Management of the Brain: Essential Oils as Promising Neuroinflammation Modulator in Neurodegenerative Diseases
Abstract
1. Introduction
2. Main Mediators of Neuroinflammation: The Role of Microglia and Astrocytes
2.1. Microglia
2.2. Astrocytes
3. Neuroinflammation in Neurodegenerative Diseases: An Immunological Perspective
4. Targeting Neuroinflammation and Oxidative Stress in Preclinical Models: Neuroprotective Role of Essential Oils
4.1. Pinus Halepensis EO
4.2. Citrus Bergamia EO
4.3. Origanum vulgare EO
4.4. Rosmarinus Officinalis EO
4.5. Lavandula Augustifolia EO
4.6. Thymus Vulgaris EO
4.7. Satureja Khuzistanica EO
4.8. Jasminum Grandiflorum EO
4.9. Acorus Tatarinowii EO
| Essential Oil (EO) | Major Constituent | Preclinical Model | EO Preparation | Effect | References |
|---|---|---|---|---|---|
| Pinus halepensis | α-pynene, myrcene, β-caryophyllene | Aβ-induced AD in rats | 1% Tween 80 solution | AChE inhibitor, antioxidant, anti-inflammatory, DNA fragmentation protector, nootropic | [9,77] |
| Citrus bergamia | limonene | Titanium dioxide- or aluminum-induced neurotoxicity in rats | soybean oil | Antioxidant, anti- inflammatory | [85,86] |
| Origanum vulgare | thymol, carvacrol | Scopolamine-induced neurotoxicity in zebrafish | 1% Tween 80 solution | Antioxidant, nootropic, AChE inhibitor | [8] |
| Rosmarinus officinalis | eucalyptol | Scopolamine-induced neurotoxicity in zebrafish | n.a. | Antioxidant, AChE inhibitor, nootropic | [94] |
| Lavandula angustifolia | linalool | H2O2-treated SH-SY5Y cells, Aβ-treated NGF- differentiated PC-12 cells, Scopolamine-induced dementia in rats, Corticosterone-treated rats | 1% Tween 80 or 20 solution | Antioxidant, NMDA receptor inhibitor, DNA fragmentation protector, neurogenesis promoter | [99,100,101] |
| Thymus vulgaris | linalool, geraniol, thujanol | LPS-treated BV-2 cells Chronologically aged mice Scopolamine-induced neurotoxicity in zebrafish | DMSO or 1% Tween 80 solution | Anti-inflammatory, decrease brain inflammaging, antioxidant, nootropic, AChE inhibitor | [108,109,110] |
| Satureja khuzistanica | carvacrol | Traumatic brain injury in rats | 1% Tween 20 | Anti-inflammatory, anti-apoptotic | [116,117,118] |
| Jasminum grandiflorum | α-hexylcinnamaldehyde nerolidol, hexahydrofarnesyl acetone, decanal, dodecanal (in silico-predicted key compounds in targeting neuroinflammation) | BV-2 microglial cell line | n.a. | Anti-inflammatory, antioxidant | [122] |
| Acorus tatarinowii | β-Asarone, α-Asarone | APPSwe/PSENM146V/MAPTP301L triple transgenic mice | n.a. | Anti-inflammatory (NRLP3-inflammasome inhibition), nootropic | [124] |
| EO Major Constituent | Chemical Structure | Molecular Mechanism | Experimental Model | Drug Preparation | References |
|---|---|---|---|---|---|
| β-caryophyllene | 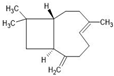 | ↑Nrf2 ↑CB2 ↑TGFβ ↑IL-10 ↑Arg1 ↑CD206 ↑SOD ↑CAT ↑GSH ↓TLR4 ↓iNOS ↓TNF-α ↓IL-1β ↓PGE2 | Ischemic stroke in mice, Experimental autoimmune encephalomyelitis mice (multiple sclerosis in vivo model) | Dissolved in olive or corn oil | [9,79,80] |
| Myrcene | 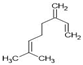 | ↑Nrf2/Keap1 ↑Autophagy ↑GSH ↑SOD ↑CAT ↓Iba1 (activated microglia) ↓GFAP (activated astrocytes) ↓iNOS ↓COX-2 ↓TNF-α ↓IL-1β ↓IL-6 ↓MMP-9 | Rotenone-induced PD in rats | Dissolved in olive oil | [9,83] |
| Limonene | 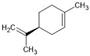 | ↑BDNF ↑GSH ↑SOD ↑CAT ↓NF-κB ↓p38 ↓JNK ↓α-Syn ↓Iba1 (activated microglia) ↓GFAP (activated astrocytes) ↓iNOS ↓COX-2 ↓TNF-α ↓IL-1β ↓IL-6 | Rotenone-induced PD in rats | Dissolved in olive oil | [87] |
| Thymol |  | ↑Nrf2/HO-1 ↑SOD ↑GSH ↑CAT ↓TLR4 ↓NLRP3 ↓NF-κB ↓IL-1 ↓TNFα ↓GFAP (activated astrocytes) ↓IL-6 ↓COX-2 ↓iNOS | Glutamate-induced excitotoxicity in rats, Rotenone-induced PD in rats | Dissolved in sunflower oil | [88,89] |
| Carvacrol | 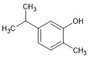 | ↑TGF-β ↑IL-10 ↑BDNF ↑SOD ↑BBB integrity ↓IFN-γ ↓IL-6 ↓IL-17 ↓NF-κB ↓TLR4 ↓iNOS ↓COX-2 ↓MMP-9 ↓TRPM7 | LPS-treated rats, Experimental autoimmune encephalomyelitis, Traumatic brain injury in rats | Dissolved in 0.9% saline solution or 2% Tween 80 or 0.1% DMSO | [90,91,117,118,119] |
| Eucalyptol | 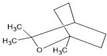 | ↑Nrf2 ↑SOD ↑GSH-Px ↓NF-κB ↓COX-2 ↓NOS-2 ↓TNFα ↓IL-6 ↓IL-1 | Brain injury after subarachnoid hemorrhage in mice, Hyperammonemic rats, Aβ-toxicated PC-12 cells | Dissolved in corn oil | [95,96,97] |
| Linalool |  | ↑Nrf2/HO-1 ↓NMDA ↓PGE2 ↓NF-κB ↓TNFα ↓IL1β | PC-12 cells treated with Aβ, LPS-induced BV-2, Triple transgenic and Aβ-induced AD mice | Dissolved in PBS or saline solution with 2% Tween 80 and 1% DMSO | [100,104,105,106] |
| Geraniol | 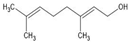 | ↑Autophagy ↑GSH ↑SOD ↓IL-6 ↓TNFα ↓α-Syn ↓PERK ↓IRE1α ↓ATF6α JAK1/2 | Rotenone-toxicated SK-N-SH, Mice fed with high fat diet In silico prediction | Dissolved in saline solution | [111,113,114] |
