Ganoderma lucidum Spore Powder Alleviates Metabolic-Associated Fatty Liver Disease by Improving Lipid Accumulation and Oxidative Stress via Autophagy
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of GLSP
2.3. Preparation of Palmitic Acid (PA)
2.4. Animals
2.5. Histological Analysis and Immunohistochemical Staining of Liver Tissue
2.6. Cell Culture and Treatments
2.7. Cell Viability
2.8. Oil Red O Staining
2.9. Triglyceride and Total Cholesterol
2.10. Reactive Oxygen Species (ROS)
2.11. Antioxidant Enzyme
2.12. Measurement of Mitochondrial Membrane Potential
2.13. Immunofluorescence
2.14. Western Blot
2.15. Quantification and Statistical Analysis
3. Results
3.1. GLSP Alleviated High-Fat-Induced MAFLD in Mice
3.2. GLSP Alleviated PA-Induced Hepatic Lipid Accumulation by Reducing Lipogenesis and Promoting Lipid Oxidation
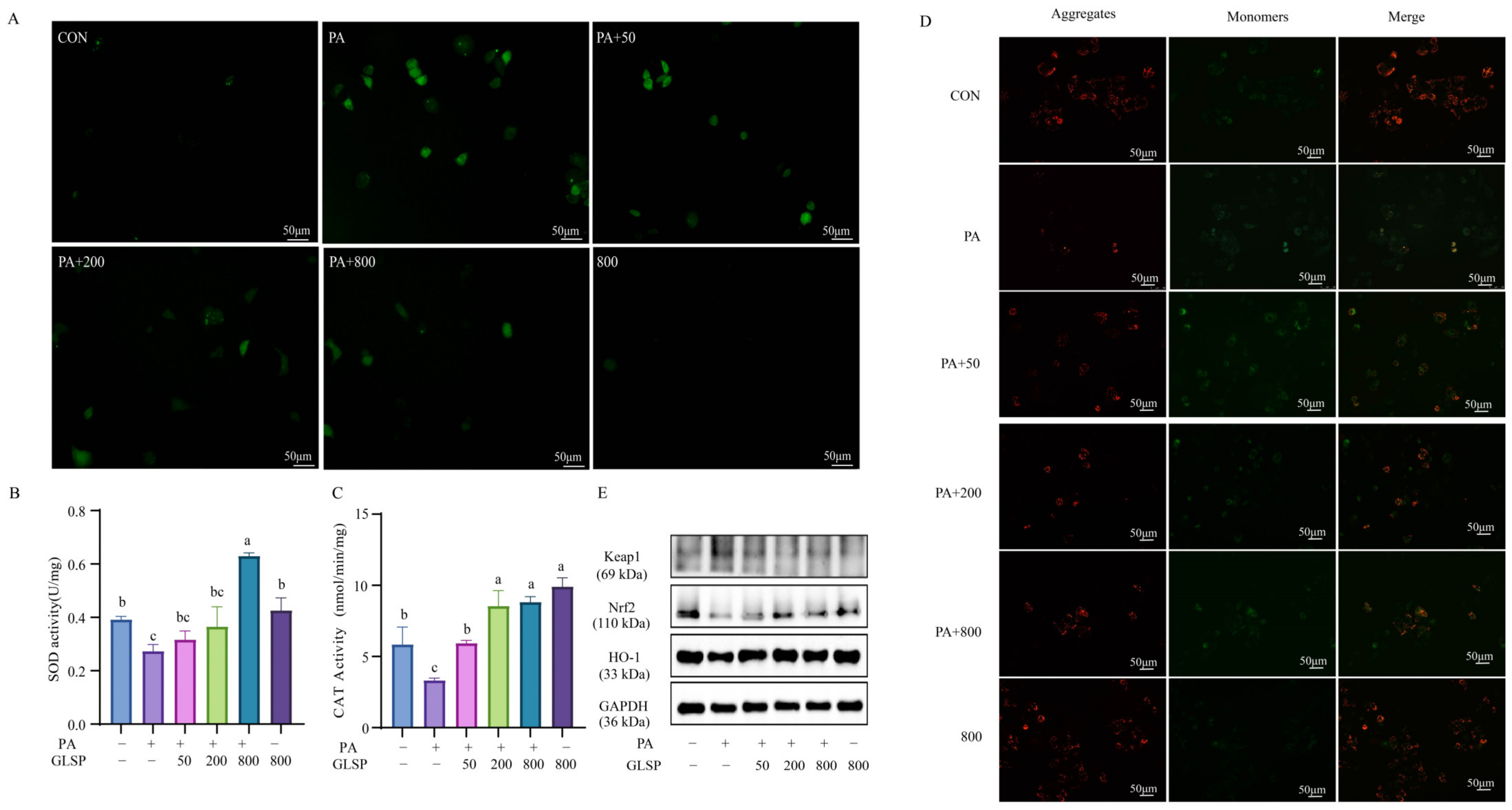
3.3. GLSP Improved PA-Induced Oxidative Stress Through Keap1-Nrf2 Pathway Induced by PA
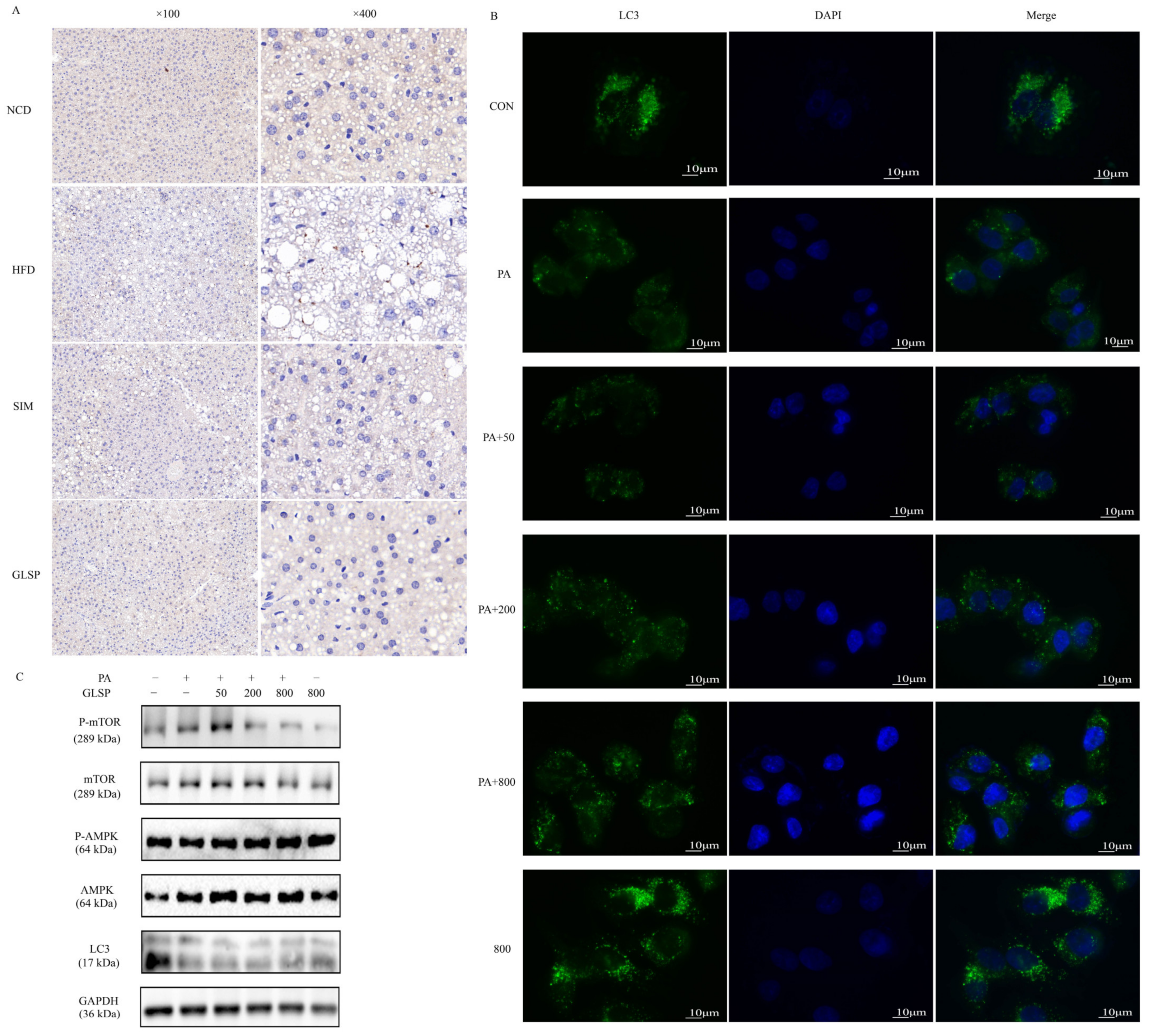
3.4. GLSP Induced Autophagy via AMPK Signal Pathway
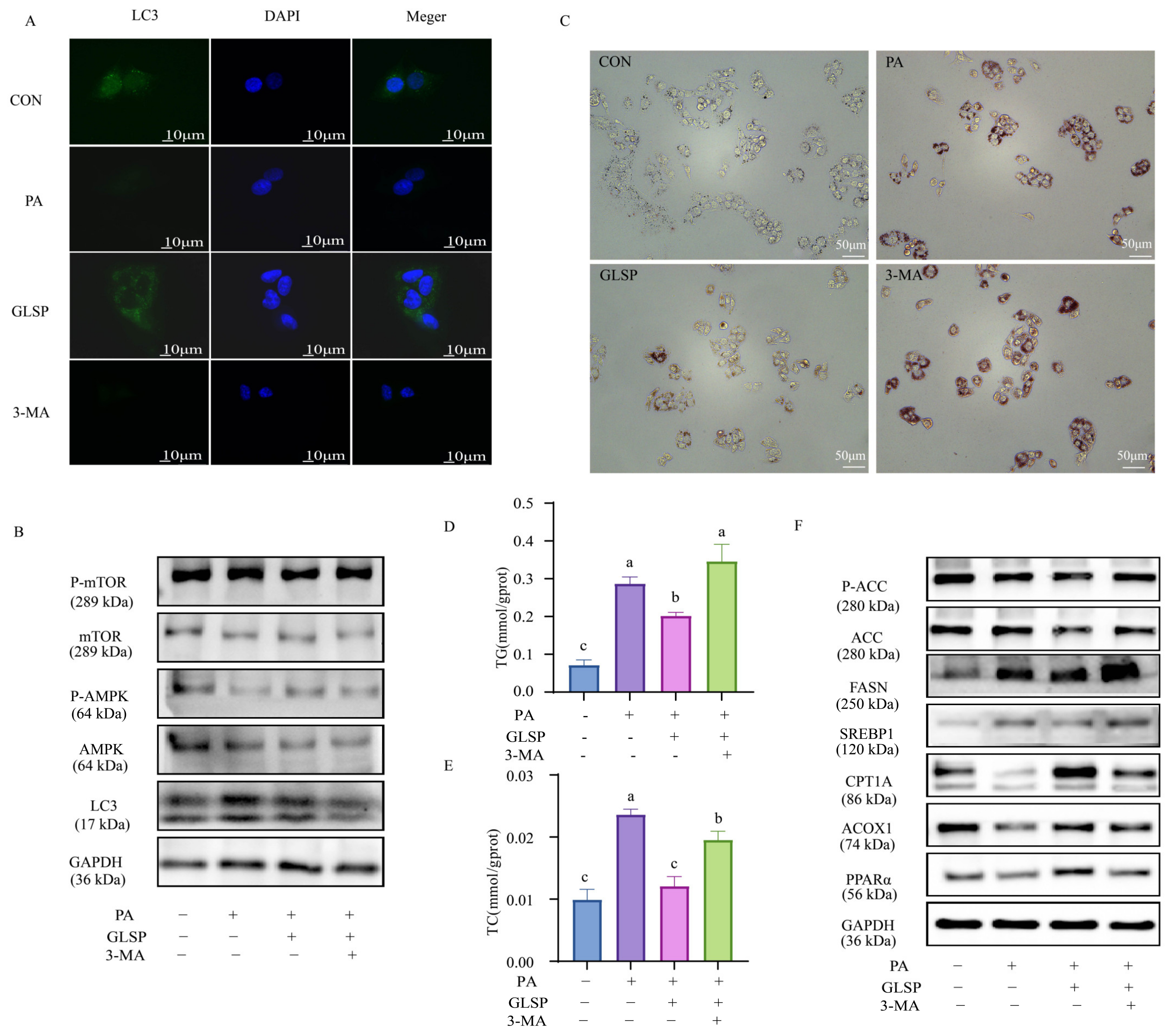
3.5. GLSP Improved Lipid Accumulation in HepG2 Cells Dependent on Autophagy
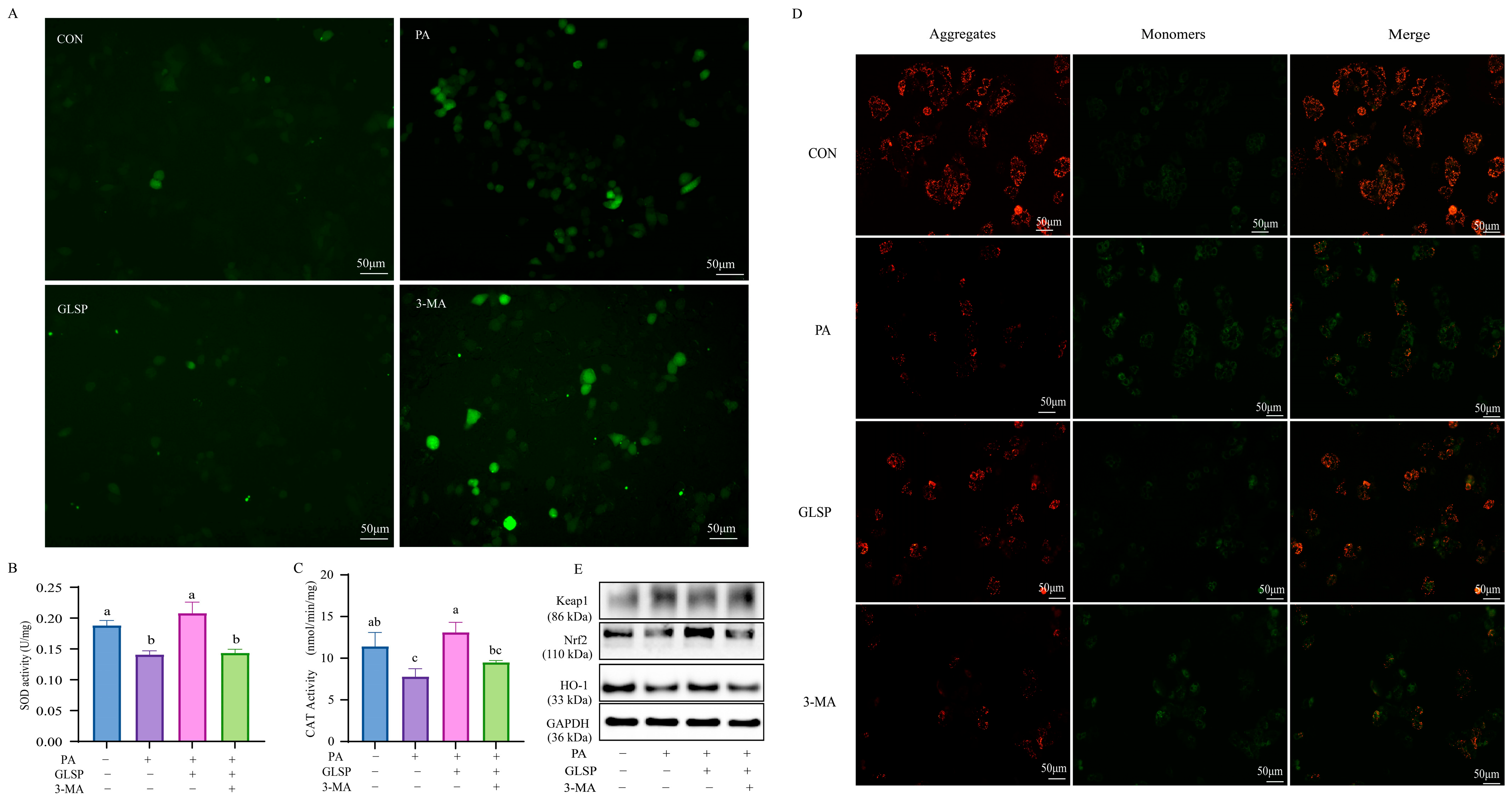
3.6. Autophagy Contributed to Effect of GLSP Protecting HepG2 Cells from Oxidative Stress Under PA Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kaya, E.; Yilmaz, Y. Metabolic-associated Fatty Liver Disease (MAFLD): A Multi-systemic Disease Beyond the Liver. J. Clin. Transl. Hepatol. 