3-Bromo-4,5-dihydroxybenzaldehyde Protects Keratinocytes from Particulate Matter 2.5-Induced Damages
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Cell Culture
2.3. Animal Experiment
2.4. ROS Scavenging Ability
2.5. Lipid Peroxidation Assay
2.6. Analysis of Mitochondria Function
2.7. Detection of 8-Oxoguanine (8-OxoG)
2.8. Comet Assay
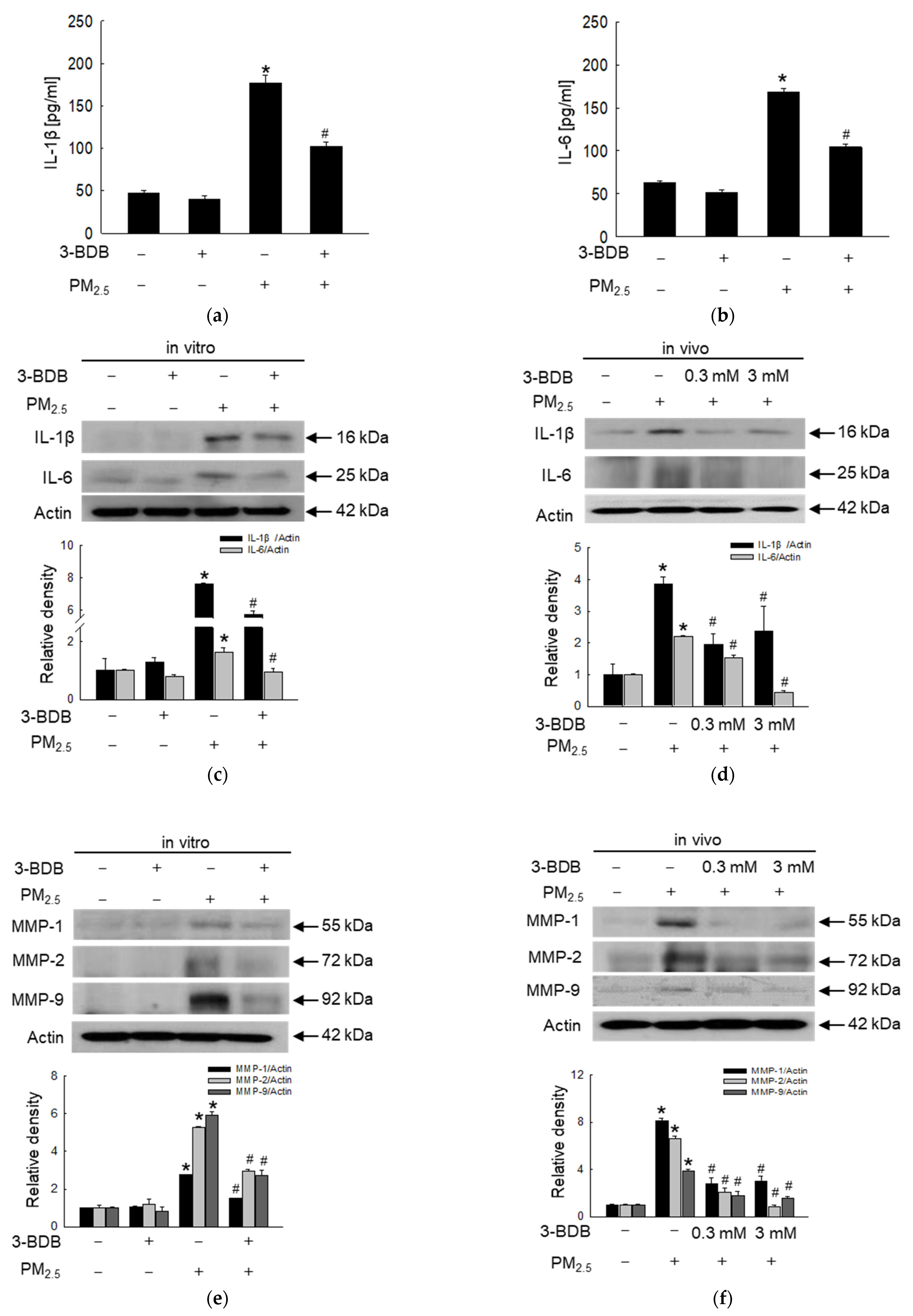
2.9. Detection of IL-1β and IL-6
2.10. Western Blot
2.11. Cell Cycle Analysis
2.12. Hoechst 33342 Staining
2.13. β-Galactosidase Staining Activity
2.14. Statistical Analysis
3. Results
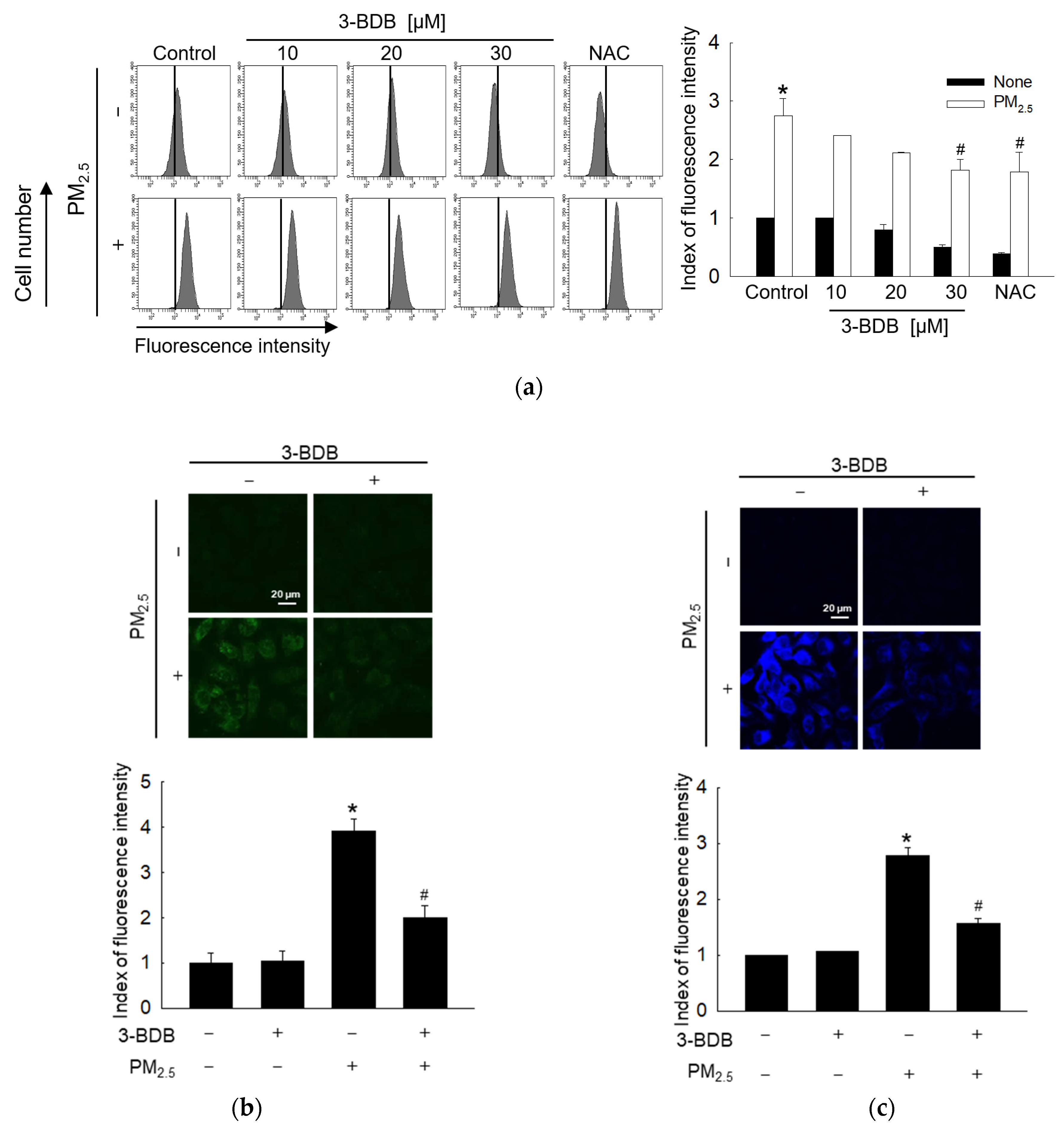
3.1. Antioxidant Effect of 3-BDB against PM2.5-Induced Intracellular ROS and Lipid Peroxidation
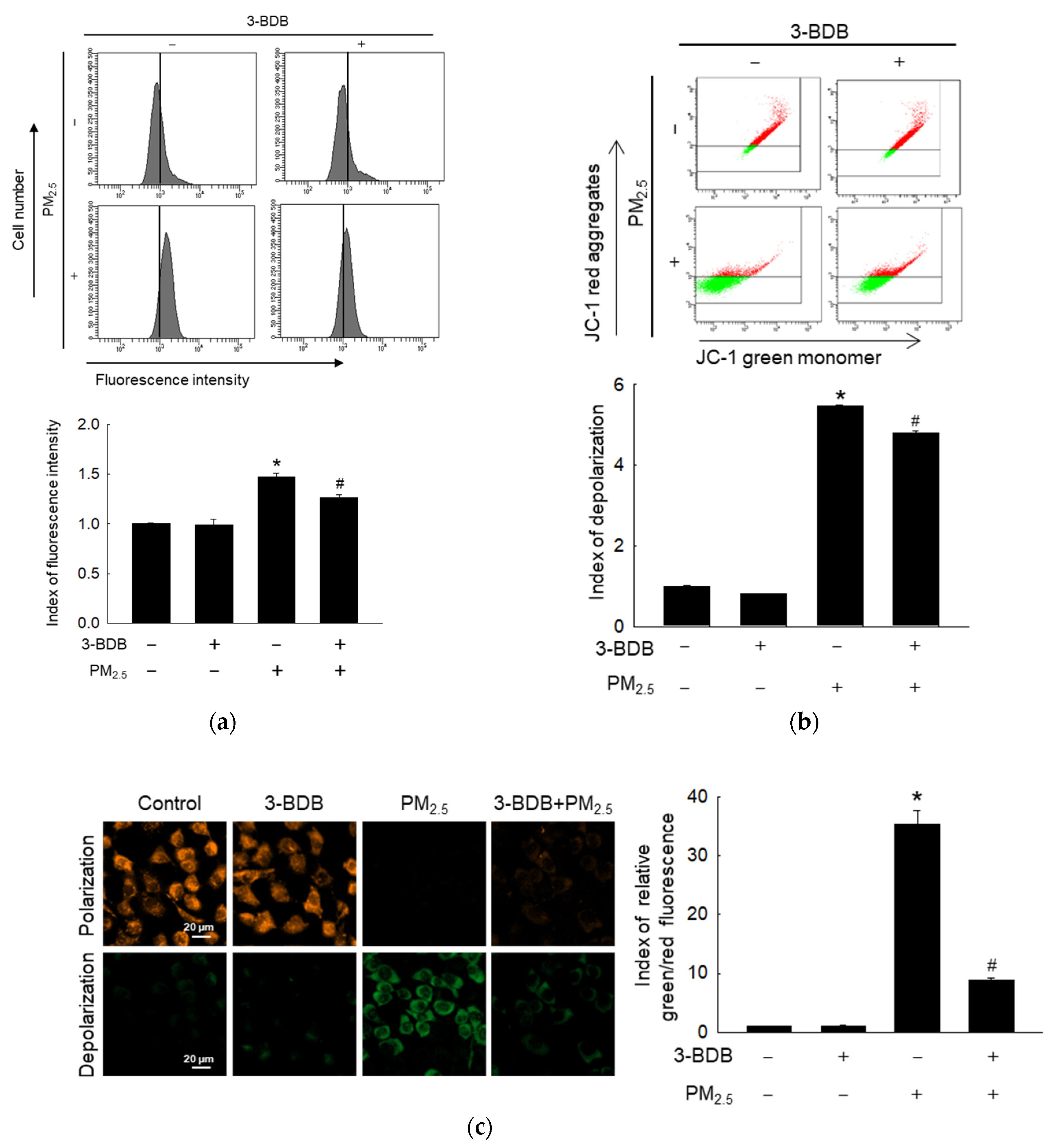
3.2. Preventive Effect of 3-BDB against PM2.5-Induced Mitochondrial Dysfunction
3.3. Inhibitory Effect of 3-BDB against PM2.5-Induced DNA Damage
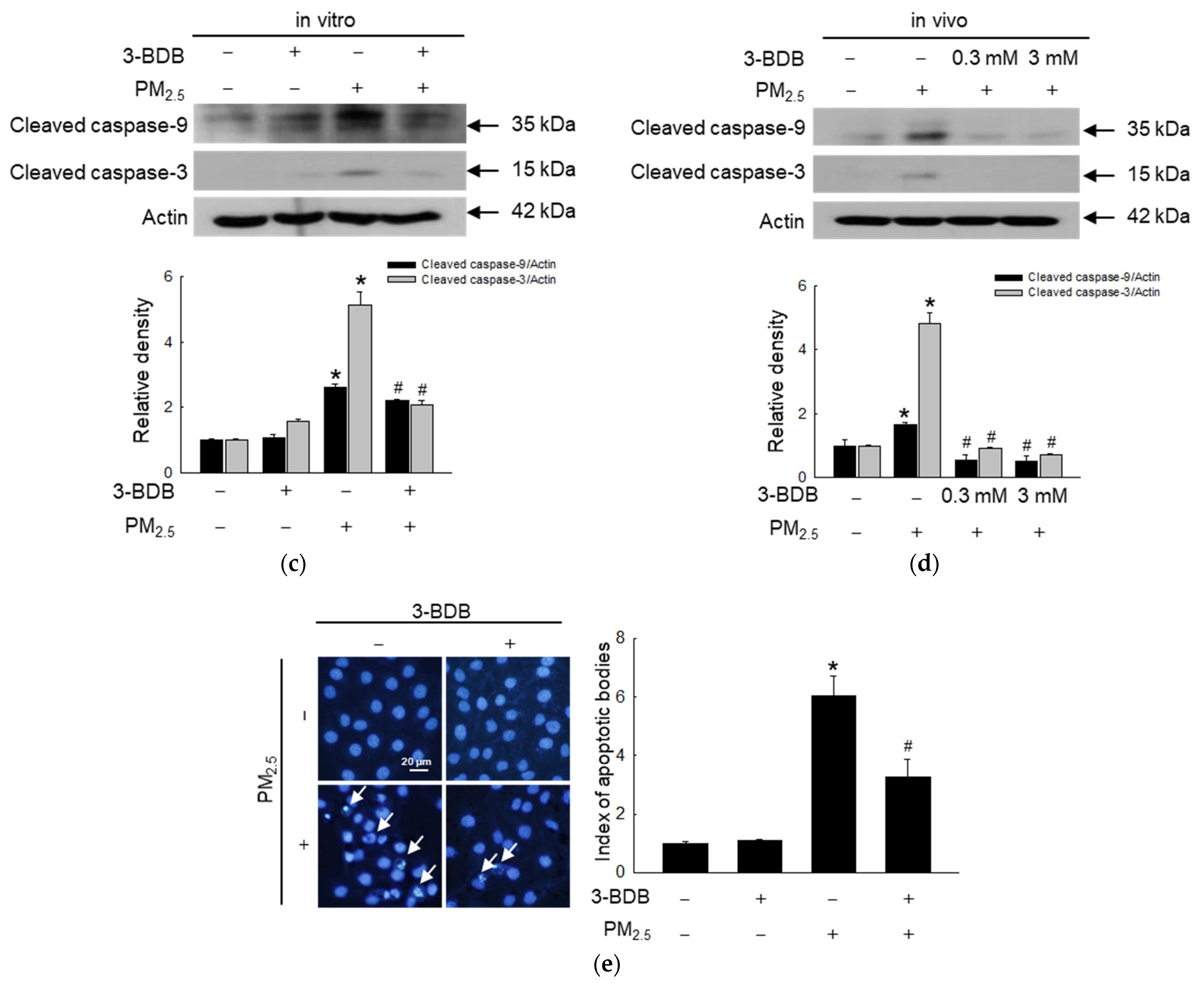
3.4. Anti-Apoptotic Effect of 3-BDB against PM2.5-Induced Apoptosis
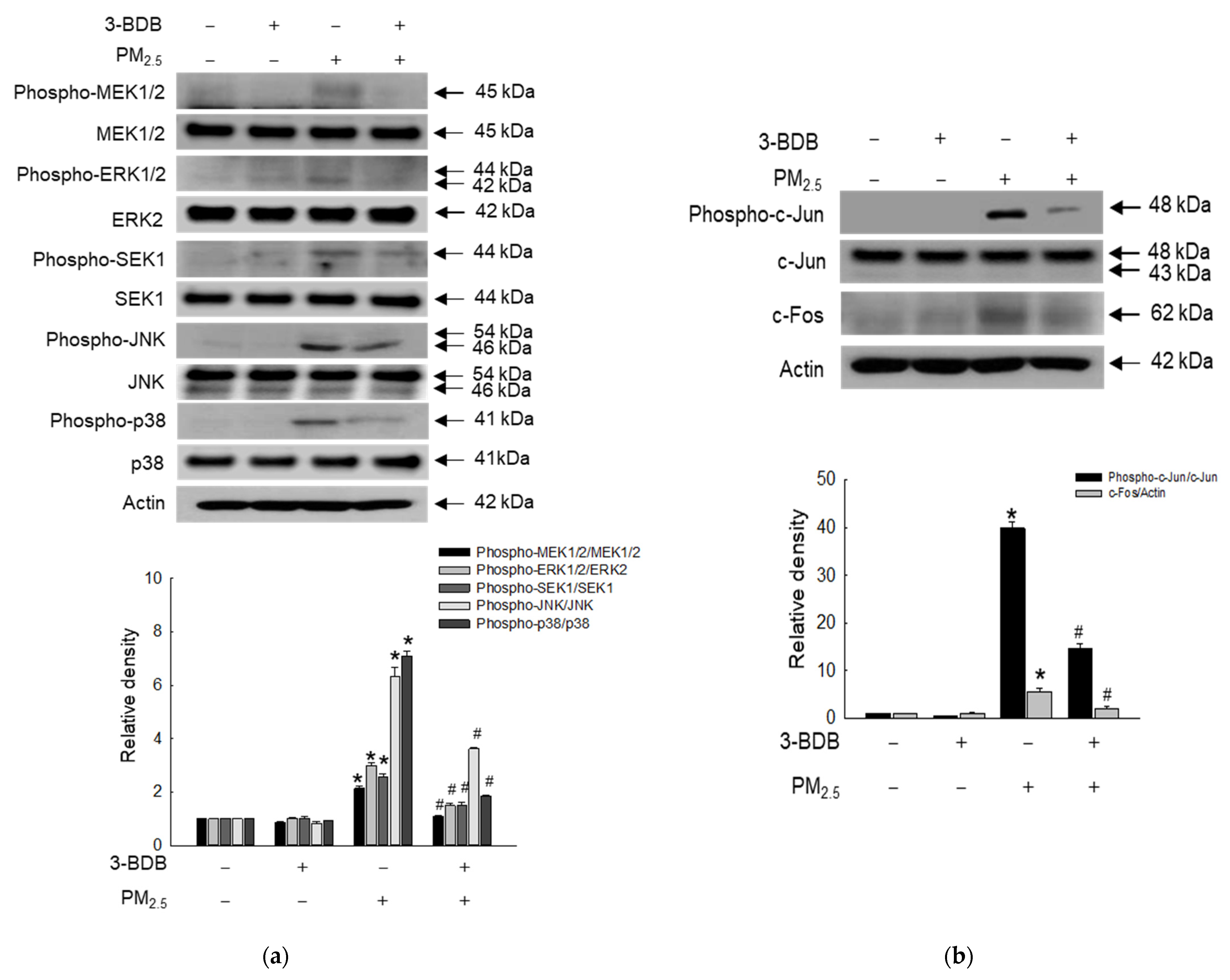
3.5. Inactivating Effect of 3-BDB against PM2.5-Induced Activator Protein (AP)-1 via Mitogen- Activated Protein Kinase (MAPK) Signaling Pathway
3.6. Antagonizing Effect of 3-BDB against PM2.5-Induced Senescence
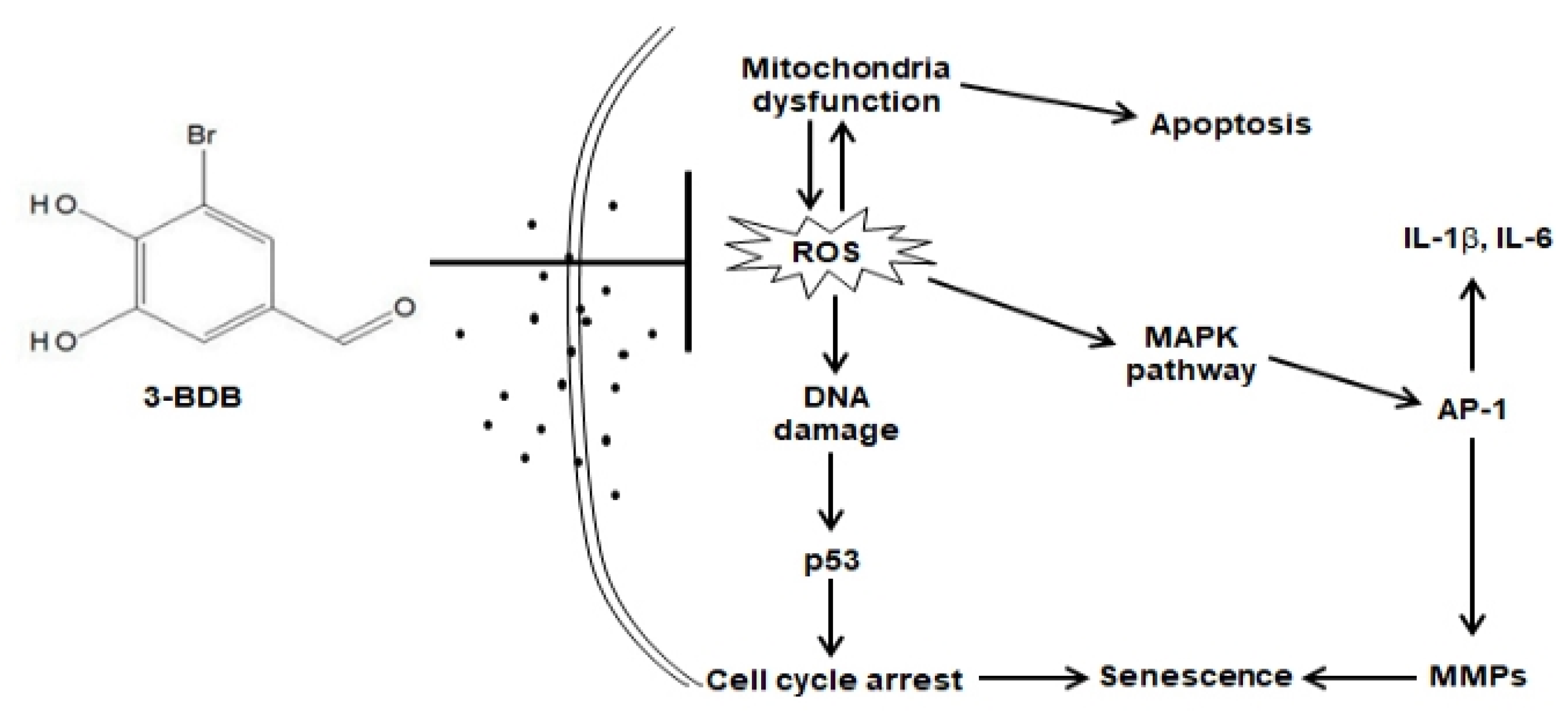
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, S.; Wang, X.; Zhang, X.; Guo, M.; Miura, M.; Xiao, Y. Influence of biomass burning on local air pollution in mainland Southeast Asia from 2001 to 2016. Environ. Pollut. 2019, 254, 112949. [Google Scholar] [CrossRef]
- Rembiesa, J.; Ruzgas, T.; Engblom, J.; Holefors, A. The impact of pollutants on skin and proper efficacy testing for anti-pollution claims. Cosmetics 2018, 5, 4. [Google Scholar] [CrossRef]
