Abstract
WRKY transcription factors (TFs) participate in plant defense mechanisms against biological and abiotic stresses. However, their regulatory role in heat resistance is still unclear in non-heading Chinese cabbage. Here, we identified the WRKY-IIe gene BcWRKY22(BraC09g001080.1), which is activated under high temperatures and plays an active role in regulating thermal stability, through transcriptome analysis. We further discovered that the BcWRKY22 protein is located in the nucleus and demonstrates transactivation activity in both the yeast and plant. Additionally, our studies showed that the transient overexpression of BcWRKY22 in non-heading Chinese cabbage activates the expression of catalase 2 (BcCAT2), enhances CAT enzyme activity, and reduces Hydrogen Peroxide (H2O2) accumulation under heat stress conditions. In addition, compared to its wild-type (WT) counterparts, Arabidopsis thaliana heterologously overexpresses BcWRKY22, improving thermotolerance. When the BcWRKY22 transgenic root was obtained, under heat stress, the accumulation of H2O2 was reduced, while the expression of catalase 2 (BcCAT2) was upregulated, thereby enhancing CAT enzyme activity. Further analysis revealed that BcWRKY22 directly activates the expression of BcCAT2 (BraC08g016240.1) by binding to the W-box element distributed within the promoter region of BcCAT2. Collectively, our findings suggest that BcWRKY22 may serve as a novel regulator of the heat stress response in non-heading Chinese cabbage, actively contributing to the establishment of thermal tolerance by upregulating catalase (CAT) activity and downregulating H2O2 accumulation via BcCAT2 expression.
1. Introduction
Heat stress, caused by high temperatures, is a widely concerning agricultural problem. Short-term or sustained high temperatures can lead to physiological, morphological, and biochemical changes in plants, thus affecting a plant’s growth and development and causing sharp declines in economic yield. By using various genetic methods to develop crop plants with improved heat resistance, the adverse effects of heat stress can be mitigated [1]. Numerous vital cellular elements and metabolic processes involved in thermal responsive growth and the acquisition of heat resistance in plants have been identified through genetic, physiological, molecular, and biochemical research [2]. Plants develop defense mechanisms with which to cope with high temperatures, incorporating the buildup of heat shock proteins (HSPs) and the establishment of complex regulatory networks through transcription factors (TFs). Many mechanisms have developed in plants that exhibit extreme complexity and play essential roles in enhancing heat tolerance [1,3,4,5].
Elevated temperatures cause harm to plants through several mechanisms, such as inducing membrane damage, inactivating proteins, overproducing reactive oxygen species (ROS), and disrupting key metabolic functions [6,7]. ROS synthesis and clearance are balanced in healthy plants. However, under various stress conditions, this equilibrium is often disrupted, leading to the generation of excessive ROS and causing oxidative stress. This can be mitigated by ROS-detoxifying enzymes, such as catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX), peroxide-reducing protein (PRX), and glutathione peroxidase (GPX). Alternatively, antioxidants like ascorbic acid and glutathione can also help clear excess ROS [8,9,10,11,12]. Although some transcription factors, such as HsfA1a, directly detect hydrogen peroxide and control the heat shock response gene expression in plants, our understanding of the relationship between hydrogen peroxide and various transcription factors that control thermotolerance is limited [13,14].
WRKY TFs can participate in a variety of adverse reactions during plant growth and development, and play an essential role in maintaining stress resistance in plants [15,16,17]. Previous research on WRKYs has focused on their role in coping with drought, cold, salt stress, and nutrient deficiencies [18,19,20,21,22,23]. Research on the heat resistance of WRKY transcription factors is relatively limited. After exposure to 45 °C for different periods of time, wrky25 null mutants exhibited moderate heat sensitivity, lower germination rates, reduced hypocotyl and root growth, and increased electrical conductivity compared to their wild-type (WT) counterparts. In contrast, transgenic seeds overexpressing AtWRKY25 showed stronger heat resistance [24]. AtWRKY26, AtWRKY33, and AtWRKY25 positively regulate the co-operation between ethylene-activating proteins and heat-shock-protein-related signaling pathways in mediating the heat stress response in plants. These three proteins co-operate in a functional way to increase a plant’s ability to withstand heat [25]. The overexpression of LlWRKY22 in lilies enhanced their heat resistance and upregulated the expression of the heat-responsive gene LlDREB2B. Conversely, the downregulation of LlWRKY22 had the opposite effect [26]. LlWRKY39 can bind to the activation region of LlMBF1c and induce its expression, thereby enhancing the heat resistance of transgenic plants [27]. The positive regulation of the heat shock response (HSR) by AtWRKY39 involves the mediation of co-operative signaling pathways activated by salicylic acid and jasmonic acid [28]. A conserved WRKYGQK sequence and a zinc finger motif (CX45CX22-23HXH or CX7CX23HXC) are the distinguishing features of WRKY transcription factors, and WRKY proteins have the capacity to bind to the W-box (TGACC (A/T)), situated in the promoter region of their target genes, thereby regulating the downstream gene expression and modulating the stress response [15,29,30,31,32,33,34,35,36,37].
Non-heading Chinese cabbage (NHCC, Brassica campestris ssp. chinensis) is a significant vegetable that is grown extensively worldwide. High temperature is the main limiting factor for vegetable production, and non-heading Chinese cabbage does not grow well at high temperatures (over 25 °C) [38]. Exposure to high temperatures directly leads to a decrease in yield and affects the quality of the food, while heat stress also affects photosynthesis and can even affect downy mildew, soft rot, viral diseases, and other diseases [39].
In this study, a heat-induced member of the WRKY-IIe family, BcWRKY22(BraC09g001080.1), was isolated and identified from NHCC. According to subcellular localization studies, BcWRKY22 is situated in the nucleus, and BcWRKY22 exhibits activation in yeast. In addition, the transient overexpression of BcWRKY22 improved the heat tolerance of NHCC leaves, and the overexpression in the roots enhanced root heat tolerance. Moreover, Arabidopsis thaliana also exhibited increased heat tolerance when BcWRKY22 was overexpressed. Further analysis showed that BcWRKY22 directly activates the expression of BcCAT2(BraC08g016240.1), which improves CAT activity and reduces H2O2 content, thereby positively regulating thermotolerance.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
The cultivated variety of non-heading Chinese cabbage, ‘NHCC001’, was utilized in this experiment under a 16 h light/8 h darkness cycle at a temperature of 24 °C/18 °C with a humidity level of 55%. Arabidopsis thaliana (Col) and N. benthamiana were raised in potting medium within a growth room set to maintain a temperature of 24 °C, with a photoperiod lasting for 16 h.
2.2. Clone BcWRKY22 from NHCC001
Total RNA was isolated from the leaves of NHCC001 plants four weeks after germination. The NHCC’s total RNA was isolated using the RNAsimple Total RNA Kit (TIANGEN, Beijing, China) and reverse-transcribed with Hifair® III 1st strand cDNA synthesis superMIX for qPCR (11141ES60, Yeasen, Shanghai, China). A designed primer was used to amplify the open reading frame (ORF) of BcWRKY22. Primers for gene cloning were shown in Supplementary Table S1.
2.3. Subcellular Localization of BcWRKY22
To create the 35S:BcWRKY22-GFP construct, the coding region of BcWRKY22 was inserted into the pRI101-GFP vector. The reconstructed plasmid and empty vector were introduced into Agrobacterium GV3101, and individual colonies were grown in Luria broth with 50 µg·mL−1 rifampicin and 50 µg·mL−1 kanamycin supplemented at 28 °C for 20 h. Following PCR identification, the bacterial strains were cultured in 5 mL of Luria broth supplemented with 50 µg·mL−1 rifampicin and 50 µg·mL−1 kanamycin at 28 °C for 16–20 h. The bacterial solutions were then centrifuged, resuspended in infiltration medium (10 mM MgCl2, 10 mM MES, and 150 µM Acetosyringone; pH adjusted to 5.7), and incubated at room temperature for a minimum of four hours until they reached OD600 values between 0.8 and 1.0. Finally, the Agrobacterium solution was injected into the backside of the tobacco leaves using pressure osmosis. After a 48 h infiltration period, the leaves were excised and observed for GFP and RFP fluorescence using a laser scanning confocal microscope (LSM800, Zeiss, Oberkochen, Germany). Three independent replicate experiments were performed to obtain a representative image.
