Exogenous GABA-Ca Alleviates Growth Inhibition Induced by a Low-P Environment in Peanuts (Arachis hypogaea)
Abstract
1. Introduction
2. Materials and Methods
3. Results
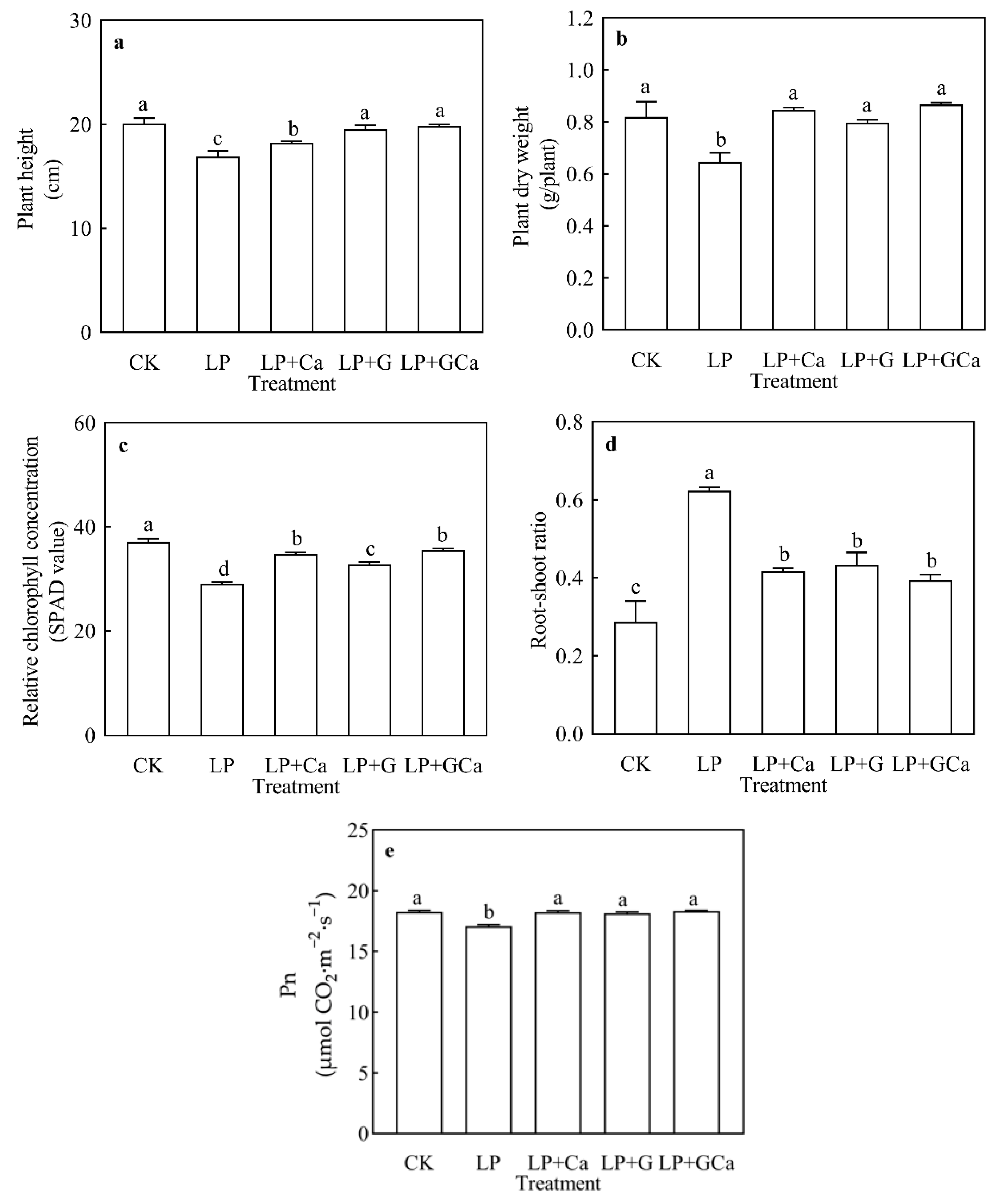
3.1. Growth
3.2. Mineral Element
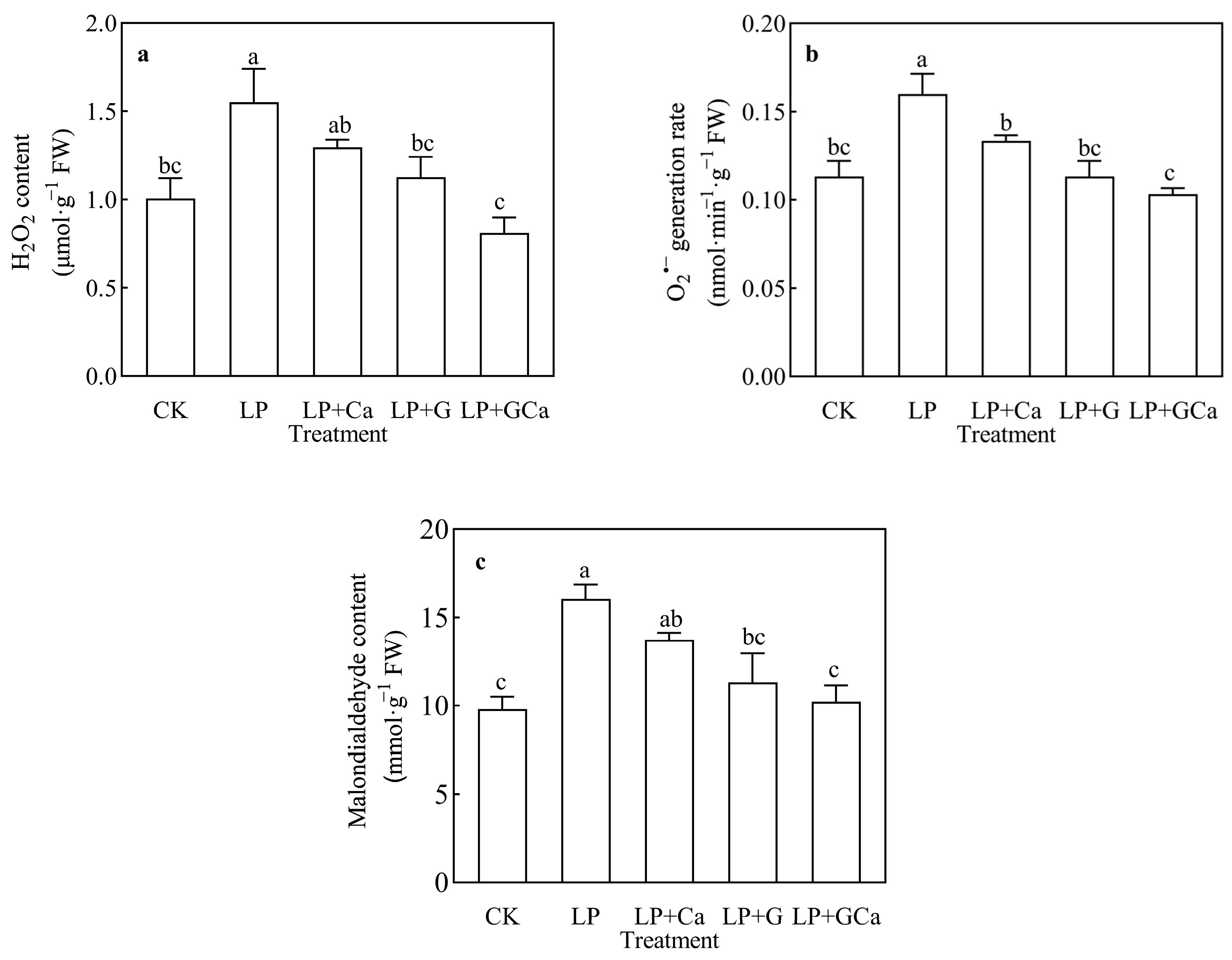
3.3. ROS
3.4. Antioxidant Enzyme Activity
3.5. Photosystem I and II Activity
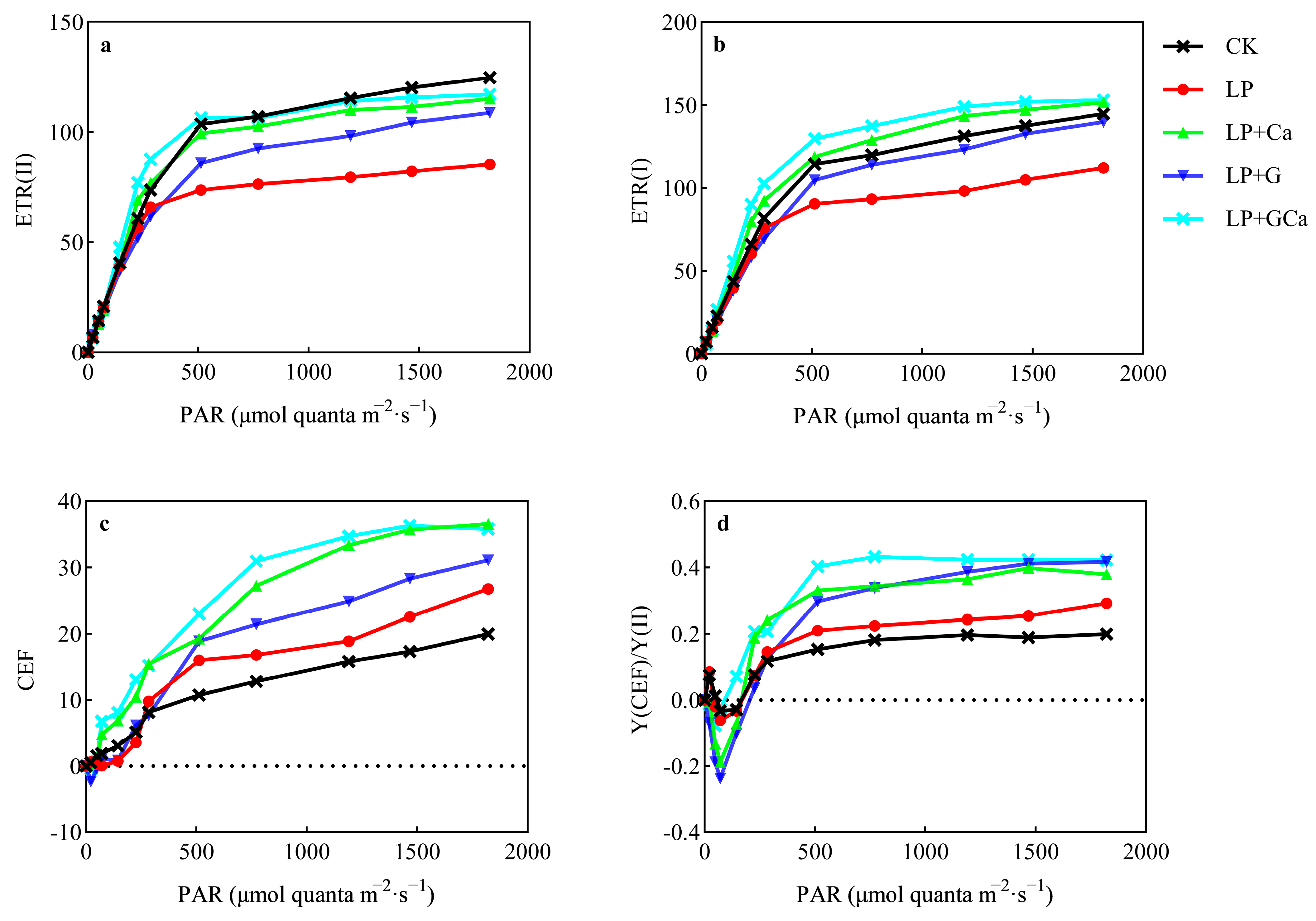
3.6. ETR and CEF
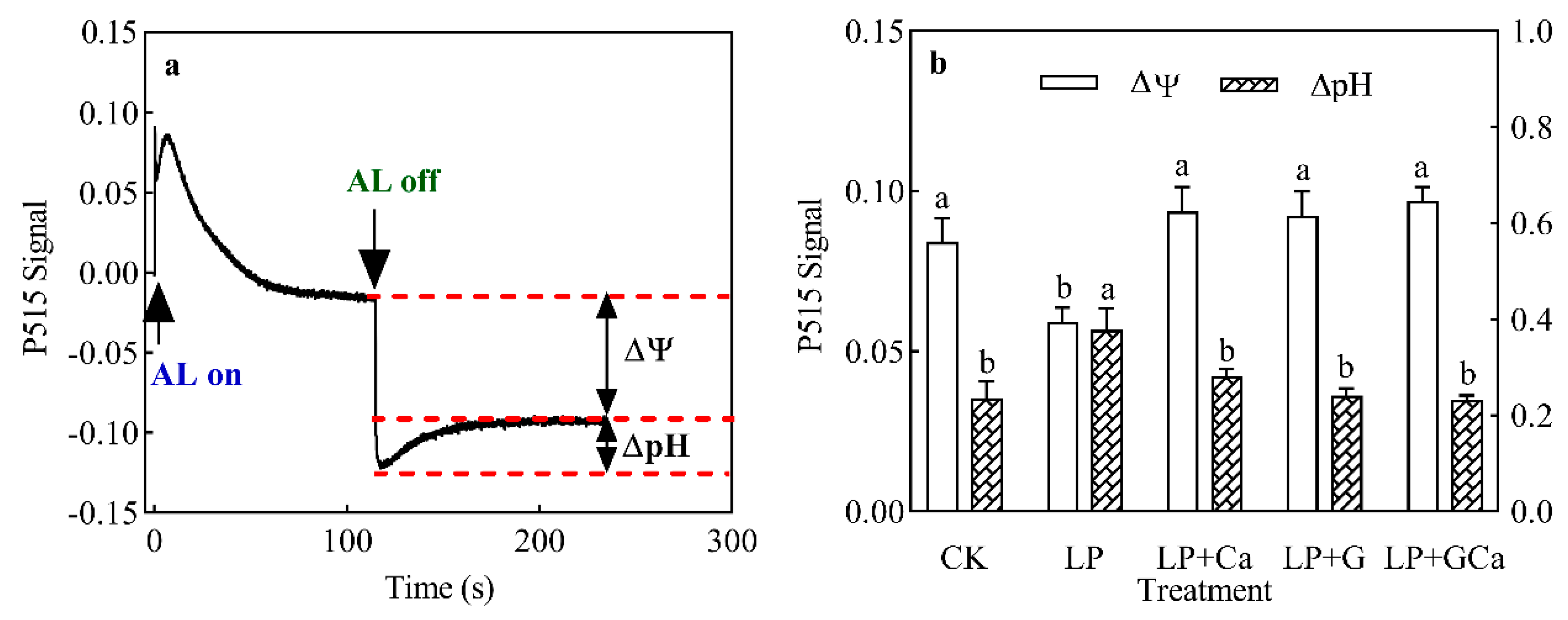
3.7. ∆pH and ∆Ψ
3.8. Thylakoid Membrane Integrity and ATP Synthase Activity
4. Discussion
4.1. Effects of GABA-Ca on Growth, Leaf Nutrients, and ROS in Peanut Under Low-P Stress
4.2. GABA-Ca Enhances CEF and Alleviates Photoinhibition in Peanut Leaves Under Low-P Stress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hou, E.; Luo, Y.; Kuang, Y.; Chen, C.; Lu, X.; Jiang, L.; Luo, X.; Wen, D. Global meta-analysis shows pervasive phosphorus limitation of aboveground plant production in natural terrestrial ecosystems. Nat. Commun. 2020, 11, 637. [Google Scholar] [CrossRef] [PubMed]
- López-Arredondo, D.L.; Leyva-González, M.A.; González-Morales, S.I.; López-Bucio, J.; Herrera-Estrella, L. Phosphate nutrition: Improving low-phosphate tolerance in crops. Annu. Rev. Plant Biol. 2014, 65, 95–123. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Hu, W.; Bu, L.; Cheng, H.; Liu, G. Legume cover crops alter soil phosphorus availability and microbial community composition in mango orchards in karst areas. Agric. Ecosyst. Environ. 2024, 364, 108906. [Google Scholar] [CrossRef]
- Yu, X.; Keitel, C.; Dijkstra, F.A. Global analysis of phosphorus fertilizer use efficiency in cereal crops. Glob. Food Sec. 2021, 29, 100545. [Google Scholar] [CrossRef]
