α-Terpineol Induces Shelterin Components TRF1 and TRF2 to Mitigate Senescence and Telomere Integrity Loss via A Telomerase-Independent Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Induction of Oxidative Stress
2.3. Relative Telomere Length
2.4. Quantification of Telomerase Activity
2.5. Immunoblot Analysis
2.6. Oxidized Protein Levels
2.7. Detection of Oxidized TRF1
2.8. Immunofluorescence
2.9. Inhibition of PI3K/AKT Pathway
3. Results
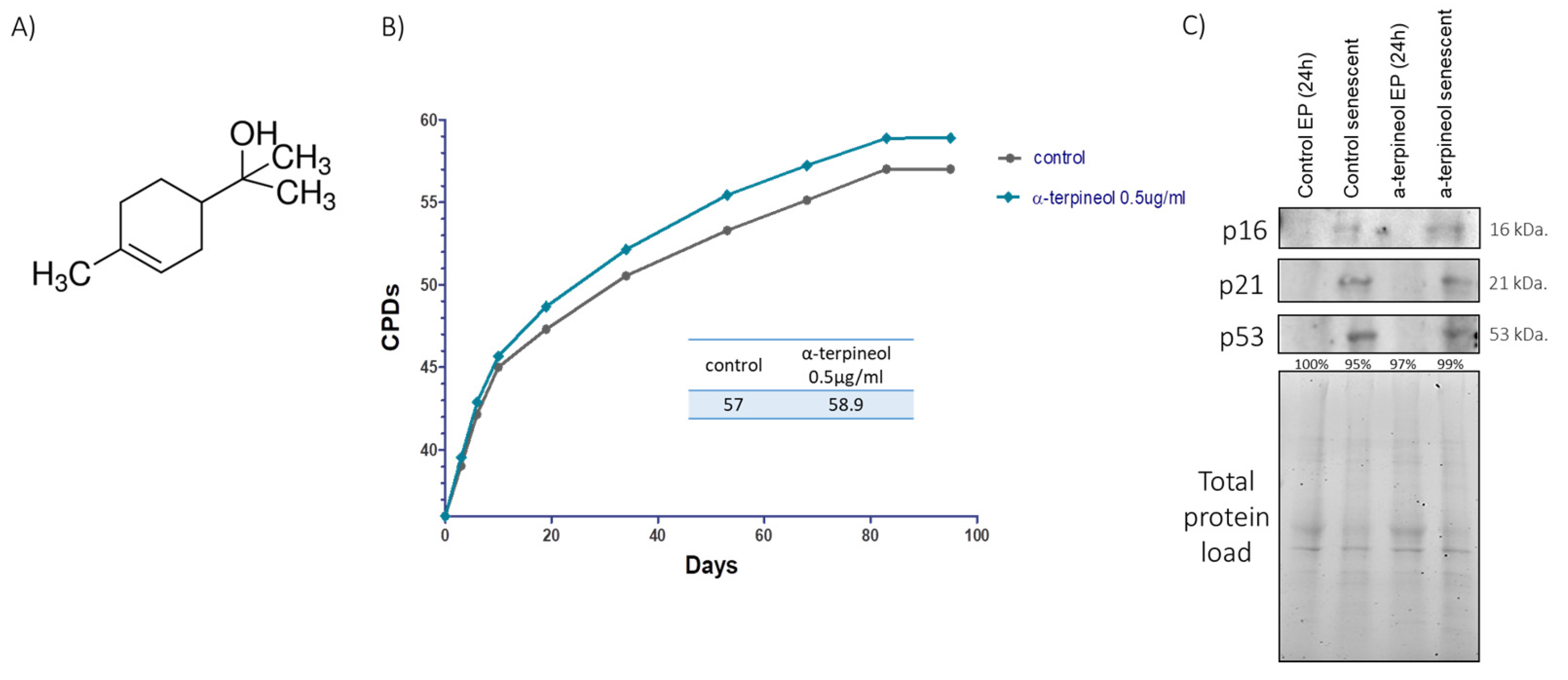
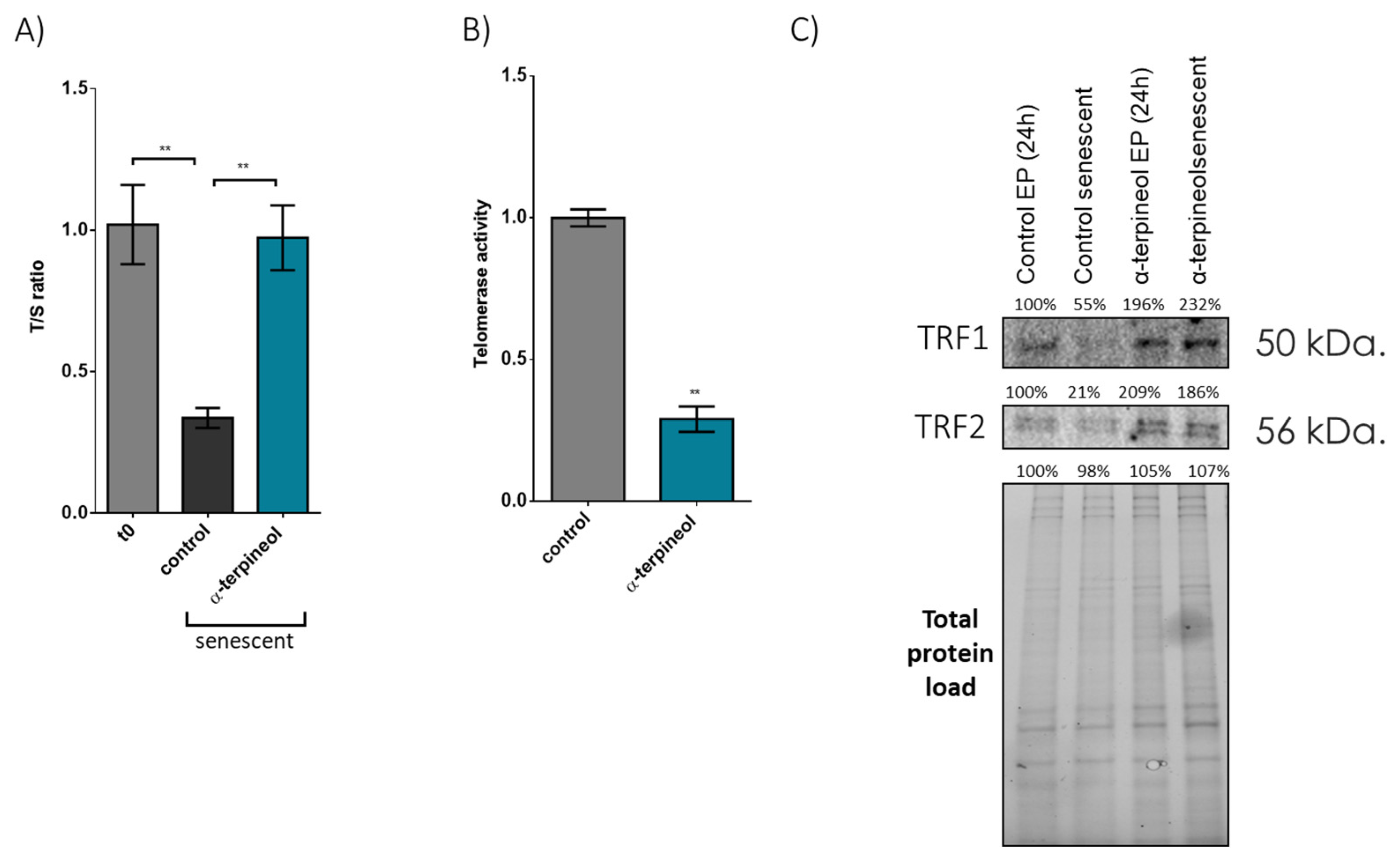
3.1. Induction of the Shelterin Components TRF1 and TRF2 by an Activator Enhances Cellular Lifespan and Preserves Telomere Length During Senescence
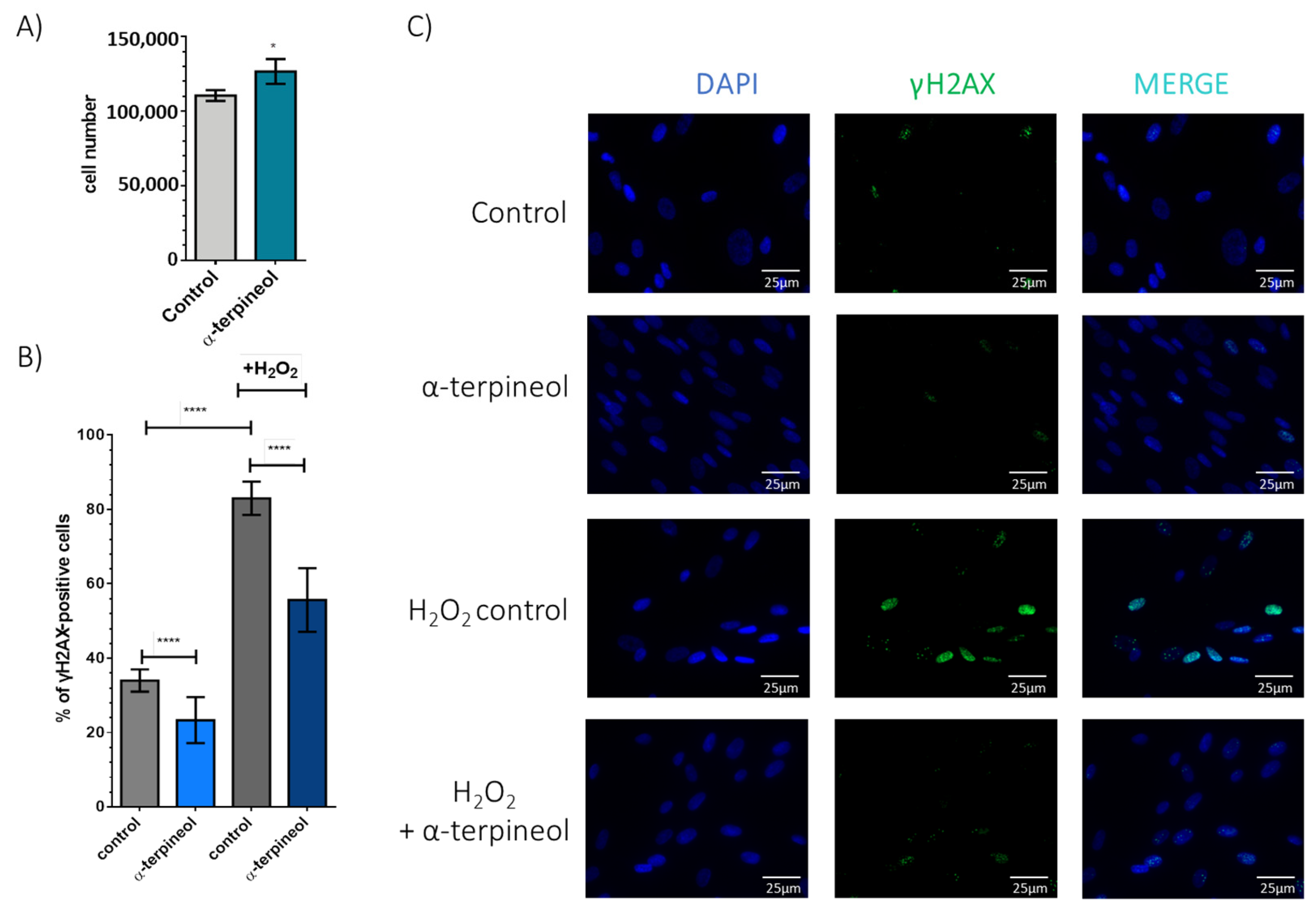
3.2. Treatment with α-Terpineol Reduces Oxidative Stress-Induced DNA Damage
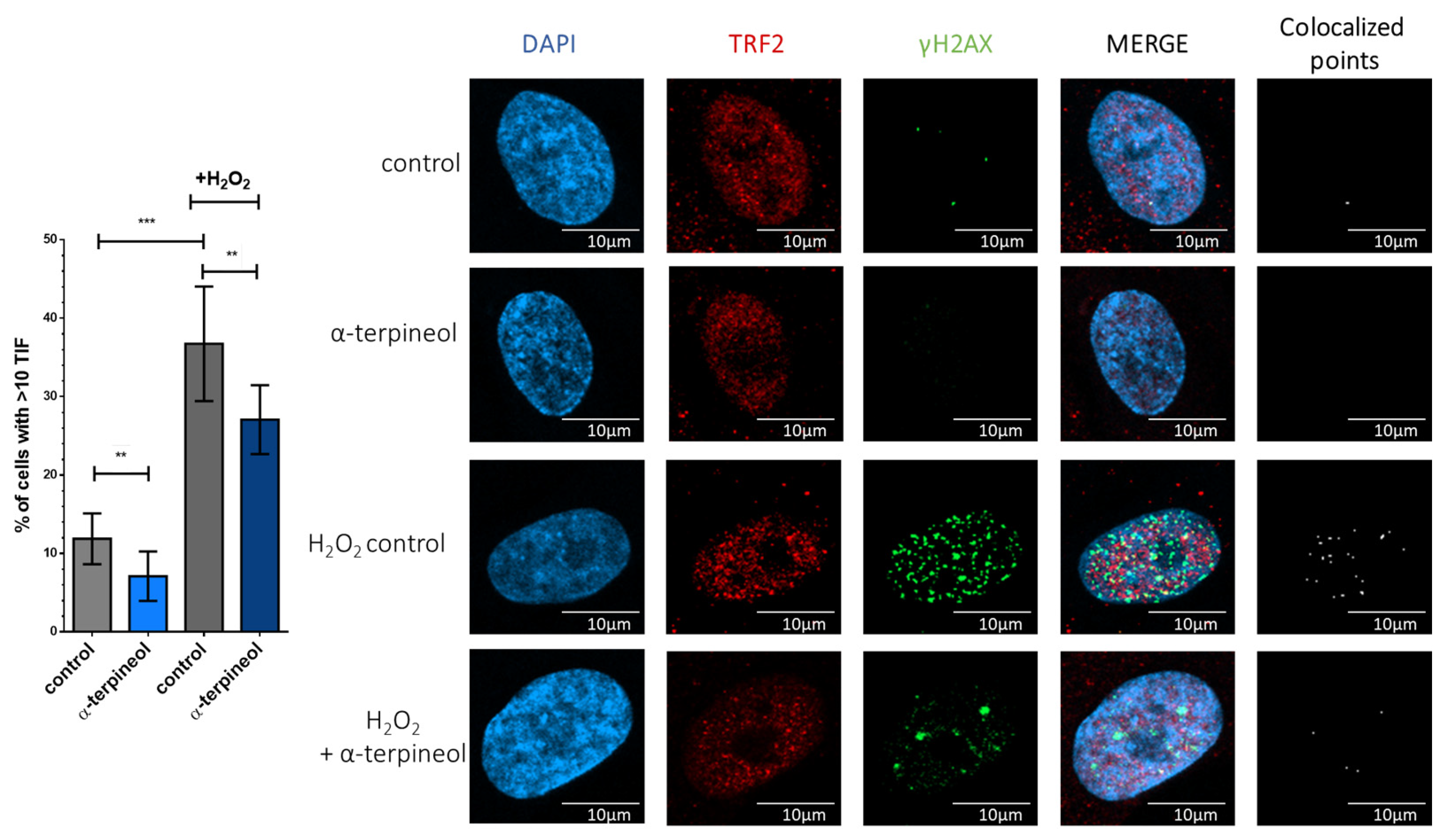
3.3. α-Terpineol Protects from Oxidative Stress-Induced Telomere-Specific DNA Damage
3.4. α-Terpineol Reduces Oxidative Damage of Nuclear Proteins and Shelterin Components During Senescence
3.5. PI3K/AKT Signaling Mediates the Effects of α-Terpineol in Shelterin Components
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Faragher, R.G.A.; Heidari, N.; Ostler, E.L. Therapeutic Opportunities Presented by Modulation of Cellular Senescence. Sub-Cell Biochem. 2023, 102, 175–193. [Google Scholar]
- Cox, L.S.; Lord, J.M. Targeting aging cells improves survival. Science 2021, 373, 281–282. [Google Scholar] [CrossRef]
