The Effect of Multilayer Nanoemulsion on the In Vitro Digestion and Antioxidant Activity of β-Carotene
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of β-Carotene-Loaded Nanoemulsions
2.2.1. Preparation of the Primary Nanoemulsion
2.2.2. Preparation of the Secondary Nanoemulsion
2.2.3. Preparation of Tertiary Nanoemulsions
2.3. Physical Properties of the Nanoemulsions
2.4. Determination of Entrapment Efficiency
2.5. Cytotoxicity
2.6. In Vitro Lipid Digestion
2.7. In Vitro Bioaccessibility of β-Carotene
2.8. Antioxidant Activity
2.8.1. DPPH Radical Scavenging Assay
2.8.2. Cellular Antioxidant Activity Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physical Properties of the Nanoemulsions
3.1.1. Characterization of PRI-NE
3.1.2. Influence of CS Concentration on the Characteristics of SEC-CS
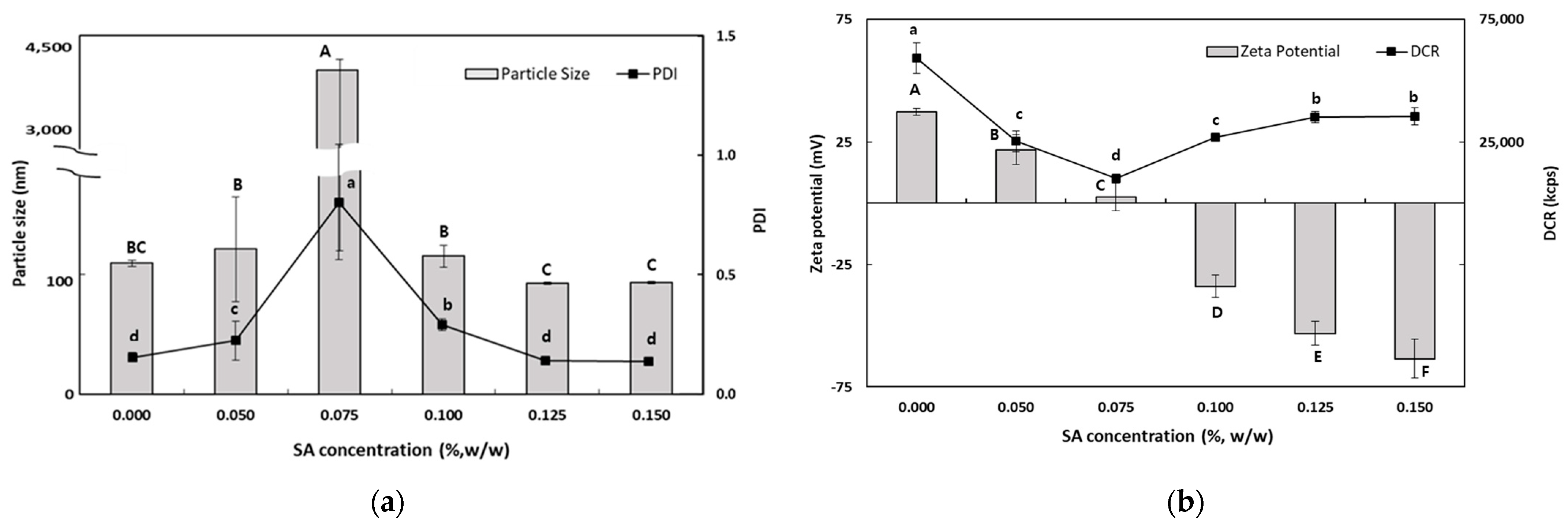
3.1.3. Influence of DS and SA Concentrations on the Characteristics of TER-DS and TER-SA
3.1.4. Characterization of the Nanoemulsions
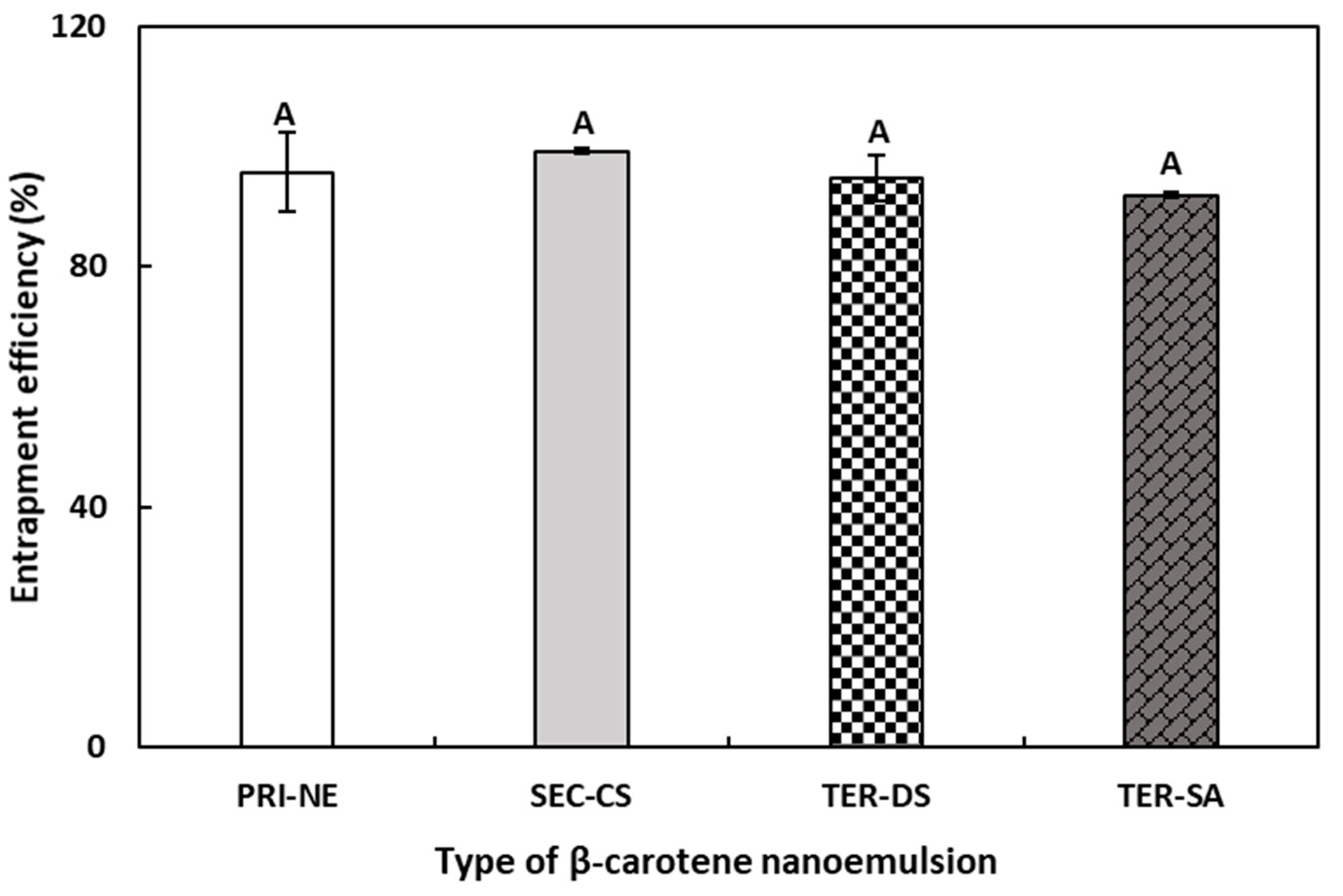
3.2. Entrapment Efficiency
3.3. Cytotoxicity of the Nanoemulsions
3.4. In Vitro Digestion
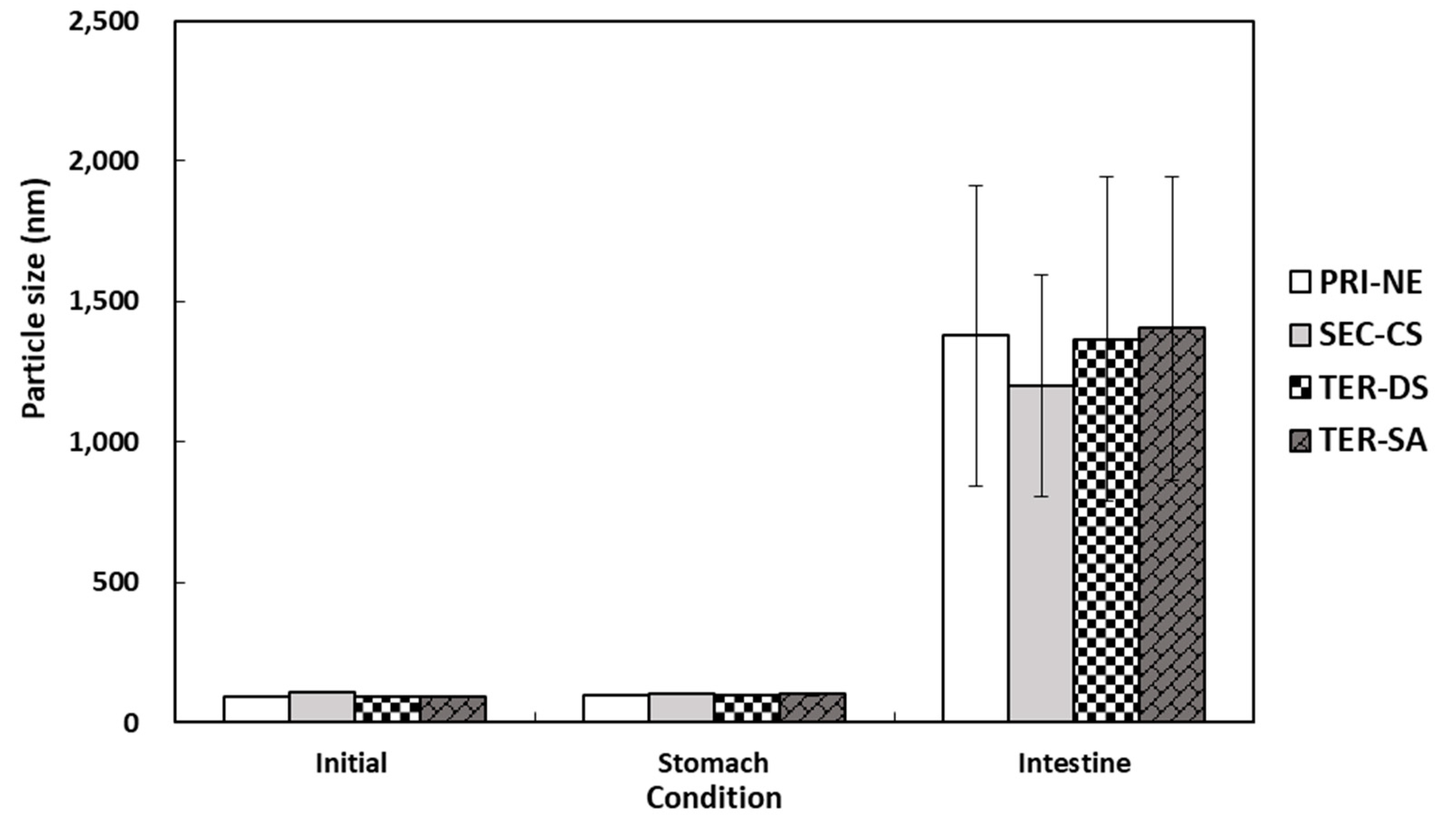
3.4.1. Influence of Initial Nanoemulsion Type on the Size and Electrical Characteristics
3.4.2. Influence of Initial Nanoemulsion Type on Lipid Digestion
3.4.3. Influence of Initial Nanoemulsion Types on β-Carotene Bioaccessibility
3.5. Antioxidant Activity
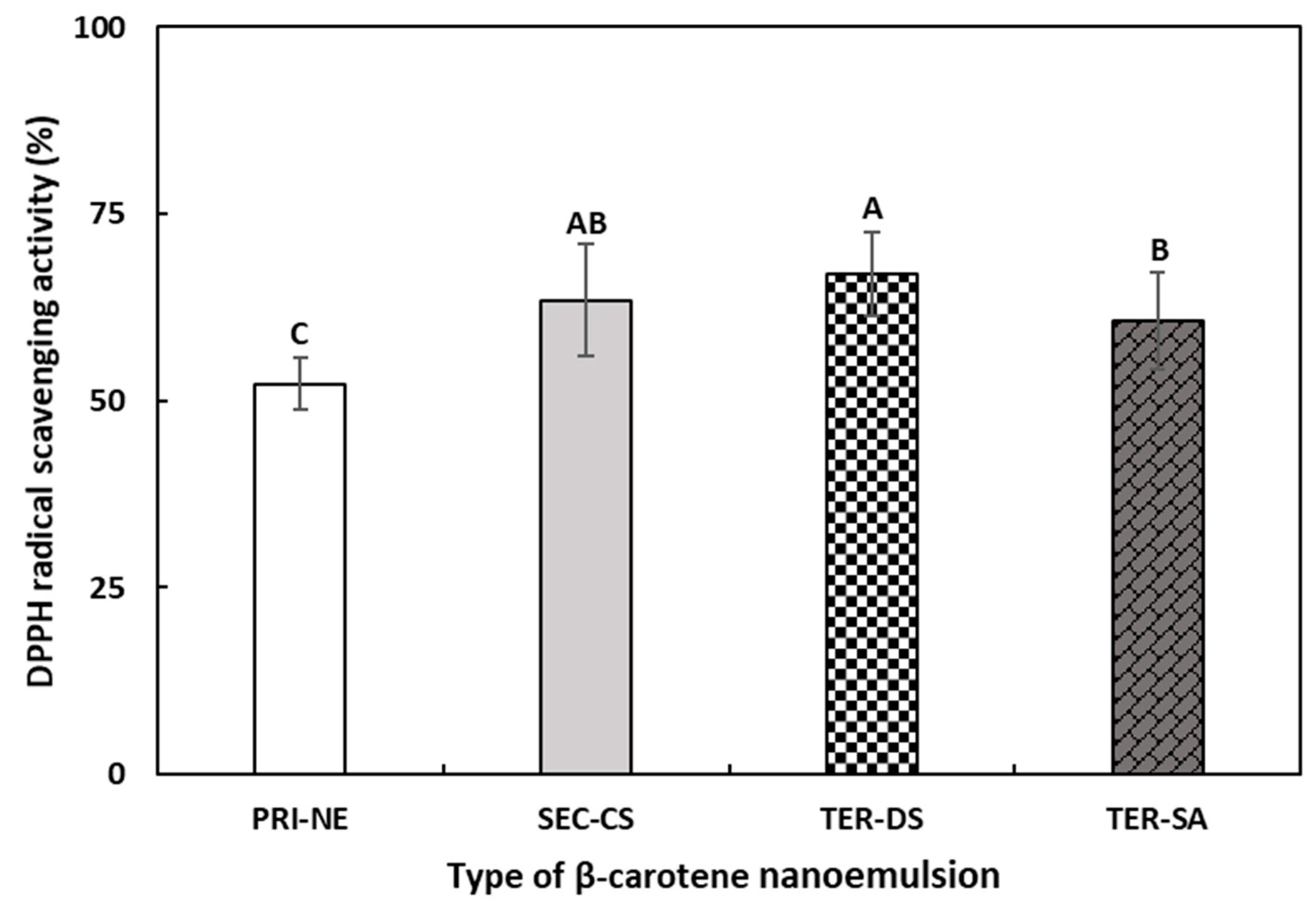
3.5.1. DPPH Radical Scavenging Activity
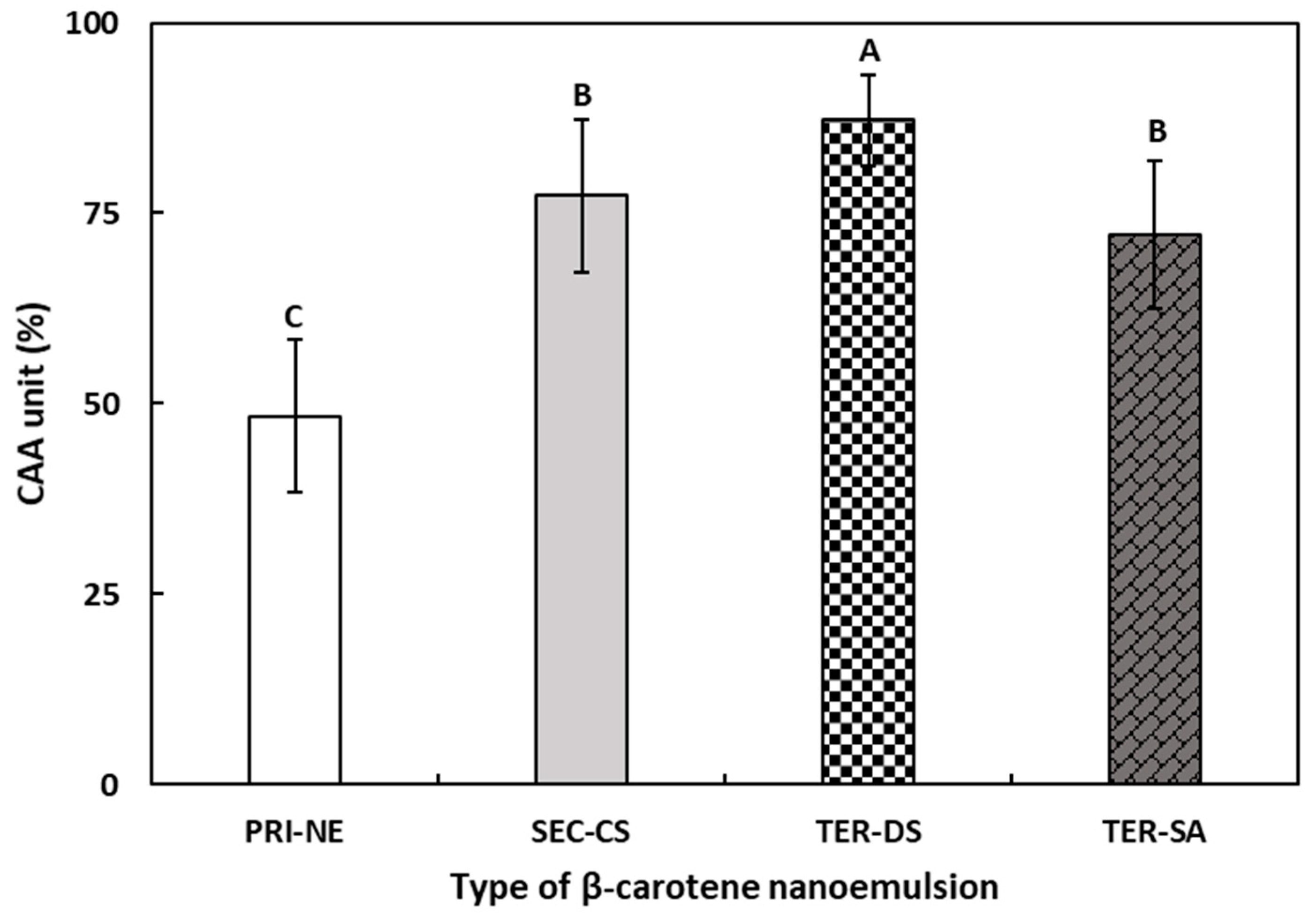
3.5.2. Cellular Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sheng, B.; Li, L.; Zhang, X.; Jiao, W.; Zhao, D.; Wang, X.; Wan, L.; Li, B.; Rong, H. Physicochemical properties and chemical stability of β-carotene bilayer emulsion coated with bovine serum albumin and Arabic gum compared to monolayer emulsions. Molecules 2018, 23, 495. [Google Scholar] [CrossRef]