- Roy, E.D.; Richards, P.D.; Martinelli, L.A.; Coletta, L.D.; Lins, S.R.M.; Vazquez, F.F.; Willig, E.; Spera, S.A.; VanWey, L.K.; Porder, S. The phosphorus cost of agricultural intensification in the tropics. Nat. Plants 2016, 2, 16043. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, X.; Zhang, J.-Y.; Xi, B.; Lu, Q. Progress on preventing and controlling strategies of lake eutrophication in China. Environ. Technol. 2010, 33, 92–98. [Google Scholar]
- Smil, V. Phosphorus in the environment: Natural flows and human interferences. Annu. Rev. Environ. Resour. 2000, 25, 53–88. [Google Scholar] [CrossRef]
- Tilman, D.; Fargione, J.E.; Wolff, B.G.; D’Antonio, C.M.; Dobson, A.P.; Howarth, R.W.; Schindler, D.W.; Schlesinger, W.H.; Simberloff, D.; Swackhamer, D.L. Forecasting agriculturally driven global environmental change. Science 2001, 292, 281–284. [Google Scholar] [CrossRef]
- Chowdhury, R.B.; Moore, G.A.; Weatherley, A.J.; Arora, M. Key sustainability challenges for the global phosphorus resource, their implications for global food security, and options for mitigation. J. Clean. Prod. 2017, 140, 945–963. [Google Scholar] [CrossRef]
- Lambers, H.; Oliveira, R. Plant Physiological Ecology, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Lambers, H. Phosphorus Acquisition and Utilization in Plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef]
- Shi, Q.; Pang, J.; Yong, J.W.H.; Bai, C.; Pereira, C.G.; Song, Q.; Wu, D.; Dong, Q.; Cheng, X.; Wang, F.; et al. Phosphorus-fertilisation has differential effects on leaf growth and photosynthetic capacity of Arachis hypogaea L. Plant Soil. 2020, 447, 99–116. [Google Scholar] [CrossRef]
