibrant colors ranging from red to blue. Key types include pelargonidin, cyanidin, peonidin, delphinidin, malvidin, and petunidin [57]. Chalcones are a class of compounds that serve as precursors to flavonoids, including marene, phloretin, lycochalcone-A, isobachalcone, and xanthohumol [58,59]. Flavonols are a subgroup of flavonoids characterized by the 3-hydroxyflavone backbone, comprising quercetin, kaempferol, rutin, myricetin, isoquercetin, and isorhamnetin [60]. Flavanones are various aromatic, colorless ketones derived from flavone, commonly occurring as glycosides in plants. Examples include naringenin, hesperidin, hesperetin, eriodictyol, and sakuranetin [61]. Flavanonols are a group of flavonoids that use the 3-hydroxy-2,3-dihydro-2-phenylchromen-4-one backbone [62]. This category includes taxifolin, aromadendrin, and engeletin. Flavones are a class of flavonoids based on the 2-phenylchromen-4-one backbone [63]. Well-known flavones include apigenin, wogonin, luteolin, baicalein, tangeretin, and chrysin [64]. While flavonoids have the 2-phenylchromen-4-one backbone, isoflavonoids, such as genistein and daidzein, feature the 3-phenylchromen-4-one backbone without hydroxyl group substitution at position 2 (isoflavones) or the 3-phenylchroman (isoflavan) backbone [65]. These varied flavonoid classes are abundant in various plant-based foods and have garnered significant scientific interest due to their potential health benefits and bioactive properties.
Considering that flavonoids, being xenobiotics, undergo metabolism in the liver, it becomes crucial to explore their specific impacts on liver health comprehensively. The following sections aim to delve into their mechanisms of action and interactions within the liver to enhance our understanding of how flavonoids may alleviate benzopyrene-induced liver damage and aid in maintaining optimal liver health.
3.2. Antioxidant Effects of Flavonoids and B[a]P in the Liver
Oxidative stress is a pivotal factor contributing to various liver diseases, including drug-induced liver injury, viral hepatitis, and alcoholic hepatitis. These conditions are triggered by the detrimental effects of drugs, viruses, and alcohol on the liver [66], thereby underscoring the significance of understanding and addressing the mechanisms underlying oxidative stress to prevent and manage these diverse liver conditions. Flavonoids are widely recognized as antioxidant compounds, prompting extensive research into their antioxidant potential [52]. Their antioxidant effects are achieved through two main mechanisms. First, they involve a chemical radical-scavenging effect that neutralizes radicals [67]. Second, they regulate the complex system of antioxidant enzymes, serving as a defense mechanism against intracellular ROS. Regarding the radical scavenging activity of flavonoids, previous reports have highlighted the influence of superoxide anion scavenging on their antioxidant properties [68]. However, a more recent study has introduced exceptions to the correlation between the antioxidant effect and scavenging activities of flavonoids [69]. This study explored the superoxide anion-scavenging potency and antioxidation capacity of seven specific flavonoids—quercetin, rutin, morin, acacetin, hispidulin, hesperidin, and naringin—and revealed distinct variations in scavenging ability. Rutin emerged as the most potent scavenger, closely followed by quercetin. Conversely, naringin and hesperidin exhibited no antioxidative effects. Furthermore, other research demonstrated variations in the roles of flavonoids, indicating that while flavonoids with scavenging effects exhibit antioxidant activity, those lacking such effects also possess antioxidant properties. This suggests that radical-scavenging properties are not universal among all flavonoids. Rather, not all flavonoids have radical-scavenging properties; different flavonoids exhibit antioxidant effects through different mechanisms.
Flavonoids act as a defense mechanism against intracellular ROS by regulating a complex system of antioxidant enzymes. Numerous studies have explored the nuclear factor E2-related factor 2 (NRF2)-HO antioxidant enzyme pathway (Table 1). Nrf2 serves as a transcription factor responsive to oxidative stress, binding to the antioxidant response element in the promoters of genes encoding antioxidant enzymes [70]. Under homeostatic conditions, KEAP1 cooperates with an E3 ubiquitin ligase to tightly regulate the activity of the transcription factor NRF2 activity through ubiquitination and subsequent proteasome-dependent degradation. In response to stress responses, complex molecular mechanisms involving sensor cysteines within KEAP1 prevent NRF2 ubiquitination, allowing its intracellular accumulation and translocation to the nucleus. There, NRF2 promotes the transcription of antioxidant enzymes such as NQO1, heme oxygenase 1 (HO1), catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPX) [71,72] all of which function as reductases with the capability to diminish ROS and reduce oxidative stress induced by ROS. SOD acts as a CAT, converting superoxide into O2) [73]. CAT catalyzes the breakdown of hydrogen peroxide (H2O2). HO1 catalyzes heme breakdown. GPX exhibits peroxide activity, protecting against oxidative damage and acting as a reductase to convert H2O2 to water. NQO1 acts as a detoxification system, removing quinone compounds from the biological environment. Notably, B[a]P triggers ROS generation through redox cycles involving benzoquinones and benzohydroquinones. In this context, the clearance of quinones becomes crucial, and NQO1 might potentially mitigate B[a]P-induced ROS.
The NRF2 pathway is intricately regulated by various signaling pathways involving transcriptional control, post-translational modifications, and maintenance of NRF2 protein stability [74]. As previously mentioned, Keap1 regulates the NRF2 protein stability, while post-translational modifications of NRF2 influence its binding partners and cellular localization, impacting its stability [75]. Several kinases, including ERK, JNK, PI3K-AKT, and PKC, contribute to NRF2 phosphorylation, enhancing its stability and subsequent transcriptional activity [76]. Conversely, phosphorylation mediated by p38 and GSK3 leads to reduced NRF2 stability. The NFE2L2 gene integrates a positive feedback mechanism, amplifying NRF2 effects [74]. Moreover, NFE2L2 transcription is regulated by various transcription factors, such as AhR and NFkB, with the NFE2L2 promoter containing an NFkB-binding site, allowing regulation by this transcription factor [75].
Numerous studies have demonstrated the regulatory role of flavonoids in modulating NRF2 via diverse mechanisms, leading to reduced ROS levels in the liver. Table 1 provides a comprehensive overview of the hepatic antioxidant mechanisms mediated by flavonoids. These compounds effectively diminish ROS and malondialdehyde, a marker reflecting oxidative stress levels (Table 1). Considering the antioxidant mechanisms of flavonoids in the liver, it is evident that they possess the potential to mitigate the hepatotoxic effects induced by B[a]P or counteract the detrimental impact of ROS-induced liver damage.

Table 1.
Antioxidant properties and the mechanism of flavonoids in the liver.
Table 1.
Antioxidant properties and the mechanism of flavonoids in the liver.
| Flavonoid | ROS | MDA | Antioxidant Enzyme | NRF2 Regulator | Refs. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRF2 | HO1 | NQO | SOD | CAT | GSH | GPX | ||||||
| Anthocyanidin | Pelargonidin | ↓ | ↓ | ↑ | ↑ | ↑ | [77] | |||||
| Cyanidin | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | [78] | |||
| ↓ | ↑ | ↑ | JNK↓ | [79] | ||||||||
| Delphinidin | ↓ | ↑ | ↑ | ↑ | Keap1↓ | [80] | ||||||
| ↓ | ↑ | PI3K/Akt↑ | [81] | |||||||||
| ↑ | ↑ | [82] | ||||||||||
| Chalcone | Phloretin | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | AMPK↑ Keap1↓ | [83] | |||
| ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | AMPK↑ | [84] | |||||
| ↓ | ↑ | ↑ | ↑ | ERK↑ | [85] | |||||||
| Xanthohumol | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | AKT↑ AMPK↑ | [86] | ||||
| ↓ | ↓ | ↑ | ↑ | ↑ | [87] | |||||||
| Flavonols | Quercetin | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | Keap1↓ | [88] | ||
| ↓ | ↓ | ↑ | NF-kB↓ | [89] | ||||||||
| ↓ | ↓ | ↑ | ↑ | ↑ | NF-kB↓ | [90] | ||||||
| Kaempferol | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | [91] | |||||
| ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | NF-kB↓ | [92] | |||||
| ↓ | ↓ | ↑ | ↑ | ↑ | AMPK↑ | [93] | ||||||
| ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | AKT↑ | [94] | ||||
| ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | NF-kB↓ | [95] | |||||
| Rutin | ↓ | ↑ | ↑ | [96] | ||||||||
| ↓ | ↑ | ↑ | ↑ | [97] | ||||||||
| ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | [98] | |||||
| Myricetin | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | NF-kB↓ | [99] | |||
| ↓ | ↓ | ↑ | ↑ | [100] | ||||||||
| ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | AMPK↑ | [101] | |||||
| Isorhamnetin | ↓ | ↑ | [102] | |||||||||
| Flavanone | Naringenin | ↓ | ↑ | ↑ | ↑ | ↑ | NF-kB↓ | [103] | ||||
| ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | SIRT1↑ | [104] | |||||
| ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | NF-kB↓ | [105] | |||||
| Hesperidin | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | Keap1↓ | [106] | ||||
| ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | NF-kB↓ | [107] | ||||
| ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | NF-kB↓ | [108] | |||||
| ↓ | ↑ | ↑ | MAPK↑ | [109] | ||||||||
| Eriodictyol | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | [110] | ||||
| Flavanonols | Taxifolin | ↓ | ↓ | ↑ | ↑ | NF-kB↓ | [111] | |||||
| Flavones | Apigenin | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | NF-kB↓ | [112] | |
| ↓ | ↓ | ↑ | ↑ | NF-kB↓ | [113] | |||||||
| Wogonin | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | NF-kB↓ | [114] | ||||
| Luteolin | ↓ | ↑ | ↑ | ↑ | ↑ | NF-kB↓ | [115] | |||||
| ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | KEAP↓ | [116] | ||
| ↓ | ↑ | [117] | ||||||||||
| Tangeretin | ↓ | ↑ | ↑ | ↑ | ↑ | MAPK↑ | [118] | |||||
| Chrysin | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | [119] | |||||
| Isoflavonoids | Genistein | ↓ | ↑ | ↑ | [120] | |||||||
| Daidzein | ↓ | ↑ | [121] | |||||||||
| ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | [122] | ||||||
ROS: reactive oxygen species, MDA: malondialdehyde, NRF2: nuclear factor E2-related factor 2, HO1: heme oxygenase 1, SOD: superoxide dismutase, GSH: glutathione, GPX: glutathione peroxidase, NQO: NAD(P)H quinone oxidoreductase 1, CAT: catalase, ↑; Upregulation of gene expression, ↓; Downregulation of gene expression.
