Radical Scavenging Capacity and In Vitro Cytoprotective Effects of Great Salt Lake-Derived Processed Mineral Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Material and Reagents
2.2. Electron Spin Resonance (ESR)-Spin Trapping Technique for Determination of Superoxide Anion Radicals (O2•−) Generated by the HPX–XOD Cell-Free System
2.3. ESR-Spin Trapping Technique for Determination of Superoxide Anion Radicals (O2•−) Generated by the Photoexcitation of Riboflavin
2.4. ESR-Spin Trapping Technique for Determination of Hydroxyl Radicals (·OH) Generated by Fenton Reaction
2.5. Scavenging of the Stable Radical DPPH
2.6. hGFs, Culture Conditions, and Cell Viability Assay
2.7. Cell Proliferation of Subconfluent hGFs After 3-min Pretreatment with GSL-MW
2.8. Cell Proliferation of Subconfluent hGFs Exposed to Pure Water or 100 μM Hydrogen Peroxide (H2O2)
2.9. Cell Viability of Confluent hGFs Exposed to 12.5–100 mM Hydrogen Peroxide (H2O2)
2.10. Statistical Analyses
3. Results
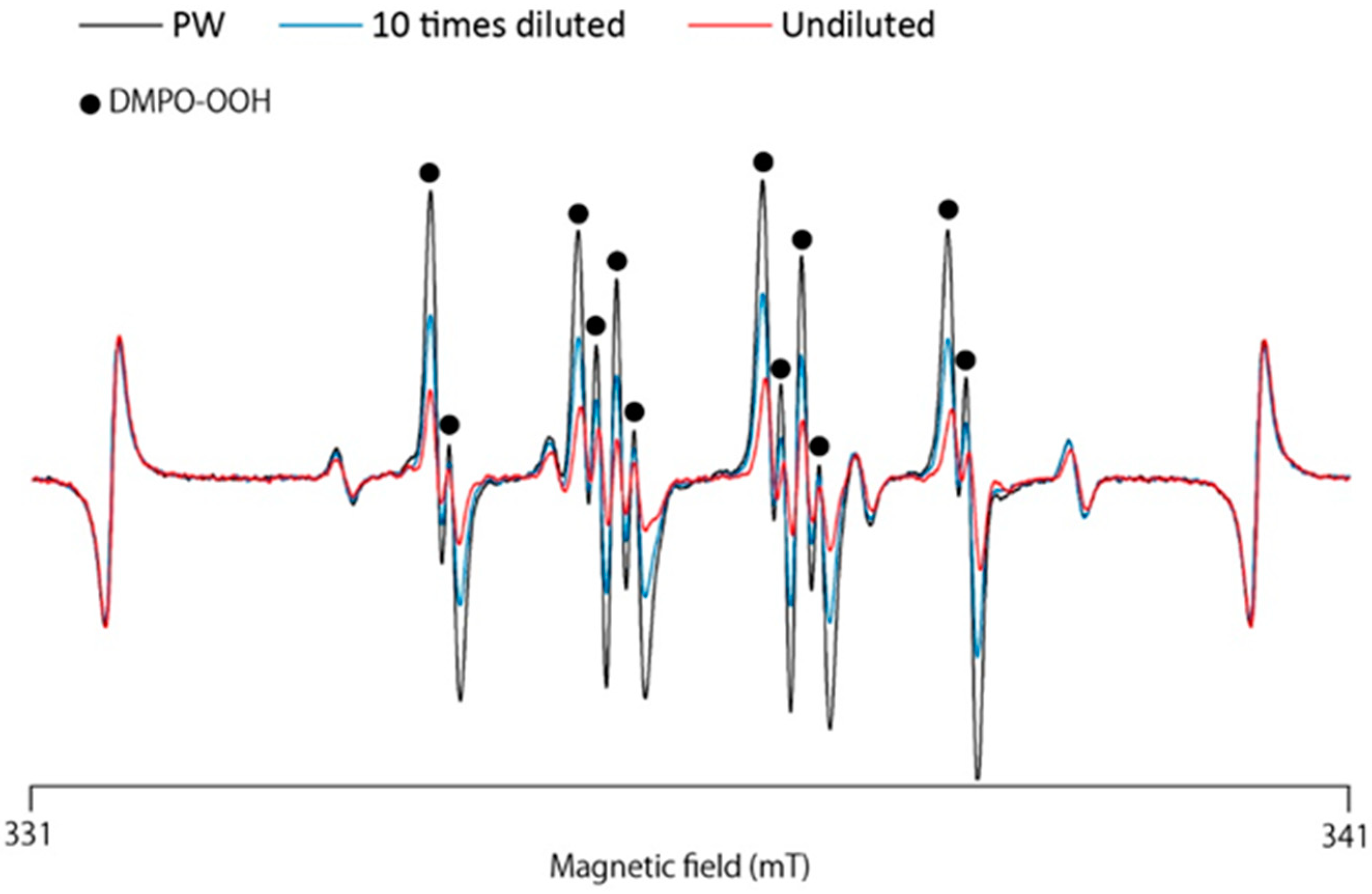
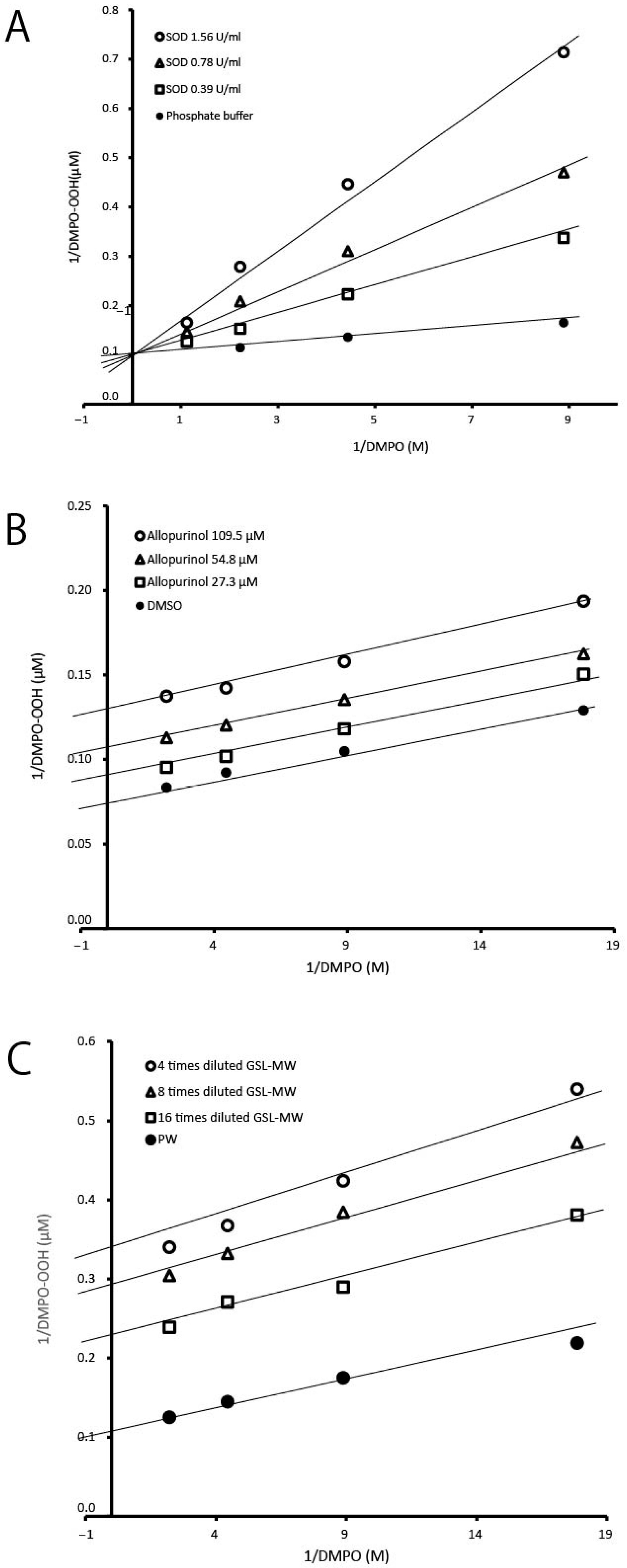
3.1. ESR-Spin Trapping Technique for Determination of Superoxide Anion Radicals (O2•−) Generated by the HPX–XOD Cell-Free System
3.2. ESR-Spin Trapping Technique for Determination of Superoxide Anion Radicals (O2•−) Generated by the Photoexcitation of Riboflavin
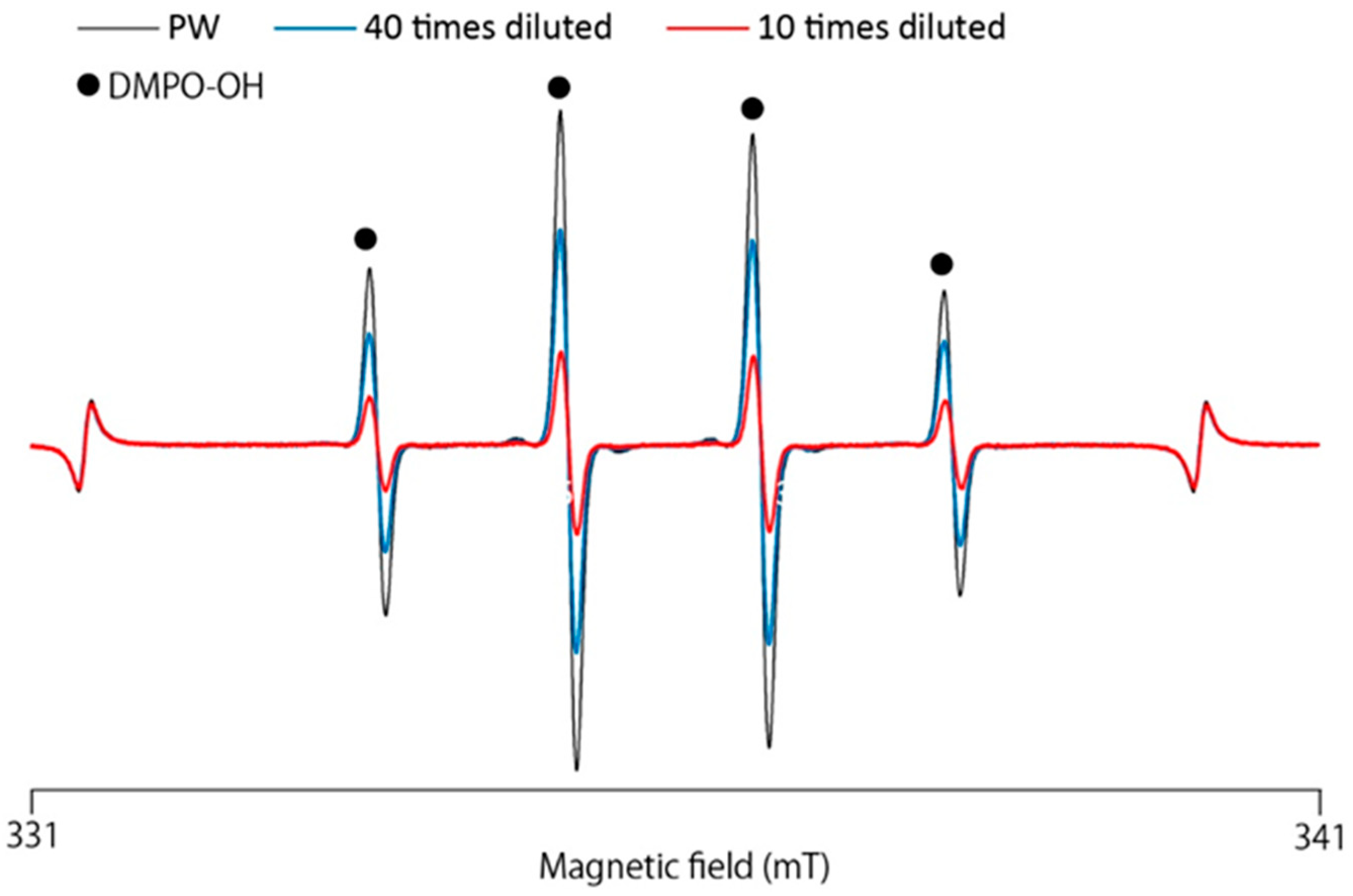
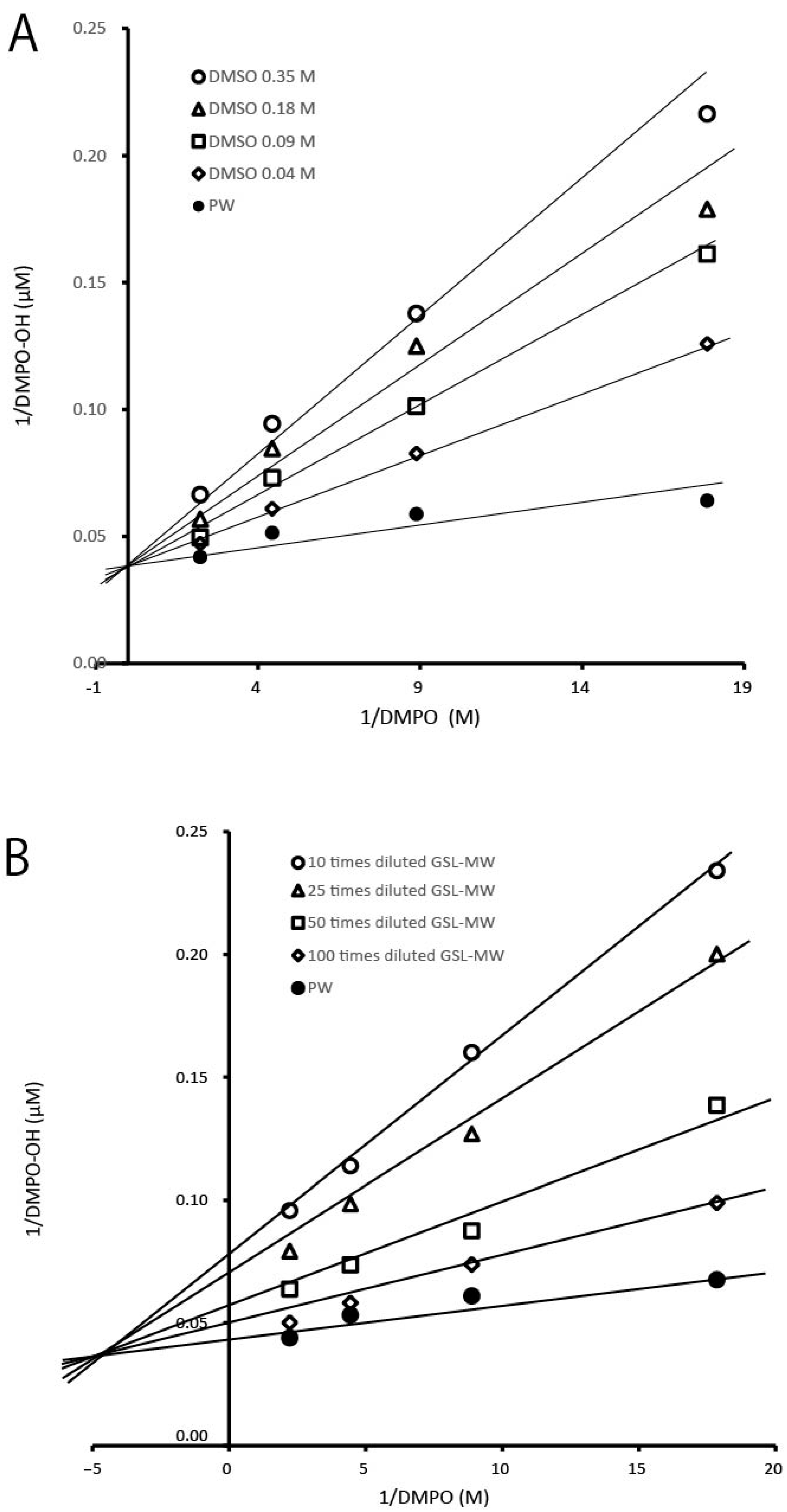
3.3. ESR-Spin Trapping Technique for Determinations of Hydroxyl Radicals (·OH) Generated by Fenton Reaction
