Chemical Constituents from the Fruit of Melia azedarach and Their Anti-Inflammatory Activity
Abstract
1. Introduction
2. Material and Methods
2.1. General Experimental Procedure
2.2. Plant Materials
2.3. Extraction and Separation
2.4. Cell Culture
2.5. Cell Viability Test
2.6. Anti-Inflammatory Effect Test
2.7. Flow Cytometry Test
2.8. Cytokine Detection
2.9. Western Blotting Analysis
2.10. Immunofluorescence Assay
2.11. Statistical Analyses
3. Results
3.1. Chemical Structural Determination of Compounds 1–5
3.2. Effects of Isolate on Nitrite Levels in Macrophages
3.3. Inhibition of the Inflammatory Response Through Limonoid 2
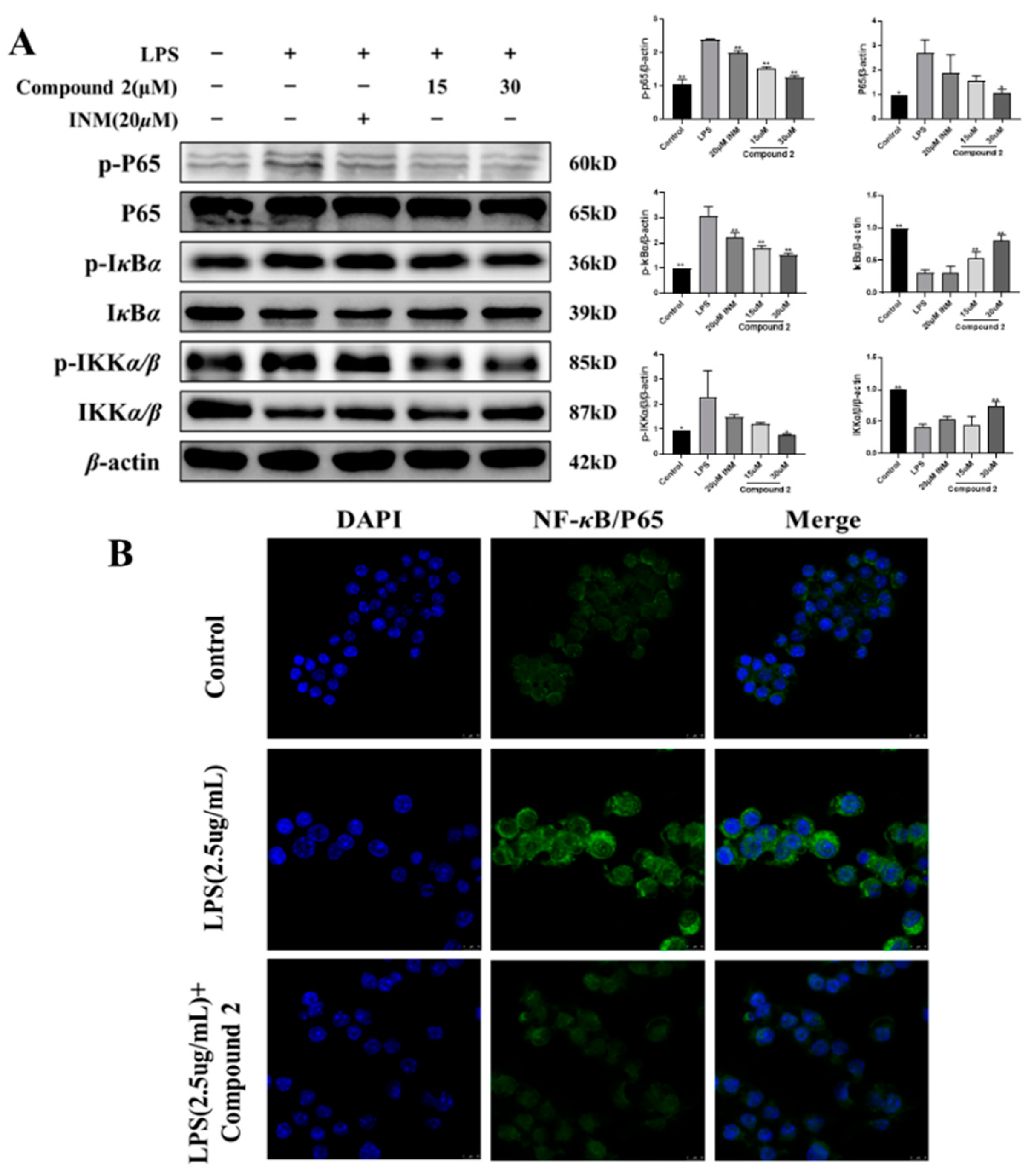
3.4. Exploring the Role of Limonoid 2 in the NF-κB Cascade
3.5. Exploring the Role of Limonoid 2 in iNOS and JAK2 Cascades
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Tang, J.Y.; Peng, S.Y.; Cheng, Y.B.; Wang, C.L.; Farooqi, A.A.; Yu, T.J.; Hou, M.F.; Wang, S.C.; Yen, C.H.; Chan, L.P.; et al. Ethyl acetate extract of Nepenthes adrianii x clipeata induces antiproliferation, apoptosis, and DNA damage against oral cancer cells through oxidative stress. Environ. Toxicol. 2019, 34, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Schuller Levis, G.B.; Lee, E.B.; Levis, W.R.; Lee, D.W.; Kim, B.S.; Park, S.Y.; Park, E. Platycodin D and D3 isolated from the root of Platycodon grandiflorum modulate the production of nitric oxide and secretion of TNF-alpha in activated RAW 264.7 cells. Int. Immunopharmacol. 2004, 4, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Pemmari, A.; Paukkeri, E.L.; Hämäläinen, M.; Leppänen, T.; Korhonen, R.; Moilanen, E. MKP-1 promotes anti-inflammatory M(IL-4/IL-13) macrophage phenotype and mediates the anti-inflammatory effects of glucocorticoids. Basic Clin. Pharmacol. Toxicol. 2019, 124, 404–415. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Rodríguez-Coello, A.; Latire, T.; Bourgougnon, N.; Torres, M.D.; Buján, M.; Muíños, A.; Muiños, A.; Meijide-Faílde, R.; Blanco, F.J.; et al. Anti-inflammatory potential of ulvan. Int. J. Biol. Macromol. 2023, 253 Pt 4, 126936. [Google Scholar] [CrossRef]
- Hermanns, H.M.; Wohlfahrt, J.; Mais, C.; Hergovits, S.; Jahn, D.; Geier, A. Endocytosis of pro-inflammatory cytokine receptors and its relevance for signal transduction. Biol. Chem. 2016, 397, 695–708. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, C.Y.; Deng, W.M. The role of pro-inflammatory cytokines in lipid metabolism of metabolic diseases. Int. Rev. Immunol. 2019, 38, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.C.O.; Costa, A.S.; Luz, P.B.; Soares, P.M.G.; Alencar, N.M.N.; Oliveira, H.D. Morindacitrifolia lipid transfer protein 1 exhibits anti-inflammatory activity by modulation of pro- and anti-inflammatory cytokines. Int. J. Biol. Macromol. 2017, 103, 1121–1129. [Google Scholar] [CrossRef]
