A Pectic Polysaccharide from Codonopsis pilosula Alleviates Inflammatory Response and Oxidative Stress of Aging Mice via Modulating Intestinal Microbiota-Related Gut–Liver Axis
Abstract
:1. Introduction
2. Methods
2.1. Plant Source
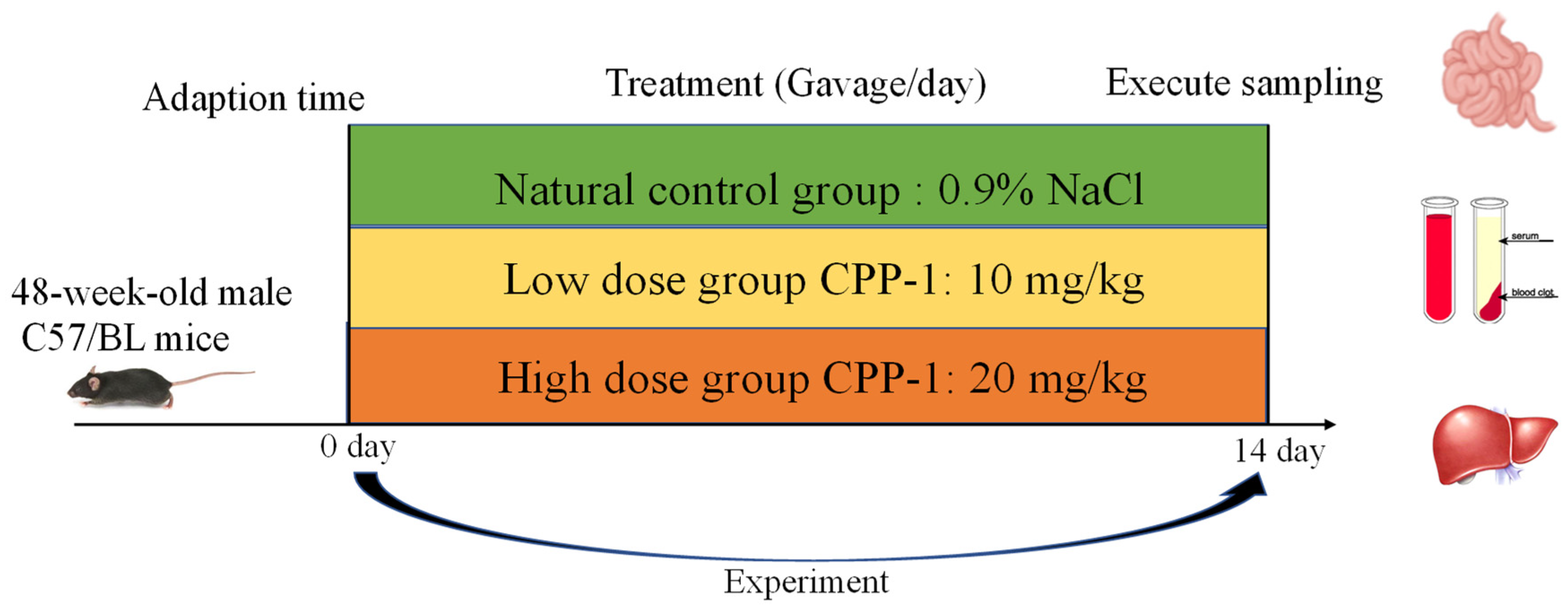
2.2. Animal Care and Experimental Design
2.3. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.4. Hematoxylin and Eosin Staining of Liver and Jejunum (H&E Staining)
2.5. The Determination of Anti-Oxidative Effect of CPP-1 during Aging Process in Mice
2.6. The Determination of Anti-Inflammatory Effect of CPP-1 during Aging Process in Mice
2.7. Determination of LPS in Serum, Liver, and sIgA in the Jejunum
2.8. Serum Biochemical Parameter Analysis
2.9. Gut Microbiota Analysis
2.10. Statistical Analysis
3. Results
3.1. CPP-1 Protected Naturally Aging Mice from Oxidative Stress and Inflammation
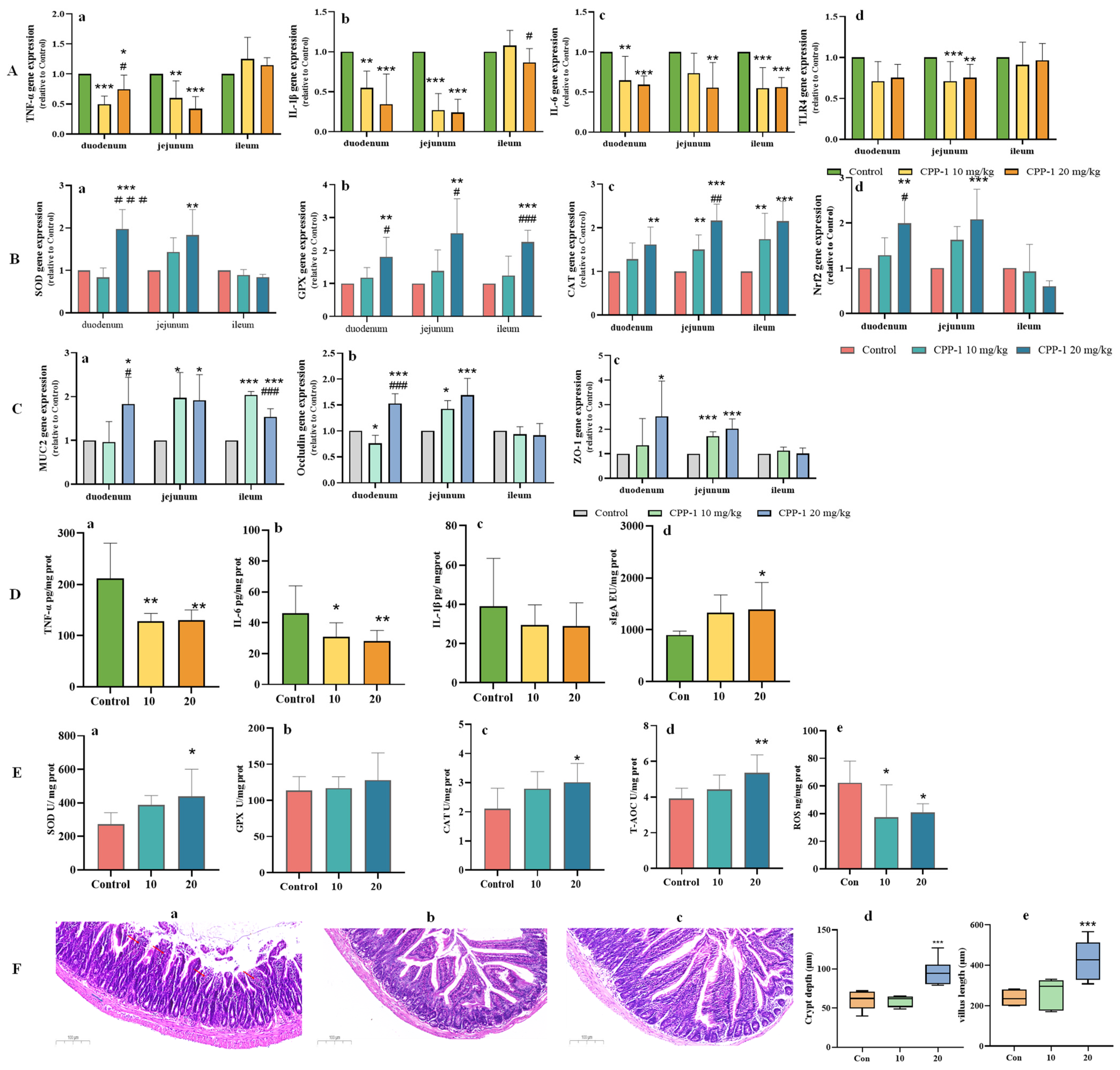
3.2. CPP-1 Attenuated Oxidative Stress and Inflammation in the Gut of Naturally Aging Mice
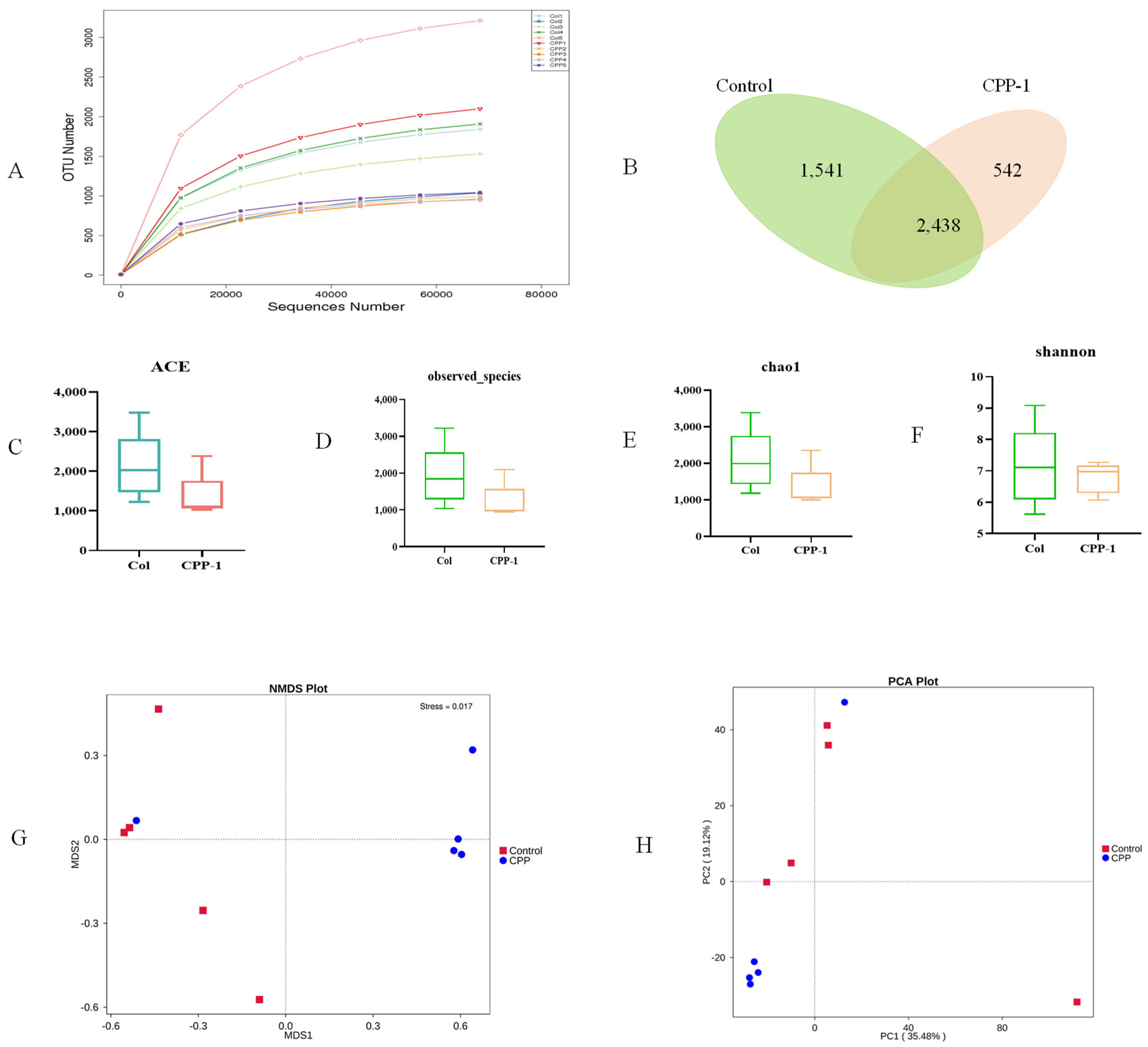
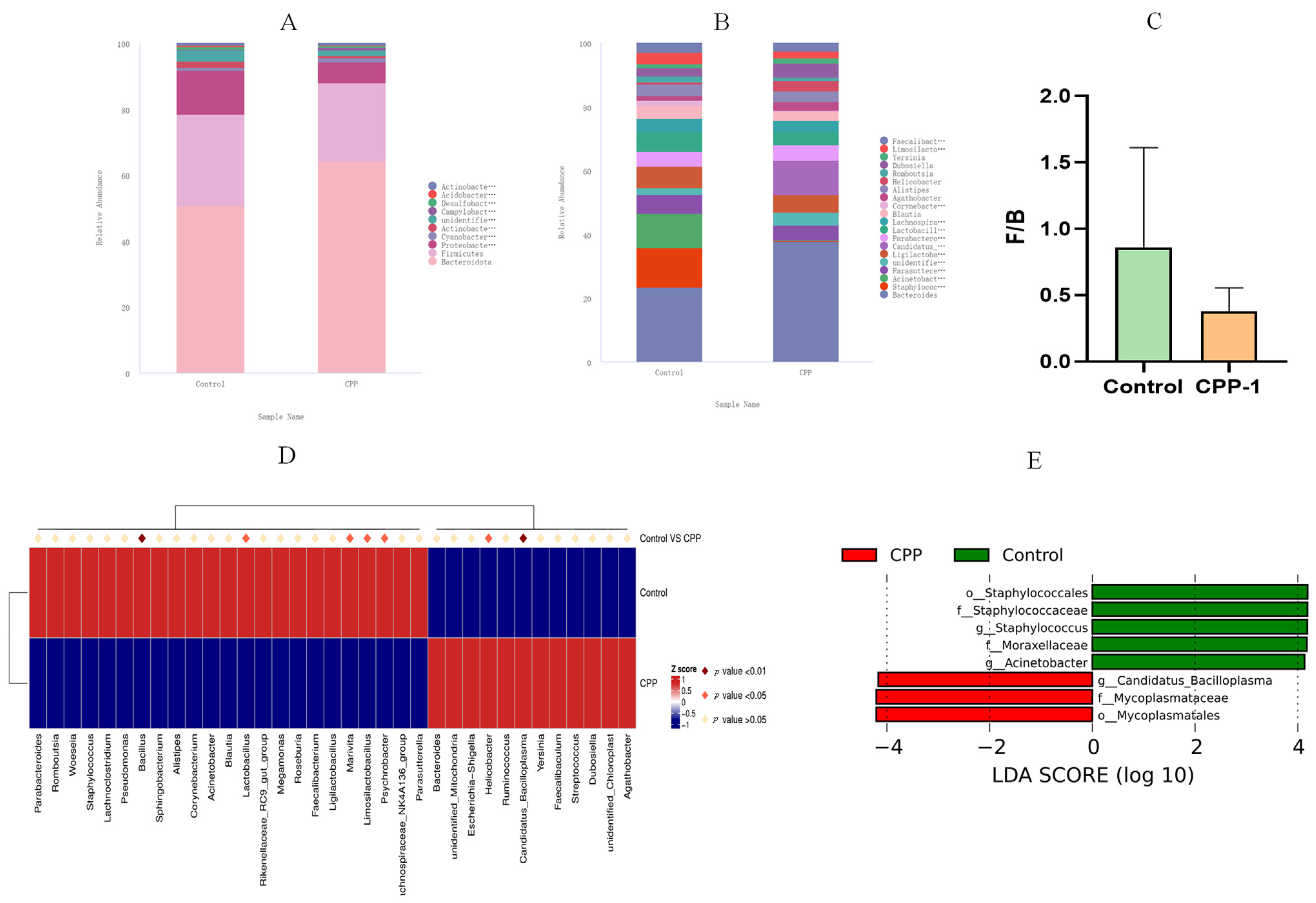
3.3. CPP-1 Modulated the Diversity and Composition of Intestinal Microbiota of Aging Mice
3.4. CPP-1 Alleviated the Oxidative Stress, Inflammatory Responses, and Lipid Metabolism in Liver of Naturally Aging Mice by Intestinal Microbe-Related Gut–Liver Axis
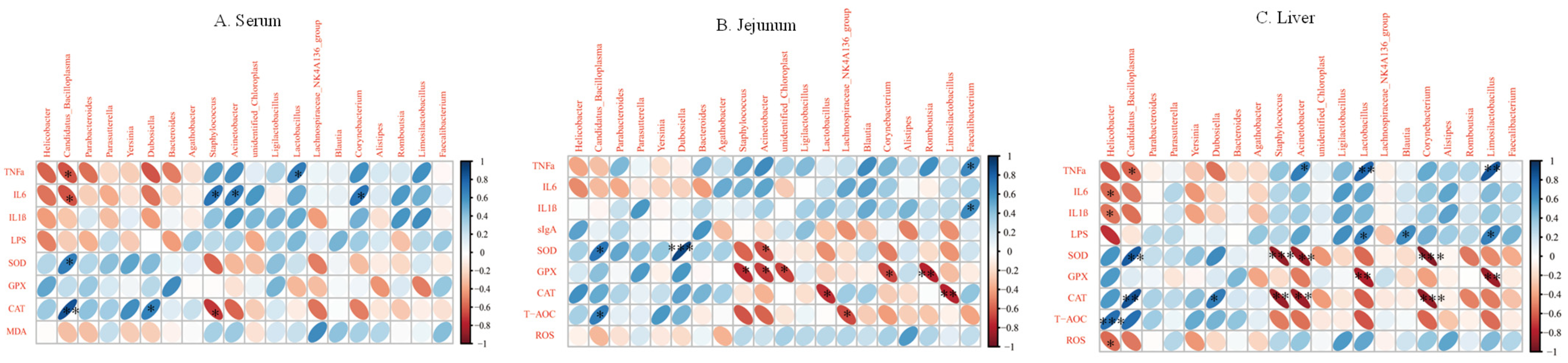
3.5. Correlation Analysis between Inflammatory Factors and Oxidative Enzymes and Intestinal Microbiota
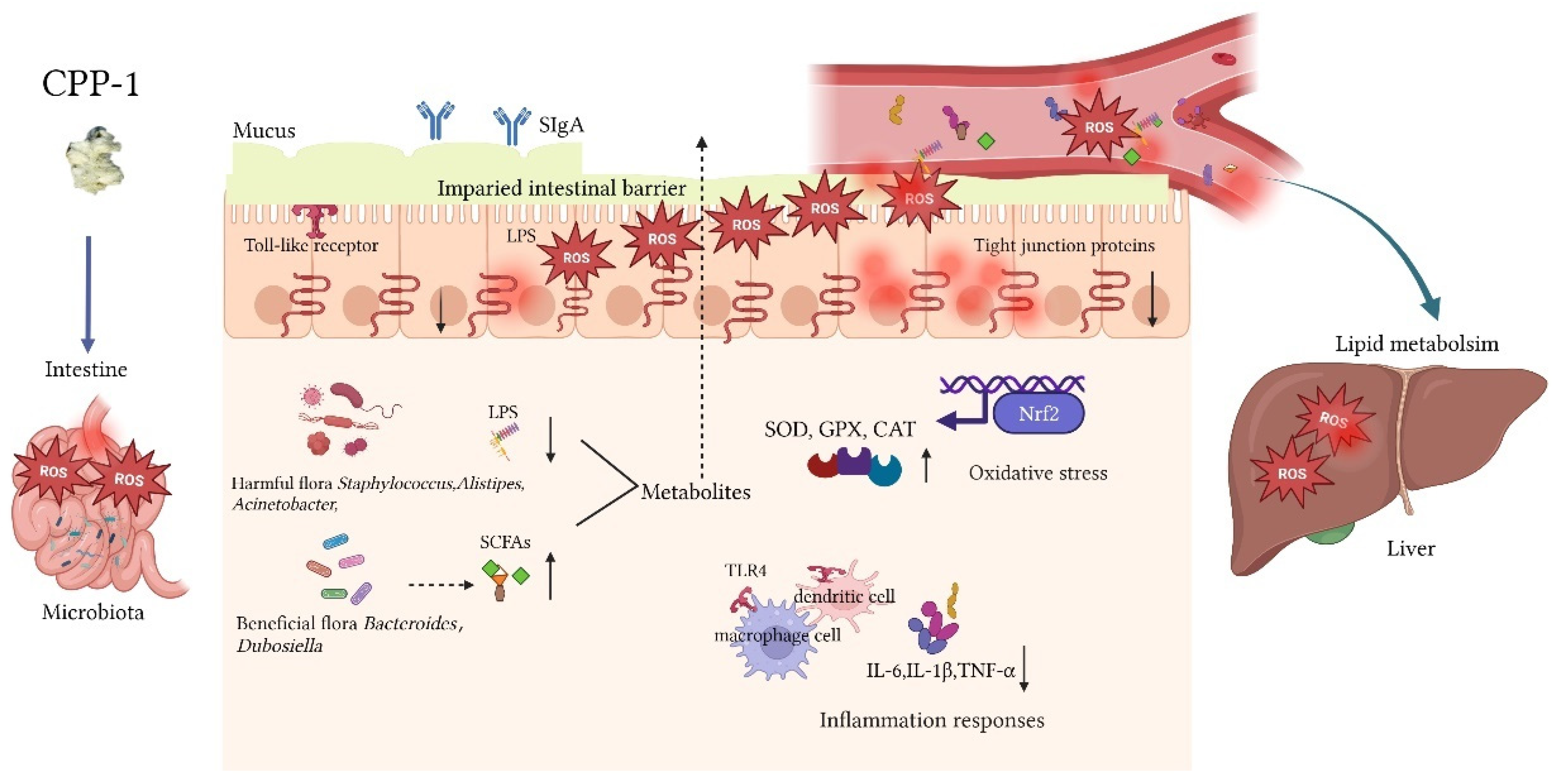
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fatemi, I.; Khaluoi, A.; Kaeidi, A.; Shamsizadeh, A.; Heydari, S.; Allahtavakoli, M.A. Protective effect of metformin on D-galactose-induced aging model in mice. Iran. J. Basic Med. Sci. 2018, 21, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Sang, G.L.; Lim, K.T.; Kim, H.R. Potential anti-aging substances derived from seaweeds. Mar. Drugs 2020, 18, 564. [Google Scholar] [CrossRef]
- Ashok, B.T.; Ali, R. The aging paradox: Free radical theory of aging. Exp. Gerontol. 1999, 34, 293–303. [Google Scholar] [CrossRef]
- Vatner, S.F.; Zhang, J.; Oydanich, M.; Berkman, T.; Vatner, D.E. Healthful aging mediated by inhibition of oxidative stress. Ageing Res. Rev. 2020, 64, 101194. [Google Scholar] [CrossRef] [PubMed]
