Effects of α-Lipoic Acid Supplementation on Growth Performance, Liver Histology, Antioxidant and Related Genes Expression of Hybrid Grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Diets
2.2. Fish and Feeding Trial
2.3. Sample Collection
2.4. Methods of Analyses
2.4.1. Growth Performance Formula
2.4.2. Measurement of Enzyme Activities
2.4.3. Hepatic Histological Structures
2.4.4. Analysis of Antioxidant and Immune-Related Gene Expression in Liver
2.5. Statistical Analysis
3. Results
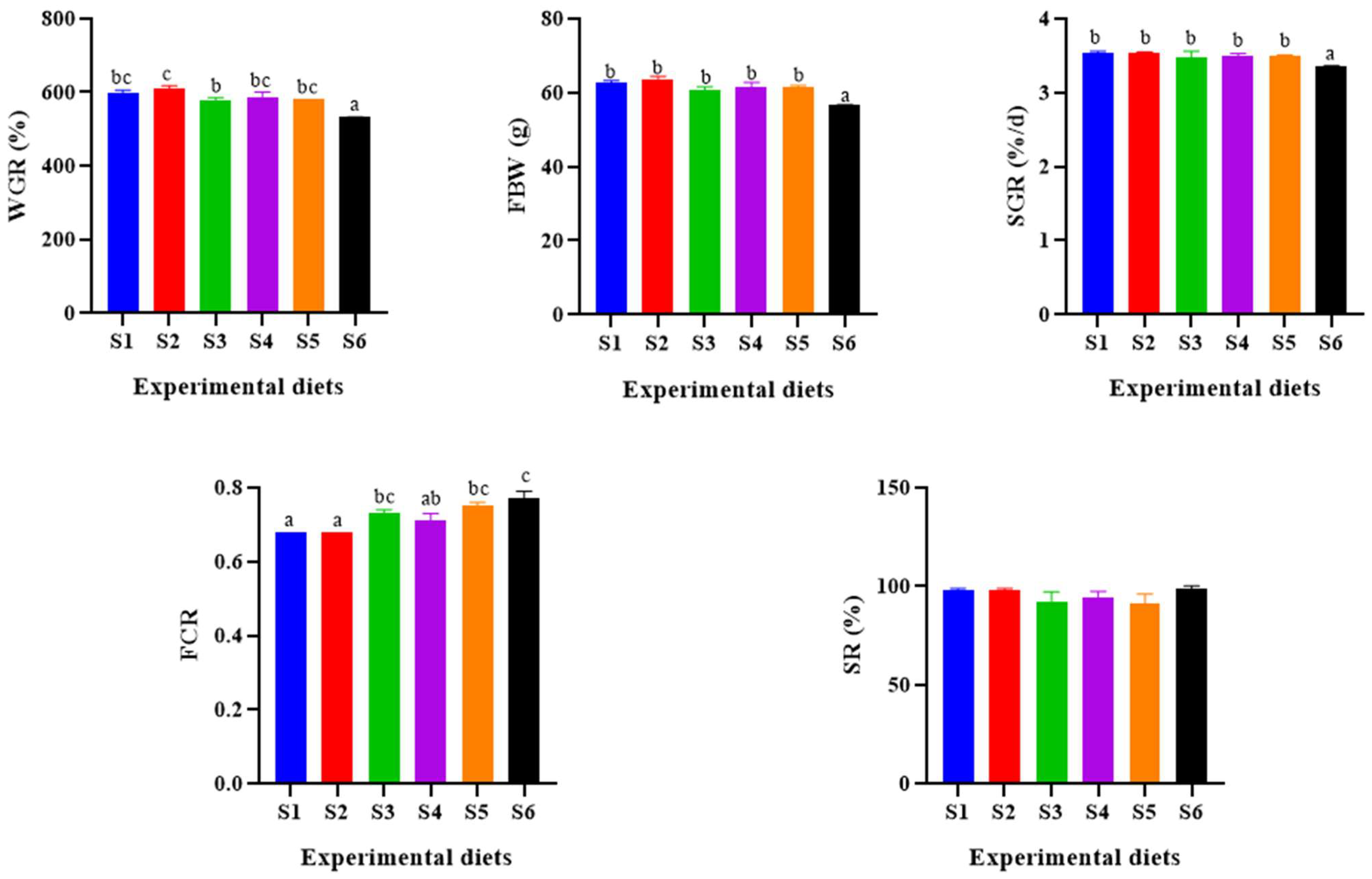
3.1. Growth Performance
3.2. Serum Antioxidant Indexes
3.3. Liver Antioxidant and Immune Indexes
3.4. Liver Histology
3.5. Antioxidant and Immune-Related Gene Expression in Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; He, X.; Huang, G.; Zhou, Y.; Lai, J. Bioremediation by the mullet mugil cephalus feeding on organic deposits produced by intensive shrimp mariculture. Aquaculture 2021, 541, 736674. [Google Scholar] [CrossRef]
- Lu, K.; Xu, W.; Li, X.; Liu, W.; Wang, L.; Zhang, C. Hepatic triacylglycerol secretion, lipid transport and tissue lipid uptake in blunt snout bream (Megalobrama amblycephala) fed high-fat diet. Aquaculture 2013, 408–409, 160–168. [Google Scholar] [CrossRef]
- Zhe, T.; Dong, L.; Ming, C.; Jun, Z.; Bo, Z.; Yong, S.; Lei, Z.; Yi, H. Low-fishmeal and high-fat diet supplement with soybean lecithin on growth, serum biochemical indexes and intestinal flora of rice field eel (Monopterus albus). Acta Hydrobiol. Sin. 2024, 48, 361–371. [Google Scholar] [CrossRef]
- Wu, L.; Liang, H.; Hamunjo, C.M.K.; Ge, X.; Ji, K.; Yu, H.; Huang, D.; Xu, H.; Ren, M. Culture salinity alters dietary protein requirement, whole body composition and nutrients metabolism related genes expression in juvenile genetically improved farmed tilapia (GIFT) (Oreochromis niloticus). Aquaculture 2021, 531, 735961. [Google Scholar] [CrossRef]
- Wang, L.; Ma, B.; Chen, D.; Lou, B.; Zhan, W.; Chen, R.; Tan, P.; Xu, D.; Liu, F.; Xie, Q. Effect of dietary level of vitamin e on growth performance, antioxidant ability, and resistance to vibrio alginolyticus challenge in yellow drum Nibea albiflora. Aquaculture 2019, 507, 119–125. [Google Scholar] [CrossRef]
- Zhang, C.X.; Huang, F.; Li, J.; Wang, L.; Song, K.; Mai, K.S. Interactive effects of dietary magnesium and vitamin e on growth performance, body composition, blood parameters and antioxidant status in japanese seabass (Lateolabrax japonicus) Fed Oxidized Oil. Aquac. Nutr. 2016, 22, 708–722. [Google Scholar] [CrossRef]
- Wei, B.; Yang, Z.; Cheng, Y.; Wang, J.; Zhou, J. Effects of the complete replacement of fish oil with linseed oil on growth, fatty acid composition, and protein expression in the chinese mitten crab (Eriocheir sinensis). Proteome Sci. 2018, 16, 6. [Google Scholar] [CrossRef]
