Protective Role of High-Density Lipoprotein in Multiple Sclerosis
Abstract
1. Introduction
2. Methods
3. Lipid Profile Alteration and Their Association with MS
4. HDL’s Role in MS Pathophysiology
5. Mechanisms of HDL Action in MS
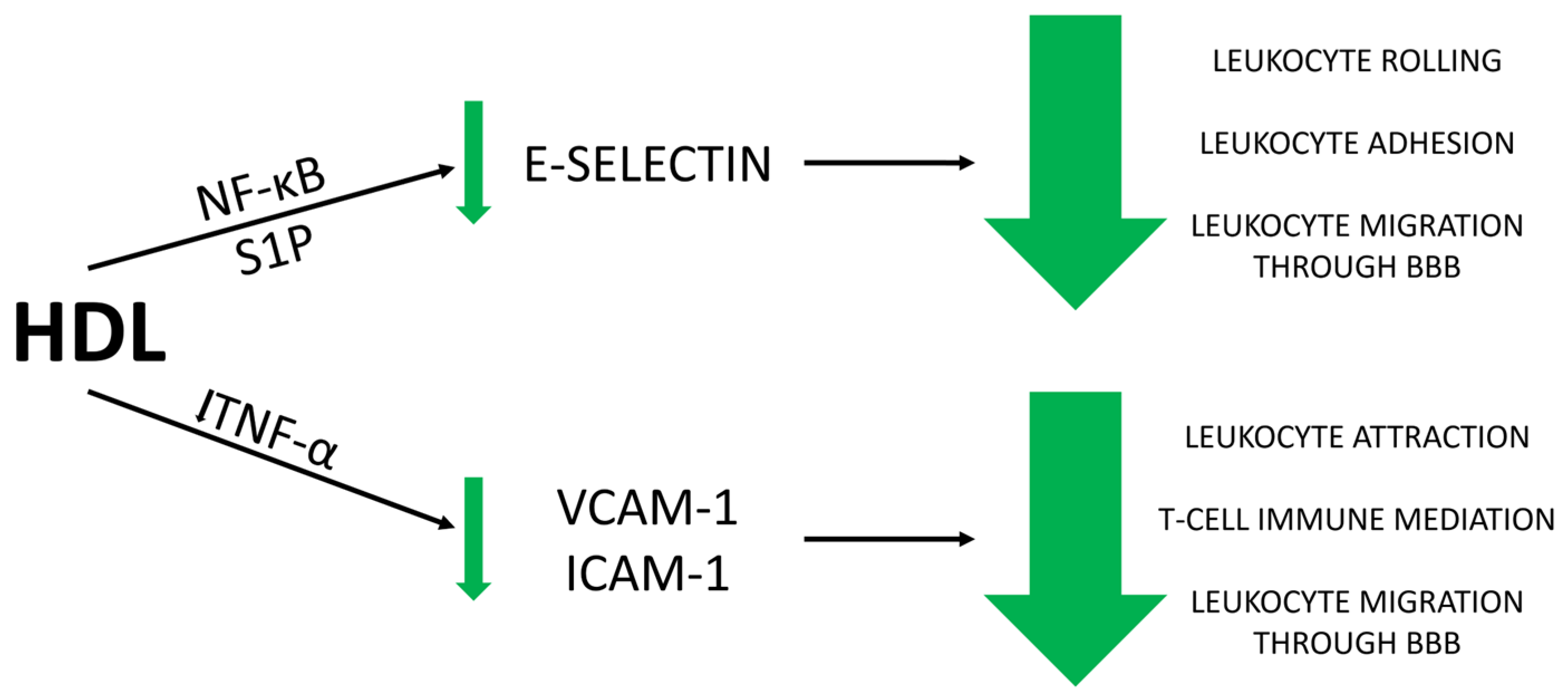
5.1. Anti-Inflammatory Effect of HDL
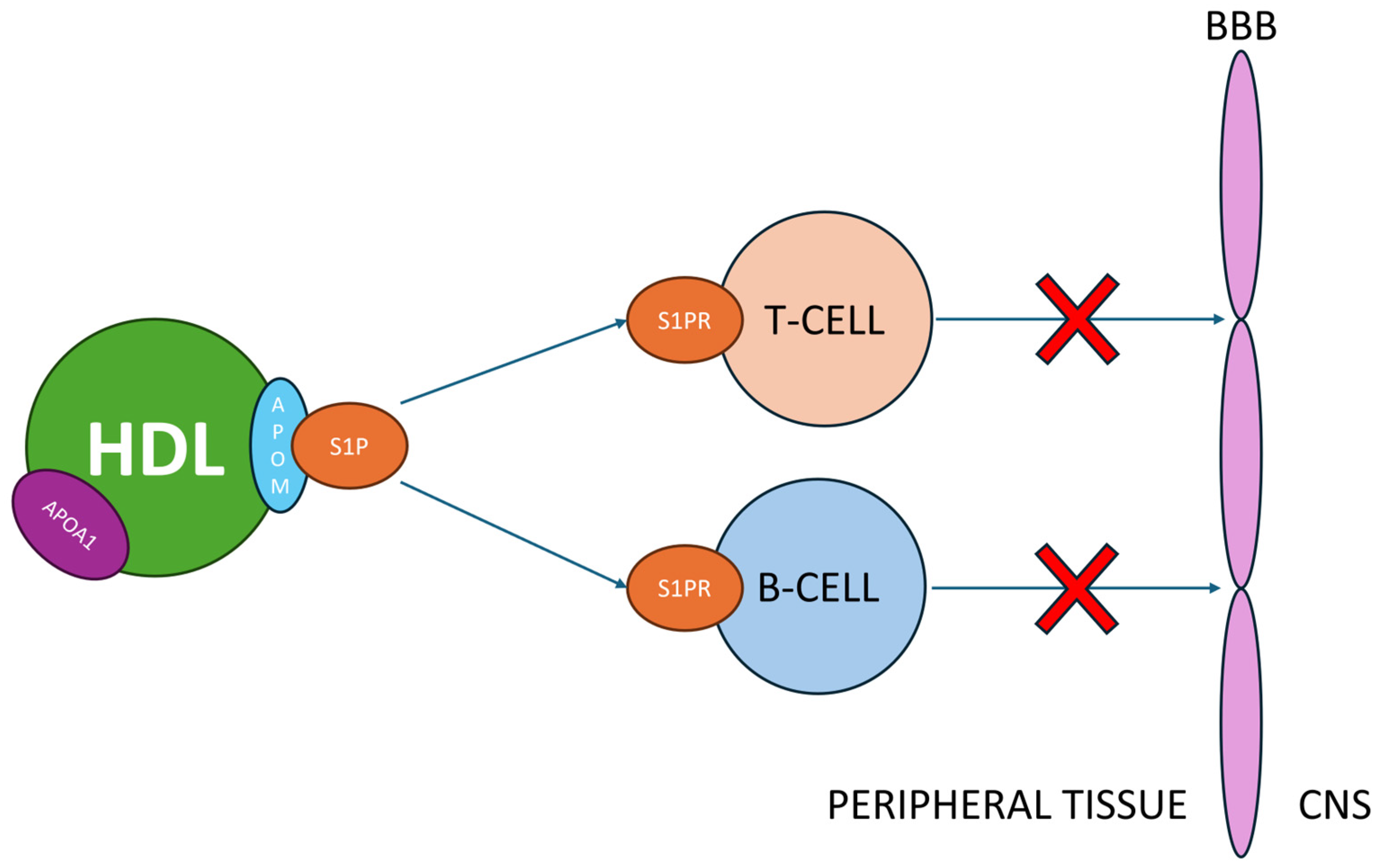
5.2. S1P Interactions with HDL
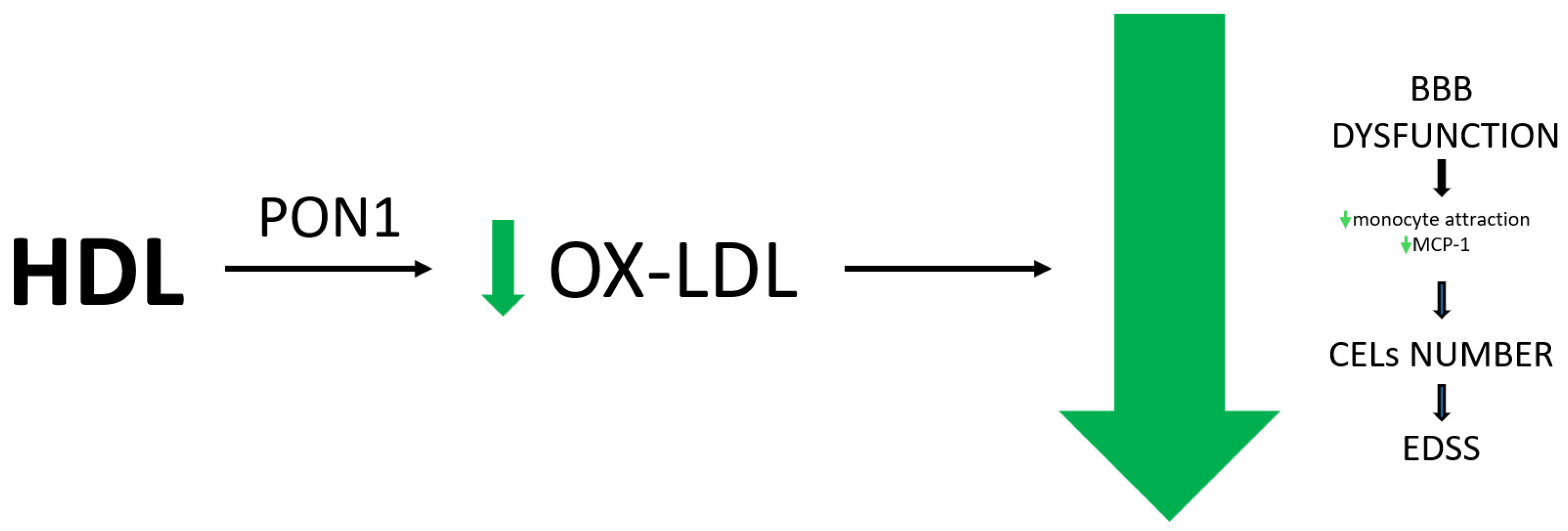
5.3. Antioxidant Effect of HDL
5.4. Ox-HDL in MS
6. Conclusions
- HDL’s Role in MS Pathophysiology: HDL plays a potentially critical role in MS by influencing BBB integrity and vascular endothelium through its anti-inflammatory, antioxidant, and nitric oxide synthesis properties.
- Mechanisms of HDL Action: HDL protects the endothelial cells forming the BBB by interacting with adhesion molecules like VCAM-1, ICAM-1, and E-selectin, thereby reducing leukocyte migration and BBB permeability. HDL also modulates endothelial activation through NF-κB and sphingosine-1-phosphate (S1P) pathways, enhancing its protective effect.