- De Martinis, M.; Franceschi, C.; Monti, D.; Ginaldi, L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 2005, 579, 2035–2039. [Google Scholar] [CrossRef]
- Soenen, S.; Rayner, C.K.; Jones, K.L.; Horowitz, M. The ageing gastrointestinal tract. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 12–18. [Google Scholar] [CrossRef]
- Kim, M.; Benayoun, B.A. The microbiome: An emerging key player in aging and longevity. Transl. Med. Aging 2020, 4, 103–116. [Google Scholar] [CrossRef]
- Marshall, J.C. The gut as a potential trigger of exercise-induced inflammatory responses. Can. J. Physiol. Pharmacol. 1998, 76, 479–484. [Google Scholar] [CrossRef]
- Vaiserman, A.M.; Koliada, A.K.; Marotta, F. Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Res. Rev. 2017, 35, 36–45. [Google Scholar] [CrossRef]
- Kraig, E.; Linehan, L.A.; Liang, H.; Romo, T.Q.; Liu, Q.; Wu, Y.; Benavides, A.D.; Curiel, T.J.; Javors, M.A.; Musi, N. A randomized control trial to establish the feasibility and safety of rapamycin treatment in an older human cohort: Immunological, physical performance, and cognitive effects. Exp. Gerontol. 2018, 105, 53–69. [Google Scholar] [CrossRef]
- De Kreutzenberg, S.V.; Ceolotto, G.; Cattelan, A.; Pagnin, E.; Mazzucato, M.; Garagnani, P.; Borelli, V.; Bacalini, M.G.; Franceschi, C.; Fadini, G.P.J.N.; et al. Metformin improves putative longevity effectors in peripheral mononuclear cells from subjects with prediabetes. A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Soukas, A.A.; Hao, H.; Wu, L. Metformin as Anti-Aging Therapy: Is It for Everyone? Trends Endocrinol. Metab. 2019, 30, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Kim, S.G.; Blenis, J. Rapamycin: One drug, many effects. Cell Metab. 2014, 19, 373–379. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. National Commission of Chinese Pharmacopoeia; Chinese Medicine and Technology Publishing House: Beijing, China, 2020.
- Liu, K.H.; Tian, W.J.; Gao, Z.Z.; Hou, R.R.; Yue, C.J.; Wang, D.Y.; Liu, J.G.; Yi, W.U.; Yuan-Liang, H.U. Selenizing polysaccharide and garlic polysaccharide synergistically enhance immunological efficacy of chicken lymphocyte and newcastle disease vaccine. Chin. J. Anim. Vet. Sci. 2017, 48, 1349–1356. [Google Scholar]
- Wu, C.; Qian, H.; Feng, H.; Fan, Z.J.P.; Gansu College of Traditional Chinese Medicine. Effect of Codonopsis pilosula var. modesta on anti-oxidation of skin tissue of aged mice induced by D-galactose. Pharmacol. Clin. Chin. Mater. Med. 2014, 30, 92–96. [Google Scholar] [CrossRef]
