Advancements in Biosensors for Lipid Peroxidation and Antioxidant Protection in Food: A Critical Review
Abstract
1. Introduction
2. Lipid Peroxidation and Antioxidants
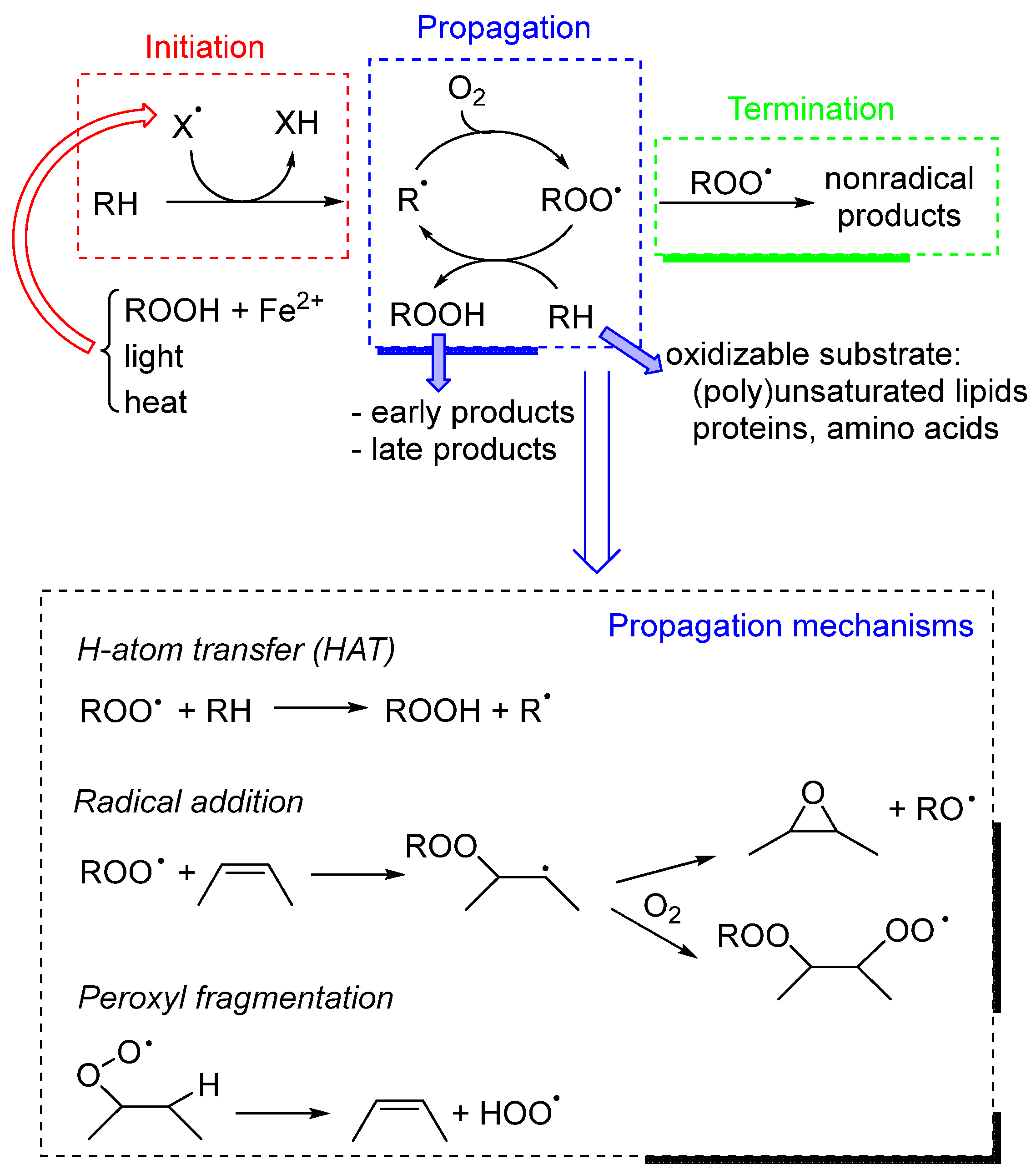
2.1. Chemical Mechanism of Lipid Peroxidation
- Initiation consists of the formation of radicals in the systems. In food, this process can be due to various mechanisms: direct reaction of O2 with bisallylic C-H groups (usually accelerated by heat) [7], photosensitization [8], and metal-catalyzed decomposition of hydroperoxides [9,10]. It should be remembered that, because of the radical chain nature of peroxidation, several substrate molecules are destroyed by one radical, and even a very small initiation rate is able to produce a dramatic effect over long periods.
- Propagation is based on the reaction of the (alkyl)peroxyl radical ROO• with the substrate, with the subsequent formation of a hydroperoxide (ROOH) and a carbon-centered radical that reacts with O2 forming a new ROO•. In lipids, propagation mainly involves polyunsaturated fatty acids (PUFAs), because the bisallylic position is (at room temperature) hundreds of times more reactive toward ROO• than allylic or aliphatic ones [5]. However, the propagation mechanism is in many cases more complex than the one described here; for instance, it may involve ROO• fragmentation to produce HOO• or RO•, together with closed-shell oxygenated early products like epoxides and carbonyls [11].
- Termination is the step in which radicals disappear from the system by reacting with each other (i.e., by bimolecular decay). Typically, two secondary ROO• radicals (like in the case of lipids) generate a carbonyl and an alcohol. While radical–radical reactions are typically fast, the low concentration of radicals nonetheless makes termination a statistically rare event, which may be further slowed down by lipid compartmentalization strategies (i.e., emulsified lipids) [5].
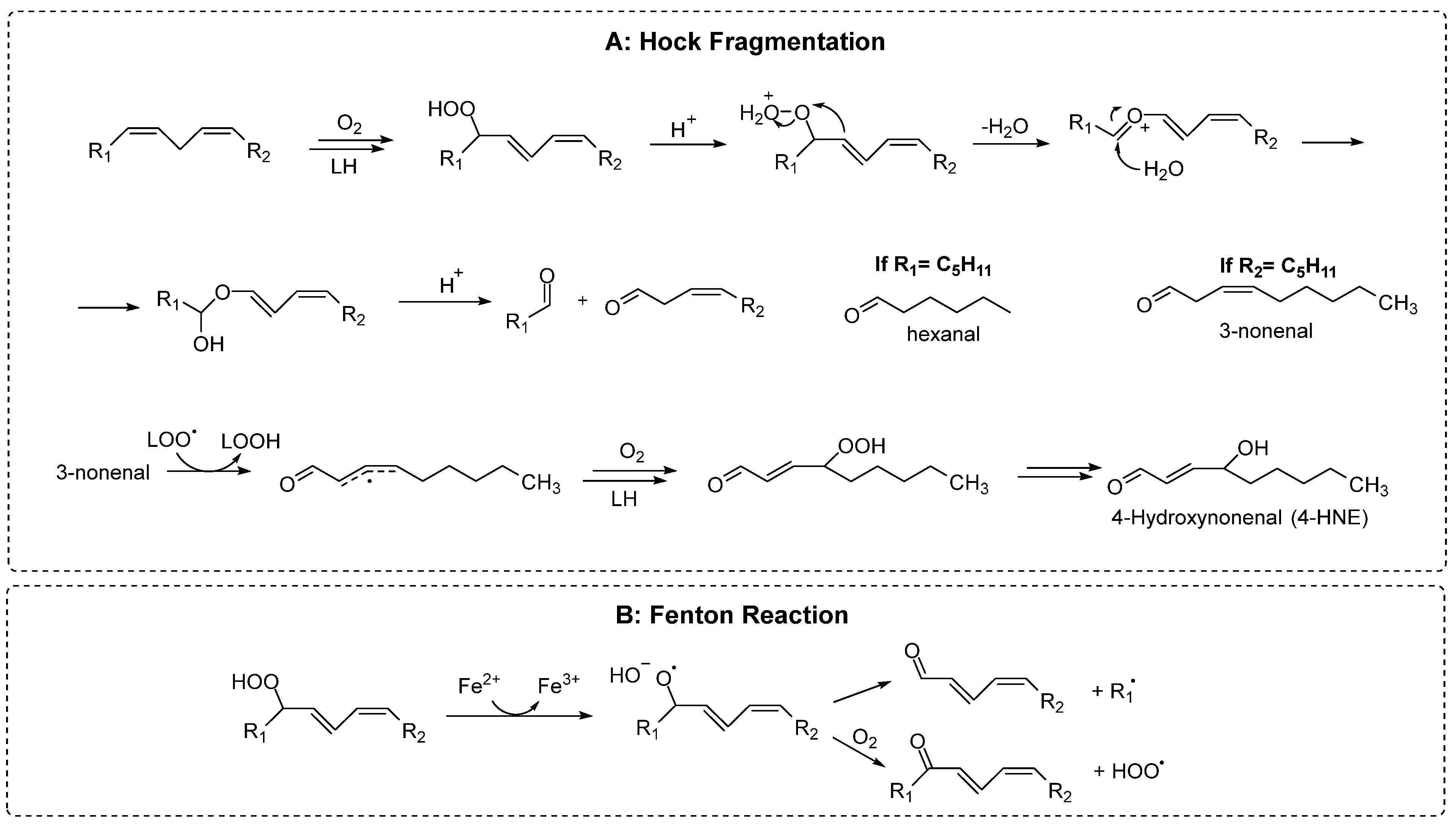
2.2. Products
2.2.1. Early Products
2.2.2. Late Products
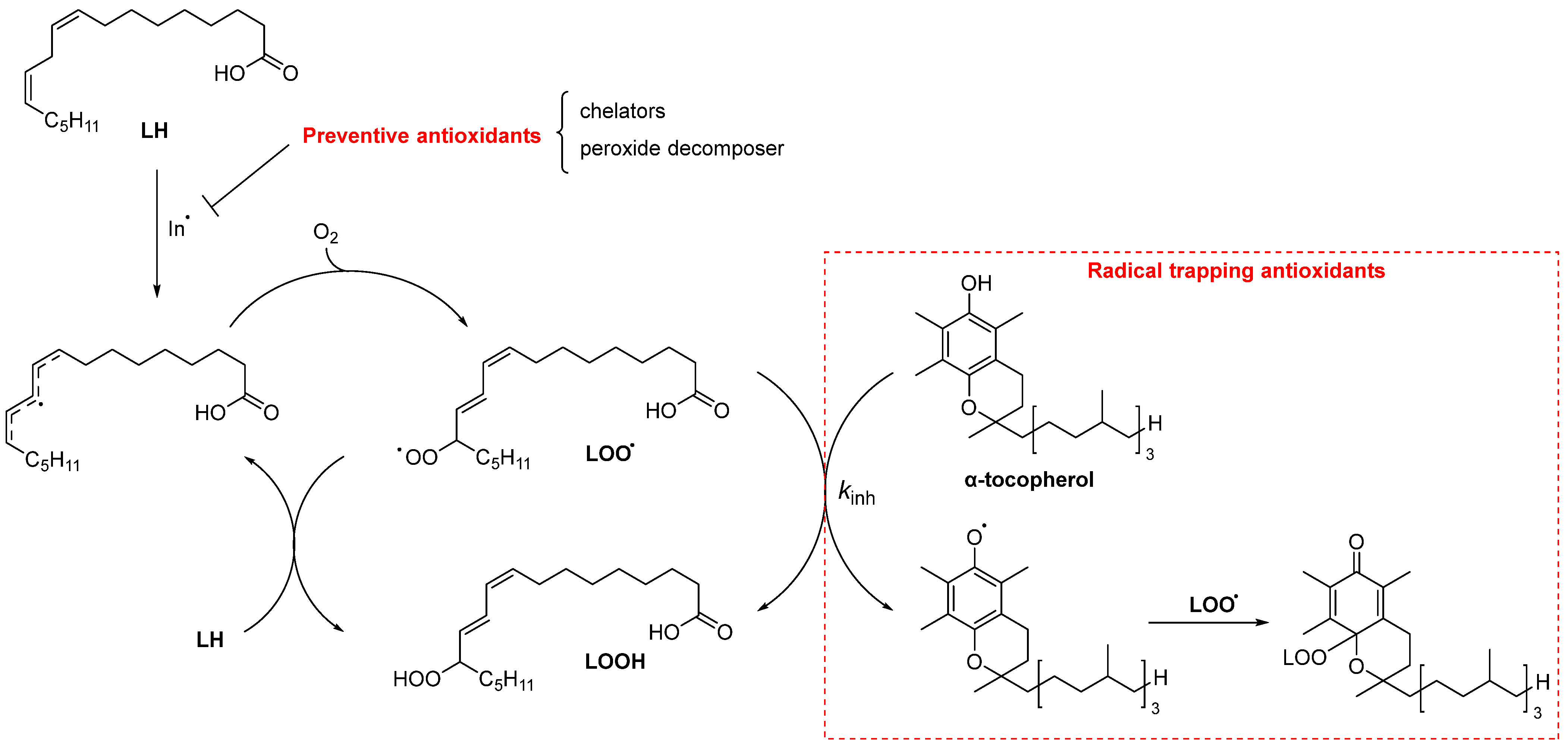
3. Antioxidants
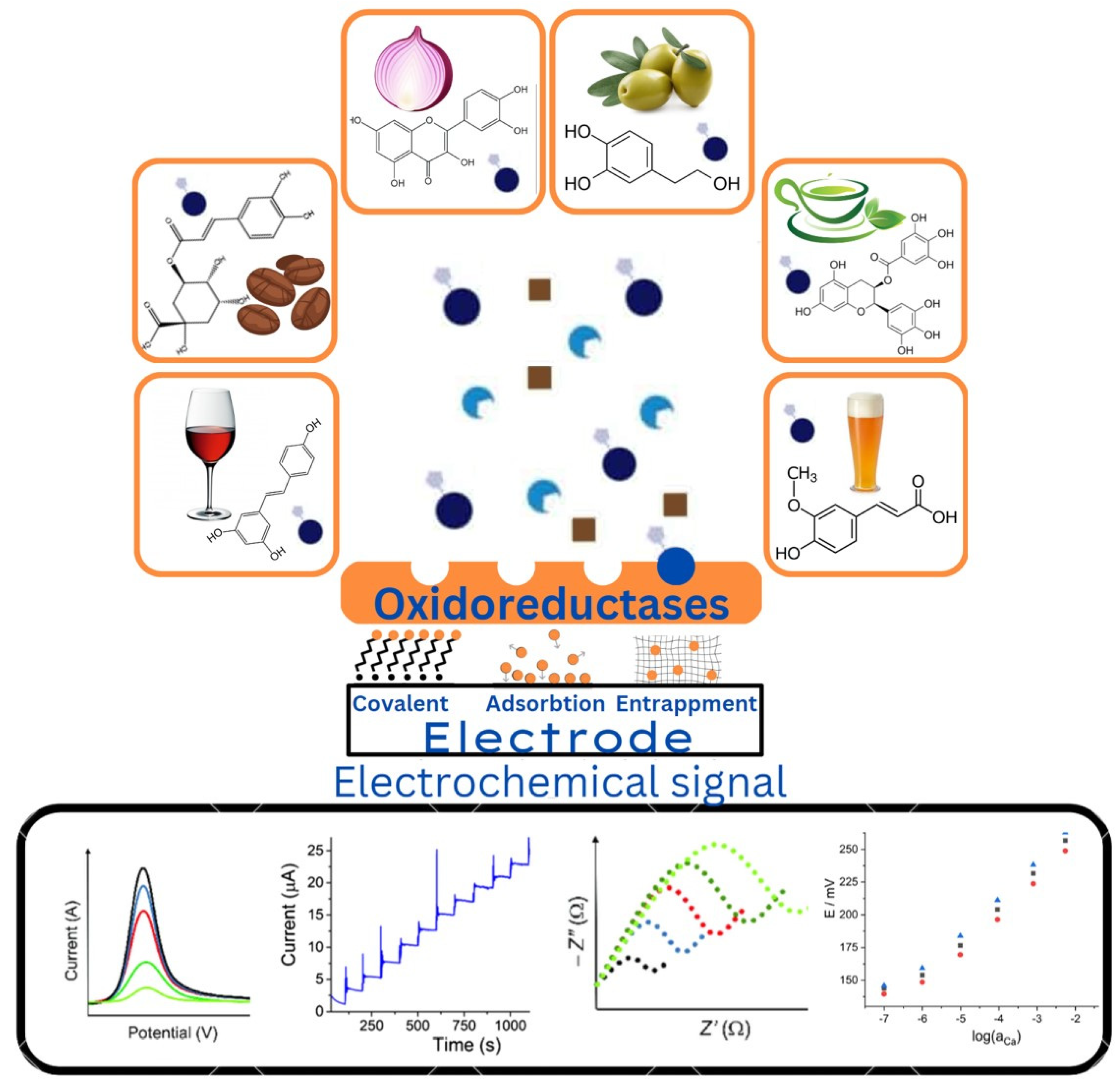
4. Biosensors for Lipid Peroxidation Analysis
4.1. What Are Biosensors
4.1.1. Enzymatic Biosensors
4.1.2. Aptasensors
4.1.3. Immunosensors
4.1.4. Molecularly Imprinted Polymers (MIP)
4.2. Antioxidant Analysis
4.2.1. Electrochemical Biosensors for Polyphenol Detection
4.2.2. Enzyme-Based Biosensors
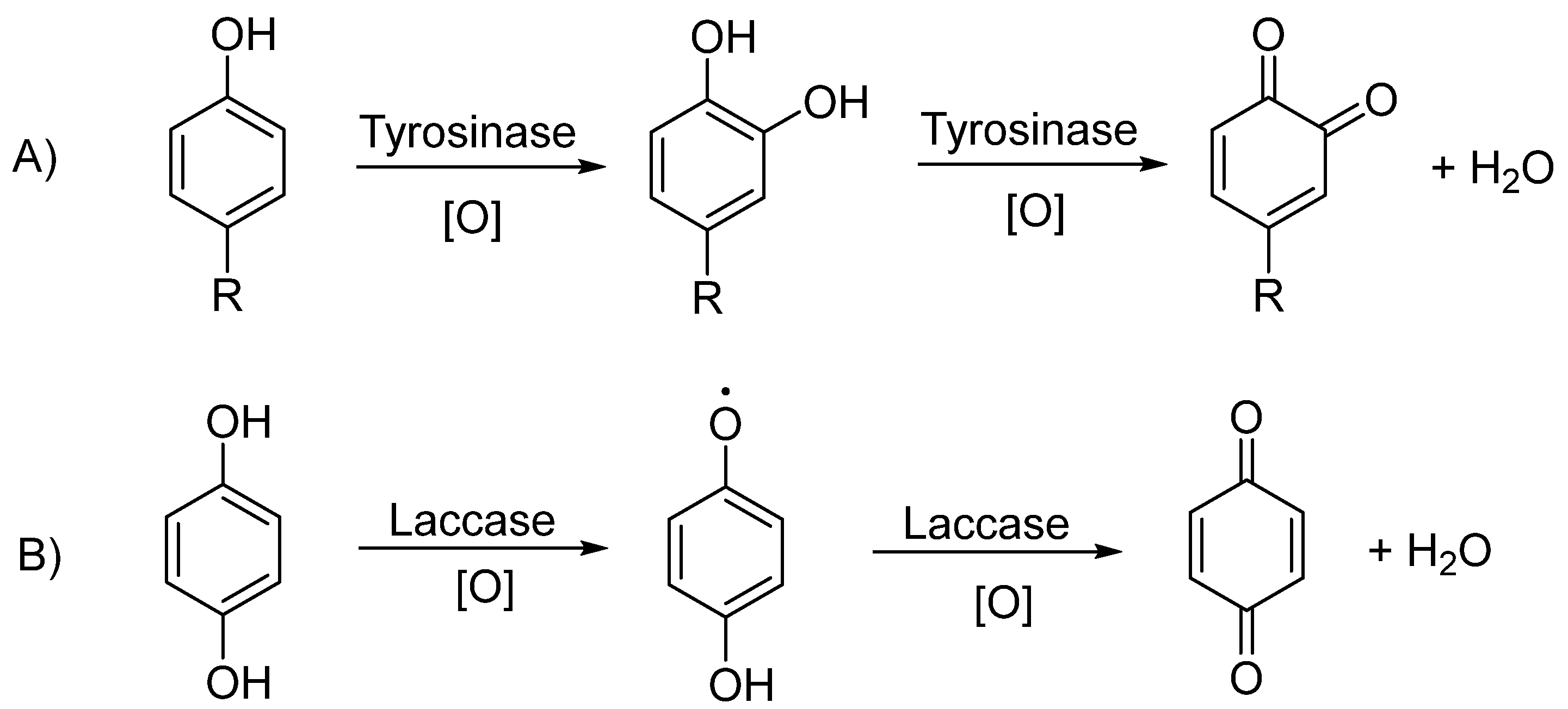
4.2.3. Tyrosinase-Based Biosensors
4.2.4. Laccase-Based Biosensors
4.2.5. Metal–Organic Framework
4.2.6. Nanozymes
4.2.7. Molecular Imprinted Polymers (MIPs)
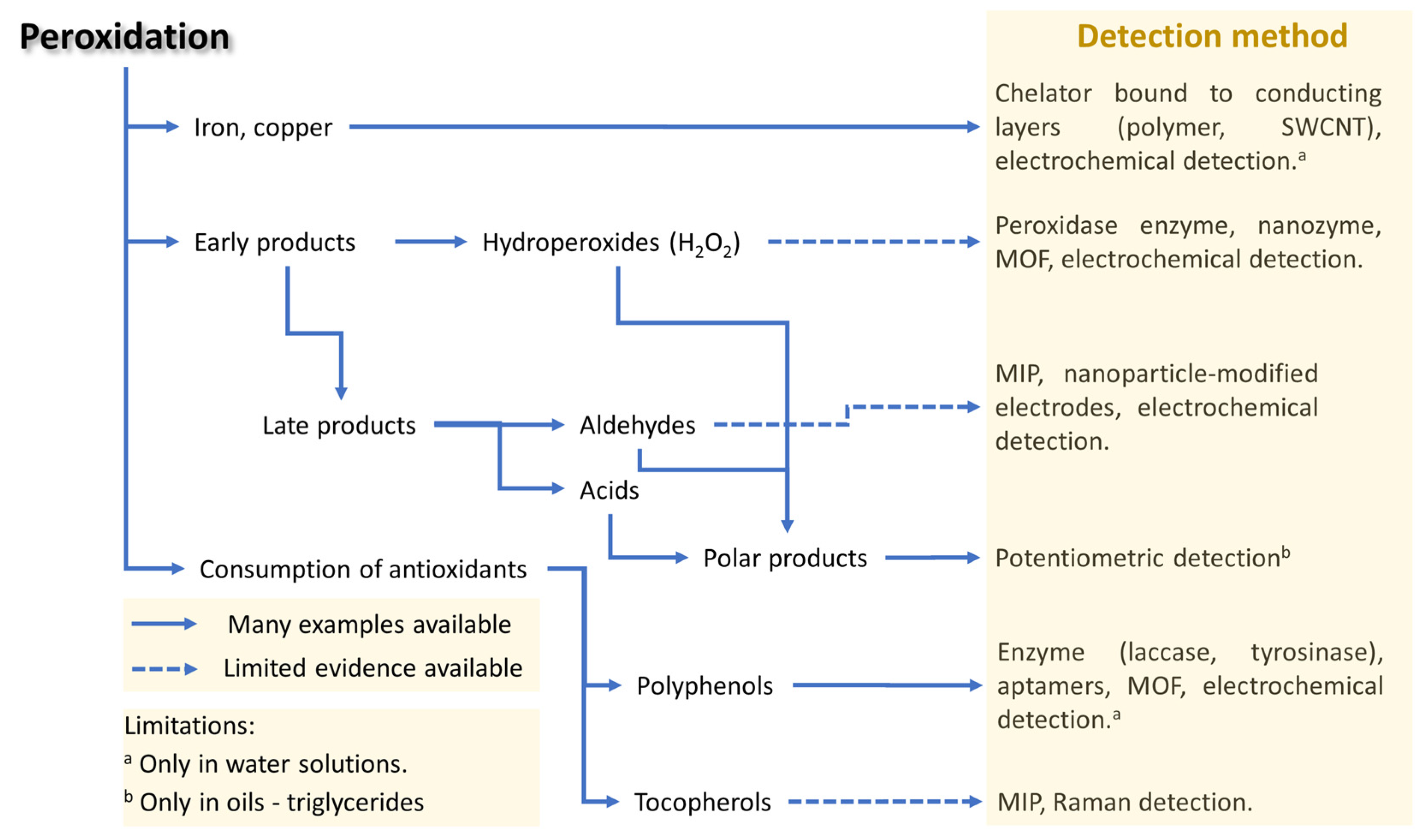
4.3. Peroxidation Analysis
4.3.1. Hydroperoxides
4.3.2. Aldehydes
4.3.3. Volatile Aldehydes (VA) and Protein Carbonyls (PC)
4.3.4. Metals
4.3.5. Polar Compounds
5. Overview of Biosensors Applications
Examples of Applications in Food: Milk and Plant-Based Milk Alternatives
6. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Antioxidants: Reviewing the Chemistry, Food Applications, Legislation and Role as Preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Advantages and Limitations of Common Testing Methods for Antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Ghelichi, S.; Hajfathalian, M.; Yesiltas, B.; Sørensen, A.M.; García-Moreno, P.J.; Jacobsen, C. Oxidation and Oxidative Stability in Emulsions. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1864–1901. [Google Scholar] [CrossRef] [PubMed]
- Suhag, R.; Ferrentino, G.; Morozova, K.; Zatelli, D.; Scampicchio, M.; Amorati, R. Antioxidant Efficiency and Oxidizability of Mayonnaise by Oximetry and Isothermal Calorimetry. Food Chem. 2024, 433, 137274. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, L. Lipid Peroxidation and Antioxidant Protection. Biomolecules 2023, 13, 1291. [Google Scholar] [CrossRef] [PubMed]
- Helberg, J.; Pratt, D.A. Autoxidation vs. Antioxidants—The Fight for Forever. Chem. Soc. Rev. 2021, 50, 7343–7358. [Google Scholar] [CrossRef]
- Mollica, F.; Bonoldi, L.; Amorati, R. Kinetic Analysis of High-Temperature Sunflower Oil Peroxidation Inhibited by the Major Families of Phenolic Antioxidants Unveils the Extraordinary Activity of 1,4-Hydroquinones. Antioxidants 2022, 11, 2142. [Google Scholar] [CrossRef]
- Lopes, C.R.B.; Courrol, L.C. Evaluation of Steady-State and Time-Resolved Fluorescence Spectroscopy as a Method for Assessing the Impact of Photo-Oxidation on Refined Soybean Oils. Foods 2023, 12, 1862. [Google Scholar] [CrossRef]
- Mallais, M.; Hanson, C.S.; Giray, M.; Pratt, D.A. General Approach to Identify, Assess, and Characterize Inhibitors of Lipid Peroxidation and Associated Cell Death. ACS Chem. Biol. 2023, 18, 561–571. [Google Scholar] [CrossRef]
- Scurti, S.; Caretti, D.; Mollica, F.; Di Antonio, E.; Amorati, R. Chain-Breaking Antioxidant and Peroxyl Radical Trapping Activity of Phenol-Coated Magnetic Iron Oxide Nanoparticles. Antioxidants 2022, 11, 1163. [Google Scholar] [CrossRef]
- Baschieri, A.; Jin, Z.; Amorati, R. Hydroperoxyl Radical (HOO•) as a Reducing Agent: Unexpected Synergy with Antioxidants. A Review. Free Radic. Res. 2023, 57, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, Z.A.M.; Pratt, D.A. Lipid Peroxidation: Kinetics, Mechanisms, and Products. J. Org. Chem. 2017, 82, 2817–2825. [Google Scholar] [CrossRef] [PubMed]
- Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Analysis of the Efficiency of Antioxidants in Inhibiting Lipid Oxidation in Terms of Characteristic Kinetic Parameters. Antioxidants 2024, 13, 593. [Google Scholar] [CrossRef]
- Saraev, D.D.; Wu, Z.; Kim, H.-Y.H.; Porter, N.A.; Pratt, D.A. Intramolecular H-Atom Transfers in Alkoxyl Radical Intermediates Underlie the Apparent Oxidation of Lipid Hydroperoxides by Fe(II). ACS Chem. Biol. 2023, 18, 2073–2081. [Google Scholar] [CrossRef]
- Caño-Ochoa, S.D.; Ruiz-Aracama, A.; Guillén, M.D. Individual and Joint Effect of Alpha-Tocopherol and Hydroxytyrosol Acetate on the Oxidation of Sunflower Oil Submitted to Oxidative Conditions: A Study by Proton Nuclear Magnetic Resonance. Antioxidants 2022, 11, 1156. [Google Scholar] [CrossRef]
- Pignoli, G.; Bou, R.; Rodriguez-Estrada, M.T.; Decker, E.A. Suitability of Saturated Aldehydes as Lipid Oxidation Markers in Washed Turkey Meat. Meat Sci. 2009, 83, 412–416. [Google Scholar] [CrossRef]
- Ten Klooster, S.; Takeuchi, M.; Schroën, K.; Tuinier, R.; Joosten, R.; Friedrich, H.; Berton-Carabin, C. Tiny, yet Impactful: Detection and Oxidative Stability of Very Small Oil Droplets in Surfactant-Stabilized Emulsions. J. Colloid Interface Sci. 2023, 652, 1994–2004. [Google Scholar] [CrossRef]
