Protective Effects of 17-βE2 on the Primary Hepatocytes of Rainbow Trout (Oncorhynchus mykiss) Under Acute Heat Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary Hepatocyte Separation and Culture
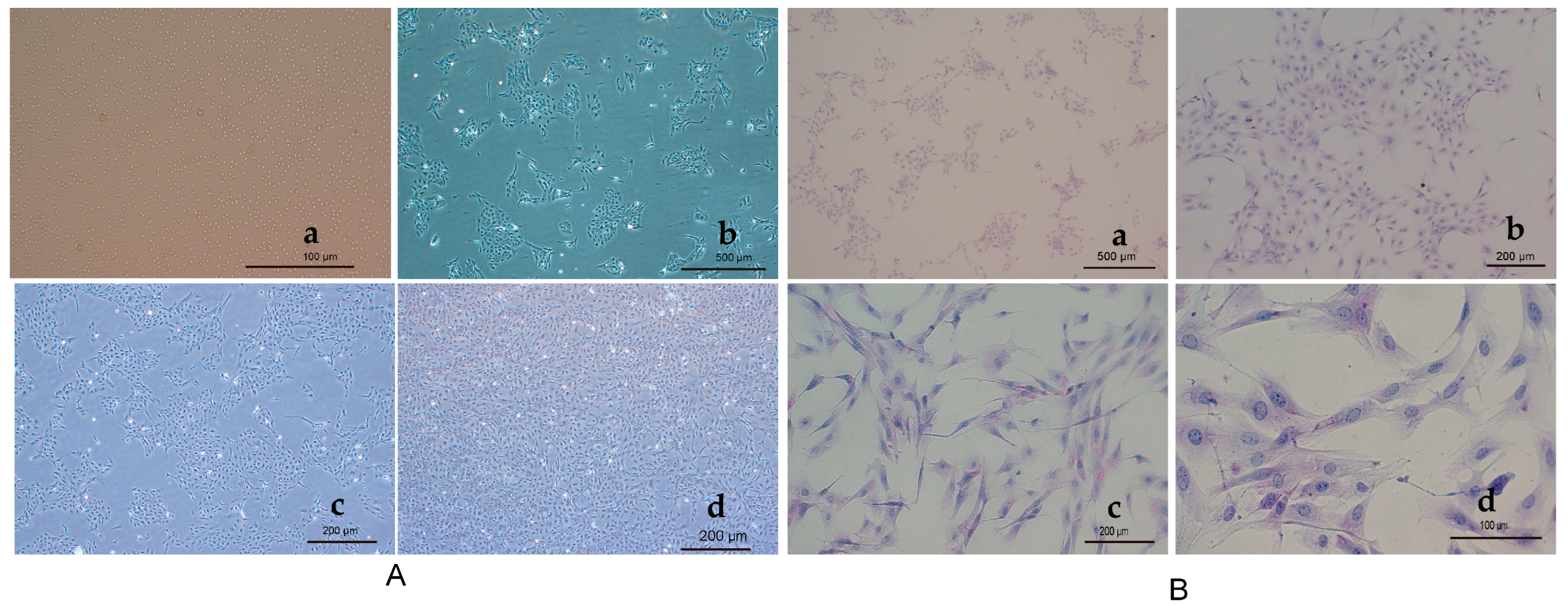
2.2. Hepatocyte Glycogen Staining Glycogen Periodic Acid Schiff (PAS) Staining
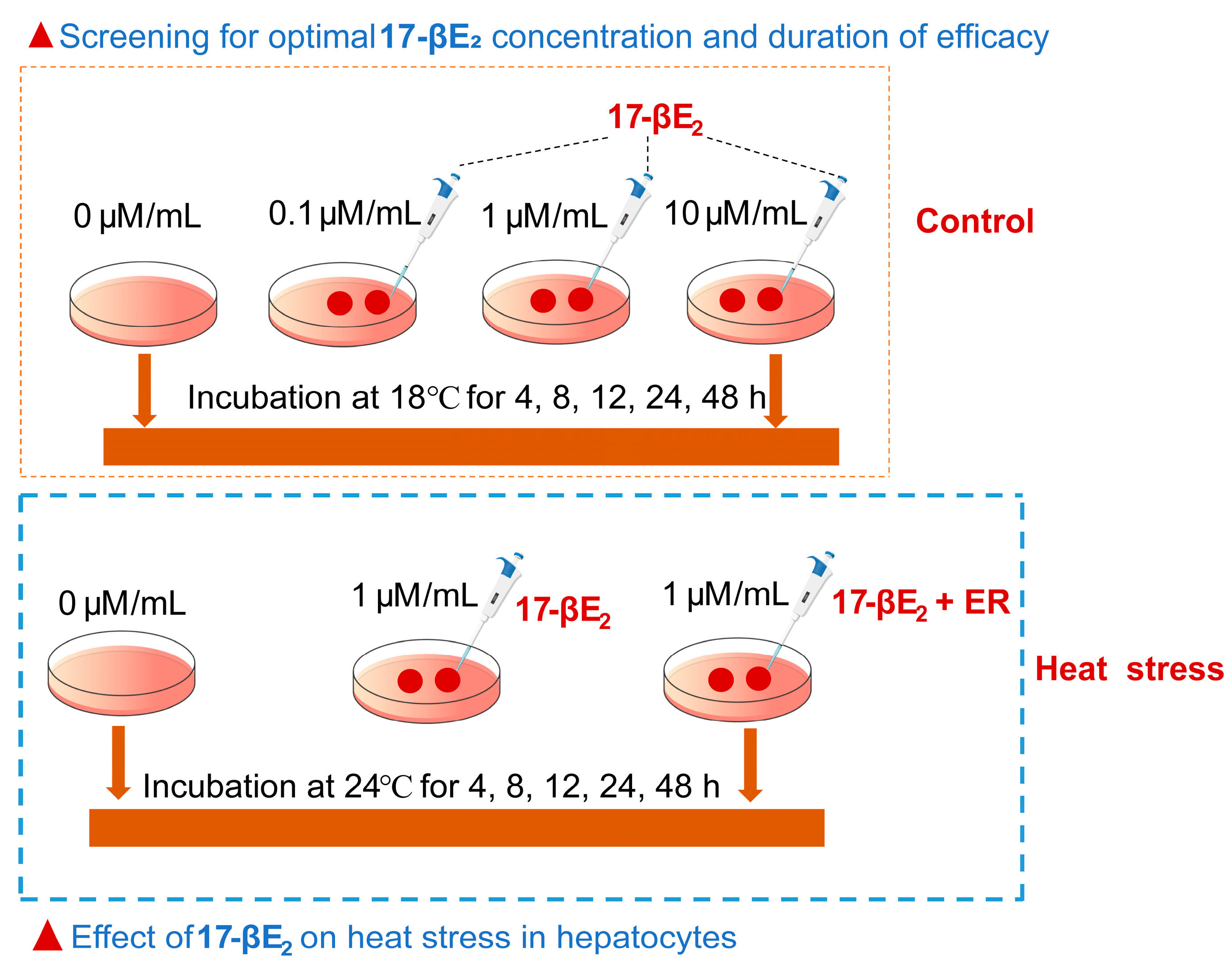
2.3. Treatment with17-βE2
2.4. Treatment with 17-βE2 and Heat Stress
2.5. Cell Viability Assay
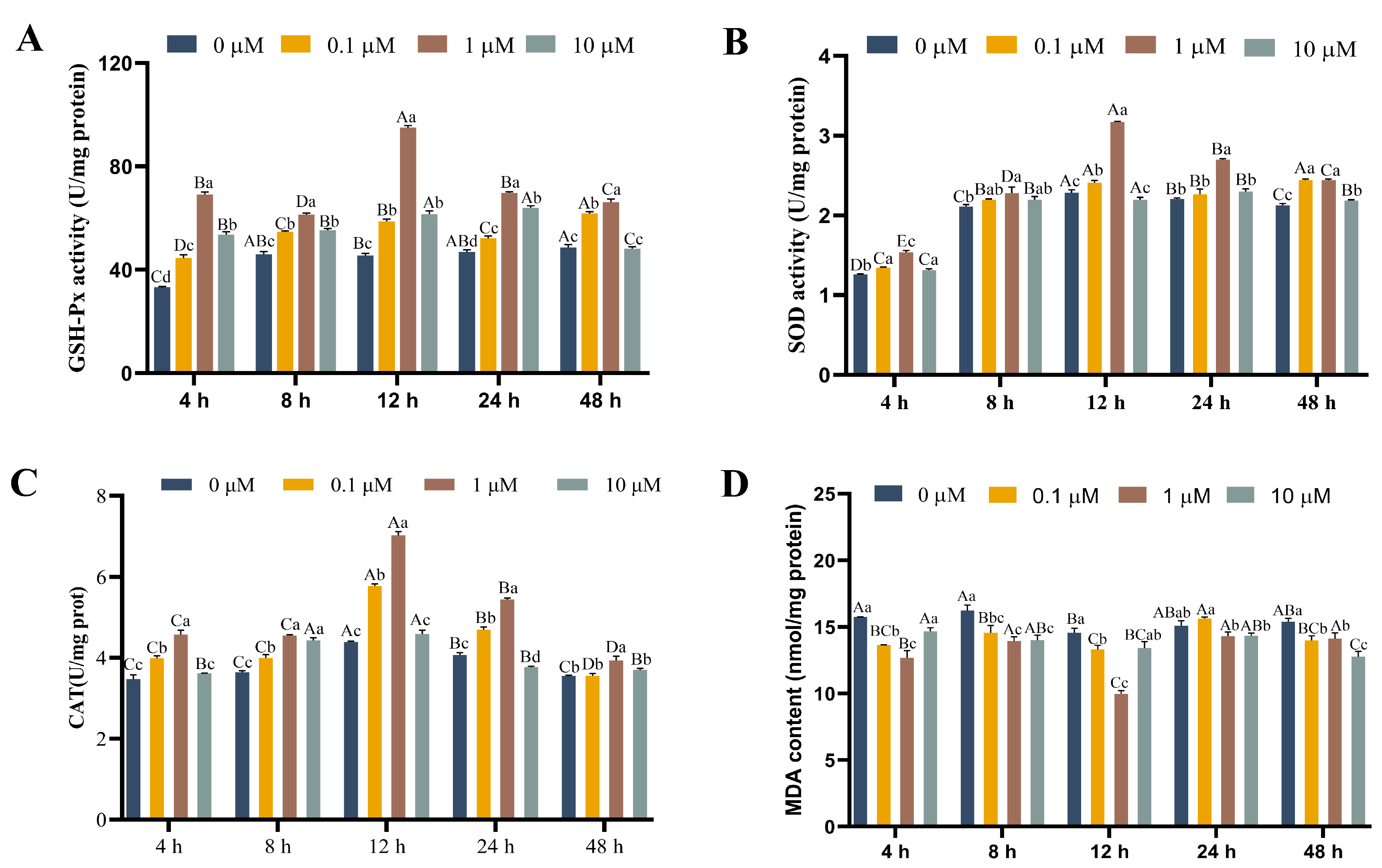
2.6. Oxidative Stress Indicator Testing
2.7. Apoptosis Detection
2.8. Ultrastructural Observation
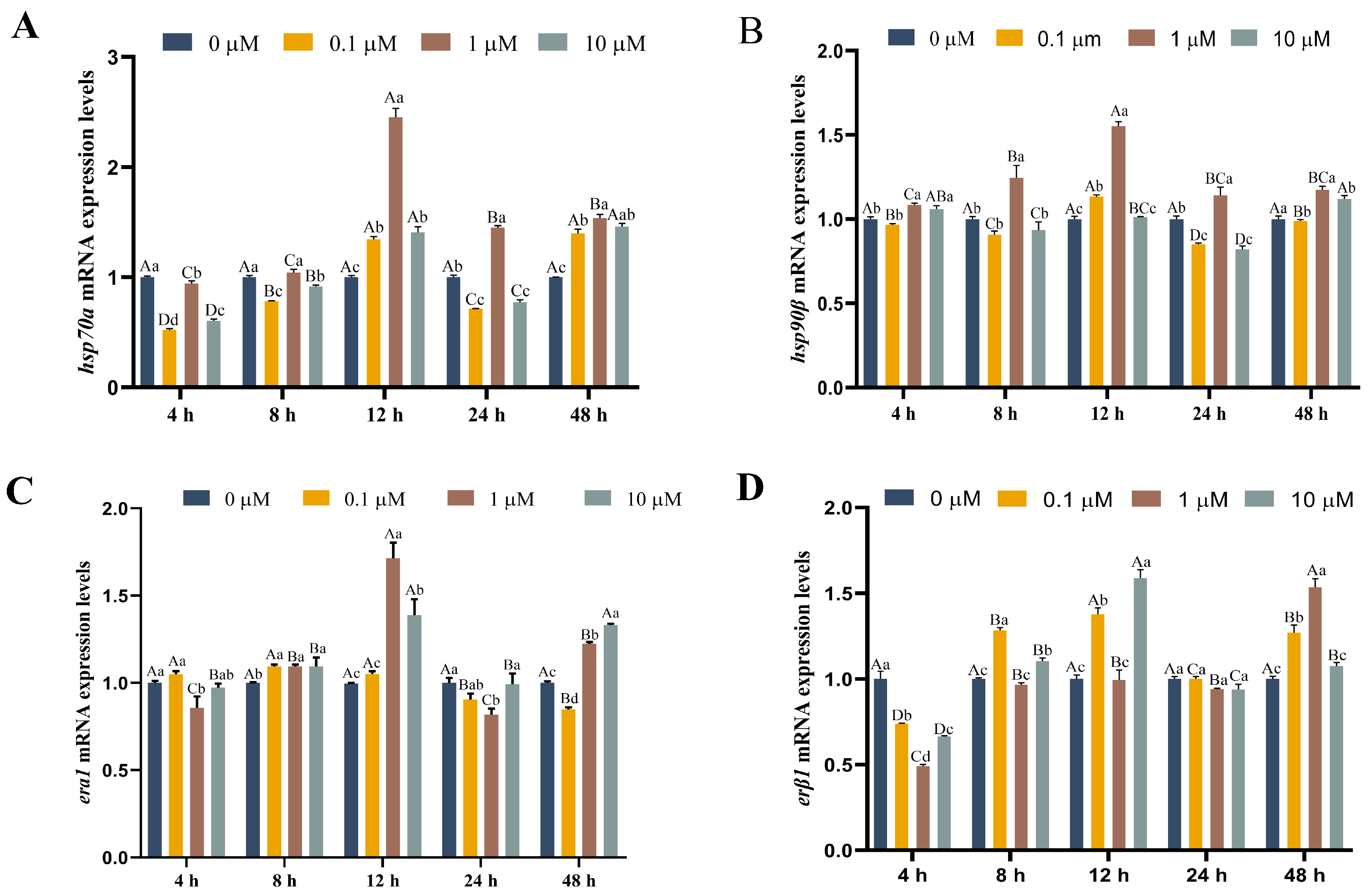
2.9. RNA Isolation and qRT-PCR Assay
2.10. Statistical Analysis
3. Results
3.1. Morphological Observations of Primary Hepatocytes
3.2. Effects of 17-βE2 on Hepatocyte Viability
3.3. Effects of 17-βE2 on the Thermal Stress Parameters
3.4. Functions of 17-βE2 in Thermal Stress-Related Gene Expression
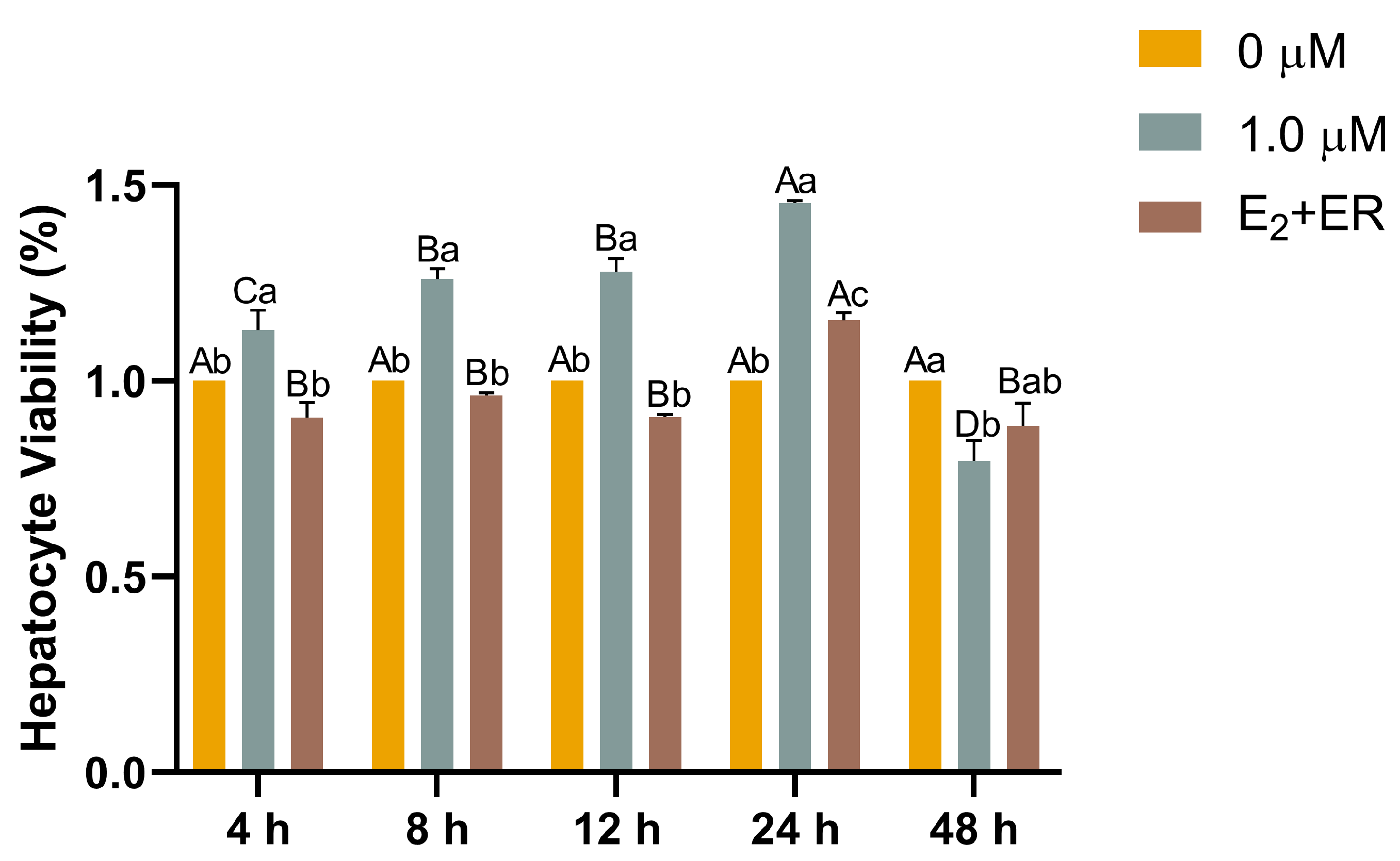
3.5. Functions of 17-βE2 in Heat-Stressed Hepatocyte Viability
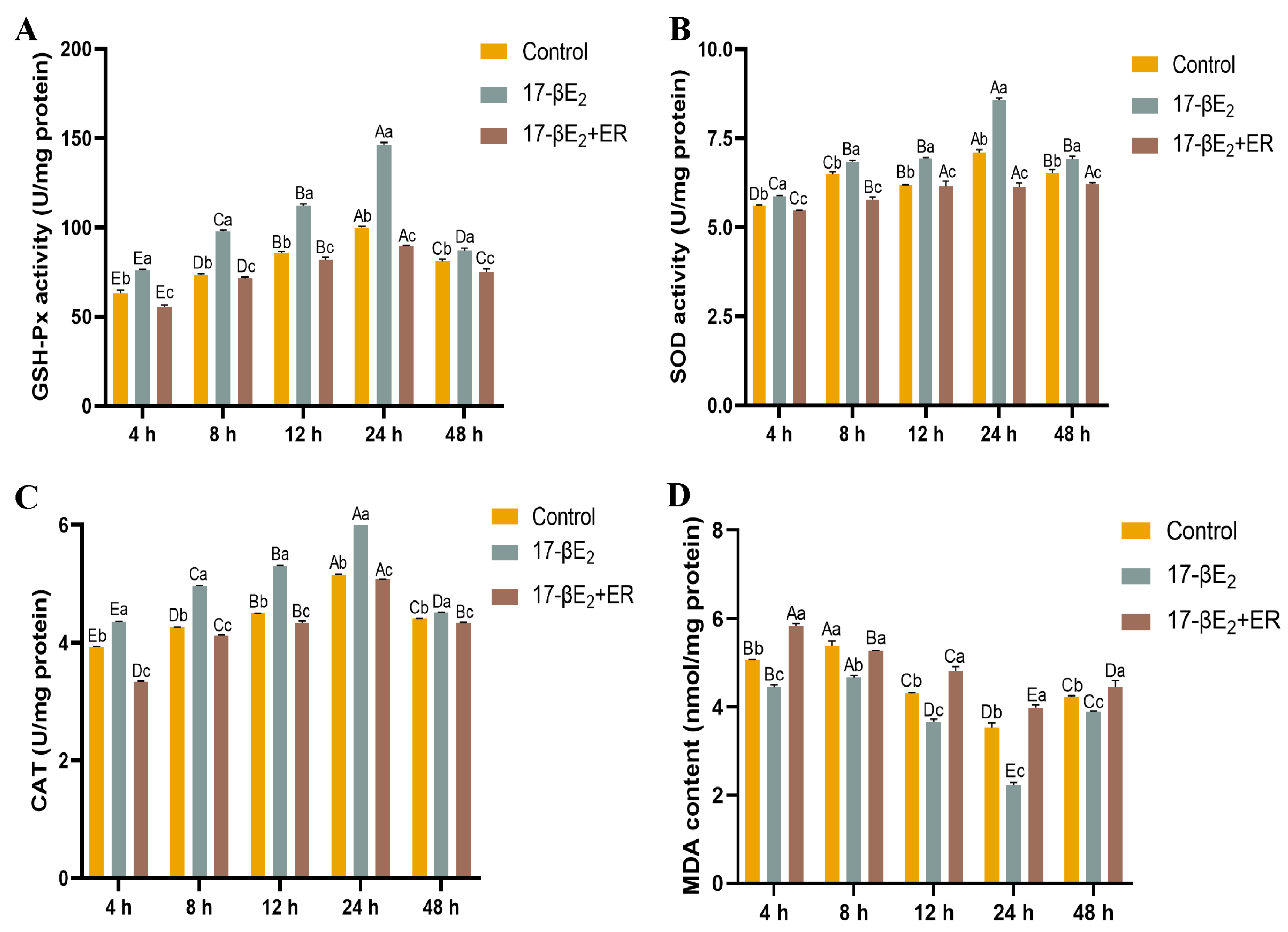
3.6. Functions of 17-βE2 and Heat Stress in the Thermal Stress Parameters
3.7. Effects of 17-βE2 and Heat Stress on Thermal Stress-Related Gene Expression
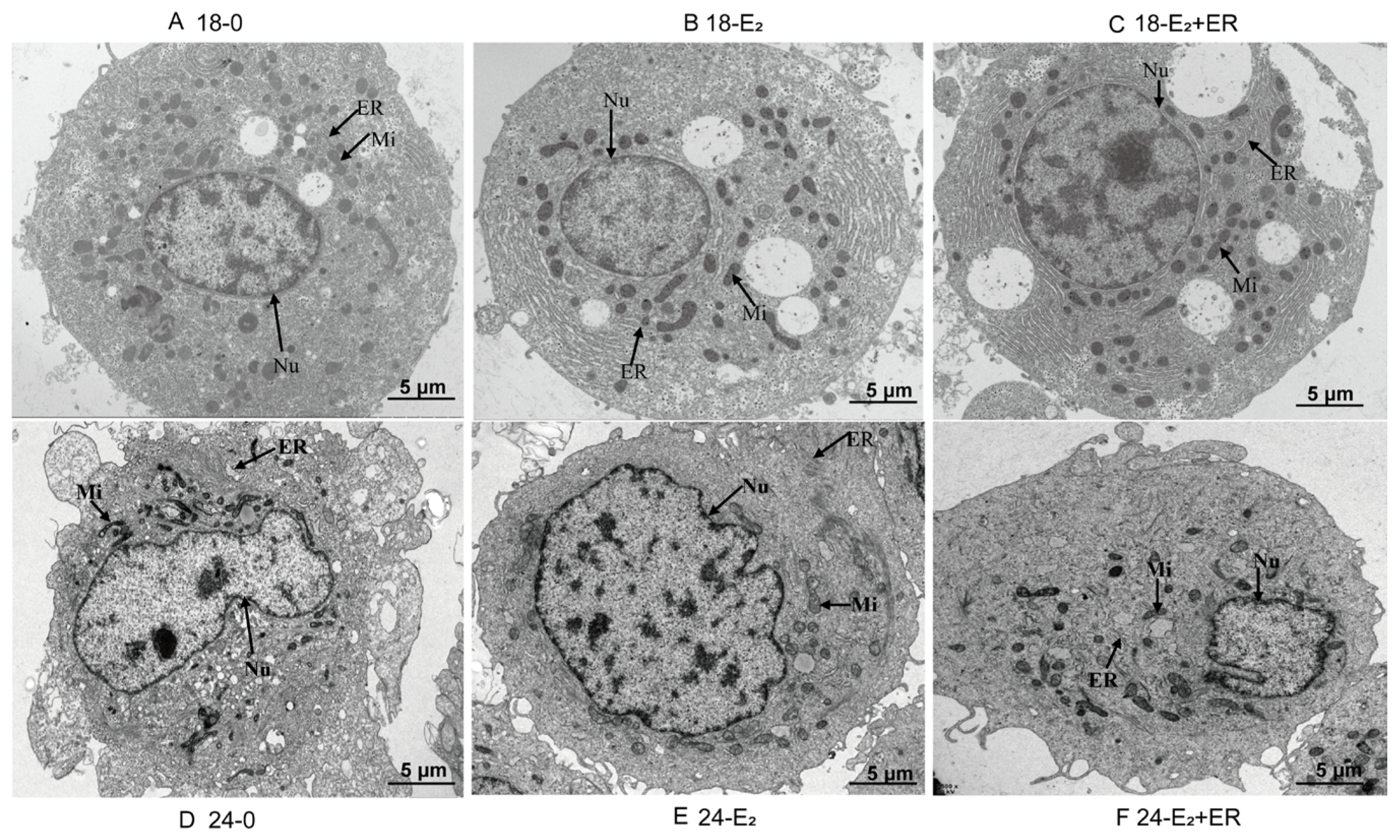
3.8. Effects of 17-βE2 on Hepatocyte Histopathological Alterations upon Heat Stress
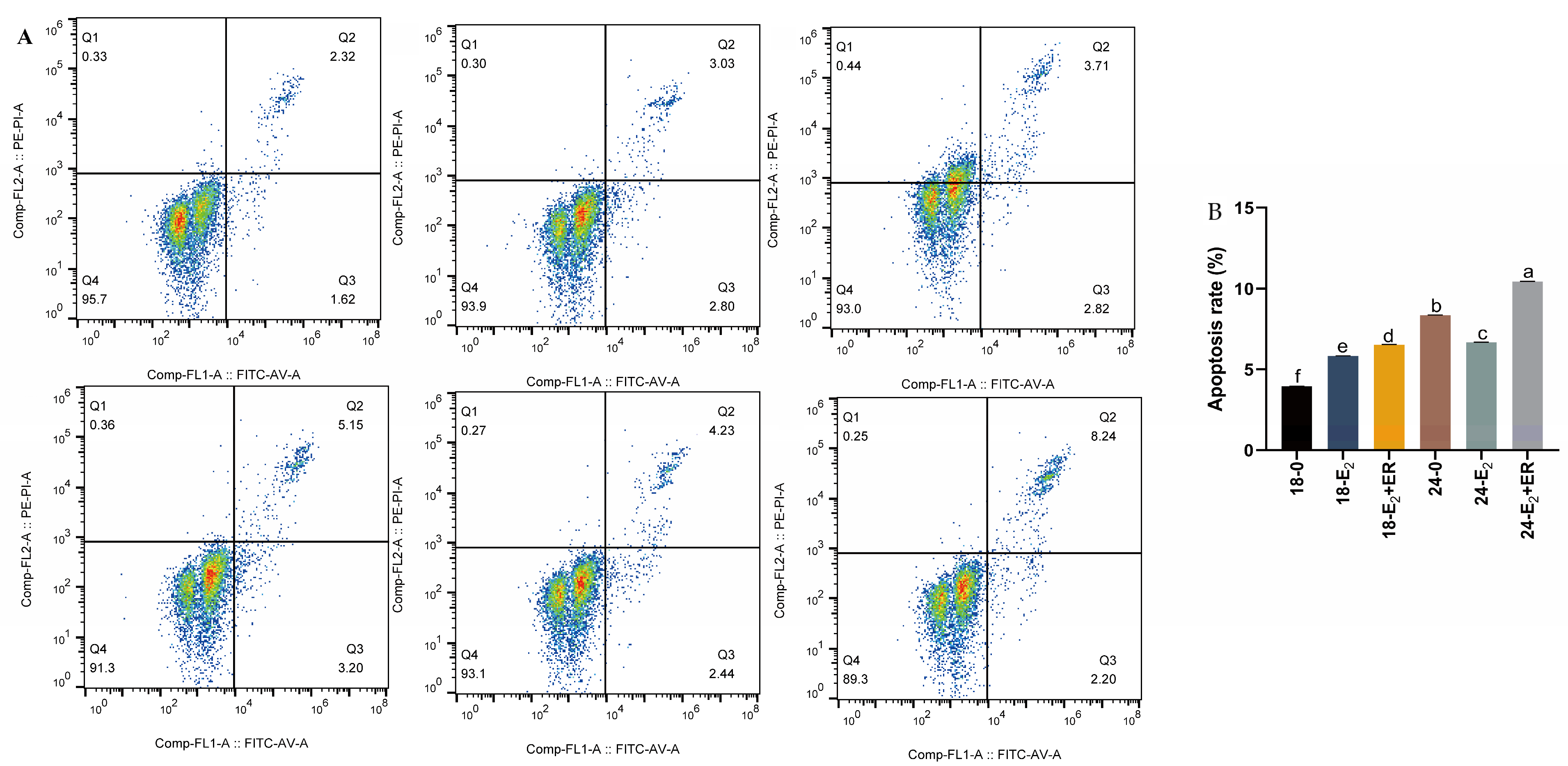
3.9. 17-βE2 Reduces Heat Stress-Mediated Rainbow Trout Hepatocyte Apoptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2021, 98, 1496–1508. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.H.; Xie, J.; Xu, P.; Ge, X.P.; Liu, W.B.; Ye, J.Y. Effects of emodin and vitamin C on growth performance, biochemical parameters and two HSP70s mRNA expression of Wuchang bream (Megalobrama amblycephala Yih) under high temperature stress. Fish Shellfish Immunol. 2012, 32, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.; Sabry, A.; Abdelaziz, A.; Shukry, M. Deleterious impacts of heat stress on steroidogenesis markers, immunity status and ovarian tissue of Nile tilapia (Oreochromis niloticus). J. Therm. Biol. 2020, 91, 102578. [Google Scholar] [CrossRef]
