Abstract
Retinopathy of prematurity (ROP) is a proliferative vascular ailment affecting the retina. It is the main risk factor for visual impairment and blindness in infants and young children worldwide. If left undiagnosed and untreated, it can progress to retinal detachment and severe visual impairment. Geographical variations in ROP epidemiology have emerged over recent decades, attributable to differing levels of care provided to preterm infants across countries and regions. Our understanding of the causes of ROP, screening, diagnosis, treatment, and associated risk factors continues to advance. This review article aims to present the pathophysiological mechanisms of ROP, including its treatment. Specifically, it delves into the latest cutting-edge treatment approaches targeting hypoxia and redox signaling pathways for this condition.
1. Introduction
Retinopathy of prematurity (ROP) is a developmental vascular proliferative disease affecting the retina characterized by abnormal capillary growth in infants born preterm [1]. Key risk factors encompass low gestational age, low birth weight, and the utilization of prolonged mechanical, and particularly fluctuating, ventilation, often employed as a therapeutic measure in preterm infants [2,3,4]. The surge in premature births globally has led to a dramatically increased incidence of ROP. If left undetected and untreated, it can result in severe consequences for the visual system, including retinal detachment and irreversible vision impairment. ROP ranks among the primary causes of childhood blindness worldwide [5].
In the 1940s and 1950s, the unrestrained use of oxygen (O2) in industrialized nations sparked the initial epidemic of retinal disease in premature births, termed retrolental fibroplasia [6,7,8,9]. Subsequently, advancements in neonatal care and perinatal monitoring in the 1960s and 1970s increased the survival rate of extremely premature infants, causing a second surge in retinal disease [10,11]. This trend continued with a third peak affecting middle-income countries and regions like China, Southeast Asia, South Asia, South America, and Eastern Europe due to enhanced survival rates of very premature infants through improved neonatal care [12,13,14,15,16,17,18,19,20]. More recently, European countries and the US have witnessed a rising trend in ROP incidence. For instance, a study in the UK revealed a 4% incidence of ROP requiring treatment among preterm babies weighing < 1500 g [21]. Similarly, studies in Greece and Norway reported incidences of around 18.4% and 39.6%, respectively, among preterm infants [22]. In the US, the incidence rose from 11% in 2009 to 15% in 2018 among neonates meeting ROP screening criteria [23]. Currently, developed regions like the US and the UK tend to show a lower incidence, while developing regions such as India and Africa demonstrate slightly higher ROP incidence rates [24].
Despite advancements in ROP research and treatment, its incidence continues to rise, leading to escalated healthcare costs. Gyllensten et al. conducted a meta-analysis highlighting that the costs of ROP screening (ranging between USD 324–USD 1072 per child) and therapy (ranging between USD 38–USD 6500 per child) are considerably lower than the societal costs of resulting blindness (ranging between USD 26,686–USD 224,295), emphasizing the pivotal role of screening tools in combating this disorder [25]. Similarly, a cost-effectiveness analysis in Mexico and the US estimated substantial annual benefits of around USD 206 million and USD 205 million, respectively, by implementing national ROP screening and treatment programs [26]. Another systematic review reaffirmed the high cost-effectiveness of ROP screening and treatment in the UK, Canada, and the US [27].
Given the imperative to prevent ROP-associated blindness in children, ophthalmologists and public health experts must prioritize therapies and screening. Understanding the pathomechanisms of ROP is crucial for proposing innovative treatments targeting new pathways. This review aims to provide an extensive overview of advancements in understanding ROP’s etiopathogenesis, delving into the molecular cascades responsible for disease onset and progression. Furthermore, it discusses experimental studies testing novel treatment approaches targeting hypoxic and redox signaling pathways, paving the way for innovative therapeutic strategies to enhance ROP management.
2. General Aspects of Retinopathy of Prematurity
2.1. Classification, Diagnosis, and Risk Factors
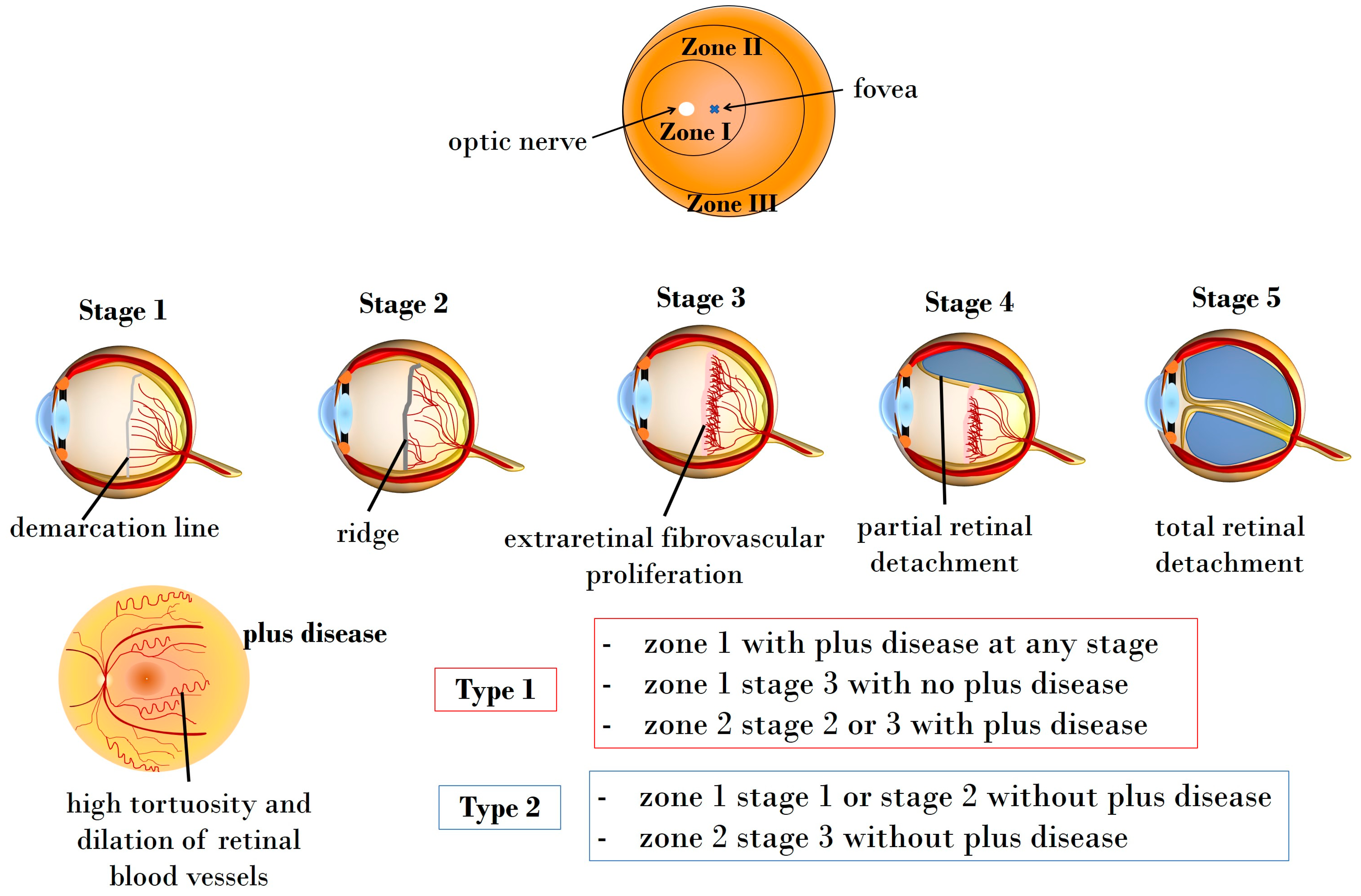
The 2021 International Classification of Retinopathy of Prematurity (ICROP3) enables ophthalmologists to classify ROP through four fundamental parameters: zone, plus disease, stage, and extent [28]. Additionally, ICROP highlights the clinical significance of an aggressive ROP variant, aggressive retinopathy of prematurity (A-ROP), characterized by rapid pathologic neovascularization [28]. Retinal vascularization initiates at the 13th gestational week, completing at birth. The extent and location of retinal vascularization may mirror infant maturity and the risk of ROP development. The zones indicating vascularization status are [29,30]:
- Zone I: circle centered on the optic nerve head, having a radius equal to twice the distance between the optic nerve and the fovea.
- Zone II: circle centered on the optic nerve head, presenting a radius equal to the distance between the optic nerve and nasal ora serrata.
- Zone III: peripherical retinal area extending over Zone II.
Additionally, the terms “plus” and “pre-plus” disease are employed to characterize vascular abnormalities observed in ROP [31]. To elaborate, plus disease is characterized by discernible vascular changes in the posterior pole, manifesting as tortuous arterioles and dilated venules. On the other hand, pre-plus disease signifies noteworthy vascular changes that, while not as advanced as in plus disease, indicate abnormal development that may progress to the latter stage [28]. It is noteworthy that the assessment of plus disease has gained increasing significance as a prognostic factor, contributing to both diagnosis and determination of severity [31].
Beyond the differentiation between plus and pre-plus diseases, ROP is further classified into five stages. The first three stages (stage 1, stage 2, and stage 3) denote acute forms, and detailed presentations of these stages are provided below in Figure 1. The last two stages are characterized as partial and total retinal detachment, labeled stage 4 and stage 5, respectively. Disease extent is categorized using 30 sectors based on clock-hour positions [28]. Furthermore, clinical trials conducted by the Early Treatment for Retinopathy of Prematurity Cooperative Group have identified the advantages of managing ROP at threshold stages, leading to the classification of pre-threshold ROP into two types [32].
Figure 1.
Illustration depicting the zones, stages, and types of ROP. Zone I encompasses the circular area centered on the optic nerve head, having a radius equal to twice the distance between the optic nerve and the fovea, while Zone II extends as a circle centered on the optic nerve head, presenting a radius equal to the distance between the optic nerve and nasal ora serrata. Zone III covers the peripherical retinal area, extending over Zone II. Stage 1 manifests as a demarcation line, delineating the boundary between the physiologically vascularized retina and the peripheral avascular retina. In Stage 2, this line progresses into a distinct ridge. Stage 3 marks the onset of extraretinal neovascularization and hemorrhages. Stage 4 indicates partial and stage 5 total retinal detachment, respectively. The plus disease is characterized by pronounced vascular dilation and tortuosity.
- Type 1: high-risk pre-threshold ROP includes Zone 1 with + disease at any stage, Zone 1 stage 3 without + disease, or Zone 2 stage 2 or 3 with + disease, necessitating prompt therapy.
- Type 2: low-risk pre-threshold ROP comprises Zone 1 stage 1 or stage 2 without + disease, and Zone 2 stage 3 without + disease, recommended for follow-up.
Figure 1 illustrates the classification of ROP based on fundus zones, ROP stages, and types.
Various maternal prenatal conditions, including hypertensive disorders, gestational diabetes, maternal age, and smoking, are recognized factors associated with prematurity. Additionally, prenatal and perinatal factors such as assisted conception, mode of delivery, premature rupture of membranes, and chorioamnionitis contribute to the risk of preterm birth [5]. Notably, specific risk factors associated with ROP include prematurity itself, prolonged postnatal oxygen supplementation, apnea, sepsis, necrotizing enterocolitis, anemia, blood transfusions, and intraventricular hemorrhage [5,33,34,35]. Key predictors of risk are postulated to be low birth weight (BW), low gestational age (GA), and the rate of weight gain, with lower BW and GA posing a higher risk for severe ROP [36,37,38,39,40]. For example, the American Academy of Pediatrics (AAP) and the American Academy of Ophthalmology (AAO) recommend screening for infants born at or before 30 weeks GA or those with a BW less than 1500 g [41]. However, universal screening guidelines are lacking, as the Royal College of Paediatrics and Child Health (RCPCH) suggests screening for infants born under 32 weeks GA or those weighing less than 1501 g. In Canada, screening is recommended for any infant born at or before 30 weeks GA, regardless of birth weight, and for infants with a BW of ≤1250 g [42,43]. Moreover, different birth weight percentiles, such as small-for-gestational-age (below the 10th percentile), appropriate-for-gestational-age, or large-for-gestational-age, need to be considered during screening, as they correlate with diverse risk probabilities for developing ROP [44].
Infant factors including ethnicity, gender, multiple births, Apgar score, postnatal weight gain, insulin-like growth factor I (IGF-1) levels, hyperglycemia, insulin levels, comorbidities, and treatments such as pulmonary complications, anemia, transfusion, erythropoietin (EPO), and thrombocytopenia also play a role in ROP pathogenesis [5].
2.2. Screening and Diagnostic Tools
Guidelines for ROP screening in preterm infants vary across countries [45,46,47,48,49,50,51,52,53]. While ROP screening is crucial for mitigating the visual complications in preterm infants, it presents drawbacks such as elevating scores in assessment tools like “Crying, Requires O2, Increased vital signs, Expression, and Sleeplessness” (CRIES) and the “Premature Infant Pain Profile” (PIPP) [54]. Additionally, the use of mydriatic agents like phenylephrine and cyclopentolate during ROP screening can trigger tachycardia and systemic hypertension in preterm infants [55]. Hence, there is a pressing need to refine ROP screening methods to minimize these undesirable effects.
In recent years, concerted scientific efforts have aimed to develop evidence-based ROP screening algorithms to identify preterm infants at a high risk of developing ROP. For instance, recognizing the crucial role of Insulin-like Growth Factor-1 (IGF-1) in retinal blood vessel growth, Lofqvist et al. proposed the WINROP online prediction model. This algorithm relies on low postnatal weight gain as an indirect indicator of slower serum IGF-1 increase and impaired retinal vascular growth, aiding in identifying infants at risk of ROP requiring treatment [56]. By integrating birth weight, gestational age, and weekly weight measurements, this model estimates the risk of severe ROP development [57].
Several studies evaluating the WINROP algorithm showcased high sensitivity (around 90–100%) but relatively lower specificity (around 30–50%) [58,59,60,61,62,63].
An alternative predictive model, the Children’s Hospital of Philadelphia (CHOP) ROP model, developed by Binenbaum and colleagues, employs three parameters—birth weight, gestational age, and daily weight gain rate—to assess severe ROP risk [64]. Subsequently, the same research group further refined this with the Postnatal Growth and ROP (G-ROP) criteria. This advancement aimed to reduce the number of infants requiring examinations and demonstrated increased sensitivity and specificity, particularly for type 1 ROP, compared with currently recommended guidelines [65,66]. These evolving algorithms hold promise for enhancing ROP screening by offering more accurate risk assessments and potentially minimizing unnecessary interventions.
Interestingly, a recent investigation led by Bortea and colleagues unveiled a significant association between the development of ROP in extremely premature and very premature neonates and inflammatory markers [67]. Specifically, the study, encompassing neonates, extremely premature infants (GA < 28 weeks), and very premature infants (GA between 28 and 32 weeks), revealed markedly higher levels of interleukin (IL)-6 and lactate dehydrogenase levels at birth and three days postnatally in the extremely premature group compared with the other groups. Conversely, C-reactive protein (CRP) levels at three days were higher in the very premature infants’ group. Additionally, a positive correlation was established between umbilical cord inflammation and ROP severity. Furthermore, elevated CRP and IL-6 levels were linked to an increased risk of developing ROP stage 2 or above, underscoring their potential as biomarkers for predicting ROP risk [67]. In summary, this investigation highlights the significance of assessing inflammatory markers in predicting the risk of ROP in extremely premature and very premature infants.