3.3. Regulation of B[a]P Metabolism by Flavonoids in the Liver
The mitigation of B[a]P toxicity fundamentally relies on the xenobiotic detoxification process [123]. As previously discussed, B[a]P treatment can upregulate phase I CYP enzymes while inhibiting phase II enzymes. In such cases, the phase II step acts as the rate-limiting step, ultimately suppressing detoxification. Consequently, intracellular accumulation of the carcinogenic compound BPDE and the generation of ROS precursor, such as B[a]P quinone, occur, amplifying the overall toxicity. Therefore, precise regulation of the rate and activity of phases I, II, and III is crucial in reducing B[a]P toxicity.
Studies suggest that flavonoids exert regulatory effects on these phase enzymes [124]. Reduced activity in phase I enzymes results in reduced B[a]P metabolism, thereby inhibiting the formation of the carcinogenic compound BPDE [125]. Conversely, increased phase II enzyme activity accelerates the detoxification of substances such as BPDE and B[a]P-quinones [51]. Elevated phase III enzyme activity facilitates the swift excretion of detoxified compounds from the cell to the external environment. When both phase I and phase II enzyme activities increase, the metabolism and detoxification processes accelerate, expediting detoxification mechanisms.
Table 2 provides a comprehensive overview of flavonoid-mediated regulation of phase enzymes and their influence on B[a]P metabolism. For instance, quercetin demonstrates the ability to enhance the expression of all three phases: I, II, and III [51]. This results in a significant enhancement of B[a]P metabolism, facilitating detoxification and efficient excretion of harmful BPDE substances from within the cell. In contrast, kaempferol appears to decelerate B[a]P metabolism and subsequent detoxification [126]. Myricetin, while inhibiting the expression of phase I enzymes, promotes detoxification through phase II [108]. This dual action not only suppresses the production of the carcinogenic BPDE in phase I but also aids in its detoxification during phase II. Similarly, quercetin-like flavonol isorhamnetin accelerates the expression of phase I, II, and III enzymes, resulting in expedited metabolism, detoxification, and excretion [51]. On the other hand, flavones, such as apigenin, wogonin, and luteolin, inhibit the expression of phase I enzymes, reducing the formation of carcinogenic BPDE [126,127,128,129]. In contrast, chrysin enhances the expression of both phase I and II enzymes, suggesting a rapid metabolism and detoxification process [130]. Notably, these flavonols, including quercetin, kaempferol, isorhamnetin, and myricetin, each exhibit distinct mechanisms for mitigating B[a]P toxicity within their shared category.
Furthermore, flavonoids intricately regulate various CYP enzymes, each exerting distinct regulatory effects. Among these, certain CYPs hold pivotal roles in controlling B[a]P metabolism, while others contribute modestly. Through an analysis of B[a]P metabolites generated during incubation with different CYP types, one study revealed that CYP1A1 and CYP1B1 significantly influence B[a]P metabolism [131]. Additionally, essential CYPs involved in B[a]P metabolism in the liver, such as CYP3A4 and CYP2C19, were identified. Moreover, several other CYPs exhibited minor involvement in B[a]P metabolism. Therefore, Table 2 details the regulation of CYPs by flavonoids, categorized according to the type of flavonoid.
Mitigating the toxicity of B[a]P relies heavily on modulating xenobiotic detoxification processes. Flavonoid treatments can significantly influence the expression of phase I, II, and III enzymes, with phase III enzymes often acting as the rate-limiting step in detoxification. Precise regulation of the rates and activities of all three phases is pivotal to reducing B[a]P toxicity effectively. Flavonoids’ regulation of the detoxification metabolic process directly affects the regulation of carcinogenic compounds such as BPDE and ROS precursors. Table 2 provides a comprehensive overview of how flavonoids influence phase enzymes and CYPs, demonstrating their potential to alleviate B[a]P toxicity through distinct mechanisms. The varied actions of flavonoids within the same category highlight the complexity and adaptability of these compounds in addressing B[a]P-induced liver problems.

Table 2.
Effect of flavonoids on xenobiotic metabolism enzymes in the liver.
Table 2.
Effect of flavonoids on xenobiotic metabolism enzymes in the liver.
| Flavonoid | Phase I Enzyme | Phase II Enzyme | Phase III Enzyme | B[a]P | Refs. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AhR | CYP1A1 | CYP1B1 | Other CYPs | NRF2 | PXR | GST | UGT | Metabolism | Detoxification | ||||
| Anthocyanidin | Pelargonidin | ↑ | ↑ | CYP1A2↑ | [132] | ||||||||
| Chalcone | Phloretin | CYP1A2↓ CYP3A4↓ | ↑ | ↑ | [133] | ||||||||
| ↑ | [134] | ||||||||||||
| Flavonols | Quercetin | ↑ | [135] | ||||||||||
| ↑ | CYP2↑ CYP3↑ | [136] | |||||||||||
| ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ABCC1↑ | ↑ | ↑ | [51] | ||||
| Kaempferol | ↓ | ↓ | CYP1A2↓ | [137] | |||||||||
| CYP2E1↓ | [138] | ||||||||||||
| ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | [126] | |||||||
| Rutin | ↑ | ↑ | [139] | ||||||||||
| ↑ | CYP1A2↑ | [140] | |||||||||||
| CYP2E1↑ | ↑ | [141] | |||||||||||
| Myricetin | CYP2C8↓ | [142] | |||||||||||
| ↓ | ↑ | ↓ | ↑ | [108] | |||||||||
| Isorhamnetin | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ABCC1↑ | ↑ | ↑ | [51] | |||
| Flavanone | Eriodictyol | CYP2E1↓ CYP3A11↓ | ↑ | ↑ | [143] | ||||||||
| Sakuranetin | CYP1A2↓ CYP2C9↓ | ↑ | ↓ | [144] | |||||||||
| Flavanonols | Taxifolin | CYP2E1↓ CYP1A2↓ CYP3A4↓ | ↑ | [145] | |||||||||
| Flavones | Apigenin | CYP4F2↓ | [146] | ||||||||||
| ↓ | ↓ | ↓ | Total contents↓ | ↑ | ↓ | ↑ | [127] | ||||||
| Wogonin | ↓ | ↓ | [129] | ||||||||||
| ↓ | CYP2E1↓ | n.s. | ↓ | n.s. | [128] | ||||||||
| Luteolin | CYP1A2↓ CYP3A4↓ | ↓ | [147] | ||||||||||
| ABCA1↑ | [148] | ||||||||||||
| ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | [126] | |||||||
| Tangeretin | ↓ | ↓ | CYPs↓ | ↑ | [149] | ||||||||
| Chrysin | ↑ | [150] | |||||||||||
| CYP2E1↑ | ↑ | [151] | |||||||||||
| ↑ | n.s. | ↑ | ↑ | ↑ | [130] | ||||||||
| Isoflavonoids | Genistein | ↑ | ↑ | [152] | |||||||||
| ↓ | ↓ | [153] | |||||||||||
| ↑ | ↑ | [154] | |||||||||||
| ↑ | [155] | ||||||||||||
| Daidzein | ↑ | ↑ | [154] | ||||||||||
| ↑ | ↑ | [156] | |||||||||||
AhR: aryl hydrocarbon receptor, NRF2: nuclear factor E2-related factor 2, PXR: Pregnane X receptor, GST: glutathione S-transferases, UGT: uridine diphosphate glucuronosyltransferases, CYP1A1: cytochrome P450 family 1 subfamily A member 1, CYP1B1: cytochrome P450 family 1 subfamily B member 1, B[a]P: benzo[a]pyrene, ↑; Upregulation of gene expression, ↓; Downregulation of gene expression. n.s.: not significant.