- Wang, Y.F.; Li, C.J.; Pan, C.L.; Liu, E.G.; Zhao, X.Q.; Ling, Q.F. Alterations to transcriptomic profile, histopathology, and oxidative stress in liver of pikeperch (Sander lucioperca) under heat stress. Fish Shellfish Immunol. 2019, 95, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Huang, J.Q.; Liu, Z.; Zhou, Y.J.; Xia, B.P.; Wang, Y.J.; Kang, Y.J.; Wang, J.F. Transcriptome analysis provides insights into hepatic responses to moderate heat stress in the rainbow trout (Oncorhynchus mykiss). Gene 2017, 619, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Liu, Z.; Quan, J.Q.; Sun, J.; Lu, J.H.; Zhao, G.Y. Comprehensive proteomic analysis to elucidate the anti-heat stress effects of nano-selenium in rainbow trout (Oncorhynchus mykiss). Ecotoxicol. Environ. Saf. 2022, 241, 113736. [Google Scholar] [CrossRef]
- Quan, J.Q.; Kang, Y.J.; Luo, Z.C.; Zhao, G.Y.; Ma, F.; Li, L.L.; Liu, Z. Identification and characterization of long noncoding RNAs provide insight into the regulation of gene expression in response to heat stress in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. Part D Genom. Proteom. 2020, 36, 100707. [Google Scholar] [CrossRef]
- Rendell, J.L.; Currie, S. Intracellular localization of hsp90 is influenced by developmental stage and environmental estrogens in rainbow trout Oncorhynchus mykiss. Physiol. Biochem. Zool. 2005, 78, 937–946. [Google Scholar] [CrossRef]
- Osborne, N.; Sherry, J.; Rendell, J.L.; Currie, S. The role of hsp90 in 17alpha-ethynylestradiol-induced endocrine disruption in rainbow trout hepatocytes. Ecotoxicol. Environ. Saf. 2007, 68, 13–19. [Google Scholar] [CrossRef]
- Kang, Y.J.; Liu, Z.; Shi, H.N.; Wang, J.F.; Huang, J.Q.; Li, Y.J.; Li, J.; Wang, Y.J. Label-free quantification of protein expression in the rainbow trout (Oncorhynchus mykiss) in response to short-term exposure to heat stress. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 30, 158–168. [Google Scholar] [CrossRef]