2.4. Transactivation Activity Assay of BcWRKY22 in Yeast
The open reading frame of BcWRKY22 was cloned into the pGBKT7 vector. The recombinant constructs, pGBKT7-BcWRKY22 and pGBKT7-GAL4, were altered into an AH109 yeast strain. Transformants were selected on SD medium lacking tryptophan at 28 °C for 3 days. Positive clones were screened on SD medium, without tryptophan and histidine, containing X-α-gal at 28 °C for 3 days. Three independent replicates were conducted to make the results representative. Supplementary Table S1 showed the primers used for vector construction.
2.5. Promoter Activity Analysis of BcWRKY22
The 2000 bp fragment of the BcWRKY22 promoter was isolated and ligated to a pGreenII-0800-LUC vector using recombinant ligase (Vazyme, Nanjing, China). The final construct and its control were transformed into A. tumefaciens strain GV3101 (pSoup) for infiltration into tobacco leaves. After 48 h, half of the infiltrated leaves underwent heat stress at 40 °C for 1.5 h, followed by recovery at 24 °C for 12 h. Subsequently, the fluorescence signal was detected using a plant living imaging system (Berthold, Stuttgart, Germany). The activities of firefly luciferase (LUC) and Renilla luciferase (REN) were measured using a luciferase reporter assay system (Yeasen, Shanghai, China). Three replicate experiments were conducted to obtain representative results. Supplementary Table S1 shows the primers used for vector construction.
2.6. Heat Stress Treatment of NHCC001
To assess gene expression levels in response to heat shock (HS), 4-week-old ‘NHCC001’ plants were subjected to a temperature of 40 °C for varying durations of 0, 1, 3, 6, 12, and 24 h, a set of experiments treated at room temperature for the same time under dark conditions as a parallel control. The heat treatments were administered in a light-free incubator (DRX-160B-LED, Ningbo, Nanjing, China). After the treatment, leaf samples were collected for qRT-PCR analysis. We subtracted the corresponding room temperature data from all heat treatment data, and then performed calculations according to the formula 2−ΔΔCT with 0 h as the basis. Three independent replicate experiments were used for statistical analysis via Student’s t-test. Supplementary Table S1 shows the primers used for vector construction.
2.7. Transient Overexpression Assay in NHCC001 Leaves
Bacterial cultures expressing pRI101-GFP or pRI101-BcWRKY22-GFP were resuspended using an infiltration buffer (10 mM MgCl2, 10 mM MES, and 150 µM Acetosyringone; pH adjusted to 5.7) and incubated at 24 °C for 4 h under dark conditions. NHCC001 was employed for transient overexpression. Subsequently, leaf discs, each with a diameter of 1 cm, were excised using a hole punch and infiltrated with bacterial solutions under negative pressure (−0.8 MPa, 30 min). The infiltrated discs were rinsed with sterile water and placed on 0.4% agar medium at 24 °C for 72 h. For heat shock treatment, the discs were exposed to 40 °C for 16 h. Heat stress can impact chlorophyll content, resulting in a reduction thereof. The extent of damage to chlorophyll is indicative of heat tolerance [40,41,42,43,44,45,46,47,48,49,50]. The activities of SOD and CAT enzymes were assessed following a 3 h heat treatment, while the levels of Malondialdehyde (MDA) and H2O2 were determined after a 16 h period. The SOD activities of treated and untreated leaves were determined with the NBT method using a Superoxide Dismutase (SOD) Activity Assay Kit (Solarbio, Beijing, China). Using the Titanium sulfate method, the H2O2 concentration was measured with a Hydrogen Peroxide (H2O2) Content Assay Kit (Solarbio, Beijing, China). A Malondialdehyde (MDA) Content Assay Kit (Solarbio, Beijing, China) was used with the TBA method to measure the MDA content of treated and untreated leaves, and the CAT activities of treated and untreated leaves were determined using the ultraviolet absorption method with a Catalase (CAT) Activity Assay Kit (Solarbio, Beijing, China). Three independent technical replicate experiments were conducted for each of the three biological replicates.
2.8. Agrobacterium-Mediated Transformation of Arabidopsis
The lack of a stable genetic transformation system for NHCC has hindered the acquisition of NHCC transgenic plants. Therefore, transgenic Arabidopsis plants were generated using the Agrobacterium-mediated floral dipping method [51]. The CDS of BcWRKY22 was cloned into the pRI101 vector and subsequently transformed into Agrobacterium GV3101 for recombinant plasmid construction.
The T0 seeds were examined on MS medium with timentin and hygromycin, and positive T1 lines were identified using PCR. Subsequent generations (T2/T3) were identified using the same methods, and the stable T3 generation was used to study BcWRKY22 function. Three independent replicate experiments were performed to assess reliable results. Supplementary Table S1 shows the primers used for vector construction.
2.9. Agrobacterium-rhizogenes-Mediated Transformation of Roots
Transgenic roots were obtained according to the methods reported by Chen [52]. The ‘NHCC001’ seeds were treated with 75% ethanol for 5 min and 10% sodium hypochlorite for 12 min, washed three times with ddH2O, and then placed on germination medium (1/2 MS, pH 5.8) and kept in a cultural space at 24 ± 2 °C under a light cycle of 16 h light and 8 h dark for 5–7 days (light intensity: 3000 lx). BcWRKY22 overexpression vector 2300-BcWRKY22 was introduced into the Agrobacterium rhizogenes MSU440 strain, which was developed overnight. The bacterial solution was then centrifuged at 6000 rpm for 10 min and the supernatant was abandoned. A co-culture liquid medium (MS-diluted tenfold (pH5.2) + acetosyringone (100 μM)) was added to make OD600 = 1.0 and placed at 28 °C and 200 rpm for three hours to form the infection medium. We removed the main roots of the seedling and soaked it in infection medium for 10 min. After washing it three times with ddH2O, we placed it in MS medium containing timentin (250 mg/L). The hairy roots grew out after 25–35 days and were used for subsequent experiments following identification. The H2O2 content, MDA content, and CAT activity of treated and untreated leaves were determined using a kit (Solarbio, Beijing, China). Following a 3 h heat treatment, the activity of CAT enzymes was assessed. Additionally, the levels of MDA and H2O2 were examined after a 16 h duration. With the titanium sulfate method, a Hydrogen peroxide (H2O2) Content Assay Kit (Solarbio, Beijing, China) was used to measure the H2O2 concentration; a Malondialdehyde (MDA) Content Assay Kit (Solarbio, Beijing, China) was used to measure the MDA content of treated and untreated leaves using the TBA method. Using ultraviolet absorption method, the CAT activity of treated and untreated leaves was determined using a microplate reader with a Catalase (CAT) Activity Assay Kit (Solarbio, Beijing, China). Supplementary Table S1 shows the primers used for vector construction. Each of the three biological replicates was subjected to three independent technical replicates.
2.10. Quantitative Real-Time PCR Analysis
The RNAsimple Total RNA Kit (TIANGEN, Beijing, China) was chosen for extracting total RNA from NHCC and Arabidopsis thaliana, which were subsequently reverse-transcribed using a Hifair® Ⅲ 1st strand cDNA synthesis superMIX for qPCR (11141ES60, Yeasen, Shanghai, China). The Hieff® qPCR SYBR Green Master Mix (11202ES03, Yeasen, Shanghai, China) was used for real-time quantification PCR on QuantStudio 5 (ABI, Los Angeles, CA, USA), with BcGAPC and AtActin as internal standards. Data analysis was performed using the 2−ΔΔCT method with three repeats. For each of the three biological replicates, three separate technical replicates were employed. Primers used for qRT-PCR are listed in Supplementary Table S1.
2.11. Y1H Assay
The BcCAT2 promoters were cloned into the pLacZi vector, while the BcWRKY22 ORF was fused into the pJG vector to create pJG-BcWRKY22. The empty pJG vector served as a negative control. Reconstructed vectors were co-transformed with Yeast EGY48 strain cells for a Y1H assay. Transformants were selected on Ura-/Trp-deficient SD medium at 28 °C, and binding activity was assessed through x-gal color change. To evaluate the reliability of the results, three independent replicate experiments were conducted. Supplementary Table S1 shows the primers used for vector construction, and the analysis of promoter elements was shown in Supplementary Table S2.
2.12. Electrophoretic Mobility Shift Assay (EMSA)
The open reading frame of BcWRKY22 was inserted into the pMAL-MBP vector and introduced into Escherichia coli Rosetta (DE3). Upon induction with isopropyl-β-D-thiogalactoside (IPTG), the MBP-BcWRKY22 fusion protein was generated. The EMSA probe primers were biotin-labeled.