2022, 10, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Shiha, G.; Alswat, K.; Al Khatry, M.; Sharara, A.I.; Örmeci, N.; Waked, I.; Benazzouz, M.; Al-Ali, F.; Hamed, A.E.; Hamoudi, W.; et al. Nomenclature and definition of metabolic-associated fatty liver disease: A consensus from the Middle East and north Africa. Lancet Gastroenterol. Hepatol. 2021, 6, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.L.; Chen, H.; Wang, C.L.; Liang, L. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: From “two hit theory” to “multiple hit model”. World J. Gastroenterol. 2018, 24, 2974–2983. [Google Scholar] [CrossRef] [PubMed]
- Clare, K.; Dillon, J.F.; Brennan, P.N. Reactive Oxygen Species and Oxidative Stress in the Pathogenesis of MAFLD. J. Clin. Transl. Hepatol. 2022, 10, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Pierantonelli, I.; Svegliati-Baroni, G. Nonalcoholic Fatty Liver Disease: Basic Pathogenetic Mechanisms in the Progression from NAFLD to NASH. Transplantation 2019, 103, e1–e13. [Google Scholar] [CrossRef]
- Khawar, M.B.; Gao, H.; Li, W. Autophagy and Lipid Metabolism. Adv. Exp. Med. Biol. 2019, 1206, 359–374. [Google Scholar] [CrossRef]
- Pietrocola, F.; Bravo-San Pedro, J.M. Targeting Autophagy to Counteract Obesity-Associated Oxidative Stress. Antioxidants 2021, 10, 102. [Google Scholar] [CrossRef]
- Fukuo, Y.; Yamashina, S.; Sonoue, H.; Arakawa, A.; Nakadera, E.; Aoyama, T.; Uchiyama, A.; Kon, K.; Ikejima, K.; Watanabe, S. Abnormality of autophagic function and cathepsin expression in the liver from patients with non-alcoholic fatty liver disease. Hepatol. Res. 2014, 44, 1026–1036. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef]