- Sun, Y.X.; Liu, J. Function and Interactions, Structural characterization of a water-soluble polysaccharide from the roots of Codonopsis pilosula and its immunity activity. J. Biol. Macromol. 2008, 43, 279–282. [Google Scholar] [CrossRef]
- Zou, Y.F.; Li, C.Y.; Fu, Y.P.; Feng, X.; Peng, X.; Feng, B.; Li, L.X.; Jia, R.Y.; Huang, C.; Song, X.; et al. Restorative Effects of Inulin From Codonopsis pilosula on Intestinal Mucosal Immunity, Anti-Inflammatory Activity and Gut Microbiota of Immunosuppressed Mice. Front. Pharmacol. 2022, 13, 786141. [Google Scholar] [CrossRef]
- Xin, T.; Zhang, F.; Jiang, Q.; Chen, C.; Huang, D.; Li, Y.; Shen, W.; Jin, Y.; Sui, G. The inhibitory effect of a polysaccharide from Codonopsis pilosula on tumor growth and metastasis in vitro. Int. J. Biol. Macromol. 2012, 51, 788–793. [Google Scholar] [CrossRef]
- Zou, Y.F.; Zhang, Y.Y.; Fu, Y.P.; Inngjerdingen, K.T.; Paulsen, B.S.; Feng, B.; Zhu, Z.K.; Li, L.X.; Jia, R.Y.; Huang, C.; et al. A Polysaccharide Isolated from Codonopsis pilosula with Immunomodulation Effects Both In Vitro and In Vivo. Molecules 2019, 24, 3632. [Google Scholar] [CrossRef]
- Meng, Y.; Xu, Y.; Chang, C.; Qiu, Z.; Zheng, B. Extraction, characterization and anti-inflammatory activities of an inulin-type fructan from Codonopsis pilosula. Int. J. Biol. Macromol. 2020, 163, 1677–1686. [Google Scholar] [CrossRef]
- Zou, Y.F.; Zhang, Y.Y.; Paulsen, B.S.; Rise, F.; Chen, Z.L.; Jia, R.Y.; Li, L.X.; Song, X.; Feng, B.; Tang, H.Q.; et al. New Pectic Polysaccharides from Codonopsis Pilosula and Codonopsis Tangshen: Structural Characterization and Cellular Antioxidant Activities. J. Sci. Food Agric. 2021, 101, 6043–6052. [Google Scholar] [CrossRef] [PubMed]
- Salyers, A.A.; Palmer, J.K.; Wilkins, T.D. Degradation of polysaccharides by intestinal bacterial enzymes. Am. J. Clin. Nutr. 1978, 31, S128–S130. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Cockburn, D.W.; Koropatkin, N.M. Polysaccharide Degradation by the Intestinal Microbiota and Its Influence on Human Health and Disease. J. Mol. Biol. 2016, 428, 3230–3252. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Wang, J.; Yu, H.; Wang, J.; Zhu, W. Effects of galacto-oligosaccharides on growth and gut function of newborn suckling piglets. J. Anim. Sci. Biotechnol. 2018, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zhao, Z. Intestinal aging is alleviated by uridine via regulating inflammation and oxidative stress in vivo and in vitro. Cell Cycle 2022, 21, 1519–1531. [Google Scholar] [CrossRef]