Then, EMSA was conducted using the LightShift Chemiluminescent EMSA Kit (Beyotime, Shanghai, China). The LightShift Chemiluminescent EMSA Kit (Beyotime, Shanghai, China) was utilized to perform EMSA. Three independent replicate tests were conducted to evaluate a representative image. Supplementary Table S1 displays the primers employed for generating the EMSA probe.
2.13. Dual-Luciferase Reporter Assay
The BcWRKY22 ORF was subsequently inserted into the pRI101 vector, giving rise to the pRI101-BcWRKY22 effector vector, while the empty vector stood as a negative control. The BcCAT2 promoters were fused with the pGreenII0800-LUC vector to create LUC reporters, which were then introduced into A. tumefaciens GV3101 (pSoup) cells and injected into tobacco leaves with mixtures of bacteria that express various arrangements of reporters and effectors. The LUC signal was observed in the infiltrated leaves and measured 48 h later. The enzymatic activities of LUC and REN were determined using the Dual-Luciferase Reporter Gene Assay Kit (Yeasen, Shanghai, China). There were three distinct technical replicates used for each of the three biological replicates.
3. Results
3.1. A WRKY-IIe Member BcWRKY22 Transcript Increased in Heat-Tolerant Cultivar NHCC001 after Heat Treatment
The investigation of WRKY gene expression in the transcriptome (the transcriptome data have been determined by previous researchers and the data have been published (http://nhccdata.njau.edu.cn/ (accessed on 2 July 2021)) [39] of non-heading Chinese cabbage NHCC001 revealed that BcWRKY22 exhibited a differential expression in response to high-temperature conditions (Figure 1a). The cDNA sequence of BcWRKY22 contains an 897 bp Open Reading Frame (ORF) encoding 298 amino acid proteins. The phylogenetic tree containing all WRKY proteins in Arabidopsis thaliana suggests that BcWRKY22 belongs to the WRKY-IIe family and is closely related to AtWRKY22, AtWRKY29, and AtWRKY27 (Figure S1). Their sequences have similar features, including a WRKYGQK sequence and a C2H2 (C-X5-C-X23-H-X1-H) zinc-binding motif (Figure 1b).
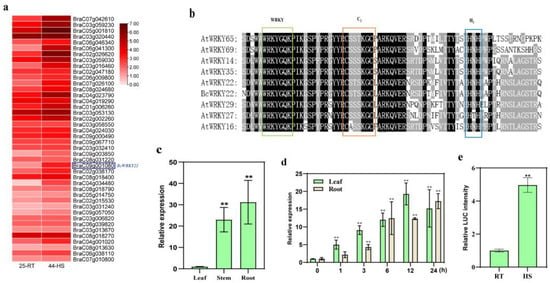
Figure 1.
The gene BcWRKY22 belongs to the WRKY-IIe subgroup and exhibits heat-inducible expression. (a) WRKY expression heat map in NHCC leaves under heat stress (HS). The heat map visualizes the expression pattern of WRKYs on the basis of the heat-treated transcriptome of NHCC leaves; the blue box indicates BcWRKY22; 25-RT: normal condition (25 °C); 44-HS: exposed to 44 °C for 4 h. (b) Multiple alignments of the conserved domains in the WRKY-IIe group. Analogous amino acids are tinted in gray, while identical amino acids are displayed with a black backdrop. The WRKY motif is exhibited with a green border, while the zinc-finger structures are depicted with orange and blue borders. (c) The expression of BcWRKY22 in NHCC leaves, stems, and roots. The values are expressed as the means ± SD of three replicates (Student’s t-test, ** p < 0.01). (d) The expression of BcWRKY22 in NHCC leaves and roots under heat stress (RT: 24 °C; HS: 40 °C) exposure of differing durations. Bars indicate means ± SD from three replicates (Student’s t-test, ** p < 0.01; all treatments compared with 0 h). (e) Quantitation of LUC intensity in tobacco leaves. The BcWRKY22 promoter activity was assessed using the LUC reporter assay in tobacco leaves under room temperature (24 °C) and heat stress (HS: 40 °C, 3 h; followed by recovery at 24 °C for 12 h). Bars indicate means ± SD from three replicates (Student’s t-test, ** p < 0.01).
In order to investigate the expression level of BcWRKY22 in various tissues, RT-qPCR was performed on the roots, stems, and leaves of NHCC001; the results showed that the expression levels of BcWRKY22 in the roots and stems were significantly higher than that in the leaves, and the expression level was the highest in the roots (Figure 1c). Meanwhile, to detect the BcWRKY22 expression under high-temperature conditions, NHCC001 plants were exposed to 40 °C for heat stress (HS) treatment (44 °C can cause irreversible damage to plants; below 40 °C, the heat damage phenotype is not obvious, and there is no significant difference in gene and indicator changes compared to 40 °C, so we used 40 °C instead of 44 °C for the transcriptome screening genes). Based on the RT-qPCR results, and compared with the treatment at 24 °C, BcWRKY22 expression was rapidly induced in the leaves after 1 h of HS and in the roots after 3 h, and both stayed at high levels even 24 h after HS (Figure 1d). Subsequently, the promoter activity of BcWRKY22 was analyzed using the LUC reporter assay. The results showed that the BcWRKY22 promoter-driven LUC reporter signal in tobacco leaves was significantly increased after HS treatment (Figure 1e). Thus, BcWRKY22 is a heat-responsive WRKY-IIe gene in NHCC001.
3.2. Localization in the Subcellular Region and Transactivation Activity of BcWRKY22
Through the analysis of a BcWRKY22-GFP fusion protein, the subcellular localization of BcWRKY22 was examined. The full-length ORF of BcWRKY22 was amplified to the N-terminal of the GFP reporter protein of the pRI101 vector, driven by the CaMV 35S promoter, generating a recombinant construct BcWRKY22-GFP. We used an RFP nuclear signaling peptide as a positive control. Laser confocal microscopy showed that BcWRKY22 fusion protein fluorescence overlaps with RFP fluorescence, suggesting that BcWRKY22 is located in the nucleus (Figure 2a,b). In addition, BcWRKY22 showed transactivation activity in yeast cells (Figure 2c).
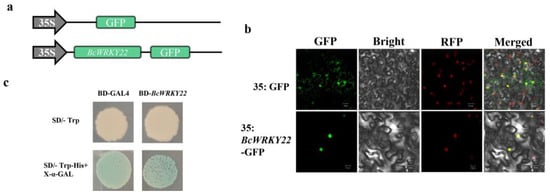
Figure 2.
Transactivation activation assay and subcellular localization of BcWRKY22. (a) The constructs for the subcellular localization assay. (b) Confocal microscopy was used to examine BcWRKY22 localization, which was found in the nucleus. BcWRKY22-GFP and the nuclear marker mCherry overlapped in the nucleus. Bars = 20 μm. (c) Transcriptional activation activity of BcWRKY22 in yeast. The transformants were selected on SD-W medium (devoid of Trp), while the culture of transformants was assessed on SD-WH medium (lacking Trp/His) supplemented with X-α-GAL.
3.3. BcWRKY22 Overexpression Increases the Thermotolerance of Non-Heading Chinese Cabbage
To investigate the function of BcWRKY22 in vivo, we overexpressed BcWRKY22 in NHCC001 leaves with a transient transformation assay. The RT-qPCR results showed that the expression level of BcWRKY22 in leaves was significantly higher than that in the control (Figure 3a). Then, after HS treatment, we found that the green degree of BcWRKY22 transiently transformed round hole leaves was higher than that in the control (Figure 3b). Interestingly, the H2O2 content of the overexpressed BcWRKY22 was lower at 24 °C than that of the control; meanwhile, after HS, the increased degree of H2O2 content in the BcWRKY22-overexpressed leaves was significantly lower than that of the control (Figure 3c). Additionally, the overexpression of BcWRKY22 in leaves had no impact on the MDA content or SOD activity at 24 °C. After HS, the value for MDA was significantly higher in the control leaves than in the BcWRKY22-overexpressed leaves, and the SOD activity was significantly lower in the control leaves than in the BcWRKY22-overexpressed leaves (Figure 3d,e). Furthermore, the CAT activity of the overexpressed BcWRKY22 was higher at 24 °C than in the control. After HS, the CAT activity of the BcWRKY22-overexpressed leaves increased more (Figure 3f). Therefore, we determined the expression level of BcCAT2, a gene related to catalase (CAT) activity, and the expression level of BcCAT2 in transient BcWRKY22-overexpressed leaves was noticeably higher than those of the control (Figure 3g). These results suggested that BcWRKY22 overexpression protects NHCC001 leaves from high-temperature stress and improves their heat resistance; moreover, BcWRKY22 may improve heat resistance by increasing CAT activity to reduce H2O2 content in NHCC001.