- Han, X.; Zhuang, Y. PM2.5 induces autophagy-mediated cell apoptosis via PI3K/AKT/mTOR signaling pathway in mice bronchial epithelium cells. Exp. Ther. Med. 2021, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Zhu, P.; Liu, Y.; Zhu, H.; Geng, J.; Wang, B.; Yuan, G.; Peng, Y.; Xu, B. PM2.5 induces endothelial dysfunction via activating NLRP3 inflammasome. Environ. Toxicol. 2021, 36, 1886–1893. [Google Scholar] [CrossRef]
- Zhou, J.; Zou, H.; Liu, Y.; Chen, Y.; Du, Y.; Liu, J.; Huang, Z.; Liang, L.; Xie, R.; Yang, Q. Acute cytotoxicity test of PM2.5, NNK and BPDE in human normal bronchial epithelial cells: A comparison of a co-culture model containing macrophages and a mono-culture model. Toxicol. In Vitro 2022, 85, 105480. [Google Scholar] [CrossRef]
- Lin, C.I.; Tsai, C.H.; Sun, Y.L.; Hsieh, W.Y.; Lin, Y.C.; Chen, C.Y.; Lin, C.S. Instillation of particulate matter 2.5 induced acute lung injury and attenuated the injury recovery in ACE2 knockout mice. Int. J. Biol. Sci. 2018, 14, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Du, L.; Sun, W.; Yu, Z.; He, F.; Chen, J.; Li, X.; Yu, L.; Chen, D. Maternal exposure to fine particulate air pollution induces epithelial-to-mesenchymal transition resulting in postnatal pulmonary dysfunction mediated by transforming growth factor-β/Smad3 signaling. Toxicol. Lett. 2017, 267, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Wang, N.; Huang, L.; Zhao, Y.; Shao, H.; Jin, Y.; Zhang, R.; Li, C.; Wu, W.; Wang, J.; et al. NLRP3 inflammasome activation is associated with PM2.5 -induced cardiac functional and pathological injury in mice. Environ. Toxicol. 2019, 34, 1246–1254. [Google Scholar] [CrossRef]
- Zhang, J.; Fulgar, C.C.; Mar, T.; Young, D.E.; Zhang, Q.; Bein, K.J.; Cui, L.; Castañeda, A.; Vogel, C.F.A.; Sun, X.; et al. TH17-induced neutrophils enhance the pulmonary allergic response following BALB/c exposure to house dust mite allergen and fine particulate matter from California and China. Toxicol. Sci. 2018, 164, 627–643. [Google Scholar] [CrossRef]