- Montiel Rojas, D.; Nilsson, A.; Ponsot, E.; Brummer, R.J.; Fairweather-Tait, S.; Jennings, A.; de Groot, L.; Berendsen, A.; Pietruszka, B.; Madej, D.; et al. Short Telomere Length Is Related to Limitations in Physical Function in Elderly European Adults. Front. Physiol. 2018, 9, 1110. [Google Scholar] [CrossRef]
- Gilson, E.; Géli, V. How telomeres are replicated. Nat. Rev. Mol. Cell Biol. 2007, 8, 825–838. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef]
- Ghilain, C.; Gilson, E.; Giraud-Panis, M.-J. Multifunctionality of the Telomere-Capping Shelterin Complex Explained by Variations in Its Protein Composition. Cells 2021, 10, 1753. [Google Scholar] [CrossRef]
- Ye, J.; Wu, Y.; Gilson, E. Dynamics of telomeric chromatin at the crossroads of aging and cancer. Essays Biochem. 2010, 48, 147–164. [Google Scholar]
- Von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002, 27, 339–344. [Google Scholar] [CrossRef]
- Fleming, A.M.; Ding, Y.; Burrows, C.J. Oxidative DNA damage is epigenetic by regulating gene transcription via base excision repair. Proc. Natl. Acad. Sci. USA 2017, 114, 2604–2609. [Google Scholar] [CrossRef]
- Van Houten, B.; Santa-Gonzalez, G.A.; Camargo, M. DNA repair after oxidative stress: Current challenges. Curr. Opin. Toxicol. 2018, 7, 9–16. [Google Scholar] [CrossRef]
- Bhargava, R.; Fischer, M.; O’Sullivan, R.J. Genome rearrangements associated with aberrant telomere maintenance. Curr. Opin. Genet. Dev. 2020, 60, 31–40. [Google Scholar] [CrossRef]
- Ruis, P.; Boulton, S.J. The end protection problem-an unexpected twist in the tail. Genes Dev. 2021, 35, 1–21. [Google Scholar] [CrossRef]