2.3. Natural Course, Long-Term Sequelae, and Prognosis
The natural regression of stage 1 ROP occurs in approximately 90% of cases, but as the stages progress, the regression rates decline significantly to about 50–60% in stage 2 and roughly 6% in stage 3 [68]. Advancement to stages 4 and 5, with retinal detachment, leads to irreversible visual loss. The CRYO-ROP study indicated that therapy significantly reduces the risk of adverse visual outcomes from 52% to 30% over a 15-year follow-up period, displaying improved visual acuity outcomes in treated patients at 3, 10, and 15 years [69]. Recent studies of our own revealed that extremely preterm adults, with or without postnatal ROP, tend to undergo more frequent ophthalmological check-ups throughout their lives, likely due to long-term complications in eye development and subsequent retinal disorders [70,71]. In the Gutenberg Prematurity Eye Study, individuals with postnatal ROP and particularly participants who needed treatment had a higher risk of impaired stereopsis, amblyopia, and reduced vision-related quality of life in adulthood [72]. Moreover, preterm delivery was shown to have little effect on absolute refractive error but was associated with anisometropia in adulthood. Notably, ROP treatment using cryo- and laser coagulation was shown to increase the risk of refractive error, lens opacification, and impaired accommodation [73]. In addition, corneal morphology was reported to be influenced by gestational age and birth weight percentile, while anterior chamber depth and lens thickness were shown to be affected by ROP treatment [74]. Additionally, a thicker foveal and a higher prevalence of foveal hypoplasia, potentially affecting visual acuity, were observed in cases of ROP requiring treatment compared with less severe ROP or preterm individuals without ROP. Moreover, individuals born extremely preterm or those with untreated ROP showed an increased vertical cup-to-disc ratio in adulthood, while all people born preterm revealed thinner peripapillary retinal nerve fiber layer thickness compared with individuals born full-term [75,76]. This might predispose these individuals to degenerative optic neuropathies [75,77]. These results collectively suggest that extreme preterm birth and postnatal ROP occurrence may lead to various anomalies in retinal development, impacting visual acuity in adulthood.
Despite advancements in treatment options, at least 50,000 children worldwide suffer blindness due to ROP annually, with approximately 600 preterm infants affected in the US each year [78]. The occurrence of ROP-related blindness varies by country and is influenced by regional socioeconomic development [79]. In high-income countries, ROP-related blindness is relatively rare, constituting less than 10% of cases of irreversible visual loss [80,81]. Conversely, middle- and low-income countries, constrained by limited public health resources, may exhibit higher rates of ROP-related blindness, accounting for up to 40% of blindness cases in these regions [16,79].
3. Insights into the Pathophysiology of Retinopathy of Prematurity
3.1. Retinal Development and Disease Pathogenesis
During physiological retinal angiogenesis, blood vessels begin to form around the 14–15th week of gestation, originating from the optic nerve head and expanding centrifugally towards the retinal periphery [82]. By 36 weeks of gestation, the nasal portion of the retina becomes vascularized, while the temporal area completes this process by the 40th week. Consequently, preterm infants exhibit incompletely vascularized retinas, with the extent of the avascular zone contingent upon their gestational age [83].
Under normal circumstances, the hypoxic conditions typical of the intrauterine environment stimulate retinal vascularization by prompting the expression of hypoxia-inducible factor 1α (HIF-1α). This factor regulates the expression of various oxygen-sensing genes, including crucial proangiogenic factors like vascular endothelial growth factor (VEGF) [84]. Although the VEGF family encompasses several members, including VEGF-A, -B, -C, -D, and placental growth factor (PlGF), it is VEGF-A that predominantly drives retinal angiogenesis [85,86].
VEGF, primarily released by neuroglia, initiates retinal blood vessel formation through the migration of vascular endothelial cells in a paracrine manner [87]. As retinal angiogenesis progresses, hypoxic conditions diminish, leading to the cessation of HIF-1 activation and its target genes. However, premature exposure to oxygen (hyperoxia) in an immature retina significantly suppresses HIF-1 and VEGF activity, resulting in oxidative stress and the emergence of avascular retinal regions [82]. Nitro-oxidative stress, characterized by an imbalance between the abundant generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) and antioxidative defense mechanisms, leads to excess ROS and RNS. This imbalance triggers dramatic structural molecular changes and the activation of inflammatory and cell death pathways, a condition detectable in various eye diseases [88,89]. It is noteworthy that infants possess reduced antioxidant defenses [90]. In this context, Buhimish and colleagues reported that preterm births exhibit a lack of compensatory upregulation of nonenzymatic antioxidant reserves, such as glutathione (GSH) and plasma total free radical-trapping antioxidant potential [91]. Given the diminished antioxidative capability and increased vulnerability to oxidative stress in preterm neonates, it is not surprising that several oxidative biomarkers, such as malondialdehyde (MDA), 8-hydroxy 2-deoxyguanosine (8-OHdG), and the GSH/GSSG ratio, have been discussed as potential diagnostic tools for ROP [92].
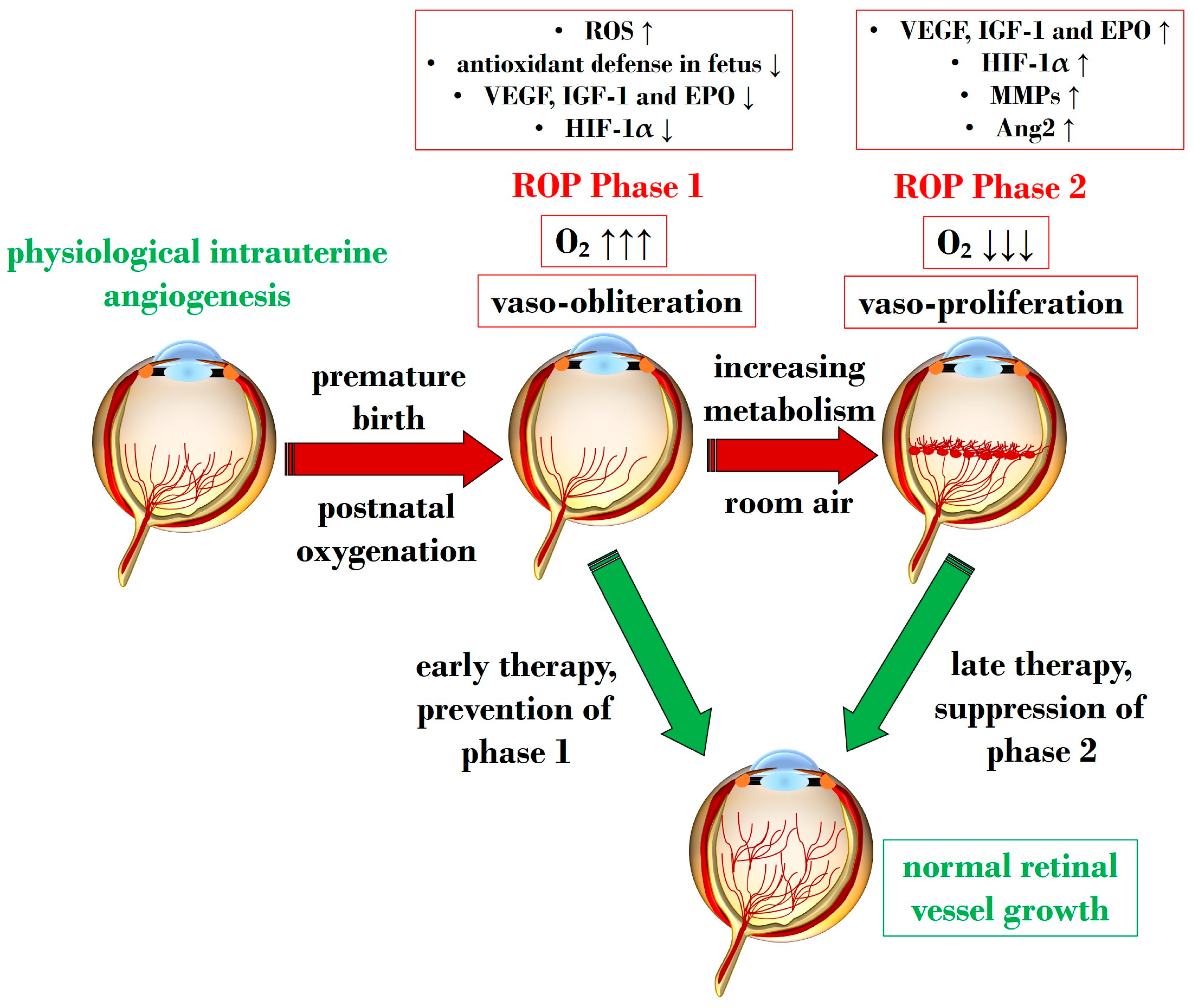
An overabundance of ROS is described during the first phase of ROP, a status of hyperoxia that manifests as vaso-obliteration, characterized by decreased levels of HIF-1α, VEGF, and IGF-1 [93]. Subsequently, an ischemic phase ensues, gradually progressing into a proliferative stage marked by abnormal and dysfunctional neoangiogenesis. This phase ultimately results in intravitreal fibrosis, retinal traction, and detachment [82,93].
Ashton et al. introduced the two-phase hypothesis on ROP pathogenesis, demonstrating that exposing healthy cats to 70–80% oxygen for four days induces newly formed capillaries, leading to a process of “vaso-obliteration.” Upon returning to normal air exposure, a phase of “vasoproliferation” is observed [94]. In the initial phase (phase I), physiological retinal angiogenesis is delayed due to high oxygen exposure, resulting in vascular occlusion, reduced serum IGF-1, and delayed expression of VEGF receptors 2 [95]. Subsequently (phase II), retinal and vitreous neoangiogenesis occurs alongside increased levels of HIF-1α, VEGF, IGF-1, placental growth factor, erythropoietin (EPO), metalloproteinase (MMP)-2, MMP-9, and angiopoietin (Ang)-2 [96]. Another model, the oxygen-induced retinopathy (OIR) mouse model, mimics high oxygen levels similar to Ashton’s experiments. In this model, exposure to constant high oxygen (75% O2) causes newly formed capillaries to regress, leading to central areas of vaso-obliteration. Upon returning to room air, relative hypoxia triggers the release of angiogenic factors, promoting the vasoproliferation of blood vessels into the vitreous [97].
Figure 2 illustrates Phases 1 and 2 in the pathogenesis of ROP, focusing on the distinct expression of key mediators contributing to the onset of the disease.
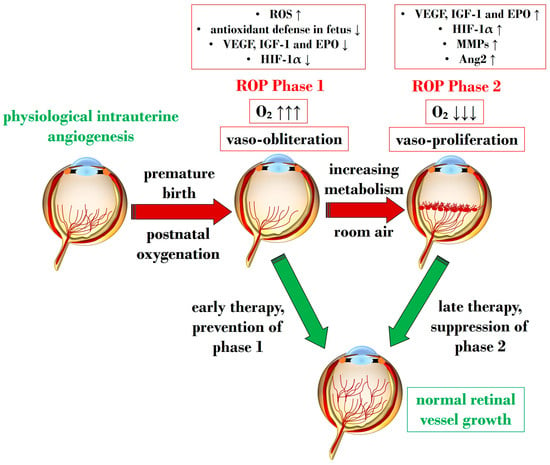
Figure 2.
Schematic representation depicting the two pathogenetic phases in ROP, highlighting distinct expression levels of key mediators in the retina. ROP: retinopathy of prematurity; ROS: reactive oxygen species; O2: oxygen; VEGF: vascular endothelial growth factor; IGF-1: insulin-like growth factor 1; EPO: erythropoietin; HIF-1α: hypoxia-inducible factor 1 alpha; MMP: metalloproteinase; Ang-2: angiopoietin 2. Upward black arrows indicate upregulation or increased concentration, downward black arrows indicate downregulation or decreased concentration.
3.2. Exploring Molecular Cascades in Retinopathy of Prematurity
Throughout the various pathological stages of ROP, a multitude of molecular signaling pathways emerge, contributing to inflammatory processes and an abundance of ROS and RNS—two crucial factors in the early stages of ROP [83,98].
Subsequent sections delve into the primary pathways responsible for vaso-obliteration and vaso-proliferation during ROP pathogenesis.
3.2.1. The Central Role of Nitro-Oxidative Stress and Inflammatory Factors
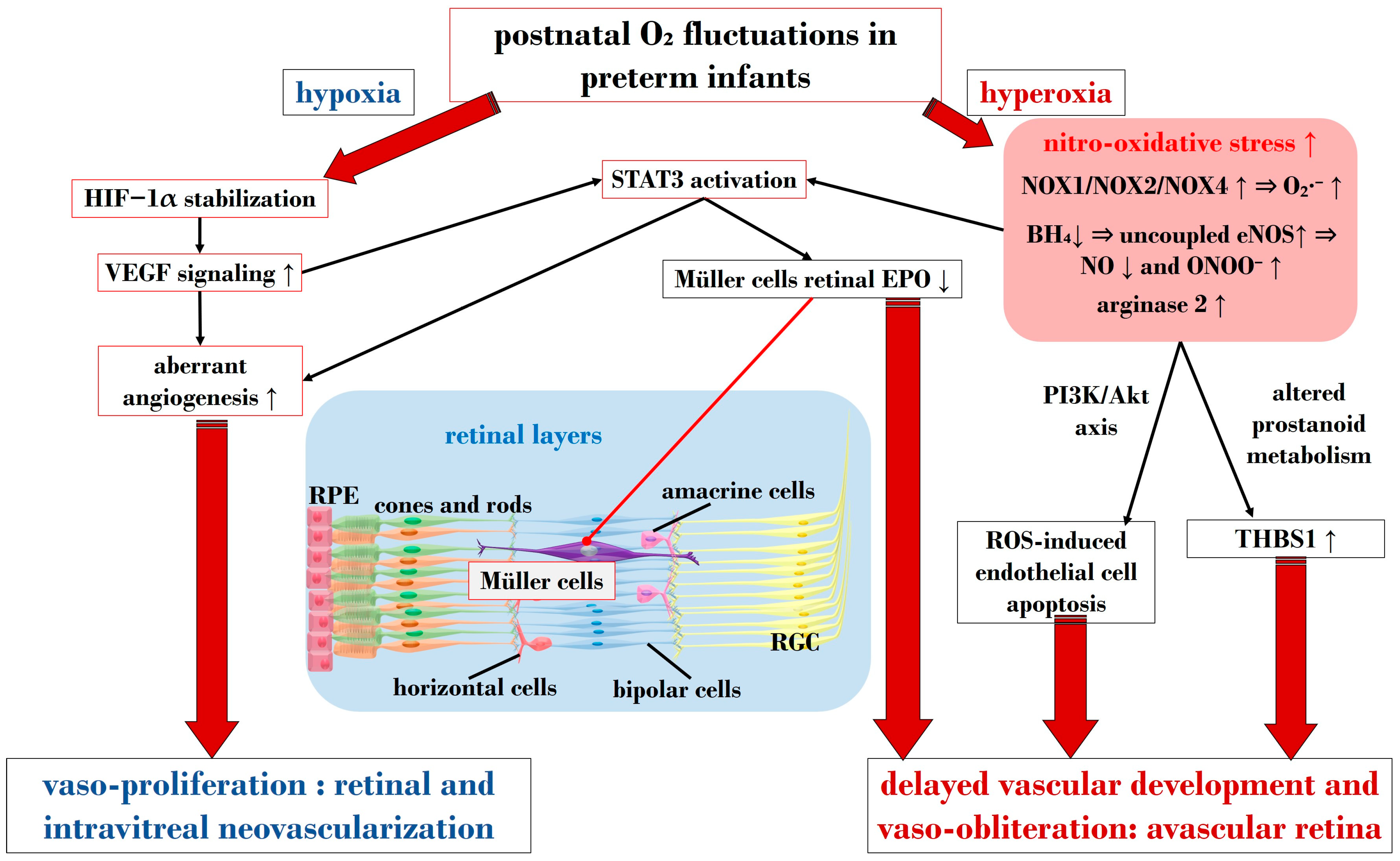
In ROP, endothelial cell apoptosis triggered by oxidative stress is implicated in the process of vaso-obliteration that occurs in the retina during the initial phase of the disorder. Gu et al. demonstrated in bovine retinal endothelial cells that hyperoxia-induced nitro-oxidative stress leads to retinal capillary endothelial cell apoptosis, potentially by impeding growth factor-induced activation of the PI3K/Akt signaling pathway [99]. The pro-oxidative enzyme nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX), present in isoforms NOX1, NOX2, and NOX4, generates high levels of superoxide (O2∙−), one of the most detrimental ROS, and has been associated with oxidative stress and vascular neoangiogenesis in OIR models [100,101,102]. Wang et al. reported in a rodent model of OIR that NOX4 might regulate intravitreal neovascularization mediated by VEGFR2 via activated signal transducer and activator of transcription (STAT) 3 within endothelial cells [101]. Studies by Saito et al. on animal models of ROP showcased that hyperoxia activates NOX, leading to an excess of ROS, culminating in apoptosis and neoangiogenesis independently of VEGF [103,104]. Furthermore, Byfield et al. demonstrated in a rodent model subjected to repeated oxygen fluctuations that hyperoxia-induced NOX activation leads to intravitreal VEGF-associated vascularization through a Janus tyrosine kinase (JAK)2/STAT3 pathway, and inhibiting this signaling pathway reduced neoangiogenesis [105]. However, VEGF signaling pathways seem to play a role in both phases of ROP, influencing pro-inflammatory and pro-oxidative processes. The same research group later revealed that VEGF-induced STAT3 activation blocked retinal angiogenesis by downregulating local expression of erythropoietin (EPO) in Müller cells during phase 1 of OIR [106]. Ren et al.’s investigation in a rodent model exposed to hyperoxia demonstrated that hyperoxia-induced STAT3 signaling enhances hepcidin expression—a key protein involved in iron balance—suggesting a potential compensatory mechanism to counteract iron overload linked to neoangiogenesis and proposing targeting molecules to regulate iron regulation [107].