- Mao, L.; Wang, D.; Liu, F.; Gao, Y. Emulsion design for the delivery of β-carotene in complex food systems. Crit. Rev. Food Sci. Nutr. 2018, 58, 770–784. [Google Scholar] [CrossRef]
- Rodríduez-Huezo, M.; Pedroza-Islas, R.; Prado-Barragán, L.; Beristain, C.; Vernon-Carter, E. Microencapsulation by spray drying of multiple emulsions containing carotenoids. J. Food Sci. 2004, 69, 351–359. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Food Carotenoids: Chemistry, Biology and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Mao, L.; Xu, D.; Yang, J.; Yuan, F.; Gao, Y.; Zhao, J. Effects of small and large molecule emulsifiers on the characteristics of β-carotene nanoemulsions prepared by high pressure homogenization. Food Technol. Biotechnol. 2009, 47, 336–342. [Google Scholar]
- Donhowe, E.G.; Kong, F. Beta-carotene: Digestion, microencapsulation, and in vitro bioavailability. Food Bioprocess Technol. 2014, 7, 338–354. [Google Scholar] [CrossRef]
- Hu, M.; Li, Y.; Decker, E.A.; Xiao, H.; McClements, D.J. Impact of layer structure on physical stability and lipase digestibility of lipid droplets coated by biopolymer nanolaminated coatings. Food Biophys. 2011, 6, 37–48. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A.; Weiss, D.J. Emulsion-based delivery systems for lipophilic bioactive components. J. Food Sci. 2007, 72, R109–R124. [Google Scholar] [CrossRef]
- Mun, S.; Decker, E.A.; McClements, D.J. Influence of droplet characteristics on the formation of oil-in-water emulsions stabilized by surfactant—Chitosan layers. Langmuir 2005, 21, 6228–6234. [Google Scholar] [CrossRef]
- Guzey, D.; Kim, H.; McClements, D. Factors influencing the production of o/w emulsions stabilized by β-lactoglobulin–pectin membranes. Food Hydrocoll. 2004, 18, 967–975. [Google Scholar] [CrossRef]