- Impact of HDL on S1P and Nitric Oxide: HDL modulates S1P, a crucial molecule in MS pathogenesis, by binding it to apolipoprotein M (apoM). This interaction impacts leukocyte migration and is linked to therapeutic effects observed with fingolimod, an agonist drug that targets S1P receptors.
- Antioxidant Properties: HDL’s antioxidant properties, linked to enzymes such as PON1 and GSPx, contribute to its protective role. Increased ox-HDL levels are associated with MS, potentially worsening disease outcomes. Due to the reduced PON1 activity, elevated ox-LDL levels are associated with increased disease severity, higher EDSS scores, and endothelial dysfunction
Author Contributions
Funding
Conflicts of Interest
References
- Denimal, D. Antioxidant and Anti-Inflammatory Functions of High-Density Lipoprotein in Type 1 and Type 2 Diabetes. Antioxidants 2023, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Heinecke, J.W. HDL, lipid peroxidation, and atherosclerosis. J. Lipid Res. 2009, 50, 599–601. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Xu, Y.; Zheng, L. HDL Structure. In HDL Metabolism and Diseases; Advances in Experimental Medicine and Biology; Springer: Singapore, 2022; Volume 1377, pp. 1–11. [Google Scholar] [CrossRef]
- Denimal, D. Rethinking ‘good cholesterol’ for cardiovascular risk stratification. QJM Int. J. Med. 2024, 117, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Mertens, A.; Holvoet, P. Oxidized LDL and HDL: Antagonists in atherothrombosis. FASEB J. 2001, 15, 2073–2084. [Google Scholar] [CrossRef]
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef]
- Kuhlmann, T.; Moccia, M.; Coetzee, T.; Cohen, J.A.; Correale, J.; Graves, J.; Marrie, R.A.; Montalban, X.; Yong, V.W.; Thompson, A.J.; et al. Multiple sclerosis progression: Time for a new mechanism-driven framework. Lancet Neurol. 2023, 22, 78–88. [Google Scholar] [CrossRef]
- Bar-Or, A.; Darlington, P.J. The immunology of multiple sclerosis. In Multiple Sclerosis Therapeutics, 4th ed.; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar] [CrossRef]
- Krumbholz, M.; Derfuss, T.; Hohlfeld, R.; Meinl, E. B cells and antibodies in multiple sclerosis pathogenesis and therapy. Nat. Rev. Neurol. 2012, 8, 613–623. [Google Scholar] [CrossRef]
- Greenfield, A.L.; Hauser, S.L. B-cell Therapy for Multiple Sclerosis: Entering an era. Ann. Neurol. 2018, 83, 13–26. [Google Scholar] [CrossRef]
- Jurewicz, A.; Domowicz, M.; Galazka, G.; Raine, C.S.; Selmaj, K. Multiple sclerosis: Presence of serum antibodies to lipids and predominance of cholesterol recognition. J. Neurosci. Res. 2017, 95, 1984–1992. [Google Scholar] [CrossRef]
- Leech, S.; Kirk, J.; Plumb, J.; McQuaid, S. Persistent endothelial abnormalities and blood–brain barrier leak in primary and secondary progressive multiple sclerosis. Neuropathol. Appl. Neurobiol. 2007, 33, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Liebner, S.; Dijkhuizen, R.M.; Reiss, Y.; Plate, K.H.; Agalliu, D.; Constantin, G. Functional morphology of the blood–brain barrier in health and disease. Acta Neuropathol. 2018, 135, 311–336. [Google Scholar] [CrossRef] [PubMed]
- Vestweber, D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 2015, 15, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, C.; Timmler, S.; Barrantes-Freer, A.; Simons, M. Myelin in the Central Nervous System: Structure, Function, and Pathology. Physiol. Rev. 2019, 99, 1381–1431. [Google Scholar] [CrossRef]
- Loving, B.A.; Bruce, K.D. Lipid and Lipoprotein Metabolism in Microglia. Front. Physiol. 2020, 11, 393. [Google Scholar] [CrossRef]
- Ho, W.Y.; Hartmann, H.; Ling, S. Central nervous system cholesterol metabolism in health and disease. IUBMB Life 2022, 74, 826–841. [Google Scholar] [CrossRef]
- Raulin, A.-C.; Martens, Y.A.; Bu, G. Lipoproteins in the Central Nervous System: From Biology to Pathobiology. Annu. Rev. Biochem. 2022, 91, 731–759. [Google Scholar] [CrossRef]
- Koch, S.; Donarski, N.; Goetze, K.; Kreckel, M.; Stuerenburg, H.-J.; Buhmann, C.; Beisiegel, U. Characterization of four lipoprotein classes in human cerebrospinal fluid. J. Lipid Res. 2001, 42, 1143–1151. [Google Scholar] [CrossRef]
- Wahrle, S.E.; Jiang, H.; Parsadanian, M.; Legleiter, J.; Han, X.; Fryer, J.D.; Kowalewski, T.; Holtzman, D.M. ABCA1 Is Required for Normal Central Nervous System ApoE Levels and for Lipidation of Astrocyte-secreted apoE. J. Biol. Chem. 2004, 279, 40987–40993. [Google Scholar] [CrossRef]