3.4. B[a]P-Induced Liver Disease and Flavonoid
Chronic and acute exposure to B[a]P has been associated with an array of health issues. Recognized as a carcinogen, it significantly contributes to the development of various cancers, particularly lung and liver cancers. B[a]P exposure not only promotes processes such as cell migration, invasion, angiogenesis, and metastasis but also contributes to cancer progression [157,158]. One study reports that B[a]P induces oxidative damage in the liver and kidney of Swiss albino mice, emphasizing its carcinogenic effects [159]. Moreover, B[a]P plays a role in the pathological progression of hepatic steatosis, characterized by liver lipid accumulation, a key factor in fatty liver disease [160]. Even a singular exposure to B[a]P can induce fatty liver disease, and in combination with ethanol, it not only induces fatty liver but also exacerbates the associated damage [161]. Reports suggest that co-treatment with B[a]P and ethanol leads to oxidative stress and mitochondrial dysfunction, amplifying fatty liver disease progression [162]. The collaborative impact of B[a]P and ethanol on primary rat hepatocytes leads to plasma membrane remodeling, elevating oxidative stress, and inducing cell death [163]. Beyond its associations with cancer and fatty liver, B[a]P triggers a cascade of interconnected responses in the liver and lymphatic regions. It disrupts the morphological composition of the blood–lymph barrier, impeding lymphatic drainage in organs [164]. Despite B[a]P’s potential to induce various diseases, limited research focuses on therapeutic strategies for addressing these conditions.
The efficacy of flavonoids in mitigating diseases induced by B[a]P has been proposed. Curcumin, for instance, exhibits a protective effect against B[a]P-induced liver toxicity in rats. Its administration counteracted the toxic effects of B[a]P, restoring the normal histological architecture of the liver tissues [165]. Isoorientin, a bioactive flavonoid, displayed significant antitumor and antioxidant properties, effectively mitigating the stimulatory effect of B[a]P on ROS levels. Moreover, isoorientin demonstrated promise in alleviating B[a]P-induced febrile hepatocyte injury through the inhibition of the ROS/NF-κB/NLRP3/Caspase-1 signaling pathway [166]. This flavonoid also demonstrated positive inhibitory effects on B[a]P-induced autophagic and pyroptotic liver injury both in vitro and in vivo [167]. In a study by Hao Li et al., the adverse effects of dietary whole grains on B[a]P-induced genotoxic, oxidative, and thermogenic damage in mouse liver were validated [168]. Naringenin, another flavonoid, exhibited preventive actions against hepatocellular carcinoma by inhibiting growth factors such as TGF-β and vascular endothelial growth factor, inducing cell apoptosis, and regulating the MAPK pathway. Its selective on various proteins ensures safety in preventing liver cell carcinoma [169]. Xanthohumol is recognized as a potent inhibitor of cytochrome P450 enzymes and a stimulator of NAD(P)H:quinone reductase. One study showed that it inhibits the metabolic activation of pro-carcinogens and induces enzymes involved in carcinogen detoxification and antioxidation. Their findings substantiate the anti-genotoxic activity of xanthohumol in human cells with metabolic competence [170]. Moreover, 5,7-dimethoxyflavone demonstrated remarkable potency as an inhibitor of B[a]P-induced DNA binding and suppressed CYP1A1 protein expression and activity in Hep G2 cells. These findings suggest the potential of 5,7-dimethoxyflavone as an effective chemoprotectant against chemical-induced liver cancer [171].
Ongoing research delves into mitigating the toxicity induced by B[a]P through flavonoids, while concurrent auxiliary studies focus on augmenting treatment efficacy. Piperine, an adjunctive agent, amplifies the effectiveness of curcumin in alleviating B[a]P toxicity [172]. When used together, curcumin and piperine exhibit more pronounced effects than curcumin alone, notably reducing oxidative damage and chromosome aberrations induced by B[a]P.
This comprehensive evidence not only provides insights into potential therapeutic strategies but also underscores the protective effects of flavonoids against the diverse impacts of B[a]P on liver health.
4. Conclusions
Following its absorption into the body, B[a]P initiates a metabolic process that generates ROS, potentially leading to diverse clinical pathologies. Flavonoids exhibit distinct antioxidant effects in countering ROS induction by B[a]P. These investigations propose a potential role for flavonoids in reducing intracellular ROS levels, thereby alleviating oxidative stress. Flavonoids demonstrate their efficacy by neutralizing ROS through interactions with free radicals and inhibiting ROS generation, highlighting their significance in countering cellular damage. In the context of B[a]P-associated research, the antioxidant properties of flavonoids serve as a natural defense mechanism against oxidative stress in hepatic cells. Moreover, flavonoids can shield against B[a]P-induced liver toxicity by regulating the xenobiotic metabolism of B[a]P. Notably, reports indicate that flavonoids have the potential to alleviate liver diseases caused by B[a]P, suggesting their role in cellular protection by preventing or minimizing B[a]P-induced liver cell damage.
Author Contributions
Writing—original draft preparation, M.K. and S.-C.J.; writing—review and editing, M.K. and J.-S.S.; project administration, J.-S.S.; funding acquisition, J.-S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant (2022R1F1A1064538) from the National Research Foundation of Korea (NRF).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bukowska, B.; Mokra, K.; Michalowicz, J. Benzo[a]pyrene-Environmental Occurrence, Human Exposure, and Mechanisms of Toxicity. Int. J. Mol. Sci. 2022, 23, 6348. [Google Scholar] [CrossRef]
- Uno, S.; Dalton, T.P.; Shertzer, H.G.; Genter, M.B.; Warshawsky, D.; Talaska, G.; Nebert, D.W. Benzo[a]pyrene-induced toxicity: Paradoxical protection in Cyp1a1(−/−) knockout mice having increased hepatic BaP-DNA adduct levels. Biochem. Biophys. Res. Commun. 2001, 289, 1049–1056. [Google Scholar] [CrossRef]
- Cavalieri, E.; Rogan, E. Role of radical cations in aromatic hydrocarbon carcinogenesis. Environ. Health Perspect. 1985, 64, 69–84. [Google Scholar] [CrossRef]
- Francis, P.; Navarro, V.J. Drug-Induced Hepatotoxicity; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Chen, S.Y.; Wang, L.Y.; Lunn, R.M.; Tsai, W.Y.; Lee, P.H.; Lee, C.S.; Ahsan, H.; Zhang, Y.J.; Chen, C.J.; Santella, R.M. Polycyclic aromatic hydrocarbon-DNA adducts in liver tissues of hepatocellular carcinoma patients and controls. Int. J. Cancer 2002, 99, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, W.; Xu, X.; Li, B.; He, W.; Padilla, M.T.; Jang, J.H.; Nyunoya, T.; Amin, S.; Wang, X.; et al. RIP1 potentiates BPDE-induced transformation in human bronchial epithelial cells through catalase-mediated suppression of excessive reactive oxygen species. Carcinogenesis 2013, 34, 2119–2128. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; Perez de la Lastra, J.M.; Plou, F.J.; Perez-Lebena, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Li, Y.; Xi, H.; Niu, Z.; Chen, N.; Wang, R.; Yan, Y.; Gan, X.; Wang, M.; Zhang, W.; et al. Benzo(a)pyrene and cardiovascular diseases: An overview of pre-clinical studies focused on the underlying molecular mechanism. Front. Nutr. 2022, 9, 978475. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Huang, P.; Zhang, L.; Qiu, Y.; Qi, H.; Leng, A.; Shang, D. Hepatoprotective effect of plant polysaccharides from natural resources: A review of the mechanisms and structure-activity relationship. Int. J. Biol. Macromol. 2020, 161, 24–34. [Google Scholar] [CrossRef]