- Zielinski, Z.A.M.; Pratt, D.A. H-Atom Abstraction vs Addition: Accounting for the Diverse Product Distribution in the Autoxidation of Cholesterol and Its Esters. J. Am. Chem. Soc. 2019, 141, 3037–3051. [Google Scholar] [CrossRef] [PubMed]
- Grebenteuch, S.; Kroh, L.W.; Drusch, S.; Rohn, S. Formation of Secondary and Tertiary Volatile Compounds Resulting from the Lipid Oxidation of Rapeseed Oil. Foods 2021, 10, 2417. [Google Scholar] [CrossRef]
- Barani, M.; Bonetti, R.; Parker, W.O. Thermal Oxidation of Model Molecules to Reveal Vegetable Oil Polymerization Studied by NMR Spectroscopy and Self-Diffusion. J. Am. Oil Chem. Soc. 2023, 100, 551–560. [Google Scholar] [CrossRef]
- Flitsch, S.; Neu, P.M.; Schober, S.; Kienzl, N.; Ullmann, J.; Mittelbach, M. Quantitation of Aging Products Formed in Biodiesel during the Rancimat Accelerated Oxidation Test. Energy Fuels 2014, 28, 5849–5856. [Google Scholar] [CrossRef]
- Mavridi-Printezi, A.; Menichetti, A.; Mordini, D.; Amorati, R.; Montalti, M. Recent Applications of Melanin-like Nanoparticles as Antioxidant Agents. Antioxidants 2023, 12, 863. [Google Scholar] [CrossRef]
- Pei, Y.; Deng, Q.; McClements, D.J.; Li, J.; Li, B. Impact of Phytic Acid on the Physical and Oxidative Stability of Protein-Stabilized Oil-in-Water Emulsions. Food Biophys. 2020, 15, 433–441. [Google Scholar] [CrossRef]
- Daoud, S.; Bou-Maroun, E.; Waschatko, G.; Cayot, P. Lipid Oxidation in Oil-in-Water Emulsions: Iron Complexation by Buffer Ions and Transfer on the Interface as a Possible Mechanism. Food Chem. 2021, 342, 128273. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and Polyphenols as Natural Antioxidant Agents in Food Preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef]
- Baschieri, A.; Amorati, R. Methods to Determine Chain-Breaking Antioxidant Activity of Nanomaterials beyond DPPH•. A Review. Antioxidants 2021, 10, 1551. [Google Scholar] [CrossRef]
- Lozano-Castellón, J.; Rinaldi De Alvarenga, J.F.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Cooking with Extra-Virgin Olive Oil: A Mixture of Food Components to Prevent Oxidation and Degradation. Trends Food Sci. Technol. 2022, 123, 28–36. [Google Scholar] [CrossRef]
- Shah, R.; Farmer, L.A.; Zilka, O.; Van Kessel, A.T.M.; Pratt, D.A. Beyond DPPH: Use of Fluorescence-Enabled Inhibited Autoxidation to Predict Oxidative Cell Death Rescue. Cell Chem. Biol. 2019, 26, 1594–1607.e7. [Google Scholar] [CrossRef]
- Li, J.; Yuan, F.; Teng, J.; Li, F.; Zhou, P.; Bi, Y. Effects of Tea Polyphenols and Tertiary Butylhydroquinone on Quality of Palm Oils and Losses of Endogenous Vitamin E during Batch Frying and Oxidative Stability of Fried Instant Noodles. Food Chem. X 2023, 20, 101049. [Google Scholar] [CrossRef]
- Bayram, I.; Parra-Escudero, C.; Decker, E.A.; Lu, J. Mathematical Modeling of Alpha-Tocopherol Early Degradation Kinetics to Predict the Shelf-Life of Bulk Oils. J. Agric. Food Chem. 2024, 72, 4939–4946. [Google Scholar] [CrossRef]
- Nagel, B.; Dellweg, H.; Gierasch, L.M. Glossary for Chemists of Terms Used in Biotechnology (IUPAC Recommendations 1992). Pure Appl. Chem. 1992, 64, 143–168. [Google Scholar] [CrossRef]
- Lobnik, A.; Turel, M.; Urek, Š.K.; Košak, A. Nanostructured Materials Use in Sensors: Their Benefits and Drawbacks. In Carbon and Oxide Nanostructures; Advanced Structured Materials; Springer: Berlin/Heidelberg, Germany, 2010; Volume 5, pp. 307–354. ISBN 978-3-642-14672-5. [Google Scholar]
- Clark, L.C.; Lyons, C. Electrode Systems for Continuous Monitoring in Cardiovascular Surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Guilbault, G.G.; Lubrano, G.J. An Enzyme Electrode for the Amperometric Determination of Glucose. Anal. Chim. Acta 1973, 64, 439–455. [Google Scholar] [CrossRef]
- Kalinke, C.; De Oliveira, P.R.; Marcolino-Júnior, L.H.; Bergamini, M.F. Nanostructures of Prussian Blue Supported on Activated Biochar for the Development of a Glucose Biosensor. Talanta 2024, 274, 126042. [Google Scholar] [CrossRef]
- Li, Z.; Cao, L.; Sui, J.; Wang, L.; Lin, H.; Wang, K. Bimetallic Fe/Ni Metal Organic Framework-Based Hypoxanthine Biosensor for Early Monitoring of Freshness Changes of Aquatic Products. Food Chem. 2024, 447, 138902. [Google Scholar] [CrossRef]
- Mohtar, L.G.; Aranda, P.; Messina, G.A.; Nazareno, M.A.; Pereira, S.V.; Raba, J.; Bertolino, F.A. Amperometric Biosensor Based on Laccase Immobilized onto a Nanostructured Screen-Printed Electrode for Determination of Polyphenols in Propolis. Microchem. J. 2019, 144, 13–18. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, J.; Liu, Z.; Chu, Z.; Jin, W. Nanomaterial-Based Electrochemical Enzymatic Biosensors for Recognizing Phenolic Compounds in Aqueous Effluents. Environ. Res. 2022, 214, 113858. [Google Scholar] [CrossRef]
- Bi, R.; Ma, X.; Miao, K.; Ma, P.; Wang, Q. Enzymatic Biosensor Based on Dendritic Gold Nanostructure and Enzyme Precipitation Coating for Glucose Sensing and Detection. Enzyme Microb. Technol. 2023, 162, 110132. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Urbiola, I.R.; Reséndiz-Jaramillo, A.Y.; Willars-Rodriguez, F.J.; Martinez-Saucedo, G.; Arriaga, L.G.; Alcantar-Peña, J.; Escalona-Villalpando, R.A.; Ledesma-García, J. Glucose Biosensor Based on a Flexible Au/ZnO Film to Enhance the Glucose Oxidase Catalytic Response. J. Electroanal. Chem. 2022, 926, 116941. [Google Scholar] [CrossRef]
- Estrada-Osorio, D.V.; Escalona-Villalpando, R.A.; Gutiérrez, A.; Arriaga, L.G.; Ledesma-García, J. Poly-L-Lysine-Modified with Ferrocene to Obtain a Redox Polymer for Mediated Glucose Biosensor Application. Bioelectrochemistry 2022, 146, 108147. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Yang, T.; Zhang, Y.; Tao, D.; Hasebe, Y.; Zhang, Z. Electrochemical Evaluation of Sulfide Mineral Modified Glassy Carbon Electrode as Novel Mediated Glucose Biosensor. J. Electroanal. Chem. 2021, 894, 115357. [Google Scholar] [CrossRef]
- Jung, J.; Lim, S. ZnO Nanowire-Based Glucose Biosensors with Different Coupling Agents. Appl. Surf. Sci. 2013, 265, 24–29. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Q.; Guan, Q.-M.; Wu, J.; Li, H.-N.; Yan, J.-J. Enhanced Direct Electrochemistry of Glucose Oxidase and Biosensing for Glucose via Synergy Effect of Graphene and CdS Nanocrystals. Biosens. Bioelectron. 2011, 26, 2252–2257. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, L.; Li, M.; Xu, S.; Gao, F. Multifunctional Carbon Nanotubes for Direct Electrochemistry of Glucose Oxidase and Glucose Bioassay. Biosens. Bioelectron. 2011, 30, 107–111. [Google Scholar] [CrossRef]
- Liang, M.; Yan, X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef]