- Kim, W.S.; Guillemin, G.J.; Glaros, E.N.; Lim, C.K.; Garner, B. Quantitation of ATP-binding cassette subfamily-A transporter gene expression in primary human brain cells. NeuroReport 2006, 17, 891–896. [Google Scholar] [CrossRef]
- Kim, W.S.; Rahmanto, A.S.; Kamili, A.; Rye, K.-A.; Guillemin, G.J.; Gelissen, I.C.; Jessup, W.; Hill, A.F.; Garner, B. Role of ABCG1 and ABCA1 in Regulation of Neuronal Cholesterol Efflux to Apolipoprotein E Discs and Suppression of Amyloid-β Peptide Generation. J. Biol. Chem. 2007, 282, 2851–2861. [Google Scholar] [CrossRef] [PubMed]
- Podbielska, M.; O’keeffe, J.; Pokryszko-Dragan, A. New Insights into Multiple Sclerosis Mechanisms: Lipids on the Track to Control Inflammation and Neurodegeneration. Int. J. Mol. Sci. 2021, 22, 7319. [Google Scholar] [CrossRef] [PubMed]
- Saher, G.; Stumpf, S.K. Cholesterol in myelin biogenesis and hypomyelinating disorders. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2015, 1851, 1083–1094. [Google Scholar] [CrossRef]
- Luo, J.; Yang, H.; Song, B.-L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Q. Cholesterol metabolism and homeostasis in the brain. Protein Cell 2015, 6, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Vejux, A.; Ghzaiel, I.; Nury, T.; Schneider, V.; Charrière, K.; Sghaier, R.; Zarrouk, A.; Leoni, V.; Moreau, T.; Lizard, G. Oxysterols and multiple sclerosis: Physiopathology, evolutive biomarkers and therapeutic strategy. J. Steroid Biochem. Mol. Biol. 2021, 210, 105870. [Google Scholar] [CrossRef] [PubMed]
- Leoni, V.; Masterman, T.; Diczfalusy, U.; De Luca, G.; Hillert, J.; Björkhem, I. Changes in human plasma levels of the brain specific oxysterol 24S-hydroxycholesterol during progression of multiple sclerosis. Neurosci. Lett. 2002, 331, 163–166. [Google Scholar] [CrossRef]
- van de Kraats, C.; Killestein, J.; Popescu, V.; Rijkers, E.; Vrenken, H.; Lütjohann, D.; Barkhof, F.; Polman, C.; Teunissen, C. Oxysterols and cholesterol precursors correlate to magnetic resonance imaging measures of neurodegeneration in multiple sclerosis. Mult. Scler. J. 2014, 20, 412–417. [Google Scholar] [CrossRef]
- Ma, X.; Bi, E.; Huang, C.; Lu, Y.; Xue, G.; Guo, X.; Wang, A.; Yang, M.; Qian, J.; Dong, C.; et al. Cholesterol negatively regulates IL-9–producing CD8+ T cell differentiation and antitumor activity. J. Exp. Med. 2018, 215, 1555–1569. [Google Scholar] [CrossRef]
- Pineda-Torra, I.; Siddique, S.; Waddington, K.E.; Farrell, R.; Jury, E.C. Disrupted Lipid Metabolism in Multiple Sclerosis: A Role for Liver X Receptors? Front. Endocrinol. 2021, 12, 639757. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Fitzner, D.; Bosch-Queralt, M.; Weil, M.-T.; Su, M.; Sen, P.; Ruhwedel, T.; Mitkovski, M.; Trendelenburg, G.; Lütjohann, D.; et al. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science 2018, 359, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Hichor, M.; Sundaram, V.K.; Eid, S.A.; Abdel-Rassoul, R.; Petit, P.X.; Borderie, D.; Bastin, J.; Eid, A.A.; Manuel, M.; Grenier, J.; et al. Liver X Receptor exerts a protective effect against the oxidative stress in the peripheral nerve. Sci. Rep. 2018, 8, 2524. [Google Scholar] [CrossRef] [PubMed]
- Uher, T.; Fellows, K.; Horakova, D.; Zivadinov, R.; Vaneckova, M.; Sobisek, L.; Tyblova, M.; Seidl, Z.; Krasensky, J.; Bergsland, N.; et al. Serum lipid profile changes predict neurodegeneration in interferon-β1a-treated multiple sclerosis patients. J. Lipid Res. 2017, 58, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Zhornitsky, S.; McKay, K.A.; Metz, L.M.; Teunissen, C.E.; Rangachari, M. Cholesterol and markers of cholesterol turnover in multiple sclerosis: Relationship with disease outcomes. Mult. Scler. Relat. Disord. 2015, 5, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Nunes, V.S.; da Silva, E.J.; Ferreira, G.d.S.; de Assis, S.I.S.; Cazita, P.M.; Nakandakare, E.R.; Zago, V.H.d.S.; de Faria, E.C.; Quintão, E.C.R. The Plasma Distribution of Non-cholesterol Sterol Precursors and Products of Cholesterol Synthesis and Phytosterols Depend on HDL Concentration. Front. Nutr. 2022, 9, 723555. [Google Scholar] [CrossRef]
- Yang, T.-M.; Miao, M.; Yu, W.-Q.; Wang, X.; Xia, F.-J.; Li, Y.-J.; Guo, S.-D. Targeting macrophages in atherosclerosis using nanocarriers loaded with liver X receptor agonists: A narrow review. Front. Mol. Biosci. 2023, 10, 1147699. [Google Scholar] [CrossRef]