Nitric oxide (NO) is pivotal in regulating vasodilation processes. Three main isoforms of NO synthases (NOS)—neural (n)NOS, inducible (i)NOS, and endothelial (e)NOS—are described in the literature. To counteract vaso-obliteration, vasodilation via NO synthesis occurs during the early phases of ROP [108]. However, differential NO responses are associated with the redox state [109]. As the disease progresses, excess NO synthesis becomes detrimental, favoring neoangiogenesis [110]. Importantly, normal NOS activity relies on the availability of the cofactor (6R)-5,6,7,8-tetrahydrobiopterin (BH4). Edgar et al. assessed in a murine model of OIR that exposure to oxygen led to decreased BH4 levels in the retinas, lungs, and aortas of mice, resulting in increased NOS-related ROS [111]. In circumstances of reduced BH4, uncoupled eNOS activity leads to nitro-oxidative stress in ROP pathogenesis. Specifically, in hyperoxia, impaired eNOS generates peroxynitrite (ONOO−) rather than physiological NO. Alongside eNOS, iNOS has also been reported as a pathogenetic factor in ROP. For instance, hypoxia-induced activation of iNOS in a murine model of OIR was linked to HIF-1α activation, VEGF expression, and PI3K/Akt signaling during neoangiogenesis, and inhibiting iNOS reduced the expression of these mediators [112]. However, NO also plays a significant role in neoangiogenesis processes during the vaso-proliferative phase, being fundamental in events of vascular permeability and leakage observed in retinopathies, affecting adherent junctions and endothelial cell polarity [113,114].
Notably, nitro-oxidative stress can interfere with prostanoid metabolism, exacerbating vaso-obliteration and contributing to avascular retinal onset. Reactive nitrogen species were observed in a murine OIR model to promote an isomerization of arachidonic acid to trans-arachidonic acid, involved in upregulating the anti-angiogenic factor thrombospondin-1 [115]. In this context, the role of phospholipase A2 (PLA-2) becomes noteworthy—an enzyme triggered by hypoxia and ROS abundance, influencing prostanoid metabolism via arachidonic acid release [116]. This molecule acts as a substrate for cyclooxygenase (COX), which converts it into proangiogenic eicosanoids, such as prostaglandins, prostacyclin, and thromboxane. Barnett et al. showed that suppressing PLA-2 decreased proangiogenic prostaglandins and intravitreal neoangiogenesis in an OIR model [116].
Arginase, present in isoforms arginase 1 and 2, hydrolyzes L-arginine to ornithine and urea, also producing glutamate [117]. This enzyme has been implicated in neural regeneration and protection [118]. Preterm infants exhibit low arginine levels, a crucial amino acid for retinal vascular development [119,120]. Arginine administration, particularly intravitreally with glutamine, countered neoangiogenesis in an OIR mouse model [121]. Importantly, arginase functionality and expression are heightened in contexts of inflammation and ROS excess, potentially interfering with NOS activity by competing for the substrate L-arginine. This competition indirectly triggers NOS uncoupling, leading to an overproduction of ONOO− [122,123]. Arginase 1 has been associated with neuroprotection [124], while arginase 2 might play a role in the events leading to retinal injuries, closely linked with nitro-oxidative stress and inflammation [125]. Shosha et al. proposed that NOX2-related O2∙− induces an upregulation of arginase 2 in ischemia/reperfusion injury, contributing to neurovascular degeneration [126].
In addition to oxidative stress, inflammatory events play a crucial role in the pathogenesis of ROP [127]. Cytokines such as IL-1β, tumor necrosis factor-α (TNF-α), and IL-6 are identified as primary drivers of inflammation, capable of inducing the overexpression of various inflammatory mediators, including chemokines and adhesion molecules. Specifically, within the hypoxic neonatal retina, retinal microglia can produce substantial amounts of IL-1β and TNF-α, ultimately promoting the death of retinal ganglion cells [128]. Furthermore, a study by Rivera and colleagues demonstrated in an OIR model that IL-1β is associated with retinal microvascular degeneration, triggering the release of the proapoptotic/repulsive factor semaphorin-3A from neurons [129]. Subsequent investigations by the same research group on the same model revealed the early pivotal role of IL-1β in the choroid, contributing to the involution of choroidal blood vessels and causing a loss of retinal pigment epithelium and photoreceptors [130]. As a consequence of cytokine downstream activation, chemokines also play a role in the pathogenesis of ROP, facilitating chemotaxis and the recruitment of immune cells to sites of inflammation. Specifically, chemokines implicated in ROP include IL-8, “RANTES” (Regulated and Normal T-cell Expressed and Secreted), and monocyte chemotactic protein 1 [131,132,133,134,135,136]. Taken together, inflammatory factors are pivotal in the pathophysiology of ROP, considering their role in orchestrating, together with oxidative stress, an amplification of the aberrant immune-mediated activation that leads to retinal cell death and choroidal degeneration.
3.2.2. The Crucial Involvement of HIF-1α and VEGF
HIF is a transcription factor composed of two subunits: HIF-1α (or its analogs HIF-2α and HIF-3α) and HIF-1β [137]. In normoxic conditions, HIF-1α undergoes hydroxylation by a prolyl hydroxylase domain (PHD) in the cytosol [138]. However, under hypoxic conditions, the enzymatic activities of PHD are inhibited, resulting in an increase in HIF-1α expression. Subsequently, HIF-1α binds to HIF-1β in the nucleus, forming the HIF-1 complex, which activates angiogenic mechanisms to help cells adapt to hypoxia. HIF-1α orchestrates the expression of several neoangiogenic mediators, including VEGF, EPO, angiopoietin (Ang)-1, and Ang-2, all observed to be upregulated in phase 2 of ROP [139]. Notably, under hypoxic conditions, HIF-1α is pivotal in reprogramming cellular metabolism, enhancing glycolysis, and increasing mitochondrial NADPH synthesis [140]. Even under normoxic conditions, HIF-1α activity can be induced by ROS and stabilized by inflammatory cytokines and growth factors like IGF-1 and TGF-β [141,142]. Enhanced HIF-1α activity due to relative intrauterine hypoxia is critical for physiological retinal vascular development [143]. However, in premature births, postnatal hyperoxia suppresses HIF-1α activity, leading to reduced VEGF release and consequent retinal capillary obliteration [144,145]. Stabilizing HIF-1α might represent a potential molecular target to halt the progression to phase 2 of ROP, as discussed in Section 4.2.4.
As mentioned earlier, VEGF plays a crucial role in both phases of ROP. Reduced VEGF levels under hyperoxia contribute to vaso-obliteration via endothelial cell apoptosis [146,147]. Conversely, in phase 2 under hypoxia, increased retinal VEGF levels act on endothelial cells through a paracrine route [148]. VEGF’s signaling involves the downregulation of retinal EPO in Müller cells via STAT3 activation. Furthermore, retinal endothelium expresses VEGFR-2, a receptor pivotal in neoangiogenic events and responsible for directing dividing endothelial cells in the developing retina [149]. Upregulation of VEGFR-2 disrupts dividing endothelial cells, potentially driving them to develop outside the retina, as observed in models of intravitreal neovascularization [150]. Inhibiting VEGFR-2 has shown promise in reducing intravitreal neoangiogenesis in preclinical investigations using the OIR model [151]. In Section 4.2.4, we delve into the primary experimental strategies targeting the VEGF/VEGFR axis in ROP.
Figure 3 provides a schematic overview illustrating the primary molecular pathways that unfold during hyperoxia and hypoxia in ROP.
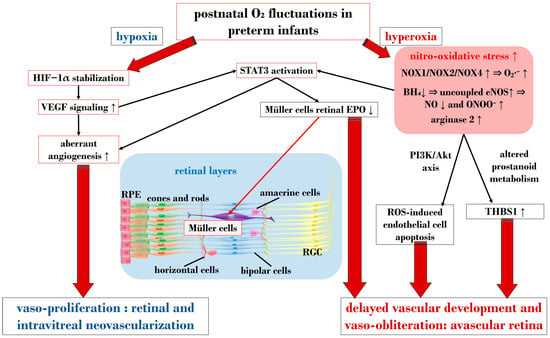
Figure 3.
Schematic overview depicting the primary molecular pathways activated during phases 1 and 2 in ROP. ROS: reactive oxygen species; NOX: nicotinamide adenine dinucleotide phosphate oxidase; O2: oxygen; O2∙−: superoxide; ONOO−: peroxynitrite; VEGF: vascular endothelial growth factor; EPO: erythropoietin; HIF-1α: hypoxia-inducible factor 1 alpha; BH4: tetrahydrobiopterin; NO: nitric oxide; eNOS: endothelial nitric oxide synthase; STAT3: signal transducer and activator of transcription 3; RGC: retinal ganglion cell; RPE: retinal pigment epithelium; THBS1: thrombospondin 1. Upward black arrows indicate upregulation or increased concentration, downward black arrows indicate downregulation or decreased concentration.
4. Treatment Intervention in Retinopathy of Prematurity
4.1. Established Therapy Options
The primary aim of managing ROP is to prevent vision loss and safeguard retinal structures. Cryotherapy emerged in the 1980s as a routine treatment, preventing fibrovascular retinal detachment by eliminating the avascular retina and halting abnormal angiogenesis in the vitreous [152]. Dedicated clinical trials have demonstrated the benefits of cryotherapy in improving eye anatomy and visual development [153]. However, studies employing indirect laser delivery systems for the eye suggested that cryotherapy outcomes were less favorable compared with laser treatment, potentially resulting in more severe myopia [154].
Laser photocoagulation therapy has notably reduced the progression of ROP to retinal detachment [155]. This approach targets the peripheral retina, aiming to decrease the risk of further angiogenesis and disease recurrence. Laser photocoagulation offers the benefit of a one-session therapy with a long-lasting effect. However, it is not without drawbacks. There is a possibility of skip areas following laser treatment, leading to disease reactivation [156]. Furthermore, both cryotherapy and laser photocoagulation may induce long-term sequelae, resulting in structural abnormalities and functional deficits such as reduced visual acuity, diminished visual fields, and significant myopia [156]. Additionally, laser therapy carries significant adverse effects, including the development of cataracts, anterior segment ischemia, and glaucoma [73,74,157,158,159]. Furthermore, although it is a rare complication, exudative retinal detachment has also been documented as a potential consequence of laser photocoagulation, a procedure employed in the treatment of infants affected by ROP [160].
Biologics represent the new pharmacological frontier for ROP, with current use including bevacizumab, aflibercept, and pegaptanib, albeit off-label, while ranibizumab has been the first licensed drug for ROP in Europe [156]. Notably, the long-term ocular and systemic effects of this therapy remain unclear [161]. The two most commonly used biologics for ROP are bevacizumab and ranibizumab [162]. Bevacizumab, the initial anti-VEGF agent used for ROP treatment, is a humanized monoclonal antibody that blocks all VEGF isoforms [163,164]. Findings from the Bevacizumab Eliminates the Angiogenic Threat (BEAT)-ROP study indicate a significantly lower recurrence rate of zone I ROP with intravitreal bevacizumab treatment compared with conventional laser treatment [165]. Unlike traditional laser treatment, intravitreal bevacizumab allows for continued blood vessel growth [166]. Ranibizumab, a monoclonal antibody fragment targeting VEGF-A, neutralizes VEGF-A to restrict the proliferation of abnormal retinal vasculature. The RAINBOW trial, involving 87 neonates across 26 countries, demonstrated higher treatment success rates at 24 weeks with intravitreal ranibizumab (80% of patients) compared with the laser treatment group (66%) in ROP infants [162]. Importantly, ranibizumab treatment did not induce systemic VEGF inhibition, positioning it as a promising alternative to ROP laser therapy.
Crucially, the benefits of anti-VEGF therapy lie in its relative speed and simplicity of administration, as well as a rapid therapy response. Biologics can protect the peripheral visual field and cause less myopia. Consequently, these drugs are specifically indicated in cases of zone I ROP and aggressive posterior ROP [156]. According to a report by the American Academy of Ophthalmology, intravitreal anti-VEGF intervention proves as effective as laser photocoagulation in inducing regression of acute ROP [167]. However, disadvantages include a higher recurrence of ROP, necessitating prolonged follow-up to monitor incomplete retinal vascularization [167]. Moreover, anti-VEGF therapy leads to a reduction in systemic VEGF levels in preterm neonates, and the impact on the developing organ systems of premature neonates remains substantially unknown. Therefore, the use of biologics for ROP is cautiously recommended, with an awareness of potential unknown adverse effects.
4.2. Exploring Emerging Molecular Targets
4.2.1. Exploring Antioxidant Strategies
Vitamin C, vitamin E, and lutein play pivotal roles as components in antioxidant defense mechanisms [168]. These substances have been investigated as potential strategies for antioxidant treatment in ROP. Vitamin C acts as a free radical scavenger and contributes to the regeneration of the antioxidant form of vitamin E. Despite promising results in preclinical studies, clinical trials have not consistently demonstrated significant advantages in managing ROP through vitamin C supplementation [92,169]. Clinical studies on vitamin E supplementation have yielded conflicting findings regarding its impact on ROP progression and the occurrence of adverse effects [170]. While preclinical studies have shown decreased avascular zones under hyperoxia [171], dedicated meta-analyses have reported benefits alongside increased rates of sepsis and necrotizing enterocolitis [172,173]. A more recent clinical study on vitamin E supplementation did not report associations with adverse effects but confirmed benefits in reducing ROP rates [174].
