To Prevent Oxidative Stress, What about Protoporphyrin IX, Biliverdin, and Bilirubin?
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Free Radical Scavengers, Antioxidants, Antireductants, or Antiradicals
3.2. Interaction with Ca2+
3.3. Excited States and Photoactivation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hauber, M.E. The Book of Eggs; Chicago University Press: Chicago, IL, USA, 2014; ISBN 9781782400479. [Google Scholar]
- Hanley, D.; Grim, T.; Cassey, P.; Hauber, M.E. Not so colourful after all: Eggshell pigments constrain avian eggshell colour space. Biol. Lett. 2015, 11, 20150087. [Google Scholar] [CrossRef]
- Kennedy, G.; Vevers, H. A survey of avian eggshell pigments. Comp. Biochem. Physiol. Part B Comp. Biochem. 1976, 55, 117–123. [Google Scholar] [CrossRef] [PubMed]
- López-Rull, I.; Miksik, I.; Gil, D. Egg pigmentation reflects female and egg quality in the spotless starling Sturnus unicolor. Behav. Ecol. Sociobiol. 2008, 62, 1877–1884. [Google Scholar] [CrossRef]
- López-Rull, I.; Celis, P.; Gil, D. Egg Colour Covaries with female expression of a male ornament in the spotless starling (Sturnus unicolor). Ethology 2007, 113, 926–933. [Google Scholar] [CrossRef]
- Sparks, N.H.C. Eggshell pigments—From formation to deposition. Avian Biol. Res. 2011, 4, 162–167. [Google Scholar] [CrossRef]
- Florczyk, U.M.; Jozkowicz, A.; Dulak, J. Biliverdin reductase: New features of an old enzyme and its potential therapeutic significance. Pharmacol. Rep. 2008, 60, 38–48. [Google Scholar]
- Stocker, R.; Yamamoto, Y.; McDonagh, A.F.; Glazer, A.N.; Ames, B.N. Bilirubin Is an Antioxidant of Possible Physiological Importance. Science 1987, 235, 1043–1046. [Google Scholar] [CrossRef]
- Asad, S.; Singh, S.; Ahmad, A.; Khan, N.U.; Hadi, S. Prooxidant and antioxidant activities of bilirubin and its metabolic precursor biliverdin: A structure—Activity study. Chem. Interactions 2001, 137, 59–74. [Google Scholar] [CrossRef]
- Baird, T.; Solomon, S.E.; Tedstone, D.R. Localisation and characterisation of egg shell porphyrins in several avian species. Br. Poult. Sci. 1975, 16, 201–208. [Google Scholar] [CrossRef]
- Hargitai, R.; Boross, N.; Hámori, S.; Neuberger, E.; Nyiri, Z. Eggshell Biliverdin and Protoporphyrin Pigments in a Songbird: Are They Derived from Erythrocytes, Blood Plasma, or the Shell Gland? Physiol. Biochem. Zool. 2017, 90, 613–626. [Google Scholar] [CrossRef]
- Wang, X.-T.; Deng, X.-M.; Zhao, C.-J.; Li, J.-Y.; Xu, G.-Y.; Lian, L.-S.; Wu, C.-X. Study of the Deposition Process of Eggshell Pigments Using an Improved Dissolution Method. Poult. Sci. 2007, 86, 2236–2238. [Google Scholar] [CrossRef] [PubMed]
- Gautron, J.; Stapane, L.; Le Roy, N.; Nys, Y.; Rodriguez-Navarro, A.B.; Hincke, M.T. Avian eggshell biomineralization: An update on its structure, mineralogy and protein tool kit. BMC Cell Biol. 2021, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, M.C.; Kupán, K.; Eyster, H.N.; Rojas-Abreu, W.; Cruz-López, M.; Serrano-Meneses, M.A.; Küpper, C. Camouflage and Clutch Survival in Plovers and Terns. Sci. Rep. 2016, 6, 32059. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, M.C.; Marshall, K.L.A.; Kilner, R.M. Imperfectly camouflaged avian eggs: Artefact or adaptation? Avian Biol. Res. 2011, 4, 196–213. [Google Scholar] [CrossRef]