- Beikoghli Kalkhoran, S.; Kararigas, G. Oestrogenic Regulation of Mitochondrial Dynamics. Int. J. Mol. Sci. 2022, 23, 1118. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Steenbergen, C. Estrogen regulation of protein expression and signaling pathways in the heart. Biol. Sex Differ. 2014, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Banerjee, A.; Singh, D.; Thakur, G.; Kasarpalkar, N.; Gavali, S.; Gadkar, S.; Madan, T.; Mahale, S.D.; Balasinor, N.H.; et al. Estradiol: A Steroid with Multiple Facets. Horm. Metab. Res. 2018, 50, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Picard, D. Chaperoning steroid hormone action. Trends Endocrinol. Metab. 2006, 17, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Chambraud, B.; Berry, M.; Redeuilh, G.; Chambon, P.; Baulieu, E.E. Several regions of human estrogen receptor are involved in the formation of receptor-heat shock protein 90 complexes. J. Biol. Chem. 1990, 265, 20686–20691. [Google Scholar] [CrossRef] [PubMed]
- Pratt, W.B.; Toft, D.O. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr. Rev. 1997, 18, 306–360. [Google Scholar]
- Gething, M.J.; Sambrook, J. Protein folding in the cell. Nature 1992, 355, 33–45. [Google Scholar] [CrossRef]
- Kohno, S.; Katsu, Y.; Urushitani, H.; Ohta, Y.; Iguchi, T.; Guillette, L.J. Potential contributions of heat shock proteins to temperature-dependent sex determination in the American alligator. Sex Dev. 2010, 4, 73–87. [Google Scholar] [CrossRef]
- Cheung, J.; Smith, D.F. Molecular chaperone interactions with steroid receptors: An update. Mol. Endocrinol. 2000, 14, 939–946. [Google Scholar] [CrossRef]
- Fliss, A.E.; Benzeno, S.; Rao, J.; Caplan, A.J. Control of estrogen receptor ligand binding by Hsp90. J. Steroid Biochem. Mol. Biol. 2000, 72, 223–230. [Google Scholar] [CrossRef]