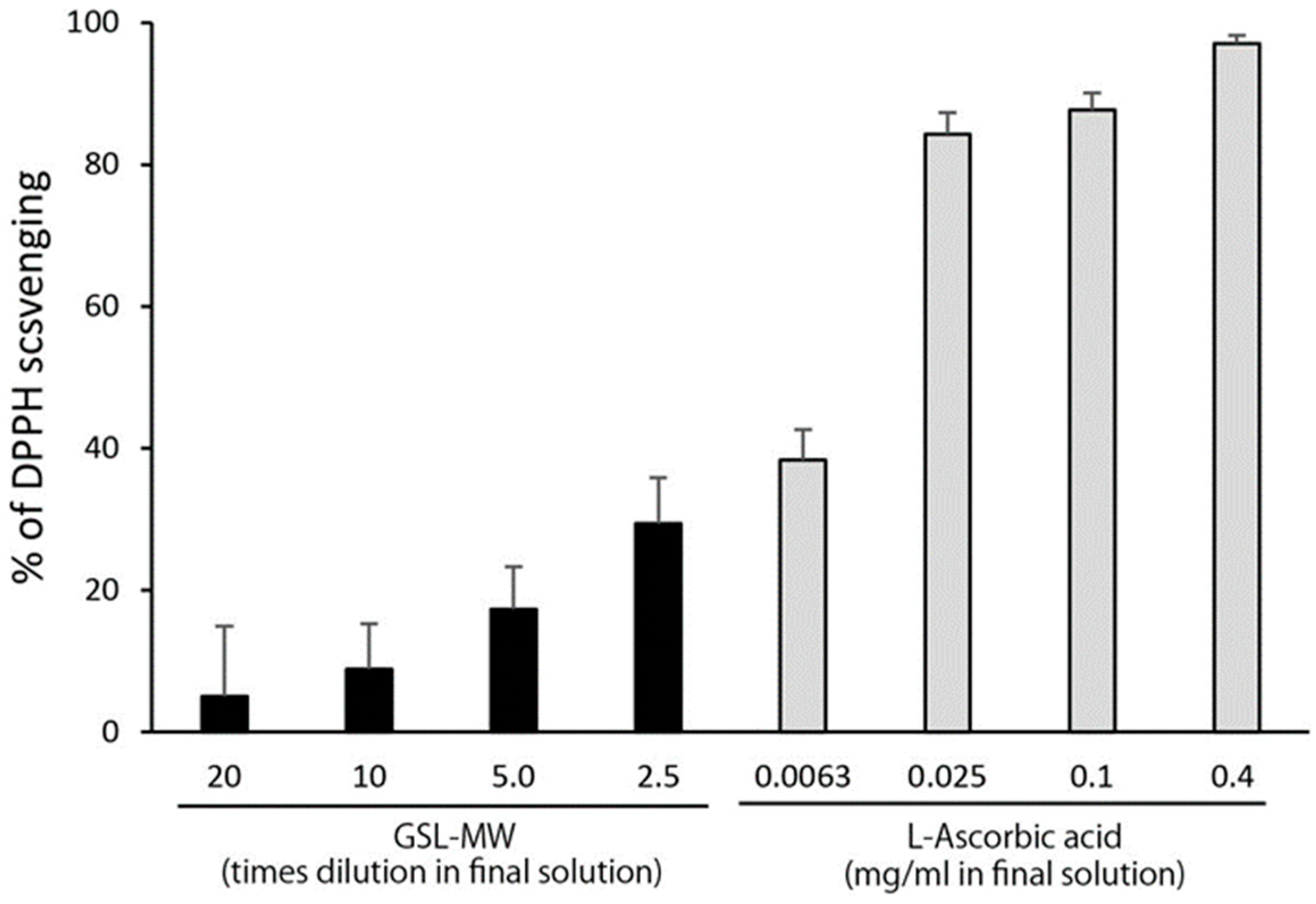
3.4. Scavenging of the Stable Radical DPPH
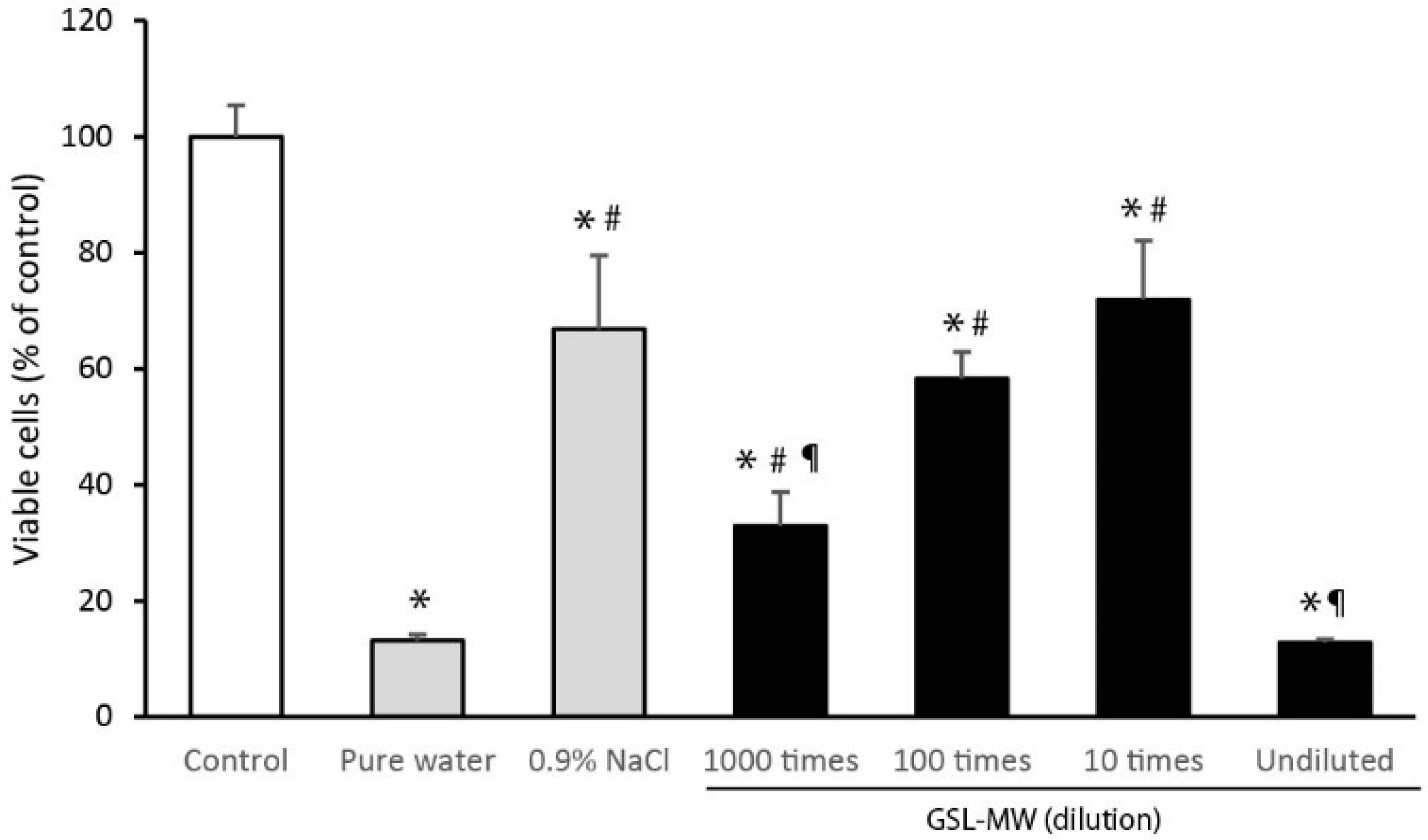
3.5. Cell Proliferation After 3-min Pretreatment with GSL-MW
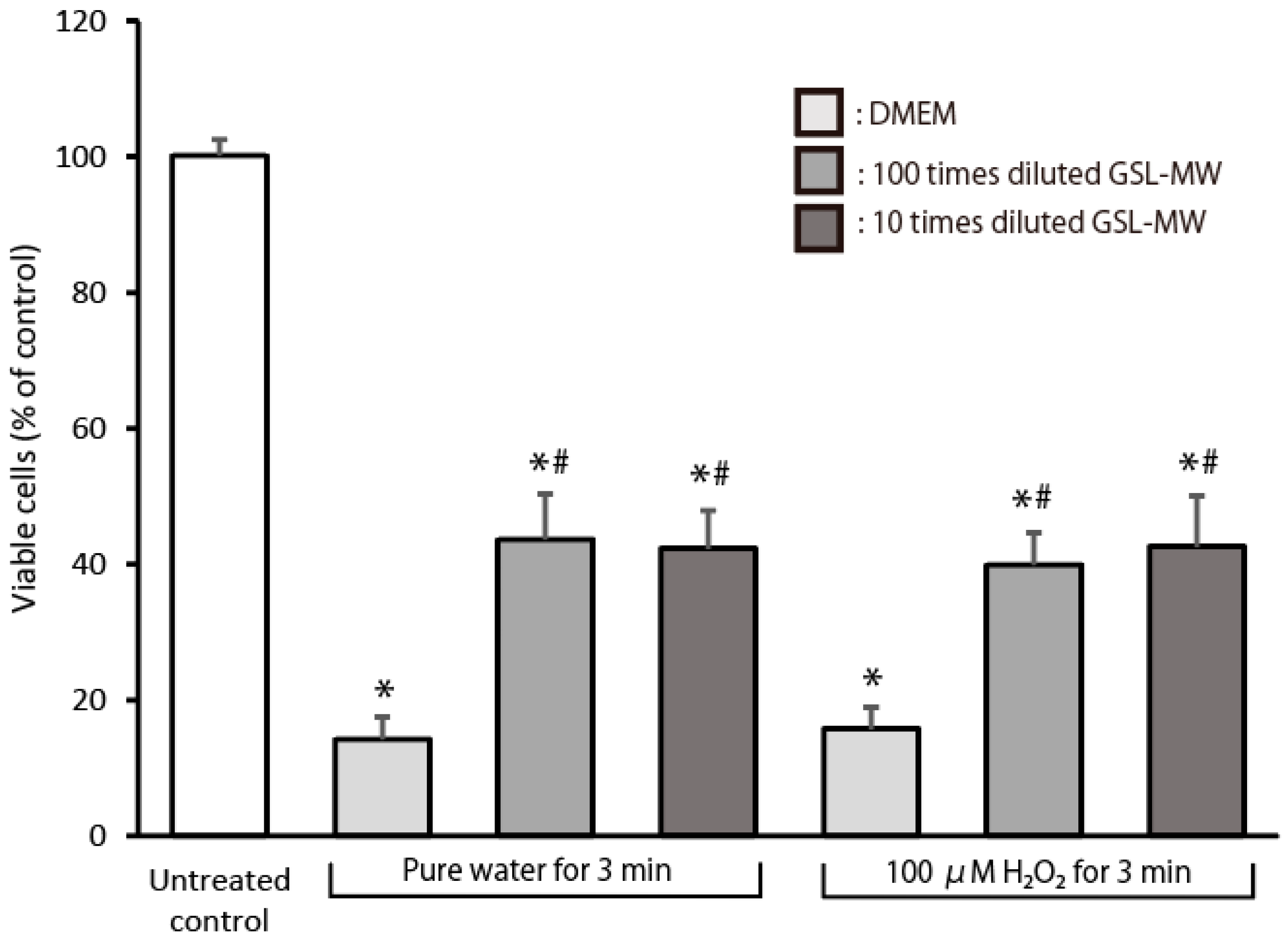
3.6. Cell Proliferation After Exposure to Pure Water or 100 μM Hydrogen Peroxide (H2O2)
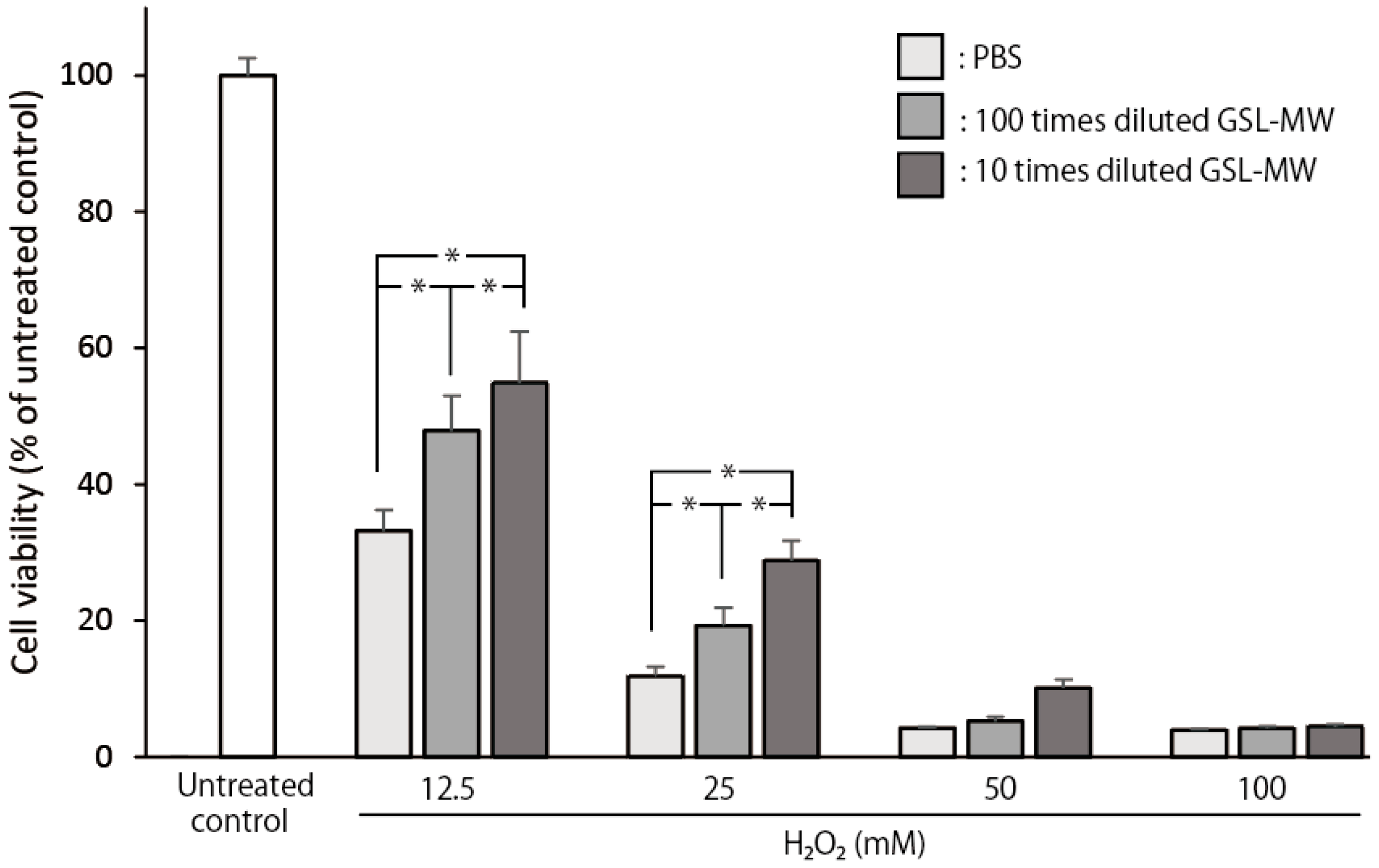
3.7. Cell Viability of Confluent hGFs Exposed to 12.5–100 mM Hydrogen Peroxide (H2O2)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bender, T.; Bariska, J.; Vághy, R.; Gomez, R.; Imre, K. Effect of balneotherapy on the antioxidant system—A controlled pilot study. Arch. Med. Res. 2007, 38, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Prandelli, C.; Parola, C.; Buizza, L.; Delbarba, A.; Marziano, M.; Salvi, V.; Zacchi, V.; Memo, M.; Sozzani, S.; Calza, S.; et al. Sulphurous thermal water increases the release of the anti-inflammatory cytokine IL-10 and modulates antioxidant enzyme activity. Int. J. Immunopathol. Pharmacol. 2013, 26, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Bajgai, J.; Fadriquela, A.; Ara, J.; Begum, R.; Ahmed, M.F.; Kim, C.S.; Kim, S.K.; Shim, K.Y.; Lee, K.J. Balneotherapeutic effects of high mineral spring water on the atopic dermatitis-like inflammation in hairless mice via immunomodulation and redox balance. BMC Complement. Altern. Med. 2017, 17, 481. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, S.; Benvenuti, F.; Nappi, G.; Fortunati, N.A.; Marino, L.; Aureli, T.; De Luca, S.; Pagliarani, S.; Canestrari, F. Antioxidative effects of sulfurous mineral water: Protection against lipid and protein oxidation. Eur. J. Clin. Nutr. 2009, 63, 106–112. [Google Scholar] [CrossRef]
- Costantino, M.; Giuberti, G.; Caraglia, M.; Lombardi, A.; Misso, G.; Abbruzzese, A.; Ciani, F.; Lampa, E. Possible antioxidant role of SPA therapy with chlorine-sulphur-bicarbonate mineral water. Amino Acids 2009, 36, 161–165. [Google Scholar] [CrossRef]
- Melgar-Sánchez, L.M.; García-Ruiz, I.; Pardo-Marqués, V.; Agulló-Ortuño, M.T.; Martínez-Galán, I. Influence of mineral waters on in vitro proliferation, antioxidant response and cytokine production in a human lung fibroblasts cell line. Int. J. Biometeorol. 2019, 63, 1171–1180. [Google Scholar] [CrossRef]
- Kurauchi, M.; Niwano, Y.; Shirato, M.; Kanno, T.; Nakamura, K.; Egusa, H.; Sasaki, K. Cytoprotective effect of short-term pretreatment with proanthocyanidin on human gingival fibroblasts exposed to harsh environmental conditions. PLoS ONE 2014, 9, e113403. [Google Scholar] [CrossRef]
- Katsuda, Y.; Niwano, Y.; Nakashima, T.; Mokudai, T.; Nakamura, K.; Oizumi, S.; Kanno, T.; Kanetaka, H.; Egusa, H. Cytoprotective effects of grape seed extract on human gingival fibroblasts in relation to its antioxidant potential. PLoS ONE 2015, 10, e0134704. [Google Scholar] [CrossRef]