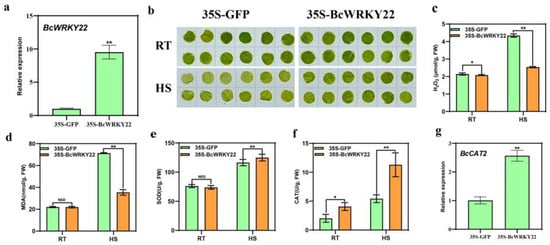
Figure 3.
BcWRKY22 transient expression positively regulates NHCC thermotolerance. (a) Relative expression levels of BcWRKY22 in transient transferred lines. Values are presented as the means ± SD of three replicates (Student’s t-test, ** p < 0.01); the 35S-BcWRKY22 compared with the control (35S-GFP). (b) The leaf disc phenotypes of NHCC were observed at room temperature (RT: 24 °C), and after exposure to heat stress (HS: 40 °C, 16 h). (c) H2O2 content determined at RT and under heat stress (HS: 40 °C, 16 h)). Values are presented as the means ± SD of three replicates (Student’s t-test, * p < 0.05; ** p < 0.01; the 35S-BcWRKY22 compared with the control (35S-GFP) at RT or under the HS condition). (d) The MDA content of discs at RT and under HS. The data are presented as the means ± SD of three repeated experiments (Student’s t-test, ** p < 0.01; NSD, no significant difference; the 35S-BcWRKY22 compared with the control (35S-GFP) at RT or under the HS condition). (e) The SOD content of discs at 24 °C (RT) and under HS (40 °C, 3 h). The data are represented as the means ± SD of three replicates (Student’s t-test, ** p < 0.01; NSD, no significant difference; the 35S-BcWRKY22 compared with the control (35S-GFP) at RT or under the HS condition). (f) The CAT content of discs at 24 °C (RT) and under HS (40 °C, 3 h). The data are presented as the means ± SD of three replicated experiments (Student’s t-test, * p < 0.05; ** p < 0.01; the 35S-BcWRKY22 compared with the control (35S-GFP) at RT or under the HS condition). (g) Relative expression levels of BcCAT2 in transient transferred lines. All data are the averages of three independent experiments; each error bar indicates mean ± SD from three replicates; ** p < 0.01 (Student’s t-test).
3.4. Overexpression of BcWRKY22 Increased the Thermotolerance of Transgenic Arabidopsis thaliana
We allogenically transformed the BcWRKY22 gene into Arabidopsis thaliana, and three homozygous T3 transgenic Arabidopsis lines were identified (Figure S2). Then, three transgenic lines (OE-1, OE-3, and OE-4) and a WT control were treated at 40 °C for 16 h and recovered for 3 days (Figure 4a). Under heat stress (HS) conditions, the WT control’s survival rate was noticeably lower than that of the three transgenic lines (Figure 4b). Meanwhile, the overexpression of BcWRKY22 in Arabidopsis thaliana did not affect the SOD activity or MDA content at 24 °C (RT). After HS, the SOD activity was significantly lower in the WT control than in those of the three transgenic lines, and the value for MDA was significantly higher in the WT control than in those of the three transgenic lines (Figure 4c,d). This outcome was consistent with the result of transient overexpression BcWRKY22, in that the H2O2 content values were lower at 24 °C in the three transgenic lines than in the WT control. After HS, the increased degree of H2O2 content values in the three transgenic lines was significantly lower than that of the WT (Figure 4e). Furthermore, the expression levels of HS-induced genes AtHSP101, AtGolS1, AtHSFA1, and AtDREB2A were significantly increased in transgenic Arabidopsis (Figure 4f). These results suggested that BcWRKY22 overexpression improved the heat tolerance of transgenic Arabidopsis.
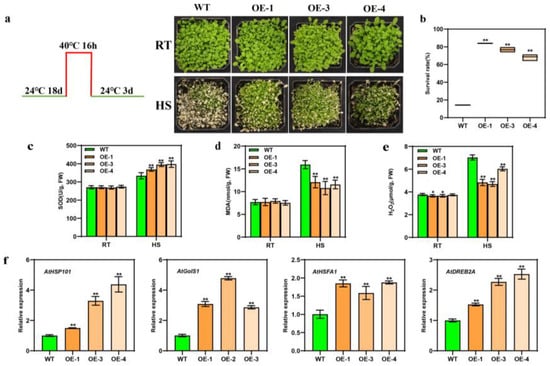
Figure 4.
Overexpressing BcWRKY22 enhances thermotolerance in Arabidopsis thaliana and upregulates the expression of heat-responsive genes. (a) Culture temperature and time setting for Arabidopsis thaliana, and the comparison between overexpressed Arabidopsis and WT phenotypes at RT (24 °C) and HS (40 °C, 16 h; restored for 3 days at 24 °C). (b) The survival rates of overexpressed Arabidopsis specimens and the WT control after HS (40 °C, 16 h) and restoration over 3 daysat 24 °C. Values are presented as the means ± SD of three replicates (Student’s t-test, ** p < 0.01). (c) The SOD content values of the WT control and the three transgenic lines at RT (24 °C) and under HS (40 °C, 3 h). The data are indicated as the means ± SD of three replicates (Student’s t-test, ** p < 0.01). (d) The MDA content values of the WT control and the three transgenic lines at RT (24 °C) and under HS (40 °C, 16 h). The data are displayed as the means ± SD of three replicates. (Student’s t-test, ** p < 0.01). (e) H2O2 content values of the WT control and the three transgenic lines, determined at 24 °C (room temperature (RT)) and under heat stress (HS, 40 °C, 16 h)). The data are presented as the means ± SD of three replicates (Student’s t-test, * p < 0.05; ** p < 0.01). (f) Relative expressions of AtHSP101, AtGolS1, AtHSFA1, and AtDREB2A in the three transgenic lines and the WT control. All data are represented as the means ± SD of three replicates (Student’s t-test, ** p < 0.01).
3.5. BcWRKY22 Expression in NHCC Roots Increased the Thermotolerance of Roots
Since the expression of BcWRKY22 was at its highest in roots (Figure 1c), we obtained transgenic roots using Agrobacterium rhizogenes in NHCC, and the GFP fluorescence of roots was observed with LUYOR 3415, a fluorescent protein excitation source, to ensure that BcWRKY22-overexpressed roots were obtained (Figure 5a). An RT-qPCR analysis showed that BcWRKY22 was overexpressed in the three root systems (OE-BcWRKY22-1, OE-BcWRKY22-2, and OE-BcWRKY22-3), and their gene expression levels were higher than that of the WT control (Figure 5b). Then, we treated the WT control and the three overexpressed roots with heat stress (HS). The overexpression of BcWRKY22 in the three root systems did not affect their MDA content values at 24 °C, while the values of MDA were significantly higher in the WT control than in the BcWRKY22-overexpressed roots under HS (Figure 5c). Meanwhile, we found that the H2O2 content values of the three transgenic roots were lower than that of the WT control at 24 °C, and the increased degree of H2O2 content values in the three transgenic roots was markedly lower than that of the WT control after HS (Figure 5d). This conclusion is compatible with the results of the transient expression and overexpression in Arabidopsis thaliana, indicating that the overexpression of BcWRKY22 can affect the content of H2O2. Furthermore, the CAT activity of the BcWRKY22-overexpressed root systems was higher at 24 °C than that of the WT control. After HS, compared with the WT control, the increase in CAT activity in the BcWRKY22-overexpressed roots was significantly upregulated (Figure 5e). In addition, the expression of BcCAT2 was significantly increased in the BcWRKY22-overexpressed roots (Figure 5f). In order to further confirm that the overexpression of BcWRKY22 can improve the thermotolerance of roots, we determined the expression levels of the heat response genes BcDREB2A, BcHSFA2, and BcGolS1. Their expression levels increased significantly in transgenic roots. Moreover, after HS, the expression of the three genes increased even more (Figure 5g). In summary, these results suggest that BcWRKY22 overexpression improves the thermotolerance of transgenic roots. At the same time, BcWRKY22 may regulate the expression level of BcCAT2 to improve CAT activity and reduce the accumulation of H2O2.