- Sun, Z.; Bai, C.; Liu, Y.; Ma, M.; Zhang, S.; Liu, H.; Bai, R.; Han, X.; Yong, J.W.H. Resilient and sustainable production of peanut (Arachis hypogaea) in phosphorus-limited environment by using exogenous gamma-aminobutyric acid to sustain photosynthesis. Ecotox. Environ. Safe 2023, 263, 115388. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Chen, T.; Li, X.; Cao, J.; Li, J.; Xu, X.; Zhang, L.; Chen, Y. Integrated physiological, transcriptomic and metabolomic analyses reveal the mechanism of peanut kernel weight reduction under waterlogging stress. Plant Cell Environ. 2024, 47, 3198–3214. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liu, X.; Zhang, S.; Ma, M.; Bai, R.; Liu, H.; Yi, B.; Han, X.; Liu, Y. Exogenous calcium alleviates phosphorus deficiency-induced photosynthetic inhibition in peanuts (Arachis hypogaea). J. Plant Nutr. 2022, 28, 1055–1066. [Google Scholar]
- Foster, A.C.; Kemp, J.A. Glutamate- and GABA-based CNS therapeutics. Curr. Opin. Pharmacol. 2006, 6, 7–17. [Google Scholar] [CrossRef]
- Sun, Y.; Mehmood, A.; Battino, M.; Xiao, J.; Chen, X. Enrichment of gamma-aminobutyric acid in foods: From conventional methods to innovative technologies. Int. Food Res. J. 2022, 162, 111801. [Google Scholar] [CrossRef]
- Hou, D.; Tang, J.; Feng, Q.; Niu, Z.; Shen, Q.; Wang, L.; Zhou, S. Gamma-aminobutyric acid (GABA): A comprehensive review of dietary sources, enrichment technologies, processing effects, health benefits, and its applications. Crit. Rev. Food Sci. Nutr. 2023, 64, 8852–8874. [Google Scholar] [CrossRef]
- Boonstra, E.; de Kleijn, R.; Colzato, L.S.; Alkemade, A.; Forstmann, B.U.; Nieuwenhuis, S. Neurotransmitters as food supplements: The effects of GABA on brain and behavior. Front. Psychol. 2015, 6, 01520. [Google Scholar] [CrossRef]
