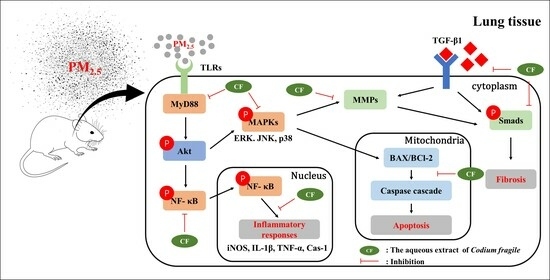
Codium fragile Suppressed Chronic PM2.5-Exposed Pulmonary Dysfunction via TLR/TGF-β Pathway in BALB/c Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Gas Chromatography–Tandem Mass Spectrometry (GC/MS2)
2.3. Ultra-Performance Liquid Chromatography–Quadrupole Time-of-Flight Mass Spectrometry (UPLC-Q-TOF/MSE)
2.4. High-Performance Liquid Chromatography (HPLC)
2.5. Animal Experimental Design
2.6. Antioxidant System Activity
2.6.1. Preparation of Lung Tissues
2.6.2. Superoxide Dismutase (SOD) Activity
2.6.3. Reduced Glutathione (GSH) Contents
2.6.4. Malondialdehyde (MDA) Contents
2.7. Mitochondrial Function Activity
2.7.1. Isolation of Mitochondria
2.7.2. Mitochondrial Reactive Oxygen Species (ROS) Content
2.7.3. Mitochondrial Membrane Potential (MMP) Levels
2.8. Western Blot
2.9. Statistical Analysis
3. Results
3.1. Physiological Compounds in Codium fragile
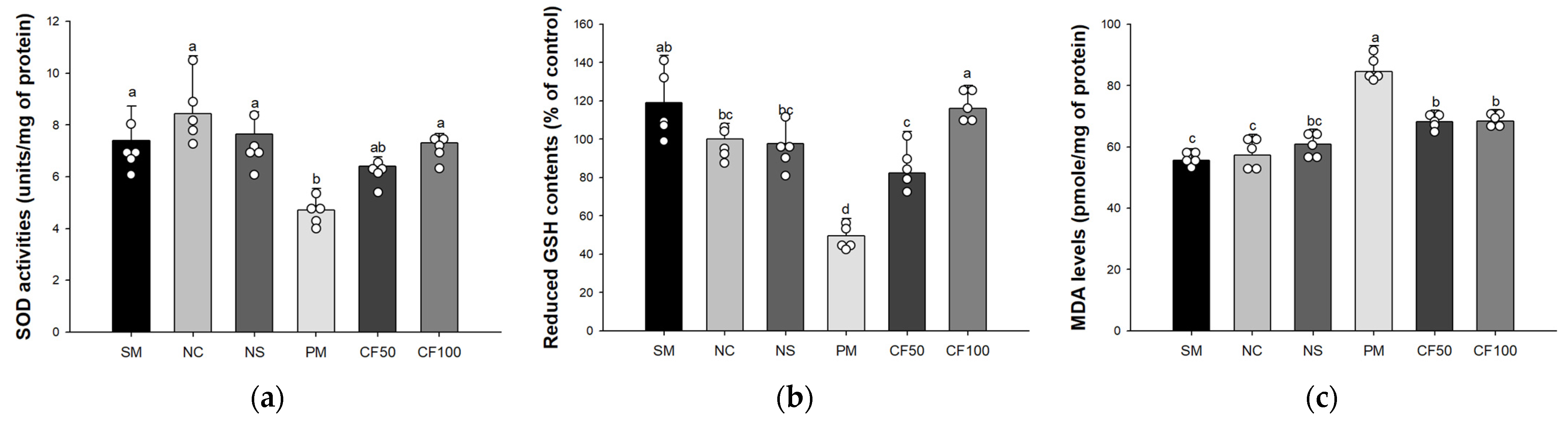
3.2. Effect of Codium fragile on Antioxidant System Biomarkers
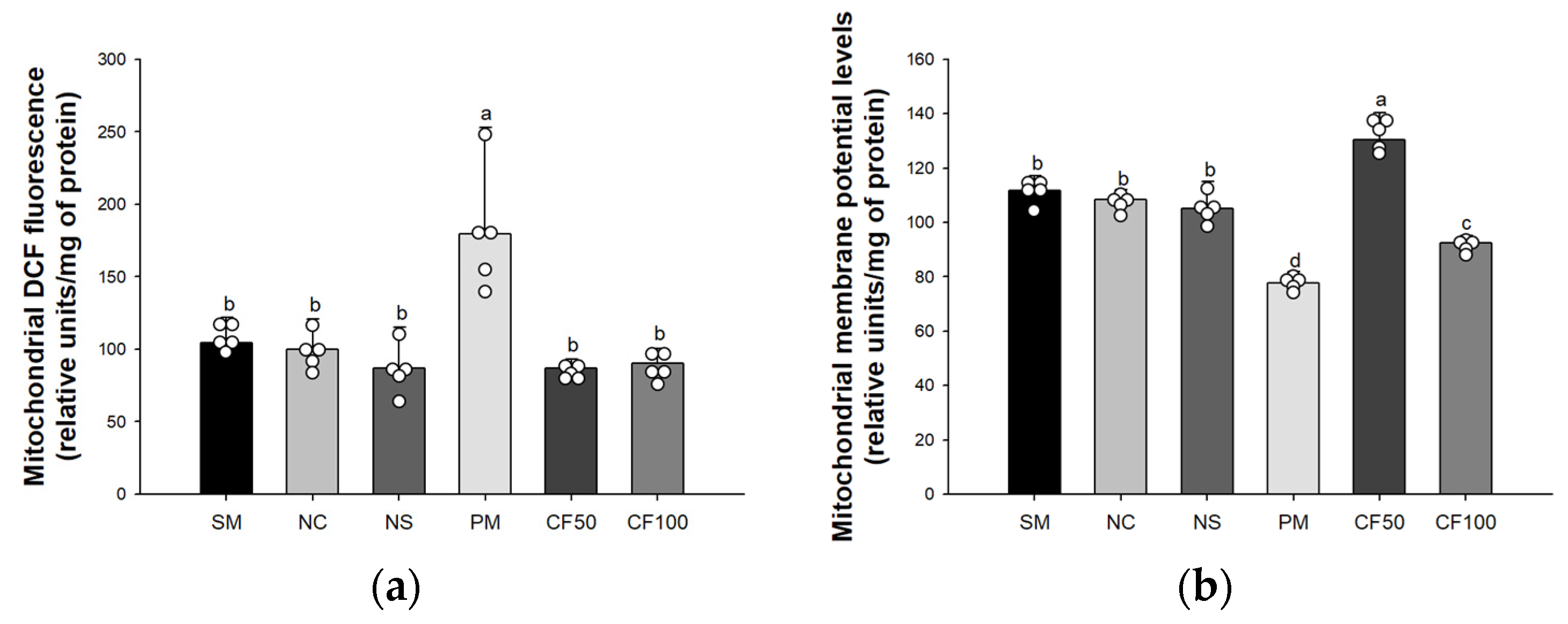
3.3. Effect of Codium fragile on Mitochondrial Activity
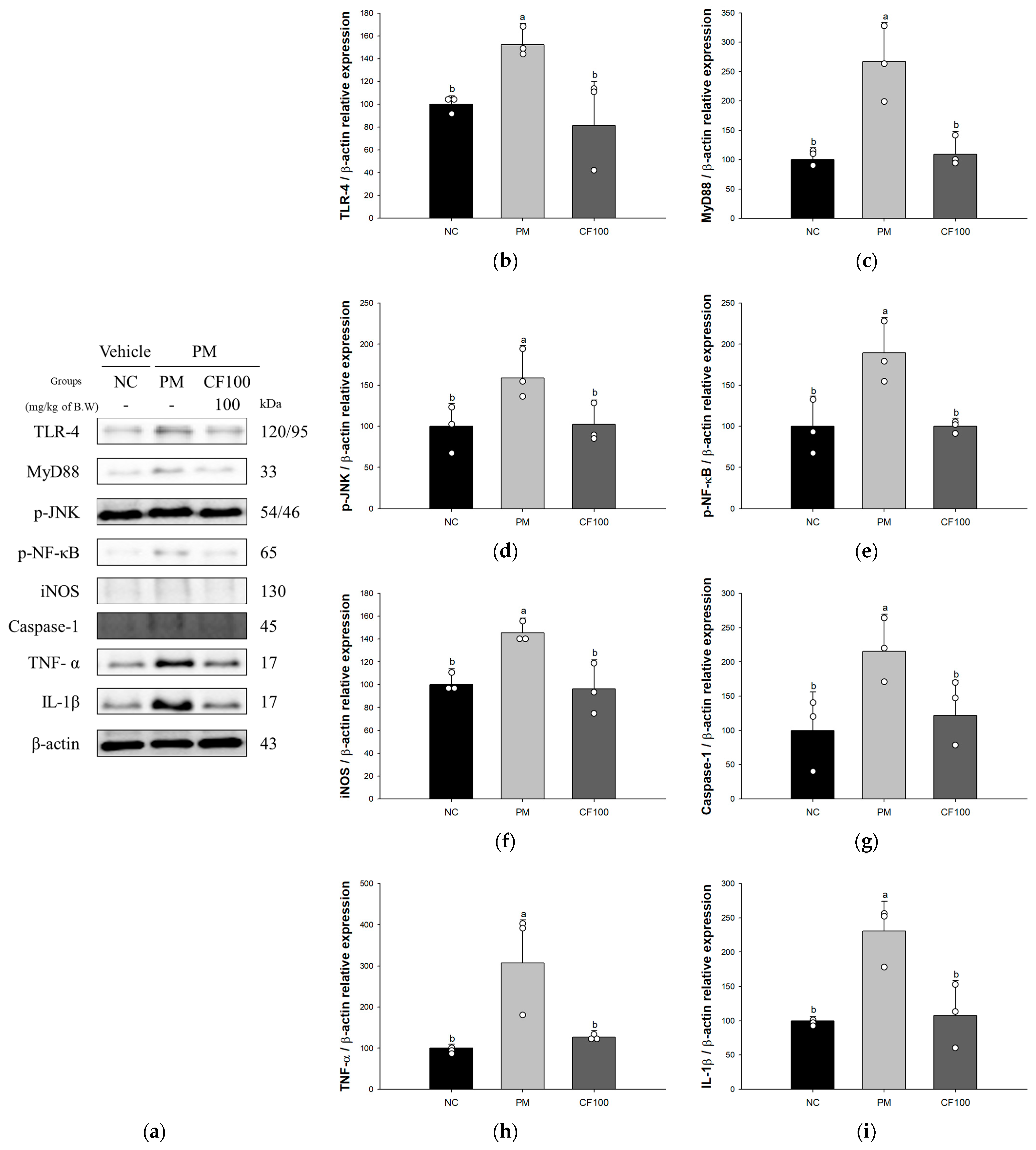
3.4. Effect of Codium fragile on PM2.5-Induced Pulmonary Inflammatory-Related Factors
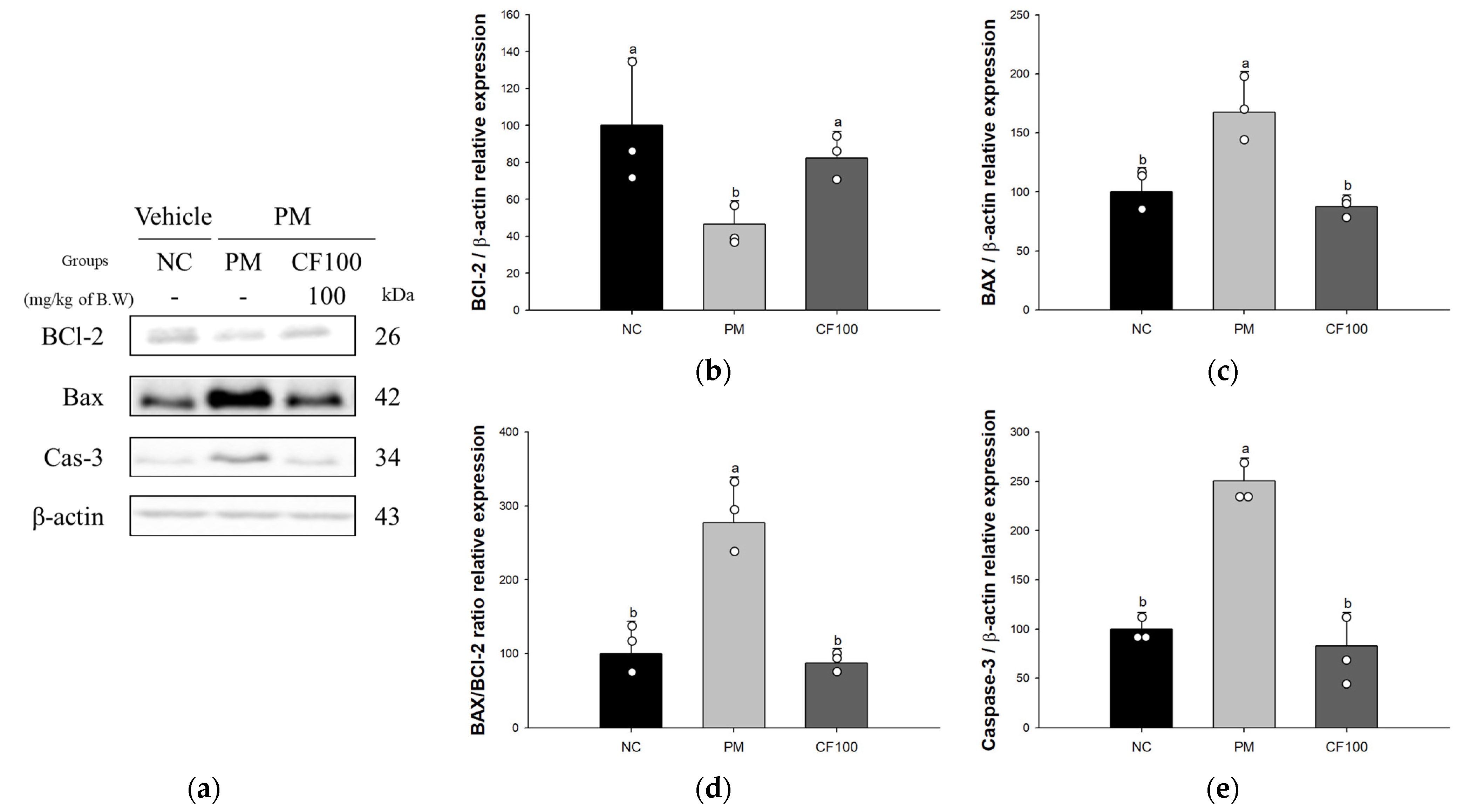
3.5. Effect of Codium fragile on PM2.5-Induced Pulmonary Apoptosis-Related Factors
3.6. Effect of Codium fragile on PM2.5-Induced Pulmonary Fibrosis-Related Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BAX | BCl-2 associated X |
| BCl-2 | B-cell lymphoma 2 |
| GSH | Glutathione |
| IL-1β | interleukin-1β |
| iNOS | inducible nitric oxide synthase |
| MDA | Malondialdehyde |
| MMP-1 | matrix metalloproteinase-1 |
| MMP-2 | matrix metalloproteinase-2 |
| MMP | mitochondrial membrane potential |
| MyD88 | myeloid differentiation primary response 88 |
| NF-κB | nuclear factor kappa-light-chain-enhancer of the activated B cell |
| p-JNK | phosphorylated c-Jun N-terminal kinase |
| p-Smad | phosphorylated small mothers against decapentaplegic |
| PM | particulate matter |
| PM2.5 | particulate matter, which classified as a size smaller than 2.5 μm |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| TGF-β1 | transforming growth factor-β1 |