- Li, P.; Kalambate, P.K.; Harris, K.D.; Jemere, A.B.; Tang, X. (Shirley) Robust and Flexible Electrochemical Lactate Sensors for Sweat Analysis Based on Nanozyme-Enhanced Electrode. Biosens. Bioelectron. X 2024, 17, 100455. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, Y.; Zhao, P.; Chen, Y.; Lei, J.; Hou, J.; Hou, C.; Huo, D. An Electrochemical Sensor Based on FeCo Bimetallic Single-Atom Nanozyme for Sensitive Detection of H2O2. Anal. Chim. Acta 2023, 1281, 341867. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Z.; Guan, J. Single-Atom Nanozyme-Based Electrochemical Sensors for Health and Food Safety Monitoring. Food Chem. 2023, 425, 136518. [Google Scholar] [CrossRef]
- Wu, X.; Chen, J.; Wu, M.; Zhao, J.X. Aptamers: Active Targeting Ligands for Cancer Diagnosis and Therapy. Theranostics 2015, 5, 322–344. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, Y.; Xu, H.; Lu, X.; Yuan, Y.; Zhang, W. A Ratiometric Electrochemical Aptasensor for Sensitive Detection of Kanamycin in Food Based on Entropy-Driven Strand Displacement Reaction. Food Control 2024, 161, 110390. [Google Scholar] [CrossRef]
- Khoshbin, Z.; Moeenfard, M.; Abnous, K.; Taghdisi, S.M. A Label-Free Aptasensor for Colorimetric Detection of Food Toxin: Mediation of Catalytically Active Gold Nanozymes and Smartphone Imaging Strategy. Food Chem. 2024, 433, 137355. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Roy, S.; Israel Shaikh, N.; Malave, P.; Mishra, A.; Anish Alam, M.; Ghorpade, Y.; Rahil Hasan, M.; Nizam, A. Recent Advances in Multiplex Aptasensor Detection Techniques for Food-Borne Pathogens: A Comprehensive Review of Novel Approaches. Biosens. Bioelectron. X 2024, 16, 100417. [Google Scholar] [CrossRef]
- Figueroa-Miranda, G.; Chen, S.; Neis, M.; Zhou, L.; Zhang, Y.; Lo, Y.; Tanner, J.A.; Kreidenweiss, A.; Offenhäusser, A.; Mayer, D. Multi-Target Electrochemical Malaria Aptasensor on Flexible Multielectrode Arrays for Detection in Malaria Parasite Blood Samples. Sens. Actuators B Chem. 2021, 349, 130812. [Google Scholar] [CrossRef]
- Hamdi, F.; Roushani, M.; Nasibipour, M.; Hoseini, S.J. Aptasensor Based on High Surface Area Covalent Organic Framework for Simple and Ultrasensitive Detection of Sarcosine in the Diagnosis of Prostate Cancer. Anal. Chim. Acta 2024, 1291, 342235. [Google Scholar] [CrossRef]
- Tseng, C.-C.; Lu, S.-Y.; Chen, S.-J.; Wang, J.-M.; Fu, L.-M.; Wu, Y.-H. Microfluidic Aptasensor POC Device for Determination of Whole Blood Potassium. Anal. Chim. Acta 2022, 1203, 339722. [Google Scholar] [CrossRef]
- Lee, K.; Ha, S.M.; Gurudatt, N.G.; Heo, W.; Hyun, K.-A.; Kim, J.; Jung, H.-I. Machine Learning-Powered Electrochemical Aptasensor for Simultaneous Monitoring of Di(2-Ethylhexyl) Phthalate and Bisphenol A in Variable pH Environments. J. Hazard. Mater. 2024, 462, 132775. [Google Scholar] [CrossRef]
- Tian, C.; Zhao, L.; Zhu, J.; Zhang, S. Ultrasensitive Detection of Trace Hg2+ by SERS Aptasensor Based on Dual Recycling Amplification in Water Environment. J. Hazard. Mater. 2021, 416, 126251. [Google Scholar] [CrossRef]
- Wang, X.; Xu, M.; Kuang, Y.; Liu, X.; Yuan, J. A Novel Ratiometric Electrochemical Aptasensor Based on M-Shaped Functional DNA Complexes for Simultaneous Detection of Trace Lead and Mercury Ions in Series Aquatic Edible Vegetables. J. Hazard. Mater. 2024, 465, 133169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Luan, Y.; Ru, S.; Teng, H.; Li, Y.; Liu, M.; Wang, J. A Novel Electrochemical Aptasensor for Ultrasensitive Detection of Herbicide Prometryn Based on Its Highly Specific Aptamer and Ag@Au Nanoflowers. Talanta 2023, 265, 124838. [Google Scholar] [CrossRef]
- Köhler, G.; Milstein, C. Continuous Cultures of Fused Cells Secreting Antibody of Predefined Specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, Y.; Li, Y.; Lv, J.; Chen, H. Electrochemical Detection of Lactobacillus Rhamnosus in Fermented Food Using Magnetic Immunosensor Based on Au-Fe3O4. Int. J. Electrochem. Sci. 2022, 17, 220329. [Google Scholar] [CrossRef]
- Chomthong, K.; Kunpatee, K.; Pimpitak, U.; Puthong, S.; Komolpis, K.; Wonsawat, W.; Nuanualsuwan, S.; Yakoh, A.; Khongchareonporn, N.; Ruecha, N.; et al. Label-Free Simultaneous Detection of Quinolone Antibiotic Residues Using an Origami Paper–Based Electrochemical Immunosensor. Sens. Actuators B Chem. 2024, 410, 135667. [Google Scholar] [CrossRef]
- Hu, J.; Wen, P.; Wang, Y.; Yang, J.; Xiao, Z.; Xu, Z.; Shen, Y.; Wang, H.; Hammock, B.D. Fabrication of a Label-Free Electrochemical Immunosensor by Functionalized Nanofiber Membrane for the Ultrasensitive Detection of Quinalphos. Food Control 2024, 162, 110423. [Google Scholar] [CrossRef]
- Subjakova, V.; Oravczova, V.; Tatarko, M.; Hianik, T. Advances in Electrochemical Aptasensors and Immunosensors for Detection of Bacterial Pathogens in Food. Electrochim. Acta 2021, 389, 138724. [Google Scholar] [CrossRef]
- Wu, C.-C.; Lin, C.-C.; Yin, H.-Y.; Tsai, W.-C.; Yang, P.-F.; Liu, H.-J.; Wen, H.-W. Rapid Detection of Mango Allergen in Processed Foods Using an Immunomagnetic Nanoparticle-Based Electrochemical Immunosensor. Microchem. J. 2024, 198, 110070. [Google Scholar] [CrossRef]
- Ayerdurai, V.; Cieplak, M.; Kutner, W. Molecularly Imprinted Polymer-Based Electrochemical Sensors for Food Contaminants Determination. TrAC Trends Anal. Chem. 2023, 158, 116830. [Google Scholar] [CrossRef]
- Ni, X.; Tang, X.; Wang, D.; Zhang, J.; Zhao, L.; Gao, J.; He, H.; Dramou, P. Research Progress of Sensors Based on Molecularly Imprinted Polymers in Analytical and Biomedical Analysis. J. Pharm. Biomed. Anal. 2023, 235, 115659. [Google Scholar] [CrossRef]
- Sundhoro, M.; Agnihotra, S.R.; Amberger, B.; Augustus, K.; Khan, N.D.; Barnes, A.; BelBruno, J.; Mendecki, L. An Electrochemical Molecularly Imprinted Polymer Sensor for Rapid and Selective Food Allergen Detection. Food Chem. 2021, 344, 128648. [Google Scholar] [CrossRef]
- Meng, F.; Duan, M.; Wu, W.; Shao, S.; Qin, Y.; Zhang, M. Enzymatic Construction Au NPs-rGO Based MIP Electrochemical Sensor for Adulteration Detection of Bovine-Derived Allergen in Camel Milk. Food Chem. 2024, 436, 137638. [Google Scholar] [CrossRef]
- Lu, Z.; Wei, K.; Ma, H.; Xiong, Q.; Li, Y.; Sun, M.; Wang, X.; Wang, Y.; Wu, C.; Su, G.; et al. Nanoarchitectonics of on–off Ratiometric Signal Amplified Electrochemical Sensor for Chlorpromazine with Molecularly Imprinted Polymer Based on Ni-MOF/Fe-MOF-5 Hybrid Au Nanoparticles. Sep. Purif. Technol. 2023, 327, 124858. [Google Scholar] [CrossRef]