- Weinstock-Guttman, B.; Zivadinov, R.; Mahfooz, N.; Carl, E.; Drake, A.; Schneider, J.; Teter, B.; Hussein, S.; Mehta, B.; Weiskopf, M.; et al. Serum lipid profiles are associated with disability and MRI outcomes in multiple sclerosis. J. Neuroinflamm. 2011, 8, 127. [Google Scholar] [CrossRef]
- Weinstock-Guttman, B.; Zivadinov, R.; Horakova, D.; Havrdova, E.; Qu, J.; Shyh, G.; Lakota, E.; O’Connor, K.; Badgett, D.; Tamaño-Blanco, M.; et al. Lipid profiles are associated with lesion formation over 24 months in interferon-β treated patients following the first demyelinating event. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1186–1191. [Google Scholar] [CrossRef]
- Wicks, T.R.; Nehzat, N.; Wolska, A.; Shalaurova, I.; Browne, R.W.; Weinstock-Guttman, B.; Jakimovski, D.; Zivadinov, R.; Remaley, A.T.; Otvos, J.; et al. Dyslipidemias in multiple sclerosis. Mult. Scler. Relat. Disord. 2024, 91, 105841. [Google Scholar] [CrossRef]
- Swank, R.L.; Grimsgaard, A. Multiple sclerosis: The lipid relationship. Am. J. Clin. Nutr. 1988, 48, 1387–1393. [Google Scholar] [CrossRef]
- Wahls, T.L.; Titcomb, T.J.; Bisht, B.; Eyck, P.T.; Rubenstein, L.M.; Carr, L.J.; Darling, W.G.; Hoth, K.F.; Kamholz, J.; Snetselaar, L.G. Impact of the Swank and Wahls elimination dietary interventions on fatigue and quality of life in relapsing-remitting multiple sclerosis: The WAVES randomized parallel-arm clinical trial. Mult. Scler. J.—Exp. Transl. Clin. 2021, 7, 20552173211035400. [Google Scholar] [CrossRef] [PubMed]
- Ortí, J.E.d.l.R.; Armero, J.L.P.; Cuerda-Ballester, M.; Sanchis-Sanchis, C.E.; Navarro-Illana, E.; Lajara-Romance, J.M.; Benlloch, M.; Ceron, J.J.; Tvarijonaviciute, A.; Proaño, B. Lipid Profile in Multiple Sclerosis: Functional Capacity and Therapeutic Potential of Its Regulation after Intervention with Epigallocatechin Gallate and Coconut Oil. Foods 2023, 12, 3730. [Google Scholar] [CrossRef] [PubMed]
- Mandoj, C.; Renna, R.; Plantone, D.; Sperduti, I.; Cigliana, G.; Conti, L.; Koudriavtseva, T. Anti-annexin antibodies, cholesterol levels and disability in multiple sclerosis. Neurosci. Lett. 2015, 606, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Ďurfinová, M.; Procházková, Ľ.; Petrleničová, D.; Bystrická, Z.; Orešanská, K.; Kuračka, Ľ.; Líška, B. Cholesterol level correlate with disability score in patients with relapsing-remitting form of multiple sclerosis. Neurosci. Lett. 2018, 687, 304–307. [Google Scholar] [CrossRef]
- Murali, N.; Browne, R.W.; Fellows Maxwell, K.; Bodziak, M.L.; Jakimovski, D.; Hagemeier, J.; Bergsland, N.; Weinstock-Guttman, B.; Zivadinov, R.; Ramanathan, M. Cholesterol and neurodegeneration: Longitudinal changes in serum cholesterol biomarkers are associated with new lesions and gray matter atrophy in multiple sclerosis over 5 years of follow-up. Eur. J. Neurol. 2020, 27, 188-e4. [Google Scholar] [CrossRef]
- Rhoads, J.P.; Major, A.S. How Oxidized Low-Density Lipoprotein Activates Inflammatory Responses. Crit. Rev. Immunol. 2018, 38, 333–342. [Google Scholar] [CrossRef]
- Navab, M.; Berliner, J.A.; Subbanagounder, G.; Hama, S.; Lusis, A.J.; Castellani, L.W.; Reddy, S.; Shih, D.; Shi, W.; Watson, A.D.; et al. HDL and the Inflammatory Response Induced by LDL-Derived Oxidized Phospholipids. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 481–488. [Google Scholar] [CrossRef]
- Newcombe, J.; Li, H.; Cuzner, M.L. Low density lipoprotein uptake by macrophages in multiple sclerosis plaques: Implications for pathogenesis. Neuropathol. Appl. Neurobiol. 1994, 20, 152–162. [Google Scholar] [CrossRef]
- Giubilei, F.; Antonini, G.; Di Legge, S.; Sormani, M.P.; Pantano, P.; Antonini, R.; Sepe-Monti, M.; Caramia, F.; Pozzilli, C. Blood cholesterol and MRI activity in first clinical episode suggestive of multiple sclerosis. Acta Neurol. Scand. 2002, 106, 109–112. [Google Scholar] [CrossRef]
- Yuan, S.; Xiong, Y.; Larsson, S.C. An atlas on risk factors for multiple sclerosis: A Mendelian randomization study. J. Neurol. 2021, 268, 114–124. [Google Scholar] [CrossRef]