| TLR | Toll-like receptors |
| TNF-α | tumor necrosis factor-α |
References
- Wei, T.; Tang, M. Biological effects of airborne fine particulate matter (PM2.5) exposure on pulmonary immune system. Environ. Toxicol. Pharmacol. 2018, 60, 195–201. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; Zeng, H. Interactions of particulate matter and pulmonary surfactant: Implications for human health. Adv. Colloid Interface Sci. 2020, 284, 102244. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Hong, W.; Li, B.; Zhou, Y.; Ran, P. Chronic exposure to biomass ambient particulate matter triggers alveolar macrophage polarization and activation in the rat lung. J. Cell Mol. Med. 2022, 26, 1156–1168. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, F.; Zhang, A. Arsenic-induced lung inflammation and fibrosis in a rat model: Contribution of the HMGB1/RAGE, PI3K/AKT, and TGF-β1/SMAD pathways. Toxicol. Appl. Pharmacol. 2021, 432, 115757. [Google Scholar] [CrossRef] [PubMed]
- Cutroneo, K.R.; White, S.L.; Phan, S.H.; Ehrlich, H.P. Therapies for bleomycin induced lung fibrosis through regulation of TGF-β1 induced collagen gene expression. J. Cell Physiol. 2007, 211, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Durham, A.L.; Adcock, I.M. The relationship between COPD and lung cancer. Lung Cancer 2015, 90, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Vijay, K. Toll-like receptors in immunity and inflammatory diseases: Past, present, and future. Int. Immunopharmacol. 2018, 59, 391–412. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, K.; Zhang, H.; Yuan, M.; An, N.; Wei, Y.; Wang, L.; Sun, Y.; Xing, Y.; Gao, Y. Anti-inflammatory and immunomodulatory effects of baicalin in cerebrovascular and neurological disorders. Brain Res. Bull. 2020, 164, 314–324. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, X. A review of oil-suspended particulate matter aggregation—A natural process of cleansing spilled oil in the aquatic environment. J. Environ. Monit. 2009, 11, 1801–1809. [Google Scholar] [CrossRef]