- Xu, J.; Li, P. Researches and Application of Ganoderma Spores Powder. Adv. Exp. Med. Biol. 2019, 1181, 157–186. [Google Scholar] [CrossRef]
- Chen, J.; He, X.; Song, Y.; Tu, Y.; Chen, W.; Yang, G. Sporoderm-broken spores of Ganoderma lucidum alleviates liver injury induced by DBP and BaP co-exposure in rat. Ecotoxicol. Environ. Saf. 2022, 241, 113750. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Wang, F.; Chen, C.; Wan, X.; Li, X.; Wang, H.; Wang, S. Protective Effect of Ganoderma lucidum Spore Powder on Acute Liver Injury in Mice and its Regulation of Gut Microbiota. Front. Biosci. Landmark Ed. 2023, 28, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Li, F.L.; Zhao, J.Y.; Fu, Y.; Peng, C. Sporoderm-broken spore powder of Ganoderma lucidum ameliorate obesity and inflammation process in high-fat diet-induced obese mice. Food Nutr. Res. 2022, 66. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Zhou, J.; Chen, H.; Chen, X.; Wang, L.; Liu, D.; Kang, X. Synthesis, characterization and MAFLD prevention potential of Ganoderma lucidum spore polysaccharide-stabilized selenium nanoparticles. Int. J. Biol. Macromol. 2024, 282, 136962. [Google Scholar] [CrossRef]
- Fang, L.; Zhao, Q.; Guo, C.; Guo, D.; Li, Z.; Xu, J.; Guo, C.; Sang, T.; Wang, Y.; Chen, J.; et al. Removing the sporoderm from the sporoderm-broken spores of Ganoderma lucidum improves the anticancer and immune-regulatory activity of the water-soluble polysaccharide. Front. Nutr. 2022, 9, 1006127. [Google Scholar] [CrossRef]