- Wu, Z.; Yu, X.; Guo, J.; Fu, Y.; Guo, Y.; Pan, M.; Zhang, W.; Mai, K. Replacement of dietary fish meal with clostridium autoethanogenum protein on growth performance, digestion, mtor pathways and muscle quality of abalone Haliotis Discus Hannai. Aquaculture 2022, 553, 738070. [Google Scholar] [CrossRef]
- Presa, N.; Clugston, R.D.; Lingrell, S.; Kelly, S.E.; Merrill, A.H.; Jana, S.; Kassiri, Z.; Gómez-Muñoz, A.; Vance, D.E.; Jacobs, R.L.; et al. Vitamin E alleviates non-alcoholic fatty liver disease in phosphatidylethanolamine n-methyltransferase deficient mice. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 14–25. [Google Scholar] [CrossRef]
- Suo, X.; Yan, X.; Tan, B.; Pan, S.; Li, T.; Liu, H.; Huang, W.; Zhang, S.; Yang, Y.; Dong, X. Lipid metabolism disorders of hybrid grouper (♀ Epinephelus fuscointestinestatus × ♂ E. lanceolatu) induced by high-lipid diet. Front. Mar. Sci. 2022, 9, 990193. [Google Scholar] [CrossRef]
- Jia, Y.; Jing, Q.; Niu, H.; Huang, B. Ameliorative Effect of vitamin E on hepatic oxidative stress and hypoimmunity induced by high-fat diet in turbot (Scophthalmus maximus). Fish Shellfish Immunol. 2017, 67, 634–642. [Google Scholar] [CrossRef]
- Xu, F.; Xu, C.; Xiao, S.; Lu, M.; Limbu, S.M.; Wang, X.; Du, Z.; Qin, J.G.; Chen, L. Effects of α-lipoic acid on growth performance, body composition, antioxidant profile and lipid metabolism of the GIFT tilapia (Oreochromis niloticus) fed high-fat diets. Aquac. Nutr. 2019, 25, 585–596. [Google Scholar] [CrossRef]
- Packer, L.; Witt, E.H.; Tritschler, H.J. Alpha-lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 1995, 19, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Han, S.; Zhou, Q.; Wu, J.; Chen, T. Effect of dietary antioxidant on microcystin-induced toxicosis in crucian. Jiangsu J. Agric. Sci. 2020, 36, 417–422. [Google Scholar] [CrossRef]
- Lu, D.L.; Limbu, S.M.; Lv, H.B.; Ma, Q.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y. The Comparisons in protective mechanisms and efficiencies among dietary α-lipoic acid, β-glucan and l-carnitine on nile tilapia infected by Aeromonas Hydrophila. Fish Shellfish Immunol. 2019, 86, 785–793. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Q.; Mai, K.; Xu, W.; Wang, X.; Liufu, Z. Effects of dietary α-lipoic acid on the growth and antioxidative responses of juvenile abalone Haliotis Discus Hannai Ino. Aquac. Res. 2010, 41, e781–e787. [Google Scholar] [CrossRef]
- Lei, Y.; Wu, W.; Zhang, Y.; Zhou, H.; Zhang, W.; Mai, K. Protective effect of dietary α-lipoic acid on abalone Haliotis discus hannai ino against the toxicity of waterborne copper. Period. Ocean. Univ. China 2008, 45, 39–45. [Google Scholar] [CrossRef]
- Weijun, W.; Xili, L.; Weipeng, C.; Yuhao, H.; Xiuping, F.; Xiaoming, Q. Stress-relieving effect of basil essential oil on temporarily cultured pearl gentian grouper (Epinephelus fuscoguttatusi ♀ × Epinephelus lanceolatus ♂) before transportation. J. Guangdong Ocean Univ. 2023, 43, 43–50. [Google Scholar]