- Rossiello, F.; Jurk, D.; Passos, J.F.; d’Adda di Fagagna, F. Telomere dysfunction in ageing and age-related diseases. Nat. Cell Biol. 2022, 24, 135–147. [Google Scholar] [CrossRef]
- Trendelenburg, A.U.; Scheuren, A.C.; Potter, P.; Müller, R.; Bellantuono, I. Geroprotectors: A role in the treatment of frailty. Mech. Ageing Dev. 2019, 180, 11–20. [Google Scholar] [CrossRef]
- Athanasopoulou, S.; Kapetanou, M.; Magouritsas, M.G.; Mougkolia, N.; Taouxidou, P.; Papacharalambous, M.; Sakellaridis, F.; Gonos, E. Antioxidant and Antiaging Properties of a Novel Synergistic Nutraceutical Complex: Readouts from an In Cellulo Study and an In Vivo Prospective, Randomized Trial. Antioxidants 2022, 11, 468. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009, 37, e21. [Google Scholar] [CrossRef]
- Palmer, H.M. Using Antibodies: A Laboratory Manual; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Tsolou, A.; Nelson, G.; Trachana, V.; Chondrogianni, N.; Saretzki, G.; von Zglinicki, T.; Gonos, E.S. The 19S proteasome subunit Rpn7 stabilizes DNA damage foci upon genotoxic insult. IUBMB Life 2012, 64, 432–442. [Google Scholar] [CrossRef]
- Méndez-Pertuz, M.; Martínez, P.; Blanco-Aparicio, C.; Gómez-Casero, E.; Belen García, A.; Martínez-Torrecuadrada, J.; Palafox, M.; Cortés, J.; Serra, V.; Pastor, J.; et al. Modulation of telomere protection by the PI3K/AKT pathway. Nat. Commun. 2017, 8, 1278. [Google Scholar] [CrossRef]
- Liu, Y.; Bloom, S.I.; Donato, A.J. The role of senescence, telomere dysfunction and shelterin in vascular aging. Microcirculation 2019, 26, e12487. [Google Scholar] [CrossRef]