| α-Hexylcinnamaldehyde | 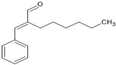 | SRC, VEGFA, EGFR, HSP90AA1, ESR1 | I In silico-predicted targets; Docking binding energies ≤ −3.9 kJ/mol | n.a. | [122] |
| Nerolidol |  | ||||
| Hexahydrofarnesyl acetone |  | ||||
| Decanal | 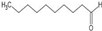 | ||||
| Dodecanal | 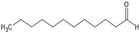 | ||||
| α-Asarone | 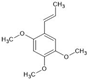 | ↑PPARγ-Glutamate transporter 1 ↑IL-10 ↑IL-4 ↑Arg1 ↓p-PERK (ER stress) ↓IL-6 ↓TNFα ↓IL-1β ↓iNOS ↓GFAP ↓MCP1 ↓MIP2 | Hypoxia-ischemia neonatal rats, HT-22 cells, Spinal cord injury in rats | Dissolved in 0.5% carboxymethylcellulose | [125,126,128] |
| β-Asarone |  | ↑PI3K/Akt/Nrf2 ↑HO-1 ↑SOD ↑CAT ↑GSH-Px ↓TNFα (promoter DNA methylation) ↓JNK/c-JUN ↓RELA (NF-κB subunit) | Scratch-injured primary cortical mice neurons, Aβ-treated PC-12 cells, Vascular dementia mice | Dissolved in DMSO or 0.9% saline solution | [129,130,131] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwon, H.S.; Koh, S.H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The Role and Consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef]
- Subhramanyam, C.S.; Wang, C.; Hu, Q.; Dheen, S.T. Microglia-Mediated Neuroinflammation in Neurodegenerative Diseases. Semin. Cell Dev. Biol. 2019, 94, 112–120. [Google Scholar] [CrossRef]
- Palpagama, T.H.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. The Role of Microglia and Astrocytes in Huntington’s Disease. Front. Mol. Neurosci. 2019, 12, 258. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M. How Neuroinflammation Contributes to Neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Fanaro, G.B.; Marques, M.R.; Calaza, K.d.C.; Brito, R.; Pessoni, A.M.; Mendonça, H.R.; Lemos, D.E.d.A.; de Brito Alves, J.L.; de Souza, E.L.; Cavalcanti Neto, M.P. New Insights on Dietary Polyphenols for the Management of Oxidative Stress and Neuroinflammation in Diabetic Retinopathy. Antioxidants 2023, 12, 1237. [Google Scholar] [CrossRef] [PubMed]
- Rahimifard, M.; Maqbool, F.; Moeini-Nodeh, S.; Niaz, K.; Abdollahi, M.; Braidy, N.; Nabavi, S.M.; Nabavi, S.F. Targeting the TLR4 Signaling Pathway by Polyphenols: A Novel Therapeutic Strategy for Neuroinflammation. Ageing Res. Rev. 2017, 36, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Capatina, L.; Napoli, E.M.; Ruberto, G.; Hritcu, L. Origanum vulgare ssp. Hirtum (Lamiaceae) Essential Oil Prevents Behavioral and Oxidative Stress Changes in the Scopolamine Zebrafish Model. Molecules 2021, 26, 7085. [Google Scholar] [CrossRef] [PubMed]
- Postu, P.A.; Mihasan, M.; Gorgan, D.L.; Sadiki, F.Z.; El Idrissi, M.; Hritcu, L. Pinus Halepensis Essential Oil Ameliorates Aβ1-42-Induced Brain Injury by Diminishing Anxiety, Oxidative Stress, and Neuroinflammation in Rats. Biomedicines 2022, 10, 2300. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, J.-X.; Du, Y.-H.; Liu, Y.; Zhang, W.; Chen, J.-F.; Liu, Y.-J.; Zheng, M.; Wang, K.-J.; He, G.-Q. Dihydromyricetin Inhibits Microglial Activation and Neuroinflammation by Suppressing NLRP3 Inflammasome Activation in APP/PS1 Transgenic Mice. CNS Neurosci. Ther. 2018, 24, 1207–1218. [Google Scholar] [CrossRef]
- Liu, P.; Zhou, Y.; Shi, J.; Wang, F.; Yang, X.; Zheng, X.; Wang, Y.; He, Y.; Xie, X.; Pang, X. Myricetin Improves Pathological Changes in 3×Tg-AD Mice by Regulating the Mitochondria-NLRP3 Inflammasome-Microglia Channel by Targeting P38 MAPK Signaling Pathway. Phytomedicine 2023, 115, 154801. [Google Scholar] [CrossRef]
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization from M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Gong, F.; Rong, Y.; Luo, Y.; Tang, P.; Zhou, Z.; Zhou, Z.; Xu, T.; Jiang, T.; et al. Exosomes Derived from Bone Mesenchymal Stem Cells Repair Traumatic Spinal Cord Injury by Suppressing the Activation of A1 Neurotoxic Reactive Astrocytes. J. Neurotrauma 2019, 36, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Bordt, E.A.; Polster, B.M. NADPH Oxidase- and Mitochondria-Derived Reactive Oxygen Species in Proinflammatory Microglial Activation: A Bipartisan Affair? Free Radic. Biol. Med. 2014, 76, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Zecca, L.; Hong, J.-S. Microglia-Mediated Neurotoxicity: Uncovering the Molecular Mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Mosley, R.L.; Benner, E.J.; Kadiu, I.; Thomas, M.; Boska, M.D.; Hasan, K.; Laurie, C.; Gendelman, H.E. Neuroinflammation, Oxidative Stress and the Pathogenesis of Parkinson’s Disease. Clin. Neurosci. Res. 2006, 6, 261–281. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid Peroxidation in Cell Death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Jin, S.; Wang, J.; Zhang, G.; Kawanokuchi, J.; Kuno, R.; Sonobe, Y.; Mizuno, T.; Suzumura, A. Tumor Necrosis Factor-Alpha Induces Neurotoxicity via Glutamate Release from Hemichannels of Activated Microglia in an Autocrine Manner. J. Biol. Chem. 2006, 281, 21362–21368. [Google Scholar] [CrossRef]