- Jia, Y.; Cavileer, T.D.; Nagler, J.J. Acute hyperthermic responses of heat shock protein and estrogen receptor mRNAs in rainbow trout hepatocytes. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 201, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.; Wang, Y.; Shapiro, D.J.; Xu, W. Differential requirements of Hsp90 and DNA for the formation of estrogen receptor homodimers and heterodimers. J. Biol. Chem. 2010, 285, 16125–16134. [Google Scholar] [CrossRef] [PubMed]
- Beato, M.; Klug, J. Steroid hormone receptors: An update. Hum. Reprod. Update 2000, 6, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Pratt, W.B.; Galigniana, M.D.; Morishima, Y.; Murphy, P.J. Role of molecular chaperones in steroid receptor action. Essays Biochem. 2004, 40, 41–58. [Google Scholar]
- Vina, J.; Borras, C.; Gambini, J.; Sastre, J.; Pallardo, F.V. Why females live longer than males? Importance of the upregulation of longevity-associated genes by oestrogenic compounds. FEBS Lett. 2005, 579, 2541–2545. [Google Scholar] [CrossRef]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J. Cell Mol. Med. 2017, 21, 1024–1032. [Google Scholar] [CrossRef]
- Brunelli, E.; Domanico, F.; La Russa, D.; Pellegrino, D. Sex differences in oxidative stress biomarkers. Curr. Drug Targets. 2014, 15, 811–815. [Google Scholar] [CrossRef]
- Vina, J.; Gambini, J.; Lopez-Grueso, R.; Abdelaziz, K.M.; Jove, M.; Borras, C. Females live longer than males: Role of oxidative stress. Curr. Pharm. Des. 2011, 17, 3959–3965. [Google Scholar] [CrossRef]
- Sobocanec, S.; Saric, A.; Macak, S.Z.; Popovic Hadzija, M.; Abramic, M.; Balog, T. The role of 17β-estradiol in the regulation of antioxidant enzymes via the Nrf2-Keap1 pathway in the livers of CBA/H mice. Life Sci. 2015, 130, 57–65. [Google Scholar] [CrossRef]