- Sfeir, A.; Kosiyatrakul, S.T.; Hockemeyer, D.; MacRae, S.L.; Karlseder, J.; Schildkraut, C.L.; de Lange, T. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell 2009, 138, 90–103. [Google Scholar] [CrossRef]
- Martínez, P.; Thanasoula, M.; Muñoz, P.; Liao, C.; Tejera, A.; McNees, C.; Flores, J.M.; Fernández-Capetillo, O.; Tarsounas, M.; Blasco, M.A. Increased telomere fragility and fusions resulting from TRF1 deficiency lead to degenerative pathologies and increased cancer in mice. Genes Dev. 2009, 23, 2060–2075. [Google Scholar] [CrossRef]
- Storchova, R.; Palek, M.; Palkova, N.; Veverka, P.; Brom, T.; Hofr, C.; Macurek, L. Phosphorylation of TRF2 promotes its interaction with TIN2 and regulates DNA damage response at telomeres. Nucleic Acids Res. 2023, 51, 1154–1172. [Google Scholar] [CrossRef]
- García-Beccaria, M.; Martínez, P.; Méndez-Pertuz, M.; Martínez, S.; Blanco-Aparicio, C.; Cañamero, M.; Mulero, F.; Ambrogio, C.; Flores, J.M.; Megias, D.; et al. Therapeutic inhibition of TRF1 impairs the growth of p53-deficient K-RasG12V-induced lung cancer by induction of telomeric DNA damage. EMBO Mol. Med. 2015, 7, 930–949. [Google Scholar] [CrossRef]
- Hohensinner, P.J.; Kaun, C.; Buchberger, E.; Ebenbauer, B.; Demyanets, S.; Huk, I.; Eppel, W.; Maurer, G.; Huber, K.; Wojta, J. Age intrinsic loss of telomere protection via TRF1 reduction in endothelial cells. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2016, 1863, 360–367. [Google Scholar] [CrossRef]