- Plotegher, N.; Filadi, R.; Pizzo, P.; Duchen, M.R. Excitotoxicity Revisited: Mitochondria on the Verge of a Nervous Breakdown. Trends Neurosci. 2021, 44, 342–351. [Google Scholar] [CrossRef]
- Leri, M.; Vasarri, M.; Carnemolla, F.; Oriente, F.; Cabaro, S.; Stio, M.; Degl’Innocenti, D.; Stefani, M.; Bucciantini, M. EVOO Polyphenols Exert Anti-Inflammatory Effects on the Microglia Cell through TREM2 Signaling Pathway. Pharmaceuticals 2023, 16, 933. [Google Scholar] [CrossRef]
- Blevins, H.M.; Xu, Y.; Biby, S.; Zhang, S. The NLRP3 Inflammasome Pathway: A Review of Mechanisms and Inhibitors for the Treatment of Inflammatory Diseases. Front. Aging Neurosci. 2022, 14, 879021. [Google Scholar] [CrossRef]
- Chen, W.; Guo, C.; Huang, S.; Jia, Z.; Wang, J.; Zhong, J.; Ge, H.; Yuan, J.; Chen, T.; Liu, X.; et al. MitoQ Attenuates Brain Damage by Polarizing Microglia towards the M2 Phenotype through Inhibition of the NLRP3 Inflammasome after ICH. Pharmacol. Res. 2020, 161, 105122. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yuan, Y.-H.; Chen, N.-H.; Wang, H.-B. The Mechanisms of NLRP3 Inflammasome/Pyroptosis Activation and Their Role in Parkinson’s Disease. Int. Immunopharmacol. 2019, 67, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Kann, O.; Almouhanna, F.; Chausse, B. Interferon γ: A Master Cytokine in Microglia-Mediated Neural Network Dysfunction and Neurodegeneration. Trends Neurosci. 2022, 45, 913–927. [Google Scholar] [CrossRef] [PubMed]
- Rock, R.B.; Hu, S.; Deshpande, A.; Munir, S.; May, B.J.; Baker, C.A.; Peterson, P.K.; Kapur, V. Transcriptional Response of Human Microglial Cells to Interferon-Gamma. Genes. Immun. 2005, 6, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.-Y.; Li, R.; Guo, Y.-J.; Zhao, Y.; Guo, J.-Z.; Ai, Q.-L.; Zhong, L.-M.; Lu, D. Gastrodin Attenuates Lipopolysaccharide-Induced Inflammatory Response and Migration via the Notch-1 Signaling Pathway in Activated Microglia. Neuromol. Med. 2022, 24, 139–154. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Wang, T.; Li, Y.-F.; Deng, Y.-N.; Shen, F.-G. Roles of the Notch Signaling Pathway and Microglia in Autism. Behav. Brain Res. 2023, 437, 114131. [Google Scholar] [CrossRef]
- Capiralla, H.; Vingtdeux, V.; Zhao, H.; Sankowski, R.; Al-Abed, Y.; Davies, P.; Marambaud, P. Resveratrol Mitigates Lipopolysaccharide- and Aβ-Mediated Microglial Inflammation by Inhibiting the TLR4/NF-κB/STAT Signaling Cascade. J. Neurochem. 2012, 120, 461–472. [Google Scholar] [CrossRef]
- Cui, W.; Sun, C.; Ma, Y.; Wang, S.; Wang, X.; Zhang, Y. Inhibition of TLR4 Induces M2 Microglial Polarization and Provides Neuroprotection via the NLRP3 Inflammasome in Alzheimer’s Disease. Front. Neurosci. 2020, 14, 444. [Google Scholar] [CrossRef]
- Hu, X.; Leak, R.K.; Shi, Y.; Suenaga, J.; Gao, Y.; Zheng, P.; Chen, J. Microglial and Macrophage Polarization—New Prospects for Brain Repair. Nat. Rev. Neurol. 2015, 11, 56–64. [Google Scholar] [CrossRef]
- Zhang, J.-M.; An, J. Cytokines, Inflammation, and Pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, L.; Ren, Y.; Huang, Y.; Liu, W.; Lv, Z.; Qian, L.; Yu, Y.; Xiong, Y. Arginase: Shedding Light on the Mechanisms and Opportunities in Cardiovascular Diseases. Cell Death Discov. 2022, 8, 413. [Google Scholar] [CrossRef]
- Tang, Y.; Li, T.; Li, J.; Yang, J.; Liu, H.; Zhang, X.J.; Le, W. Jmjd3 Is Essential for the Epigenetic Modulation of Microglia Phenotypes in the Immune Pathogenesis of Parkinson’s Disease. Cell Death Differ. 2014, 21, 369–380. [Google Scholar] [CrossRef]
- Gazi, U.; Martinez-Pomares, L. Influence of the Mannose Receptor in Host Immune Responses. Immunobiology 2009, 214, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Rahimian, R.; Belliveau, C.; Chen, R.; Mechawar, N. Microglial Inflammatory-Metabolic Pathways and Their Potential Therapeutic Implication in Major Depressive Disorder. Front. Psychiatry 2022, 13, 871997. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Gao, Y.; Zhang, Q.; Zhou, G.; Cao, F.; Yao, S. IL-4 Switches Microglia/Macrophage M1/M2 Polarization and Alleviates Neurological Damage by Modulating the JAK1/STAT6 Pathway Following ICH. Neuroscience 2020, 437, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, Y.; Wang, L.; Zhan, H.; Luo, X.; Zeng, Y.; Wu, W.; Zhang, X.; Wang, F. TREM2 Ameliorates Neuroinflammatory Response and Cognitive Impairment via PI3K/AKT/FoxO3a Signaling Pathway in Alzheimer’s Disease Mice. Aging 2020, 12, 20862–20879. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Luo, Y.; Du, Y.; Zhang, X.; Fu, J. Curcumin Inhibits LPS-Induced Neuroinflammation by Promoting Microglial M2 Polarization via TREM2/ TLR4/ NF-κB Pathways in BV2 Cells. Mol. Immunol. 2019, 116, 29–37. [Google Scholar] [CrossRef]
- Liu, W.; Rong, Y.; Wang, J.; Zhou, Z.; Ge, X.; Ji, C.; Jiang, D.; Gong, F.; Li, L.; Chen, J.; et al. Exosome-Shuttled miR-216a-5p from Hypoxic Preconditioned Mesenchymal Stem Cells Repair Traumatic Spinal Cord Injury by Shifting Microglial M1/M2 Polarization. J. Neuroinflamm. 2020, 17, 47. [Google Scholar] [CrossRef]