- Renes, E.; Linares, D.M.; González, L.; Fresno, J.M.; Tornadijo, M.E.; Stanton, C. Production of conjugated linoleic acid and gamma-aminobutyric acid by autochthonous lactic acid bacteria and detection of the genes involved. J. Funct. Food 2017, 34, 340–346. [Google Scholar] [CrossRef]
- Bown, A.W.; Shelp, B.J. Plant GABA: Not just a metabolite. Trends Plant Sci. 2016, 21, 811–813. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Zhang, X.; Ma, F.; Guo, T.; Li, C. Activation of the ABA signal pathway mediated by GABA improves the drought resistance of apple seedlings. Int. J. Mol. Sci. 2021, 22, 12676. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.R.; Campbell, A.K.; Smith, S.M.; Trewavas, A.J. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 1991, 352, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Kinnersley, A.M.; Turano, F.J. Gamma aminobutyric acid (GABA) and plant responses to stress. Crit. Rev. Plant Sci. 2000, 19, 479–509. [Google Scholar] [CrossRef]
- Braam, J.; Davis, R.W. Rain-, wind-, and touch-induced expression of calmodulin and calmodulin-related genes in Arabidopsis. Cell 1990, 60, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Liu, Y.; Pang, J.; Yong, J.W.H.; Chen, Y.; Bai, C.; Han, X.; Liu, X.; Sun, Z.; Zhang, S.; et al. Exogenous calcium alleviates nocturnal chilling-induced feedback inhibition of photosynthesis by improving sink demand in peanut (Arachis hypogaea). Front. Plant Sci. 2020, 11, 607029. [Google Scholar] [CrossRef]
- Song, Q.; Liu, Y.; Pang, J.; Yong, J.W.H.; Chen, Y.; Bai, C.; Gille, C.; Shi, Q.; Wu, D.; Han, X.; et al. Supplementary calcium restores peanut (Arachis hypogaea) growth and photosynthetic capacity under low nocturnal temperature. Front. Plant Sci. 2020, 10, 1637. [Google Scholar] [CrossRef]