- Yang, X.W.; Nan, W.; Li, W.; Wei, X.; Wu, S. Biotransformation of 4,5-O-dicaffeoylquinic acid methyl ester by human intestinal flora and evaluation on their inhibition of NO production and antioxidant activity of the products. Food Chem. Toxicol. 2013, 55, 297–303. [Google Scholar] [CrossRef]
- Cui, L.; Guan, X.; Ding, W.; Luo, Y.; Wang, W.; Bu, W.; Song, J.; Tan, X.; Sun, E.; Ning, Q.; et al. Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol. 2021, 166, 1035–1045. [Google Scholar] [CrossRef]
- Vemuri, R.; Shinde, T.; Gundamaraju, R.; Gondalia, S.V.; Karpe, A.V.; Beale, D.J.; Martoni, C.J.; Eri, R. Lactobacillus acidophilus DDS-1 modulates the gut microbiota and improves metabolic profiles in aging mice. Nutrients 2018, 10, 1255. [Google Scholar] [CrossRef]
- Zhao, J.; Tian, F.; Yan, S.; Zhai, Q.; Zhang, H.; Chen, W. Lactobacillus plantarum CCFM10 alleviating oxidative stress and restoring the gut microbiota in D-galactose-induced aging mice. Food Funct. 2018, 9, 917–924. [Google Scholar] [CrossRef]
- Walrath, T.; Dyamenahalli, K.U.; Hulsebus, H.J.; McCullough, R.L.; Idrovo, J.P.; Boe, D.M.; McMahan, R.H.; Kovacs, E.J. Age-related changes in intestinal immunity and the microbiome. J. Leukoc. Biol. 2021, 109, 1045–1061. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Li, J.; Zhang, J.; Wang, W.; Li, S.; Ren, Z.; Gao, Z.; Song, X.; Wang, X.; Jia, L. The antioxidative and anti-aging effects of acidic- and alkalic-extractable mycelium polysaccharides by Agrocybe aegerita (Brig.) Sing. Int. J. Biol. Macromol. 2018, 106, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Han, S.; Huang, M.; Yin, J.; Yang, F.; Luo, F. Antiaging effects of dietary polysaccharides: Advance and mechanisms. Oxidative Med. Cell. Longev. 2022, 2022, 4362479. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Peng, X.; Pang, D.J.; Li, J.; Paulsen, B.S.; Rise, F.; Chen, Y.L.; Chen, Z.L.; Jia, R.Y.; Li, L.X.; et al. Pectic polysaccharide from Nelumbo nucifera leaves promotes intestinal antioxidant defense in vitro and in vivo. Food Funct. 2021, 12, 10828–10841. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Nenkov, M.; Chen, Y.; Press, A.T.; Kaemmerer, E.; Gassler, N. Fatty acid metabolism and acyl-CoA synthetases in the liver-gut axis. World J. Hepatol. 2021, 13, 1512–1533. [Google Scholar] [CrossRef] [PubMed]