- Vergadi, E.; Ieronymaki, E.; Lyroni, K.; Vaporidi, K.; Tsatsanis, C. Akt Signaling Pathway in Macrophage Activation and M1/M2 Polarization. J. Immunol. 2017, 198, 1006–1014. [Google Scholar] [CrossRef]
- Bellot-Saez, A.; Stevenson, R.; Kékesi, O.; Samokhina, E.; Ben-Abu, Y.; Morley, J.W.; Buskila, Y. Neuromodulation of Astrocytic K+ Clearance. Int. J. Mol. Sci. 2021, 22, 2520. [Google Scholar] [CrossRef] [PubMed]
- Gomolka, R.S.; Hablitz, L.M.; Mestre, H.; Giannetto, M.; Du, T.; Hauglund, N.L.; Xie, L.; Peng, W.; Martinez, P.M.; Nedergaard, M.; et al. Loss of Aquaporin-4 Results in Glymphatic System Dysfunction via Brain-Wide Interstitial Fluid Stagnation. eLife 2023, 12, e82232. [Google Scholar] [CrossRef]
- Phatnani, H.; Maniatis, T. Astrocytes in Neurodegenerative Disease. Cold Spring Harb. Perspect. Biol. 2015, 7, a020628. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Liu, L.-R.; Liu, J.-C.; Bao, J.-S.; Bai, Q.-Q.; Wang, G.-Q. Interaction of Microglia and Astrocytes in the Neurovascular Unit. Front. Immunol. 2020, 11, 1024. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhang, N.; Zhang, Q.; Li, C.; Sandhu, A.F.; Iii, G.W.; Lin, S.; Lv, P.; Liu, Y.; Wu, Q.; et al. Inflammatory Factors and Amyloid β-Induced Microglial Polarization Promote Inflammatory Crosstalk with Astrocytes. Aging 2020, 12, 22538–22549. [Google Scholar] [CrossRef]
- Li, S.; Fang, Y.; Zhang, Y.; Song, M.; Zhang, X.; Ding, X.; Yao, H.; Chen, M.; Sun, Y.; Ding, J.; et al. Microglial NLRP3 Inflammasome Activates Neurotoxic Astrocytes in Depression-like Mice. Cell Rep. 2022, 41, 111532. [Google Scholar] [CrossRef]
- Xiao, T.; Ji, H.; Shangguan, X.; Qu, S.; Cui, Y.; Xu, J. NLRP3 Inflammasome of Microglia Promotes A1 Astrocyte Transformation, Neo-Neuron Decline and Cognition Impairment in Endotoxemia. Biochem. Biophys. Res. Commun. 2022, 602, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Molecular Dissection of Reactive Astrogliosis and Glial Scar Formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef]
- Wang, H.; Song, G.; Chuang, H.; Chiu, C.; Abdelmaksoud, A.; Ye, Y.; Zhao, L. Portrait of Glial Scar in Neurological Diseases. Int. J. Immunopathol. Pharmacol. 2018, 31, 2058738418801406. [Google Scholar] [CrossRef]
- Hamby, M.E.; Sofroniew, M.V. Reactive Astrocytes as Therapeutic Targets for CNS Disorders. Neurotherapeutics 2010, 7, 494–506. [Google Scholar] [CrossRef]
- Qian, D.; Li, L.; Rong, Y.; Liu, W.; Wang, Q.; Zhou, Z.; Gu, C.; Huang, Y.; Zhao, X.; Chen, J.; et al. Blocking Notch Signal Pathway Suppresses the Activation of Neurotoxic A1 Astrocytes after Spinal Cord Injury. Cell Cycle 2019, 18, 3010–3029. [Google Scholar] [CrossRef]
- Wu, M.; Wang, L.; Li, F.; Hu, R.; Ma, J.; Zhang, K.; Cheng, X. Resveratrol Downregulates STAT3 Expression and Astrocyte Activation in Primary Astrocyte Cultures of Rat. Neurochem. Res. 2020, 45, 455–464. [Google Scholar] [CrossRef]
- Chang, J.; Qian, Z.; Wang, B.; Cao, J.; Zhang, S.; Jiang, F.; Kong, R.; Yu, X.; Cao, X.; Yang, L.; et al. Transplantation of A2 Type Astrocytes Promotes Neural Repair and Remyelination after Spinal Cord Injury. Cell Commun. Signal 2023, 21, 37. [Google Scholar] [CrossRef]
- Li, T.; Liu, T.; Chen, X.; Li, L.; Feng, M.; Zhang, Y.; Wan, L.; Zhang, C.; Yao, W. Microglia Induce the Transformation of A1/A2 Reactive Astrocytes via the CXCR7/PI3K/Akt Pathway in Chronic Post-Surgical Pain. J. Neuroinflamm. 2020, 17, 211. [Google Scholar] [CrossRef] [PubMed]
- Neal, M.; Luo, J.; Harischandra, D.S.; Gordon, R.; Sarkar, S.; Jin, H.; Anantharam, V.; Désaubry, L.; Kanthasamy, A.; Kanthasamy, A. Prokineticin-2 Promotes Chemotaxis and Alternative A2 Reactivity of Astrocytes. Glia 2018, 66, 2137–2157. [Google Scholar] [CrossRef]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS Neurodegenerative Diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and Aging-Related Diseases: From Molecular Mechanisms to Interventions and Treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Scheiblich, H.; Trombly, M.; Ramirez, A.; Heneka, M.T. Neuroimmune Connections in Aging and Neurodegenerative Diseases. Trends Immunol. 2020, 41, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Li, M.D.; Burns, T.C.; Morgan, A.A.; Khatri, P. Integrated Multi-Cohort Transcriptional Meta-Analysis of Neurodegenerative Diseases. Acta Neuropathol. Commun. 2014, 2, 93. [Google Scholar] [CrossRef]
- Ross, C.A.; Poirier, M.A. Protein Aggregation and Neurodegenerative Disease. Nat. Med. 2004, 10 (Suppl. S7), S10–S17. [Google Scholar] [CrossRef]
- Hinkle, J.T.; Patel, J.; Panicker, N.; Karuppagounder, S.S.; Biswas, D.; Belingon, B.; Chen, R.; Brahmachari, S.; Pletnikova, O.; Troncoso, J.C.; et al. STING Mediates Neurodegeneration and Neuroinflammation in Nigrostriatal α-Synucleinopathy. Proc. Natl. Acad. Sci. USA 2022, 119, e2118819119. [Google Scholar] [CrossRef]
- Gulen, M.F.; Samson, N.; Keller, A.; Schwabenland, M.; Liu, C.; Glück, S.; Thacker, V.V.; Favre, L.; Mangeat, B.; Kroese, L.J.; et al. cGAS-STING Drives Ageing-Related Inflammation and Neurodegeneration. Nature 2023, 620, 374–380. [Google Scholar] [CrossRef]