Lutein, recognized as a safe antioxidant agent and used in managing eye diseases such as age-related macular degeneration, has been tested in a murine model of OIR. In this model, lutein demonstrated the ability to diminish vascular leakage and promote normal endothelial tip cell formation, contributing to retinal vascular development [171]. Omega-3 long-chain polyunsaturated fatty acids, known for their effectiveness in countering various neurodegenerative disorders and suppressing apoptosis through the reduction of oxidative stress [171], have shown promise in a recent clinical trial. The trial reported that enteral lipid supplementation with docosahexaenoic acid and arachidonic acid, primary polyunsaturated fatty acids (PUFAs), effectively reduced the risk of severe ROP by 50% for neonates born at less than 28 weeks gestational age [175]. In summary, the effects of potential antioxidant supplements on managing ROP are subject to ongoing discussion, and further research is warranted to elucidate their efficacy and potential benefits.
4.2.2. Targeting Nuclear Factor-Erythroid 2-Related Factor 2 (Nrf2) for ROP Management
The transcription factor nuclear factor-erythroid 2-related factor 2 (Nrf2) plays a crucial role in modulating the expression of essential antioxidative agents, providing fundamental protection against ROS [176]. Uno and colleagues investigated the impact of hyperoxia in Nrf2 knockout compared with wild-type mice, revealing a beneficial effect of Nrf2 in the retina exposed to hyperoxia-related oxidative stress, crucial for promoting vascular endothelial cell survival [177]. Notably, the authors also noted that the protective role of Nrf2 during normal retinal development aligns with a specific window of time, coinciding with angiogenesis. This suggests that precise intervention timing, such as the use of Nrf2 modulators, may be critical in the treatment of ROP [177]. Consistent with these findings, Deliyanti et al. explored the effect of a Nrf2 activator, dh404, in a murine model of OIR. They observed a reduction in ROS levels, suppression of vaso-obliteration in phase I, and mitigation of neovascularization, vascular leakage, and inflammation in phase II [178]. In contrast, Liang and Wang investigated the impact of brusatol, a naturally occurring Nrf2 inhibitor extracted from Brucea javanica, in a rodent model of OIR. They found that brusatol could mitigate retinal microglial activation, neovascularization, and inflammation. This included a downregulation of VEGFR1, VEGFR2, TNF-α, and iNOS [179].
The existing literature suggests that the effect of modulating Nrf2 in ROP models is still not fully understood. While Nrf2 activation enhances the antioxidant response, its downregulation may also be beneficial by decreasing angiogenic and inflammatory agents regulated by Nrf2. Considering the interconnected and overlapping events of inflammation, neoangiogenesis, and oxidative stress in ROP, the timing of administering Nrf2 modulators may be essential to achieve the desired beneficial effects in counteracting ROP development.
4.2.3. Targeting the STAT3 Signaling Pathway in ROP Management
Activation of the STAT3 signaling pathway represents a potential convergence point between two pivotal pathogenetic cascades in ROP: oxidative stress and the VEGF signaling pathway. Therefore, exploring molecules with the capability to suppress this pathway becomes of particular interest. In a murine model of OIR, Bartoli and colleagues demonstrated that the administration of fluvastatin, a 3-hydroxy-3-methylglutaryl-Coenzyme A (HMG-CoA) reductase inhibitor, preserved retinal neovascularization. This effect was achieved through the prevention of the upregulation of key factors such as VEGF, HIF-1α, phosphorylated STAT3, and ICAM-1. Additionally, fluvastatin exhibited antioxidant effects by reducing O2∙− formation and lipid peroxidation in the ischemic retina [180]. In a more recent preclinical study, Chen et al. shed light on the role of the C-CBL protein, an E3 ubiquitin-protein ligase, in a murine model of OIR. They emphasized its crucial function in negatively controlling JAK2/STAT3/VEGF-related angiogenesis, highlighting that its upregulation suppresses neoangiogenesis in the retinas of OIR mice [181]. Ren and colleagues, in a rodent model of ROP, demonstrated that S3I-201, a STAT3 pathway inhibitor, downregulated the expression of STAT3 and VEGF mRNA levels, effectively countering neoangiogenesis in ROP [107]. In another investigation, the E3 ubiquitin ligase synoviolin (SYVN1) was examined for its potential in ROP management. SYVN1, known for its physiological functions in recognizing misfolded proteins in endoplasmic reticulum-associated degradation (ERAD), was upregulated through adenoviral vectors in a mouse model of OIR. This led to the ubiquitination and degradation of STAT3, reduced levels of phosphorylated STAT3, and a decrease in the release of VEGF. Collectively, these actions effectively countered neovascularization in the context of ROP [182].
4.2.4. Targeting HIF-1α and VEGF
Jiang and colleagues demonstrated that inhibition of HIF-1α suppresses the production of pro-angiogenic factors that cause the neovascular phase [183]. Usui-Ouchi and associates demonstrated that intravitreal injection of peptides derived from the intrinsically disordered protein CITED2, an endogenous negative feedback regulator of HIF-1α prevented ROP in a mouse model of OIR [184]. Huang et al. recently showed in an animal model of OIR that recombinant thrombomodulin domain 1 (rTMD1) significantly decreased retinal neovascularization and contributed to normal physiological vessel growth through inhibition of the HIF-1α-VEGF pathway [185]. Zhao and coworkers demonstrated in a murine model of OIR that celastrol, a naturally occurring molecule extracted from Tripterygium wilfordii Hook F., has been assessed to be capable of mitigating retinal neovascularization via inhibition of miR-17-5p/HIF-1α/VEGF signaling pathway, thereby being suggested as a therapeutic candidate for prevention and treatment in ROP [186].
Caffeine, a common treatment for premature infants with apnea, has shown potential for preventing ROP. Research by Aranda et al. revealed that caffeine could prevent ROP by upregulating genes associated with the sonic hedgehog signaling pathways, involving VEGF and IGF-1, and contributing to neuroprotection and angiogenesis [187]. Additionally, a meta-analysis indicated that early administration of caffeine reduces the likelihood of requiring laser photocoagulation therapy for managing ROP [188].
Vitamin A, a crucial fat-soluble organic compound in eye homeostasis [189], has been explored in systemic retinoic acid administration during hyperoxia, showing an increase in VEGF expression [190]. Studies have suggested that vitamin A reduces retinal angiogenesis in animal models of OIR by inhibiting VEGF production, potentially impacting the progression and incidence of ROP [191,192,193].
A compelling new molecule currently under investigation for its anti-inflammatory properties in the context of ROP is stanniocalcin-1. Dalvin and colleagues conducted studies using rodent models of OIR by comparing knockout mice for stanniocalcin-1 with wild-type controls. Their findings revealed a more severe establishment of ROP, characterized by increased avascular retina and vaso-proliferative areas in the absence of stanniocalcin-1. Notably, when stanniocalcin-1 was expressed, it demonstrated the capability to reduce the levels of VEGF-A. This observation suggests that stanniocalcin-1 holds promise as a potential molecular target for mitigating the progression of ROP [194].
4.2.5. The Role of Steroids in Managing ROP
Within the realm of steroidal drugs, triamcinolone stands out as a molecule under investigation in preclinical studies for its potential to counteract inflammatory events in ROP. For example, intravitreal injection of triamcinolone acetonide in a neonatal rat model of OIR has shown efficacy in reducing neovascularization by decreasing IGF-1 receptor phosphorylation [195]. In a more recent investigation, Öhnell et al. compared the incidence of type 2 ROP versus type 1 ROP among infants treated with dexamethasone eye drops and those untreated. They observed a treatment frequency of around 74% in infants with type 2 ROP who did not receive dexamethasone before laser ablation, compared with 24% in infants with type 2 ROP who received dexamethasone eye drops for type 2 ROP. The study concluded that such eye drops could substantially prevent these infants from developing type 1 ROP [196]. Nevertheless, the use of steroids in ROP remains a subject of debate. Prenatal exposure to dexamethasone has been associated with significantly lower odds of stage 2 or higher ROP [197]. However, the timing of postnatal corticosteroid administration is crucial. Early postpartum use can be beneficial in reducing ROP incidence, while late administration (more than seven days after birth) increases the risk of severe ROP [198,199]. Long-term corticosteroid use in ROP patients can also elevate the risk of progressing to severe stages [200]. A recent retrospective cohort study by Shekhawat and colleagues on 1695 infants with gestational age ≤ 32 weeks and/or birth weight ≤ 1500 g demonstrated that cumulative dose and duration of postnatal steroid use were independently associated with the severity of ROP and peripheral avascular retina [201].
In summary, based on the existing literature, the use of steroids in the context of ROP needs to be approached with great prudence and caution.
4.2.6. Exploring Matrix Metalloproteinases (MMPs) in ROP Treatment
Matrix Metalloproteinases (MMPs) constitute a group of endopeptidases critical for the breakdown of components within the extracellular matrix, with activation triggered by factors such as ROS, low pH, and heat treatment [202,203,204]. In response to angiogenic stimuli such as VEGF, fibronectin, and TNF-α, retinal pigment epithelial cells exhibit an increased secretion of MMP-2 and MMP-9 [205]. Das et al. demonstrated that heightened expression of MMP-9 contributes to elevated VEGF levels and retinal neovascularization during phase 2 of retinopathy in an OIR model. Significantly, intravitreal injection of TIMP-1, an inhibitor of MMP, successfully prevented the expression levels of MMP-9, VEGF, and retinal neovascularization. This underscores the potential efficacy of MMP-9-targeted interventions in the treatment of ROP [206]. More recently, Patnaik and colleagues unveiled that the decreased level of opticin, an anti-angiogenic factor, is induced through the release of MMP-9 by activated microglia under hypoxia in the vitreous of ROP eyes. They demonstrated that the use of doxycycline and EDTA to suppress MMP-9 can rescue the expression of opticin, potentially countering vaso-proliferative processes [207].
4.2.7. Exploring Potential β-Adrenoceptor Targets: Focus on Propranolol
Propranolol, a non-selective beta-adrenergic blocker commonly employed in pediatric care for conditions such as arterial hypertension and arrhythmias, demonstrates generally good tolerance in humans [208,209]. There is growing interest in the potential use of prophylactic oral beta-blockers, such as propranolol, to limit the progression to stage 3 ROP and reduce the need for anti-VEGF drugs or laser therapy [210]. This effect is attributed to evidence suggesting that β2-adrenoceptors play a role in upregulating vascular endothelial growth factor VEGF and IGF-1 levels, making them relevant in the pathogenesis of various neovascular retinal diseases [211,212].
Studies on preterm newborns with stage 2 ROP who received oral propranolol have indicated a reduction in the risk of advancing to stage 3 or 4. However, it is crucial to note that some preterm infants may experience severe adverse reactions, such as hypotension and bradycardia, particularly in the presence of sepsis episodes, anesthesia induction, or tracheal irritation [213]. To mitigate these risks, alternative routes of propranolol administration have been proposed. In a multicenter clinical trial, the topical application of 0.2% propranolol eye drops in newborns at stage 1 ROP significantly reduced disease progression to stage 2 or 3 plus [214]. Although promising for preventing severe ROP, further randomized controlled studies are essential to assess the long-term effects, determine optimal dosages, and establish the duration of therapy for propranolol treatment.
4.2.8. Targeting Succinate and Adenosine Pathways
During episodes of ischemic hypoxia, oxidative metabolites such as succinate serve as signaling factors that respond to compromised energy states, leading to an increase in retinal vascularization [215]. Similarly, hypoxia induces the accumulation of purine products (ATP, ADP, AMP, and adenosine) and activates purinergic receptors, further promoting neovascularization [216,217]. Elevated levels of succinate and adenosine during hypoxia activate their cognate G protein-coupled receptors (GPCRs), restoring adequate blood flow by dilating blood vessels and stimulating angiogenesis [218]. In an OIR model, succinate and adenosine contribute to the proliferative phase of ROP by modulating the expression or activity of their respective GPCRs. Furthermore, the down-regulation of GPR91 disrupts normal retinal vascular development and reduces abnormal intravitreal neovascularization [219]. Antagonists targeting the A2B adenosine receptor have shown efficacy in reducing pre-retinal neovascularization [216]. GPR91 plays a crucial role in preventing excessive growth factor secretion and decreasing intravitreal neovascularization [219,220], making it a promising target for the development of molecular interventions [221].
5. Concluding Remarks
As our understanding of ROP has evolved since the initial discovery of retrolental fibroplasia, advancements across technology, scientific research, and clinical medicine have profoundly influenced our comprehension of its pathogenesis. Technological strides in regulating the environmental conditions for preterm infants have notably enhanced the survival rates of extremely premature infants.
Scientific and technological progress in unraveling the molecular mechanisms underlying pathophysiology has shed light on both normal and aberrant developmental angiogenesis. Exploring neurovascular interactions and their role in cognitive function and angiogenesis represents an emerging frontier. Significant clinical trials have contributed crucial insights into oxygenation, screening, classification, and treatment modalities for severe ROP. There is a growing awareness that ROP manifests variably across different regions globally, prompting the consideration of tailored approaches to screening and treatment. Despite these strides, the treatment of ROP remains an active area of research. There is a critical need for targeted therapies that mitigate abnormal vascular proliferation while fostering the physiological development of the retinal vasculature and safeguarding the delicate health of the developing infant. Of note, numerous treatment approaches targeting hypoxia and redox signaling pathways in the context of ROP have shown promising results in preclinical studies. Hence, more clinical studies are warranted in this field.