- Lovell, P.G.; Ruxton, G.D.; Langridge, K.V.; Spencer, K.A. Egg-laying substrate selection for optimal camouflage by quail. Curr. Biol. 2013, 23, 260–264. [Google Scholar] [CrossRef]
- Moreno, J.; Osorno, J.L. Avian egg colour and sexual selection: Does eggshell pigmentation reflect female condition and genetic quality? Ecol. Lett. 2003, 6, 803–806. [Google Scholar] [CrossRef]
- Wisocki, P.A.; Kennelly, P.; Rojas Rivera, I.; Cassey, P.; Burkey, M.L.; Hanley, D. The global distribution of avian eggshell colours suggest a thermoregulatory benefit of darker pigmentation. Nat. Ecol. Evol. 2020, 4, 148–155. [Google Scholar] [CrossRef]
- Gosler, A.G.; Connora, O.R.; Bonserb, R.H.C. Protoporphyrin and eggshell strength: Preliminary findings from a passerine bird. Avian Biol. Res. 2011, 4, 214–223. [Google Scholar] [CrossRef]
- García-Navas, V.; Sanz, J.J.; Merino, S.; Martínez de la Fuente, J.; Lobato, E.; del Cerro, S.; Rivero, J.; Ruíz de Casteñeda, R.; Moreno, J. Experimental evidence for the role of calcium in eggshell pigmentation pattern and breeding performance in Blue Tits Cyanistes caeruleus. J. Ornithol. 2011, 152, 71–82. [Google Scholar] [CrossRef][Green Version]
- Hargitai, R.; Nagy, G.; Herényi, M.; Török, J. Effects of experimental calcium availability, egg parameters and laying order on Great Tit Parus major eggshell pigmentation patterns. Ibis 2013, 155, 561–570. [Google Scholar] [CrossRef]
- Ishikawa, S.I.; Suzuki, K.; Fukuda, E.; Arihara, K.; Yamamoto, Y.; Mukai, T.; Itoh, M. Photodynamic antimicrobial activity of avian eggshell pigments. FEBS Lett. 2010, 584, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Fargallo, J.A.; López-Rull, I.; Mikšík, I.; Eckhardt, A.; Peralta-Sánchez, J.M. Eggshell pigmentation has no evident effects on offspring viability in common kestrels. Evol. Ecol. 2014, 28, 627–637. [Google Scholar] [CrossRef]
- Maurer, G.; Portugal, S.J.; Cassey, P. Review: An embryo’s eye view of avian eggshell pigmentation. J. Avian Biol. 2011, 42, 494–504. [Google Scholar] [CrossRef]
- Morales, J. Eggshell biliverdin as an antioxidant maternal effect biliverdin as an antioxidant resource in oviparous animals. BioEssays 2020, 42, 2000010. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; McDonagh, A.F.; Glazer, A.N.; Ames, B.N. Antioxidant activities of bile pigments: Biliverdin and bilirubin. Methods Enzymol. 1990, 186, 301–309. [Google Scholar] [PubMed]
- Jansen, T.; Daiber, A. Direct antioxidant properties of bilirubin and biliverdin. Is there a role for biliverdin reductase? Front. Pharmacol. 2012, 3, 30. [Google Scholar] [CrossRef]
- Jayanti, S.; Vítek, L.; Tiribelli, C.; Gazzin, S. The Role of Bilirubin and the Other “Yellow Players” in Neurodegenerative Diseases. Antioxidants 2020, 9, 900. [Google Scholar] [CrossRef]