- Gordon, R.; Albornoz, E.A.; Christie, D.C.; Langley, M.R.; Kumar, V.; Mantovani, S.; Robertson, A.A.B.; Butler, M.S.; Rowe, D.B.; O’Neill, L.A.; et al. Inflammasome Inhibition Prevents α-Synuclein Pathology and Dopaminergic Neurodegeneration in Mice. Sci. Transl. Med. 2018, 10, eaah4066. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Hwang, I.; Park, S.; Hong, S.; Hwang, B.; Cho, Y.; Son, J.; Yu, J.-W. MPTP-Driven NLRP3 Inflammasome Activation in Microglia Plays a Central Role in Dopaminergic Neurodegeneration. Cell Death Differ. 2019, 26, 213–228. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, Y.; Li, Q.; Chen, C.; Chen, H.; Song, Y.; Hua, F.; Zhang, Z. Beta-Amyloid Activates NLRP3 Inflammasome via TLR4 in Mouse Microglia. Neurosci. Lett. 2020, 736, 135279. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.S.; Suh, K.; Han, J.; Kim, H.; Kang, H.-S.; Choi, W.-S.; Mook-Jung, I. Amyloid-β Activates NLRP3 Inflammasomes by Affecting Microglial Immunometabolism through the Syk-AMPK Pathway. Aging Cell 2022, 21, e13623. [Google Scholar] [CrossRef] [PubMed]
- Moonen, S.; Koper, M.J.; Van Schoor, E.; Schaeverbeke, J.M.; Vandenberghe, R.; von Arnim, C.A.F.; Tousseyn, T.; De Strooper, B.; Thal, D.R. Pyroptosis in Alzheimer’s Disease: Cell Type-Specific Activation in Microglia, Astrocytes and Neurons. Acta Neuropathol. 2023, 145, 175–195. [Google Scholar] [CrossRef]
- McKenzie, B.A.; Mamik, M.K.; Saito, L.B.; Boghozian, R.; Monaco, M.C.; Major, E.O.; Lu, J.-Q.; Branton, W.G.; Power, C. Caspase-1 Inhibition Prevents Glial Inflammasome Activation and Pyroptosis in Models of Multiple Sclerosis. Proc. Natl. Acad. Sci. USA 2018, 115, E6065–E6074. [Google Scholar] [CrossRef]
- Van Schoor, E.; Ospitalieri, S.; Moonen, S.; Tomé, S.O.; Ronisz, A.; Ok, O.; Weishaupt, J.; Ludolph, A.C.; Van Damme, P.; Van Den Bosch, L.; et al. Increased Pyroptosis Activation in White Matter Microglia Is Associated with Neuronal Loss in ALS Motor Cortex. Acta Neuropathol. 2022, 144, 393–411. [Google Scholar] [CrossRef]
- Bartolini, M.; Bertucci, C.; Cavrini, V.; Andrisano, V. Beta-Amyloid Aggregation Induced by Human Acetylcholinesterase: Inhibition Studies. Biochem. Pharmacol. 2003, 65, 407–416. [Google Scholar] [CrossRef]
- Avola, R.; Granata, G.; Geraci, C.; Napoli, E.; Graziano, A.C.E.; Cardile, V. Oregano (Origanum vulgare L.) Essential Oil Provides Anti-Inflammatory Activity and Facilitates Wound Healing in a Human Keratinocytes Cell Model. Food Chem. Toxicol. 2020, 144, 111586. [Google Scholar] [CrossRef] [PubMed]
- de Lavor, É.M.; Fernandes, A.W.C.; de Andrade Teles, R.B.; Leal, A.E.B.P.; de Oliveira Júnior, R.G.; Gama E Silva, M.; de Oliveira, A.P.; Silva, J.C.; de Moura Fontes Araújo, M.T.; Coutinho, H.D.M.; et al. Essential Oils and Their Major Compounds in the Treatment of Chronic Inflammation: A Review of Antioxidant Potential in Preclinical Studies and Molecular Mechanisms. Oxid. Med. Cell. Longev. 2018, 2018, 6468593. [Google Scholar] [CrossRef]
- Mladenović, M.; Astolfi, R.; Tomašević, N.; Matić, S.; Božović, M.; Sapienza, F.; Ragno, R. In Vitro Antioxidant and In Vivo Antigenotoxic Features of a Series of 61 Essential Oils and Quantitative Composition-Activity Relationships Modeled through Machine Learning Algorithms. Antioxidants 2023, 12, 1815. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, J.T.; Shropshire, B.C.; Nagy, T.R.; Chambers, K.D.; Li, Y.; Korach, K.S. Essential Oils and Health. Yale J. Biol. Med. 2020, 93, 291–305. [Google Scholar]
- Russo, A.; Bruno, M.; Avola, R.; Cardile, V.; Rigano, D. Chamazulene-Rich Artemisia Arborescens Essential Oils Affect the Cell Growth of Human Melanoma Cells. Plants 2020, 9, 1000. [Google Scholar] [CrossRef] [PubMed]
- Postu, P.A.; Sadiki, F.Z.; El Idrissi, M.; Cioanca, O.; Trifan, A.; Hancianu, M.; Hritcu, L. Pinus Halepensis Essential Oil Attenuates the Toxic Alzheimer’s Amyloid Beta (1-42)-Induced Memory Impairment and Oxidative Stress in the Rat Hippocampus. Biomed. Pharmacother. 2019, 112, 108673. [Google Scholar] [CrossRef] [PubMed]
- Irrera, N.; D’Ascola, A.; Pallio, G.; Bitto, A.; Mannino, F.; Arcoraci, V.; Rottura, M.; Ieni, A.; Minutoli, L.; Metro, D.; et al. β-Caryophyllene Inhibits Cell Proliferation through a Direct Modulation of CB2 Receptors in Glioblastoma Cells. Cancers 2020, 12, 1038. [Google Scholar] [CrossRef]
- Tian, X.; Liu, H.; Xiang, F.; Xu, L.; Dong, Z. β-Caryophyllene Protects against Ischemic Stroke by Promoting Polarization of Microglia toward M2 Phenotype via the TLR4 Pathway. Life Sci. 2019, 237, 116915. [Google Scholar] [CrossRef]
- Askari, V.R.; Baradaran Rahimi, V.; Shafiee-Nick, R. Low Doses of β-Caryophyllene Reduced Clinical and Paraclinical Parameters of an Autoimmune Animal Model of Multiple Sclerosis: Investigating the Role of CB2 Receptors in Inflammation by Lymphocytes and Microglial. Brain Sci. 2023, 13, 1092. [Google Scholar] [CrossRef]