- Razmara, A.; Sunday, L.; Stirone, C.; Wang, X.B.; Krause, D.N.; Duckles, S.P.; Procaccio, V. Mitochondrial effects of estrogen are mediated by estrogen receptor alpha in brain endothelial cells. J. Pharmacol. Exp. Ther. 2008, 325, 782–790. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Z.; Quan, J.Q.; Li, L.L.; Zhao, G.Y.; Lu, J.H. Protective effects of different concentrations of selenium nanoparticles on rainbow trout (Oncorhynchus mykiss) primary hepatocytes under heat stress. Ecotoxicol. Environ. Saf. 2022, 230, 113121. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.Y.; Liu, Z.; Quan, J.Q.; Sun, J.; Li, L.L.; Lu, J.H. Potential role of miR-8159-x in heat stress response in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2023, 268, 110877. [Google Scholar] [CrossRef]
- Wojnarowski, K.; Cholewinska, P.; Palic, D.; Bednarska, M.; Jarosz, M.; Wisniewska, I. Estrogen Receptors Mediated Negative Effects of Estrogens and Xenoestrogens in Teleost Fishes-Review. Int. J. Mol. Sci. 2022, 23, 2605. [Google Scholar] [CrossRef]
- Green, S.; Chambon, P. Nuclear receptors enhance our understanding of transcription regulation. Trends Genet. 1988, 4, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Abdrrabba, M. Heat stress effects on livestock: Molecular, cellular and metabolic aspects, a review. J. Anim. Physiol. Anim. Nutr. (Berl.) 2016, 100, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Portner, H.O.; Knust, R. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 2007, 315, 95–97. [Google Scholar] [CrossRef]
- Bai, Z.; Harvey, L.M.; McNeil, B. Elevated temperature effects on the oxidant/antioxidant balance in submerged batch cultures of the filamentous fungus Aspergillus niger B1-D. Biotechnol. Bioeng. 2003, 83, 772–779. [Google Scholar] [CrossRef]
- Mujahid, A.; Yoshiki, Y.; Akiba, Y.; Toyomizu, M. Superoxide radical production in chicken skeletal muscle induced by acute heat stress. Poult. Sci. 2005, 84, 307–314. [Google Scholar] [CrossRef]
- Lin, H.; Decuypere, E.; Buyse, J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 144, 11–17. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Wang, L.; Sanjeewa, K.K.; Kang, S.I.; Lee, J.S.; Jeon, Y.J. Antioxidant Potential of Sulfated Polysaccharides from Padina boryana; Protective Effect against Oxidative Stress in In Vitro and In Vivo Zebrafish Model. Mar. Drugs 2020, 18, 212. [Google Scholar] [CrossRef]