- Yin, L.-J.; Chu, B.-S.; Kobayashi, I.; Nakajima, M. Performance of selected emulsifiers and their combinations in the preparation of β-carotene nanodispersions. Food Hydrocoll. 2009, 23, 1617–1622. [Google Scholar] [CrossRef]
- McClements, D.J. Enhanced delivery of lipophilic bioactives using emulsions: A review of major factors affecting vitamin, nutraceutical, and lipid bioaccessibility. Food Funct. 2018, 9, 22–41. [Google Scholar] [CrossRef]
- Hou, Z.; Zhang, M.; Liu, B.; Yan, Q.; Yuan, F.; Xu, D.; Gao, Y. Effect of chitosan molecular weight on the stability and rheological properties of β-carotene emulsions stabilized by soybean soluble polysaccharides. Food Hydrocoll. 2012, 26, 205–211. [Google Scholar] [CrossRef]
- Klinkesorn, U.; Sophanodora, P.; Chinachoti, P.; Decker, E.A.; McClements, D.J. Encapsulation of emulsified tuna oil in two-layered interfacial membranes prepared using electrostatic layer-by-layer deposition. Food Hydrocoll. 2005, 19, 1044–1053. [Google Scholar] [CrossRef]
- Hong, S.E.; Lee, J.S.; Lee, H.G. α-Tocopherol-loaded multi-layer nanoemulsion using chitosan, and dextran sulfate: Cellular uptake, antioxidant activity, and in vitro bioaccessibility. Int. J. Biol. Macromol. 2024, 254, 127819. [Google Scholar] [CrossRef]
- Giacone, D.V.; Dartora, V.F.; de Matos, J.K.; Passos, J.S.; Miranda, D.A.; de Oliveira, E.A.; Lopes, L.B. Effect of nanoemulsion modification with chitosan and sodium alginate on the topical delivery and efficacy of the cytotoxic agent piplartine in 2D and 3D skin cancer models. Int. J. Biol. Macromol. 2020, 165, 1055–1065. [Google Scholar] [CrossRef]