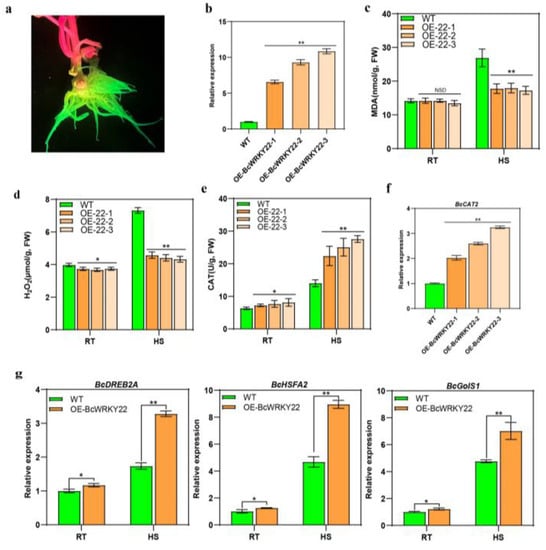
Figure 5.
Overexpression of BcWRKY22 in NHCC roots enhance heat resistance and induces the upregulation of genes associated with heat response. (a) LUYOR-3415-observed GFP fluorescence photograph of the NHCC roots. (b) The expression of BcWRKY22 in the three overexpressed root systems and in the WT control. The error bars indicate the means ± SD from three repetitions (Student’s t-test, ** p < 0.01). (c) The MDA content values in the WT control and the three overexpressed root systems at RT (24 °C) and under HS (40 °C, 16 h). The values are presented as the means ± SD of three repetitions (Student’s t-test, ** p < 0.01; NSD, no significant difference). (d) H2O2 content values of the WT control and the three overexpressed root systems were determined at 24 °C (room temperature (RT) and under heat stress (HS, 40 °C, 16 h)). Values are represented as the means ± SD of three repeated experiments (Student’s t-test, * p < 0.05; ** p < 0.01). (e) The CAT content values of the three overexpressed root systems and the WT control at RT (24 °C) and under HS (40 °C, 3 h). The data are represented as the means ± SD of three repeated experiments (Student’s t-test, * p < 0.05; ** p < 0.01). (f) Relative expression level of BcCAT2 in the three overexpressed root systems and the WT control. All data are the averages of three independent experiments, and error bars indicate the means ± SD from three replicates, ** p < 0.01 (Student’s t-test). (g) BcDREB2A, BcHSFA2, and BcGolS1 relative expression levels in the three overexpressed root systems and the WT control. (RT, 24 °C; HS, 40 °C). All data are the averages of three independent experiments, and error bars indicate the means ± SD from three replicates (Student’s t-test, * p < 0.05; ** p < 0.01).
3.6. BcWRKY22 Binds to the Promoter of BcCAT2 and Activates Its Expression
Based on our findings that BcCAT2 being activated in both BcWRKY22-overexpressed NHCC leaves and BcWRKY22-overexpressed transgenic roots (Figure 3g and Figure 5f), we speculated that BcWRKY22 might increase CAT activity and reduce H2O2 accumulation by regulating the expression level of BcCAT2. Meanwhile, we found that BcCAT2 had two common W-boxes in its promoter (Figure 6a). A yeast one-hybrid (Y1H) assay showed that BcWRKY22 could strongly activate 3 × W-box-2; however, the activation ability of 3 × W-box-1 is weak (Figure 6b). Therefore, we chose W-box-2 for the “Electrophoretic mobility shift assay” (EMSA). The EMSA results also revealed that MBP-BcWRKY22 could bind to the W-box-2 tandem element of the BcCAT2 promoter, while adding W-box-2, mutated from AGTCAA to AAAAAA, would not affect the binding, thus suggesting that BcWRKY22 may play a direct regulatory role in the expression of BcCAT2 (Figure 6c). In addition, we constructed vectors for a dual-luciferase reporter assay (Figure 6d); in this experiment, we used the full-length promoter of BcCAT2. Effector reporter tests showed a lower LUC signal in the leaves of N. benthamiana co-transformed with the control combination, but a significantly increased LUC signal and a significantly higher LUC/REN ratio after co-transformation with BcWRKY22 and proBcCAT2-LUC (Figure 6e,f). Taken together, these results suggest that BcWRKY22 can directly activate BcCAT2 by binding to its promoter.
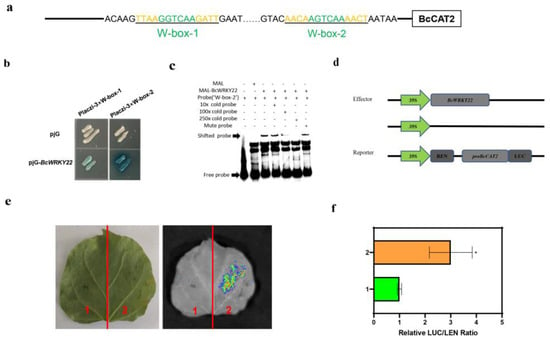
Figure 6.
BcWRKY22 activated BcCAT2 expression directly. (a) The BcCAT2 promoter is shown in this diagram, in which the W-box elements are highlighted in green. Black underlining indicates truncated fragments for the yeast one-hybrid (Y1H) assay. (b) A Y1H assay for BcWRKY22 and the fragments of the BcCAT2 promoter. A color change on Ura-/Trp-deficient SD medium was used to measure fragment activity following the addition of x-gal. Based on three replications, this is a representative image. (c) EMSA was employed to identify the direct interaction between BcWRKY22 and the W-box-2 region within the BcCAT2 promoter. The EMSA of the biotin-labeled oligonucleotide was derived from the putative BcWRKY22 binding site of the BcCAT2 promoter in the presence or absence of a cold competitor and mutated probe. Crude BcWRKY22 protein (3 μg) was incubated with 100 nM biotin-labeled probes. To assess the competition, cold competitor and mutated probes were added at different concentrations (10×, 100×, and 250×) during the experiment. Presence (+) or absence (−) of the components is indicated at the top. (d) A dual-luciferase reporter assay’s supporting constructs. (e) Finding the LUC signal in tobacco leaves. Based on three experiments, this is a representative image. 1: pro-BcCAT2+pRI101 empty; 2: pro-BcCAT2+pRI101-BcWRKY22. (f) Measurement of LUC intensity in the dual-luciferase reporter assay. Values are represented as the means ± SD of three replications (Student’s t-test, * p < 0.05).
4. Discussion
Previous research found that the AtWRKY22 transcription factor may be involved in multiple regulatory pathways, which play important roles in regulating the growth and development of Arabidopsis thaliana. Dark-treated AtWRKY22 overexpression and knockout lines caused age-related genes, and increased and decreased expression levels, which led to accelerated and delayed aging phenotypes, respectively [53]. Meanwhile, in coping with biostress, AtWRKY22 not only acts downstream of the flagellin receptor FLS2 and participates in activating the MAPK cascade and confers resistance against bacterial and fungal pathogens [54], but also enhances the susceptibility to aphids and modulates the signaling pathways of salicylic acid and jasmonic acid [55]. Additionally, among other species, NbWRKY22 and NbWRKY25 fully activate immunity against bacterial patterns, effectors, and non-bacterial defense triggers [56], while overexpressed LlWRKY22 in lily induced the expression of heat-related gene LlDREB2B, thereby enhancing heat tolerance [26]. However, the role of BcWRKY22 in NHCC remains unexplored. We identified BcWRKY22, a member of the WRKY-IIe subgroup with a classical WRKY domain. Its expression is induced by high temperature, and it exerts a function in the nucleus in response to HS. Transcriptomic analysis revealed that BcWRKY22 is a heat-responsive gene in NHCC. Furthermore, we observed a significant upregulation of both AtDREB2A and BcDREB2A subsequent to the overexpression of BcWRKY22 (Figure 4f and Figure 5g). BcWRKY22 has the potential to enhance heat resistance by regulating BcDREB2A in NHCC. Meanwhile, we observed that the overexpression of BcWRKY22 in NHCC cells resulted in an increased activity of the CAT enzyme (Figure 3f and Figure 5e), indicating its potential to mitigate H2O2 toxicity and enhance heat resistance by modulating CAT activity.
Our findings that heat shock induces the expression of BcWRKY22 suggests that BcWRKY22 may be involved in regulating the heat shock response (HSR) (Figure 1). Therefore, we elucidated the role of BcWRKY22 in HSR. The overexpression of BcWRKY22 enhanced the thermotolerance of NHCC leaves and roots, as well as those of Arabidopsis (Figure 3, Figure 4 and Figure 5). Several heat shock response genes, including AtHSFA1, AtDREB2A, AtHSP101, and AtGolS1, exhibited significant upregulation in the transgenic line (Figure 4). Similarly, BcHSFA1, BcDREB2A, and BcGolS1 were also significantly upregulated in transgenic roots (Figure 5). Given their active roles in enhancing heat tolerance mechanisms within plants, it is plausible that the increased expression of these genes may contribute to an overall improvement in thermal stress resistance [57,58,59,60,61,62,63,64,65]. At the same time, the MDA content, H2O2 content, and CAT enzyme activity are also important indicators of heat resistance in plants [57,66,67,68,69,70,71,72,73,74,75]. The overexpression of BcWRKY22 in plants demonstrated an enhancement in heat resistance through the observed physiological changes.