- Noailles, A.; Maneu, V.; Campello, L.; Lax, P.; Cuenca, N. Systemic inflammation induced by lipopolysaccharide aggravates inherited retinal dystrophy. Cell Death Dis. 2018, 9, 350. [Google Scholar] [CrossRef]
- Shin, W.B.; Dong, X.; Kim, Y.S.; Park, J.S.; Kim, S.J.; Go, E.A.; Kim, E.K.; Park, P.J. Anti-inflammatory Effects of Batillariamultiformis Water Extracts via NF-κB and MAPK Signaling Pathways in LPS-Induced RAW 264.7 Cells. Adv. Exp. Med. Biol. 2019, 1155, 1001–1014. [Google Scholar]
- Diao, J.; Chi, Z.; Guo, Z.; Zhang, L. Mung Bean Protein Hydrolysate Modulates the Immune Response Through NF-κB Pathway in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. J. Food Sci. 2019, 84, 2652–2657. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Oh, Y.C.; Cho, W.K.; Yim, N.H.; Ma, J.Y. Hoveniae Semen Seu Fructus Ethanol Extract Exhibits Anti-Inflammatory Activity via MAPK, AP-1, and STAT Signaling Pathways in LPS-Stimulated RAW 264.7 and Mouse Peritoneal Macrophages. Mediators Inflamm. 2019, 2019, 9184769. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Shin, J.S.; Chung, K.S.; Lee, Y.G.; Baek, N.I.; Lee, K.T. Anti-Inflammatory Mechanisms of Koreanaside A, a Lignan Isolated from the Flower of Forsythia koreana, against LPS-Induced Macrophage Activation and DSS-Induced Colitis Mice: The Crucial Role of AP-1, NF-κB, and JAK/STAT Signaling. Cells 2019, 8, 1163. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, M.; Ahmed, M.; Naz, S.; Ayaz, M. Cytotoxic, antibacterial and antioxidant activities of extracts of the bark of Melia azedarach (China Berry). Nat. Prod. Res. 2015, 29, 1170–1172. [Google Scholar] [CrossRef] [PubMed]
- Descalzo, A.M.; Coto, C. Inhibición del virus de pseudorrabia (Suid herpesvirus 1) poracción de un antiviral aislado de hojas de Melia azedarach [Inhibition of the pseudorabies virus (Suis herpesvirus 1) by an antiviral agent isolated from the leaves of Melia azedarach]. Rev. Argent. Microbiol. 1989, 21, 133–140. (In Spanish) [Google Scholar]
- D’Ambrosio, M.; Guerriero, A. Degraded limonoids from Melia azedarach and biogenetic implications. Phytochemistry 2002, 60, 419–424. [Google Scholar] [CrossRef]
- Su, Z.S.; Yang, S.P.; Zhang, S.; Dong, L.; Yue, J.M. Meliarachins A-K: Eleven limonoids from the twigs and leaves of Melia azedarach. Helv. Chim. Acta 2011, 94, 1515–1526. [Google Scholar] [CrossRef]