- Kolsi, R.B.A.; Jardak, N.; Hajkacem, F.; Chaaben, R.; El Feki, A.; Rebai, T.; Moussi, K.; Fki, L.; Belghith, H. Anti-obesity effect and protection of liver-kidney functions by Codium fragile sulphated polysaccharide on high fat diet induced obese rats. Int. J. Biol. Macromol. 2017, 102, 119–129. [Google Scholar] [CrossRef]
- Keskinkaya, H.B.; Deveci, E.; Güneş, E.; Okudan, E.Ş.; Akköz, C.; Gümüş, N.E.; Karakurt, S. Chemical composition, in vitro antimicrobial and antioxidant activities of marine macroalgae Codium fragile (Suringar) Hariot. Commagene J. Biol. 2022, 6, 94–104. [Google Scholar] [CrossRef]
- Magwaza, S.T.N.; Islam, M.S. Roles of marine macroalgae or seaweeds and their bioactive compounds in combating overweight, obesity and diabetes: A comprehensive review. Mar. Drugs 2023, 21, 258. [Google Scholar] [CrossRef]
- Moon, S.M.; Lee, S.A.; Han, S.H.; Park, B.R.; Choi, M.S.; Kim, J.S.; Kim, S.G.; Kim, H.J.; Chun, H.S.; Kim, D.K.; et al. Aqueous extract of Codium fragile alleviates osteoarthritis through the MAPK/NF-κB pathways in IL-1β-induced rat primary chondrocytes and a rat osteoarthritis model. Biomed. Pharmacother. 2018, 97, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Park, S.K.; Kang, J.Y.; Kim, J.M.; Shin, E.J.; Moon, J.H.; Kim, M.J.; Lee, H.L.; Jeong, H.R.; Heo, H.J. Protective effect of Codium fragile extract on fine dust (PM2.5)-induced toxicity in nasal cavity, lung, and brain cells. Korean J. Food Sci. Technol. 2021, 53, 223–229. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kim, J.M.; Kang, J.Y.; Park, S.K.; Moon, J.H.; Kim, M.J.; Lee, H.L.; Jeong, H.R.; Kim, J.C.; Heo, H.J. Powdered green tea (matcha) attenuates the cognitive dysfunction via the regulation of systemic inflammation in chronic PM2.5-exposed BALB/c mice. Antioxidants 2021, 10, 1932. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lee, U.; Kang, J.Y.; Park, S.K.; Kim, J.C.; Heo, H.J. Matcha improves metabolic imbalance-induced cognitive dysfunction. Oxidative Med. Cell. Longev. 2020, 2020, 8882763. [Google Scholar] [CrossRef]