- Kim, M.K.; Lee, J.-S.; Kim, K.Y.; Lee, H.G. Ascorbyl palmitate-loaded chitosan nanoparticles: Characteristic and polyphenol oxidase inhibitory activity. Colloids Surf. B Biointerfaces 2013, 103, 391–394. [Google Scholar] [CrossRef]
- Shi, M.; Cai, Q.; Yao, L.; Mao, Y.; Ming, Y.; Ouyang, G. Antiproliferation and apoptosis induced by curcumin in human ovarian cancer cells. Cell Biol. Int. 2006, 30, 221–226. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Qian, C.; Martín-Belloso, O.; McClements, D.J. Influence of particle size on lipid digestion and β-carotene bioaccessibility in emulsions and nanoemulsions. Food Chem. 2013, 141, 1472–1480. [Google Scholar] [CrossRef]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Nanoemulsion delivery systems: Influence of carrier oil on β-carotene bioaccessibility. Food Chem. 2012, 135, 1440–1447. [Google Scholar] [CrossRef]
- Wan, H.; Liu, D.; Yu, X.; Sun, H.; Li, Y. A Caco-2 cell-based quantitative antioxidant activity assay for antioxidants. Food Chem. 2015, 175, 601–608. [Google Scholar] [CrossRef]
- Carbone, C.; Tomasello, B.; Ruozi, B.; Renis, M.; Puglisi, G. Preparation and optimization of PIT solid lipid nanoparticles via statistical factorial design. Eur. J. Med. Chem. 2012, 49, 110–117. [Google Scholar] [CrossRef]
- Gaumet, M.; Vargas, A.; Gurny, R.; Delie, F. Nanoparticles for drug delivery: The need for precision in reporting particle size parameters. Eur. J. Pharm. Biopharm. 2008, 69, 1–9. [Google Scholar] [CrossRef]
- O’Brien, R.W. Electroacoustic studies of moderately concentrated colloidal suspensions. Faraday Discuss. Chem. Soc. 1990, 90, 301–312. [Google Scholar] [CrossRef]
- Lee, H.-K.; Lee, H.-Y.; Jeon, J.-M. Codeposition of micro-and nano-sized SiC particles in the nickel matrix composite coatings obtained by electroplating. Surf. Coat. Technol. 2007, 201, 4711–4717. [Google Scholar] [CrossRef]