- Liu, J.; Hu, J.; Li, Y.; Li, G.; Wu, H. Chapter 10-Calcium channels and transporters in plants under salinity stress. In Calcium Transport Elements in Plants; Upadhyay, S.K., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 157–169. [Google Scholar]
- Ma, Y.; Wang, P.; Gu, Z.; Tao, Y.; Shen, C.; Zhou, Y.; Han, Y.-B.; Yang, R. Ca2+ involved in GABA signal transduction for phenolics accumulation in germinated hulless barley under NaCl stress. Food Chem. X 2019, 2, 100023. [Google Scholar] [CrossRef]
- Guo, Z.; Gong, J.; Luo, S.; Zuo, Y.; Shen, Y. Role of gamma-aminobutyric acid in plant defense response. Metabolites 2023, 13, 741. [Google Scholar] [CrossRef]
- Xie, C.; Sun, M.; Wang, P.; Yang, R. Interaction of Gamma-Aminobutyric Acid and Ca2+ on Phenolic Compounds Bioaccumulation in Soybean Sprouts under NaCl Stress. Plants 2022, 11, 3503. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Gupta, P.; Srivastava, S.; Seth, C.S. 24-Epibrassinolide and Sodium Nitroprusside alleviate the salinity stress in Brassica juncea L. cv. Varuna through cross talk among proline, nitrogen metabolism and abscisic acid. Plant Soil 2017, 411, 483–498. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxylammoniumchloride: A simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Uthairatanakij, A.; Srilaong, V.; Laohakunjit, N.; Kato, M.; Jitareerat, P. Impact of electron beam irradiation on the chlorophyll degradation and antioxidant capacity of mango fruit. Appl. Biol. Chem. 2021, 64, 19. [Google Scholar] [CrossRef] [PubMed]