- Wei, M.; Bao, G.; Li, S.; Yang, Z.; Cheng, S.; Le, W. PM2.5 exposure triggers cell death through lysosomal membrane permeabilization and leads to ferroptosis insensitivity via the autophagy dysfunction/p62-KEAP1-NRF2 activation in neuronal cells. Ecotoxicol. Environ. Saf. 2022, 248, 114333. [Google Scholar] [CrossRef]
- Liu, K.; Hua, S.; Song, L. PM2.5 exposure and asthma development: The key role of oxidative stress. Oxidative Med. Cell. Longev. 2022, 2022, 3618806. [Google Scholar] [CrossRef]
- Liu, X.; Meng, Z. Effects of airborne fine particulate matter on antioxidant capacity and lipid peroxidation in multiple organs of rats. Inhal. Toxicol. 2005, 17, 467–473. [Google Scholar] [CrossRef]
- Kim, J.M.; Heo, H.J. The roles of catechins in regulation of systemic inflammation. Food Sci. Biotechnol. 2022, 31, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, P.; Murphy, M.E.; Sies, H. Antioxidant defense systems: The role of carotenoids, tocopherols, and thiols. Am. J. Clin. Nutr. 1991, 53, 194–200. [Google Scholar] [CrossRef]
- Reyes-Soto, C.Y.; Villaseca-Flores, M.; Ovalle-Noguez, E.A.; Nava-Osorio, J.; Galván-Arzate, S.; Rangel-López, E.; Maya-López, M.; Retana-Márquez, S.; Túnez, I.; Tinkov, A.A.; et al. Oleamide reduces mitochondrial dysfunction and toxicity in rat cortical slices through the combined action of cannabinoid receptors activation and induction of antioxidant activity. Neurotox. Res. 2022, 140, 2167–2178. [Google Scholar] [CrossRef] [PubMed]
- Kalita, P.; Ahmed, A.B.; Sen, S.; Chakraborty, R. A comprehensive review on polysaccharides with hypolipidemic activity: Occurrence, chemistry and molecular mechanism. Int. J. Biol. Macromol. 2022, 206, 681–698. [Google Scholar] [CrossRef]
- Li, R.; Kou, X.; Geng, H.; Xie, J.; Tian, J.; Cai, Z.; Dong, C. Mitochondrial damage: An important mechanism of ambient PM2.5 exposure-induced acute heart injury in rats. J. Hazard. Mater. 2015, 287, 392–401. [Google Scholar] [CrossRef]
- Li, R.; Kou, X.; Geng, H.; Xie, J.; Yang, Z.; Zhang, Y.; Cai, Z.; Dong, C. Effect of ambient PM2.5 on lung mitochondrial damage and fusion/fission gene expression in rats. Chem. Res. Toxicol. 2015, 28, 408–418. [Google Scholar] [CrossRef]