- Yang, Y.; Nakada, N.; Nakajima, R.; Yasojima, M.; Wang, C.; Tanaka, H. pH, ionic strength and dissolved organic matter alter aggregation of fullerene C60 nanoparticles suspensions in wastewater. J. Hazard. Mater. 2013, 244, 582–587. [Google Scholar] [CrossRef]
- Lee, J.S.; Suh, J.W.; Kim, E.S.; Lee, H.G. Preparation and characterization of mucoadhesive nanoparticles for enhancing cellular uptake of coenzyme Q10. J. Agric. Food Chem. 2017, 65, 8930–8937. [Google Scholar] [CrossRef]
- Xu, D.; Aihemaiti, Z.; Cao, Y.; Teng, C.; Li, X. Physicochemical stability, microrheological properties and microstructure of lutein emulsions stabilized by multilayer membranes consisting of whey protein isolate, flaxseed gum and chitosan. Food Chem. 2016, 202, 156–164. [Google Scholar] [CrossRef]
- Teo, A.; Goh, K.K.; Wen, J.; Oey, I.; Ko, S.; Kwak, H.-S.; Lee, S.J. Physicochemical properties of whey protein, lactoferrin and Tween 20 stabilised nanoemulsions: Effect of temperature, pH and salt. Food Chem. 2016, 197, 297–306. [Google Scholar] [CrossRef]
- Hou, Z.; Gao, Y.; Yuan, F.; Liu, Y.; Li, C.; Xu, D. Investigation into the physicochemical stability and rheological properties of β-carotene emulsion stabilized by soybean soluble polysaccharides and chitosan. J. Agric. Food Chem. 2010, 58, 8604–8611. [Google Scholar] [CrossRef]
- Nguyen, M.-H.; Hwang, I.-C.; Park, H.-J. Enhanced photoprotection for photo-labile compounds using double-layer coated corn oil-nanoemulsions with chitosan and lignosulfonate. J. Photochem. Photobiol. B Biol. 2013, 125, 194–201. [Google Scholar] [CrossRef]
- Wooster, T.J.; Augustin, M.A. β-Lactoglobulin–dextran Maillard conjugates: Their effect on interfacial thickness and emulsion stability. J. Colloid Interface Sci. 2006, 303, 564–572. [Google Scholar] [CrossRef]