- Liang, S.; Lu, Y. Combined Effects of temperature and salinity on activity of serum immune indicators in pearl gentian grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂). J. Guangdong Ocean Univ. 2023, 43, 8–16. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.Y.; Li, B.S.; Qiao, H.J.; Liu, X.D.; Hao, T.T.; Wang, X.Y. Dietary manganese requirement of juvenile hybrid grouper, Epinephelus lanceolatus × E. fuscoguttatus. Aquac. Nutr. 2018, 24, 215–223. [Google Scholar] [CrossRef]
- Liu, H.; Li, L.; Akiku, S.; Tang, Z.; Fang, W.; Tan, B.; Dong, X.; Chi, S.; Yang, Q.; Zhang, S.; et al. Effects of dietary yeast culture supplementation on growth, intestinal morphology, immunity, and disease resistance in Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatu ♂. J. Guangdong Ocean Univ. 2021, 41, 1–11. [Google Scholar] [CrossRef]
- Hirwitz, W.; Latimer, G. Official methods of analysis of AOAC international (16th Edn). Off. Methods Anal. AOAC Int. 1995, 6, 382. [Google Scholar]
- Yang, T.; Huang, Y.; Ru, X.; Li, J.; Zhu, K.; Yang, J.; Chen, P.; Zhu, C. Effects of formula feed replacing chilled fish on growth and liver transcriptome of juvenile Seriola dumerili. J. Guangdong Ocean Univ. 2023, 43, 92–99. [Google Scholar] [CrossRef]
- Kütter, M.T.; Monserrat, J.M.; Primel, E.G.; Caldas, S.S.; Tesser, M.B. Effects of dietary α-lipoic acid on growth, body composition and antioxidant status in the plata pompano trachinotus marginatus (Pisces, carangidae). Aquaculture 2012, 368–369, 29–35. [Google Scholar] [CrossRef]
- Huang, C.; Sun, J.; Ji, H.; Oku, H.; Chang, Z.; Tian, J.; Yu, E.; Xie, J. Influence of dietary alpha-lipoic acid and lipid level on the growth performance, food intake and gene expression of peripheral appetite regulating factors in juvenile grass carp (Ctenopharyngodon idellus). Aquaculture 2019, 505, 412–422. [Google Scholar] [CrossRef]
- Ungvari, Z.; Bagi, Z.; Feher, A.; Recchia, F.A.; Sonntag, W.E.; Pearson, K.; De Cabo, R.; Csiszar, A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am. J. Physiol. Hear. Circ. Physiol. 2010, 299, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wu, X.Q.; Zheng, L.J.; Dai, Z.Y.; Wu, L.F. Effect of acute exposure to ammonia and bft alterations on rhynchocypris lagowski: Digestive enzyme, inflammation response, oxidative stress and immunological parameters. Environ. Toxicol. Pharmacol. 2020, 78, 103380. [Google Scholar] [CrossRef]
- Yaghoubi, M.; Mozanzadeh, M.T.; Marammazi, J.G.; Safari, O.; Gisbert, E. Dietary replacement of fish meal by soy products (soybean meal and isolated soy protein) in silvery-black porgy juveniles (Sparidentex hasta). Aquaculture 2016, 464, 50–59. [Google Scholar] [CrossRef]
- Koruk, M.; Taysi, S.; Savas, M.C.; Yilmaz, O.; Akcay, F.; Karakok, M. Oxidative stress enzymatic antioxidant status in patients with nonalcoholic steatohepatitis. Ann. Clin. Lab. Sci. 2004, 34, 57–62. [Google Scholar]