- Kabuto, H.; Nishizawa, M.; Tada, M.; Higashio, C.; Shishibori, T.; Kohno, M. Zingerone [4-(4-hydroxy-3-methoxyphenyl)-2-butanone] prevents 6-hydroxydopamine-induced dopamine depression in mouse striatum and increases superoxide scavenging activity in serum. Neurochem. Res. 2005, 30, 325–332. [Google Scholar] [CrossRef]
- Sato, E.; Kohno, M.; Hamano, H.; Niwano, Y. Increased anti-oxidative potency of garlic by spontaneous short-term fermentation. Plant Foods Hum. Nutr. 2006, 61, 157–160. [Google Scholar] [CrossRef]
- Kohno, M.; Mizuta, Y.; Kusai, M.; Masumizu, T.; Makino, K. Measurements of superoxide anion radical and superoxide anion scavenging activity by electron spin resonance spectroscopy coupled with DMPO spin trapping. Bull. Chem. Soc. Jpn. 1994, 67, 1085–1090. [Google Scholar] [CrossRef]
- Niwano, Y.; Sato, E.; Kohno, M.; Matsuyama, Y.; Kim, D.; Oda, T. Antioxidant properties of aqueous extracts from red tide plankton cultures. Biosci. Biotechnol. Biochem. 2007, 71, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Buettner, G.R. Spin trapping: ESR parameters of spin adducts. Free Radic. Biol. Med. 1987, 3, 259–303. [Google Scholar] [CrossRef]
- Saito, K.; Kohno, M.; Yoshizaki, F.; Niwano, Y. Extensive screening for edible herbal extracts with potent scavenging activity against superoxide anions. Plant Foods Hum. Nutr. 2008, 63, 65–70. [Google Scholar] [CrossRef]
- Watts, R.W.; Watts, J.E.; Seegmiller, J.E. Xanthine oxidase activity in human tissues and its inhibition by allopurinol (4-hydroxypyrazolo[3,4-d] pyrimidine). J. Lab. Clin. Med. 1965, 66, 688–697. [Google Scholar]