- Youssef, D.A.; El-Fayoumi, H.M.; Mahmoud, M.F. Beta-Caryophyllene Alleviates Diet-Induced Neurobehavioral Changes in Rats: The Role of CB2 and PPAR-γ Receptors. Biomed. Pharmacother. 2019, 110, 145–154. [Google Scholar] [CrossRef]
- Fumagalli, M.; Lombardi, M.; Gressens, P.; Verderio, C. How to Reprogram Microglia toward Beneficial Functions. Glia 2018, 66, 2531–2549. [Google Scholar] [CrossRef]
- Azimullah, S.; Jayaraj, R.L.; Meeran, M.F.N.; Jalal, F.Y.; Adem, A.; Ojha, S.; Beiram, R. Myrcene Salvages Rotenone-Induced Loss of Dopaminergic Neurons by Inhibiting Oxidative Stress, Inflammation, Apoptosis, and Autophagy. Molecules 2023, 28, 685. [Google Scholar] [CrossRef]
- Nauman, M.C.; Johnson, J.J. Clinical Application of Bergamot (Citrus bergamia) for Reducing High Cholesterol and Cardiovascular Disease Markers. Integr. Food Nutr. Metab. 2019, 6. [Google Scholar] [CrossRef]
- Cui, Y.; Che, Y.; Wang, H. Bergamot Essential Oil Attenuate Aluminum-Induced Anxiety-like Behavior through Antioxidation, Anti-Inflammatory and GABA Regulation in Rats. Food Chem. Toxicol. 2020, 145, 111766. [Google Scholar] [CrossRef]
- Cui, Y.; Che, Y.; Wang, H. Nono-Titanium Dioxide Exposure during the Adolescent Period Induces Neurotoxicities in Rats: Ameliorative Potential of Bergamot Essential Oil. Brain Behav. 2021, 11, e02099. [Google Scholar] [CrossRef]
- Eddin, L.B.; Azimullah, S.; Jha, N.K.; Nagoor Meeran, M.F.; Beiram, R.; Ojha, S. Limonene, a Monoterpene, Mitigates Rotenone-Induced Dopaminergic Neurodegeneration by Modulating Neuroinflammation, Hippo Signaling and Apoptosis in Rats. Int. J. Mol. Sci. 2023, 24, 5222. [Google Scholar] [CrossRef]
- Abu-Elfotuh, K.; Abdel-Sattar, S.A.; Abbas, A.N.; Mahran, Y.F.; Alshanwani, A.R.; Hamdan, A.M.E.; Atwa, A.M.; Reda, E.; Ahmed, Y.M.; Zaghlool, S.S.; et al. The Protective Effect of Thymoquinone or/and Thymol against Monosodium Glutamate-Induced Attention-Deficit/Hyperactivity Disorder (ADHD)-like Behavior in Rats: Modulation of Nrf2/HO-1, TLR4/NF-κB/NLRP3/Caspase-1 and Wnt/β-Catenin Signaling Pathways in Rat Model. Biomed. Pharmacother. 2022, 155, 113799. [Google Scholar] [CrossRef]
- Javed, H.; Azimullah, S.; Meeran, M.F.N.; Ansari, S.A.; Ojha, S. Neuroprotective Effects of Thymol, a Dietary Monoterpene Against Dopaminergic Neurodegeneration in Rotenone-Induced Rat Model of Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 1538. [Google Scholar] [CrossRef]
- Lee, B.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.-H. Inhibitory Effect of Carvacrol on Lipopolysaccharide-Induced Memory Impairment in Rats. Korean J. Physiol. Pharmacol. 2020, 24, 27–37. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Amiri, H.; Ayoobi, F.; Rahmani, M.; Taghipour, Z.; Ghavamabadi, R.T.; Jafarzadeh, A.; Sankian, M. Carvacrol Ameliorates Experimental Autoimmune Encephalomyelitis through Modulating Pro- and Anti-Inflammatory Cytokines. Life Sci. 2019, 219, 257–263. [Google Scholar] [CrossRef]
- Borges, R.S.; Keita, H.; Ortiz, B.L.S.; Dos Santos Sampaio, T.I.; Ferreira, I.M.; Lima, E.S.; de Jesus Amazonas da Silva, M.; Fernandes, C.P.; de Faria Mota Oliveira, A.E.M.; da Conceição, E.C.; et al. Anti-Inflammatory Activity of Nanoemulsions of Essential Oil from Rosmarinus officinalis L.: In Vitro and in Zebrafish Studies. Inflammopharmacology 2018, 26, 1057–1080. [Google Scholar] [CrossRef]
- Oualdi, I.; Brahmi, F.; Mokhtari, O.; Abdellaoui, S.; Tahani, A.; Oussaid, A. Rosmarinus Officinalis from Morocco, Italy and France: Insight into Chemical Compositions and Biological Properties. Mater. Today Proc. 2021, 45, 7706–7710. [Google Scholar] [CrossRef]
- Capatina, L.; Boiangiu, R.S.; Dumitru, G.; Napoli, E.M.; Ruberto, G.; Hritcu, L.; Todirascu-Ciornea, E. Rosmarinus Officinalis Essential Oil Improves Scopolamine-Induced Neurobehavioral Changes via Restoration of Cholinergic Function and Brain Antioxidant Status in Zebrafish (Danio rerio). Antioxidants 2020, 9, 62. [Google Scholar] [CrossRef]
- Xu, G.; Guo, J.; Sun, C. Eucalyptol Ameliorates Early Brain Injury after Subarachnoid Haemorrhage via Antioxidant and Anti-Inflammatory Effects in a Rat Model. Pharm. Biol. 2021, 59, 114–120. [Google Scholar] [CrossRef]
- Bahrami, T.; Yaghmaei, P.; Yousofvand, N. The Effects of Ibuprofen and 1, 8- Cineol on Anxiety and Spatial Memory in Hyperammonemic Rats. Metab. Brain Dis. 2023, 38, 613–620. [Google Scholar] [CrossRef]
- Khan, A.; Vaibhav, K.; Javed, H.; Tabassum, R.; Ahmed, M.E.; Khan, M.M.; Khan, M.B.; Shrivastava, P.; Islam, F.; Siddiqui, M.S.; et al. 1,8-Cineole (Eucalyptol) Mitigates Inflammation in Amyloid Beta Toxicated PC12 Cells: Relevance to Alzheimer’s Disease. Neurochem. Res. 2014, 39, 344–352. [Google Scholar] [CrossRef]
- Habán, M.; Korczyk-Szabó, J.; Čerteková, S.; Ražná, K. Lavandula Species, Their Bioactive Phytochemicals, and Their Biosynthetic Regulation. Int. J. Mol. Sci. 2023, 24, 8831. [Google Scholar] [CrossRef]