- Ngoc, L.T.N.; Park, D.; Lee, Y.; Lee, Y.C. Systematic review and meta-analysis of human skin diseases due to particulate matter. Int. J. Environ. Res. Public Health 2017, 14, 1458. [Google Scholar] [CrossRef]
- Kim, K.E.; Cho, D.; Park, H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin dis eases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Ahn, M.J.; Kang, K.A.; Ryu, Y.S.; Hyun, Y.; Shilnikova, K.; Zhen, A.X.; Jeong, J.W.; Choi, Y.H.; Kang, H.K.; et al. Partic ulate matter 2.5 damages skin cells by inducing oxidative stress, subcellular organelle dysfunction, and apoptosis. Arch. Toxicol. 2018, 92, 2077–2091. [Google Scholar] [CrossRef] [PubMed]
- Hyun, Y.J.; Piao, M.J.; Kang, K.A.; Zhen, A.X.; Madushan Fernando, P.D.S.; Kang, H.K.; Ahn, Y.S.; Hyun, J.W. Effect of fer-mented fish oil on fine particulate matter-induced skin aging. Mar. Drugs 2019, 17, 61. [Google Scholar] [CrossRef]
- Ryu, Y.S.; Kang, K.A.; Piao, M.J.; Ahn, M.J.; Yi, J.M.; Hyun, Y.M.; Kim, S.H.; Ko, M.K.; Park, C.O.; Hyun, J.W. Particulate matter induces inflammatory cytokine production via activation of NFκB by TLR5-NOX4-ROS signaling in human skin keratinocyte and mouse skin. Redox Biol. 2019, 21, 101080. [Google Scholar] [CrossRef]
- Ryu, Y.S.; Kang, K.A.; Piao, M.J.; Ahn, M.J.; Yi, J.M.; Bossis, G.; Hyun, Y.M.; Park, C.O.; Hyun, J.W. Particulate matter-induced senescence of skin keratinocytes involves oxidative stress-dependent epigenetic modifications. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, L.; Tuo, J.; Liu, Q.; Zhang, X.; Xu, Z.; Liu, S.; Sui, G. Analysis of PM2.5-induced cytotoxicity in human HaCaT cells based on a microfluidic system. Toxicol. In Vitro 2017, 43, 1–8. [Google Scholar] [CrossRef]
- Nguyen, L.T.H.; Nguyen, U.T.; Kimb, Y.H.; Shin, H.M.; Yang, I.J. Astragali radix and its compound formononetin ameliorate diesel particulate matter-induced skin barrier disruption by regulation of keratinocyte proliferation and apoptosis. J. Ethnopharmacol. 2019, 228, 132–141. [Google Scholar] [CrossRef]