- Wang, L.; Gallagher, E.P. Role of nrf2 antioxidant defense in mitigating cadmium-induced oxidative stress in the olfactory system of zebrafish. Toxicol. Appl. Pharmacol. 2013, 266, 177–186. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Z.; Zhong, H.; Yin, Q.; Xiao, J.; Wang, F.; Zhou, Y.; Luo, Y. Regulation of triglyceride synthesis by estradiol in the livers of hybrid tilapia (Oreochromis niloticus ♀ × O. aureus ♂). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2019, 238, 110335. [Google Scholar] [CrossRef]
- Zeng, F.; Tee, C.; Liu, M.; Sherry, J.P.; Dixon, B.; Duncker, B.P.; Bols, N.C. The p53/hsp70 inhibitor, 2-phenylethynesulfonamide, causes oxidative stress, unfolded protein response and apoptosis in rainbow trout cells. Aquat. Toxicol. 2014, 146, 45–51. [Google Scholar] [CrossRef]
- Sun, S.; Gu, Z.; Fu, H.; Zhu, J.; Ge, X.; Xuan, F. Molecular cloning, characterization, and expression analysis of p53 from the oriental river prawn, Macrobrachium nipponense, in response to hypoxia. Fish Shellfish Immunol. 2016, 54, 68–76. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Liu, H.; Tan, B.; Dong, X.; Yang, Q.; Chi, S.; Zhang, S.; Xie, R. Effects of clostridium butyricum on growth, antioxidant capacity and non-specific immunology of Litopenaeus vannamei fed with concentrated cottonseed protein replacement of fishmeal. J. Guangdong Ocean Univ. 2022, 42, 29–37. [Google Scholar] [CrossRef]
- Mohd Faudzi, N.; Yong, A.S.K.; Shapawi, R.; Senoo, S.; Biswas, A.; Takii, K. Soy Protein concentrate as an alternative in replacement of fish meal in the feeds of hybrid grouper, brown-marbled grouper (Epinephelus fuscoguttatus) × giant grouper (E. lanceolatus) juvenile. Aquac. Res. 2018, 49, 431–441. [Google Scholar] [CrossRef]
- Feng, L.; Chen, Y.P.; Jiang, W.D.; Liu, Y.; Jiang, J.; Wu, P.; Zhao, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; et al. Modulation of immune response, physical barrier and related signaling factors in the gills of juvenile grass carp (Ctenopharyngodon idella) fed supplemented diet with phospholipids. Fish Shellfish Immunol. 2016, 48, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Q.; Liu, W.B.; Zhou, M.; Dai, Y.J.; Xu, C.; Tian, H.Y.; Xu, W.N. Effects of berberine on the growth and immune performance in response to ammonia stress and high-fat dietary in blunt snout bream Megalobrama amblycephala. Fish Shellfish Immunol. 2016, 55, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Jin, A.; Sun, J.; Yang, Z.; Tian, J.; Ji, H.; Yu, H.; Li, Y.; Zhou, J.; Du, Z.; et al. α-lipoic acid ameliorates n-3 highly-unsaturated fatty acids induced lipid peroxidation via regulating antioxidant defenses in grass carp (Ctenopharyngodon idellus). Fish Shellfish Immunol. 2017, 67, 359–367. [Google Scholar] [CrossRef]