- Szilard, L. On the nature of the aging process. Proc. Natl. Acad. Sci. USA 1959, 45, 30–45. [Google Scholar] [CrossRef]
- Carter, E.A. Spectrochimica acta part A: Molecular and biomolecular spectroscopy. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2011, 80, 1. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Skalska, J.; Dąbrowska-Bouta, B.; Strużyńska, L. Oxidative stress in rat brain but not in liver following oral administration of a low dose of nanoparticulate silver. Food Chem. Toxicol. 2016, 97, 307–315. [Google Scholar] [CrossRef]
- Sla, B.; Xz, A.; Xz, A.; Hya, B.; Bing, H.A.; Yl, A.; Yan, L.A.; Xw, A.; Zza, B. Exploring the liver fibrosis induced by deltamethrin exposure in quails and elucidating the protective mechanism of resveratrol–ScienceDirect. Ecotoxicol. Environ. Saf. 2021, 207, 111501. [Google Scholar] [CrossRef]
- Chung, H.Y.; Sung, B.; Jung, K.J.; Zou, Y.; Yu, B.P. The molecular inflammatory process in aging. Antioxid. Redox Signal. 2006, 8, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.W.; Roach, J.; Fabela, S.; Moorfield, E.; Ding, S.; Blue, E.; Dagher, S.; Magness, S.; Tamayo, R.; Bruno-Barcena, J.M.; et al. The pleiotropic effects of prebiotic galacto-oligosaccharides on the aging gut. Microbiome 2021, 9, 31. [Google Scholar] [CrossRef]
- de Gonzalo-Calvo, D.; Neitzert, K.; Fernández, M.; Vega-Naredo, I.; Caballero, B.; García-Macía, M.; Suárez, F.M.; Rodríguez-Colunga, M.J.; Solano, J.J.; Coto-Montes, A. Differential inflammatory responses in aging and disease: TNF-alpha and IL-6 as possible biomarkers. Free Radic. Biol. Med. 2010, 49, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Baiocchi, L.; Glaser, S.; Francis, H.; Kennedy, L.; Felli, E.; Alpini, G.; Gracia-Sancho, J. Impact of aging on liver cells and liver disease: Focus on the biliary and vascular compartments. Hepatol. Commun. 2021, 5, 1125–1137. [Google Scholar] [CrossRef]