- Palavra, F.; Marado, D.; Mascarenhas-Melo, F.; Sereno, J.; Teixeira-Lemos, E.; Nunes, C.C.; Gonçalves, G.; Teixeira, F.; Reis, F. New Markers of Early Cardiovascular Risk in Multiple Sclerosis Patients: Oxidized-LDL Correlates with Clinical Staging. Dis. Markers 2013, 34, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Salomonsson, L. Oxidised LDL decreases VEGFR-1 expression in human monocyte-derived macrophages. Atherosclerosis 2003, 169, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.N.; Hanni, K.B.; Gabbita, S.; Friebe, V.; Mattson, M.P.; Kindy, M.S. Oxidized lipoproteins increase reactive oxygen species formation in microglia and astrocyte cell lines. Brain Res. 1999, 830, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Haider, L.; Fischer, M.T.; Frischer, J.M.; Bauer, J.; Höftberger, R.; Botond, G.; Esterbauer, H.; Binder, C.J.; Witztum, J.L.; Lassmann, H. Oxidative damage in multiple sclerosis lesions. Brain 2011, 134, 1914–1924. [Google Scholar] [CrossRef] [PubMed]
- Van der Vorst, E.P.C. High-Density Lipoproteins and Apolipoprotein A1. In Vertebrate and Invertebrate Respiratory Proteins, Lipoproteins and Other Body Fluid Proteins; Springer: Cham, Switzerland, 2020; pp. 399–420. [Google Scholar] [CrossRef]
- Jakimovski, D.; Zivadinov, R.; Dwyer, M.G.; Bergsland, N.; Ramasamy, D.P.; Browne, R.W.; Weinstock-Guttman, B.; Ramanathan, M. High density lipoprotein cholesterol and apolipoprotein A-I are associated with greater cerebral perfusion in multiple sclerosis. J. Neurol. Sci. 2020, 418, 117120. [Google Scholar] [CrossRef]
- Tettey, P.; Simpson, S.; Taylor, B.; Blizzard, L.; Ponsonby, A.-L.; Dwyer, T.; Kostner, K.; van der Mei, I. An adverse lipid profile is associated with disability and progression in disability, in people with MS. Mult. Scler. J. 2014, 20, 1737–1744. [Google Scholar] [CrossRef]
- Meyers, L.; Groover, C.J.; Douglas, J.; Lee, S.; Brand, D.; Levin, M.C.; Gardner, L.A. A role for Apolipoprotein A-I in the pathogenesis of multiple sclerosis. J. Neuroimmunol. 2014, 277, 176–185. [Google Scholar] [CrossRef]
- Gardner, L.A.; Levin, M.C. Importance of Apolipoprotein A-I in Multiple Sclerosis. Front. Pharmacol. 2015, 6, 278. [Google Scholar] [CrossRef]
- McComb, M.; Parambi, R.; Browne, R.W.; Bodziak, M.L.; Jakimovski, D.; Bergsland, N.; Maceski, A.; Weinstock-Guttman, B.; Kuhle, J.; Zivadinov, R.; et al. Apolipoproteins AI and E are associated with neuroaxonal injury to gray matter in multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 45, 102389. [Google Scholar] [CrossRef]
- Browne, R.W.; Weinstock-Guttman, B.; Horakova, D.; Zivadinov, R.; Bodziak, M.L.; Tamaño-Blanco, M.; Badgett, D.; Tyblova, M.; Vaneckova, M.; Seidl, Z.; et al. Apolipoproteins are associated with new MRI lesions and deep grey matter atrophy in clinically isolated syndromes. J. Neurol. Neurosurg. Psychiatry 2014, 85, 859–864. [Google Scholar] [CrossRef]
- Fellows, K.; Uher, T.; Browne, R.W.; Weinstock-Guttman, B.; Horakova, D.; Posova, H.; Vaneckova, M.; Seidl, Z.; Krasensky, J.; Tyblova, M.; et al. Protective associations of HDL with blood-brain barrier injury in multiple sclerosis patients. J. Lipid Res. 2015, 56, 2010–2018. [Google Scholar] [CrossRef] [PubMed]
- Bowman, G.L.; Kaye, J.A.; Quinn, J.F. Dyslipidemia and Blood-Brain Barrier Integrity in Alzheimer’s Disease. Curr. Gerontol. Geriatr. Res. 2012, 2012, 184042. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.J.; Nicholls, S.; Rye, K.-A.; Anantharamaiah, G.; Navab, M.; Fogelman, A.M. Antiinflammatory Properties of HDL. Circ. Res. 2004, 95, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Biomarkers of endothelial activation and dysfunction in cardiovascular diseases. Rev. Cardiovasc. Med. 2022, 23, 73. [Google Scholar] [CrossRef]
- Calabresia, L.; Franceschinia, G.; Sirtori, C.R.; De Palma, A.; Saresellac, M.; Ferrantec, P.; Taramellib, D. Inhibition of VCAM-1 Expression in Endothelial Cells by Reconstituted High Density Lipoproteins. Biochem. Biophys. Res. Commun. 1997, 238, 61–65. [Google Scholar] [CrossRef]
- Siddiqui, K.; George, T.P.; Mujammami, M.; Isnani, A.; Alfadda, A.A. The association of cell adhesion molecules and selectins (VCAM-1, ICAM-1, E-selectin, L-selectin, and P-selectin) with microvascular complications in patients with type 2 diabetes: A follow-up study. Front. Endocrinol. 2023, 14, 1072288. [Google Scholar] [CrossRef]
- Gauberti, M.; Fournier, A.P.; Docagne, F.; Vivien, D.; de Lizarrondo, S.M. Molecular Magnetic Resonance Imaging of Endothelial Activation in the Central Nervous System. Theranostics 2018, 8, 1195–1212. [Google Scholar] [CrossRef]
- Cockerill, G.W.; Saklatvala, J.; Ridley, S.H.; Yarwood, H.; Miller, N.E.; Oral, B.; Nithyanathan, S.; Taylor, G.; Haskard, D.O. High-Density Lipoproteins Differentially Modulate Cytokine-Induced Expression of E-Selectin and Cyclooxygenase-2. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 910–917. [Google Scholar] [CrossRef]