- Cho, S.H.; Heo, S.J.; Yang, H.W.; Ko, E.Y.; Jung, M.S.; Cha, S.H.; Ahn, G.; Jeon, Y.J.; Kim, K.N. Protective effect of 3-Bromo-4,5-dihydroxybenzaldehyde from Polysiphonia morrowii harvey against hydrogen peroxide-induced oxidative stress in vitro and in vivo. J. Microbiol. Biotechnol. 2019, 29, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, S.R.; Oh, M.J.; Jung, S.J.; Kang, S.Y. In vitro antiviral activity of red alga, Polysiphonia morrowii extract and its bromophenols against fish pathogenic infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus. J. Microbiol. 2011, 49, 102–106. [Google Scholar] [CrossRef]
- Jayasinghe, A.M.K.; Han, E.J.; Kirindage, K.G.I.S.; Fernando, I.P.S.; Kim, E.A.; Kim, J.; Jung, K.; Kim, K.N.; Heo, S.J.; Ahn, G. 3-bromo-4,5-dihydroxybenzaldehyde isolated from Polysiphonia morrowii suppresses TNF-α/IFN-γ-stimulated inflammation and deterioration of skin barrier in HaCaT keratinocytes. Mar. Drugs 2022, 20, 563. [Google Scholar] [CrossRef]
- Ryu, Y.S.; Fernando, P.D.S.M.; Kang, K.A.; Piao, M.J.; Zhen, A.X.; Kang, H.K.; Koh, Y.S.; Hyun, J.W. Marine compound 3-bromo-4,5-dihydroxybenzaldehyde protects skin cells against oxidative damage via the Nrf2/HO-1 pathway. Mar. Drugs 2019, 17, 234. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Hyun, Y.J.; Hewage, S.R.K.M.; Piao, M.J.; Kang, K.A.; Kang, H.K.; Koh, Y.S.; Ahn, M.J.; Hyun, J.W. 3-Bromo-4,5-dihy droxybenzaldehyde enhances the level of reduced glutathione via the Nrf2-mediated pathway in human keratinocytes. Mar. Drugs 2017, 15, 291. [Google Scholar] [CrossRef]
- Piao, M.J.; Kang, K.A.; Ryu, Y.S.; Shilnikova, K.; Park, J.E.; Hyun, Y.J.; Zhen, A.X.; Kang, H.K.; Koh, Y.S.; Ahn, M.J.; et al. The red algae compound 3-bromo-4,5-dihydroxybenzaldehyde protects human keratinocytes on oxidative stress-related molecules and pathways activated by UVB irradiation. Mar. Drugs 2017, 15, 268. [Google Scholar] [CrossRef]