- Gajender; Mazumder, A.; Sharma, A.; Azad, M.A.K. A Comprehensive Review of the Pharmacological Importance of Dietary Flavonoids as Hepatoprotective Agents. Evid. Based Complement. Alternat Med. 2023, 2023, 4139117. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Hrdina, A.I.H.; Kohale, I.N.; Kaushal, S.; Kelly, J.; Selin, N.E.; Engelward, B.P.; Kroll, J.H. The Parallel Transformations of Polycyclic Aromatic Hydrocarbons in the Body and in the Atmosphere. Environ. Health Perspect. 2022, 130, 25004. [Google Scholar] [CrossRef]
- McQueen, C.A. Comprehensive Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Chakraborty, R.; Ghosh, D. The effect of sequence on the ionization of guanine in DNA. Phys. Chem. Chem. Phys. 2016, 18, 6526–6533. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T.; Gasparutto, D.; Ravanat, J.L. Oxidative damage to DNA: Formation, measurement and biochemical features. Mutat. Res. 2003, 531, 5–23. [Google Scholar] [CrossRef]
- Jee, S.C.; Kim, M.; Kim, K.S.; Kim, H.S.; Sung, J.S. Protective Effects of Myricetin on Benzo[a]pyrene-Induced 8-Hydroxy-2′-Deoxyguanosine and BPDE-DNA Adduct. Antioxidants 2020, 9, 446. [Google Scholar] [CrossRef]
- Hahm, J.Y.; Park, J.; Jang, E.S.; Chi, S.W. 8-Oxoguanine: From oxidative damage to epigenetic and epitranscriptional modification. Exp. Mol. Med. 2022, 54, 1626–1642. [Google Scholar] [CrossRef]
- Li, C.; Xue, Y.; Ba, X.; Wang, R. The Role of 8-oxoG Repair Systems in Tumorigenesis and Cancer Therapy. Cells 2022, 11, 3798. [Google Scholar] [CrossRef] [PubMed]
- Chiorcea-Paquim, A.M. 8-oxoguanine and 8-oxodeoxyguanosine Biomarkers of Oxidative DNA Damage: A Review on HPLC-ECD Determination. Molecules 2022, 27, 1620. [Google Scholar] [CrossRef] [PubMed]
- Qing, X.; Shi, D.; Lv, X.; Wang, B.; Chen, S.; Shao, Z. Prognostic significance of 8-hydroxy-2′-deoxyguanosine in solid tumors: A meta-analysis. BMC Cancer 2019, 19, 997. [Google Scholar] [CrossRef] [PubMed]
- Lagadu, S.; Lechevrel, M.; Sichel, F.; Breton, J.; Pottier, D.; Couderc, R.; Moussa, F.; Prevost, V. 8-oxo-7,8-dihydro-2′-deoxyguanosine as a biomarker of oxidative damage in oesophageal cancer patients: Lack of association with antioxidant vitamins and polymorphism of hOGG1 and GST. J. Exp. Clin. Cancer Res. 2010, 29, 157. [Google Scholar] [CrossRef] [PubMed]
- Iida, T.; Furuta, A.; Kawashima, M.; Nishida, J.; Nakabeppu, Y.; Iwaki, T. Accumulation of 8-oxo-2′-deoxyguanosine and increased expression of hMTH1 protein in brain tumors. Neuro Oncol. 2001, 3, 73–81. [Google Scholar] [CrossRef]
- Larosche, I.; Letteron, P.; Berson, A.; Fromenty, B.; Huang, T.T.; Moreau, R.; Pessayre, D.; Mansouri, A. Hepatic mitochondrial DNA depletion after an alcohol binge in mice: Probable role of peroxynitrite and modulation by manganese superoxide dismutase. J. Pharmacol. Exp. Ther. 2010, 332, 886–897. [Google Scholar] [CrossRef]
- Ji, X.; Li, Y.; He, J.; Shah, W.; Xue, X.; Feng, G.; Zhang, H.; Gao, M. Depletion of mitochondrial enzyme system in liver, lung, brain, stomach and kidney induced by benzo(a)pyrene. Environ. Toxicol. Pharmacol. 2016, 43, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. Mitochondrial dysfunction indirectly elevates ROS production by the endoplasmic reticulum. Cell Metab. 2013, 18, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Dang, F.; Gao, J.; Zhao, H.; Qi, S.; Gao, M. Acute benzo[a]pyrene treatment causes different antioxidant response and DNA damage in liver, lung, brain, stomach and kidney. Heliyon 2018, 4, e00898. [Google Scholar] [CrossRef]
- Cichoz-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroentero 2014, 20, 8082–8091. [Google Scholar] [CrossRef]
- Baillie, T.A. Metabolism and toxicity of drugs. Two decades of progress in industrial drug metabolism. Chem. Res. Toxicol. 2008, 21, 129–137. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Q.; Cui, Q.; Huang, G.; Pan, X.; Li, S. Flavonoids and Naphthoflavonoids: Wider Roles in the Modulation of Cytochrome P450 Family 1 Enzymes. ChemMedChem 2016, 11, 2102–2118. [Google Scholar] [CrossRef]
- Kozack, R.E.; Loechler, E.L. Molecular modeling of the major adduct of (+)-anti-B[a]PDE (N2-dG) in the eight conformations and the five DNA sequences most relevant to base substitution mutagenesis. Carcinogenesis 1999, 20, 85–94. [Google Scholar] [CrossRef]
- Guo, L.; Jiang, X.; Tian, H.Y.; Yao, S.J.; Li, B.Y.; Zhang, R.J.; Zhang, S.S.; Sun, X. Detection of BPDE-DNA adducts in human umbilical cord blood by LC-MS/MS analysis. J. Food Drug Anal. 2019, 27, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Menzies, G.E.; Prior, I.A.; Brancale, A.; Reed, S.H.; Lewis, P.D. Carcinogen-induced DNA structural distortion differences in the RAS gene isoforms; the importance of local sequence. BMC Chem. 2021, 15, 51. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Sayer, J.M.; Plosky, B.S.; Yagi, H.; Boudsocq, F.; Woodgate, R.; Jerina, D.M.; Yang, W. Crystal structure of a benzo[a]pyrene diol epoxide adduct in a ternary complex with a DNA polymerase. Proc. Natl. Acad. Sci. USA 2004, 101, 2265–2269. [Google Scholar] [CrossRef]
- Barnes, J.L.; Zubair, M.; John, K.; Poirier, M.C.; Martin, F.L. Carcinogens and DNA damage. Biochem. Soc. Trans. 2018, 46, 1213–1224. [Google Scholar] [CrossRef]
- Nebert, D.W.; Karp, C.L. Endogenous functions of the aryl hydrocarbon receptor (AHR): Intersection of cytochrome P450 1 (CYP1)-metabolized eicosanoids and AHR biology. J. Biol. Chem. 2008, 283, 36061–36065. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Zhang, L. The Role of the Aryl Hydrocarbon Receptor (AhR) and Its Ligands in Breast Cancer. Cancers 2022, 14, 5574. [Google Scholar] [CrossRef] [PubMed]
- Tew, K.D.; Townsend, D.M. Glutathione-s-transferases as determinants of cell survival and death. Antioxid. Redox Signal. 2012, 17, 1728–1737. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Hu, X.; Xia, H.; Awasthi, S.; Amin, S.; Singh, S.V. Metabolic fate of glutathione conjugate of benzo[a]pyrene-(7R,8S)-diol (9S,10R)-epoxide in human liver. Arch. Biochem. Biophys. 1999, 371, 340–344. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, M.; Blair, I.A.; Penning, T.M. Interception of benzo[a]pyrene-7,8-dione by UDP glucuronosyltransferases (UGTs) in human lung cells. Chem. Res. Toxicol. 2013, 26, 1570–1578. [Google Scholar] [CrossRef]
- Jancova, P.; Anzenbacher, P.; Anzenbacherova, E. Phase II drug metabolizing enzymes. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2010, 154, 103–116. [Google Scholar] [CrossRef]
- Garg, R.; Gupta, S.; Maru, G.B. Dietary curcumin modulates transcriptional regulators of phase I and phase II enzymes in benzo[a]pyrene-treated mice: Mechanism of its anti-initiating action. Carcinogenesis 2008, 29, 1022–1032. [Google Scholar] [CrossRef]
- Stoddard, E.G.; Killinger, B.J.; Nag, S.A.; Martin, J.; Corley, R.; Smith, J.N.; Wright, A.T. Benzo[a]pyrene Induction of Glutathione S-Transferases: An Activity-Based Protein Profiling Investigation. Chem. Res. Toxicol. 2019, 32, 1259–1267. [Google Scholar] [CrossRef]
- Konig, J.; Nies, A.T.; Cui, Y.; Leier, I.; Keppler, D. Conjugate export pumps of the multidrug resistance protein (MRP) family: Localization, substrate specificity, and MRP2-mediated drug resistance. Biochim. Biophys. Acta 1999, 1461, 377–394. [Google Scholar] [CrossRef]