In plants, reactive oxygen species (ROS) are considered a pivotal signaling component for the crosstalk between biological and abiotic stress responses. Among the ROS, hydrogen peroxide (H2O2) stands out as the most stable one, acting as a crucial signaling molecule in defense responses against pathogens and abiotic stresses such as heat [76,77,78,79]. Previous research claimed that reduced activity of the antioxidant enzymes, particularly catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidases (GXP), also contributes to the increased buildup of ROS in plant cells [80]. We observed that HS induced increased cellular H2O2 levels; however, NHCC leaves and roots, and Arabidopsis with BcWRKY22 overexpression, exhibited the reduced accumulation of H2O2 (Figure 3c, Figure 4e, and Figure 5d). In Arabidopsis, a series of tiny proteins called CAT1, CAT2, and CAT3 are produced by the CAT gene. These proteins catalyze the clearing of hydrogen peroxide (H2O2) and are crucial for controlling ROS homeostasis. Specifically, under various environmental stresses, CAT2 is a crucial participant in H2O2 removal [81,82,83,84]. Interestingly, in NHCC leaves and roots overexpressing BcWRKY22, we observed an enhancement in CAT activity. Moreover, under HS conditions, the overexpressed tissues exhibited higher levels of CAT enzyme activity to effectively eliminate accumulated H2O2 than did the control (Figure 3f and Figure 5e). Meanwhile, the upregulation of BcCAT2 gene expression, which governs CAT enzyme activity, was significantly enhanced in both the overexpressed leaves and the overexpressed roots (Figure 3g and Figure 5f). Although the regulation of CAT genes by other WRKY members in response to HS is unknown, our research demonstrates that BcWRKY22 directly stimulates the expression of BcCAT2 by binding to its promoter, suggesting a possible regulatory pathway involving HS-BcWRKY22-BcCAT2-CAT-H2O2.
5. Conclusions
In conclusion, our findings demonstrate that the activation of BcWRKY22, a member of the WRKY-IIe family, under heat stress conditions leads to the increased activity of the CAT enzyme through the upregulation of the expression of BcCAT2, a key regulator for CAT enzyme activity. This ultimately results in scavenging ROS being generated due to heat stress and enhancing the thermotolerance capacity of NHCC. The explanation of this mechanism can serve as a reference for the impact of antioxidant enzymes on plant heat tolerance, and also provide guidance for breeders in selecting heat-resistant crops, thereby addressing the issue of low yield in summer Chinese cabbage cultivation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox12091710/s1. Figure S1: A phylogenetic tree constructed based on WRKYs of Arabidopsis thaliana and BcWRKY22; Figure S2: Identification of BcWRKY22 overexpressed Arabidopsis DNA; Table S1: The primer list used in this article; Table S2: Analysis of the BcCAT2 promoter elements by PlantCare.
Author Contributions
Conceptualization, X.H.; formal analysis, H.W.; investigation, Z.G. and X.C.; data curation, X.H.; writing—original draft preparation, H.W. and Z.G.; writing-review and editing, X.H. and C.Z.; visualization, X.C. and E.L.; funding acquisition, Y.L., C.Z. and X.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Plan, 2022YFD1200502, and supported by “the Fundamental Research Funds for the Central Universities, CGPY2023009”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ohama, N.; Sato, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional Regulatory Network of Plant Heat Stress Response. Trends Plant. Sci. 2017, 22, 53–65. [Google Scholar] [CrossRef]
- Li, B.; Gao, K.; Ren, H.; Tang, W. Molecular mechanisms governing plant responses to high temperatures. J. Integr. Plant. Biol. 2018, 60, 757–779. [Google Scholar] [CrossRef]
- Mishra, R.C.; Grover, A. ClpB/Hsp100 proteins and heat stress tolerance in plants. Crit. Rev. Biotechnol. 2016, 36, 862–874. [Google Scholar] [CrossRef]
- Friedrich, T.; Oberkofler, V.; Trindade, I.; Altmann, S.; Brzezinka, K.; Lämke, J.; Gorka, M.; Kappel, C.; Sokolowska, E.; Skirycz, A.; et al. Heteromeric HSFA2/HSFA3 complexes drive transcriptional memory after heat stress in Arabidopsis. Nat. Commun. 2021, 12, 3426. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Z.; Wang, L.; Jin, B. Plant Responses to Heat Stress: Physiology, Transcription, Noncoding RNAs, and Epigenetics. Int. J. Mol. Sci. 2020, 22, 117. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A. Physiological implications of metabolite biosynthesis for net assimilation and heat-stress tolerance of sugarcane (Saccharum officinarum) sprouts. J. Plant Res. 2007, 120, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Iba, K. Acclimative response to temperature stress in higher plants: Approaches of gene engineering for temperature tolerance. Annu. Rev. Plant. Biol. 2002, 53, 225–245. [Google Scholar] [CrossRef]
- Salvi, P.; Kamble, N.U.; Majee, M. Stress-Inducible Galactinol Synthase of Chickpea (CaGolS) is Implicated in Heat and Oxidative Stress Tolerance Through Reducing Stress-Induced Excessive Reactive Oxygen Species Accumulation. Plant Cell Physiol. 2018, 59, 155–166. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2018, 162, 2–12. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant. Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Morales, J.; Kadota, Y.; Zipfel, C.; Molina, A.; Torres, M.A. The Arabidopsis NADPH oxidases RbohD and RbohF display differential expression patterns and contributions during plant immunity. J. Exp. Bot. 2016, 67, 1663–1676. [Google Scholar] [CrossRef]
- Shi, X.; Jiang, F.; Wen, J.; Wu, Z. Overexpression of Solanum habrochaites microRNA319d (sha-miR319d) confers chilling and heat stress tolerance in tomato (S. lycopersicum). BMC Plant Biol. 2019, 19, 214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, X.C.; Cao, J.J.; Yin, L.L.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Heat Shock Factor HsfA1a Is Essential for R Gene-Mediated Nematode Resistance and Triggers H2O2 Production. Plant Physiol. 2018, 176, 2456–2471. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ma, S.; Ye, N.; Jiang, M.; Cao, J.; Zhang, J. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Kumar, S.R.; Dwivedi, V.; Rai, A.; Pal, S.; Shasany, A.K.; Nagegowda, D.A. A WRKY transcription factor from Withania somnifera regulates triterpenoid withanolide accumulation and biotic stress tolerance through modulation of phytosterol and defense pathways. New Phytol. 2017, 215, 1115–1131. [Google Scholar] [CrossRef]
- Wani, S.H.; Anand, S.; Singh, B.; Bohra, A.; Joshi, R. WRKY transcription factors and plant defense responses: Latest discoveries and future prospects. Plant Cell Rep. 2021, 40, 1071–1085. [Google Scholar] [CrossRef]
- Luo, X.; Li, C.; He, X.; Zhang, X.; Zhu, L. ABA signaling is negatively regulated by GbWRKY1 through JAZ1 and ABI1 to affect salt and drought tolerance. Plant Cell Rep. 2020, 39, 181–194. [Google Scholar] [CrossRef]
- Zhu, D.; Hou, L.; Xiao, P.; Guo, Y.; Deyholos, M.K.; Liu, X. VvWRKY30, a grape WRKY transcription factor, plays a positive regulatory role under salinity stress. Plant Sci. 2019, 280, 132–142. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, T.; Lin, Z.; Gu, B.; Xing, C.; Zhao, L.; Dong, H.; Gao, J.; Xie, Z.; Zhang, S.; et al. A WRKY transcription factor PbrWRKY53 from Pyrus betulaefolia is involved in drought tolerance and AsA accumulation. Plant Biotechnol. J. 2019, 17, 1770–1787. [Google Scholar] [CrossRef]