- Fang, X.; Yue, M.; Wei, J.; Wang, Y.; Hong, D.; Wang, B.; Zhou, X.; Chen, T. Evaluation of the anti-Aging effects of a probiotic combination isolated from centenarians in a SAMP8 mouse model. Front. Immunol. 2021, 12, 792746. [Google Scholar] [CrossRef]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise alters gut microbiota composition and function in lean and obese humans. Med. Sci. Sport. Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef]
- Kirjavainen, P.V.; Karvonen, A.M.; Adams, R.I.; Täubel, M.; Roponen, M.; Tuoresmäki, P.; Loss, G.; Jayaprakash, B.; Depner, M.; Ege, M.J.; et al. Farm-like indoor microbiota in non-farm homes protects children from asthma development. Nat. Med. 2019, 25, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, J.; Luo, Y.; Lei, Y.; Long, W.; Wang, K.; Zhou, J.; Lei, M.; Yang, N.; Zou, H.; et al. Various fractions of alcoholic extracts from dendrobium nobile functionalized antioxidation and antiaging in D-Galactose-induced aging mice. Front. Biosci. 2022, 27, 315. [Google Scholar] [CrossRef]
- Wang, Z.; Ammar, E.M.; Zhang, A.; Wang, L.; Lin, M.; Yang, S.T. Engineering Propionibacterium freudenreichii subsp. Shermanii for enhanced propionic acid fermentation. Metab. Eng. 2015, 27, 46–56. [Google Scholar] [CrossRef]
- Zafar, H.; Saier, M.H., Jr. Gut Bacteroides species in health and disease. Gut Microbes 2021, 13, 1848158. [Google Scholar] [CrossRef]
- Lan, Y.; Sun, Q.; Ma, Z.; Peng, J.; Zhang, M.; Wang, C.; Zhang, X.; Yan, X.; Chang, L.; Hou, X.; et al. Seabuckthorn polysaccharide ameliorates high-fat diet-induced obesity by gut microbiota-SCFAs-liver axis. Food Funct. 2022, 13, 2925–2937. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria with emerging Implications to inflammation, cancer, and mental health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Li, Y.; Du, Y.; Guo, L.; Chen, M.; Huang, X.; Yang, F.; Hong, J.; Kong, X. Konjaku flour reduces obesity in mice by modulating the composition of the gut microbiota. Int. J. Obes. 2019, 43, 1631–1643. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Lv, H.; Wang, H.; Yang, H.; Li, Y.; Qian, J. Aging increases the severity of colitis and the related changes to the gut barrier and gut microbiota in humans and mice. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020, 75, 1284–1292. [Google Scholar] [CrossRef]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef]
- Shintouo, C.M.; Mets, T.; Beckwee, D.; Bautmans, I.; Ghogomu, S.M.; Souopgui, J.; Leemans, L.; Meriki, H.D.; Njemini, R. Is inflammageing influenced by the microbiota in the aged gut? A systematic review. Exp. Gerontol. 2020, 141, 111079. [Google Scholar] [CrossRef]