- Dornhof, R.; Maschowski, C.; Osipova, A.; Giere, R.; Seidl, M.; Merfort, I.; Humar, M. Stress fibers, autophagy and necrosis by persistent exposure to PM2.5 from biomass combustion. PLoS ONE 2017, 12, 0180291. [Google Scholar] [CrossRef]
- Lin, Q.; Zhang, C.F.; Guo, J.L.; Su, J.L.; Guo, Z.K.; Li, H.Y. Involvement of NEAT1/PINK1-mediated mitophagy in chronic obstructive pulmonary disease induced by cigarette smoke or PM2.5. J. Transl. Med. 2022, 10, 277. [Google Scholar] [CrossRef]
- Maya-López, M.; Rubio-López, L.C.; Rodríguez-Alvarez, I.V.; Orduño-Piceno, J.; Flores-Valdivia, Y.; Colonnello, A.; López, E.R.; Túnez, I.; Prospéro-García, O.; Santamaría, A. A cannabinoid receptor-mediated mechanism participates in the neuroprotective effects of oleamide against excitotoxic damage in rat brain synaptosomes and cortical slices. Neurotox. Res. 2020, 37, 126–135. [Google Scholar] [CrossRef]
- Ahn, J.; Kim, M.J.; Ahn, J.; Ha, T.Y.; Jung, C.H.; Seo, H.D.; Jang, Y.J. Identifying Codium fragile extract components and their effects on muscle weight and exercise endurance. Food Chem. 2021, 353, 129463. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Harwanto, D.; Choi, J.S. Seaweed exhibits therapeutic properties against chronic diseases: An overview. Appl. Sci. 2022, 12, 2638. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, W.; Feng, F.; Qin, L.; Ji, W.; Li, D.; Liang, R.; Zhang, Y.; Wang, Y.; Li, M.; et al. Role of angiotensin-converting enzyme 2 in fine particulate matter-induced acute lung injury. Sci. Total Environ. 2022, 825, 153964. [Google Scholar] [CrossRef]
- Lee, S.A.; Moon, S.M.; Choi, Y.H.; Han, S.H.; Park, B.R.; Choi, M.S.; Kim, J.S.; Kim, Y.H.; Kim, D.K.; Kim, C.S. Aqueous extract of Codium fragile suppressed inflammatory responses in lipopolysaccharide-stimulated RAW264.7 cells and carrageenan-induced rats. Biomed. Pharmacother. 2017, 93, 1055–1064. [Google Scholar] [CrossRef]
- Patel, S. Therapeutic importance of sulfated polysaccharides from seaweeds: Updating the recent findings. 3 Biotech. 2012, 2, 171–185. [Google Scholar] [CrossRef]
- Michalak, I.; Tiwari, R.; Dhawan, M.; Alagawany, M.; Farag, M.R.; Sharun, K.; Emran, T.B.; Dhama, K. Antioxidant effects of seaweeds and their active compounds on animal health and production—A review. Vet. Q. 2022, 42, 48–67. [Google Scholar] [CrossRef]