- Liu, M.T.; Chen, L.X.; Zhao, J.; Li, S.P. Ganoderma spore powder contains little triterpenoids. Chin. Med. 2020, 15, 111. [Google Scholar] [CrossRef]
- Li, H.N.; Zhao, L.L.; Zhou, D.Y.; Chen, D.Q. Ganoderma Lucidum Polysaccharides Ameliorates Hepatic Steatosis and Oxidative Stress in db/db Mice via Targeting Nuclear Factor E2 (Erythroid-Derived 2)-Related Factor-2/Heme Oxygenase-1 (HO-1) Pathway. Med. Sci. Monit. 2020, 26, e921905. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Wang, Z.; Zhang, J.; Jia, L. Ganoderma lucidum polysaccharides improve lipid metabolism against high-fat diet-induced dyslipidemia. J. Ethnopharmacol. 2023, 309, 116321. [Google Scholar] [CrossRef]
- Peng, H.; Zhong, L.; Cheng, L.; Chen, L.; Tong, R.; Shi, J.; Bai, L. Ganoderma lucidum: Current advancements of characteristic components and experimental progress in anti-liver fibrosis. Front. Pharmacol. 2022, 13, 1094405. [Google Scholar] [CrossRef]
- Teng, X.; Zhang, W.; Song, Y.; Wang, H.; Ge, M.; Zhang, R. Protective effects of Ganoderma lucidum triterpenoids on oxidative stress and apoptosis in the spleen of chickens induced by cadmium. Environ. Sci. Pollut. Res. Int. 2019, 26, 23967–23980. [Google Scholar] [CrossRef]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell. Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef] [PubMed]
- Bordoloi, J.; Ozah, D.; Bora, T.; Kalita, J.; Manna, P. Gamma-glutamyl carboxylated Gas6 mediates the beneficial effect of vitamin K on lowering hyperlipidemia via regulating the AMPK/SREBP1/PPARα signaling cascade of lipid metabolism. J. Nutr. Biochem. 