- Akihisa, T.; Pan, X.; Nakamura, Y.; Kikuchi, T.; Takahashi, N.; Matsumoto, M.; Ogihara, E.; Fukatsu, M.; Koike, K.; Tokuda, H. Limonoids from the fruits of Melia azedarach and their cytotoxic activities. Phytochemistry 2013, 89, 59–70. [Google Scholar] [CrossRef]
- Pan, X.; Matsumoto, M.; Nakamura, Y.; Kikuchi, T.; Zhang, J.; Ukiya, M.; Suzuki, T.; Koike, K.; Akihisa, R.; Akihisa, T. Three new and other limonoids from the hexane extract of Melia azedarach fruits and their cytotoxic activities. Chem. Biodivers. 2014, 11, 987–1000. [Google Scholar] [CrossRef]
- Zhou, F.; Ma, X.H.; Li, Z.J.; Li, W.; Zheng, W.M.; Wang, Z.B.; Zeng, X.M.; Sun, K.H.; Zhang, Y.H. Four New Tirucallane Triterpenoids from the Fruits of Melia azedarach and Their Cytotoxic Activities. Chem. Biodivers. 2016, 13, 1738–1746. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, Y.; Li, X.; Sun, X.; Wang, Z.; Wang, H.; Nie, R.; Yu, W.; Zhou, Y. Coniferyl Aldehyde Inhibits the Inflammatory Effects of Leptomeningeal Cells by Suppressing the JAK2 Signaling. BioMed Res. Int. 2020, 2020, 4616308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.N.; Huang, L.; Ma, R.J.; Yang, M.F.; Wei, B.F.; Song, H.Z.; Wang, H.S.; Tan, Q.G. Chemical constituents from the barks of Melia azedarach and their PTP1B inhibitory activity. Nat. Prod. Res. 2021, 35, 4442–4447. [Google Scholar] [CrossRef]
- Song, M.; Luo, H.J.; Li, Z.W.; Qiu, L.; Zhao, Y.X.; He, C.W.; Zhang, X.Q.; Ye, W.C.; Lin, L.G.; Zhang, Q.W. Limonoids from the roots of Melia azedarach and their anti-inflammatory activity. Phytochemistry 2023, 216, 113869. [Google Scholar] [CrossRef] [PubMed]
- Hieu, T.T.; Thuy, P.T.; Duc, D.X. Isolation and Bioactivities of Limonoids from Meliaceae Family: A Review. Curr. Org. Chem. 2022, 26, 1359–1430. [Google Scholar] [CrossRef]
- Zhou, H.; Hamazaki, A.; Fontana, J.D.; Takahashi, H.; Wandscheer, C.B.; Fukuyama, Y. Cytotoxic limonoids from Brazilian Melia azedarach. Chem. Pharm. Bull. 2005, 53, 1362–1365. [Google Scholar] [CrossRef]
- Huang, R.C.; Okamura, H.; Iwagawa, T.; Nakatani, M. The Structures of Azedarachins, Limonoid Antifeedants from ChineseMeliaazedarachLinn. Bull. Chem. Soc. Jpn. 1994, 67, 2468–2472. [Google Scholar] [CrossRef]
- Nakatani, M.; Chun Huang, R.; Okamura, H.; Naoki, H.; Iwagawa, T. Limonoid antifeedants from chinese Melia azedarach. Phytochemistry 1994, 36, 39–41. [Google Scholar] [CrossRef]