- Lai, Y.; Chu, X.; Di, L.; Gao, W.; Guo, Y.; Liu, X.; Lu, C.; Mao, J.; Shen, H.; Tang, H.; et al. Recent advances in the translation of drug metabolism and pharmacokinetics science for drug discovery and development. Acta Pharm. Sin. B 2022, 12, 2751–2777. [Google Scholar] [CrossRef]
- Yuan, L.; Lv, B.; Zha, J.; Wang, W.; Wang, Z. Basal and benzo[a]pyrene-induced expression profile of phase I and II enzymes and ABC transporter mRNA in the early life stage of Chinese rare minnows (Gobiocypris rarus). Ecotoxicol. Environ. Saf. 2014, 106, 86–94. [Google Scholar] [CrossRef]
- Guo, B.Y.; Xu, Z.T.; Yan, X.J.; Buttino, I.; Li, J.J.; Zhou, C.; Qi, P.Z. Novel ABCB1 and ABCC Transporters Are Involved in the Detoxification of Benzo(alpha)pyrene in Thick Shell Mussel, Mytilus coruscus. Front. Mar. Sci. 2020, 7, 119. [Google Scholar] [CrossRef]
- Bak, Y.; Jang, H.J.; Seo, J.H.; No, S.H.; Chae, J.I.; Hong, J.; Yoon, D.Y. Benzo[a]pyrene Alters the Expression of Genes in A549 Lung Cancer Cells and Cancer Stem Cells. J. Microbiol. Biotechnol. 2018, 28, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jee, S.C.; Kim, K.S.; Kim, H.S.; Yu, K.N.; Sung, J.S. Quercetin and Isorhamnetin Attenuate Benzo[a]pyrene-Induced Toxicity by Modulating Detoxification Enzymes through the AhR and NRF2 Signaling Pathways. Antioxidants 2021, 10, 787. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M.R. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. BioMed Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Kahkonen, M.P.; Heinonen, M. Antioxidant activity of anthocyanins and their aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khan, J.; Dukhyil, A.A.B.; Alarousy, R.; Attah, E.I.; Sharma, T.; Khairnar, S.J.; Bendale, A.R. Chalcone Scaffolds, Bioprecursors of Flavonoids: Chemistry, Bioactivities, and Pharmacokinetics. Molecules 2021, 26, 7177. [Google Scholar] [CrossRef] [PubMed]
- Budziak-Wieczorek, I.; Kaminski, D.; Skrzypek, A.; Ciolek, A.; Skrzypek, T.; Janik-Zabrotowicz, E.; Arczewska, M. Naturally Occurring Chalcones with Aggregation-Induced Emission Enhancement Characteristics. Molecules 2023, 28, 3412. [Google Scholar] [CrossRef]
- Chagas, M.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Goncalves-de-Albuquerque, C.F. Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxid. Med. Cell Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef]
- Rencoret, J.; Rosado, M.J.; Kim, H.; Timokhin, V.I.; Gutierrez, A.; Bausch, F.; Rosenau, T.; Potthast, A.; Ralph, J.; Del Rio, J.C. Flavonoids naringenin chalcone, naringenin, dihydrotricin, and tricin are lignin monomers in papyrus. Plant Physiol. 2022, 188, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Koosha, S.; Alshawsh, M.A.; Looi, C.Y.; Seyedan, A.; Mohamed, Z. An Association Map on the Effect of Flavonoids on the Signaling Pathways in Colorectal Cancer. Int. J. Med. Sci. 2016, 13, 374–385. [Google Scholar] [CrossRef]
- Yi, Y.S. Regulatory Roles of Flavonoids in Caspase-11 Non-Canonical Inflammasome-Mediated Inflammatory Responses and Diseases. Int. J. Mol. Sci. 2023, 24, 10402. [Google Scholar] [CrossRef]
- Jeong, S.H.; Kim, H.H.; Ha, S.E.; Park, M.Y.; Bhosale, P.B.; Abusaliya, A.; Park, K.I.; Heo, J.D.; Kim, H.W.; Kim, G.S. Flavones: Six Selected Flavones and Their Related Signaling Pathways That Induce Apoptosis in Cancer. Int. J. Mol. Sci. 2022, 23, 10965. [Google Scholar] [CrossRef]
- Lorent, K.; Gong, W.; Koo, K.A.; Waisbourd-Zinman, O.; Karjoo, S.; Zhao, X.; Sealy, I.; Kettleborough, R.N.; Stemple, D.L.; Windsor, P.A.; et al. Identification of a plant isoflavonoid that causes biliary atresia. Sci. Transl. Med. 2015, 7, 286ra267. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, Q.; Chen, Z. The Nrf2 Pathway in Liver Diseases. Front. Cell Dev. Biol. 2022, 10, 826204. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Robak, J.; Gryglewski, R.J. Flavonoids are scavengers of superoxide anions. Biochem. Pharmacol. 1988, 37, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Zheng, R.L.; Jia, Z.J.; Ju, Y. Flavonoids as superoxide scavengers and antioxidants. Free Radic. Biol. Med. 1990, 9, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Vomhof-Dekrey, E.E.; Picklo, M.J., Sr. The Nrf2-antioxidant response element pathway: A target for regulating energy metabolism. J. Nutr. Biochem. 2012, 23, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Moratilla-Rivera, I.; Sanchez, M.; Valdes-Gonzalez, J.A.; Gomez-Serranillos, M.P. Natural Products as Modulators of Nrf2 Signaling Pathway in Neuroprotection. Int. J. Mol. Sci. 2023, 24, 3748. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noe, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Liu, H.; Johnston, L.J.; Wang, F.; Ma, X. Triggers for the Nrf2/ARE Signaling Pathway and Its Nutritional Regulation: Potential Therapeutic Applications of Ulcerative Colitis. Int. J. Mol. Sci. 2021, 22, 11411. [Google Scholar] [CrossRef]
- Shi, Y.S.; Li, X.X.; Li, H.T.; Zhang, Y. Pelargonidin ameliorates CCl4-induced liver fibrosis by suppressing the ROS-NLRP3-IL-1beta axis via activating the Nrf2 pathway. Food Funct. 2020, 11, 5156–5165. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Cui, S.; Mao, B.; Zhang, Q.; Tian, F.; Zhao, J.; Tang, X.; Chen, W. Cyanidin Alleviated CCl4-Induced Acute Liver Injury by Regulating the Nrf2 and NF-kappaB Signaling Pathways. Antioxidants 2022, 11, 2383. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Jia, Q.; Wang, Y.; Zhang, Y.; Xia, M. The anthocyanin cyanidin-3-O-beta-glucoside, a flavonoid, increases hepatic glutathione synthesis and protects hepatocytes against reactive oxygen species during hyperglycemia: Involvement of a cAMP-PKA-dependent signaling pathway. Free Radic. Biol. Med. 2012, 52, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Ren, G.; Yang, R.; Chen, J.; Xiang, X.; Qin, H.; Chen, J. Inhibitory Effect of Delphinidin on Oxidative Stress Induced by H2O2 in HepG2 Cells. Oxid. Med. Cell Longev. 2020, 2020, 4694760. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, H.Y.; Wang, D.; Guo, X.Z. Delphinidin protects beta2m-/Thy1+ bone marrow-derived hepatocyte stem cells against TGF-beta1-induced oxidative stress and apoptosis through the PI3K/Akt pathway in vitro. Chem. Biol. Interact. 2019, 297, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Maeda-Yamamoto, M.; Nesumi, A.; Murakami, A. Delphinidin-3-O-galactoside protects mouse hepatocytes from (-)-epigallocatechin-3-gallate-induced cytotoxicity via up-regulation of heme oxygenase-1 and heat shock protein 70. Nutr. Res. 2012, 32, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, Q.; Han, L.; Pan, C.; Lei, C.; Chen, H.; Lan, X. C2C12 Mouse Myoblasts Damage Induced by Oxidative Stress Is Alleviated by the Antioxidant Capacity of the Active Substance Phloretin. Front. Cell Dev. Biol. 2020, 8, 541260. [Google Scholar] [CrossRef]
- Yang, Q.; Han, L.; Li, J.; Xu, H.; Liu, X.; Wang, X.; Pan, C.; Lei, C.; Chen, H.; Lan, X. Activation of Nrf2 by Phloretin Attenuates Palmitic Acid-Induced Endothelial Cell Oxidative Stress via AMPK-Dependent Signaling. J. Agric. Food Chem. 2019, 67, 120–131. [Google Scholar] [CrossRef]
- Yang, Y.C.; Lii, C.K.; Lin, A.H.; Yeh, Y.W.; Yao, H.T.; Li, C.C.; Liu, K.L.; Chen, H.W. Induction of glutathione synthesis and heme oxygenase 1 by the flavonoids butein and phloretin is mediated through the ERK/Nrf2 pathway and protects against oxidative stress. Free Radic. Biol. Med. 2011, 51, 2073–2081. [Google Scholar] [CrossRef]
- Zhu, L.; Fan, X.; Cao, C.; Li, K.; Hou, W.; Ci, X. Xanthohumol protect against acetaminophen-induced hepatotoxicity via Nrf2 activation through the AMPK/Akt/GSK3beta pathway. Biomed. Pharmacother. 2023, 165, 115097. [Google Scholar] [CrossRef]