- Yan, J.Y.; Li, C.X.; Sun, L.; Ren, J.Y.; Li, G.X.; Ding, Z.J.; Zheng, S.J. A WRKY Transcription Factor Regulates Fe Translocation under Fe Deficiency. Plant Physiol. 2016, 171, 2017–2027. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Jiang, W.; Yu, D. Male gametophyte-specific WRKY34 transcription factor mediates cold sensitivity of mature pollen in Arabidopsis. J. Exp. Bot. 2010, 61, 3901–3914. [Google Scholar] [CrossRef]
- Kasajima, I.; Ide, Y.; Yokota Hirai, M.; Fujiwara, T. WRKY6 is involved in the response to boron deficiency in Arabidopsis thaliana. Physiol. Plant 2010, 139, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fu, Q.; Huang, W.; Yu, D. Functional analysis of an Arabidopsis transcription factor WRKY25 in heat stress. Plant Cell Rep. 2009, 28, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fu, Q.; Chen, L.; Huang, W.; Yu, D. Arabidopsis thaliana WRKY25, WRKY26, and WRKY33 coordinate induction of plant thermotolerance. Planta 2011, 233, 1237–1252. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, T.; Cao, X.; Zhang, D.; Teng, N. Lily WRKY factor LlWRKY22 promotes thermotolerance through autoactivation and activation of LlDREB2B. Hortic. Res. 2022, 9, uhac186. [Google Scholar] [CrossRef]
- Ding, L.; Wu, Z.; Teng, R.; Xu, S.; Cao, X.; Yuan, G.; Zhang, D.; Teng, N. LlWRKY39 is involved in thermotolerance by activating LlMBF1c and interacting with LlCaM3 in lily (Lilium longiflorum). Hortic. Res. 2021, 8, 36. [Google Scholar] [CrossRef]
- Li, S.; Zhou, X.; Chen, L.; Huang, W.; Yu, D. Functional characterization of Arabidopsis thaliana WRKY39 in heat stress. Mol. Cells 2010, 29, 475–483. [Google Scholar] [CrossRef]
- Khoso, M.A.; Hussain, A.; Ritonga, F.N.; Ali, Q.; Channa, M.M.; Alshegaihi, R.M.; Meng, Q.; Ali, M.; Zaman, W.; Brohi, R.D.; et al. WRKY transcription factors (TFs): Molecular switches to regulate drought, temperature, and salinity stresses in plants. Front. Plant Sci. 2022, 13, 1039329. [Google Scholar] [CrossRef]
- Huang, J.; Liu, F.; Chao, D.; Xin, B.; Liu, K.; Cao, S.; Chen, X.; Peng, L.; Zhang, B.; Fu, S.; et al. The WRKY Transcription Factor OsWRKY54 Is Involved in Salt Tolerance in Rice. Int. J. Mol. Sci. 2022, 23, 11999. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, J.; Li, Y.; Song, L.; Chen, D.; Liu, L.; Jiang, C.Z. A WRKY Protein, MfWRKY40, of Resurrection Plant Myrothamnus flabellifolia Plays a Positive Role in Regulating Tolerance to Drought and Salinity Stresses of Arabidopsis. Int. J. Mol. Sci. 2022, 23, 8145. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, H.; Liu, M.; Chen, B.; Dong, N.; Chang, X.; Wang, J.; Xing, S.; Peng, H.; Zha, L.; et al. Transcriptome-wide identification of WRKY transcription factors and their expression profiles in response to methyl jasmonate in Platycodon grandiflorus. Plant. Signal. Behav. 2022, 17, 2089473. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Luan, Y.; Meng, J.; Sun, J.; Tao, J.; Zhao, D. WRKY Transcription Factor Response to High-Temperature Stress. Plants 2021, 10, 2211. [Google Scholar] [CrossRef]
- Hussain, A.; Noman, A.; Khan, M.I.; Zaynab, M.; Aqeel, M.; Anwar, M.; Ashraf, M.F.; Liu, Z.; Raza, A.; Mahpara, S.; et al. Molecular regulation of pepper innate immunity and stress tolerance: An overview of WRKY TFs. Microb. Pathog. 2019, 135, 103610. [Google Scholar] [CrossRef] [PubMed]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant. Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Ulker, B.; Somssich, I.E. WRKY transcription factors: From DNA binding towards biological function. Curr. Opin. Plant Biol. 2004, 7, 491–498. [Google Scholar] [CrossRef]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Liu, G.; Xia, Y.; Liu, T.; Dai, S.; Hou, X. The DNA Methylome and Association of Differentially Methylated Regions with Differential Gene Expression during Heat Stress in Brassica rapa. Int. J. Mol. Sci. 2018, 19, 1414. [Google Scholar] [CrossRef]
- Song, X.; Liu, G.; Huang, Z.; Duan, W.; Tan, H.; Li, Y.; Hou, X. Temperature expression patterns of genes and their coexpression with LncRNAs revealed by RNA-Seq in non-heading Chinese cabbage. BMC Genom. 2016, 17, 297. [Google Scholar] [CrossRef]
- Zhu, H.; Wu, Y.; Zheng, Y. Effects of heat shock on photosynthesis-related characteristics and lipid profile of Cycas multipinnata and C. panzhihuaensis. BMC Plant Biol. 2022, 22, 442. [Google Scholar] [CrossRef]
- Bi, A.; Wang, T.; Wang, G.; Zhang, L.; Wassie, M.; Amee, M.; Xu, H.; Hu, Z.; Liu, A.; Fu, J.; et al. Stress memory geneFaHSP17.8-CII controls thermotolerance via remodeling PSII and ROS signaling in tall fescue. Plant Physiol. 2021, 187, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Lin, J.; Liu, J.Z.; Wang, X.; Lim, W.; Oh, M.; Park, J.; Rajashekar, C.B.; Whitham, S.A.; Cheng, N.H.; et al. Ectopic expression of Arabidopsis glutaredoxin AtGRXS17 enhances thermotolerance in tomato. Plant Biotechnol. J. 2012, 10, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Amano, M.; Iida, S.; Kosuge, K. Comparative studies of thermotolerance: Different modes of heat acclimation between tolerant and intolerant aquatic plants of the genus Potamogeton. Ann. Bot. 2012, 109, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, G.; Zhang, H.; Zhang, Y.; Zhang, Y.; Duan, S.; Sheteiwy, M.S.A.; Zhang, H.; Shao, H.; Guo, X. TaHsfA2-1, a new gene for thermotolerance in wheat seedlings: Characterization and functional roles. J. Plant Physiol. 2020, 246–247, 153135. [Google Scholar] [CrossRef]
- Chaudhary, S.; Devi, P.; Bhardwaj, A.; Jha, U.C.; Sharma, K.D.; Prasad, P.V.V.; Siddique, K.H.M.; Bindumadhava, H.; Kumar, S.; Nayyar, H. Identification and Characterization of Contrasting Genotypes/Cultivars for Developing Heat Tolerance in Agricultural Crops: Current Status and Prospects. Front. Plant Sci. 2020, 11, 587264. [Google Scholar] [CrossRef]
- Tsai, W.A.; Sung, P.H.; Kuo, Y.W.; Chen, M.C.; Jeng, S.T.; Lin, J.S. Involvement of microRNA164 in responses to heat stress in Arabidopsis. Plant Sci. 2023, 329, 111598. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.M.; Kim, K.M.; Lee, I.J. Thermotolerance effect of plant growth-promoting Bacillus cereus SA1 on soybean during heat stress. BMC Microbiol. 2020, 20, 175. [Google Scholar] [CrossRef]
- Burke, J.J.; O’Mahony, P.J.; Oliver, M.J. Isolation of Arabidopsis mutants lacking components of acquired thermotolerance. Plant Physiol. 2000, 123, 575–588. [Google Scholar] [CrossRef]
- Wang, G.; Cai, G.; Xu, N.; Zhang, L.; Sun, X.; Guan, J.; Meng, Q. Novel DnaJ Protein Facilitates Thermotolerance of Transgenic Tomatoes. Int. J. Mol. Sci. 2019, 20, 367. [Google Scholar] [CrossRef]