- Xu, C.; Li, E.; Liu, S.; Huang, Z.; Qin, J.G.; Chen, L. Effects of α-lipoic acid on growth performance, body composition, antioxidant status and lipid catabolism of juvenile chinese mitten crab Eriocheir sinensis fed different lipid percentage. Aquaculture 2018, 484, 286–292. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, B.; Liu, L.; Yang, R.; Liu, H. Effects of feed protein levels on growth, digestive enzyme activities, non-specific immunity and protein metabolism of Schizothorax O’Connori. Acta Hydrobiol. Sin. 2020, 44, 693–706. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, H.; He, H.; Ma, C. Assays for alkaline phosphatase activity: Progress and prospects. TrAC Trends Anal. Chem. 2019, 113, 32–43. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Guo, J.L.; Tang, R.J.; Ma, H.J.; Chen, Y.J.; Lin, S.M. High dietary lipid level alters the growth, hepatic metabolism enzyme, and anti-oxidative capacity in juvenile largemouth bass Micropterus salmoides. Fish Physiol. Biochem. 2020, 46, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Lallès, J.P. Intestinal alkaline phosphatase in the gastrointestinal tract of fish: Biology, ontogeny, and environmental and nutritional modulation. Rev. Aquac. 2020, 12, 555–581. [Google Scholar] [CrossRef]
- Dossou, S.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Dawood, M.A.O.; El Basuini, M.F.; El-Hais, A.M.; Olivier, A. Effect of partial replacement of fish meal by fermented rapeseed meal on growth, immune response and oxidative condition of red sea bream juvenile, Pagrus Major. Aquaculture 2018, 490, 228–235. [Google Scholar] [CrossRef]
- Zhuo, L.C.; Chen, C.F.; Lin, Y.H. Dietary supplementation of fermented lemon peel enhances lysozyme activity and susceptibility to photobacterium damselae for orange-spotted grouper, Epinephelus coioides. Fish Shellfish Immunol. 2021, 117, 248–252. [Google Scholar] [CrossRef]
- Niu, H.; Jia, Y.; Hu, P.; Meng, Z.; Lei, J. Effect of dietary vitamin E on the growth performance and nonspecific immunity in sub-adult turbot (Scophthalmus maximus). Fish Shellfish Immunol. 2014, 41, 501–506. [Google Scholar] [CrossRef]
- Novriadi, R.; Ilham, I.; Roigé, O.; Segarra, S. Effects of dietary nucleotides supplementation on growth, total haemocyte count, lysozyme activity and survival upon challenge with Vibrio harveyi in pacific white shrimp, Litopenaeus vannamei. Aquac. Rep. 2021, 21, 100840. [Google Scholar] [CrossRef]
- Ma, F.; Li, X.Q.; Li, B.A.; Leng, X.J. Effects of extruded and pelleted diets with differing lipid levels on growth, nutrient retention and serum biochemical indices of tilapia (Oreochromis aureus × Tilapia nilotica). Aquac. Nutr. 2016, 22, 61–71. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Wang, L.; Zhao, Z.; Luo, L.; Du, X.; Yin, J.; Xu, Q. Effects of dietary phosphorus on growth, body composition and immunity of young taimen Hucho taimen (Pallas, 1773). Aquac. Res. 2017, 48, 3066–3079. [Google Scholar] [CrossRef]