Author Contributions
Conceptualization A.G.; writing—original draft preparation, L.Z.; writing—review and editing, A.G., F.B., A.F. and N.P.; visualization, F.B.; supervision, A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rowe, L.W.; Belamkar, A.; Antman, G.; Hajrasouliha, A.R.; Harris, A. Vascular imaging findings in retinopathy of prematurity. Acta Ophthalmol. 2023, 00, 1–21. [Google Scholar] [CrossRef]
- Schaffer, D.B.; Palmer, E.A.; Plotsky, D.F.; Metz, H.S.; Flynn, J.T.; Tung, B.; Hardy, R.J.; Cryotherapy for Retinopathy of Prematurity Cooperative Group. Prognostic factors in the natural course of retinopathy of prematurity. Ophthalmology 1993, 100, 230–237. [Google Scholar] [CrossRef]
- Bossi, E.; Koerner, F.; Flury, B.; Zulauf, M. Retinopathy of prematurity: A risk factor analysis with univariate and multivariate statistics. Helv. Paediatr. Acta 1984, 39, 307–317. [Google Scholar] [PubMed]
- Enomoto, H.; Miki, A.; Matsumiya, W.; Honda, S. Evaluation of oxygen supplementation status as a risk factor associated with the development of severe retinopathy of prematurity. Ophthalmologica 2015, 234, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Port, A.D.; Swan, R.; Campbell, J.P.; Chan, R.V.P.; Chiang, M.F. Retinopathy of prematurity: A review of risk factors and their clinical significance. Surv. Ophthalmol. 2018, 63, 618–637. [Google Scholar] [CrossRef] [PubMed]
- Patz, A. Retrolental fibroplasia. Surv. Ophthalmol. 1969, 14, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Patz, A. The role of oxygen in retrolental fibroplasia. Trans. Am. Ophthalmol. Soc. 1968, 66, 940–985. [Google Scholar] [PubMed]
- Patz, A.; Hoeck, L.E.; De La Cruz, E. Studies on the effect of high oxygen administration in retrolental fibroplasia. I. Nursery observations. Am. J. Ophthalmol. 1952, 35, 1248–1253. [Google Scholar] [CrossRef]
- Campbell, K. Intensive oxygen therapy as a possible cause of retrolental fibroplasia; a clinical approach. Med. J. Aust. 1951, 2, 48–50. [Google Scholar] [CrossRef]
- Gibson, D.L.; Sheps, S.B.; Uh, S.H.; Schechter, M.T.; McCormick, A.Q. Retinopathy of prematurity-induced blindness: Birth weight-specific survival and the new epidemic. Pediatrics 1990, 86, 405–412. [Google Scholar] [CrossRef]
- Phelps, D.L. Retinopathy of prematurity: An estimate of vision loss in the United States—1979. Pediatrics 1981, 67, 924–925. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X. Characteristics of severe retinopathy of prematurity patients in China: A repeat of the first epidemic? Br. J. Ophthalmol. 2006, 90, 268–271. [Google Scholar] [CrossRef]
- Phan, M.H.; Nguyen, P.N.; Reynolds, J.D. Incidence and severity of retinopathy of prematurity in Vietnam, a developing middle-income country. J. Pediatr. Ophthalmol. Strabismus 2003, 40, 208–212. [Google Scholar] [CrossRef]
- Azad, R.V.; Chandra, P. Retinopathy of prematurity—Screening and management. J. Indian Med. Assoc. 2003, 101, 593–596. [Google Scholar]
- Jalali, S.; Anand, R.; Kumar, H.; Dogra, M.R.; Azad, R.; Gopal, L. Programme planning and screening strategy in retinopathy of prematurity. Indian J. Ophthalmol. 2003, 51, 89–99. [Google Scholar] [PubMed]
- Gilbert, C.; Fielder, A.; Gordillo, L.; Quinn, G.; Semiglia, R.; Visintin, P.; Zin, A. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: Implications for screening programs. Pediatrics 2005, 115, e518–e525. [Google Scholar] [CrossRef] [PubMed]
- Lermann, V.L.; Fortes Filho, J.B.; Procianoy, R.S. The prevalence of retinopathy of prematurity in very low birth weight newborn infants. J. Pediatr. (Rio J) 2006, 82, 27–32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gilbert, C.E.; Anderton, L.; Dandona, L.; Foster, A. Prevalence of visual impairment in children: A review of available data. Ophthalmic Epidemiol. 1999, 6, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, C.; Foster, A. Childhood blindness in the context of VISION 2020—The right to sight. Bull. World Health Organ. 2001, 79, 227–232. [Google Scholar] [PubMed]
- Gilbert, C.; Rahi, J.; Eckstein, M.; O’Sullivan, J.; Foster, A. Retinopathy of prematurity in middle-income countries. Lancet 1997, 350, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.G.; Bunce, C.; Xing, W.; Butler, L.; Long, V.; Reddy, A.; Dahlmann-Noor, A.H. Treatment trends for retinopathy of prematurity in the UK: Active surveillance study of infants at risk. BMJ Open 2017, 7, e013366. [Google Scholar] [CrossRef] [PubMed]
- Moutzouri, S.; Haidich, A.B.; Seliniotaki, A.K.; Tsakalidis, C.; Soubasi, V.; Ziakas, N.; Mataftsi, A. Optimization of retinopathy of prematurity screening in a tertiary neonatal unit in Northern Greece based on 16-year data. J. Perinatol. 2022, 42, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Thangamathesvaran, L.; Wang, J.; Repka, M.X.; Scott, A.W. Trends in Retinopathy of Prematurity Care in the United States 2009–2018: A Nationwide Analysis Using National Inpatient Sample. Ophthalmol. Retin. 2023, 7, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.C.; Lam, P.Y.; Lam, W.C.; Fung, N.S.K. The role of anti-vascular endothelial growth factor in treatment of retinopathy of prematurity-a current review. Eye 2022, 36, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- Gyllensten, H.; Humayun, J.; Sjöbom, U.; Hellström, A.; Löfqvist, C. Costs associated with retinopathy of prematurity: A systematic review and meta-analysis. BMJ Open 2022, 12, e057864. [Google Scholar] [CrossRef]
- Rothschild, M.I.; Russ, R.; Brennan, K.A.; Williams, C.J.; Berrones, D.; Patel, B.; Martinez-Castellanos, M.A.; Fernandes, A.; Hubbard, G.B.; Chan, R.V.P.; et al. The Economic Model of Retinopathy of Prematurity (EcROP) Screening and Treatment: Mexico and the United States. Am. J. Ophthalmol. 2016, 168, 110–121. [Google Scholar] [CrossRef]
- Smith, A.F.; Sadeq, A.; Kinzel, E.; Bhambhwani, V. A Systematic Review of Economic Evaluations Conducted for Interventions to Screen, Treat, and Manage Retinopathy of Prematurity (ROP) in the United States, United Kingdom, and Canada. Ophthalmic Epidemiol. 2023, 30, 113–120. [Google Scholar] [CrossRef]
- Chiang, M.F.; Quinn, G.E.; Fielder, A.R.; Ostmo, S.R.; Paul Chan, R.V.; Berrocal, A.; Binenbaum, G.; Blair, M.; Peter Campbell, J.; Capone, A., Jr.; et al. International Classification of Retinopathy of Prematurity, Third Edition. Ophthalmology 2021, 128, e51–e68. [Google Scholar] [CrossRef]
- Molinari, A.; Weaver, D.; Jalali, S. Classifying retinopathy of prematurity. Community Eye Health 2017, 30, 55–56. [Google Scholar]
- Al Rashaed, S. Retinopathy of prematurity—A brief review. Dr. Sulaiman Al Habib Med. J. 2019, 1, 58–64. [Google Scholar] [CrossRef]
- Solarte, C.E.; Awad, A.H.; Wilson, C.M.; Ells, A. Plus Disease: Why is it Important in Retinopathy of Prematurity? Middle East Afr. J. Ophthalmol. 2010, 17, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: Results of the early treatment for retinopathy of prematurity randomized trial. Arch. Ophthalmol. 2003, 121, 1684–1694. [Google Scholar] [CrossRef] [PubMed]
- Stoltz Sjöström, E.; Lundgren, P.; Öhlund, I.; Holmström, G.; Hellström, A.; Domellöf, M. Low energy intake during the first 4 weeks of life increases the risk for severe retinopathy of prematurity in extremely preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F108–F113. [Google Scholar] [CrossRef]
- Alajbegovic-Halimic, J.; Zvizdic, D.; Alimanovic-Halilovic, E.; Dodik, I.; Duvnjak, S. Risk Factors for Retinopathy of Prematurity in Premature Born Children. Med. Arch. 2015, 69, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Binenbaum, G. Postnatal weight gain and retinopathy of prematurity. Semin. Perinatol. 2019, 43, 352–359. [Google Scholar] [CrossRef]
- Darlow, B.A.; Hutchinson, J.L.; Henderson-Smart, D.J.; Donoghue, D.A.; Simpson, J.M.; Evans, N.J. Prenatal risk factors for severe retinopathy of prematurity among very preterm infants of the Australian and New Zealand Neonatal Network. Pediatrics 2005, 115, 990–996. [Google Scholar] [CrossRef]
- Kermorvant-Duchemin, E.; Sapieha, P.; Sirinyan, M.; Beauchamp, M.; Checchin, D.; Hardy, P.; Sennlaub, F.; Lachapelle, P.; Chemtob, S. Understanding ischemic retinopathies: Emerging concepts from oxygen-induced retinopathy. Doc. Ophthalmol. 2010, 120, 51–60. [Google Scholar] [CrossRef]
- Poets, C.F.; Roberts, R.S.; Schmidt, B.; Whyte, R.K.; Asztalos, E.V.; Bader, D.; Bairam, A.; Moddemann, D.; Peliowski, A.; Rabi, Y.; et al. Association between Intermittent Hypoxemia or Bradycardia and Late Death or Disability in Extremely Preterm Infants. JAMA 2015, 314, 595–603. [Google Scholar] [CrossRef]
- Di Fiore, J.M.; Bloom, J.N.; Orge, F.; Schutt, A.; Schluchter, M.; Cheruvu, V.K.; Walsh, M.; Finer, N.; Martin, R.J. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J. Pediatr. 2010, 157, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Binenbaum, G.; Ying, G.-s.; Quinn, G.E.; Dreiseitl, S.; Karp, K.; Roberts, R.S.; Kirpalani, H.; Premature Infants in Need of Transfusion Study Group. A clinical prediction model to stratify retinopathy of prematurity risk using postnatal weight gain. Pediatrics 2011, 127, e607–e614. [Google Scholar] [CrossRef] [PubMed]
- Fierson, W.M.; American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus; American Association of Certified Orthoptists. Screening Examination of Premature Infants for Retinopathy of Prematurity. Pediatrics. 2018;142(6):e20183061. Pediatrics 2019, 143, e20183810. [Google Scholar] [CrossRef]
- Berrocal, A.M.; Fan, K.C.; Al-Khersan, H.; Negron, C.I.; Murray, T. Retinopathy of Prematurity: Advances in the Screening and Treatment of Retinopathy of Prematurity Using a Single Center Approach. Am. J. Ophthalmol. 2022, 233, 189–215. [Google Scholar] [CrossRef]
- Jefferies, A.L. Retinopathy of prematurity: An update on screening and management. Paediatr. Child Health 2016, 21, 101–108. [Google Scholar] [CrossRef]
- Dhaliwal, C.A.; Fleck, B.W.; Wright, E.; Graham, C.; McIntosh, N. Retinopathy of prematurity in small-for-gestational age infants compared with those of appropriate size for gestational age. Arch. Dis. Child. Fetal Neonatal Ed. 2009, 94, F193–F195. [Google Scholar] [CrossRef]
- Grupo de Trabajo Colaborativo Multicéntrico para la Prevención de la Ceguera en la Infancia por Retinopatía del Prematuro. Recommendations for Retinopathy of Prematurity screening in at-risk populations. Arch. Argent. Pediatr. 2008, 106, 71–76. [Google Scholar]
- Zin, A.; Florêncio, T.; Fortes Filho, J.B.; Nakanami, C.R.; Gianini, N.; Graziano, R.M.; Moraes, N. Brazilian guidelines proposal for screening and treatment of retinopathy of prematurity (ROP). Arq. Bras. Oftalmol. 2007, 70, 875–883. [Google Scholar] [CrossRef]
- Jefferies, A. Retinopathy of prematurity: Recommendations for screening. Paediatr. Child Health 2010, 15, 667–670. [Google Scholar] [CrossRef][Green Version]
- Fierson, W.M.; Chiang, M.F.; Good, W.; Phelps, D.; Reynolds, J.; Robbins, S.L.; Karr, D.J.; Bradford, G.E.; Nischal, K.; Roarty, J. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2018, 142, e20183061. [Google Scholar] [CrossRef]
- Wilkinson, A.; Haines, L.; Head, K.; Fielder, A. UK retinopathy of prematurity guideline. Eye 2009, 23, 2137–2139. [Google Scholar] [CrossRef]
- Bas, A.Y.; Demirel, N.; Koc, E.; Isik, D.U.; Hirfanoglu, İ.M.; Tunc, T. Incidence, risk factors and severity of retinopathy of prematurity in Turkey (TR-ROP study): A prospective, multicentre study in 69 neonatal intensive care units. Br. J. Ophthalmol. 2018, 102, 1711–1716. [Google Scholar] [CrossRef]
- Ells, A.; Hicks, M.; Fielden, M.; Ingram, A. Severe retinopathy of prematurity: Longitudinal observation of disease and screening implications. Eye 2005, 19, 138–144. [Google Scholar] [CrossRef]
- Fielder, A.R.; Hildebrand, P.L.; Ells, A.; Lorenz, B.; Trese, M.T.; Capone, A., Jr.; Gordon, R.A.; Wilson, C.; Fleck, B.W.; Chiang, M.F. Systematic review of digital imaging screening strategies for retinopathy of prematurity. Pediatrics 2009, 123, e360–e361. [Google Scholar] [CrossRef] [PubMed]
- Bowe, T.; Nyamai, L.; Ademola-Popoola, D.; Amphornphruet, A.; Anzures, R.; Cernichiaro-Espinosa, L.A.; Duke, R.; Duran, F.; Martinez-Castellanos, M.A.; Multani, P.K. The current state of retinopathy of prematurity in India, Kenya, Mexico, Nigeria, Philippines, Romania, Thailand, and Venezuela. Digit. J. Ophthalmol. DJO 2019, 25, 49. [Google Scholar] [CrossRef]
- Moral-Pumarega, M.T.; Caserío-Carbonero, S.; De-La-Cruz-Bértolo, J.; Tejada-Palacios, P.; Lora-Pablos, D.; Pallás-Alonso, C.R. Pain and stress assessment after retinopathy of prematurity screening examination: Indirect ophthalmoscopy versus digital retinal imaging. BMC Pediatr. 2012, 12, 132. [Google Scholar] [CrossRef]
- Hered, R.W.; Gyland, E.A. The retinopathy of prematurity screening examination: Ensuring a safe and efficient examination while minimizing infant discomfort. Neonatal Netw. 2010, 29, 143–151. [Google Scholar] [CrossRef]
- Löfqvist, C.; Andersson, E.; Sigurdsson, J.; Engström, E.; Hård, A.L.; Niklasson, A.; Smith, L.E.; Hellström, A. Longitudinal postnatal weight and insulin-like growth factor I measurements in the prediction of retinopathy of prematurity. Arch. Ophthalmol. 2006, 124, 1711–1718. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Athikarisamy, S.E.; Lundgren, P.; Simmer, K.; Lam, G.C. Validation of WINROP (online prediction model) to identify severe retinopathy of prematurity (ROP) in an Australian preterm population: A retrospective study. Eye 2021, 35, 1334–1339. [Google Scholar] [CrossRef]