- Yuan, L.X.; Zhang, R.; Ma, X.Z.; Yang, L.; Zheng, Q.; Chen, D.; Li, M.; Fan, T.; Liu, Y.X.; Pan, L.P.; et al. Selenium Accumulation, Antioxidant Enzyme Levels, and Amino Acids Composition in Chinese Mitten Crab (Eriocheir sinensis) Fed Selenium-Biofortified Corn. Nutrients 2018, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Munuswamy, N. Impact of metals on histopathology and expression of HSP 70 in different tissues of Milk fish (Chanos chanos) of Kaattuppalli Island, South East Coast, India. Chemosphere 2011, 83, 415–421. [Google Scholar] [CrossRef]
- Ye, Z.F.; Zhao, T.T.; Wei, Q.H.; Lin, H.R.; Zhang, Y.; Li, S.S. Distinct Roles of Estrogen Receptors in the Regulation of Vitellogenin Expression in Orange-Spotted Grouper (Epinephelus coioides). Int. J. Mol. Sci. 2022, 23, 8632. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tang, H.P.; He, J.A.; Wu, X.; Wang, L.; Liu, X.C.; Lin, H.R. Interaction of nuclear ERs and GPER in vitellogenesis in zebrafish. J. Steroid Biochem. Mol. Biol. 2019, 189, 10–18. [Google Scholar] [CrossRef]
- Ma, F.; Luo, L.T. Genome-wide identification of Hsp70/110 genes in rainbow trout and their regulated expression in response to heat stress. Peer J. 2020, 8, e10022. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lee, S.; Hung, S.S.; Deng, D.F. Responses of heat shock protein 70 and caspase-3/7 to dietary selenomethionine in juvenile white sturgeon. Anim. Nutr. 2016, 2, 45–50. [Google Scholar] [CrossRef]
- Yu, H.P.; Choudhry, M.A.; Shimizu, T.; Hsieh, Y.C.; Schwacha, M.G.; Yang, S.; Chaudry, I.H. Mechanism of the salutary effects of flutamide on intestinal myeloperoxidase activity following trauma-hemorrhage: Up-regulation of estrogen receptor-{beta}-dependent HO-1. J. Leukoc. Biol. 2006, 79, 277–284. [Google Scholar] [CrossRef]