- Zeeb, B.; Thongkaew, C.; Weiss, J. Theoretical and practical considerations in electrostatic depositioning of charged polymers. J. Appl. Polym. Sci. 2014, 131, 40099. [Google Scholar] [CrossRef]
- Li, J.; Hwang, I.-C.; Chen, X.; Park, H.J. Effects of chitosan coating on curcumin loaded nano-emulsion: Study on stability and in vitro digestibility. Food Hydrocoll. 2016, 60, 138–147. [Google Scholar] [CrossRef]
- Li, Y.Y.; Chen, X.G.; Liu, C.S.; Cha, D.S.; Park, H.J.; Lee, C.M. Effect of the molecular mass and degree of substitution of oleoylchitosan on the structure, rheological properties, and formation of nanoparticles. J. Agric. Food Chem. 2007, 55, 4842–4847. [Google Scholar] [CrossRef]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef]
- Hsu, J.-P.; Nacu, A. Behavior of soybean oil-in-water emulsion stabilized by nonionic surfactant. J. Colloid Interface Sci. 2003, 259, 374–381. [Google Scholar] [CrossRef]
- Pouton, C.W.; Porter, C.J. Formulation of lipid-based delivery systems for oral administration: Materials, methods and strategies. Adv. Drug Deliv. Rev. 2008, 60, 625–637. [Google Scholar] [CrossRef]
- Reis, P.; Holmberg, K.; Watzke, H.; Leser, M.E.; Miller, R. Lipases at interfaces: A review. Adv. Colloid Interface Sci. 2009, 147, 237–250. [Google Scholar] [CrossRef]
- Waraho, T.; McClements, D.J.; Decker, E.A. Impact of free fatty acid concentration and structure on lipid oxidation in oil-in-water emulsions. Food Chem. 2011, 129, 854–859. [Google Scholar] [CrossRef]
- Yao, M.; Xiao, H.; McClements, D.J. Delivery of lipophilic bioactives: Assembly, disassembly, and reassembly of lipid nanoparticles. Annu. Rev. Food Sci. Technol. 2014, 5, 53–81. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, Z.; Lei, F.; Chang, Y.; Gao, Y. Investigation into the bioaccessibility and microstructure changes of β-carotene emulsions during in vitro digestion. Innov. Food Sci. Emerg. Technol. 2012, 15, 86–95. [Google Scholar] [CrossRef]