- Lim, Z.D.; Oo, K.T.; Tai, E.L.M.; Shatriah, I. Efficacy of WINROP as a Screening Tool for Retinopathy of Prematurity in the East Coast of Malaysia. Clin. Ophthalmol. 2020, 14, 1101–1106. [Google Scholar] [CrossRef]
- Jung, J.L.; Wagner, B.D.; McCourt, E.A.; Palestine, A.G.; Cerda, A.; Cao, J.H.; Enzenauer, R.W.; Singh, J.K.; Braverman, R.S.; Wymore, E.; et al. Validation of WINROP for detecting retinopathy of prematurity in a North American cohort of preterm infants. J. AAPOS 2017, 21, 229–233. [Google Scholar] [CrossRef]
- Thomas, D.; Madathil, S.; Thukral, A.; Sankar, M.J.; Chandra, P.; Agarwal, R.; Deorari, A. Diagnostic Accuracy of WINROP, CHOP-ROP and ROPScore in Detecting Type 1 Retinopathy of Prematurity. Indian Pediatr. 2021, 58, 915–921. [Google Scholar] [CrossRef]
- Raffa, L.H.; Alessa, S.K.; Alamri, A.S.; Malaikah, R.H. Prediction of retinopathy of prematurity using the screening algorithm WINROP in a Saudi cohort of preterm infants. Saudi Med. J. 2020, 41, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.C.; Wu, R.; Chen, S.Z.; Wei, S.Y.; Chen, H.J.; Chen, Y.C.; Feng, S.F.; Lu, X.H. Efficacy of the WINROP algorithm for retinopathy of prematurity screening in Southern China. Int. J. Ophthalmol. 2021, 14, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Kesting, S.J.; Nakwa, F.L. Prediction of Retinopathy of Prematurity Using the WINROP (Weight, IGF-1, Neonatal Retinopathy of Prematurity) Algorithm in a South African Population. Front. Pediatr. 2022, 10, 812404. [Google Scholar] [CrossRef] [PubMed]
- Binenbaum, G.; Ying, G.-s.; Quinn, G.E.; Huang, J.; Dreiseitl, S.; Antigua, J.; Foroughi, N.; Abbasi, S. The CHOP postnatal weight gain, birth weight, and gestational age retinopathy of prematurity risk model. Arch. Ophthalmol. 2012, 130, 1560–1565. [Google Scholar] [CrossRef] [PubMed]
- Binenbaum, G.; Bell, E.F.; Donohue, P.; Quinn, G.; Shaffer, J.; Tomlinson, L.A.; Ying, G.-s.; G-ROP Study Group. Development of modified screening criteria for retinopathy of prematurity: Primary results from the postnatal growth and retinopathy of prematurity study. JAMA Ophthalmol. 2018, 136, 1034–1040. [Google Scholar] [CrossRef]
- Binenbaum, G.; Tomlinson, L.A.; de Alba Campomanes, A.G.; Bell, E.F.; Donohue, P.; Morrison, D.; Quinn, G.E.; Repka, M.X.; Rogers, D.; Yang, M.B. Validation of the postnatal growth and retinopathy of prematurity screening criteria. JAMA Ophthalmol. 2020, 138, 31–37. [Google Scholar] [CrossRef]
- Borțea, C.I.; Enatescu, I.; Dima, M.; Pantea, M.; Iacob, E.R.; Dumitru, C.; Popescu, A.; Stoica, F.; Heredea, R.E.; Iacob, D. A Prospective Analysis of the Retinopathy of Prematurity Correlated with the Inflammatory Status of the Extremely Premature and Very Premature Neonates. Diagnostics 2023, 13, 2105. [Google Scholar] [CrossRef]
- Ju, R.H.; Zhang, J.Q.; Ke, X.Y.; Lu, X.H.; Liang, L.F.; Wang, W.J. Spontaneous regression of retinopathy of prematurity: Incidence and predictive factors. Int. J. Ophthalmol. 2013, 6, 475–480. [Google Scholar] [CrossRef]
- Palmer, E.A.; Hardy, R.J.; Dobson, V.; Phelps, D.L.; Quinn, G.E.; Summers, C.G.; Krom, C.P.; Tung, B. 15-year outcomes following threshold retinopathy of prematurity: Final results from the multicenter trial of cryotherapy for retinopathy of prematurity. Arch. Ophthalmol. 2005, 123, 311–318. [Google Scholar] [CrossRef]
- Fieß, A.; Wacker, A.; Gißler, S.; Fauer, A.; Mildenberger, E.; Urschitz, M.S.; Elflein, H.; Stoffelns, B.; Pfeiffer, N.; Schuster, A.K. Ophthalmic care of adults born preterm and full-term-results from the Gutenberg Prematurity Eye Study (GPES) : Premature birth and ophthalmological care. Ophthalmologie 2023, 120, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Fieß, A.; Mildenberger, E.; Pfeiffer, N.; Schuster, A.K. Ophthalmological long-term sequelae of premature birth-Persisting into adulthood: Eye development and premature birth anamnesis. Ophthalmologie 2023, 120, 597–607. [Google Scholar] [CrossRef]
- Fieß, A.; Greven, K.; Mildenberger, E.; Urschitz, M.S.; Elflein, H.M.; Zepp, F.; Stoffelns, B.; Pfeiffer, N.; Schuster, A.K. Visual acuity, amblyopia, and vision-related quality of life in preterm adults with and without ROP: Results from the Gutenberg prematurity eye study. Eye 2023, 37, 1794–1801. [Google Scholar] [CrossRef]
- Fieß, A.; Fauer, A.; Mildenberger, E.; Urschitz, M.S.; Elflein, H.M.; Zepp, F.; Stoffelns, B.; Pfeiffer, N.; Schuster, A.K. Refractive error, accommodation and lens opacification in adults born preterm and full-term: Results from the Gutenberg Prematurity Eye Study (GPES). Acta Ophthalmol. 2022, 100, e1439–e1450. [Google Scholar] [CrossRef]
- Fieß, A.; Nauen, H.; Mildenberger, E.; Zepp, F.; Urschitz, M.S.; Pfeiffer, N.; Schuster, A.K. Ocular geometry in adults born extremely, very and moderately preterm with and without retinopathy of prematurity: Results from the Gutenberg Prematurity Eye Study. Br. J. Ophthalmol. 2023, 107, 1125–1131. [Google Scholar] [CrossRef]
- Fieß, A.; Gißler, S.; Mildenberger, E.; Urschitz, M.S.; Zepp, F.; Hoffmann, E.M.; Brockmann, M.A.; Stoffelns, B.; Pfeiffer, N.; Schuster, A.K. Optic Nerve Head Morphology in Adults Born Extreme, Very, and Moderate Preterm with and without Retinopathy of Prematurity: Results From the Gutenberg Prematurity Eye Study. Am. J. Ophthalmol. 2022, 239, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Fieß, A.; Schäffler, A.; Mildenberger, E.; Urschitz, M.S.; Wagner, F.M.; Hoffmann, E.M.; Zepp, F.; Pfeiffer, N.; Schuster, A.K. Peripapillary Retinal Nerve Fiber Layer Thickness in Adults Born Extremely, Very, and Moderately Preterm with and without Retinopathy of Prematurity: Results from the Gutenberg Prematurity Eye Study (GPES). Am. J. Ophthalmol. 2022, 244, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Fieß, A.; Pfisterer, A.; Gißler, S.; Korb, C.; Mildenberger, E.; Urschitz, M.S.; Zepp, F.; Stoffelns, B.; Pfeiffer, N.; Schuster, A.K. RETINAL THICKNESS AND FOVEAL HYPOPLASIA IN ADULTS BORN PRETERM WITH AND WITHOUT RETINOPATHY OF PREMATURITY: The Gutenberg Prematurity Eye Study. Retina 2022, 42, 1716–1728. [Google Scholar] [CrossRef] [PubMed]
- Wood, E.H.; Chang, E.Y.; Beck, K.; Hadfield, B.R.; Quinn, A.R.; Harper, C.A. 80 Years of vision: Preventing blindness from retinopathy of prematurity. J. Perinatol. 2021, 41, 1216–1224. [Google Scholar] [CrossRef]
- Quinn, G.E. Retinopathy of prematurity blindness worldwide: Phenotypes in the third epidemic. Eye Brain 2016, 8, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Rahi, J.S.; Cable, N. Severe visual impairment and blindness in children in the UK. Lancet 2003, 362, 1359–1365. [Google Scholar] [CrossRef]
- Good, W.V.; Early Treatment for Retinopathy of Prematurity Cooperative Group. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans. Am. Ophthalmol. Soc. 2004, 102, 233. [Google Scholar]
- Lucchesi, M.; Marracci, S.; Amato, R.; Filippi, L.; Cammalleri, M.; Dal Monte, M. Neurosensory Alterations in Retinopathy of Prematurity: A Window to Neurological Impairments Associated to Preterm Birth. Biomedicines 2022, 10, 1603. [Google Scholar] [CrossRef] [PubMed]
- Fevereiro-Martins, M.; Marques-Neves, C.; Guimarães, H.; Bicho, M. Retinopathy of prematurity: A review of pathophysiology and signaling pathways. Surv. Ophthalmol. 2023, 68, 175–210. [Google Scholar] [CrossRef]
- Krock, B.L.; Skuli, N.; Simon, M.C. Hypoxia-induced angiogenesis: Good and evil. Genes Cancer 2011, 2, 1117–1133. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef]
- Stone, J.; Itin, A.; Alon, T.; Pe’Er, J.; Gnessin, H.; Chan-Ling, T.; Keshet, E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J. Neurosci. 1995, 15, 4738–4747. [Google Scholar] [CrossRef]
- Böhm, E.W.; Buonfiglio, F.; Voigt, A.M.; Bachmann, P.; Safi, T.; Pfeiffer, N.; Gericke, A. Oxidative stress in the eye and its role in the pathophysiology of ocular diseases. Redox Biol. 2023, 68, 102967. [Google Scholar] [CrossRef]
- Buonfiglio, F.; Böhm, E.W.; Pfeiffer, N.; Gericke, A. Oxidative Stress: A Suitable Therapeutic Target for Optic Nerve Diseases? Antioxidants 2023, 12, 1465. [Google Scholar] [CrossRef]
- Hartnett, M.E. Advances in understanding and management of retinopathy of prematurity. Surv. Ophthalmol. 2017, 62, 257–276. [Google Scholar] [CrossRef]
- Buhimschi, I.A.; Buhimschi, C.S.; Pupkin, M.; Weiner, C.P. Beneficial impact of term labor: Nonenzymatic antioxidant reserve in the human fetus. Am. J. Obstet. Gynecol. 2003, 189, 181–188. [Google Scholar] [CrossRef]
- Graziosi, A.; Perrotta, M.; Russo, D.; Gasparroni, G.; D’Egidio, C.; Marinelli, B.; Di Marzio, G.; Falconio, G.; Mastropasqua, L.; Li Volti, G.; et al. Oxidative Stress Markers and the Retinopathy of Prematurity. J. Clin. Med. 2020, 9, 2711. [Google Scholar] [CrossRef]
- Hellström, A.; Smith, L.E.; Dammann, O. Retinopathy of prematurity. Lancet 2013, 382, 1445–1457. [Google Scholar] [CrossRef]
- Ashton, N.; Ward, B.; Serpell, G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br. J. Ophthalmol. 1954, 38, 397–432. [Google Scholar] [CrossRef]
- Simmons, A.B.; Bretz, C.A.; Wang, H.; Kunz, E.; Hajj, K.; Kennedy, C.; Yang, Z.; Suwanmanee, T.; Kafri, T.; Hartnett, M.E. Gene therapy knockdown of VEGFR2 in retinal endothelial cells to treat retinopathy. Angiogenesis 2018, 21, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J. New aspects on the treatment of retinopathy of prematurity: Currently available therapies and emerging novel therapeutics. Int. J. Mol. Sci. 2022, 23, 8529. [Google Scholar] [CrossRef]
- Smith, L.E.H.; Wesolowski, E.; McLellan, A.; Kostyk, S.K.; D’Amato, R.J.; Sullivan, R.; D’Amore, P.A. Oxygen-induced retinopathy in the mouse. Investig. Ophthalmol. Vis. Sci. 1994, 35, 101–111. [Google Scholar]
- Wang, H.; Zhang, S.X.; Hartnett, M.E. Signaling pathways triggered by oxidative stress that mediate features of severe retinopathy of prematurity. JAMA Ophthalmol. 2013, 131, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; El-Remessy, A.B.; Brooks, S.E.; Al-Shabrawey, M.; Tsai, N.-T.; Caldwell, R.B. Hyperoxia induces retinal vascular endothelial cell apoptosis through formation of peroxynitrite. Am. J. Physiol.-Cell Physiol. 2003, 285, C546–C554. [Google Scholar] [CrossRef]
- Wilkinson-Berka, J.L.; Deliyanti, D.; Rana, I.; Miller, A.G.; Agrotis, A.; Armani, R.; Szyndralewiez, C.; Wingler, K.; Touyz, R.M.; Cooper, M.E.; et al. NADPH Oxidase, NOX1, Mediates Vascular Injury in Ischemic Retinopathy. Antioxid. Redox Signal. 2013, 20, 2726–2740. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Z.; Jiang, Y.; Hartnett, M.E. Endothelial NADPH oxidase 4 mediates vascular endothelial growth factor receptor 2-induced intravitreal neovascularization in a rat model of retinopathy of prematurity. Mol. Vis. 2014, 20, 231–241. [Google Scholar]
- Chan, E.C.; van Wijngaarden, P.; Liu, G.S.; Jiang, F.; Peshavariya, H.; Dusting, G.J. Involvement of Nox2 NADPH oxidase in retinal neovascularization. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7061–7067. [Google Scholar] [CrossRef]
- Saito, Y.; Uppal, A.; Byfield, G.; Budd, S.; Hartnett, M.E. Activated NAD(P)H oxidase from supplemental oxygen induces neovascularization independent of VEGF in retinopathy of prematurity model. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Geisen, P.; Uppal, A.; Hartnett, M.E. Inhibition of NAD(P)H oxidase reduces apoptosis and avascular retina in an animal model of retinopathy of prematurity. Mol. Vis. 2007, 13, 840–853. [Google Scholar] [PubMed]
- Byfield, G.; Budd, S.; Hartnett, M.E. The role of supplemental oxygen and JAK/STAT signaling in intravitreous neovascularization in a ROP rat model. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3360–3365. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Byfield, G.; Jiang, Y.; Smith, G.W.; McCloskey, M.; Hartnett, M.E. VEGF-Mediated STAT3 Activation Inhibits Retinal Vascularization by Down-Regulating Local Erythropoietin Expression. Am. J. Pathol. 2012, 180, 1243–1253. [Google Scholar] [CrossRef]
- Ren, J.; Jiang, J.; Ou, W.; Luo, X.; Xiang, J.; Liu, G.; Huang, S.; He, L.; Gan, J.; Li, H.; et al. The Effect of STAT3 Signal Pathway Activation on Retinopathy of Prematurity. Front. Pediatr. 2021, 9, 638432. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Filippi, L.; Bagnoli, P.; La Marca, G.; Cristofori, G.; Raffaeli, G.; Padrini, L.; Araimo, G.; Fumagalli, M.; Groppo, M.; et al. The pathophysiology of retinopathy of prematurity: An update of previous and recent knowledge. Acta Ophthalmol. 2014, 92, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Toda, N.; Nakanishi-Toda, M. Nitric oxide: Ocular blood flow, glaucoma, and diabetic retinopathy. Prog. Retin. Eye Res. 2007, 26, 205–238. [Google Scholar] [CrossRef]
- Opatrilova, R.; Kubatka, P.; Caprnda, M.; Büsselberg, D.; Krasnik, V.; Vesely, P.; Saxena, S.; Ruia, S.; Mozos, I.; Rodrigo, L.; et al. Nitric oxide in the pathophysiology of retinopathy: Evidences from preclinical and clinical researches. Acta Ophthalmol. 2018, 96, 222–231. [Google Scholar] [CrossRef]
- Edgar, K.S.; Matesanz, N.; Gardiner, T.A.; Katusic, Z.S.; McDonald, D.M. Hyperoxia depletes (6R)-5,6,7,8-tetrahydrobiopterin levels in the neonatal retina: Implications for nitric oxide synthase function in retinopathy. Am. J. Pathol. 2015, 185, 1769–1782. [Google Scholar] [CrossRef]