- Baker, P.W.; Rye, K.-A.; Gamble, J.R.; Vadas, M.A.; Barter, P.J. Ability of reconstituted high density lipoproteins to inhibit cytokine-induced expression of vascular cell adhesion molecule-1 in human umbilical vein endothelial cells. J. Lipid Res. 1999, 40, 345–353. [Google Scholar] [CrossRef]
- Gilmore, T.D. Introduction to NF-κB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef]
- Christoffersen, C.; Obinata, H.; Kumaraswamy, S.B.; Galvani, S.; Ahnström, J.; Sevvana, M.; Egerer-Sieber, C.; Muller, Y.A.; Hla, T.; Nielsen, L.B.; et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl. Acad. Sci. USA 2011, 108, 9613–9618. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Vadas, M.A.; Rye, K.-A.; Barter, P.J.; Gamble, J.R. High Density Lipoproteins (HDL) Interrupt the Sphingosine Kinase Signaling Pathway. J. Biol. Chem. 1999, 274, 33143–33147. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Park, J.H.Y.; Kang, J.-S.; Kang, Y.-H. Involvement of transcription factors in plasma HDL protection against TNF-α-induced vascular cell adhesion molecule-1 expression. Int. J. Biochem. Cell Biol. 2003, 35, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Frenette, P.S.; Wagner, D.D. Insights into Selectin Function from Knockout Mice. Thromb. Haemost. 1997, 78, 060–064. [Google Scholar] [CrossRef]
- van Kruining, D.; Luo, Q.; van Echten-Deckert, G.; Mielke, M.M.; Bowman, A.; Ellis, S.; Oliveira, T.G.; Martinez-Martinez, P. Sphingolipids as prognostic biomarkers of neurodegeneration, neuroinflammation, and psychiatric diseases and their emerging role in lipidomic investigation methods. Adv. Drug Deliv. Rev. 2020, 159, 232–244. [Google Scholar] [CrossRef]
- Podbielska, M.; Ariga, T.; Pokryszko-Dragan, A. Sphingolipid Players in Multiple Sclerosis: Their Influence on the Initiation and Course of the Disease. Int. J. Mol. Sci. 2022, 23, 5330. [Google Scholar] [CrossRef]
- Obinata, H.; Hla, T. Sphingosine 1-phosphate and inflammation. Int. Immunol. 2019, 31, 617–625. [Google Scholar] [CrossRef]
- Roy, R.; Alotaibi, A.A.; Freedman, M.S. Sphingosine 1-Phosphate Receptor Modulators for Multiple Sclerosis. CNS Drugs 2021, 35, 385–402. [Google Scholar] [CrossRef]
- Jozefczuk, E.; Guzik, T.; Siedlinski, M. Significance of sphingosine-1-phosphate in cardiovascular physiology and pathology. Pharmacol. Res. 2020, 156, 104793. [Google Scholar] [CrossRef]
- Parra, S.; Castro, A.; Masana, L. The pleiotropic role of HDL in autoimmune diseases. Clin. Investig. Arterioscler. 2015, 27, 97–106. [Google Scholar] [CrossRef]
- Levkau, B. HDL-S1P: Cardiovascular functions, disease-associated alterations, and therapeutic applications. Front. Pharmacol. 2015, 6, 243. [Google Scholar] [CrossRef] [PubMed]
- Kan, S.B.; Staun-Ram, E.; Golan, D.; Miller, A. HDL-cholesterol elevation associated with fingolimod and dimethyl fumarate therapies in multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2019, 5, 205521731988272. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, F.J.; Alonso-Navarro, H.; Salgado-Cámara, P.; García-Martín, E.; Agúndez, J.A.G. Oxidative Stress Markers in Multiple Sclerosis. Int. J. Mol. Sci. 2024, 25, 6289. [Google Scholar] [CrossRef] [PubMed]
- Pegoretti, V.; Swanson, K.A.; Bethea, J.R.; Probert, L.; Eisel, U.L.M.; Fischer, R. Inflammation and Oxidative Stress in Multiple Sclerosis: Consequences for Therapy Development. Oxidative Med. Cell. Longev. 2020, 2020, 7191080. [Google Scholar] [CrossRef] [PubMed]
- Van Horssen, J.; Schreibelt, G.; Drexhage, J.; Hazes, T.; Dijkstra, C.; van der Valk, P.; de Vries, H. Severe oxidative damage in multiple sclerosis lesions coincides with enhanced antioxidant enzyme expression. Free Radic. Biol. Med. 2008, 45, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Penkowa, M.; Espejo, C.; Ortega-Aznar, A.; Hidalgo, J.; Montalban, X.; Cáceres, E.M.M. Metallothionein expression in the central nervous system of multiple sclerosis patients. Cell. Mol. Life Sci. 2003, 60, 1258–1266. [Google Scholar] [CrossRef]
- Essenburg, C.; Browne, R.W.; Ghazal, D.; Tamaño-Blanco, M.; Jakimovski, D.; Weinstock-Guttman, B.; Zivadinov, R.; Ramanathan, M. Antioxidant defense enzymes in multiple sclerosis: A 5-year follow-up study. Eur. J. Neurol. 2023, 30, 2338–2347. [Google Scholar] [CrossRef]