- Liu, H.X.; Zhou, X.Q.; Jiang, W.D.; Wu, P.; Liu, Y.; Zeng, Y.Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Feng, L. Optimal α-lipoic acid strengthen immunity of young grass carp (Ctenopharyngodon idella) by enhancing immune function of head kidney, spleen and skin. Fish Shellfish Immunol. 2018, 80, 600–617. [Google Scholar] [CrossRef]
- Barja-Fernández, S.; Míguez, J.M.; Álvarez-Otero, R. Histopathological effects of 2,2’,4,4’-tetrabromodiphenyl ether (bde-47) in the gills, intestine and liver of turbot (Psetta maxima). Ecotoxicol. Environ. Saf. 2013, 95, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.W.; Chang, C.L.; Kao, E.S.; Lin, J.H. Effect of hibiscus sabdariffa extract on high fat diet-induced obesity and liver damage in hamsters. Food Nutr. Res. 2015, 59, 29018. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Yan, X.; Li, T.; Suo, X.; Liu, H.; Tan, B.; Huang, W.; Yang, Y.; Zhang, H.; Dong, X. Impacts of tea polyphenols on growth, antioxidant capacity and immunity in juvenile hybrid grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂) fed high-lipid diets. Fish Shellfish Immunol. 2022, 128, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Pan, S.; Dong, X.; Tan, B.; Li, T.; Huang, W.; Suo, X.; Li, Z.; Yang, Y. Vitamin E amelioration of oxidative stress and low immunity induced by high-lipid diets in hybrid grouper (♀ Epinephelus fuscoguttatus × ♂ E. lanceolatu). Fish Shellfish Immunol. 2022, 124, 156–163. [Google Scholar] [CrossRef]
- Vazirzadeh, A.; Marhamati, A.; Rabiee, R.; Faggio, C. Immunomodulation, antioxidant enhancement and immune genes up-regulation in rainbow trout (Oncorhynchus mykiss) fed on seaweeds included diets. Fish Shellfish Immunol. 2020, 106, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Enamorado, A.D.; Martins, A.C.; Flores, J.A.; Tesser, M.B.; Caldas, S.S.; Primel, E.G.; Monserrat, J.M. Biochemical responses over time in common carp cyprinus carpio (Teleostei, Cyprinidae) during fed supplementation with α-lipoic acid. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 188, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Asea, A.; Rehli, M.; Kabingu, E.; Boch, J.A.; Baré, O.; Auron, P.E.; Stevenson, M.A.; Calderwood, S.K. Novel signal transduction pathway utilized by extracellular hsp70. role of toll-like receptor (tlr) 2 and tlr4. J. Biol. Chem. 2002, 277, 15028–15034. [Google Scholar] [CrossRef]
- Kucukbay, F.Z.; Yazlak, H.; Karaca, I.; Sahin, N.; Tuzcu, M.; Cakmak, M.N.; Sahin, K. The effects of dietary organic or inorganic selenium in rainbow trout (Oncorhynchus mykiss) under crowding conditions. Aquac. Nutr. 2009, 15, 569–576. [Google Scholar] [CrossRef]
- Wang, S.; Shi, J.; Yang, J.; Xing, J.; Wu, J. Effect of dietary alpha-lipoic acid on thyroid hormones, inflammatory cytokines and antioxidant ability in finishing pigs under oxidative stress. Feed Res. 2020, 45, 56–60. [Google Scholar] [CrossRef]