- Vajrabhaya, L.O.; Korsuwannawong, S. Cytotoxicity evaluation of a Thai herb using tetrazolium (MTT) and sulforhodamine B (SRB) assays. J. Anal. Sci. Technol. 2018, 9, 15. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Liang, R.; Liu, C.; Chen, M.; Chen, J. Synergistic Anti-Inflammatory Effects of Lipophilic Grape Seed Proanthocyanidin and Camellia Oil Combination in LPS-Stimulated RAW264.7 Cells. Antioxidants 2022, 11, 289. [Google Scholar] [CrossRef]
- Robles, V.; Riesco, M.F.; Martínez-Vázquez, J.M.; Valcarce, D.G. Flow Cytometry and Confocal Microscopy for ROS Evaluation in Fish and Human Spermatozoa. Methods Mol. Biol. 2021, 2202, 93–102. [Google Scholar]
- Kim, S.H.; Kang, I.C. Induction of TNF-α by Filifactoralocis in THP-1 macrophagic cells. Arch. Oral Biol. 2023, 155, 105806. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Hong, Y.; Wan, Y.; He, X.; Geng, B.; Yang, X.; Xiang, J.; Cai, J.; Zeng, Z.; Liu, Z.; et al. PVB exerts anti-inflammatory effects by inhibiting the activation of MAPK and NF-κB signaling pathways and ROS generation in neutrophils. Int. Immunopharmacol. 2024, 126, 111271. [Google Scholar] [CrossRef] [PubMed]
- Vieira, I.J.; Azevedo Ode, A.; de Souza, J.J.; Braz-Filho, R.; Gonçalves Mdos, S.; de Araújo, M.F. Hirtinone, a Novel cycloartane-type triterpene and other compounds from Trichiliahirta L. (Meliaceae). Molecules 2013, 18, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Jolad, S.D.; Hoffmann, J.J.; Cole, J.R.; Tempesta, M.S.; Bates, R.B. Constituents of Trichilia hispida (Meliaceae). 2. A new triterpenoid, hispidone, and bourjotinolone A. J. Org. Chem. 1980, 45, 3132–3135. [Google Scholar] [CrossRef]
- Pescitelli, G.; Bruhn, T. Good Computational Practice in the Assignment of Absolute Configurations by TDDFT Calculations of ECD Spectra. Chirality 2016, 28, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Pescitelli, G. ECD exciton chirality method today: A modern tool for determining absolute configurations. Chirality 2022, 34, 333–363. [Google Scholar] [CrossRef]
- Manosroi, A.; Kitdamrongtham, W.; Ishii, K.; Shinozaki, T.; Tachi, Y.; Takagi, M.; Ebina, K.; Zhang, J.; Manosroi, J.; Akihisa, R.; et al. Limonoids from Azadirachta indica var. siamensis extracts and their cytotoxic and melanogenesis-inhibitory activities. Chem. Biodivers. 2014, 11, 505–531. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Xu, R.; Kong, L.Y.; Luo, J. New meliacarpin-type (C-seco) and C-ring intact limonoids from the fruits of Melia toosendan. Fitoterapia 2020, 144, 104605. [Google Scholar] [CrossRef]
- Zhang, H.P.; Bao, G.H.; Wang, H.B.; Qin, G.W. Two new limonoids from Munroniahenryi. Nat. Prod. Res. 2004, 18, 415–419. [Google Scholar] [CrossRef]