- Misra, S.; Niyogi, S. Selenite causes cytotoxicity in rainbow trout (Oncorhynchus mykiss) hepatocytes by inducing oxidative stress. Toxicol. In Vitro 2009, 23, 1249–1258. [Google Scholar] [CrossRef]
- Webb, A.C.; Iverson, J.B.; Knapp, C.R.; DeNardo, D.F.; French, S.S. Energetic investment associated with vitellogenesis induces an oxidative cost of reproduction. J. Anim. Ecol. 2019, 88, 461–472. [Google Scholar] [CrossRef]
- Nagler, J.J.; Davis, T.L.; Modi, N.; Vijayan, M.M.; Schultz, I. Intracellular, not membrane, estrogen receptors control vitellogenin synthesis in the rainbow trout. Gen. Comp. Endocrinol. 2010, 167, 326–330. [Google Scholar] [CrossRef]
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular stress responses: Cell survival and cell death. Int. J. Cell Biol. 2010, 2010, 214074. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.K.; Katsu, Y.; Iguchi, T.; Lerner, D.T.; Hirano, T.; Grau, E.G. Transcriptional activity and biological effects of mammalian estrogen receptor ligands on three hepatic estrogen receptors in Mozambique tilapia. J. Steroid Biochem. Mol. Biol. 2010, 122, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Chandra, K.; Bosker, T.; Hogan, N.; Lister, A.; MacLatchy, D.; Currie, S. Sustained high temperature increases the vitellogenin response to 17α-ethynyl17β-estradiol in mummichog (Fundulus heteroclitus). Aquat. Toxicol. 2012, 118–119, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Jacobs, A.T.; Marnett, L.J. Heat shock factor 1 attenuates 4-Hydroxynonenal-mediated apoptosis: Critical role for heat shock protein 70 induction and stabilization of Bcl-XL. J. Biol. Chem. 2007, 282, 33412–33420. [Google Scholar] [CrossRef]
- Voss, M.R.; Stallone, J.N.; Li, M.; Cornelussen, R.N.; Knuefermann, P.; Knowlton, A.A. Gender differences in the expression of heat shock proteins: The effect of estrogen. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H687–H692. [Google Scholar] [CrossRef] [PubMed]