- López, V.; Nielsen, B.; Solas, M.; Ramírez, M.J.; Jäger, A.K. Exploring Pharmacological Mechanisms of Lavender (Lavandula angustifolia) Essential Oil on Central Nervous System Targets. Front. Pharmacol. 2017, 8, 280. [Google Scholar] [CrossRef]
- Caputo, L.; Piccialli, I.; Ciccone, R.; de Caprariis, P.; Massa, A.; De Feo, V.; Pannaccione, A. Lavender and Coriander Essential Oils and Their Main Component Linalool Exert a Protective Effect against Amyloid-β Neurotoxicity. Phytother. Res. 2021, 35, 486–493. [Google Scholar] [CrossRef]
- Hancianu, M.; Cioanca, O.; Mihasan, M.; Hritcu, L. Neuroprotective Effects of Inhaled Lavender Oil on Scopolamine-Induced Dementia via Anti-Oxidative Activities in Rats. Phytomedicine 2013, 20, 446–452. [Google Scholar] [CrossRef]
- Sánchez-Vidaña, D.I.; Po, K.K.-T.; Fung, T.K.-H.; Chow, J.K.-W.; Lau, W.K.-W.; So, P.-K.; Lau, B.W.-M.; Tsang, H.W.-H. Lavender Essential Oil Ameliorates Depression-like Behavior and Increases Neurogenesis and Dendritic Complexity in Rats. Neurosci. Lett. 2019, 701, 180–192. [Google Scholar] [CrossRef]
- Ouanes, S.; Popp, J. High Cortisol and the Risk of Dementia and Alzheimer’s Disease: A Review of the Literature. Front. Aging Neurosci. 2019, 11, 43. [Google Scholar] [CrossRef]
- Li, Y.; Lv, O.; Zhou, F.; Li, Q.; Wu, Z.; Zheng, Y. Linalool Inhibits LPS-Induced Inflammation in BV2 Microglia Cells by Activating Nrf2. Neurochem. Res. 2015, 40, 1520–1525. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Osorio, E.; Cardona-Gómez, G.P. Linalool Reverses Neuropathological and Behavioral Impairments in Old Triple Transgenic Alzheimer’s Mice. Neuropharmacology 2016, 102, 111–120. [Google Scholar] [CrossRef]
- Xu, P.; Wang, K.; Lu, C.; Dong, L.; Gao, L.; Yan, M.; Aibai, S.; Yang, Y.; Liu, X. Protective Effects of Linalool against Amyloid Beta-Induced Cognitive Deficits and Damages in Mice. Life Sci. 2017, 174, 21–27. [Google Scholar] [CrossRef]
- Galovičová, L.; Borotová, P.; Valková, V.; Vukovic, N.L.; Vukic, M.; Štefániková, J.; Ďúranová, H.; Kowalczewski, P.Ł.; Čmiková, N.; Kačániová, M. Thymus vulgaris Essential Oil and Its Biological Activity. Plants 2021, 10, 1959. [Google Scholar] [CrossRef]
- Horváth, G.; Horváth, A.; Reichert, G.; Böszörményi, A.; Sipos, K.; Pandur, E. Three Chemotypes of Thyme (Thymus vulgaris L.) Essential Oil and Their Main Compounds Affect Differently the IL-6 and TNFα Cytokine Secretions of BV-2 Microglia by Modulating the NF-κB and C/EBPβ Signalling Pathways. BMC Complement. Med. Ther. 2021, 21, 148. [Google Scholar] [CrossRef]
- Warman, D.J.; Jia, H.; Kato, H. Effects of Thyme (Thymus vulgaris L.) Essential Oil on Aging-Induced Brain Inflammation and Blood Telomere Attrition in Chronologically Aged C57BL/6J Mice. Antioxidants 2023, 12, 1178. [Google Scholar] [CrossRef]
- Capatina, L.; Todirascu-Ciornea, E.; Napoli, E.M.; Ruberto, G.; Hritcu, L.; Dumitru, G. Thymus vulgaris Essential Oil Protects Zebrafish against Cognitive Dysfunction by Regulating Cholinergic and Antioxidants Systems. Antioxidants 2020, 9, 1083. [Google Scholar] [CrossRef] [PubMed]
- Rekha, K.R.; Inmozhi Sivakamasundari, R. Geraniol Protects Against the Protein and Oxidative Stress Induced by Rotenone in an In Vitro Model of Parkinson’s Disease. Neurochem. Res. 2018, 43, 1947–1962. [Google Scholar] [CrossRef]
- Uddin, M.S.; Yu, W.S.; Lim, L.W. Exploring ER Stress Response in Cellular Aging and Neuroinflammation in Alzheimer’s Disease. Ageing Res. Rev. 2021, 70, 101417. [Google Scholar] [CrossRef] [PubMed]
- El Azab, E.F.; Abdulmalek, S. Amelioration of Age-Related Multiple Neuronal Impairments and Inflammation in High-Fat Diet-Fed Rats: The Prospective Multitargets of Geraniol. Oxid. Med. Cell. Longev. 2022, 2022, 4812993. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, S.; Huang, X.; Rehman, H.M. Mechanistic Insight of the Potential of Geraniol against Alzheimer’s Disease. Eur. J. Med. Res. 2022, 27, 93. [Google Scholar] [CrossRef]
- Hadian, J.; Hossein Mirjalili, M.; Reza Kanani, M.; Salehnia, A.; Ganjipoor, P. Phytochemical and Morphological Characterization of Satureja Khuzistanica Jamzad Populations from Iran. Chem. Biodivers. 2011, 8, 902–915. [Google Scholar] [CrossRef]
- Abbasloo, E.; Dehghan, F.; Khaksari, M.; Najafipour, H.; Vahidi, R.; Dabiri, S.; Sepehri, G.; Asadikaram, G. The Anti-Inflammatory Properties of Satureja Khuzistanica Jamzad Essential Oil Attenuate the Effects of Traumatic Brain Injuries in Rats. Sci. Rep. 2016, 6, 31866. [Google Scholar] [CrossRef] [PubMed]
- Abbasloo, E.; Amiresmaili, S.; Shirazpour, S.; Khaksari, M.; Kobeissy, F.; Thomas, T.C. Satureja Khuzistanica Jamzad Essential Oil and Pure Carvacrol Attenuate TBI-Induced Inflammation and Apoptosis via NF-κB and Caspase-3 Regulation in the Male Rat Brain. Sci. Rep. 2023, 13, 4780. [Google Scholar] [CrossRef] [PubMed]
- Abbasloo, E.; Khaksari, M.; Sanjari, M.; Kobeissy, F.; Thomas, T.C. Carvacrol Decreases Blood-Brain Barrier Permeability Post-Diffuse Traumatic Brain Injury in Rats. Sci. Rep. 2023, 13, 14546. [Google Scholar] [CrossRef]