- Tsai, P.; Pou, S.; Straus, R.; Rosen, G.M. Evaluation of various spin traps for the in vivo in situ detection of hydroxyl radical. J. Chem. Soc. Perkin Trans. 2 1999, 1999, 1759–1764. [Google Scholar] [CrossRef]
- Villamena, F.A.; Zweier, J.L. Superoxide radical trapping and spin adduct decay of 5-tert-butoxycarbonyl-5-methyl-1-pyrroline N-oxide (BocMPO†): Kinetics and theoretical analysis. J. Chem. Soc. Perkin Trans. 2 2002, 2002, 1340–1344. [Google Scholar] [CrossRef]
- El-Hage, A.N.; Herman, E.H.; Ferrans, V.J. Reduction in the diabetogenic effect of alloxan in mice by treatment with the antineoplastic agent ICRF-187. Res. Commun. Chem. Pathol. Pharmacol. 1981, 33, 509–523. [Google Scholar]
- Repine, J.E.; Fox, R.B.; Berger, E.M. Dimethyl sulfoxide inhibits killing of Staphylococcus aureus by polymorphonuclear leukocytes. Infect. Immun. 1981, 31, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, W. Dimethyl sulfoxide effects on platelet aggregation and vascular reactivity in pial microcirculation. Ann. N. Y. Acad. Sci. 1983, 411, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Swartz, H.M.; Clarkson, R.B. The measurement of oxygen in vivo using EPR techniques. Phys. Med. Biol. 1998, 43, 1957–1975. [Google Scholar] [CrossRef] [PubMed]
- Diepart, C.; Verrax, J.; Calderon, P.B.; Feron, O.; Jordan, B.F.; Gallez, B. Comparison of methods for measuring oxygen consumption in tumor cells in vitro. Anal. Biochem. 2010, 396, 250–256. [Google Scholar] [CrossRef]