- He, T.; Ai, M.; Zhao, X.H.; Xing, Y.Q. Inducible nitric oxide synthase mediates hypoxia-induced hypoxia-inducible factor-1 alpha activation and vascular endothelial growth factor expression in oxygen-induced retinopathy. Pathobiology 2007, 74, 336–343. [Google Scholar] [CrossRef]
- Smith, T.L.; Oubaha, M.; Cagnone, G.; Boscher, C.; Kim, J.S.; El Bakkouri, Y.; Zhang, Y.; Chidiac, R.; Corriveau, J.; Delisle, C.; et al. eNOS controls angiogenic sprouting and retinal neovascularization through the regulation of endothelial cell polarity. Cell. Mol. Life Sci. 2021, 79, 37. [Google Scholar] [CrossRef]
- Ninchoji, T.; Love, D.T.; Smith, R.O.; Hedlund, M.; Vestweber, D.; Sessa, W.C.; Claesson-Welsh, L. eNOS-induced vascular barrier disruption in retinopathy by c-Src activation and tyrosine phosphorylation of VE-cadherin. Elife 2021, 10, e64944. [Google Scholar] [CrossRef]
- Kermorvant-Duchemin, E.; Sennlaub, F.; Sirinyan, M.; Brault, S.; Andelfinger, G.; Kooli, A.; Germain, S.; Ong, H.; d’Orleans-Juste, P.; Gobeil, F., Jr. Trans-arachidonic acids generated during nitrative stress induce a thrombospondin-1–dependent microvascular degeneration. Nat. Med. 2005, 11, 1339–1345. [Google Scholar] [CrossRef][Green Version]
- Barnett, J.M.; McCollum, G.W.; Penn, J.S. Role of cytosolic phospholipase A(2) in retinal neovascularization. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.P.; Rojas, M.; Suwanpradid, J.; Toque, H.A.; Caldwell, R.W.; Caldwell, R.B. Arginase in retinopathy. Prog. Retin. Eye Res. 2013, 36, 260–280. [Google Scholar] [CrossRef]
- Lange, P.S.; Langley, B.; Lu, P.; Ratan, R.R. Novel roles for arginase in cell survival, regeneration, and translation in the central nervous system. J. Nutr. 2004, 134, 2812S–2817S. [Google Scholar] [CrossRef]
- Fouda, A.Y.; Eldahshan, W.; Narayanan, S.P.; Caldwell, R.W.; Caldwell, R.B. Arginase pathway in acute retina and brain injury: Therapeutic opportunities and unexplored avenues. Front. Pharmacol. 2020, 11, 277. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Usui-Ouchi, A.; Nilsson, A.K.; Yang, J.; Ko, M.; Hellström, A.; Fu, Z. Metabolism in retinopathy of prematurity. Life 2021, 11, 1119. [Google Scholar] [CrossRef] [PubMed]
- Neu, J.; Afzal, A.; Pan, H.; Gallego, E.; Li, N.; Calzi, S.L.; Caballero, S.; Spoerri, P.E.; Shaw, L.C.; Grant, M.B. The dipeptide Arg-Gln inhibits retinal neovascularization in the mouse model of oxygen-induced retinopathy. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3151–3155. [Google Scholar] [CrossRef] [PubMed]
- Bachetti, T.; Comini, L.; Francolini, G.; Bastianon, D.; Valetti, B.; Cadei, M.; Grigolato, P.; Suzuki, H.; Finazzi, D.; Albertini, A. Arginase pathway in human endothelial cells in pathophysiological conditions. J. Mol. Cell. Cardiol. 2004, 37, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Suwanpradid, J.; Rojas, M.; Behzadian, M.A.; Caldwell, R.W.; Caldwell, R.B. Arginase 2 deficiency prevents oxidative stress and limits hyperoxia-induced retinal vascular degeneration. PLoS ONE 2014, 9, e110604. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.Y.; Xu, Z.; Shosha, E.; Lemtalsi, T.; Chen, J.; Toque, H.A.; Tritz, R.; Cui, X.; Stansfield, B.K.; Huo, Y. Arginase 1 promotes retinal neurovascular protection from ischemia through suppression of macrophage inflammatory responses. Cell Death Dis. 2018, 9, 1001. [Google Scholar] [CrossRef] [PubMed]
- Shosha, E.; Fouda, A.Y.; Narayanan, S.P.; Caldwell, R.W.; Caldwell, R.B. Is the arginase pathway a novel therapeutic avenue for diabetic retinopathy? J. Clin. Med. 2020, 9, 425. [Google Scholar] [CrossRef] [PubMed]
- Shosha, E.; Xu, Z.; Yokota, H.; Saul, A.; Rojas, M.; Caldwell, R.W.; Caldwell, R.B.; Narayanan, S.P. Arginase 2 promotes neurovascular degeneration during ischemia/reperfusion injury. Cell Death Dis. 2016, 7, e2483. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.C.; Holm, M.; Austeng, D.; Morken, T.S.; Zhou, T.E.; Beaudry-Richard, A.; Sierra, E.M.; Dammann, O.; Chemtob, S. Retinopathy of prematurity: Inflammation, choroidal degeneration, and novel promising therapeutic strategies. J. Neuroinflamm. 2017, 14, 165. [Google Scholar] [CrossRef]
- Sivakumar, V.; Foulds, W.S.; Luu, C.D.; Ling, E.A.; Kaur, C. Retinal ganglion cell death is induced by microglia derived pro-inflammatory cytokines in the hypoxic neonatal retina. J. Pathol. 2011, 224, 245–260. [Google Scholar] [CrossRef]
- Rivera, J.C.; Sitaras, N.; Noueihed, B.; Hamel, D.; Madaan, A.; Zhou, T.; Honoré, J.C.; Quiniou, C.; Joyal, J.S.; Hardy, P.; et al. Microglia and interleukin-1β in ischemic retinopathy elicit microvascular degeneration through neuronal semaphorin-3A. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1881–1891. [Google Scholar] [CrossRef]
- Zhou, T.E.; Rivera, J.C.; Bhosle, V.K.; Lahaie, I.; Shao, Z.; Tahiri, H.; Zhu, T.; Polosa, A.; Dorfman, A.; Beaudry-Richard, A.; et al. Choroidal Involution Is Associated with a Progressive Degeneration of the Outer Retinal Function in a Model of Retinopathy of Prematurity: Early Role for IL-1β. Am. J. Pathol. 2016, 186, 3100–3116. [Google Scholar] [CrossRef]
- Sullivan, G.; Galdi, P.; Cabez, M.B.; Borbye-Lorenzen, N.; Stoye, D.Q.; Lamb, G.J.; Evans, M.J.; Quigley, A.J.; Thrippleton, M.J.; Skogstrand, K.; et al. Interleukin-8 dysregulation is implicated in brain dysmaturation following preterm birth. Brain Behav. Immun. 2020, 90, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Silveira, R.C.; Fortes Filho, J.B.; Procianoy, R.S. Assessment of the contribution of cytokine plasma levels to detect retinopathy of prematurity in very low birth weight infants. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.R.; Davies, M.H.; Eubanks, J.P. Increased expression of chemokine KC, an interleukin-8 homologue, in a model of oxygen-induced retinopathy. Curr. Eye Res. 2005, 30, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Hellgren, G.; Willett, K.; Engstrom, E.; Thorsen, P.; Hougaard, D.M.; Jacobsson, B.; Hellstrom, A.; Lofqvist, C. Proliferative retinopathy is associated with impaired increase in BDNF and RANTES expression levels after preterm birth. Neonatology 2010, 98, 409–418. [Google Scholar] [CrossRef]
- Yoshida, S.; Yoshida, A.; Ishibashi, T.; Elner, S.G.; Elner, V.M. Role of MCP-1 and MIP-1α in retinal neovascularization during postischemic inflammation in a mouse model of retinal neovascularization. J. Leucoc. Biol. 2003, 73, 137–144. [Google Scholar] [CrossRef]
- Yoshida, S.; Yoshida, A.; Ishibashi, T. Induction of IL-8, MCP-1, and bFGF by TNF-α in retinal glial cells: Implications for retinal neovascularization during post-ischemic inflammation. Graefe’s Arch. Clin. Exp. Ophthalmol. 2004, 242, 409–413. [Google Scholar] [CrossRef]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef]
- Pazos-Sanou, L.; Mata-Segreda, J.F. Effect of ionic charge on detergent-induced hemolysis. Acta Physiol. Pharmacol. Latinoam. 1989, 39, 27–31. [Google Scholar]
- Hashimoto, T.; Shibasaki, F. Hypoxia-inducible factor as an angiogenic master switch. Front. Pediatr. 2015, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Hoppe, G.; Tran, V.; McCollum, L.; Bolok, Y.; Song, W.; Sharma, A.; Brunengraber, H.; Sears, J.E. Serine and 1-carbon metabolism are required for HIF-mediated protection against retinopathy of prematurity. JCI Insight 2019, 4, e129398. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Bae, S.-H.; Jeong, J.-W.; Kim, S.-H.; Kim, K.-W. Hypoxia-inducible factor (HIF-1) α: Its protein stability and biological functions. Exp. Mol. Med. 2004, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cash, T.P.; Pan, Y.; Simon, M.C. Reactive oxygen species and cellular oxygen sensing. Free. Radic. Biol. Med. 2007, 43, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Weidemann, A.; Krohne, T.U.; Aguilar, E.; Kurihara, T.; Takeda, N.; Dorrell, M.I.; Simon, M.C.; Haase, V.H.; Friedlander, M.; Johnson, R.S. Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia 2010, 58, 1177–1185. [Google Scholar] [CrossRef]
- Arjamaa, O.; Nikinmaa, M. Oxygen-dependent diseases in the retina: Role of hypoxia-inducible factors. Exp. Eye Res. 2006, 83, 473–483. [Google Scholar] [CrossRef]
- Smith, L.E. Pathogenesis of retinopathy of prematurity. Growth Horm. IGF Res. 2004, 14, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Shih, S.-C.; Ju, M.; Liu, N.; Smith, L.E. Selective stimulation of VEGFR-1 prevents oxygen-induced retinal vascular degeneration in retinopathy of prematurity. J. Clin. Investig. 2003, 112, 50–57. [Google Scholar] [CrossRef]
- Pierce, E.A.; Foley, E.D.; Smith, L.E. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch. Ophthalmol. 1996, 114, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Pierce, E.A.; Avery, R.L.; Foley, E.D.; Aiello, L.P.; Smith, L. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc. Natl. Acad. Sci. USA 1995, 92, 905–909. [Google Scholar] [CrossRef]
- Ramshekar, A.; Hartnett, M.E. Vascular Endothelial Growth Factor Signaling in Models of Oxygen-Induced Retinopathy: Insights into Mechanisms of Pathology in Retinopathy of Prematurity. Front. Pediatr. 2021, 9, 796143. [Google Scholar] [CrossRef]
- Hartnett, M.E.; Martiniuk, D.; Byfield, G.; Geisen, P.; Zeng, G.; Bautch, V.L. Neutralizing VEGF decreases tortuosity and alters endothelial cell division orientation in arterioles and veins in a rat model of ROP: Relevance to plus disease. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3107–3114. [Google Scholar] [CrossRef]
- Budd, S.; Byfield, G.; Martiniuk, D.; Geisen, P.; Hartnett, M.E. Reduction in endothelial tip cell filopodia corresponds to reduced intravitreous but not intraretinal vascularization in a model of ROP. Exp. Eye Res. 2009, 89, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Tasman, W. Multicenter trial of cryotherapy for retinopathy of prematurity. Arch. Ophthalmol. 1988, 106, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity. Snellen visual acuity and structural outcome at 5 1/2 years after randomization. Arch. Ophthalmol. 1996, 114, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghamdi, A.; Albiani, D.A.; Hodge, W.G.; Clarke, W.N. Myopia and astigmatism in retinopathy of prematurity after treatment with cryotherapy or laser photocoagulation. Can. J. Ophthalmol. 2004, 39, 521–525. [Google Scholar] [CrossRef] [PubMed]
- McNamara, J.A. Laser treatment for retinopathy of prematurity. Curr. Opin. Ophthalmol. 1993, 4, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.S.H.; Chou, H.-D.; Ling, X.C.; Al-Khaled, T.; Valikodath, N.; Cole, E.; Yap, V.L.; Chiang, M.F.; Chan, R.V.P.; Wu, W.-C. Assessment and management of retinopathy of prematurity in the era of anti-vascular endothelial growth factor (VEGF). Prog. Retin. Eye Res. 2022, 88, 101018. [Google Scholar] [CrossRef] [PubMed]
- Fallaha, N.; Lynn, M.J.; Aaberg, T.M., Jr.; Lambert, S.R. Clinical outcome of confluent laser photoablation for retinopathy of prematurity. J. AAPOS 2002, 6, 81–85. [Google Scholar] [CrossRef]
- Trigler, L.; Weaver, R.G., Jr.; O’Neil, J.W.; Barondes, M.J.; Freedman, S.F. Case series of angle-closure glaucoma after laser treatment for retinopathy of prematurity. J. AAPOS 2005, 9, 17–21. [Google Scholar] [CrossRef]
- Koukourakis, G.V.; Sotiropoulou-Lontou, A. Targeted therapy with bevacizumab (Avastin) for metastatic colorectal cancer. Clin. Transl. Oncol. 2011, 13, 710–714. [Google Scholar] [CrossRef]
- Moinuddin, O.; Bonnafini, S.; Besirli, C.G. Exudative Retinal Detachment Following Laser Photocoagulation for Retinopathy of Prematurity: A Rare Complication. Ophthalmic Surg. Lasers Imaging Retin. 2019, 50, 242–246. [Google Scholar] [CrossRef] [PubMed]
- VanderVeen, D.K.; Cataltepe, S.U. Anti-vascular endothelial growth factor intravitreal therapy for retinopathy of prematurity. In Proceedings of the Seminars in Perinatology; WB Saunders: Philadelphia, PA, USA, 2019; pp. 375–380. [Google Scholar]
- Stahl, A.; Lepore, D.; Fielder, A.; Fleck, B.; Reynolds, J.D.; Chiang, M.F.; Li, J.; Liew, M.; Maier, R.; Zhu, Q.; et al. Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): An open-label randomised controlled trial. Lancet 2019, 394, 1551–1559. [Google Scholar] [CrossRef]
- Presta, L.G.; Chen, H.; O’Connor, S.J.; Chisholm, V.; Meng, Y.G.; Krummen, L.; Winkler, M.; Ferrara, N. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997, 57, 4593–4599. [Google Scholar]
- Mintz-Hittner, H.A.; Kennedy, K.A.; Chuang, A.Z. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N. Engl. J. Med. 2011, 364, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Dorta, P.; Kychenthal, A. Treatment of type 1 retinopathy of prematurity with intravitreal bevacizumab (Avastin). Retina 2010, 30, S24–S31. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.C.; Yeh, P.T.; Chen, S.N.; Yang, C.M.; Lai, C.C.; Kuo, H.K. Effects and complications of bevacizumab use in patients with retinopathy of prematurity: A multicenter study in taiwan. Ophthalmology 2011, 118, 176–183. [Google Scholar] [CrossRef] [PubMed]
- VanderVeen, D.K.; Melia, M.; Yang, M.B.; Hutchinson, A.K.; Wilson, L.B.; Lambert, S.R. Anti-Vascular Endothelial Growth Factor Therapy for Primary Treatment of Type 1 Retinopathy of Prematurity: A Report by the American Academy of Ophthalmology. Ophthalmology 2017, 124, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Davis, J.M. Future applications of antioxidants in premature infants. Curr. Opin. Pediatr. 2011, 23, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Darlow, B.A.; Buss, H.; McGill, F.; Fletcher, L.; Graham, P.; Winterbourn, C.C. Vitamin C supplementation in very preterm infants: A randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 2005, 90, F117–F122. [Google Scholar] [CrossRef] [PubMed]