- Lee, M.; Lee, S.H.; Choi, S.; Choi, B.Y.; Suh, S.W. Carvacrol Inhibits Expression of Transient Receptor Potential Melastatin 7 Channels and Alleviates Zinc Neurotoxicity Induced by Traumatic Brain Injury. Int. J. Mol. Sci. 2022, 23, 13840. [Google Scholar] [CrossRef]
- Ahmed, A.B.; Tahir, H.M.; Yousaf, M.S.; Munir, F.; Ali, S. Efficacy of Silk Sericin and Jasminum grandiflorum L. Leaf Extract on Skin Injuries Induced by Burn in Mice. J. Burn Care Res. 2023, 44, 58–64. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Wen, S.; Li, Q.; Chen, R.; Lai, X.; Zhang, Z.; Zhou, Z.; Xie, Y.; Zheng, X.; et al. Extract of Jasminum grandiflorum L. Alleviates CCl4-Induced Liver Injury by Decreasing Inflammation, Oxidative Stress and Hepatic CYP2E1 Expression in Mice. Biomed. Pharmacother. 2022, 152, 113255. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zeng, X.; Feng, Y.; Li, S.; Wang, Y.; Liu, Y.; Chen, F.; Guan, Z.; Chen, T.; Wei, F. Inhibitory Effects of Jasminum grandiflorum L. Essential Oil on Lipopolysaccharide-Induced Microglia Activation-Integrated Characteristic Analysis of Volatile Compounds, Network Pharmacology, and BV-2 Cell. Front. Pharmacol. 2023, 14, 1180618. [Google Scholar] [CrossRef]
- Wang, M.; Tang, H.-P.; Wang, S.; Hu, W.-J.; Li, J.-Y.; Yu, A.-Q.; Bai, Q.-X.; Yang, B.-Y.; Kuang, H.-X. Acorus Tatarinowii Schott: A Review of Its Botany, Traditional Uses, Phytochemistry, and Pharmacology. Molecules 2023, 28, 4525. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, X.; Hong, X.; Wang, S.; Wei, J.; Huang, J.; Ji, L.; Yang, Y.; Efferth, T.; Hong, C.; et al. Essential Oil of Acorus Tatarinowii Schott Inhibits Neuroinflammation by Suppressing NLRP3 Inflammasome Activation in 3 × Tg-AD Transgenic Mice. Phytomedicine 2023, 112, 154695. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Zhen, F.; Liu, Z.; Feng, Z.; Wang, G.; Zhang, C.; Wang, X.; Sun, Y.; Zheng, X.; Bai, Y.; et al. Alpha-Asaronol Alleviates Dysmyelination by Enhancing Glutamate Transport Through the Activation of PPARγ-GLT-1 Signaling in Hypoxia-Ischemia Neonatal Rats. Front. Pharmacol. 2022, 13, 766744. [Google Scholar] [CrossRef]
- Mikami, M.; Takuya, O.; Yoshino, Y.; Nakamura, S.; Ito, K.; Kojima, H.; Takahashi, T.; Iddamalgoda, A.; Inoue, S.; Shimazawa, M.; et al. Acorus Calamus Extract and Its Component α-Asarone Attenuate Murine Hippocampal Neuronal Cell Death Induced by l-Glutamate and Tunicamycin. Biosci. Biotechnol. Biochem. 2021, 85, 493–501. [Google Scholar] [CrossRef]
- Sen, T.; Saha, P.; Gupta, R.; Foley, L.M.; Jiang, T.; Abakumova, O.S.; Hitchens, T.K.; Sen, N. Aberrant ER Stress Induced Neuronal-IFNβ Elicits White Matter Injury Due to Microglial Activation and T-Cell Infiltration after TBI. J. Neurosci. 2020, 40, 424–446. [Google Scholar] [CrossRef]
- Jo, M.-J.; Kumar, H.; Joshi, H.P.; Choi, H.; Ko, W.-K.; Kim, J.M.; Hwang, S.S.S.; Park, S.Y.; Sohn, S.; Bello, A.B.; et al. Oral Administration of α-Asarone Promotes Functional Recovery in Rats with Spinal Cord Injury. Front. Pharmacol. 2018, 9, 445. [Google Scholar] [CrossRef]
- Yi, M.; Wang, D.; Chen, Y.; Xu, X.; Dai, X. β-Asarone Suppresses TNF-α Expression through DNA Methylation and c-Jun-Mediated Transcription Modulation in Scratch-Injured Neuronal Cells. J. Biochem. Mol. Toxicol. 2021, 35, e22798. [Google Scholar] [CrossRef]
- Meng, M.; Zhang, L.; Ai, D.; Wu, H.; Peng, W. β-Asarone Ameliorates β-Amyloid-Induced Neurotoxicity in PC12 Cells by Activating P13K/Akt/Nrf2 Signaling Pathway. Front. Pharmacol. 2021, 12, 659955. [Google Scholar] [CrossRef]
- Ning, Z.; Zhong, X.; Wu, Y.; Wang, Y.; Hu, D.; Wang, K.; Deng, M. β-Asarone Improves Cognitive Impairment and Alleviates Autophagy in Mice with Vascular Dementia via the cAMP/PKA/CREB Pathway. Phytomedicine 2023, 123, 155215. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avola, R.; Furnari, A.G.; Graziano, A.C.E.; Russo, A.; Cardile, V. Management of the Brain: Essential Oils as Promising Neuroinflammation Modulator in Neurodegenerative Diseases. Antioxidants 2024, 13, 178. https://doi.org/10.3390/antiox13020178
Avola R, Furnari AG, Graziano ACE, Russo A, Cardile V. Management of the Brain: Essential Oils as Promising Neuroinflammation Modulator in Neurodegenerative Diseases. Antioxidants. 2024; 13(2):178. https://doi.org/10.3390/antiox13020178
Chicago/Turabian StyleAvola, Rosanna, Alessandro Giuseppe Furnari, Adriana Carol Eleonora Graziano, Alessandra Russo, and Venera Cardile. 2024. "Management of the Brain: Essential Oils as Promising Neuroinflammation Modulator in Neurodegenerative Diseases" Antioxidants 13, no. 2: 178. https://doi.org/10.3390/antiox13020178
APA StyleAvola, R., Furnari, A. G., Graziano, A. C. E., Russo, A., & Cardile, V. (2024). Management of the Brain: Essential Oils as Promising Neuroinflammation Modulator in Neurodegenerative Diseases. Antioxidants, 13(2), 178. https://doi.org/10.3390/antiox13020178