- Ji, N.; Lou, H.; Gong, X.; Fu, T.; Ni, S. Treatment with 3-bromo-4,5-dihydroxybenzaldehyde improves cardiac function by inhib iting macrophage infiltration in mice. Korean Circ. J. 2018, 48, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.G.; Tian, H.Y.; Wei, J.; Han, Z.H.; Zhang, M.J.; Hao, G.H.; Liu, X.; Pan, L.F. 3-Bromo-4,5-dihydroxybenzaldehyde protects against myocardial ischemia and reperfusion injury through the Akt-PGC1α-Sirt3 pathway. Front. Pharmacol. 2018, 9, 722. [Google Scholar] [CrossRef]
- Kang, N.J.; Han, S.C.; Kang, H.J.; Ko, G.; Yoon, W.J.; Kang, H.K.; Yoo, E.S. Anti-inflammatory effect of 3-bromo-4,5-dihydroxyben zaldehyde, a component of Polysiphonia morrowii, in vivo and in vitro. Toxicol. Res. 2017, 33, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Chiorcea-Paquim, A.M. 8-oxoguanine and 8-oxodeoxyguanosine biomarkers of oxidative DNA damage: A review on HPLC-ECD determination. Molecules 2022, 27, 1620. [Google Scholar] [CrossRef]
- Conners, R.; Hooley, E.; Clarke, A.R.; Thomas, S.; Brady, R.L. Recognition of oxidatively modified bases within the biotin-binding site of avidin. J. Mol. Biol. 2006, 357, 263–274. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Drigeard Desgarnier, M.C.; Rochette, P.J. Enhancement of UVB-induced DNA damage repair after a chronic low-dose UVB pre-stimulation. DNA Repair 2018, 63, 56–62. [Google Scholar] [CrossRef]
- Reed, S.M.; Quelle, D.E. p53 acetylation: Regulation and consequences. Cancers 2014, 7, 30–69. [Google Scholar] [CrossRef] [PubMed]
- Keyvanloo Shahrestanaki, M.; Bagheri, M.; Ghanadian, M.; Aghaei, M.; Jafari, S.M. Centaurea cyanus extracted 13-O-acetylsolsti tialin A decrease Bax/Bcl-2 ratio and expression of cyclin D1/Cdk-4 to induce apoptosis and cell cycle arrest in MCF-7 and MDA-MB-231 breast cancer cell lines. J. Cell. Biochem. 