- Afonso, S.; Vanore, G.; Batlle, A. Protoporphyrin IX and oxidative stress. Free. Radic. Res. 1999, 31, 161–170. [Google Scholar] [CrossRef]
- Martínez, A.; Rodríguez-Gironés, M.A.; Barbosa, A.; Costas, M. Donator Acceptor Map for Carotenoids, Melatonin and Vitamins. J. Phys. Chem. A 2008, 112, 9037–9042. [Google Scholar] [CrossRef]
- Martínez, A.; Barbosa, A. Antiradical Power of Carotenoids and Vitamin E: Testing the Hydrogen Atom Transfer Mechanism. J. Phys. Chem. B 2008, 112, 16945–16951. [Google Scholar] [CrossRef]
- Martínez, A.; Vargas, R.; Galano, A. What is Important to Prevent Oxidative Stress? A Theoretical Study on Electron-Transfer Reactions between Carotenoids and Free Radicals. J. Phys. Chem. B 2009, 113, 12113–12120. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Rodríguez-Gironés, M.Á.; Barbosa, A. Can bird carotenoids play an antioxidant role oxidizing other substances? Ardeola 2009, 56, 287–294. [Google Scholar]
- Galano, A.; Vargas, R.; Martínez, A. Carotenoids can act as antioxidants by oxidizing the superoxide radical anion. Phys. Chem. Chem. Phys. 2010, 12, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.08, Wallingford, CT, 2009; D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Al-Laham, M.A.; Shirley, W.A.; Mantzaris, J. A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row elements. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]
- Petersson, G.A.; Al-Laham, M.A. A complete basis set model chemistry. II. Open-shell systems and the total energies of the first-row atoms. J. Chem. Phys. 1991, 94, 6081–6090. [Google Scholar] [CrossRef]
- Dunnning, T.H., Jr.; Hay, P.J. Modern Theoretical Chemistry; Schaefer, H.F., III, Ed.; Plenum: New York, NY, USA, 1977; Volume 3, pp. 1–28. [Google Scholar]
- Adamo, C.; Jacquemin, D. The calculations of excited-state properties with Time-Dependent Density Functional Theory. Chem. Soc. Rev. 2013, 42, 845–856. [Google Scholar] [CrossRef]
- Laurent, A.D.; Adamo, C.; Jacquemin, D. Dye chemistry with time-dependent density functional theory. Phys. Chem. Chem. Phys. 2014, 16, 14334–14356. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Geerlings, P.; Chamorro, E.; Chattaraj, P.K.; De Proft, F.; Gázquez, J.L.; Liu, S.; Morell, C.; Toro-Labbé, A.; Vela, A.; Ayers, P. Conceptual density functional theory: Status, prospects, issues. Theor. Chem. Acc. 2020, 139, 36. [Google Scholar] [CrossRef]
- Gázquez, J.L.; Cedillo, A.; Vela, A. Electrodonating and Electroaccepting Powers. J. Phys. Chem. A 2007, 111, 1966–1970. [Google Scholar] [CrossRef]
- Gázquez, J.L. Perspectives on the density functional theory of chemical reactivity. J. Mex. Chem. Soc. 2008, 52, 3–10. [Google Scholar]
- Pearson, R.G. Hard and soft acids and bases, HSAB, part 1: Fundamental principles. J. Chem. Educ. 1968, 45, 581–587. [Google Scholar] [CrossRef]
- Alfaro, R.A.D.; Gomez-Sandoval, Z.; Mammino, L. Evaluation of the antiradical activity of hyperjovinol-A utilizing donor-acceptor maps. J. Mol. Model. 2014, 20, 2337. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.M. Antireduction: An ancient strategy fit for future. Biosci. Rep. 2016, 36, e00367. [Google Scholar] [CrossRef]