- Park, S.; Nhiem, N.X.; Subedi, L.; Oh, I.; Kim, J.Y.; Kim, S.Y.; Kim, S.H. Isolation of bioactive limonoids from the fruits of Melia azedarach. J. Asian Nat. Prod. Res. 2020, 22, 830–838. [Google Scholar] [CrossRef]
- Qiu, L.; Heng, L.; Xu, R.; Luo, J.; Li, Y. Two new nimbolinin- and trichilin-class limonoids isolated from the fruits of Melia azedarach. Chin. J. Nat. Med. 2019, 17, 227–230. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, Y.; Liu, X.T.; Liang, J.Y.; Ip, N.Y.; Min, Z.D. Minor limonoids from Melia toosendan and their antibacterial activity. Planta Med. 2007, 73, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Brasier, A.R. The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc. Res. 2010, 86, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Shrestha, A.; Park, P.; Lee, E. Hydroxyl-and Halogen-containing Chalcones for the Inhibition of LPS-stimulated ROS Production in RAW 264.7 Macrophages: Design, Synthesis and Structure–Activity Relationship Study. Bull. Korean Chem. Soc. 2019, 40, 729–734. [Google Scholar] [CrossRef]
- Zong, L.; Zhang, J.; Dai, L.; Liu, J.; Yang, Y.; Xie, J.; Luo, X. The Anti-Inflammatory Properties of Rhododendron molle Leaf Extract in LPS-Induced RAW264.7. Chem. Biodivers. 2020, 17, e2000477. [Google Scholar] [CrossRef]
- Yu, S.; Chen, X.; Xiu, M.; He, F.; Xing, J.; Min, D.; Guo, F. The regulation of Jmjd3 upon the expression of NF-κB downstream inflammatory genes in LPS activated vascular endothelial cells. Biochem. Biophys. Res. Commun. 2017, 485, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Dunkhunthod, B.; Talabnin, C.; Murphy, M.; Thumanu, K.; Sittisart, P.; Hengpratom, T.; Eumkeb, G. Intracellular ROS Scavenging and Anti-Inflammatory Activities of Oroxylum indicum Kurz (L.) Extract in LPS plus IFN-γ-Activated RAW264.7 Macrophages. Evid. Based Complement. Altern. Med. 2020, 2020, 7436920. [Google Scholar] [CrossRef]
- Liu, H.; Pan, Z.; Ma, X.; Cui, J.; Gao, J.; Miao, Q.; Zhu, Z.; Chen, X.; Su, S. ROCK inhibitor fasudil reduces the expression of inflammatory factors in LPS-induced rat pulmonary microvascular endothelial cells via ROS/NF-κB pathway. BMC Pharmacol. Toxicol. 2022, 23, 24. [Google Scholar] [CrossRef]
- Singh, G.; Bhatti, R.; Mannan, R.; Singh, D.; Kesavan, A.; Singh, P. Osthole ameliorates neurogenic and inflammatory hyperalgesia by modulation of iNOS, COX-2, and inflammatory cytokines in mice. Inflammopharmacology 2019, 27, 949–960. [Google Scholar] [CrossRef]
- Wu, H.; Liu, H.; Zhao, X.; Zheng, Y.; Liu, B.; Zhang, L.; Gao, C. IKIP Negatively Regulates NF-κB Activation and Inflammation through Inhibition of IKKα/β Phosphorylation. J. Immunol. 2020, 204, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef]