- Bhusal, N.; Sharma, P.; Sareen, S.; Sarial, A.K. Mapping QTLs for chlorophyll content and chlorophyll fluorescence in wheat under heat stress. Biologia Plantarum 2018, 62, 721–731. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, Y.; Yu, Z.; Gao, Z.; Ding, Q.; Shah, S.H.A.; Lin, W.; Li, Y.; Hou, X. BcMYB111 Responds to BcCBF2 and Induces Flavonol Biosynthesis to Enhance Tolerance under Cold Stress in Non-Heading Chinese Cabbage. Int. J. Mol. Sci. 2023, 24, 8670. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jiang, Y.; Yu, D. WRKY22 transcription factor mediates dark-induced leaf senescence in Arabidopsis. Mol. Cells 2011, 31, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Tena, G.; Plotnikova, J.; Willmann, M.R.; Chiu, W.L.; Gomez-Gomez, L.; Boller, T.; Ausubel, F.M.; Sheen, J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 2002, 415, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Kloth, K.J.; Wiegers, G.L.; Busscher-Lange, J.; van Haarst, J.C.; Kruijer, W.; Bouwmeester, H.J.; Dicke, M.; Jongsma, M.A. AtWRKY22 promotes susceptibility to aphids and modulates salicylic acid and jasmonic acid signalling. J. Exp. Bot. 2016, 67, 3383–3396. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.N.; Martin, G.B.; Pombo, M.A.; Rosli, H.G. WRKY22 and WRKY25 transcription factors are positive regulators of defense responses in Nicotiana benthamiana. Plant Mol. Biol. 2021, 105, 65–82. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.S.; Shu, S.; Wang, Y.; Chen, Z.; He, M.; Tao, M.; Sun, J.; Guo, S. Melatonin alleviates heat-induced damage of tomato seedlings by balancing redox homeostasis and modulating polyamine and nitric oxide biosynthesis. BMC Plant Biol. 2019, 19, 414. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, J.; Zhang, S.; Zhang, B.; Zhao, Q.; Li, G.; Yang, X.; Wang, C.; He, J.; Yi, M. A Canonical DREB2-Type Transcription Factor in Lily Is Post-translationally Regulated and Mediates Heat Stress Response. Front. Plant Sci. 2018, 9, 243. [Google Scholar] [CrossRef]
- Burke, J.J.; Chen, J. Enhancement of reproductive heat tolerance in plants. PLoS ONE 2015, 10, e0122933. [Google Scholar] [CrossRef]
- Liu, H.C.; Liao, H.T.; Charng, Y.Y. The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant Cell. Environ. 2011, 34, 738–751. [Google Scholar] [CrossRef]
- Ogawa, D.; Yamaguchi, K.; Nishiuchi, T. High-level overexpression of the Arabidopsis HsfA2 gene confers not only increased themotolerance but also salt/osmotic stress tolerance and enhanced callus growth. J. Exp. Bot. 2007, 58, 3373–3383. [Google Scholar] [CrossRef]
- Lee, U.; Rioflorido, I.; Hong, S.-W.; Larkindale, J.; Waters, E.R.; Vierling, E. The Arabidopsis ClpB/Hsp100 family of proteins: Chaperones for stress and chloroplast development. Plant J. 2007, 49, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Charng, Y.Y.; Liu, H.C.; Liu, N.Y.; Chi, W.T.; Wang, C.N.; Chang, S.H.; Wang, T.T. A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiol. 2007, 143, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, E.C.; Lindquist, S.; Vierling, E. An Arabidopsis heat shock protein complements a thermotolerance defect in yeast. Plant Cell. 1994, 6, 1899–1909. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Zhou, Y.; Yao, L.; Yu, L.; Qiao, Z.; Tang, M.; Wei, F. Amomum tsaoko DRM1 regulate seed germination and improve heat tolerance in Arabidopsis. J. Plant Physiol. 2023, 286, 154007. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Y.; Zhang, Y.; Xiao, Y.; Liu, X.; Deng, H.; Lu, X.; Tang, W.; Zhang, G. Comparative Analysis of Heat-Tolerant and Heat-Susceptible Rice Highlights the Role of OsNCED1 Gene in Heat Stress Tolerance. Plants 2022, 11, 1062. [Google Scholar] [CrossRef]
- Wu, D.C.; Zhu, J.F.; Shu, Z.Z.; Wang, W.; Yan, C.; Xu, S.B.; Wu, D.X.; Wang, C.Y.; Dong, Z.R.; Sun, G. Physiological and transcriptional response to heat stress in heat-resistant and heat-sensitive maize (Zea mays L.) inbred lines at seedling stage. Protoplasma 2020, 257, 1615–1637. [Google Scholar] [CrossRef]
- Wang, M.; He, X.; Peng, Q.; Liang, Z.; Peng, Q.; Liu, W.; Jiang, B.; Xie, D.; Chen, L.; Yan, J.; et al. Understanding the heat resistance of cucumber through leaf transcriptomics. Funct. Plant Biol. 2020, 47, 704–715. [Google Scholar] [CrossRef]
- Huang, Y.; Xuan, H.; Yang, C.; Guo, N.; Wang, H.; Zhao, J.; Xing, H. GmHsp90A2 is involved in soybean heat stress as a positive regulator. Plant Sci. 2019, 285, 26–33. [Google Scholar] [CrossRef]
- Wang, C.T.; Ru, J.N.; Liu, Y.W.; Li, M.; Zhao, D.; Yang, J.F.; Fu, J.D.; Xu, Z.S. Maize WRKY Transcription Factor ZmWRKY106 Confers Drought and Heat Tolerance in Transgenic Plants. Int. J. Mol. Sci. 2018, 19, 3046. [Google Scholar] [CrossRef]
- Xu, Y.; Burgess, P.; Huang, B. Root Antioxidant Mechanisms in Relation to Root Thermotolerance in Perennial Grass Species Contrasting in Heat Tolerance. PLoS ONE 2015, 10, e0138268. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Li, X.; Xia, X.J.; Zhou, Y.H.; Shi, K.; Chen, Z.; Yu, J.Q. H2O2 mediates the crosstalk of brassinosteroid and abscisic acid in tomato responses to heat and oxidative stresses. J. Exp. Bot. 2014, 65, 4371–4383. [Google Scholar] [CrossRef] [PubMed]
- Taratima, W.; Chuanchumkan, C.; Maneerattanarungroj, P.; Trunjaruen, A.; Theerakulpisut, P.; Dongsansuk, A. Effect of Heat Stress on Some Physiological and Anatomical Characteristics of Rice (Oryza sativa L.) cv. KDML105 Callus and Seedling. Biology 2022, 11, 1587. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, H.S.; Alharby, H.F.; Fahad, S. Antioxidative Defense System, Hormones, and Metabolite Accumulation in Different Plant Parts of Two Contrasting Rice Cultivars as Influenced by Plant Growth Regulators Under Heat Stress. Front. Plant Sci. 2022, 13, 911846. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Jiang, T.; Zhang, C.; Li, X.; Wang, C.; Zhang, Y.; Li, T.; Dirk, L.M.A.; Downie, A.B.; Zhao, T. Maize HSFA2 and HSBP2 antagonistically modulate raffinose biosynthesis and heat tolerance in Arabidopsis. Plant J. 2019, 100, 128–142. [Google Scholar] [CrossRef]
- Fujita, M.; Fujita, Y.; Noutoshi, Y.; Takahashi, F.; Narusaka, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Crosstalk between abiotic and biotic stress responses: A current view from the points of convergence in the stress signaling networks. Curr. Opin. Plant Biol. 2006, 9, 436–442. [Google Scholar] [CrossRef]
- Levine, A.; Tenhaken, R.; Dixon, R.; Lamb, C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 1994, 79, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.E.; Pennell, R.; Meijer, P.-J.; Ishikawa, A.; Dixon, R.; Lamb, C. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 1998, 92, 773–784. [Google Scholar] [CrossRef]
- Dat, J.F.; Lopez-Delgado, H.; Foyer, C.H.; Scott, I.M. Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol. 1998, 116, 1351–1357. [Google Scholar] [CrossRef]
- Medina, E.; Kim, S.-H.; Yun, M.; Choi, W.-G. Recapitulation of the Function and Role of ROS Generated in Response to Heat Stress in Plants. Plants 2021, 10, 371. [Google Scholar] [CrossRef]
- McClung, C.R. Regulation of catalases in Arabidopsis. Free Radic. Biol. Med. 1997, 23, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.Q.; Liu, S.; Yuan, H.M.; Li, J.; Yan, D.W.; Zhang, J.F.; Lu, Y.T. Functional comparison of catalase genes in the elimination of photorespiratory H2O2 using promoter- and 3′-untranslated region exchange experiments in the Arabidopsis cat2 photorespiratory mutant. Plant Cell Environ. 2010, 33, 1656–1670. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Jia, W.; Zhang, J. AtMEK1 mediates stress-induced gene expression of CAT1 catalase by triggering H2O2 production in Arabidopsis. J. Exp. Bot. 2007, 58, 2969–2981. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.Y.; Wang, P.C.; Chen, J.; Song, C.P. Comprehensive functional analysis of the catalase gene family in Arabidopsis thaliana. J. Integr. Plant Biol. 2008, 50, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).