- Beharry, K.D.; Valencia, G.B.; Lazzaro, D.R.; Aranda, J.V. Pharmacologic interventions for the prevention and treatment of retinopathy of prematurity. Semin. Perinatol. 2016, 40, 189–202. [Google Scholar] [CrossRef]
- Tsang, J.K.W.; Liu, J.; Lo, A.C.Y. Vascular and Neuronal Protection in the Developing Retina: Potential Therapeutic Targets for Retinopathy of Prematurity. Int. J. Mol. Sci. 2019, 20, 4321. [Google Scholar] [CrossRef]
- Brion, L.P.; Bell, E.F.; Raghuveer, T.S. Vitamin E supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. 2003, 3, Cd003665. [Google Scholar] [CrossRef]
- Raju, T.N.; Langenberg, P.; Bhutani, V.; Quinn, G.E. Vitamin E prophylaxis to reduce retinopathy of prematurity: A reappraisal of published trials. J. Pediatr. 1997, 131, 844–850. [Google Scholar] [CrossRef]
- Romero-Maldonado, S.; Montoya-Estrada, A.; Reyes-Muñoz, E.; Guzmán-Grenfell, A.M.; Torres-Ramos, Y.D.; Sánchez-Mendez, M.D.; Tolentino-Dolores, M.; Salgado-Valladares, M.B.; Belmont-Gómez, A.; Najéra, N.; et al. Efficacy of water-based vitamin E solution versus placebo in the prevention of retinopathy of prematurity in very low birth weight infants: A randomized clinical trial. Medicine 2021, 100, e26765. [Google Scholar] [CrossRef] [PubMed]
- Hellström, A.; Nilsson, A.K.; Wackernagel, D.; Pivodic, A.; Vanpee, M.; Sjöbom, U.; Hellgren, G.; Hallberg, B.; Domellöf, M.; Klevebro, S.; et al. Effect of Enteral Lipid Supplement on Severe Retinopathy of Prematurity: A Randomized Clinical Trial. JAMA Pediatr. 2021, 175, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Madden, S.K.; Itzhaki, L.S. Structural and mechanistic insights into the Keap1-Nrf2 system as a route to drug discovery. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140405. [Google Scholar] [CrossRef]
- Uno, K.; Prow, T.W.; Bhutto, I.A.; Yerrapureddy, A.; McLeod, D.S.; Yamamoto, M.; Reddy, S.P.; Lutty, G.A. Role of Nrf2 in retinal vascular development and the vaso-obliterative phase of oxygen-induced retinopathy. Exp. Eye Res. 2010, 90, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Deliyanti, D.; Lee, J.Y.; Petratos, S.; Meyer, C.J.; Ward, K.W.; Wilkinson-Berka, J.L.; de Haan, J.B. A potent Nrf2 activator, dh404, bolsters antioxidant capacity in glial cells and attenuates ischaemic retinopathy. Clin. Sci. 2016, 130, 1375–1387. [Google Scholar] [CrossRef]
- Liang, X.; Wang, R. The Nrf2 inhibitor brusatol has a protective role in a rat model of oxygen-induced retinopathy of prematurity. Vis. Neurosci. 2021, 38, E002. [Google Scholar] [CrossRef]
- Bartoli, M.; Al-Shabrawey, M.; Labazi, M.; Behzadian, M.A.; Istanboli, M.; El-Remessy, A.B.; Caldwell, R.W.; Marcus, D.M.; Caldwell, R.B. HMG-CoA reductase inhibitors (statin) prevents retinal neovascularization in a model of oxygen-induced retinopathy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4934–4940. [Google Scholar] [CrossRef]
- Chen, S.; Sun, Q.; Sun, D.; Willette-Brown, J.; Anderson, M.J.; Gu, Q.; Lewandoski, M.; Hu, Y.; Zhu, F.; Wei, F.; et al. C-CBL is required for inhibition of angiogenesis through modulating JAK2/STAT3 activity in ROP development. Biomed. Pharmacother. 2020, 132, 110856. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Sun, D.; Wu, Y.; Fang, J.; Wan, X.; Li, S.; Zhang, S.; Gu, Q.; Shao, Q.; et al. SYVN1 Promotes STAT3 Protein Ubiquitination and Exerts Antiangiogenesis Effects in Retinopathy of Prematurity Development. Investig. Ophthalmol. Vis. Sci. 2023, 64, 8. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Xia, X.B.; Xu, H.Z.; Xiong, Y.; Song, W.T.; Xiong, S.Q.; Li, Y. Inhibition of retinal neovascularization by gene transfer of small interfering RNA targeting HIF-1alpha and VEGF. J. Cell Physiol. 2009, 218, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Usui-Ouchi, A.; Aguilar, E.; Murinello, S.; Prins, M.; Gantner, M.L.; Wright, P.E.; Berlow, R.B.; Friedlander, M. An allosteric peptide inhibitor of HIF-1α regulates hypoxia-induced retinal neovascularization. Proc. Natl. Acad. Sci. USA 2020, 117, 28297–28306. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Kuo, C.H.; Peng, I.C.; Chang, Y.S.; Tseng, S.H.; Conway, E.M.; Wu, H.L. Recombinant thrombomodulin domain 1 rescues pathological angiogenesis by inhibition of HIF-1α-VEGF pathway. Cell. Mol. Life Sci. 2021, 78, 7681–7692. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Jiang, Y.; Zhang, J.; Shi, J.; Zheng, P.; Yang, C.; Chen, Y. Celastrol inhibits pathologic neovascularization in oxygen-induced retinopathy by targeting the miR-17-5p/HIF-1α/VEGF pathway. Cell Cycle 2022, 21, 2091–2108. [Google Scholar] [CrossRef]
- Aranda, J.V.; Beharry, K.; Valencia, G.B.; Natarajan, G.; Davis, J. Caffeine impact on neonatal morbidities. J. Matern. Fetal Neonatal Med. 2010, 23 (Suppl. S3), 20–23. [Google Scholar] [CrossRef]
- Park, H.W.; Lim, G.; Chung, S.H.; Chung, S.; Kim, K.S.; Kim, S.N. Early Caffeine Use in Very Low Birth Weight Infants and Neonatal Outcomes: A Systematic Review and Meta-Analysis. J. Korean Med. Sci. 2015, 30, 1828–1835. [Google Scholar] [CrossRef]
- Dowling, J.E. Vitamin A: Its many roles-from vision and synaptic plasticity to infant mortality. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2020, 206, 389–399. [Google Scholar] [CrossRef]
- Wang, L.; Shi, P.; Xu, Z.; Li, J.; Xie, Y.; Mitton, K.; Drenser, K.; Yan, Q. Up-regulation of VEGF by retinoic acid during hyperoxia prevents retinal neovascularization and retinopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4276–4287. [Google Scholar] [CrossRef]
- Ozkan, H.; Duman, N.; Kumral, A.; Kasap, B.; Ozer, E.A.; Lebe, B.; Yaman, A.; Berk, T.; Yilmaz, O.; Ozer, E. Inhibition of vascular endothelial growth factor-induced retinal neovascularization by retinoic acid in experimental retinopathy of prematurity. Physiol. Res. 2006, 55, 267–275. [Google Scholar] [CrossRef]
- Garofoli, F.; Barillà, D.; Angelini, M.; Mazzucchelli, I.; De Silvestri, A.; Guagliano, R.; Decembrino, L.; Tzialla, C. Oral vitamin A supplementation for ROP prevention in VLBW preterm infants. Ital. J. Pediatr. 2020, 46, 77. [Google Scholar] [CrossRef]
- Sun, H.; Cheng, R.; Wang, Z. Early Vitamin A Supplementation Improves the Outcome of Retinopathy of Prematurity in Extremely Preterm Infants. Retina 2020, 40, 1176–1184. [Google Scholar] [CrossRef]
- Dalvin, L.A.; Hartnett, M.E.; Bretz, C.A.; Hann, C.R.; Cui, R.Z.; Marmorstein, A.D.; Sheikh-Hamad, D.; Fautsch, M.P.; Roddy, G.W. Stanniocalcin-1 is a Modifier of Oxygen-Induced Retinopathy Severity. Curr. Eye Res. 2020, 45, 46–51. [Google Scholar] [CrossRef]
- Hartnett, M.E.; Martiniuk, D.J.; Saito, Y.; Geisen, P.; Peterson, L.J.; McColm, J.R. Triamcinolone reduces neovascularization, capillary density and IGF-1 receptor phosphorylation in a model of oxygen-induced retinopathy. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4975–4982. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Öhnell, H.M.; Andreasson, S.; Gränse, L. Dexamethasone Eye Drops for the Treatment of Retinopathy of Prematurity. Ophthalmol. Retin. 2022, 6, 181–182. [Google Scholar] [CrossRef]
- Higgins, R.D.; Mendelsohn, A.L.; DeFeo, M.J.; Ucsel, R.; Hendricks-Munoz, K.D. Antenatal dexamethasone and decreased severity of retinopathy of prematurity. Arch. Ophthalmol. 1998, 116, 601–605. [Google Scholar] [CrossRef]
- Doyle, L.W.; Cheong, J.L.; Ehrenkranz, R.A.; Halliday, H.L. Early (<8 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 2017, 10, Cd001146. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.W.; Cheong, J.L.; Hay, S.; Manley, B.J.; Halliday, H.L. Late (≥7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 2021, 11, Cd001145. [Google Scholar] [CrossRef]
- Termote, J.; Schalij-Delfos, N.E.; Donders, A.R.; Cats, B.P. Do postnatal glucocorticoids and retinopathy of prematurity relate? Am. J. Perinatol. 2000, 17, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, P.S.; Ali, M.A.M.; Kannekanti, N.; Koechley, H.; Mhanna, C.; Pinto, M.; Farghaly, M.A.A.; Mhanna, M.; Aly, H.Z.; Sears, J.E. Impact of postnatal steroids on peripheral avascular retina and severity of retinopathy of prematurity. Pediatr. Res. 2023, 94, 1966–1972. [Google Scholar] [CrossRef]
- Cockle, J.V.; Gopichandran, N.; Walker, J.J.; Levene, M.I.; Orsi, N.M. Matrix metalloproteinases and their tissue inhibitors in preterm perinatal complications. Reprod. Sci. 2007, 14, 629–645. [Google Scholar] [CrossRef]
- Van Wart, H.E.; Birkedal-Hansen, H. The cysteine switch: A principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc. Natl. Acad. Sci. USA 1990, 87, 5578–5582. [Google Scholar] [CrossRef]
- Chen, L.C.; Noelken, M.E.; Nagase, H. Disruption of the cysteine-75 and zinc ion coordination is not sufficient to activate the precursor of human matrix metalloproteinase 3 (stromelysin 1). Biochemistry 1993, 32, 10289–10295. [Google Scholar] [CrossRef]
- Hoffmann, S.; He, S.; Ehren, M.; Ryan, S.J.; Wiedemann, P.; Hinton, D.R. MMP-2 and MMP-9 secretion by rpe is stimulated by angiogenic molecules found in choroidal neovascular membranes. Retina 2006, 26, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; McLamore, A.; Song, W.; McGuire, P.G. Retinal neovascularization is suppressed with a matrix metalloproteinase inhibitor. Arch. Ophthalmol. 1999, 117, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.; Rai, M.; Jalali, S.; Agarwal, K.; Badakere, A.; Puppala, L.; Vishwakarma, S.; Balakrishnan, D.; Rani, P.K.; Kekunnaya, R.; et al. An interplay of microglia and matrix metalloproteinase MMP9 under hypoxic stress regulates the opticin expression in retina. Sci. Rep. 2021, 11, 7444. [Google Scholar] [CrossRef] [PubMed]
- Drolet, B.A.; Frommelt, P.C.; Chamlin, S.L.; Haggstrom, A.; Bauman, N.M.; Chiu, Y.E.; Chun, R.H.; Garzon, M.C.; Holland, K.E.; Liberman, L.; et al. Initiation and use of propranolol for infantile hemangioma: Report of a consensus conference. Pediatrics 2013, 131, 128–140. [Google Scholar] [CrossRef]
- Love, J.N.; Sikka, N. Are 1-2 tablets dangerous? Beta-blocker exposure in toddlers. J. Emerg. Med. 2004, 26, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Kaempfen, S.; Neumann, R.P.; Jost, K.; Schulzke, S.M. Beta-blockers for prevention and treatment of retinopathy of prematurity in preterm infants. Cochrane Database Syst. Rev. 2018, 3, Cd011893. [Google Scholar] [CrossRef]
- Casini, G.; Dal Monte, M.; Fornaciari, I.; Filippi, L.; Bagnoli, P. The β-adrenergic system as a possible new target for pharmacologic treatment of neovascular retinal diseases. Prog. Retin. Eye Res. 2014, 42, 103–129. [Google Scholar] [CrossRef]
- Martini, D.; Monte, M.D.; Ristori, C.; Cupisti, E.; Mei, S.; Fiorini, P.; Filippi, L.; Bagnoli, P. Antiangiogenic effects of β2-adrenergic receptor blockade in a mouse model of oxygen-induced retinopathy. J. Neurochem. 2011, 119, 1317–1329. [Google Scholar] [CrossRef]
- Filippi, L.; Cavallaro, G.; Bagnoli, P.; Dal Monte, M.; Fiorini, P.; Donzelli, G.; Tinelli, F.; Araimo, G.; Cristofori, G.; la Marca, G.; et al. Oral propranolol for retinopathy of prematurity: Risks, safety concerns, and perspectives. J. Pediatr. 2013, 163, 1570–1577.e76. [Google Scholar] [CrossRef]
- Filippi, L.; Cavallaro, G.; Berti, E.; Padrini, L.; Araimo, G.; Regiroli, G.; Raffaeli, G.; Bozzetti, V.; Tagliabue, P.; Tomasini, B.; et al. Propranolol 0.2% Eye Micro-Drops for Retinopathy of Prematurity: A Prospective Phase IIB Study. Front. Pediatr. 2019, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.; Wilson, D.J. A study of metabolites as intermediate effectors in angiogenesis. Angiogenesis 2001, 4, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Mino, R.P.; Spoerri, P.E.; Caballero, S.; Player, D.; Belardinelli, L.; Biaggioni, I.; Grant, M.B. Adenosine receptor antagonists and retinal neovascularization in vivo. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3320–3324. [Google Scholar]
- Wells, J.A.; Glassman, A.R.; Ayala, A.R.; Jampol, L.M.; Aiello, L.P.; Antoszyk, A.N.; Arnold-Bush, B.; Baker, C.W.; Bressler, N.M.; Browning, D.J.; et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N. Engl. J. Med. 2015, 372, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Joyal, J.S.; Omri, S.; Sitaras, N.; Rivera, J.C.; Sapieha, P.; Chemtob, S. Neovascularization in retinopathy of prematurity: Opposing actions of neuronal factors GPR91 and semaphorins 3A. Acta Paediatr. 2012, 101, 819–826. [Google Scholar] [CrossRef]
- Sapieha, P.; Sirinyan, M.; Hamel, D.; Zaniolo, K.; Joyal, J.S.; Cho, J.H.; Honoré, J.C.; Kermorvant-Duchemin, E.; Varma, D.R.; Tremblay, S.; et al. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat. Med. 2008, 14, 1067–1076. [Google Scholar] [CrossRef]
- Hu, J.; Li, T.; Du, X.; Wu, Q.; Le, Y.Z. G protein-coupled receptor 91 signaling in diabetic retinopathy and hypoxic retinal diseases. Vis. Res. 2017, 139, 59–64. [Google Scholar] [CrossRef]
- Li, X.; Xie, L.; Qu, X.; Zhao, B.; Fu, W.; Wu, B.; Wu, J. GPR91, a critical signaling mechanism in modulating pathophysiologic processes in chronic illnesses. FASEB J. 2020, 34, 13091–13105. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).