- Egley, G.H.; Paul, R.N.; Vaughn, K.C.; Duke, S.O. Role of peroxidase in the development of water-impermeable seed coats in Sida spinosa L. Planta 1983, 157, 224–232. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for Superoxide Dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Hadwan, M. Simple spectrophotometric assay for measuring catalase activity in biological tissues. BMC Biochem. 2018, 19, 1. [Google Scholar] [CrossRef]
- Dahnke, W.C.; Johnson, G.V. Testing Soils for Available Nitrogen. In Soil Testing and Plant Analysis; SSSA Book Series; SSSA: Madison, WI, USA, 1990; pp. 127–139. [Google Scholar]
- Jones, J.B. Phosphorus toxicity in tomato plants: When and how does it occur? Commun. Soil Sci. Plant Anal. 1998, 29, 1779–1784. [Google Scholar] [CrossRef]
- Wu, X.; Jia, Q.; Ji, S.; Gong, B.; Li, J.; Lü, G.; Gao, H. Gamma-aminobutyric acid (GABA) alleviates salt damage in tomato by modulating Na+ uptake, the GAD gene, amino acid synthesis and reactive oxygen species metabolism. BMC Plant Biol. 2020, 20, 465. [Google Scholar] [CrossRef]
- Ramesh, S.A.; Tyerman, S.D.; Xu, B.; Bose, J.; Kaur, S.; Conn, V.; Domingos, P.; Ullah, S.; Wege, S.; Shabala, S.; et al. GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat. Commun. 2015, 6, 7879. [Google Scholar] [CrossRef]
- Ramesh, S.A.; Kamran, M.; Sullivan, W.; Chirkova, L.; Okamoto, M.; Degryse, F.; McLaughlin, M.; Gilliham, M.; Tyerman, S.D. Aluminum-activated malate transporters can facilitate GABA transport. Plant Cell 2018, 30, 1147–1164. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, W.; Meng, Y.; Xie, T.; Li, L.; Li, J.; Wei, S. Gamma-aminobutyric acid imparts partial protection from salt stress injury to maize seedlings by improving photosynthesis and upregulating osmoprotectants and antioxidants. Sci. Rep. 2017, 7, 43609. [Google Scholar] [CrossRef]