- Sun, H.; Ye, C.; Li, P.; Liu, Y.; Gao, L. Effects of Lipoic acid on intestinal redox status and digestive and absorption functions in mice fed a high-fat diet. Chin. J. Gerontol. 2020, 40, 6–9. [Google Scholar] [CrossRef]


| Ingredient | S1 | S2 | S3 | S4 | S5 | S6 |
|---|---|---|---|---|---|---|
| Fish meal | 43 | 43 | 43 | 43 | 43 | 43 |
| Wheat gluten | 10 | 10 | 10 | 10 | 10 | 10 |
| Casein | 12 | 12 | 12 | 12 | 12 | 12 |
| Wheat flour | 17 | 17 | 17 | 17 | 17 | 17 |
| Soybean lecithin | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Fish oil | 5 | 5 | 5 | 5 | 5 | 5 |
| Corn oil | 7 | 7 | 7 | 7 | 7 | 7 |
| Gelatinized starch | 0.7 | 0.66 | 0.62 | 0.58 | 0.54 | 0.5 |
| Compound premix a | 1 | 1 | 1 | 1 | 1 | 1 |
| Vitamin C | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Choline chloride | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Ca(H2PO4)2 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Antioxidant b | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Attractant c | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| CMC d | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| α-lipoic acid | 0 | 0.04 | 0.08 | 0.12 | 0.16 | 0.2 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
| Proximate composition e | ||||||
| Moisture | 9.23 | 10.09 | 10.95 | 9.8 | 9.38 | 10.44 |
| Crude protein | 50.62 | 51.18 | 51.55 | 50.94 | 50.84 | 50.06 |
| Crude lipid | 16.38 | 15.72 | 16.14 | 15.75 | 16.16 | 16.2 |
| Ash | 12.74 | 12.15 | 12.09 | 12.86 | 12.45 | 12.79 |
| Genes | 5=/3 = Forward Primer | 5=/3 = Reverse Primer | Amplicon | E-Value % | Genbank No. |
|---|---|---|---|---|---|
| β-actin | ACTGCTGCCTCCTCTTCATC | ACCGCAAGACTCCATACCAA | 135 | 93.71 | KU746361.1 |
| sod | TGGAAACACCTTTCCCCCAC | CTGACAGGGTAAAGCATGGC | 120 | 91.41 | AY735008.1 |
| cat | CGCGGGAAGCAAAGATTCAG | CCGCAGTTTCCAGTGTGTTG | 194 | 104.32 | KT884509.1 |
| il6 | AGGAAGTCTGGCTGTCAGGA | GCCCTGAGGCCTTCAAGATT | 250 | 95.06 | JN806222.1 |
| tgfβ | CGATGTCACTGACGCCCTGC | AGCCGCGGTCATCACTTATC | 107 | 90.00 | GQ205390.1 |
| il1β | CGACATGGTGCGGTTTC | TCTGTAGCGGCTGGTGG | 151 | 91.95 | EF582837.1 |
| il10 | ACACAGCGCTGCTAGACGAG | GGGCAGCACCGTGTTCAGAT | 104 | 91.86 | KJ741852.1 |
| hsp70 | CTTGCAAGAAGTGGCCAACA | AAAGCCATCTTCCTGCCTTGT | 131 | 94.03 | EU816600.1 |
| Group | CAT (U/mL) | T-AOC (U/mL) | SOD (U/mL) | GSH-Px (U/L) |
|---|---|---|---|---|
| S1 | 25.21 ± 2.06 a | 13.13 ± 0.96 | 62.06 ± 3.33 a | 62.89 ± 3.53 a |
| S2 | 29.02 ± 2.01 ab | 12.47 ± 0.34 | 83.4 ± 2.94 b | 73.79 ± 8.01 ab |
| S3 | 30.07 ± 1.91 ab | 13.01 ± 0.54 | 87.88 ± 4.63 b | 75.53 ± 4.56 abc |
| S4 | 34.51 ± 3.41 b | 14.21 ± 1.67 | 97.16 ± 6.16 b | 92.41 ± 4.99 cd |
| S5 | 44.51 ± 2.95 c | 15.61 ± 1.26 | 117.03 ± 7.68 c | 102.04 ± 5.53 d |
| S6 | 36.51 ± 1.53 b | 14.46 ± 1.04 | 90.71 ± 4.41 b | 82.18 ± 6.25 bc |
| Group | S1 | S2 | S3 | S4 | S5 | S6 |
|---|---|---|---|---|---|---|