- Singh, H.; Ye, A.; Horne, D. Structuring food emulsions in the gastrointestinal tract to modify lipid digestion. Prog. Lipid Res. 2009, 48, 92–100. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Zou, L.; Xiao, H.; Zhang, G.; Decker, E.A.; McClements, D.J. Enhancing nutraceutical bioavailability from raw and cooked vegetables using excipient emulsions: Influence of lipid type on carotenoid bioaccessibility from carrots. J. Agric. Food Chem. 2015, 63, 10508–10517. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Zou, L.; Xiao, H.; Zhang, G.; Decker, E.A.; McClements, D.J. Impact of lipid content on the ability of excipient emulsions to increase carotenoid bioaccessibility from natural sources (raw and cooked carrots). Food Biophys. 2016, 11, 71–80. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; McClements, D.J. Encapsulation of β-carotene in alginate-based hydrogel beads: Impact on physicochemical stability and bioaccessibility. Food Hydrocoll. 2016, 61, 1–10. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Nakajima, Y.; Inokuchi, Y.; Nishi, M.; Shimazawa, M.; Otsubo, K.; Hara, H. Coenzyme Q10 protects retinal cells against oxidative stress in vitro and in vivo. Brain Res. 2008, 1226, 226–233. [Google Scholar] [CrossRef]
- Carpenter, J.; George, S.; Saharan, V.K. Curcumin encapsulation in multilayer oil-in-water emulsion: Synthesis using ultrasonication and studies on stability and antioxidant and release activities. Langmuir 2019, 35, 10866–10876. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Silva, H.D.; Soliva-Fortuny, R.; Martín-Belloso, O.; Vicente, A.A. Formation, stability and antioxidant activity of food-grade multilayer emulsions containing resveratrol. Food Hydrocoll. 2017, 71, 207–215. [Google Scholar] [CrossRef]