- Sun, R.; Han, S.; Zong, W.; Chu, H.; Zhang, X.; Jiang, H. Ultrasensitive Detection of Chlortetracycline in Animal-Origin Food Using Molecularly Imprinted Electrochemical Sensor Based on SnS2/ZnCo-MOF and AuNPs. Food Chem. 2024, 452, 139537. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, H.; Gopinath, S.C.B.; Arshad, M.K.M.; Zulhaimi, H.I.; Anbu, P.; Subramaniam, S. Molecularly Imprinted Polymer Enhances Affinity and Stability over Conventional Aptasensor for Blood Clotting Biomarker Detection on Regimented Carbon Nanohorn and Gold Nanourchin Hybrid Layers. Sens. Actuators B Chem. 2022, 363, 131842. [Google Scholar] [CrossRef]
- Atta, E.M.; Mohamed, N.H.; Abdelgawad, A.A.M. Antioxidants: An Overview on the Natural and Synthetic Types. Eur. Chem. Bull. 2017, 6, 365. [Google Scholar] [CrossRef]
- Poljsak, B.; Kovač, V.; Milisav, I. Antioxidants, Food Processing and Health. Antioxidants 2021, 10, 433. [Google Scholar] [CrossRef] [PubMed]
- Arab, Z.; Jafarian, S.; Karimi-Maleh, H.; Roozbeh Nasiraie, L.; Ahmadi, M. Monitoring of Butylated Hydroxyanisole in Food and Wastewater Samples Using Electroanalytical Two-Fold Amplified Sensor. Sustainability 2022, 14, 2169. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Lorenzo, J.M.; Franco, D. Advances in Natural Antioxidants for Food Improvement. Antioxidants 2022, 11, 1825. [Google Scholar] [CrossRef]
- Cannavacciuolo, C.; Pagliari, S.; Giustra, C.M.; Carabetta, S.; Guidi Nissim, W.; Russo, M.; Branduardi, P.; Labra, M.; Campone, L. LC-MS and GC-MS Data Fusion Metabolomics Profiling Coupled with Multivariate Analysis for the Discrimination of Different Parts of Faustrime Fruit and Evaluation of Their Antioxidant Activity. Antioxidants 2023, 12, 565. [Google Scholar] [CrossRef]
- Hasan, M.R.; Haque, M.M.; Hoque, M.A.; Sultana, S.; Rahman, M.M.; Ali Shaikh, M.A.; Sarker, M.K.U. Antioxidant Activity Study and GC-MS Profiling of Camellia Sinensis Linn. Heliyon 2024, 10, e23514. [Google Scholar] [CrossRef]
- Du, J.; Zhong, B.; Subbiah, V.; Barrow, C.; Dunshea, F.; Suleria, H. LC-ESI-QTOF-MS/MS Profiling and Antioxidant Activity of Phenolics from Custard Apple Fruit and By-Products. Separations 2021, 8, 62. [Google Scholar] [CrossRef]
- Hamasaki, T.; Kashiwagi, T.; Komatsu, T.; Kabayama, S.; Nakamichi, N.; Teruya, K.; Shirahata, S. A New Colorimetric Method for Determining Antioxidant Levels Using 3,5-Dibromo-4-Nitrosobenzene Sulfonate (DBNBS). MethodsX 2022, 9, 101797. [Google Scholar] [CrossRef]
- Alam, M.W.; Najeeb, J.; Naeem, S.; Usman, S.M.; Nahvi, I.; Alismail, F.; Abuzir, A.; Farhan, M.; Nawaz, A. Electrochemical Methodologies for Investigating the Antioxidant Potential of Plant and Fruit Extracts: A Review. Antioxidants 2022, 11, 1205. [Google Scholar] [CrossRef] [PubMed]
- David, I.G.; Iorgulescu, E.E.; Popa, D.E.; Buleandra, M.; Cheregi, M.C.; Noor, H. Curcumin Electrochemistry—Antioxidant Activity Assessment, Voltammetric Behavior and Quantitative Determination, Applications as Electrode Modifier. Antioxidants 2023, 12, 1908. [Google Scholar] [CrossRef] [PubMed]
- Forzato, C.; Vida, V.; Berti, F. Biosensors and Sensing Systems for Rapid Analysis of Phenolic Compounds from Plants: A Comprehensive Review. Biosensors 2020, 10, 105. [Google Scholar] [CrossRef]
- Lzaod, S.; Dutta, T. Recent Advances in the Development of Oxidoreductase-Based Biosensors for Detection of Phenolic Antioxidants in Food and Beverages. ACS Omega 2022, 7, 47434–47448. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Silva, T.A.; Caetano, F.R.; Ribovski, L.; Zapp, E.; Brondani, D.; Bergamini, M.F.; Marcolino, L.H.; Banks, C.E.; Oliveira, O.N.; et al. Polyphenol Oxidase-Based Electrochemical Biosensors: A Review. Anal. Chim. Acta 2020, 1139, 198–221. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, A.; Stozhko, N.; Bukharinova, M.; Khamzina, E. Biosensors Based on Phenol Oxidases (Laccase, Tyrosinase, and Their Mixture) for Estimating the Total Phenolic Index in Food-Related Samples. Life 2023, 13, 291. [Google Scholar] [CrossRef]
- Wijayanti, S.D.; Tsvik, L.; Haltrich, D. Recent Advances in Electrochemical Enzyme-Based Biosensors for Food and Beverage Analysis. Foods 2023, 12, 3355. [Google Scholar] [CrossRef]
- Satchanska, G. Antibacterial Activity of Plant Polyphenols. In Secondary Metabolites—Trends and Reviews; Vijayakumar, R., Selvapuram Sudalaimuthu Raja, S., Eds.; IntechOpen: London, UK, 2022; ISBN 978-1-80355-207-1. [Google Scholar]
- Cerrato-Alvarez, M.; Bernalte, E.; Bernalte-García, M.J.; Pinilla-Gil, E. Fast and Direct Amperometric Analysis of Polyphenols in Beers Using Tyrosinase-Modified Screen-Printed Gold Nanoparticles Biosensors. Talanta 2019, 193, 93–99. [Google Scholar] [CrossRef]
- David, I.G.; Popa, D.E.; Buleandră, M.; Cheregi, M.C. Electrochemical Methods and (Bio) Sensors for Rosmarinic Acid Investigation. Chemosensors 2020, 8, 74. [Google Scholar] [CrossRef]
- García-Guzmán, J.; López-Iglesias, D.; Cubillana-Aguilera, L.; Lete, C.; Lupu, S.; Palacios-Santander, J.; Bellido-Milla, D. Assessment of the Polyphenol Indices and Antioxidant Capacity for Beers and Wines Using a Tyrosinase-Based Biosensor Prepared by Sinusoidal Current Method. Sensors 2018, 19, 66. [Google Scholar] [CrossRef]
- Kadam, A.A.; Saratale, G.D.; Ghodake, G.S.; Saratale, R.G.; Shahzad, A.; Magotra, V.K.; Kumar, M.; Palem, R.R.; Sung, J.-S. Recent Advances in the Development of Laccase-Based Biosensors via Nano-Immobilization Techniques. Chemosensors 2022, 10, 58. [Google Scholar] [CrossRef]
- Boubezari, I.; Bessueille, F.; Bonhomme, A.; Raimondi, G.; Zazoua, A.; Errachid, A.; Jaffrezic-Renault, N. Laccase-Based Biosensor Encapsulated in a Galactomannan-Chitosan Composite for the Evaluation of Phenolic Compounds. Biosensors 2020, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Salamanca-Neto, C.A.R.; Marcheafave, G.G.; Scremin, J.; Barbosa, E.C.M.; Camargo, P.H.C.; Dekker, R.F.H.; Scarminio, I.S.; Barbosa-Dekker, A.M.; Sartori, E.R. Chemometric-Assisted Construction of a Biosensing Device to Measure Chlorogenic Acid Content in Brewed Coffee Beverages to Discriminate Quality. Food Chem. 2020, 315, 126306. [Google Scholar] [CrossRef]
- Zrinski, I.; Pungjunun, K.; Martinez, S.; Zavašnik, J.; Stanković, D.; Kalcher, K.; Mehmeti, E. Evaluation of Phenolic Antioxidant Capacity in Beverages Based on Laccase Immobilized on Screen-Printed Carbon Electrode Modified with Graphene Nanoplatelets and Gold Nanoparticles. Microchem. J. 2020, 152, 104282. [Google Scholar] [CrossRef]
- Mediavilla, M.; Revenga-Parra, M.; Gutiérrez-Sánchez, C.; Hernández-Apaolaza, L.; Pariente, F.; Lorenzo, E. Fluorescent Enzymatic Assay for Direct Total Polyphenol Determination in Food-Related Samples. Talanta 2022, 247, 123576. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Mattos, G.J.; Coldibeli, B.; Dekker, R.F.H.; Barbosa Dekker, A.M.; Sartori, E.R. Covalent Attachment of Laccase to Carboxymethyl-Botryosphaeran in Aqueous Solution for the Construction of a Voltammetric Biosensor to Quantify Quercetin. Bioelectrochemistry 2020, 135, 107543. [Google Scholar] [CrossRef]