2019, 120, 18309–18319. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of cellular senescence: Cell cycle arrest and senescence associated secretory phenotype. Front Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; López, J.M. Understanding MAPK signaling pathways in apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Joo, H.S.; Lee, K.; Jang, M.; Kim, S.D.; Kim, I.; Borlaza, L.J.S.; Lim, H.; Shin, H.; Chung, K.H.; et al. Differential toxicities of fine particulate matters from various sources. Sci. Rep. 2018, 8, 17007. [Google Scholar] [CrossRef]
- Quezada-Maldonado, E.M.; Sánchez-Pérez, Y.; Chirino, Y.I.; García-Cuellar, C.M. Airborne particulate matter induces oxidative damage, DNA adduct formation and alterations in DNA repair pathways. Environ. Pollut. 2021, 287, 117313. [Google Scholar] [CrossRef]
- Zhen, A.X.; Piao, M.J.; Hyun, Y.J.; Kang, K.A.; Ryu, Y.S.; Cho, S.J.; Kang, H.K.; Koh, Y.S.; Ahn, M.J.; Kim, T.H.; et al. Purpurogallin protects keratinocytes from damage and apoptosis induced by Ultraviolet B radiation and particulate matter 2.5. Biomol. Ther. 2019, 27, 395–403. [Google Scholar] [CrossRef]
- Hyun, Y.J.; Piao, M.J.; Zhang, R.; Choi, Y.H.; Chae, S.; Hyun, J.W. Photo-protection by 3-bromo-4, 5-dihydroxybenzaldehyde against ultraviolet B-induced oxidative stress in human keratinocytes. Ecotoxicol. Environ. Saf. 2012, 83, 71–78. [Google Scholar] [CrossRef]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef]
- Cadenas, S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 940–950. [Google Scholar] [CrossRef]
- Park, C.; Cha, H.J.; Hong, S.H.; Kim, G.Y.; Kim, S.; Kim, H.S.; Kim, B.W.; Jeon, Y.J.; Choi, Y.H. Protective effect of phloroglucinol on oxidative stress-induced DNA damage and apoptosis through activation of the Nrf2/HO-1 signaling pathway in HaCaT human keratinocytes. Mar. Drugs 2019, 17, 225. [Google Scholar] [CrossRef] [PubMed]