- Pabst, O.; Hornef, M.W.; Schaap, F.G.; Cerovic, V.; Clavel, T.; Bruns, T. Gut-liver axis: Barriers and functional circuits. Nat. Reviews. Gastroenterol. Hepatol. 2023, 20, 447–461. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. The role of MUC2 mucin in intestinal homeostasis and the impact of dietary components on MUC2 expression. Int. J. Biol. Macromol. 2020, 164, 884–891. [Google Scholar] [CrossRef]
- Kuo, W.T.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef]
- Ren, W.; Wang, K.; Yin, J.; Chen, S.; Liu, G.; Tan, B.; Wu, G.; Bazer, F.W.; Peng, Y.; Yin, Y. Glutamine-induced secretion of intestinal secretory immunoglobulin A: A mechanistic perspective. Front. Immunol. 2016, 7, 503. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, G.; Huang, L.; Zhou, M.; Zhu, J.; Yi, L.; Mi, M. Pterostilbene attenuates intestinal epithelial barrier loss induced by high loading intensity of exercise. Front. Nutr. 2022, 9, 965180. [Google Scholar] [CrossRef] [PubMed]
- Wassenaar, T.M.; Zimmermann, K. Lipopolysaccharides in Food, Food Supplements, and Probiotics: Should We be Worried? Eur. J. Microbiol. Immunol. 2018, 8, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, N.; Emsenhuber, C.; Doupovec, B.; Mayer, E.; Schatzmayr, G.; Nagl, V.; Grenier, B. Endotoxin translocation and gut inflammation are increased in Broiler chickens receiving an oral Lipopolysaccharide (LPS) bolus during heat stress. Toxins 2020, 12, 622. [Google Scholar] [CrossRef] [PubMed]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef]
- den Besten, G.; Bleeker, A.; Gerding, A.; van Eunen, K.; Havinga, R.; van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.J.; et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Zhao, H.; Li, S.; Zhang, J.; Che, G.; Zhou, M.; Liu, M.; Zhang, C.; Xu, N.; Lin, L.; Liu, Y.; et al. The antihyperlipidemic activities of enzymatic and acidic intracellular polysaccharides by Termitomyces albuminosus. Carbohydr. Polym. 2016, 151, 1227–1234. [Google Scholar] [CrossRef]


| Gene | Primer Sequence 5′ to 3′ | PubMed No. | bp |
|---|---|---|---|
| β-actin | F: CATCCGTAAAGACCTCTATGCCAAC R: ATGGAGCCACCGATCCACA | NM_007393.5 | 171 |
| IL-1β | F: CCTGTGTTTTCCTCCTTGCCT R: AGTGCGGGCTATGACCAATTC | NM_008361.4 | 158 |