- Nagata, K.; Araumi, S.; Ando, D.; Ito, N.; Ando, M.; Ikeda, Y.; Takahashi, M.; Noguchi, S.; Yasuda, Y.; Nakano, N.; et al. Kaempferol Suppresses the Activation of Mast Cells by Modulating the Expression of FcεRI and SHIP1. Int. J. Mol. Sci. 2023, 24, 5997. [Google Scholar] [CrossRef]
- Talebi, H.; Farahpour, M.R.; Hamishehkar, H. The effectiveness of Rutin for prevention of surgical induced endometriosis development in a rat model. Sci. Rep. 2021, 11, 7180. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Batibawa, J.W.; Du, Z.; Liang, S.; Duan, J.; Sun, Z. Acute exposure to PM2.5 triggers lung inflammatory response and apoptosis in rat. Ecotoxicol. Environ. Saf. 2021, 222, 112526. [Google Scholar] [CrossRef]
- Yang, J.Y.; Abe, K.; Xu, N.J.; Matsuki, N.; Wu, C.F. Oleamide attenuates apoptotic death in cultured rat cerebellar granule neurons. Neurosci. Lett. 2002, 328, 165–169. [Google Scholar] [CrossRef]
- Sabitha, R.; Nishi, K.; Gunasekaran, V.P.; Agilan, B.; David, E.; Annamalai, G.; Vinothkumar, R.; Perumal, M.; Subbiah, L.; Ganeshan, M. p-Coumaric acid attenuates alcohol exposed hepatic injury through MAPKs, apoptosis and Nrf2 signaling in experimental models. Chem. Biol. Interact. 2020, 321, 109044. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, W.; Zhang, F.; Roepstorff, P.; Yang, F.; Lu, Z.; Ding, W. TMT-based quantitative proteomics analysis reveals airborne PM2.5-induced pulmonary fibrosis. Int. J. Environ. Res. Public. Health 2019, 16, 98. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Li, Y.; Yang, H.; Cui, Y.; Wang, H.; Zhou, H.; Zhang, J.; Du, B.; Zhai, Q.; Chen, X.; et al. Tamarixetin protects against cardiac hypertrophy via inhibiting NFAT and AKT pathway. J. Mol. Histol. 2019, 50, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, L.; White, J.; Fang, J. Inhibitory effect of gallic acid on CCl4-mediated liver fibrosis in mice. Cell Biochem. Biophys. 2014, 69, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ge, A.; Zhu, W.; Liu, Y.N.; Ji, N.F.; Zha, W.J.; Zhang, J.X.; Zeng, X.N.; Huang, M. Morin attenuates ovalbumin-induced airway inflammation by modulating oxidative stress-responsive MAPK signaling. Oxidative Med. Cell. Longev. 2016, 2016, 5843672. [Google Scholar] [CrossRef]