- Sterea, A.M.; EI Hiani, Y. The role of mitochondrial calcium signaling in the pathophysiology of cancer cells. Adv. Exp. Med. Biol. 2020, 1131, 747–770. [Google Scholar] [PubMed]
- Correia-Melo, C.; Marques, F.D.; Anderson, R.; Hewitt, G.; Hewitt, R.; Cole, J.; Carroll, B.M.; Miwa, S.; Birch, J.; Merz, A.; et al. Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J. 2016, 35, 724–742. [Google Scholar] [CrossRef] [PubMed]
- Herath, H.M.U.L.; Piao, M.J.; Kang, K.A.; Zhen, A.X.; Fernando, P.D.S.M.; Kang, H.K.; Yi, J.M.; Hyun, J.W. Hesperidin exhibits protective effects against PM2. 5-mediated mitochondrial damage, cell cycle arrest, and cellular senescence in human HaCaT keratinocytes. Molecules 2022, 27, 4800. [Google Scholar] [CrossRef] [PubMed]
- Zhen, A.X.; Piao, M.J.; Hyun, Y.J.; Kang, K.A.; Madushan Fernando, P.D.S.; Cho, S.J.; Ahn, M.J.; Hyun, J.W. Diphlorethohy droxycarmalol attenuates fine particulate matter-induced subcellular skin dysfunction. Mar. Drugs 2019, 17, 95. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Sobenin, I.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. Mitochondrial aging and age-related dysfunction of mitochondria. Biomed. Res. Int. 2014, 2014, 238463. [Google Scholar] [CrossRef]
- Kwon, K.R.; Alam, M.B.; Park, J.H.; Kim, T.H.; Lee, S.H. Attenuation of UVB-induced photo-aging by polyphenolic-rich spathol obus suberectus stem extract via modulation of MAPK/AP-1/MMPs signaling in human keratinocytes. Nutrients 2019, 11, 1341. [Google Scholar] [CrossRef]
- Lee, Y.H.; Seo, E.K.; Lee, S.T. Skullcapflavone II inhibits degradation of type I collagen by suppressing MMP-1 transcription in human skin fibroblasts. Int. J. Mol. Sci. 2019, 20, 2734. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, W.; Wu, J.; Qiu, S.; Yuan, S.; Fu, P.L.; Qian, Q.R.; Xu, Y.Z. Ubiquitin-specific protease 3 attenuates interleukin-1β-mediated chondrocyte senescence by deacetylating forkhead box O-3 via sirtuin-3. Bioengineered 2022, 13, 2017–2027. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of cellular senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef]