- Pinto, C.; Cestero, J.J.; Rodriguez-Galdon, B.; Macias, P. Xanthohumol, a prenylated flavonoid from hops (Humulus lupulus L.), protects rat tissues against oxidative damage after acute ethanol administration. Toxicol. Rep. 2014, 1, 726–733. [Google Scholar] [CrossRef]
- Wang, J.; Wang, K.; Ding, L.; Zhao, P.; Zhang, C.; Wang, H.; Yang, Z.; Liu, Z. Alleviating effect of quercetin on cadmium-induced oxidative damage and apoptosis by activating the Nrf2-keap1 pathway in BRL-3A cells. Front. Pharmacol. 2022, 13, 969892. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Dou, B.; Liang, J.; Hou, W.; Ma, C.; Zhang, Q. Quercetin Reduces Oxidative Stress and Apoptosis by Inhibiting HMGB1 and Its Translocation, Thereby Alleviating Liver Injury in ACLF Rats. Evid. Based Complement. Alternat Med. 2021, 2021, 2898995. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tian, L.; Chai, G.; Wen, B.; Wang, B. Targeting heme oxygenase-1 by quercetin ameliorates alcohol-induced acute liver injury via inhibiting NLRP3 inflammasome activation. Food Funct. 2018, 9, 4184–4193. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Weng, Q.; Gong, S.; Zhang, W.; Wang, J.; Huang, Y.; Li, Y.; Guo, J.; Lan, T. Kaempferol prevents acetaminophen-induced liver injury by suppressing hepatocyte ferroptosis via Nrf2 pathway activation. Food Funct. 2023, 14, 1884–1896. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, A.S.; El-Kott, A.F.; El-Gerbed, M.S.A.; El-Kenawy, A.E.; Albadrani, G.M.; Khalifa, H.S. Kaempferol prevents cadmium chloride-induced liver damage by upregulating Nrf2 and suppressing NF-kappaB and keap1. Environ. Sci. Pollut. Res. Int. 2022, 29, 13917–13929. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yoon, S.; Moon, J.O. Kaempferol Suppresses Carbon Tetrachloride-Induced Liver Damage in Rats via the MAPKs/NF-kappaB and AMPK/Nrf2 Signaling Pathways. Int. J. Mol. Sci. 2023, 24, 6900. [Google Scholar] [CrossRef]
- Rajendran, P.; Ammar, R.B.; Al-Saeedi, F.J.; Mohamed, M.E.; ElNaggar, M.A.; Al-Ramadan, S.Y.; Bekhet, G.M.; Soliman, A.M. Kaempferol Inhibits Zearalenone-Induced Oxidative Stress and Apoptosis via the PI3K/Akt-Mediated Nrf2 Signaling Pathway: In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2020, 22, 217. [Google Scholar] [CrossRef]
- Du, Y.C.; Lai, L.; Zhang, H.; Zhong, F.R.; Cheng, H.L.; Qian, B.L.; Tan, P.; Xia, X.M.; Fu, W.G. Kaempferol from Penthorum chinense Pursh suppresses HMGB1/TLR4/NF-kappaB signaling and NLRP3 inflammasome activation in acetaminophen-induced hepatotoxicity. Food Funct. 2020, 11, 7925–7934. [Google Scholar] [CrossRef]
- Saafan, S.M.; Mohamed, S.A.; Noreldin, A.E.; El Tedawy, F.A.; Elewa, Y.H.A.; Fadly, R.S.; Al Jaouni, S.K.; El-Far, A.H.; Alsenosy, A.A. Rutin attenuates D-galactose-induced oxidative stress in rats’ brain and liver: Molecular docking and experimental approaches. Food Funct. 2023, 14, 5728–5751. [Google Scholar] [CrossRef]
- Freitas, P.A.; Oliveira, K.A.; Magalhaes, L.A.; Neves, R.J.D.; Maia, C.S.C.; Silveira, L.R.; de Lima, T.T.; Vasconcelos, R.P.; Brito, L.C.; Torres-Leal, F.L.; et al. Improvement of 2,2′-Azobis(2-Methylpropionamidine) Dihydrochloride-Induced Hepatic Redox Imbalance in Swiss Mice and HepG2 Cells by Rutin. J. Med. Food 2022, 25, 630–635. [Google Scholar] [CrossRef]
- Singh, S.; Singh, D.K.; Meena, A.; Dubey, V.; Masood, N.; Luqman, S. Rutin protects t-butyl hydroperoxide-induced oxidative impairment via modulating the Nrf2 and iNOS activity. Phytomedicine 2019, 55, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; Baluchnejadmojarad, T.; Roghani, M. Hepatoprotective Effect of Myricetin following Lipopolysaccharide/DGalactosamine: Involvement of Autophagy and Sirtuin 1. Curr. Mol. Pharmacol. 2023, 16, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, J.; Zhang, H.; Bai, W.; Dong, J.; Yang, Z.; Yang, P.; Gu, Z.; Li, Y.; Chen, X.; et al. Antioxidative myricetin-enriched nanoparticles towards acute liver injury. J. Mater. Chem. B 2022, 10, 7875–7883. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; An, B.; Yu, Q.; Cao, Y.; Liu, Y.; Li, S. The hepatoprotective effect of myricetin against lipopolysaccharide and D-galactosamine-induced fulminant hepatitis. Int. J. Biol. Macromol. 2020, 155, 1092–1104. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.Z.; Lee, J.H.; Ki, S.H.; Yang, J.H.; Cho, I.J.; Kang, S.H.; Zhao, R.J.; Kim, S.C.; Kim, Y.W. AMPK activation by isorhamnetin protects hepatocytes against oxidative stress and mitochondrial dysfunction. Eur. J. Pharmacol. 2014, 740, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, A.Z.; Alhazzani, K.; Alrewily, S.Q.; Aljerian, K.; Algahtani, M.M.; Alqahtani, Q.H.; Haspula, D.; Alhamed, A.S.; Alqinyah, M.; Raish, M. The Potential Protective Role of Naringenin against Dasatinib-Induced Hepatotoxicity. Pharmaceuticals 2023, 16, 921. [Google Scholar] [CrossRef]
- Hua, Y.Q.; Zeng, Y.; Xu, J.; Xu, X.L. Naringenin alleviates nonalcoholic steatohepatitis in middle-aged Apoe(−/−)mice: Role of SIRT1. Phytomedicine 2021, 81, 153412. [Google Scholar] [CrossRef]
- Wali, A.F.; Rashid, S.; Rashid, S.M.; Ansari, M.A.; Khan, M.R.; Haq, N.; Alhareth, D.Y.; Ahmad, A.; Rehman, M.U. Naringenin Regulates Doxorubicin-Induced Liver Dysfunction: Impact on Oxidative Stress and Inflammation. Plants 2020, 9, 550. [Google Scholar] [CrossRef]
- Jia, Y.; Li, J.; Liu, P.; Si, M.; Jin, Y.; Wang, H.; Ma, D.; Chu, L. Based on Activation of p62-Keap1-Nrf2 Pathway, Hesperidin Protects Arsenic-Trioxide-Induced Cardiotoxicity in Mice. Front. Pharmacol. 2021, 12, 758670. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Mohammed, H.M.; Khadrawy, S.M.; Galaly, S.R. Hesperidin protects against chemically induced hepatocarcinogenesis via modulation of Nrf2/ARE/HO-1, PPARgamma and TGF-beta1/Smad3 signaling, and amelioration of oxidative stress and inflammation. Chem. Biol. Interact. 2017, 277, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, R.M.; Abdelazem, A.Z.; Hashem, K.S.; Attia, Y.A. Protective effects of hesperidin against MTX-induced hepatotoxicity in male albino rats. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1405–1417. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Ye, Y.Y.; Ji, G.; Liu, J.W. Hesperidin upregulates heme oxygenase-1 to attenuate hydrogen peroxide-induced cell damage in hepatic L02 cells. J. Agric. Food Chem. 2010, 58, 3330–3335. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Meng, X.; Wang, F.; Bao, Y.; Huo, J. Eriodictyol attenuates arsenic trioxide-induced liver injury by activation of Nrf2. Oncotarget 2017, 8, 68668–68674. [Google Scholar] [CrossRef]
- Okkay, U.; Ferah Okkay, I.; Cicek, B.; Aydin, I.C.; Ozkaraca, M. Hepatoprotective and neuroprotective effect of taxifolin on hepatic encephalopathy in rats. Metab. Brain Dis. 2022, 37, 1541–1556. [Google Scholar] [CrossRef]
- Al-Amarat, W.; Abukhalil, M.H.; Alruhaimi, R.S.; Alqhtani, H.A.; Aldawood, N.; Alfwuaires, M.A.; Althunibat, O.Y.; Aladaileh, S.H.; Algefare, A.I.; Alanezi, A.A.; et al. Upregulation of Nrf2/HO-1 Signaling and Attenuation of Oxidative Stress, Inflammation, and Cell Death Mediate the Protective Effect of Apigenin against Cyclophosphamide Hepatotoxicity. Metabolites 2022, 12, 648. [Google Scholar] [CrossRef]
- Pan, X.; Shao, Y.; Wang, F.; Cai, Z.; Liu, S.; Xi, J.; He, R.; Zhao, Y.; Zhuang, R. Protective effect of apigenin magnesium complex on H2O2-induced oxidative stress and inflammatory responses in rat hepatic stellate cells. Pharm. Biol. 2020, 58, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.M.; Guo, W.N.; Tan, Y.Z.; Niu, K.W.; Zhang, J.J.; Liu, C.L.; Yang, X.M.; Tao, K.S.; Chen, Z.N.; Dai, J.Y. Wogonin alleviates liver injury in sepsis through Nrf2-mediated NF-kappaB signalling suppression. J. Cell Mol. Med. 2021, 25, 5782–5798. [Google Scholar] [CrossRef]