- Gao, Y.; Lu, J.; Zhang, Y.; Chen, Y.; Gu, Z.; Jiang, X. Baicalein attenuates bleomycin-induced pulmonary fibrosis in rats through inhibition of miR-21. Pulm. Pharmacol. Ther. 2013, 26, 649–654. [Google Scholar] [CrossRef]
- Wygrecka, M.; Hadzic, S.; Potaczek, D.P.; Alexopoulos, I.; El Agha, E.; Schaefer, L. Decoding the role of fatty acids and their metabolites in lung fibrosis. Pol. Arch. Intern. Med. 2023, 133, 16520. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Guo, C.; Zhang, H. Fatty acid metabolism-related genes in bronchoalveolar lavage fluid unveil prognostic and immune infiltration in idiopathic pulmonary fibrosis. Front. Endocrinol. 2022, 13, 1001563. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.R.; Schulze, A. Lipid metabolism in cancer. FEBS J. 2012, 279, 2610–2623. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Yoo, H.J.; Lee, K.M.; Song, H.E.; Kim, S.J.; Lee, J.O.; Hwang, J.J.; Song, J.W. Stearic acid attenuates profibrotic signalling in idiopathic pulmonary fibrosis. Respirology 2021, 26, 255–263. [Google Scholar] [CrossRef] [PubMed]




| Antibody | Catalog | Concentration | Manufacturer |
|---|---|---|---|
| TLR4 | sc-52962 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| MyD88 | sc-74532 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| p-JNK | sc-6254 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| p-NF-κB | 3033 | 1:1000 | Cell Signaling Tech (Danvers, MA, USA) |
| iNOS | sc-7271 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| Caspase-1 | sc-392736 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| TNF-α | sc-393887 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| IL-1β | sc-4592 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| BCl-2 | sc-509 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| BAX | sc-7480 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| Caspase-3 | CSB-PA05689A0Rb | 1:1000 | Cusabio (Hubei, China) |