- Shin, M.Y.; Cho, Y.E.; Park, C.; Sohn, H.Y.; Lim, J.H.; Kwun, I.S. The supplementation of yam powder products can give the nutritional benefits of the antioxidant mineral (Cu, Zn, Mn, Fe and Se) intakes. Prev. Nutr. Food Sci. 2012, 17, 299–305. [Google Scholar] [CrossRef]
- Sequetto, P.L.; Oliveira, T.T.; Maldonado, I.R.; Augusto, L.E.; Mello, V.J.; Pizziolo, V.R.; Almeida, M.R.; Silva, M.E.; Novaes, R.D. Naringin accelerates the regression of pre-neoplastic lesions and the colorectal structural reorganization in a murine model of chemical carcinogenesis. Food Chem. Toxicol. 2014, 64, 200–209. [Google Scholar] [CrossRef]
- Castro Aguilar-Tablada, T.; Navarro-Alarcón, M.; Quesada Granados, J.; Samaniego Sánchez, C.; Rufián-Henares, J.; Nogueras-Lopez, F. Ulcerative colitis and Crohn's disease are associated with decreased serum selenium concentrations and increased cardiovascular risk. Nutrients 2016, 8, 780. [Google Scholar] [CrossRef]
- Dhawan, M.; Emran, T.B.; Priyanaka; Choudhary, O.P. Immunomodulatory effects of zinc and its impact on COVID-19 severity. Ann. Med. Surg. 2022, 77, 103638. [Google Scholar] [CrossRef]
- Nakanishi, I.; Miyazaki, K.; Shimada, T.; Ohkubo, K.; Urano, S.; Ikota, N.; Ozawa, T.; Fukuzumi, S.; Fukuhara, K. Effects of metal ions distinguishing between one-step hydrogen- and electron-transfer mechanisms for the radical-scavenging reaction of (+)-catechin. J. Phys. Chem. 2002, 106, 11123–11126. [Google Scholar] [CrossRef]