- Apetrei, R.-M.; Cârâc, G.; Bahrim, G.; Camurlu, P. Utilization of Enzyme Extract Self-Encapsulated within Polypyrrole in Sensitive Detection of Catechol. Enzyme Microb. Technol. 2019, 128, 34–39. [Google Scholar] [CrossRef]
- Batista, É.A.; Silva, G.N.M.; Sgobbi, L.F.; Machado, F.B.; Macedo, I.Y.; Moreno, E.K.; Neto, J.R.; Scalize, P.S.; Gil, E.S. Enzymatic Electroanalytical Biosensor Based on Maramiellus Colocasiae Fungus for Detection of Phytomarkers in Infusions and Green Tea Kombucha. Biosensors 2021, 11, 91. [Google Scholar] [CrossRef]
- Yoon, M.; Srirambalaji, R.; Kim, K. Homochiral Metal–Organic Frameworks for Asymmetric Heterogeneous Catalysis. Chem. Rev. 2012, 112, 1196–1231. [Google Scholar] [CrossRef]
- Li, C.; Shen, J.; Wu, K.; Yang, N. Metal Centers and Organic Ligands Determine Electrochemistry of Metal–Organic Frameworks. Small 2022, 18, 2106607. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Wang, C.; Zhang, L.; Yang, Y.; Chen, C.; Xie, Y.; Zhao, P.; Fei, J. An Electrochemical Sensor Based on Ce-MOF-Derived Ce-Doped Poly(3,4-Ethylenedioxythiophene) Composite for Efficient Determination of Rutin in Food. Talanta 2023, 263, 124678. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fan, J.; He, F.; Li, X.; Tang, T.; Cheng, H.; Li, L.; Hu, G. Glycosyl/MOF-5-Based Carbon Nanofibers for Highly Sensitive Detection of Anti-Bacterial Drug Quercetin. Surf. Interfaces 2021, 27, 101488. [Google Scholar] [CrossRef]
- Zhao, P.; Huang, L.; Wang, H.; Wang, C.; Chen, J.; Yang, P.; Ni, M.; Chen, C.; Li, C.; Xie, Y.; et al. An Ultrasensitive High-Performance Baicalin Sensor Based on C3N4-SWCNTs/Reduced Graphene Oxide/Cyclodextrin Metal-Organic Framework Nanocomposite. Sens. Actuators B Chem. 2022, 350, 130853. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hassan, M.; Bo, X.; Guo, L. Fumarate-Based Metal-Organic Framework/Mesoporous Carbon as a Novel Electrochemical Sensor for the Detection of Gallic Acid and Luteolin. J. Electroanal. Chem. 2019, 849, 113378. [Google Scholar] [CrossRef]
- Tan, Q.; Chen, C.; Lin, C.; Zhang, J.; Liu, S.; Zhang, J. Highly Sensitive Detection of Kaempferol Using Electrochemical Sensors Based on 3D-Ordered Mesh Interconnect C60-GO, Ni-MOF, and β-Cyclodextrin. Microchem. J. 2024, 197, 109866. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Wang, C.; Tang, J.; Chen, X.; Li, Y.; Shi, J.; Zhao, P.; Xie, Y.; Fei, J. A Novel Kaempferol Electrochemical Sensor Based on Glass Carbon Electrode Modified by Poly (3, 4-Ethylenedioxythiophene) Decorated with Green Synthesized MIL-100(Fe)-Multi- Walled Carbon Nanotubes Composites. Colloids Surf. Physicochem. Eng. Asp. 2022, 649, 129484. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, C.; Zhao, P.; Zhang, L.; Fei, J.; Xie, Y. A Novel Catechin Electrochemical Sensor Based on a Two-Dimensional MOFs Material Derivative Zn Doped Carbon Nanosheets and Multi-Walled Carbon Nanotubes Composite Film. Talanta 2022, 246, 123520. [Google Scholar] [CrossRef]
- Li, J.; Zhu, D.; Huang, H.; Xie, S.; Xu, J.; Yue, R.; Duan, X. High-Efficient Electrochemical Sensing Platform Based on MOF-Doped Au/PEDOT Composites toward Simultaneous Detection of Catechin and Sunset Yellow in Tea Beverage. Electrochim. Acta 2023, 462, 142732. [Google Scholar] [CrossRef]
- Sivasankar, K.; Devasenathipathy, R.; Wang, S.-F.; Kohila Rani, K.; Raja, D.S.; Lin, C.-H. Synthesis of Hierarchical Mesoporous Graphite Oxide/Al2O3 from MIL-100(Al) for the Electrochemical Determination of Caffeic Acid in Red Wine Samples. J. Taiwan Inst. Chem. Eng. 2018, 84, 188–195. [Google Scholar] [CrossRef]
- Venkadesh, A.; Mathiyarasu, J.; Radhakrishnan, S. MOF Mediated Synthesis of Porous Copper Oxide and Their Electrochemical Sensing of Caffeic Acid in Caffeinated Drinks. Inorg. Chem. Commun. 2021, 128, 108573. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Kurbanoglu, S.; Asadpour-Zeynali, K.; Ozkan, S.A. Preparation of Porous Cu Metal Organic Framework/ZnTe Nanorods/Au Nanoparticles Hybrid Platform for Nonenzymatic Determination of Catechol. J. Electroanal. Chem. 2020, 856, 113672. [Google Scholar] [CrossRef]
- Yan, Y.; Bo, X.; Guo, L. MOF-818 Metal-Organic Framework-Reduced Graphene Oxide/Multiwalled Carbon Nanotubes Composite for Electrochemical Sensitive Detection of Phenolic Acids. Talanta 2020, 218, 121123. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, X.; Bo, X.; Zhou, M.; Guo, L. Nickel-Based Metal-Organic Framework/Crosslinked Tubular Poly(3,4-ethylenedioxythiophene) Composite as an Electrocatalyst for the Detection of Gallic Acid and Tinidazole. ChemElectroChem 2020, 7, 4031–4037. [Google Scholar] [CrossRef]
- Şenocak, A. Fast, Simple and Sensitive Determination of Coumaric Acid in Fruit Juice Samples by Magnetite Nanoparticles-zeolitic Imidazolate Framework Material. Electroanalysis 2020, 32, 2330–2339. [Google Scholar] [CrossRef]
- Shen, T.; Liu, T.; Mo, H.; Yuan, Z.; Cui, F.; Jin, Y.; Chen, X. Cu-Based Metal–Organic Framework HKUST-1 as Effective Catalyst for Highly Sensitive Determination of Ascorbic Acid. RSC Adv. 2020, 10, 22881–22890. [Google Scholar] [CrossRef]
- Wu, X.-Q.; Ma, J.-G.; Li, H.; Chen, D.-M.; Gu, W.; Yang, G.-M.; Cheng, P. Metal–Organic Framework Biosensor with High Stability and Selectivity in a Bio-Mimic Environment. Chem. Commun. 2015, 51, 9161–9164. [Google Scholar] [CrossRef]
- Yi, Y.; Wu, S.; Luo, H.; He, L.; Yang, Y.; Xue, T.; Xu, J.; Wen, Y.; Wang, P. Soft Template Assisted Hydrothermal Synthesis of Phosphorus Doped Porous Carbon Spheres with Tunable Microstructure as Electrochemical Nanozyme Sensor for Distinguishable Detection of Two Flavonoids Coupled with Derivative Voltammetry. J. Electroanal. Chem. 2021, 897, 115563. [Google Scholar] [CrossRef]
- Facure, M.H.M.; Andre, R.S.; Cardoso, R.M.; Mercante, L.A.; Correa, D.S. Electrochemical and Optical Dual-Mode Detection of Phenolic Compounds Using MnO2/GQD Nanozyme. Electrochim. Acta 2023, 441, 141777. [Google Scholar] [CrossRef]
- Niu, Y.; Kang, K.; Wang, B.; Wang, L.; Li, C.; Gao, X.; Zhao, Z.; Ji, X. Ultrasensitive Electrochemical Sensing of Catechol and Hydroquinone via Single-Atom Nanozyme Anchored on MOF-Derived Porous Carbon. Talanta 2024, 268, 125349. [Google Scholar] [CrossRef]
- Feng, S.; Gao, F.; Chen, Z.; Grant, E.; Kitts, D.D.; Wang, S.; Lu, X. Determination of α-Tocopherol in Vegetable Oils Using a Molecularly Imprinted Polymers–Surface-Enhanced Raman Spectroscopic Biosensor. J. Agric. Food Chem. 2013, 61, 10467–10475. [Google Scholar] [CrossRef]