- Fekete, A.; Vannay, A.; Ver, A.; Rusai, K.; Müller, V.; Reusz, G.; Tulassay, T.; Szabo, A.J. Sex differences in heat shock protein 72 expression and localization in rats following renal ischemia-reperfusion injury. Am. J. Physiol. Renal Physiol. 2006, 291, F806–F811. [Google Scholar] [CrossRef]
- Hsu, J.T.; Hsieh, Y.C.; Kan, W.H.; Chen, J.G.; Choudhry, M.A.; Schwacha, M.G.; Bland, K.I.; Chaudry, I.H. Role of p38 mitogen-activated protein kinase pathway in estrogen-mediated cardioprotection following trauma-hemorrhage. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2982–H2987. [Google Scholar] [CrossRef]

| Group | 0 µM/mL | 0.1 µM/mL | 1 µM/mL | 10 µM/mL |
|---|---|---|---|---|
| 4 h | 1.00 aA ±00 | 1.12 aA ±0.11 | 1.14 aE ±0.01 | 1.02 aAB ±0.15 |
| 8 h | 1.00 bA ±00 | 0.93 cB ±0.01 | 1.27 aD ±0.01 | 0.85 dB ±0.00 |
| 12 h | 1.00 dA ±00 | 1.07 cAB ±0.01 | 1.77 aA ±0.02 | 1.12 bA ±0.01 |
| 24 h | 1.00 cA ±00 | 1.18 bA ±0.00 | 1.48 aB ±0.01 | 1.19 bA ±0.01 |
| 48 h | 1.00 dA ±00 | 1.05 cAB ±0.01 | 1.38 aC ±0.01 | 1.09 bA ±0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, G.; Liu, Z.; Lu, J.; Quan, J.; Pan, Y. Protective Effects of 17-βE2 on the Primary Hepatocytes of Rainbow Trout (Oncorhynchus mykiss) Under Acute Heat Stress. Antioxidants 2024, 13, 1316. https://doi.org/10.3390/antiox13111316
Zhao G, Liu Z, Lu J, Quan J, Pan Y. Protective Effects of 17-βE2 on the Primary Hepatocytes of Rainbow Trout (Oncorhynchus mykiss) Under Acute Heat Stress. Antioxidants. 2024; 13(11):1316. https://doi.org/10.3390/antiox13111316
Chicago/Turabian StyleZhao, Guiyan, Zhe Liu, Junhao Lu, Jinqiang Quan, and Yucai Pan. 2024. "Protective Effects of 17-βE2 on the Primary Hepatocytes of Rainbow Trout (Oncorhynchus mykiss) Under Acute Heat Stress" Antioxidants 13, no. 11: 1316. https://doi.org/10.3390/antiox13111316
APA StyleZhao, G., Liu, Z., Lu, J., Quan, J., & Pan, Y. (2024). Protective Effects of 17-βE2 on the Primary Hepatocytes of Rainbow Trout (Oncorhynchus mykiss) Under Acute Heat Stress. Antioxidants, 13(11), 1316. https://doi.org/10.3390/antiox13111316