- Nakanishi, I.; Kawashima, T.; Ohkubo, K.; Kanazawa, H.; Inami, K.; Mochizuki, M.; Fukuhara, K.; Okuda, H.; Ozawa, T.; Itoh, S.; et al. Electron-transfer mechanism in radical-scavenging reactions by a vitamin E model in a protic medium. Org. Biomol. Chem. 2005, 3, 626–629. [Google Scholar] [CrossRef]
- Nakanishi, I.; Shoji, Y.; Ohkubo, K.; Ueno, M.; Shimoda, K.; Matsumoto, K.I.; Fukuhara, K.; Hamada, H. Effect of magnesium ion on the radical-scavenging rate of pterostilbene in an aprotic medium: Mechanistic insight into the antioxidative reaction of pterostilbene. Antioxidants 2022, 11, 340. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Weisleder, N.; Collet, C.; Zhou, J.; Chu, Y.; Hirata, Y.; Zhao, X.; Pan, Z.; Brotto, M.; Cheng, H.; et al. Uncontrolled calcium sparks act as a dystrophic signal for mammalian skeletal muscle. Nat. Cell Biol. 2005, 7, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Elizondo, J.; Chiong, M.; Rojas-Rivera, D.; Olea-Azar, C.; Kwon, H.M.; Lavandero, S. Reactive oxygen species inhibit hyposmotic stress-dependent volume regulation in cultured rat cardiomyocytes. Biochem. Biophys. Res. Commun. 2006, 350, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.S.; Shkryl, V.M.; Nowycky, M.C.; Shirokova, N. Reactive oxygen species contribute to Ca2+ signals produced by osmotic stress in mouse skeletal muscle fibres. J. Physiol. 2008, 586, 197–210. [Google Scholar] [CrossRef]

| Mineral | Concentration (mg/mL) |