2019, 70, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zheng, X.; Bai, J.; Zhong, P.; Tan, S.; Zeng, W.; Chen, J.; Sun, Z.; Liu, Z.; Jin, J.; et al. Triterpenoids from the genus Ilex attenuate free fatty acid-induced lipid accumulation in HepG2 cells by regulating lipid metabolism disorder and the AMPK signalling pathway. J. Ethnopharmacol. 2023, 302, 115845. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Luo, Y.; Deng, H.; Qin, S.; Tang, W.; Zeng, L.; Zhou, B. Hugan Qingzhi medication ameliorates hepatic steatosis by activating AMPK and PPARα pathways in L02 cells and HepG2 cells. J. Ethnopharmacol. 2014, 154, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK activators: Mechanisms of action and physiological activities. Exp. Mol. Med. 2016, 48, e224. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef]
- Li, B.; Lee, D.S.; Kang, Y.; Yao, N.Q.; An, R.B.; Kim, Y.C. Protective effect of ganodermanondiol isolated from the Lingzhi mushroom against tert-butyl hydroperoxide-induced hepatotoxicity through Nrf2-mediated antioxidant enzymes. Food Chem. Toxicol. 2013, 53, 317–324. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Yu, F.; Pan, Y.; Zhang, Z.; He, Y.; Yang, H.; Zhou, P. Proteoglycan Extracted from Ganoderma lucidum Ameliorated Diabetes-Induced Muscle Atrophy via the AMPK/SIRT1 Pathway In Vivo and In Vitro. ACS Omega 2023, 8, 30359–30373. [Google Scholar] [CrossRef]
- Allameh, A.; Niayesh-Mehr, R.; Aliarab, A.; Sebastiani, G.; Pantopoulos, K. Oxidative Stress in Liver Pathophysiology and Disease. Antioxidants 2023, 12, 1653. [Google Scholar] [CrossRef]