- Cámara-Martos, F.; Da Costa, J.; Justino, C.I.L.; Cardoso, S.; Duarte, A.C.; Rocha-Santos, T. Disposable Biosensor for Detection of Iron (III) in Wines. Talanta 2016, 154, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Caglar, B.; İçer, F.; Özdokur, K.V.; Caglar, S.; Özdemir, A.O.; Guner, E.K.; Beşer, B.M.; Altay, A.; Çırak, Ç.; Doğan, B.; et al. A Novel Amperometric H2O2 Biosensor Constructed by Cress Peroxidase Entrapped on BiFeO3 Nanoparticles. Mater. Chem. Phys. 2021, 262, 124287. [Google Scholar] [CrossRef]
- Bhapkar, S.; Choudhari, U.; Jadhav, U.; Jagtap, S. Evaluation of Soybean Peroxidase—Copper Phosphate Mediated Organic-Inorganic Hybrid for Hydrogen Peroxide Biosensor Application. Sens. Int. 2023, 4, 100242. [Google Scholar] [CrossRef]
- Li, D.; Tian, R.; Kang, S.; Chu, X.-Q.; Ge, D.; Chen, X. Fabrication of Ag Nanoparticles Coupled with Ferrous Disulfide Biocatalyst as a Peroxidase Mimic for Sensitive Electrochemical and Colorimetric Dual-Mode Biosensing of H2O2. Food Chem. 2022, 393, 133386. [Google Scholar] [CrossRef]
- Kim, I.H.; Lim, J.; Kim, S.O. Discovery of Single-Atom Catalyst: Customized Heteroelement Dopants on Graphene. Acc. Mater. Res. 2021, 2, 394–406. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, T.; Zhu, X.; Zu, S.; Xie, Z.; Lu, X.; Zhang, M.; Song, L.; Jin, Y. Metal–Organic Frameworks for Electrocatalytic Sensing of Hydrogen Peroxide. Molecules 2022, 27, 4571. [Google Scholar] [CrossRef]
- Portorreal-Bottier, A.; Gutiérrez-Tarriño, S.; Calvente, J.J.; Andreu, R.; Roldán, E.; Oña-Burgos, P.; Olloqui-Sariego, J.L. Enzyme-like Activity of Cobalt-MOF Nanosheets for Hydrogen Peroxide Electrochemical Sensing. Sens. Actuators B Chem. 2022, 368, 132129. [Google Scholar] [CrossRef]
- Ma, Y.; Wei, P.; Chen, M.; Shi, X.; Lu, X.; Zhang, X.; Sun, D. Trimetallic Metal–Organic Framework Nanosheets as Nanozymes for the Electrochemical Sensing of H2O2. J. Electroanal. Chem. 2023, 940, 117490. [Google Scholar] [CrossRef]
- Tian, L.; Zhao, B.; Zhang, J.; Luo, X.; Wu, F. Magnetic Covalent Organic Framework Nanospheres with Enhanced Peroxidase-like Activity for Colorimetric Detection of H2O2 and Glucose. Colloids Surf. Physicochem. Eng. Asp. 2023, 666, 131309. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, B.; Li, L.; Song, J.; Song, L.; Zhang, M. Fewer-Layer Conductive Metal-Organic Langmuir-Blodgett Films as Electrocatalysts Enable an Ultralow Detection Limit of H2O2. Appl. Surf. Sci. 2021, 539, 148255. [Google Scholar] [CrossRef]
- Chen, S.; Xie, Y.; Guo, X.; Sun, D. Self-Supporting Electrochemical Sensors for Monitoring of Cell-Released H2O2 Based on Metal Nanoparticle/MOF Nanozymes. Microchem. J. 2022, 181, 107715. [Google Scholar] [CrossRef]
- Salis, S.; Spano, N.; Ciulu, M.; Floris, I.; Pilo, M.I.; Sanna, G. Electrochemical Determination of the “Furanic Index” in Honey. Molecules 2021, 26, 4115. [Google Scholar] [CrossRef] [PubMed]
- Pesavento, M.; Merli, D.; Biesuz, R.; Alberti, G.; Marchetti, S.; Milanese, C. A MIP-Based Low-Cost Electrochemical Sensor for 2-Furaldehyde Detection in Beverages. Anal. Chim. Acta 2021, 1142, 201–210. [Google Scholar] [CrossRef]
- Francisco, K.C.A.; Lobato, A.; Tasić, N.; Cardoso, A.A.; Gonçalves, L.M. Determination of 5-Hydroxymethylfurfural Using an Electropolymerized Molecularly Imprinted Polymer in Combination with Salle. Talanta 2022, 250, 123723. [Google Scholar] [CrossRef]
- Khonyoung, S.; Upan, J.; Mool-am-kha, P.; Lerdsri, J.; Jakmunee, J.; Reanpang, P. A Rapid and Reliable Electrochemical Determination of 5-Hydroxymethylfurfural in Honey Exploiting Nickel Oxide Nanoparticles Modified Electrode. Talanta 2024, 268, 125373. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Zhang, H.; Kahaljan, G.; Wang, M.; Mohet, A.; He, S.; Cao, X.; Zheng, H. Electro-Oxidation and Determination 5-Hydroxymethylfurfural in Food on Co-Electrodeposited Cu-Ni Bimetallic Microparticles Modified Copper Electrode. Food Chem. 2022, 367, 130659. [Google Scholar] [CrossRef]
- Laolue, P.; Lerdsri, J. Development of Square Wave Voltammetry Method Using Working Electrodes Modified with Nickel Oxide and Carbon Black for Determination of 5-Hydroxymethylfurfural in Honey. J. Food Compos. Anal. 2023, 124, 105699. [Google Scholar] [CrossRef]
- Alqahtani, N.K.; Alnemr, T.M.; Shulaybi, F.A.; Mohamed, H.A.; Gouda, M. Carboxymethyl-Cellulose-Containing Ag Nanoparticles as an Electrochemical Working Electrode for Fast Hydroxymethyl-Furfural Sensing in Date Molasses. Polymers 2022, 15, 79. [Google Scholar] [CrossRef]
- Upan, J.; Lerdsri, J.; Soongsong, J.; Mool-am-kha, P.; Sridara, T.; Reanpang, P.; Jakmunee, J. A Novel and Portable Electrochemical Sensor for 5-Hydroxymethylfurfural Detection Using Silver Microdendrite Electrodeposited Paper-Based Electrode. The Analyst 2022, 147, 2170–2179. [Google Scholar] [CrossRef]
- Musella, E.; Gualandi, I.; Scavetta, E.; Rivalta, A.; Venuti, E.; Christian, M.; Morandi, V.; Mullaliu, A.; Giorgetti, M.; Tonelli, D. Newly Developed Electrochemical Synthesis of Co-Based Layered Double Hydroxides: Toward Noble Metal-Free Electro-Catalysis. J. Mater. Chem. A 2019, 7, 11241–11249. [Google Scholar] [CrossRef]
- Turan, H.E.; Medetalibeyoglu, H.; Polat, İ.; Yola, B.B.; Atar, N.; Yola, M.L. Graphene Quantum Dots Incorporated NiAl2O4 Nanocomposite Based Molecularly Imprinted Electrochemical Sensor for 5-Hydroxymethyl Furfural Detection in Coffee Samples. Anal. Methods 2023, 15, 1932–1938. [Google Scholar] [CrossRef] [PubMed]
- Kau, N.; Jindal, G.; Kaur, R.; Rana, S. Progress in Development of Metal Organic Frameworks for Electrochemical Sensing of Volatile Organic Compounds. Results Chem. 2022, 4, 100678. [Google Scholar] [CrossRef]
- Jahangiri-Manesh, A.; Mousazadeh, M.; Nikkhah, M.; Abbasian, S.; Moshaii, A.; Masroor, M.J.; Norouzi, P. Molecularly Imprinted Polymer-Based Chemiresistive Sensor for Detection of Nonanal as a Cancer Related Biomarker. Microchem. J. 2022, 173, 106988. [Google Scholar] [CrossRef]
- Tian, H.; Fan, H.; Li, M.; Ma, L. Zeolitic Imidazolate Framework Coated ZnO Nanorods as Molecular Sieving to Improve Selectivity of Formaldehyde Gas Sensor. ACS Sens. 2016, 1, 243–250. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, Y.-P.; Zhao, J.; Dong, W.-W.; Qiao, X.-Q.; Hou, D.-F.; Bu, X.; Li, D.-S. Efficient Gas-Sensing for Formaldehyde with 3D Hierarchical Co3O4 Derived from Co5-Based MOF Microcrystals. Inorg. Chem. 2017, 56, 14111–14117. [Google Scholar] [CrossRef] [PubMed]
- Enache, T.A.; Matei, E.; Diculescu, V.C. Electrochemical Sensor for Carbonyl Groups in Oxidized Proteins. Anal. Chem. 2019, 91, 1920–1927. [Google Scholar] [CrossRef]