- Salah, A.; Zhan, M.; Cao, C.; Han, Y.; Ling, L.; Liu, Z.; Li, P.; Ye, M.; Jiang, Y. γ-Aminobutyric Acid Promotes Chloroplast Ultrastructure, Antioxidant Capacity, and Growth of Waterlogged Maize Seedlings. Sci. Rep. 2019, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Hassan, M.J.; Peng, Y.; Liu, L.; Liu, W.; Zhang, Y.; Li, Z. γ-Aminobutyric acid (GABA) priming improves seed germination and seedling stress tolerance associated with enhanced antioxidant metabolism, DREB expression, and dehydrin accumulation in white clover under water stress. Front. Plant Sci. 2021, 12, 776939. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wu, Q.; Liu, X.; Shen, Z.; Chen, J.; Liu, T.; Chen, J.; Zhu, C.; Wu, F.; Chen, L.; et al. Comparative proteomic analysis reveals the effects of exogenous calcium against acid rain stress in Liquidambar formosana Hance leaves. J. Proteome Res. 2016, 15, 216–228. [Google Scholar] [CrossRef]
- Tikkanen, M.; Mekala, N.R.; Aro, E.-M. Photosystem II photoinhibition-repair cycle protects Photosystem I from irreversible damage. Biochim. Biophys. Acta (BBA)-Bioenerg. 2014, 1837, 210–215. [Google Scholar] [CrossRef]
- Barbato, R.; Tadini, L.; Cannata, R.; Peracchio, C.; Jeran, N.; Alboresi, A.; Morosinotto, T.; Bajwa, A.A.; Paakkarinen, V.; Suorsa, M.; et al. Higher order photoprotection mutants reveal the importance of ΔpH-dependent photosynthesis-control in preventing light induced damage to both photosystem II and photosystem I. Sci. Rep. 2020, 10, 6770. [Google Scholar] [CrossRef]
- Foyer, C.H.; Neukermans, J.; Queval, G.; Noctor, G.; Harbinson, J. Photosynthetic control of electron transport and the regulation of gene expression. J. Exp. Bot. 2012, 63, 1637–1661. [Google Scholar] [CrossRef]