- Laroux, F.S.; Pavlick, K.P.; Hines, I.N.; Kawachi, S.; Harada, H.; Bharwani, S.; Hoffman, J.; Grisham, M.B. Role of nitric oxide in inflammation. Acta Physiol. Scand. 2001, 173, 113–118. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxinitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Kaushal, G.P.; Chandrashekar, K.; Juncos, L.A. Molecular interaction between reactive oxygen species and autophage in kidney disease. Int. J. Mol. Sci. 2019, 20, 3791. [Google Scholar] [CrossRef]
- Sandoval-Acuna, C.; Ferreira, J.; Speisky, H. Polyphenola and mitochondria: An update on their increasingly emerging ROS scavenging independent action. Arch. Biochem. Biophys. 2014, 559, 75–90. [Google Scholar] [CrossRef]
- Samard, T.A.; Moore, K.A.; Spirstein, A.; Billet, S.; Allchrone, A.; Poole, S.; Bonventre, J.V.; Woolf, C.J. Interleukin-1-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature 2001, 410, 471–475. [Google Scholar] [CrossRef]
- Dinarello, C.A. Proinflammatory Cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef]
- Lawrence, T.; Gilroy, D.W.; Colville-Nash, P.R.; Willoughby, D.A. Possible new role for NF-kB in the resolution of inflammation. Nat. Med. 2001, 7, 1291–1297. [Google Scholar] [CrossRef]
- Lewis, A.J.; Mamming, A.M. New targets for anti-inflammatory drugs. Curr. Opin. Chem. Biol. 1999, 3, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Naumann, M. BeyondIkBs: Alternative regulation of NF-kB activity. FASEB J. 2007, 21, 2642–2654. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; He, G.; Hao, Y.; Chen, C.; Li, M.; Wang, Y.; Zhang, G.; Yu, Z. The role of the JAK2-STAT3 pathway in pro-inflammatory responses of EMF-stimulated N9 microglial cells. J. Neuroinflamm. 2010, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Gebru, E.; Kang, E.H.; Damte, D.; Lee, J.S.; Jang, S.H.; Kim, M.H.; Cheng, H.; Park, S.C. The role of Janus kinase 2 (JAK2) activation in pneumococcal EstAprotein-induced inflammatory response in RAW 264.7 macrophages. Microb. Pathog. 2011, 51, 297–303. [Google Scholar] [CrossRef]
- Huang, F.M.; Chang, Y.C.; Lee, S.S.; Yang, M.L.; Kuan, Y.H. Expression of pro-inflammatory cytokines and mediators induced by Bisphenol A via ERK-NFκB and JAK1/2-STAT3 pathways in macrophages. Environ. Toxicol. 2019, 34, 486–494. [Google Scholar] [CrossRef]





| Proton | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 1.49(m) | 3.59(t,2.8) | 3.86(t,3.9) | 4.86(m) | 3.56(s) |
| 2.01(m) | |||||