- Lee, K.C.; Wu, P.S.; Lin, H.C. Pathogenesis and treatment of non-alcoholic steatohepatitis and its fibrosis. Clin. Mol. Hepatol. 2023, 29, 77–98. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Q.; Fu, X.; Zhu, S.; Huang, Q.; Li, C. Current Advances in the Regulatory Effects of Bioactive Compounds from Dietary Resources on Nonalcoholic Fatty Liver Disease: Role of Autophagy. J. Agric. Food Chem. 2023, 71, 17554–17569. [Google Scholar] [CrossRef] [PubMed]
- Nasiri-Ansari, N.; Nikolopoulou, C.; Papoutsi, K.; Kyrou, I.; Mantzoros, C.S.; Kyriakopoulos, G.; Chatzigeorgiou, A.; Kalotychou, V.; Randeva, M.S.; Chatha, K.; et al. Empagliflozin Attenuates Non-Alcoholic Fatty Liver Disease (NAFLD) in High Fat Diet Fed ApoE((-/-)) Mice by Activating Autophagy and Reducing ER Stress and Apoptosis. Int. J. Mol. Sci. 2021, 22, 818. [Google Scholar] [CrossRef] [PubMed]
- Doria, A.; Gatto, M.; Punzi, L. Autophagy in human health and disease. N. Engl. J. Med. 2013, 368, 1845. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, S.W.; Wang, H.; Kim, R.H.; Park, H.K.; Lee, H.; Kang, E.S. DA-1241, a Novel GPR119 Agonist, Improves Hyperglycaemia by Inhibiting Hepatic Gluconeogenesis and Enhancing Insulin Secretion in Diabetic Mice. Diabetes Metab. J. 2022, 46, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhong, P.; Wang, Y.; Zhang, Q.; Li, J.; Liu, Z.; Ji, A.; Li, Y. Hydrogen Sulfide Attenuates High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease by Inhibiting Apoptosis and Promoting Autophagy via Reactive Oxygen Species/Phosphatidylinositol 3-Kinase/AKT/Mammalian Target of Rapamycin Signaling Pathway. Front. Pharmacol. 2020, 11, 585860. [Google Scholar] [CrossRef]