- Yang, D.; Tan, X.; Lv, Z.; Liu, B.; Baiyun, R.; Lu, J.; Zhang, Z. Regulation of Sirt1/Nrf2/TNF-alpha signaling pathway by luteolin is critical to attenuate acute mercuric chloride exposure induced hepatotoxicity. Sci. Rep. 2016, 6, 37157. [Google Scholar] [CrossRef]
- Rajput, S.A.; Shaukat, A.; Wu, K.; Rajput, I.R.; Baloch, D.M.; Akhtar, R.W.; Raza, M.A.; Najda, A.; Rafal, P.; Albrakati, A.; et al. Luteolin Alleviates AflatoxinB(1)-Induced Apoptosis and Oxidative Stress in the Liver of Mice through Activation of Nrf2 Signaling Pathway. Antioxidants 2021, 10, 1268. [Google Scholar] [CrossRef]
- Janda, E.; Martino, C.; Riillo, C.; Parafati, M.; Lascala, A.; Mollace, V.; Boutin, J.A. Apigenin and Luteolin Regulate Autophagy by Targeting NRH-Quinone Oxidoreductase 2 in Liver Cells. Antioxidants 2021, 10, 776. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Fang, Y.; Cao, W.; Zhang, Z.; Pan, S.; Xu, X. Attenuation of tert-Butyl Hydroperoxide (t-BHP)-Induced Oxidative Damage in HepG2 Cells by Tangeretin: Relevance of the Nrf2-ARE and MAPK Signaling Pathways. J. Agric. Food Chem. 2018, 66, 6317–6325. [Google Scholar] [CrossRef] [PubMed]
- Tekeli, M.Y.; Cakir Bayram, L.; Eraslan, G.; Soyer Sarica, Z. The protective effect of chrysin against oxidative stress and organ toxicity in rats exposed to propetamphos. Drug Chem. Toxicol. 2022, 45, 2664–2677. [Google Scholar] [CrossRef] [PubMed]
- El-Far, Y.M.; Khodir, A.E.; Emarah, Z.A.; Ebrahim, M.A.; Al-Gayyar, M.M.H. Chemopreventive and hepatoprotective effects of genistein via inhibition of oxidative stress and the versican/PDGF/PKC signaling pathway in experimentally induced hepatocellular carcinoma in rats by thioacetamide. Redox Rep. 2022, 27, 9–20. [Google Scholar] [CrossRef]
- Kampkotter, A.; Chovolou, Y.; Kulawik, A.; Rohrdanz, E.; Weber, N.; Proksch, P.; Watjen, W. Isoflavone daidzein possesses no antioxidant activities in cell-free assays but induces the antioxidant enzyme catalase. Nutr. Res. 2008, 28, 620–628. [Google Scholar] [CrossRef]
- Mishra, P.; Kar, A.; Kale, R.K. Prevention of chemically induced mammary tumorigenesis by daidzein in pre-pubertal rats: The role of peroxidative damage and antioxidative enzymes. Mol. Cell Biochem. 2009, 325, 149–157. [Google Scholar] [CrossRef]
- Gu, X.; Manautou, J.E. Molecular mechanisms underlying chemical liver injury. Expert. Rev. Mol. Med. 2012, 14, e4. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Hu, M. Mutual interactions between flavonoids and enzymatic and transporter elements responsible for flavonoid disposition via phase II metabolic pathways. RSC Adv. 2012, 2, 7948–7963. [Google Scholar] [CrossRef] [PubMed]
- Arlt, V.M.; Stiborova, M.; Henderson, C.J.; Thiemann, M.; Frei, E.; Aimova, D.; Singh, R.; Gamboa da Costa, G.; Schmitz, O.J.; Farmer, P.B.; et al. Metabolic activation of benzo[a]pyrene in vitro by hepatic cytochrome P450 contrasts with detoxification in vivo: Experiments with hepatic cytochrome P450 reductase null mice. Carcinogenesis 2008, 29, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Kitakaze, T.; Makiyama, A.; Nakai, R.; Kimura, Y.; Ashida, H. Kaempferol modulates TCDD- and t-BHQ-induced drug-metabolizing enzymes and luteolin enhances this effect. Food Funct. 2020, 11, 3668–3680. [Google Scholar] [CrossRef]
- Khan, T.H.; Jahangir, T.; Prasad, L.; Sultana, S. Inhibitory effect of apigenin on benzo(a)pyrene-mediated genotoxicity in Swiss albino mice. J. Pharm. Pharmacol. 2006, 58, 1655–1660. [Google Scholar] [CrossRef]
- Ueng, Y.F.; Shyu, C.C.; Lin, Y.L.; Park, S.S.; Liao, J.F.; Chen, C.F. Effects of baicalein and wogonin on drug-metabolizing enzymes in C57BL/6J mice. Life Sci. 2000, 67, 2189–2200. [Google Scholar] [CrossRef]
- Ni, Z.; Ma, H.; Li, X.; Zou, L.; Liu, Z.; Wang, X.; Ma, H.; Yang, L. Wogonin alleviates BaP-induced DNA damage and oxidative stress in human airway epithelial cells by dual inhibiting CYP1A1 activity and expression. Environ. Toxicol. 2023, 38, 2717–2729. [Google Scholar] [CrossRef]
- Uhl, M.; Ecker, S.; Kassie, F.; Lhoste, E.; Chakraborty, A.; Mohn, G.; Knasmuller, S. Effect of chrysin, a flavonoid compound, on the mutagenic activity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and benzo(a)pyrene (B(a)P) in bacterial and human hepatoma (HepG2) cells. Arch. Toxicol. 2003, 77, 477–484. [Google Scholar] [CrossRef]
- Sulc, M.; Indra, R.; Moserova, M.; Schmeiser, H.H.; Frei, E.; Arlt, V.M.; Stiborova, M. The impact of individual cytochrome P450 enzymes on oxidative metabolism of benzo[a]pyrene in human livers. Environ. Mol. Mutagen. 2016, 57, 229–235. [Google Scholar] [CrossRef]
- Kamenickova, A.; Anzenbacherova, E.; Pavek, P.; Soshilov, A.A.; Denison, M.S.; Anzenbacher, P.; Dvorak, Z. Pelargonidin activates the AhR and induces CYP1A1 in primary human hepatocytes and human cancer cell lines HepG2 and LS174T. Toxicol. Lett. 2013, 218, 253–259. [Google Scholar] [CrossRef]
- Gao, S.S.; Chen, X.Y.; Zhu, R.Z.; Choi, B.M.; Kim, S.J.; Kim, B.R. Dual effects of phloretin on aflatoxin B1 metabolism: Activation and detoxification of aflatoxin B1. Biofactors 2012, 38, 34–43. [Google Scholar] [CrossRef]
- Singh, G.; Thaker, R.; Sharma, A.; Parmar, D. Therapeutic effects of biochanin A, phloretin, and epigallocatechin-3-gallate in reducing oxidative stress in arsenic-intoxicated mice. Environ. Sci. Pollut. Res. Int. 2021, 28, 20517–20536. [Google Scholar] [CrossRef] [PubMed]
- Dudka, J.; Jodynis-Liebert, J.; Korobowicz, E.; Burdan, F.; Korobowicz, A.; Szumilo, J.; Tokarska, E.; Klepacz, R.; Murias, M. Activity of NADPH-cytochrome P-450 reductase of the human heart, liver and lungs in the presence of (-)-epigallocatechin gallate, quercetin and resveratrol: An in vitro study. Basic. Clin. Pharmacol. Toxicol. 2005, 97, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Rahden-Staron, I.; Czeczot, H.; Szumilo, M. Induction of rat liver cytochrome P450 isoenzymes CYP 1A and CYP 2B by different fungicides, nitrofurans, and quercetin. Mutat. Res. 2001, 498, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.K.; Chen, J.; Yeung, E.Y. Effect of Ginkgo biloba extract on procarcinogen-bioactivating human CYP1 enzymes: Identification of isorhamnetin, kaempferol, and quercetin as potent inhibitors of CYP1B1. Toxicol. Appl. Pharmacol. 2006, 213, 18–26. [Google Scholar] [CrossRef]
- BinMowyna, M.N.; AlFaris, N.A. Kaempferol suppresses acetaminophen-induced liver damage by upregulation/activation of SIRT1. Pharm. Biol. 2021, 59, 146–156. [Google Scholar] [CrossRef]
- Karakurt, S. Modulatory effects of rutin on the expression of cytochrome P450s and antioxidant enzymes in human hepatoma cells. Acta Pharm. 2016, 66, 491–502. [Google Scholar] [CrossRef]
- Kravchenko, L.V.; Avren’eva, L.I.; Aksenov, I.V.; Balakina, A.S.; Guseva, G.V.; Trusov, N.V. Effects of rutin on protective capacity in rats. Vopr. Pitan. 2015, 84, 22–30. [Google Scholar]
- Khan, R.A.; Khan, M.R.; Sahreen, S. CCl4-induced hepatotoxicity: Protective effect of rutin on p53, CYP2E1 and the antioxidative status in rat. BMC Complement. Altern. Med. 2012, 12, 178. [Google Scholar] [CrossRef]
- Bhatt, S.; Manhas, D.; Kumar, V.; Gour, A.; Sharma, K.; Dogra, A.; Ojha, P.K.; Nandi, U. Effect of Myricetin on CYP2C8 Inhibition to Assess the Likelihood of Drug Interaction Using In Silico, In Vitro, and In Vivo Approaches. ACS Omega 2022, 7, 13260–13269. [Google Scholar] [CrossRef]