- Yamori, W.; Makino, A.; Shikanai, T. A physiological role of cyclic electron transport around photosystem I in sustaining photosynthesis under fluctuating light in rice. Sci. Rep. 2016, 6, 20147. [Google Scholar] [CrossRef]
- Ma, M.; Liu, Y.; Bai, C.; Yang, Y.; Sun, Z.; Liu, X.; Zhang, S.; Han, X.; Yong, J.W.H. The physiological functionality of PGR5/PGRL1-dependent cyclic electron transport in sustaining photosynthesis. Front. Plant Sci. 2021, 12, 702196. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Yang, X.; Wang, F.; Qi, M.; Li, T.; Liu, Y. Cyclic electron flow protects photosystem I donor side under low night temperature in tomato. Environ. Exp. Bot. 2020, 177, 104151. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Ma, M.; Liu, H.; Tao, D.; Salam, S.A.; Han, X.; Liu, Y.; Yong, J.W.H. Exogenous GABA-Ca Alleviates Growth Inhibition Induced by a Low-P Environment in Peanuts (Arachis hypogaea). Antioxidants 2024, 13, 1414. https://doi.org/10.3390/antiox13111414
Sun Z, Ma M, Liu H, Tao D, Salam SA, Han X, Liu Y, Yong JWH. Exogenous GABA-Ca Alleviates Growth Inhibition Induced by a Low-P Environment in Peanuts (Arachis hypogaea). Antioxidants. 2024; 13(11):1414. https://doi.org/10.3390/antiox13111414
Chicago/Turabian StyleSun, Zhiyu, Mingzhu Ma, Huan Liu, Dongbing Tao, Shaikh Amjad Salam, Xiaori Han, Yifei Liu, and Jean Wan Hong Yong. 2024. "Exogenous GABA-Ca Alleviates Growth Inhibition Induced by a Low-P Environment in Peanuts (Arachis hypogaea)" Antioxidants 13, no. 11: 1414. https://doi.org/10.3390/antiox13111414
APA StyleSun, Z., Ma, M., Liu, H., Tao, D., Salam, S. A., Han, X., Liu, Y., & Yong, J. W. H. (2024). Exogenous GABA-Ca Alleviates Growth Inhibition Induced by a Low-P Environment in Peanuts (Arachis hypogaea). Antioxidants, 13(11), 1414. https://doi.org/10.3390/antiox13111414