- Selvan, K.S.; Narayanan, S.S. Synthesis, Structural Characterization and Electrochemical Studies Switching of MWCNT/Novel Tetradentate Ligand Forming Metal Complexes on PIGE Modified Electrode by Using SWASV. Mater. Sci. Eng. C 2019, 98, 657–665. [Google Scholar] [CrossRef]
- Ping, J.; Wang, Y.; Wu, J.; Ying, Y. Development of an Electrochemically Reduced Graphene Oxide Modified Disposable Bismuth Film Electrode and Its Application for Stripping Analysis of Heavy Metals in Milk. Food Chem. 2014, 151, 65–71. [Google Scholar] [CrossRef]
- Challier, L.; Forget, A.; Bazin, C.; Tanniou, S.; Doare, J.L.; Davy, R.; Bernard, H.; Tripier, R.; Laes-Huon, A.; Poul, N.L. An Ultrasensitive and Highly Selective Nanomolar Electrochemical Sensor Based on an Electrocatalytic Peak Shift Analysis Approach for Copper Trace Detection in Water. Electrochim. Acta 2022, 434, 141298. [Google Scholar] [CrossRef]
- Lu, M.; Deng, Y.; Luo, Y.; Lv, J.; Li, T.; Xu, J.; Chen, S.-W.; Wang, J. Graphene Aerogel–Metal–Organic Framework-Based Electrochemical Method for Simultaneous Detection of Multiple Heavy-Metal Ions. Anal. Chem. 2019, 91, 888–895. [Google Scholar] [CrossRef]
- Pan, D.; Wang, Y.; Chen, Z.; Lou, T.; Qin, W. Nanomaterial/Ionophore-Based Electrode for Anodic Stripping Voltammetric Determination of Lead: An Electrochemical Sensing Platform toward Heavy Metals. Anal. Chem. 2009, 81, 5088–5094. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, T.; Amjadi, S. Preparation of Nano-Sized Pb2+ Imprinted Polymer and Its Application as the Chemical Interface of an Electrochemical Sensor for Toxic Lead Determination in Different Real Samples. J. Hazard. Mater. 2011, 190, 451–459. [Google Scholar] [CrossRef]
- He, X.; Su, Z.; Xie, Q.; Chen, C.; Fu, Y.; Chen, L.; Liu, Y.; Ma, M.; Deng, L.; Qin, D.; et al. Differential Pulse Anodic Stripping Voltammetric Determination of Cd and Pb at a Bismuth Glassy Carbon Electrode Modified with Nafion, Poly(2,5-Dimercapto-1,3,4-Thiadiazole) and Multiwalled Carbon Nanotubes. Microchim. Acta 2011, 173, 95–102. [Google Scholar] [CrossRef]
- Ye, W.; Li, Y.; Wang, J.; Li, B.; Cui, Y.; Yang, Y.; Qian, G. Electrochemical Detection of Trace Heavy Metal Ions Using a Ln-MOF Modified Glass Carbon Electrode. J. Solid State Chem. 2020, 281, 121032. [Google Scholar] [CrossRef]
- Wen, S.-H.; Wang, Y.; Yuan, Y.-H.; Liang, R.-P.; Qiu, J.-D. Electrochemical Sensor for Arsenite Detection Using Graphene Oxide Assisted Generation of Prussian Blue Nanoparticles as Enhanced Signal Label. Anal. Chim. Acta 2018, 1002, 82–89. [Google Scholar] [CrossRef]
- Li, S.-S.; Zhou, W.-Y.; Jiang, M.; Guo, Z.; Liu, J.-H.; Zhang, L.; Huang, X.-J. Surface Fe(II)/Fe(III) Cycle Promoted Ultra-Highly Sensitive Electrochemical Sensing of Arsenic(III) with Dumbbell-Like Au/Fe3O4 Nanoparticles. Anal. Chem. 2018, 90, 4569–4577. [Google Scholar] [CrossRef]
- Wan, H.; Sun, Q.; Li, H.; Sun, F.; Hu, N.; Wang, P. Screen-Printed Gold Electrode with Gold Nanoparticles Modification for Simultaneous Electrochemical Determination of Lead and Copper. Sens. Actuators B Chem. 2015, 209, 336–342. [Google Scholar] [CrossRef]
- Hou, X.; Cheng, Q.; Wang, H. Self-Cleaning Paper-Based Microfluidic Biosensor Employing DNAzyme and Semiconducting Single-Walled Carbon Nanotube for Copper Ion Detection. Bioelectrochemistry 2024, 155, 108602. [Google Scholar] [CrossRef]
- Soares, P.I.; Lima, T.M.; Do Nascimento, L.A.; Coelho, R.M.; Franco, D.L.; Pereira, A.C.; Ferreira, L.F. Co-detection of Copper and Lead in Artisanal Sugarcane Spirit Using Caffeic Acid-modified Graphite Electrodes. Electroanalysis 2023, 35, e202200302. [Google Scholar] [CrossRef]
- Berrabah, S.E.; Benchettara, A.; Smaili, F.; Tabti, S.; Benchettara, A. Electrodeposition of Zinc Hydroxide on Carbon Graphite Electrode for Electrochemical Determination of Trace Copper in Water Samples Using Square Wave Anodic Stripping Voltammetry. Mater. Chem. Phys. 2022, 278, 125670. [Google Scholar] [CrossRef]
- Qin, J.; Li, W.; Cai, K.; Wang, D.; Peng, C.; Luo, L.; Song, S.; Mei, Y.; Wang, Y. Simultaneous Electrochemical Detection of Zinc and Copper in Fruit Juice Using Hg/CMWCNTs@ZIF-8 Modified Glassy Carbon Electrode. Microporous Mesoporous Mater. 2023, 360, 112721. [Google Scholar] [CrossRef]
- Ismail, R.; Šeděnková, I.; Černochová, Z.; Romanenko, I.; Pop-Georgievski, O.; Hrubý, M.; Tomšík, E. Potentiometric Performance of Ion-Selective Electrodes Based on Polyaniline and Chelating Agents: Detection of Fe2+ or Fe3+ Ions. Biosensors 2022, 12, 446. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.F.; Garcia, A.B. A Study of the Evolution of the P and Structural Characteristics of Olive and Sunflower Oils after Heating at Frying Temperatures. Food Chem. 2006, 98, 214–219. [Google Scholar] [CrossRef]
- Šegatin, N.; Pajk Žontar, T.; Poklar Ulrih, N. Dielectric Properties and Dipole Moment of Edible Oils Subjected to ‘Frying’ Thermal Treatment. Foods 2020, 9, 900. [Google Scholar] [CrossRef]
- Fatima, S.; Kumar, V.; Bhadauria, G.; Verma, H. Quality Indicators Based Rapid Test Kits for Detection of Frying Oil Quality: A Review. Food Chem. Adv. 2023, 2, 100305. [Google Scholar] [CrossRef]
- Khaled, A.Y.; Aziz, S.A.; Rokhani, F.Z. Capacitive Sensor Probe to Assess Frying Oil Degradation. Inf. Process. Agric. 2015, 2, 142–148. [Google Scholar] [CrossRef]
- Liu, M.; Qin, X.; Chen, Z.; Tang, L.; Borom, B.; Cao, N.; Barnes, D.; Cheng, K.; Chen, J.; Wang, T.; et al. Frying Oil Evaluation by a Portable Sensor Based on Dielectric Constant Measurement. Sensors 2019, 19, 5375. [Google Scholar] [CrossRef] [PubMed]
- Rubalya Valantina, S. Measurement of Dielectric Constant: A Recent Trend in Quality Analysis of Vegetable Oil—A Review. Trends Food Sci. Technol. 2021, 113, 1–11. [Google Scholar] [CrossRef]
- Stevan, S.; Paiter, L.; Galvão, J.; Roque, D.; Chaves, E. Sensor and Methodology for Dielectric Analysis of Vegetal Oils Submitted to Thermal Stress. Sensors 2015, 15, 26457–26477. [Google Scholar] [CrossRef]
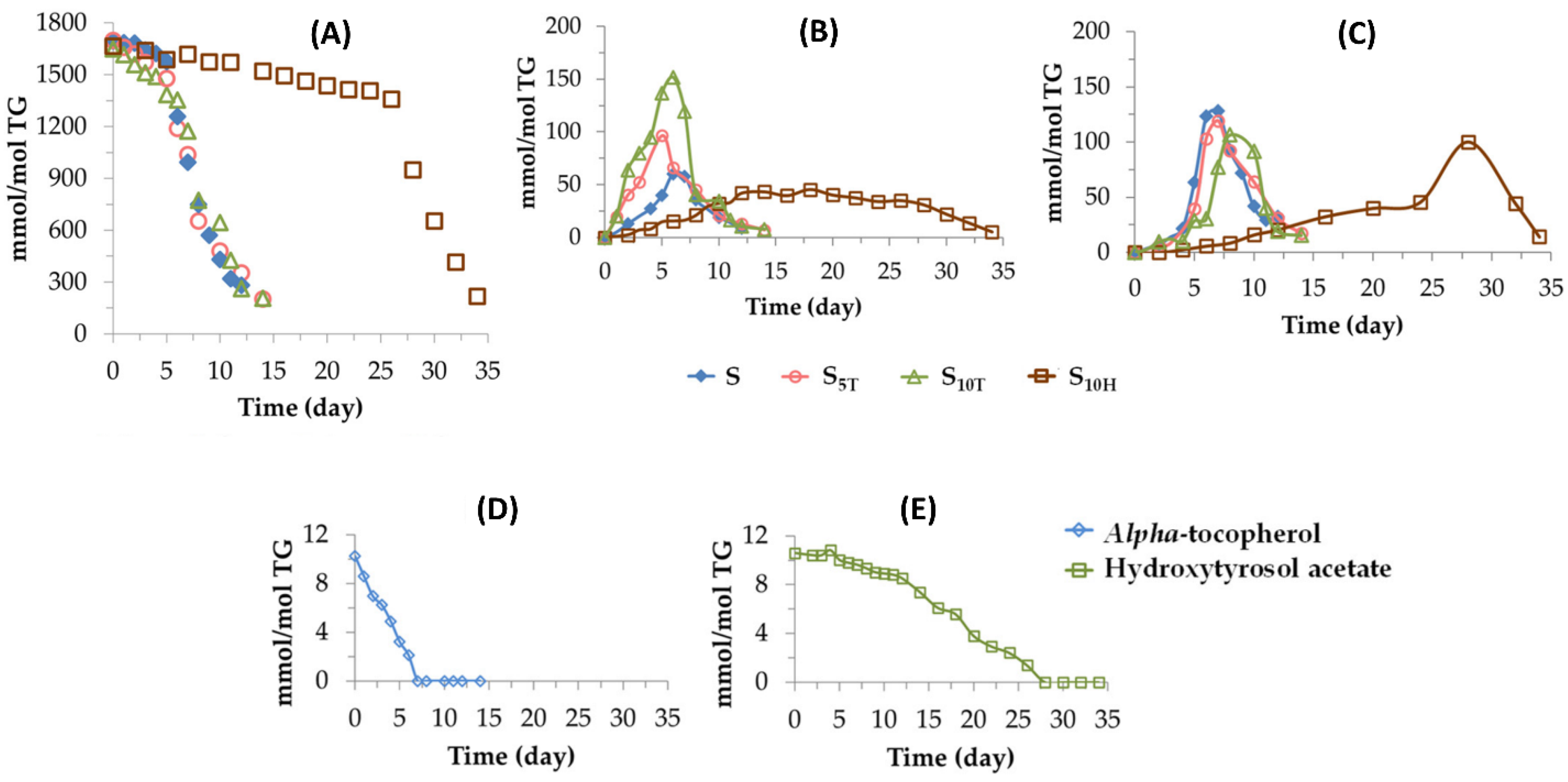
- Moretto, L.; Tonolo, F.; Folda, A.; Scalcon, V.; Bindoli, A.; Bellamio, M.; Feller, E.; Rigobello, M.P. Comparative Analysis of the Antioxidant Capacity and Lipid and Protein Oxidation of Soy and Oats Beverages. Food Prod. Process. Nutr. 2021, 3, 1. [Google Scholar] [CrossRef]
- Qiu, X.; Jacobsen, C.; Sørensen, A.-D.M. The Effect of Rosemary (Rosmarinus officinalis L.) Extract on the Oxidative Stability of Lipids in Cow and Soy Milk Enriched with Fish Oil. Food Chem. 2018, 263, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Østdal, H.; Bjerrum, M.J.; Pedersen, J.A.; Andersen, H.J. Lactoperoxidase-Induced Protein Oxidation in Milk. J. Agric. Food Chem. 2000, 48, 3939–3944. [Google Scholar] [CrossRef] [PubMed]