- Wang, Z.; Lan, Y.; Chen, M.; Wen, C.; Hu, Y.; Liu, Z.; Ye, L. Eriodictyol, Not Its Glucuronide Metabolites, Attenuates Acetaminophen-Induced Hepatotoxicity. Mol. Pharm. 2017, 14, 2937–2951. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Lee, J.; Kim, S.; Yeo, Y.Y.; So, H.; Wu, H.; Song, Y.S.; Jang, C.Y.; Kim, H.D.; Kim, M.J.; et al. Hepatic Metabolism of Sakuranetin and Its Modulating Effects on Cytochrome P450s and UDP-Glucuronosyltransferases. Molecules 2018, 23, 1542. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Ye, J.; Zhao, L.; Li, X.; Wang, Y.; Liu, X.; Pan, L.; You, L.; Chen, L.; Jia, Y.; et al. 5,7,3′,4′-flavan-on-ol (taxifolin) protects against acetaminophen-induced liver injury by regulating the glutathione pathway. Life Sci. 2019, 236, 116939. [Google Scholar] [CrossRef] [PubMed]
- Steuck, M.; Hellhake, S.; Schebb, N.H. Food Polyphenol Apigenin Inhibits the Cytochrome P450 Monoxygenase Branch of the Arachidonic Acid Cascade. J. Agric. Food Chem. 2016, 64, 8973–8976. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Kwara, A.; Greenblatt, D.J. Metabolic interactions between acetaminophen (paracetamol) and two flavonoids, luteolin and quercetin, through in-vitro inhibition studies. J. Pharm. Pharmacol. 2017, 69, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Francisco, V.; Figueirinha, A.; Costa, G.; Liberal, J.; Ferreira, I.; Lopes, M.C.; Garcia-Rodriguez, C.; Cruz, M.T.; Batista, M.T. The Flavone Luteolin Inhibits Liver X Receptor Activation. J. Nat. Prod. 2016, 79, 1423–1428. [Google Scholar] [CrossRef]
- Lakshmi, A.; Subramanian, S. Chemotherapeutic effect of tangeretin, a polymethoxylated flavone studied in 7, 12-dimethylbenz(a)anthracene induced mammary carcinoma in experimental rats. Biochimie 2014, 99, 96–109. [Google Scholar] [CrossRef]
- Chlouchi, A.; Girard, C.; Bonet, A.; Viollon-Abadie, C.; Heyd, B.; Mantion, G.; Martin, H.; Richert, L. Effect of chrysin and natural coumarins on UGT1A1 and 1A6 activities in rat and human hepatocytes in primary culture. Planta Med. 2007, 73, 742–747. [Google Scholar] [CrossRef]
- Soliman, M.M.; Aldhahrani, A.; Gaber, A.; Alsanie, W.F.; Mohamed, W.A.; Metwally, M.M.M.; Elbadawy, M.; Shukry, M. Ameliorative impacts of chrysin against gibberellic acid-induced liver and kidney damage through the regulation of antioxidants, oxidative stress, inflammatory cytokines, and apoptosis biomarkers. Toxicol. Res. 2022, 11, 235–244. [Google Scholar] [CrossRef]
- Steiner, C.; Peters, W.H.; Gallagher, E.P.; Magee, P.; Rowland, I.; Pool-Zobel, B.L. Genistein protects human mammary epithelial cells from benzo(a)pyrene-7,8-dihydrodiol-9,10-epoxide and 4-hydroxy-2-nonenal genotoxicity by modulating the glutathione/glutathione S-transferase system. Carcinogenesis 2007, 28, 738–748. [Google Scholar] [CrossRef]
- Froyen, E.B.; Steinberg, F.M. Genistein decreases basal hepatic cytochrome P450 1A1 protein expression and activity in Swiss Webster mice. Nutr. Res. 2016, 36, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Froyen, E.B.; Reeves, J.L.; Mitchell, A.E.; Steinberg, F.M. Regulation of phase II enzymes by genistein and daidzein in male and female Swiss Webster mice. J. Med. Food 2009, 12, 1227–1237. [Google Scholar] [CrossRef]
- Wiegand, H.; Wagner, A.E.; Boesch-Saadatmandi, C.; Kruse, H.P.; Kulling, S.; Rimbach, G. Effect of dietary genistein on Phase II and antioxidant enzymes in rat liver. Cancer Genom. Proteom. 2009, 6, 85–92. [Google Scholar]
- Atherton, K.M.; Mutch, E.; Ford, D. Metabolism of the soyabean isoflavone daidzein by CYP1A2 and the extra-hepatic CYPs 1A1 and 1B1 affects biological activity. Biochem. Pharmacol. 2006, 72, 624–631. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, T.; Li, L.; Wang, H.; Zhang, D.; Yang, H. Benzo(a)pyrene promotes Hep-G2 cell migration and invasion by upregulating phosphorylated extracellular signal-regulated kinase expression. Oncol. Lett. 2018, 15, 8325–8332. [Google Scholar] [CrossRef]
- Ba, Q.; Li, J.; Huang, C.; Qiu, H.; Li, J.; Chu, R.; Zhang, W.; Xie, D.; Wu, Y.; Wang, H. Effects of benzo[a]pyrene exposure on human hepatocellular carcinoma cell angiogenesis, metastasis, and NF-kappaB signaling. Environ. Health Perspect. 2015, 123, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Asha, A.; Girija, D. Benzo[a]pyrene induced liver and kidney cancer in swiss albino mice. Res. Pharm. 2015, 1, 21. [Google Scholar]
- Ge, Y.; Gu, P.; Wang, W.; Cao, L.; Zhang, L.; Li, J.; Mu, W.; Wang, H. Benzo[a]pyrene stimulates miR-650 expression to promote the pathogenesis of fatty liver disease and hepatocellular carcinoma via SOCS3/JAK/STAT3 cascades. J. Mol. Cell Biol. 2021, 13, 556–564. [Google Scholar] [CrossRef]
- Bucher, S.; Tete, A.; Podechard, N.; Liamin, M.; Le Guillou, D.; Chevanne, M.; Coulouarn, C.; Imran, M.; Gallais, I.; Fernier, M.; et al. Co-exposure to benzo[a]pyrene and ethanol induces a pathological progression of liver steatosis in vitro and in vivo. Sci. Rep. 2018, 8, 5963. [Google Scholar] [CrossRef]
- Bucher, S.; Le Guillou, D.; Allard, J.; Pinon, G.; Begriche, K.; Tete, A.; Sergent, O.; Lagadic-Gossmann, D.; Fromenty, B. Possible Involvement of Mitochondrial Dysfunction and Oxidative Stress in a Cellular Model of NAFLD Progression Induced by Benzo[a]pyrene/Ethanol CoExposure. Oxid. Med. Cell Longev. 2018, 2018, 4396403. [Google Scholar] [CrossRef]
- Collin, A.; Hardonniere, K.; Chevanne, M.; Vuillemin, J.; Podechard, N.; Burel, A.; Dimanche-Boitrel, M.T.; Lagadic-Gossmann, D.; Sergent, O. Cooperative interaction of benzo[a]pyrene and ethanol on plasma membrane remodeling is responsible for enhanced oxidative stress and cell death in primary rat hepatocytes. Free Radic. Biol. Med. 2014, 72, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Michurina, S.V.; Borodin Iu, I.; Kolesnikov, S.I.; Ischenko, I.; Konenkov, V.I. Liver and Its Lymph Region at Benzo[a]pyrene Effects in an Experiment. Vestn. Ross. Akad. Med. Nauk. 2015, 70, 242–248. [Google Scholar] [CrossRef]
- Kolade, O.Y.; Oladiji, T.A. Protective Effects of Curcumin against Benzopyrene Induced Liver Toxicity in Albino Rats. Iop C Ser. Earth Env. 2018, 210, 012013. [Google Scholar] [CrossRef]
- Li, H.; Yuan, L.; Li, X.; Luo, Y.; Zhang, Z.; Li, J. Isoorientin Attenuated the Pyroptotic Hepatocyte Damage Induced by Benzo[a]pyrene via ROS/NF-kappaB/NLRP3/Caspase-1 Signaling Pathway. Antioxidants 2021, 10, 1275. [Google Scholar] [CrossRef]
- Li, X.Y.; He, S.Y.; Gao, C.X.; Deng, H.; Liu, Y.F.; Li, C.Q.; Yuan, L.; Luo, Y. Isoorientin attenuates benzo[a]pyrene-induced liver injury by inhibiting autophagy and pyroptosis in vitro and vivo. Food Agric. Immunol. 2019, 30, 841–861. [Google Scholar] [CrossRef]
- Li, H.; Yuan, L.; Wang, Z.L.; Shi, L.; Dong, R.; Hu, X.Z. Effects of dietary whole grain buckwheat and oat on benzo[a] pyrene-induced genotoxicity, oxidative and pyroptotic injury in liver of mice. J. Funct. Foods 2022, 93, 105082. [Google Scholar] [CrossRef]
- Hernandez-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679–1707. [Google Scholar] [CrossRef] [PubMed]
- Plazar, J.; Zegura, B.; Lah, T.T.; Filipic, M. Protective effects of xanthohumol against the genotoxicity of benzo(a)pyrene (BaP), 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) and tert-butyl hydroperoxide (t-BOOH) in HepG2 human hepatoma cells. Mutat. Res. 2007, 632, 1–8. [Google Scholar] [CrossRef]
- Wen, X.; Walle, U.K.; Walle, T. 5,7-Dimethoxyflavone downregulates CYP1A1 expression and benzo[a]pyrene-induced DNA binding in Hep G2 cells. Carcinogenesis 2005, 26, 803–809. [Google Scholar] [CrossRef]
- Sehgal, A.; Kumar, M.; Jain, M.; Dhawan, D.K. Piperine as an adjuvant increases the efficacy of curcumin in mitigating benzo(a)pyrene toxicity. Hum. Exp. Toxicol. 2012, 31, 473–482. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).