- Zhou, J.; Tripathi, M.; Ho, J.P.; Widjaja, A.A.; Shekeran, S.G.; Camat, M.D.; James, A.; Wu, Y.; Ching, J.; Kovalik, J.P.; et al. Thyroid Hormone Decreases Hepatic Steatosis, Inflammation, and Fibrosis in a Dietary Mouse Model of Nonalcoholic Steatohepatitis. Thyroid 2022, 32, 725–738. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhou, J.; Yang, L.; Xiao, H.; Liu, D.; Kang, X. Ganoderma lucidum Spore Powder Alleviates Metabolic-Associated Fatty Liver Disease by Improving Lipid Accumulation and Oxidative Stress via Autophagy. Antioxidants 2024, 13, 1501. https://doi.org/10.3390/antiox13121501
Zhang Y, Zhou J, Yang L, Xiao H, Liu D, Kang X. Ganoderma lucidum Spore Powder Alleviates Metabolic-Associated Fatty Liver Disease by Improving Lipid Accumulation and Oxidative Stress via Autophagy. Antioxidants. 2024; 13(12):1501. https://doi.org/10.3390/antiox13121501
Chicago/Turabian StyleZhang, Yuxuan, Jiali Zhou, Lan Yang, Hang Xiao, Dongbo Liu, and Xincong Kang. 2024. "Ganoderma lucidum Spore Powder Alleviates Metabolic-Associated Fatty Liver Disease by Improving Lipid Accumulation and Oxidative Stress via Autophagy" Antioxidants 13, no. 12: 1501. https://doi.org/10.3390/antiox13121501
APA StyleZhang, Y., Zhou, J., Yang, L., Xiao, H., Liu, D., & Kang, X. (2024). Ganoderma lucidum Spore Powder Alleviates Metabolic-Associated Fatty Liver Disease by Improving Lipid Accumulation and Oxidative Stress via Autophagy. Antioxidants, 13(12), 1501. https://doi.org/10.3390/antiox13121501