- Kim, H.; Woo, S.M.; Choi, W.R.; Kim, H.; Yi, C.; Kim, K.; Cheng, J.; Yang, S.H.; Suh, J. Scopoletin downregulates MMP-1 expression in human fibroblasts via inhibition of p38 phosphorylation. Int. J. Mol. Med. 2018, 42, 2285–2293. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Kim, J.; Jung, S.; Sung, K.H.; Son, Y.K.; Bae, J.M.; Kim, B.H. Alleviation of ultraviolet B-induced photoaging by 7-MEGATM 500 in hairless mouse skin. Toxicol. Res. 2019, 35, 353–359. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhen, A.-X.; Piao, M.-J.; Kang, K.-A.; Fernando, P.-D.-S.-M.; Herath, H.-M.-U.-L.; Cho, S.-J.; Hyun, J.-W. 3-Bromo-4,5-dihydroxybenzaldehyde Protects Keratinocytes from Particulate Matter 2.5-Induced Damages. Antioxidants 2023, 12, 1307. https://doi.org/10.3390/antiox12061307
Zhen A-X, Piao M-J, Kang K-A, Fernando P-D-S-M, Herath H-M-U-L, Cho S-J, Hyun J-W. 3-Bromo-4,5-dihydroxybenzaldehyde Protects Keratinocytes from Particulate Matter 2.5-Induced Damages. Antioxidants. 2023; 12(6):1307. https://doi.org/10.3390/antiox12061307
Chicago/Turabian StyleZhen, Ao-Xuan, Mei-Jing Piao, Kyoung-Ah Kang, Pincha-Devage-Sameera-Madushan Fernando, Herath-Mudiyanselage-Udari-Lakmini Herath, Suk-Ju Cho, and Jin-Won Hyun. 2023. "3-Bromo-4,5-dihydroxybenzaldehyde Protects Keratinocytes from Particulate Matter 2.5-Induced Damages" Antioxidants 12, no. 6: 1307. https://doi.org/10.3390/antiox12061307
APA StyleZhen, A.-X., Piao, M.-J., Kang, K.-A., Fernando, P.-D.-S.-M., Herath, H.-M.-U.-L., Cho, S.-J., & Hyun, J.-W. (2023). 3-Bromo-4,5-dihydroxybenzaldehyde Protects Keratinocytes from Particulate Matter 2.5-Induced Damages. Antioxidants, 12(6), 1307. https://doi.org/10.3390/antiox12061307