- Brothersen, C.; McMahon, D.J.; Legako, J.; Martini, S. Comparison of Milk Oxidation by Exposure to LED and Fluorescent Light. J. Dairy Sci. 2016, 99, 2537–2544. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Xiong, Y.L. Protein Oxidation in Foods: Mechanisms, Consequences, and Antioxidant Solutions. Foods 2021, 10, 2346. [Google Scholar] [CrossRef]
- Wüst, J.; Pischetsrieder, M. Methionine Sulfoxide Profiling of Milk Proteins to Assess the Influence of Lipids on Protein Oxidation in Milk. Food Funct. 2016, 7, 2526–2536. [Google Scholar] [CrossRef]
- Deeth, H.C. Lipoprotein Lipase and Lipolysis in Milk. Int. Dairy J. 2006, 16, 555–562. [Google Scholar] [CrossRef]
- Clarke, H.J.; McCarthy, W.P.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Oxidative Quality of Dairy Powders: Influencing Factors and Analysis. Foods 2021, 10, 2315. [Google Scholar] [CrossRef]
- Lim, S.H.; Chin, N.L.; Sulaiman, A.; Tay, C.H.; Wong, T.H. Sensory Analysis for Cow Milk Product Development Using High Pressure Processing (HPP) in the Dairy Industry. Foods 2022, 11, 1233. [Google Scholar] [CrossRef]


| Sample Matrix | Nanomaterial | Target Analyte | Detection Method | LOD | Ref. |
|---|---|---|---|---|---|
| Buckwheat tea and orange | Ce-PEDOT nanocomposite | Rutin | ECD | 14.7 nM | [103] |
| Plant extract | glycosyl-MOF-5 and polyacrylonitrile | Quercetin | ECD | 83.3 nM | [104] |
| Human serum | C3N4-SWCNT/rGO/cyclodextrin MOF | Baicalin | ECD | 0.46 nM | [105] |
| Green tea and urine | Mesoporous carbon and Zr-MOF-801 | Gallic acid and luteolin | ECD | 0.15 μM | [106] |
| Pharmaceuticals | Nanocomposite of MIL-100(Fe)-MWCNTs/PEDOT | Kaempferol | ECD | 13.2 nM | [108] |
| Broccoli | Nanocomposite β-CD/C60-GO/Ni-MOF | Kaempferol | ECD | 58 nM | [107] |
| Tea beverage | MOF-doped Au/PEDOT | Catechin/sunset yellow | ECD, DPV | 10 nM/150 nM | [110] |
| Model system (water solution) | Zn CNs/MWCNTs-COOH nanocomposite | Catechin | ECD | 10 nM | [109] |
| Caffeinated drinks and wine | CuO -Cu-MOF | Caffeic acid | ECD | 40 nM | [112] |
| Wine | Mesoporous graphite oxide/Al2O3, MIT-100(Al) | Caffeic acid | ECD | 4 nM | [111] |
| Pharmaceuticals, wastewater, well water, tap water, tea samples, and biological fluids | ZnTe NRs and Cu MOF | Catechol | ECD | 16 nM | [113] |
| Human serum and urine | Bimetallic MOF-818 and RGO/MWCNTs | Caffeic acid, chlorogenic acid, and gallic acid | ECD, DPV | 5.2 nM 5.7 nM 180 nM | [114] |
| Human serum and urine | Ni-MOF/T-PEDOT | Gallic acid and tinidazole | ECD | [115] | |
| Orange juices | Magnetic nanoparticle Fe3O4-ZIF-4 | Coumaric acid | ECD | 180 nM | [116] |
| Healthcare tablets | Cu HKUST-1 | Ascorbic acid | ECD | 3 μM | [117] |
| Sample Matrix | Nanomaterial | Detection Method | LOD | Ref. |
|---|---|---|---|---|
| Milk samples | BiFeO3 and cress peroxidase | AD | 70 nM | [124] |
| Milk samples | Soybean peroxidase/OIH | CV | 190 nM | [125] |
| Real serum, milk, and orange juice | Ag NPs/FeS2/ITO nanozyme | ECD | 600 nM | [126] |
| 2D cobalt-MOF nanosheets (nanozyme) | ECD | [129] | ||
| Serum samples | 2D NiCoM nanosheets | ECD | 2.1 μM | [130] |
| 2D MOF [Co3(HOB)2]n films | ECD | 3.08 nM | [132] | |
| AgNPs/2D Zn-MOFs | ECD | 1.7 μM | [133] |
| Sample Matrix | Nanomaterial | Detection Method | LOD | Ref. |
|---|---|---|---|---|
| Beverages | MIP | SWV | 50 μM | [135] |
| Coffee | Electropolymerized MIP | DPV | 0.37 mg L−1 | [136] |
| Honey samples | NiONPs-modified SPCE | SWV | 0.24 ppm | [137] |
| Food | Cu-Ni bimetallic microparticles | ChAD | 3.5 μM | [138] |
| Honey | NiO-CB (nickel oxide and carbon black) | SWV | 5.4 mg kg−1 | [139] |
| Molasses | Ag@CMC/GCE | CV and LSV | – | [140] |
| Honey | Silver microdendrite/SPCE | ECD | 1.0 ppm | [141] |
| Model system (water solution) | Co-based layered double hydroxides | ChAD | 0.1 mM | [142] |
| Coffee | Graphene quantum dots NiAl2O4—MIP | ECD, SWV | 0.30 ng L−1 | [143] |
| Sample Matrix | Nanomaterial | Target Analyte | Detection Method | LOD | Ref. |
|---|---|---|---|---|---|
| Rice and tap water | MWCNT/carboxamide ligand PIGE | Pb(II) and Cd(II) | ECD, SWV | 2.7 nM and 0.92 nM | [149] |
| Milk | eRGO/Bi | Pb(II) and Cd(II | SWASV | 0.8 μg L−1 0.5 μg L−1 | [150] |
| Water | GE-cyclam | Cu(II) | AdASV | 1.1 nM | [151] |
| River water, soil, and vegetable (spinach) | Graphene aerogel (GA)-MOF | Cd2+, Pb2+, Cu2+, and Hg2+ | DPSV | 20 nM Cd2+, 1.5 nM Pb2+, 7 nM Cu2+, 2 nM Hg2+ | [152] |
| Water samples | Nanomaterial/ionophore | Pb2+ | ASV | 1.0 nM | [153] |
| Water samples | Nano-sized Pb2+ MIP | Pb2+ | SV | 0.6 nM | [154] |
| Water samples | PDMcT-MWCNTs (poly(2,5-dimercapto-1,3,4-thiadiazole)) | Pb2+ and Cd2+ | ASDPV | 0.05 μg L−1 0.03 μg L−1 | [155] |
| Water samples | Ln-MOF/GCE | Pb2+ and Cd2+ | SWASV | 1.10 nM 1.66 nM | [156] |
| Water samples | GO on ssDNA-PBNP Prussian blue nanoparticles | As | ECD | 0.058 ppb | [157] |
| Water samples | Au/Fe3O4 nanoparticles | As | ECD, SWASV | 0.0215 ppb | [158] |
| Water samples | SPGE/AuNP | Pb2+ and Cu2+ | SWASV | 2.2 ppb 1.6 ppb | [159] |
| Livestock feed and manure | DNAzyme-SWCNT | Cu2+ | Resistance | 0.65 nM | [160] |
| Artisanal sugarcane spirit | GE/poly(CA) caffeic acid (CA)-modified graphite electrodes | Pb2+ and Cu2+ | SWASV | 3.01 μg/L Pb(II), and 4.50 μg/L Cu(II) | [161] |
| Water samples | Zn(OH)2@CGE | Cu2+ | SWASV | 0.9 nM | [162] |
| Fruit juice beverages | Hg/CMWCNTs@ZIF-8 -GCE | Zn2+ and Cu2+ | Voltammetry | 5.23 × 10−3 and 6.52 × 10−3 mg L−1 | [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daci, M.; Berisha, L.; Mercatante, D.; Rodriguez-Estrada, M.T.; Jin, Z.; Huang, Y.; Amorati, R. Advancements in Biosensors for Lipid Peroxidation and Antioxidant Protection in Food: A Critical Review. Antioxidants 2024, 13, 1484. https://doi.org/10.3390/antiox13121484
Daci M, Berisha L, Mercatante D, Rodriguez-Estrada MT, Jin Z, Huang Y, Amorati R. Advancements in Biosensors for Lipid Peroxidation and Antioxidant Protection in Food: A Critical Review. Antioxidants. 2024; 13(12):1484. https://doi.org/10.3390/antiox13121484
Chicago/Turabian StyleDaci, Majlinda, Liridon Berisha, Dario Mercatante, Maria Teresa Rodriguez-Estrada, Zongxin Jin, Yeqin Huang, and Riccardo Amorati. 2024. "Advancements in Biosensors for Lipid Peroxidation and Antioxidant Protection in Food: A Critical Review" Antioxidants 13, no. 12: 1484. https://doi.org/10.3390/antiox13121484
APA StyleDaci, M., Berisha, L., Mercatante, D., Rodriguez-Estrada, M. T., Jin, Z., Huang, Y., & Amorati, R. (2024). Advancements in Biosensors for Lipid Peroxidation and Antioxidant Protection in Food: A Critical Review. Antioxidants, 13(12), 1484. https://doi.org/10.3390/antiox13121484