- Brouwers, J.; Brewster, M.E.; Augustijns, P. Supersaturating drug delivery systems: The answer to solubility-limited oral bioavailability? J. Pharm. Sci. 2009, 98, 2549–2572. [Google Scholar] [CrossRef]
- Keck, C.M.; Müller, R.H. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur. J. Pharm. Biopharm. 2006, 62, 3–16. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]






| Nanoemulsions | Particle Size (nm) | Polydispersity Index Value | Zeta Potential (mV) |
|---|---|---|---|
| PRI-NE | 92.2 ± 0.7 b | 0.163 ± 0.013 c | −42.4 ± 1.7 b |
| SEC-CS | 109.5 ± 2.6 a | 0.189 ± 0.020 a | 37.3 ± 1.4 a |
| TER-DS | 91.9 ± 1.1 b | 0.159 ± 0.016 c | −46.6 ± 1.9 c |
| TER-SA | 92.6 ± 1.0 b | 0.176 ± 0.009 b | −53.1 ± 4.8 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.Z.; Kim, D.-Y.; Baek, Y.; Lee, H.G. The Effect of Multilayer Nanoemulsion on the In Vitro Digestion and Antioxidant Activity of β-Carotene. Antioxidants 2024, 13, 1218. https://doi.org/10.3390/antiox13101218
Sun MZ, Kim D-Y, Baek Y, Lee HG. The Effect of Multilayer Nanoemulsion on the In Vitro Digestion and Antioxidant Activity of β-Carotene. Antioxidants. 2024; 13(10):1218. https://doi.org/10.3390/antiox13101218
Chicago/Turabian StyleSun, Mei Zi, Do-Yeong Kim, Youjin Baek, and Hyeon Gyu Lee. 2024. "The Effect of Multilayer Nanoemulsion on the In Vitro Digestion and Antioxidant Activity of β-Carotene" Antioxidants 13, no. 10: 1218. https://doi.org/10.3390/antiox13101218
APA StyleSun, M. Z., Kim, D.-Y., Baek, Y., & Lee, H. G. (2024). The Effect of Multilayer Nanoemulsion on the In Vitro Digestion and Antioxidant Activity of β-Carotene. Antioxidants, 13(10), 1218. https://doi.org/10.3390/antiox13101218