- Wang, J.; Uryga, A.K.; Reinhold, J.; Figg, N.; Baker, L.; Finigan, A.; Gray, K.; Kumar, S.; Clarke, M.; Bennett, M. Vascular Smooth Muscle Cell Senescence Promotes Atherosclerosis and Features of Plaque Vulnerability. Circulation 2015, 132, 1909–1919. [Google Scholar] [CrossRef]
- Schneider, R.P.; Garrobo, I.; Foronda, M.; Palacios, J.A.; Marión, R.M.; Flores, I.; Ortega, S.; Blasco, M.A. TRF1 is a stem cell marker and is essential for the generation of induced pluripotent stem cells. Nat. Commun. 2013, 4, 1946. [Google Scholar] [CrossRef]
- Derevyanko, A.; Whittemore, K.; Schneider, R.P.; Jiménez, V.; Bosch, F.; Blasco, M.A. Gene therapy with the TRF1 telomere gene rescues decreased TRF1 levels with aging and prolongs mouse health span. Aging Cell 2017, 16, 1353–1368. [Google Scholar] [CrossRef]
- Wang, Z.; Rhee, D.B.; Lu, J.; Bohr, C.T.; Zhou, F.; Vallabhaneni, H.; de Souza-Pinto, N.C.; Liu, Y. Characterization of oxidative guanine damage and repair in mammalian telomeres. PLoS Genet. 2010, 6, e1000951. [Google Scholar] [CrossRef]
- Opresko, P.L.; Fan, J.; Danzy, S.; Wilson, D.M., 3rd; Bohr, V.A. Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic Acids Res. 2005, 33, 1230–1239. [Google Scholar] [CrossRef]
- Rhee, D.B.; Ghosh, A.; Lu, J.; Bohr, V.A.; Liu, Y. Factors that influence telomeric oxidative base damage and repair by DNA glycosylase OGG1. DNA Repair. 2011, 10, 34–44. [Google Scholar] [CrossRef]
- Richter, T.; Saretzki, G.; Nelson, G.; Melcher, M.; Olijslagers, S.; von Zglinicki, T. TRF2 overexpression diminishes repair of telomeric single-strand breaks and accelerates telomere shortening in human fibroblasts. Mech. Ageing Dev. 2007, 128, 340–345. [Google Scholar] [CrossRef]