| 2 | 2.26(dt,11.2,3.2) | 2.02(dd,2.8,15.1) | 1.09(dd,2.0,15.1) | 1.06(dd,1.5,10.9) | 2.15(m) |
| 2.78(dt,14.7,3.8) | 2.34(dd,3.2,15.1) | 2.28(dd,2.3,15.1) | 2.38(dd,2.8,10.9) | 2.19(m) | |
| 3 | 5.08(t,2.7) | 4.94(t,4.1) | 4.89(t,4.1) | 4.86(t,2.5) | |
| 5 | 1.75(m) | 2.67(d,12.6) | 2.45(d,12.1) | 2.66(d,4.8) | 2.76(d,12.7) |
| 6 | 2.11(m) | 4.28(d,12.6) | 4.17(d,12.1) | 4.16(d,12.2) | 4.01(dd,12.5,3.0) |
| 7 | 5.34(t,3.1) | 5.90(d,3.0) | 4.19(d,2.8) | 4.22(d,3.3) | 5.68(d,2.8) |
| 9 | 2.32(m) | 3.58(dd,5.5,7.3) | 2.64(dd,5.5,6.9) | 2.94(dd,3.6,4.4) | 2.98(d,7.5) |
| 11 | 1.61(m) | 2.54(m) | 2.28(m) | 2.28(m) | 1.72(m) |
| 2.34(m) | 2.03(m) | 2.08(m) | 1.78(m) | ||
| 12 | 1.60(m) | 4.87(m) | 3.85(m) | 5.94(s) | |
| 2.03(m) | |||||
| 15 | 1.62(m) | 5.73(t,2.5) | 5.66(t,3.7) | 5.64(t,3.5) | 4.95(7.4) |
| 16 | 1.51(m) | 2.34(m) | 2.45(m) | 2.38(m) | 1.77(m) |
| 1.91(m) | 2.43(m) | 2.64(m) | 2.51(m) | 2.19(m) | |
| 17 | 1.85(m) | 3.46(m) | 2.90(m) | 2.94(m) | 3.36(m) |
| 18 | 0.84(s) | 0.97(s) | 0.95(s) | 0.91(s) | 1.79(s) |
| 19 | 1.03(s) | 0.98(s) | 1.00(s) | 1.03(s) | 0.89(s) |
| 20 | 2.03(m) | ||||
| 21 | 5.48(d,2.2) | 7.25(t,1.6) | 5.88(s) | 6.09(s) | |
| 22 | 2.02(m) | 6.48(d,1.6) | 5.85(s) | 6.85(d,1.2) | 5.82(m) |
| 1.12(m) | |||||
| 23 | 4.66(d,5.1) | 7.27(s) | 6.43(d,1.8) | ||
| 24 | 3.37(d,5.2) | ||||
| 26 | 1.18(s) | ||||
| 27 | 1.17(s) | ||||
| 28 | 1.07(s) | 3.18(d,7.8) | 3.62(d,7.6) | 3.62(d,7.6) | 3.47(d,7.6) |
| 3.46(d,7.8) | 4.12(d,7.6) | 4.11(d,8.0) | 3.56(d,7.6) | ||
| 29 | 1.14(s) | 1.17(s) | 1.15(s) | 1.15(s) | 1.12(s) |
| 30 | 1.06(s) | 1.18(s) | 1.09(s) | 1.09(s) | 1.46(s) |
| 12-OCH3 | 3.37(s) | ||||
| 1-OAC | 1.83(s) | ||||
| 3-OAC | 1.82(s) | 2.02(s) | |||
| 12-OAC | 2.03(s) | ||||
| 2’ | 6.40(d,15.9) | 6.03(d,17.9) | |||
| 3’ | 8.05(dd,7.6,1.3) | 7.72(d,16.0) | 7.71(d,15.9) | 6.88(qd,7.0,1.5) | |
| 4’ | 7.39(t,7.6) | 1.73(d,7.0) | |||
| 5’ | 7.53(t,7.5) | 7.48(dd,7.2,1.7) | 7.49(dd,4.4,1.9) | 1.78(s) | |
| 6’ | 7.39(t,7.6) | 7.38(t,4.9) | 7.39(t,6.9) | ||
| 7’ | 8.05(dd, 7.6,1.3) | 7.40(t,4.1) | 7.40(t,8.1) | ||
| 8’ | 7.38(t,4.9) | 7.39(t,6.9) | |||
| 9’ | 7.48(dd,7.2,1.7) | 7.49(dd,4.4,1.9) |
| Carbon | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 38.6 | 71.8 | 71.1 | 77.2 | 70.6 |
| 2 | 35.1 | 30.3 | 25.1 | 24.5 | 29.1 |
| 3 | 217.0 | 73.6 | 73.1 | 72.4 | 72.2 |
| 4 | 47.7 | 42.5 | 43.9 | 43.9 | 42.4 |
| 5 | 52.5 | 40.2 | 38.7 | 38.7 | 39.0 |
| 6 | 24.5 | 72.9 | 73.7 | 73.9 | 72.5 |
| 7 | 118.2 | 74.7 | 74.1 | 74.0 | 74.6 |
| 8 | 145.7 | 44.5 | 39.7 | 39.8 | 45.4 |
| 9 | 48.0 | 36.6 | 34.3 | 34.8 | 36.4 |
| 10 | 35.2 | 40.3 | 45.2 | 45.2 | 41.2 |