- Bizoń, A.; Chojdak-Łukasiewicz, J.; Kołtuniuk, A.; Budrewicz, S.; Pokryszko-Dragan, A.; Piwowar, A. Evaluation of Selected Oxidant/Antioxidant Parameters in Patients with Relapsing-Remitting Multiple Sclerosis Undergoing Disease-Modifying Therapies. Antioxidants 2022, 11, 2416. [Google Scholar] [CrossRef]
- Kirbas, A.; Kirbas, S.; Anlar, O.; Efe, H.; Yilmaz, A. Serum paraoxonase and arylesterase activity and oxidative status in patients with multiple sclerosis. J. Clin. Neurosci. 2013, 20, 1106–1109. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T.; Principi, F.; Di Ludovico, F.; Viti, B.; Angeleri, V.A.; Danni, M.; Provinciali, L. Increased levels of lipid hydroperoxides in plasma of patients with multiple sclerosis: A relationship with paraoxonase activity. Mult. Scler. J. 2005, 11, 677–682. [Google Scholar] [CrossRef]
- Jamroz-Wisniewska, A.; Beltowski, J.; Stelmasiak, Z.; Bartosik-Psujek, H. Paraoxonase 1 activity in different types of multiple sclerosis. Mult. Scler. J. 2009, 15, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Bacchetti, T.; Ferretti, G.; Carbone, F.; Ministrini, S.; Montecucco, F.; Jamialahmadi, T.; Sahebkar, A. Dysfunctional High-density Lipoprotein: The Role of Myeloperoxidase and Paraoxonase-1. Curr. Med. Chem. 2021, 28, 2842–2850. [Google Scholar] [CrossRef] [PubMed]
- Mineo, C.; Yuhanna, I.S.; Quon, M.J.; Shaul, P.W. High Density Lipoprotein-induced Endothelial Nitric-oxide Synthase Activation Is Mediated by Akt and MAP Kinases. J. Biol. Chem. 2003, 278, 9142–9149. [Google Scholar] [CrossRef] [PubMed]
- Yuhanna, I.S.; Zhu, Y.; Cox, B.E.; Hahner, L.D.; Osborne-Lawrence, S.; Lu, P.; Marcel, Y.L.; Anderson, R.G.; Mendelsohn, M.E.; Hobbs, H.H.; et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat. Med. 2001, 7, 853–857. [Google Scholar] [CrossRef]
- Nofer, J.-R.; van der Giet, M.; Tölle, M.; Wolinska, I.; Lipinski, K.v.W.; Baba, H.A.; Tietge, U.J.; Gödecke, A.; Ishii, I.; Kleuser, B.; et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J. Clin. Investig. 2004, 113, 569–581. [Google Scholar] [CrossRef]
- Jorissen, W.; Wouters, E.; Bogie, J.F.; Vanmierlo, T.; Noben, J.-P.; Sviridov, D.; Hellings, N.; Somers, V.; Valcke, R.; Vanwijmeersch, B.; et al. Relapsing-remitting multiple sclerosis patients display an altered lipoprotein profile with dysfunctional HDL. Sci. Rep. 2017, 7, srep43410. [Google Scholar] [CrossRef]
- Mehindate, K.; Sahlas, D.J.; Frankel, D.; Mawal, Y.; Liberman, A.; Corcos, J.; Dion, S.; Schipper, H.M. Proinflammatory cytokines promote glial heme oxygenase-1 expression and mitochondrial iron deposition: Implications for multiple sclerosis. J. Neurochem. 2001, 77, 1386–1395. [Google Scholar] [CrossRef]
- Jhelum, P.; Zandee, S.; Ryan, F.; Zarruk, J.G.; Michalke, B.; Venkataramani, V.; Curran, L.; Klement, W.; Prat, A.; David, S. Ferroptosis induces detrimental effects in chronic EAE and its implications for progressive MS. Acta Neuropathol. Commun. 2023, 11, 1–22. [Google Scholar] [CrossRef]
- Pennisi, G.; Cornelius, C.; Cavallaro, M.; Salinaro, A.T.; Cambria, M.; Pennisi, M.; Bella, R.; Milone, P.; Ventimiglia, B.; Migliore, M.; et al. Redox regulation of cellular stress response in multiple sclerosis. Biochem. Pharmacol. 2011, 82, 1490–1499. [Google Scholar] [CrossRef]
- Stahnke, T.; Richter-Landsberg, C.; Stadelmann, C.; Netzler, A.; Brück, W. Differential upregulation of heme oxygenase-1 (HSP32) in glial cells after oxidative stress and in demyelinating disorders. J. Mol. Neurosci. 2007, 32, 25–37. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T. Peroxidation of lipoproteins in multiple sclerosis. J. Neurol. Sci. 2011, 311, 92–97. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damiza-Detmer, A.; Pawełczyk, M.; Głąbiński, A. Protective Role of High-Density Lipoprotein in Multiple Sclerosis. Antioxidants 2024, 13, 1276. https://doi.org/10.3390/antiox13111276
Damiza-Detmer A, Pawełczyk M, Głąbiński A. Protective Role of High-Density Lipoprotein in Multiple Sclerosis. Antioxidants. 2024; 13(11):1276. https://doi.org/10.3390/antiox13111276
Chicago/Turabian StyleDamiza-Detmer, Agnieszka, Małgorzata Pawełczyk, and Andrzej Głąbiński. 2024. "Protective Role of High-Density Lipoprotein in Multiple Sclerosis" Antioxidants 13, no. 11: 1276. https://doi.org/10.3390/antiox13111276
APA StyleDamiza-Detmer, A., Pawełczyk, M., & Głąbiński, A. (2024). Protective Role of High-Density Lipoprotein in Multiple Sclerosis. Antioxidants, 13(11), 1276. https://doi.org/10.3390/antiox13111276