- Mender, I.; Shay, J.W. Telomere Dysfunction Induced Foci (TIF) Analysis. Bio-Protocol 2015, 5, e1656. [Google Scholar] [CrossRef]
- Bellantuono, I. Find drugs that delay many diseases of old age. Nature 2018, 554, 293–295. [Google Scholar] [CrossRef]
- Chondrogianni, N.; Voutetakis, K.; Kapetanou, M.; Delitsikou, V.; Papaevgeniou, N.; Sakellari, M.; Lefaki, M.; Filippopoulou, K.; Gonos, E.S. Proteasome activation: An innovative promising approach for delaying aging and retarding age-related diseases. Ageing Res. Rev. 2015, 23, 37–55. [Google Scholar] [CrossRef]
- Kapetanou, M.; Chondrogianni, N.; Petrakis, S.; Koliakos, G.; Gonos, E.S. Proteasome activation enhances stemness and lifespan of human mesenchymal stem cells. Free Radic. Biol. Med. 2017, 103, 226–235. [Google Scholar] [CrossRef]
- Mladenovic Djordjevic, A.N.; Kapetanou, M.; Loncarevic-Vasiljkovic, N.; Todorovic, S.; Athanasopoulou, S.; Jovic, M.; Prvulovic, M.; Taoufik, E.; Matsas, R.; Kanazir, S.; et al. Pharmacological intervention in a transgenic mouse model improves Alzheimer’s-associated pathological phenotype: Involvement of proteasome activation. Free Radic. Biol. Med. 2021, 162, 88–103. [Google Scholar] [CrossRef]
- Kapeta, S.; Chondrogianni, N.; Gonos, E.S. Nuclear erythroid factor 2-mediated proteasome activation delays senescence in human fibroblasts. J. Biol. Chem. 2010, 285, 8171–8184. [Google Scholar] [CrossRef]