| ROS (U/mg.pro) | 352.65 ± 4.44 c | 370.31 ± 9.79 c | 292.77 ± 27.48 b | 225.17 ± 13.39 a | 253.06 ± 10.86 ab | 269.87 ± 5.34 ab |
| MDA (nmol/mg.pro) | 15.98 ± 0.81 c | 16.03 ± 1.34 c | 13.29 ± 0.82 b | 9.65 ± 0.68 a | 7.84 ± 0.26 a | 8.93 ± 0.4 a |
| CAT (U/mg.pro) | 38.44 ± 5.69 a | 60.74 ± 6.29 b | 52.33 ± 4.51 ab | 62.3 ± 3.32 b | 79.08 ± 3.28 c | 56.45 ± 4.45 b |
| T-AOC (U/mg.pro) | 9.55 ± 0.61 a | 15.98 ± 1.19 b | 15.55 ± 1.62 b | 15.67 ± 1.17 b | 21.28 ± 1.01 c | 16.19 ± 0.67 b |
| SOD (U/mg.pro) | 118.27 ± 2.52 a | 174.78 ± 1.01 b | 168.09 ± 22.81 b | 166.15 ± 10.66 b | 179.57 ± 2.41 b | 163.09 ± 16.62 b |
| GSH-Px (mU/mg.pro) | 125.57 ± 11.45 a | 178.02 ± 18.18 b | 150.48 ± 6.5 ab | 165.11 ± 15.59 b | 178 ± 8.01 b | 168.58 ± 4.94 b |
| AKP (mIU/mg.pro) | 11.89 ± 1.95 a | 20.32 ± 2.2 b | 16.14 ± 0.8 ab | 21.02 ± 0.27 b | 17.88 ± 1.45 b | 19.13 ± 1.19 b |
| ACP (mU/mg.pro) | 14.80 ± 1.22 | 12.65 ± 1.28 | 12.6 ± 1.61 | 14.75 ± 0.17 | 14.18 ± 1.39 | 11.9 ± 1.05 |
| AST (mU/mg.pro) | 20.11 ± 1.28 | 17.18 ± 1.57 | 17.53 ± 2.54 | 17.84 ± 0.24 | 15.03 ± 2.62 | 16.31 ± 1.07 |
| ALT (mU/mg.pro) | 9.54 ± 0.6 | 10.86 ± 1.45 | 9.24 ± 1.49 | 8.85 ± 1.46 | 7.72 ± 1.01 | 9.04 ± 0.47 |
| IgM (ug/mg.pro) | 49.58 ± 3.52 | 54.84 ± 4.36 | 51.17 ± 1.85 | 52.17 ± 4.7 | 56.94 ± 5.24 | 51.14 ± 2.31 |
| LYZ (mU/mg.pro) | 6.49 ± 0.62 a | 9.87 ± 0.34 c | 8.12 ± 0.53 b | 9.00 ± 0.56 bc | 9.51 ± 0.56 bc | 9.24 ± 0.23 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Li, T.; Cai, W.; Song, H.; Liu, H.; Tan, B.; Zhang, S.; Zhou, M.; Yang, Y.; Dong, X. Effects of α-Lipoic Acid Supplementation on Growth Performance, Liver Histology, Antioxidant and Related Genes Expression of Hybrid Grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂). Antioxidants 2024, 13, 88. https://doi.org/10.3390/antiox13010088
Huang W, Li T, Cai W, Song H, Liu H, Tan B, Zhang S, Zhou M, Yang Y, Dong X. Effects of α-Lipoic Acid Supplementation on Growth Performance, Liver Histology, Antioxidant and Related Genes Expression of Hybrid Grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂). Antioxidants. 2024; 13(1):88. https://doi.org/10.3390/antiox13010088
Chicago/Turabian StyleHuang, Weibin, Tao Li, Wenshan Cai, Hengyang Song, Hao Liu, Beiping Tan, Shuang Zhang, Menglong Zhou, Yuanzhi Yang, and Xiaohui Dong. 2024. "Effects of α-Lipoic Acid Supplementation on Growth Performance, Liver Histology, Antioxidant and Related Genes Expression of Hybrid Grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂)" Antioxidants 13, no. 1: 88. https://doi.org/10.3390/antiox13010088
APA StyleHuang, W., Li, T., Cai, W., Song, H., Liu, H., Tan, B., Zhang, S., Zhou, M., Yang, Y., & Dong, X. (2024). Effects of α-Lipoic Acid Supplementation on Growth Performance, Liver Histology, Antioxidant and Related Genes Expression of Hybrid Grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂). Antioxidants, 13(1), 88. https://doi.org/10.3390/antiox13010088