| TNF-α | F: CTCTTCTCATTCCTGCTCGT R: ACCCCGAAGTTCAGTAGACA | NM_012675.3 | 62 |
| IL-6 | F: AAATATGAGACTGGGGATGTC R: TCAGTCCCAAGAAGGCAAC | NM_001314054 | 90 |
| TLR4 | F: CACTTTATTCAGAGCCGTTG R: AGGCGATACAATTCCAC | NM_021297.3 | 146 |
| CAT | F: ACCAGATACTCCAAGGCAAA R: TAAAATTTCACTGCAAACCCC | NM_009804.2 | 137 |
| SOD | F: GAACCATCCACTTCGAGCAG R: ATCACACGATCTTCAATGGAC | NM_011434.2 | 265 |
| GPX | F: TGCTTGCCTCCTAAATGCTG R: CCCAGAATGACCAAGCCAA | NM_001329860.1 | 81 |
| Nrf2 | F: AACCTCCCTGTTGATGACTTC R: CTGTCGTTTTCTCCCTTTTCTC | NM_001399226.1 | 101 |
| Mucin 2 | F: TCATCAACCTTCACTACCCCA R: TTTTGCACACTAACCCAAC | NM_023566.4 | 247 |
| ZO-1 | F: TCGATCAAATCATTACGACCCT R: GCTCTCAAAACTTCTTCGGTCAA | NM_001352638.1 | 55 |
| Occludin | F: TTGAAAGTCCACCTCCTTACAGA R: CCGGATAAAAAGAGTACGCTGG | NM_001360536.1 | 129 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Y.; Yan, H.; Li, C.; Wen, F.; Jize, X.; Zhang, C.; Liu, S.; Zhao, Y.; Fu, Y.; Li, L.; et al. A Pectic Polysaccharide from Codonopsis pilosula Alleviates Inflammatory Response and Oxidative Stress of Aging Mice via Modulating Intestinal Microbiota-Related Gut–Liver Axis. Antioxidants 2023, 12, 1781. https://doi.org/10.3390/antiox12091781
Zou Y, Yan H, Li C, Wen F, Jize X, Zhang C, Liu S, Zhao Y, Fu Y, Li L, et al. A Pectic Polysaccharide from Codonopsis pilosula Alleviates Inflammatory Response and Oxidative Stress of Aging Mice via Modulating Intestinal Microbiota-Related Gut–Liver Axis. Antioxidants. 2023; 12(9):1781. https://doi.org/10.3390/antiox12091781
Chicago/Turabian StyleZou, Yuanfeng, Hong Yan, Cenyu Li, Fang Wen, Xiaoping Jize, Chaowen Zhang, Siqi Liu, Yuzhe Zhao, Yuping Fu, Lixia Li, and et al. 2023. "A Pectic Polysaccharide from Codonopsis pilosula Alleviates Inflammatory Response and Oxidative Stress of Aging Mice via Modulating Intestinal Microbiota-Related Gut–Liver Axis" Antioxidants 12, no. 9: 1781. https://doi.org/10.3390/antiox12091781
APA StyleZou, Y., Yan, H., Li, C., Wen, F., Jize, X., Zhang, C., Liu, S., Zhao, Y., Fu, Y., Li, L., Liu, F., Chen, J., Li, R., Chen, X., & Tian, M. (2023). A Pectic Polysaccharide from Codonopsis pilosula Alleviates Inflammatory Response and Oxidative Stress of Aging Mice via Modulating Intestinal Microbiota-Related Gut–Liver Axis. Antioxidants, 12(9), 1781. https://doi.org/10.3390/antiox12091781