- Diala, I.; Wagner, N.; Magdinier, F.; Shkreli, M.; Sirakov, M.; Bauwens, S.; Schluth-Bolard, C.; Simonet, T.; Renault, V.M.; Ye, J.; et al. Telomere protection and TRF2 expression are enhanced by the canonical Wnt signalling pathway. EMBO Rep. 2013, 14, 356–363. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapetanou, M.; Athanasopoulou, S.; Goutas, A.; Makatsori, D.; Trachana, V.; Gonos, E. α-Terpineol Induces Shelterin Components TRF1 and TRF2 to Mitigate Senescence and Telomere Integrity Loss via A Telomerase-Independent Pathway. Antioxidants 2024, 13, 1258. https://doi.org/10.3390/antiox13101258
Kapetanou M, Athanasopoulou S, Goutas A, Makatsori D, Trachana V, Gonos E. α-Terpineol Induces Shelterin Components TRF1 and TRF2 to Mitigate Senescence and Telomere Integrity Loss via A Telomerase-Independent Pathway. Antioxidants. 2024; 13(10):1258. https://doi.org/10.3390/antiox13101258
Chicago/Turabian StyleKapetanou, Marianna, Sophia Athanasopoulou, Andreas Goutas, Dimitra Makatsori, Varvara Trachana, and Efstathios Gonos. 2024. "α-Terpineol Induces Shelterin Components TRF1 and TRF2 to Mitigate Senescence and Telomere Integrity Loss via A Telomerase-Independent Pathway" Antioxidants 13, no. 10: 1258. https://doi.org/10.3390/antiox13101258
APA StyleKapetanou, M., Athanasopoulou, S., Goutas, A., Makatsori, D., Trachana, V., & Gonos, E. (2024). α-Terpineol Induces Shelterin Components TRF1 and TRF2 to Mitigate Senescence and Telomere Integrity Loss via A Telomerase-Independent Pathway. Antioxidants, 13(10), 1258. https://doi.org/10.3390/antiox13101258