| 11 | 17.9 | 34.1 | 30.5 | 30.4 | 30.5 |
| 12 | 31.6 | 213.8 | 77.9 | 71.2 | 97.8 |
| 13 | 43.6 | 61.5 | 52.5 | 51.7 | 140.3 |
| 14 | 50.9 | 154.1 | 156.1 | 157.1 | 146.1 |
| 15 | 34.4 | 123.8 | 122.5 | 122.2 | 77.9 |
| 16 | 27.6 | 34.2 | 35.5 | 35.8 | 35.8 |
| 17 | 48.5 | 42.9 | 51.2 | 48.9 | 49.3 |
| 18 | 23.3 | 19.4 | 15.5 | 15.1 | 16.5 |
| 19 | 12.8 | 15.6 | 16.1 | 15.5 | 16.7 |
| 20 | 47.1 | 124.6 | 169.1 | 137.5 | _ a |
| 21 | 101.8 | 142.4 | 99.1 | 171.4 | 98.7 |
| 22 | 33.6 | 112.6 | 120.4 | 146.5 | 118.7 |
| 23 | 76.7 | 141.0 | 170.9 | 96.5 | 170.8 |
| 24 | 86.5 | ||||
| 25 | 71.1 | ||||
| 26 | 25.9 | ||||
| 27 | 25.7 | ||||
| 28 | 24.6 | 78.1 | 78.4 | 78.4 | 78.1 |
| 29 | 21.7 | 18.8 | 20.2 | 20.2 | 19.0 |
| 30 | 27.4 | 25.8 | 26.7 | 26.9 | 20.7 |
| 12-OCH3 | 55.2 | ||||
| 1-OAC | 170.8 | ||||
| 21.2 | |||||
| 3-OAC | 169.2 | 170.7 | |||
| 20.9 | 21.3 | ||||
| 12-OAC | 170.9 | ||||
| 21.5 | |||||
| 1’ | 165.1 | 165.4 | 165.4 | 166.7 | |
| 2’ | 130.5 | 117.0 | 117.2 | 128.6 | |
| 3’ | 129.6 | 146.8 | 144.8 | 137.5 | |
| 4’ | 128.6 | 133.8 | 134.1 | 14.6 | |
| 5’ | 133.2 | 128.4 | 128.5 | 12.2 | |
| 6’ | 128.6 | 129.2 | 129.2 | ||
| 7’ | 129.6 | 131. | 130.9 | ||
| 8’ | 129.2 | 129.2 | |||
| 9’ | 128.4 | 128.5 |
| Compounds | IC50 (μM) | Compounds | IC50 (μM) |
|---|---|---|---|
| 1 | 26.85 ± 1.56 | 6 | 64.95 ± 3.09 |
| 2 | 22.04 ± 0.96 | 7 | 64.95 ± 3.09 |
| 3 | >100 | 8 | 50.80 ± 4.06 |
| 4 | >100 | 9 | >100 |
| 5 | 24.06 ± 1.92 | Indomethacin a | 37.06 ± 2.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, F.; Chen, J.; Lin, Z.-T.; Lin, H.-Y.; Liu, B.; Chen, Z.-W.; Ma, X.-H.; Zhang, Y.-H. Chemical Constituents from the Fruit of Melia azedarach and Their Anti-Inflammatory Activity. Antioxidants 2024, 13, 1338. https://doi.org/10.3390/antiox13111338
Cao F, Chen J, Lin Z-T, Lin H-Y, Liu B, Chen Z-W, Ma X-H, Zhang Y-H. Chemical Constituents from the Fruit of Melia azedarach and Their Anti-Inflammatory Activity. Antioxidants. 2024; 13(11):1338. https://doi.org/10.3390/antiox13111338
Chicago/Turabian StyleCao, Fan, Jing Chen, Zheng-Tao Lin, Han-Ying Lin, Bin Liu, Zhen-Wei Chen, Xin-Hua Ma, and Yong-Hong Zhang. 2024. "Chemical Constituents from the Fruit of Melia azedarach and Their Anti-Inflammatory Activity" Antioxidants 13, no. 11: 1338. https://doi.org/10.3390/antiox13111338
APA StyleCao, F., Chen, J., Lin, Z.-T., Lin, H.-Y., Liu, B., Chen, Z.-W., Ma, X.-H., & Zhang, Y.-H. (2024). Chemical Constituents from the Fruit of Melia azedarach and Their Anti-Inflammatory Activity. Antioxidants, 13(11), 1338. https://doi.org/10.3390/antiox13111338