|---|---|
| Chloride (Cl) | 275 |
| Magnesium (Mg) | 101 |
| Sulfate (SO4) | 21.4 |
| Sodium (Na) | 3.1 |
| Potassium (K) | 2.1 |
| Osmolality (mOSM/kg) | |
|---|---|
| Physiological saline (0.9% NaCl) | 289 |
| Undiluted GSL-MW | 12,347 |
| 10-times diluted GSL-MW | 1282 |
| 100-times diluted GSL-MW | 118 |
| 1000-times diluted GSL-MW | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokudai, T.; Nakagawa, S.; Kanetaka, H.; Oda, K.; Abe, H.; Niwano, Y. Radical Scavenging Capacity and In Vitro Cytoprotective Effects of Great Salt Lake-Derived Processed Mineral Water. Antioxidants 2024, 13, 1266. https://doi.org/10.3390/antiox13101266
Mokudai T, Nakagawa S, Kanetaka H, Oda K, Abe H, Niwano Y. Radical Scavenging Capacity and In Vitro Cytoprotective Effects of Great Salt Lake-Derived Processed Mineral Water. Antioxidants. 2024; 13(10):1266. https://doi.org/10.3390/antiox13101266
Chicago/Turabian StyleMokudai, Takayuki, Seiko Nakagawa, Hiroyasu Kanetaka, Kazuo Oda, Hiroya Abe, and Yoshimi Niwano. 2024. "Radical Scavenging Capacity and In Vitro Cytoprotective Effects of Great Salt Lake-Derived Processed Mineral Water" Antioxidants 13, no. 10: 1266. https://doi.org/10.3390/antiox13101266
APA StyleMokudai, T., Nakagawa, S., Kanetaka, H., Oda, K., Abe, H., & Niwano, Y. (2024). Radical Scavenging Capacity and In Vitro Cytoprotective Effects of Great Salt Lake-Derived Processed Mineral Water. Antioxidants, 13(10), 1266. https://doi.org/10.3390/antiox13101266