| TFG-β1 | sc-130348 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| p-Smad-2 | 3108 | 1:1000 | Cell Signaling Tech (Danvers, MA, USA) |
| p-Smad-3 | sc-517575 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| MMP-1 | sc-21731 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| MMP-2 | sc-13595 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| β-actin | 66009-1-Ig | 1:1000 | Proteintech (Rosemont, IL, USA) |
| No. | RT (min) 1 | Parent Ion | Fragment (m/z) | Compound |
|---|---|---|---|---|
| 1 | 43.92 | 328 | 313, 269, 201, 117, 73, 43 | Palmitic acid |
| 2 | 48.58 | 356 | 341, 309, 241, 201, 117 | Stearic acid |
| 3 | 51.82 | 330 | 282, 249, 167, 149, 122 | Oleamide |
| No. | RT (min) 1 | m/z [M + H]+ | Fragment (m/z) | Compound |
|---|---|---|---|---|
| 1 | 8.35 | 256 | 80, 88, 184, 201 | Hexadecanamide |
| 2 | 8.44 | 282 | 135, 149, 247, 265 | Oleamide |
| 3 | 8.73 | 338 | 80, 106, 309 | 13-docosenamide |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.Y.; Kim, J.M.; Lee, H.L.; Go, M.J.; Joo, S.G.; Kim, J.H.; Lee, H.S.; Jeong, W.M.; Lee, D.Y.; Kim, H.-J.; et al. Codium fragile Suppressed Chronic PM2.5-Exposed Pulmonary Dysfunction via TLR/TGF-β Pathway in BALB/c Mice. Antioxidants 2023, 12, 1743. https://doi.org/10.3390/antiox12091743
Kim TY, Kim JM, Lee HL, Go MJ, Joo SG, Kim JH, Lee HS, Jeong WM, Lee DY, Kim H-J, et al. Codium fragile Suppressed Chronic PM2.5-Exposed Pulmonary Dysfunction via TLR/TGF-β Pathway in BALB/c Mice. Antioxidants. 2023; 12(9):1743. https://doi.org/10.3390/antiox12091743
Chicago/Turabian StyleKim, Tae Yoon, Jong Min Kim, Hyo Lim Lee, Min Ji Go, Seung Gyum Joo, Ju Hui Kim, Han Su Lee, Won Min Jeong, Dong Yeol Lee, Hyun-Jin Kim, and et al. 2023. "Codium fragile Suppressed Chronic PM2.5-Exposed Pulmonary Dysfunction via TLR/TGF-β Pathway in BALB/c Mice" Antioxidants 12, no. 9: 1743. https://doi.org/10.3390/antiox12091743
APA StyleKim, T. Y., Kim, J. M., Lee, H. L., Go, M. J., Joo, S. G., Kim, J. H., Lee, H. S., Jeong, W. M., Lee, D. Y., Kim, H.-J., & Heo, H. J. (2023). Codium fragile Suppressed Chronic PM2.5-Exposed Pulmonary Dysfunction via TLR/TGF-β Pathway in BALB/c Mice. Antioxidants, 12(9), 1743. https://doi.org/10.3390/antiox12091743