- Colominas-Ciuró, R.; Bertellotti, M.; D’amico, V.L.; Carabajal, E.; Benzal, J.; Vidal, V.; Motas, M.; Barbosa, A. Sex matters? Association between foraging behaviour, diet, and physiology in Magellanic penguins. Mar. Biol. 2022, 169, 21. [Google Scholar] [CrossRef]
- Trigo, S.; Mota, P.G. What is the value of a yellow patch? Assessing the signalling role of yellow colouration in the European serin. Behav. Ecol. Sociobiol. 2015, 69, 481–490. [Google Scholar] [CrossRef]
- Papa, T.B.R.; Pinho, V.D.; do Nascimento, E.S.P.; Santos, W.G.; Burtoloso, A.C.B.; Skibsted, L.H.; Cardoso, D.R. Astaxanthin diferulate as a bifunctional antioxidant. Free. Radic. Res. 2015, 49, 102–111. [Google Scholar] [CrossRef]
- Kopena, R.; López, P.; Martín, J. What are carotenoids signaling? Immunostimulatory effects of dietary vitamin E, but not of carotenoids, in Iberian green lizards. Naturwissenschaften 2014, 101, 1107–1114. [Google Scholar] [CrossRef]
- Runemark, A.; Gabirot, M.; Svensson, E.I. Population divergence in chemical signals and the potential for premating isolation between islet- and mainland populations of the Skyros wall lizard (Podarcis gaigeae). J. Evol. Biol. 2011, 24, 795–809. [Google Scholar] [CrossRef]
- García-de Blas, E.; Mateo, R.; Alonso-Alvarez, C. Specific carotenoid pigments in the diet and a bit of oxidative stress in the recipe for producing red carotenoid-based signals. PeerJ 2016, 4, e2237. [Google Scholar] [CrossRef] [PubMed]
- del Val, E.; Senar, J.C.; Garrido-Fernández, J.; Jarén, M.; Borràs, A.; Cabrera, J.; Negro, J.J. The liver but not the skin is the site for conversion of a red carotenoid in a passerine bird. Naturwissenschaften 2009, 96, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Skibsted, L.H. Carotenoids in Antioxidant Networks. Colorants or Radical Scavengers. J. Agric. Food Chem. 2012, 60, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Abbas, G.; Alagawany, M.; Kamboh, A.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Chao, S. Heat stress management in poultry farms: A comprehensive overview. J. Therm. Biol. 2019, 84, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, M. Current Trends in Computational Quantum Chemistry Studies on Antioxidant Radical Scavenging Activity. J. Chem. Inf. Model. 2022, 62, 2639–2658. [Google Scholar] [CrossRef]
- Spiegel, M.; Gamian, A.; Sroka, Z. A Statistically Supported Antioxidant Activity DFT Benchmark—The Effects of Hartree–Fock Exchange and Basis Set Selection on Accuracy and Resources Uptake. Molecules 2021, 26, 5058. [Google Scholar] [CrossRef]
- Kirschweng, B.; Tátraaljai, D.; Földes, E.; Pukánszky, B. Natural antioxidants as stabilizers for polymers. Polym. Degrad. Stab. 2017, 145, 25–40. [Google Scholar] [CrossRef]
- Fathia, P.; Roslenda, A.; Mehtab, K.; Moitrac, P.; Zhangb, K.; Pana, D. UV-trained and metal-enhanced fluorescence of biliverdin and biliverdin nanoparticle. Nanoscale 2021, 13, 4785–4798. [Google Scholar] [CrossRef]
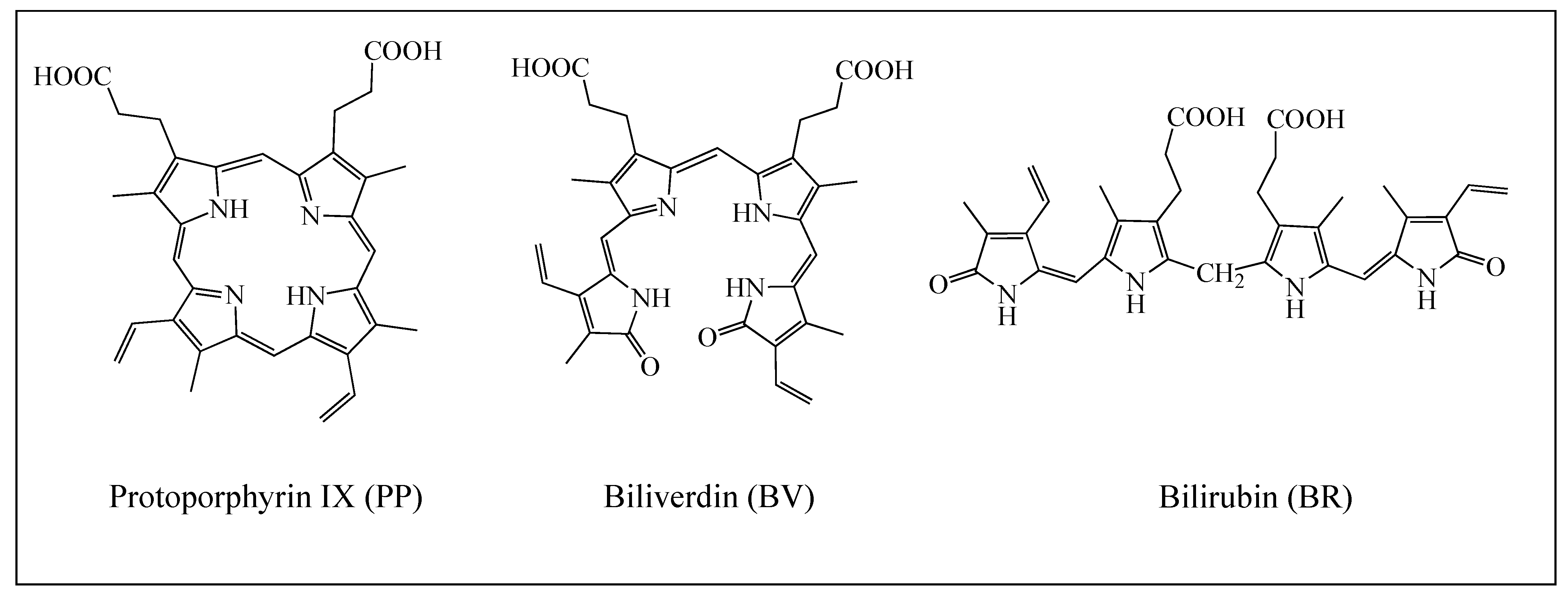


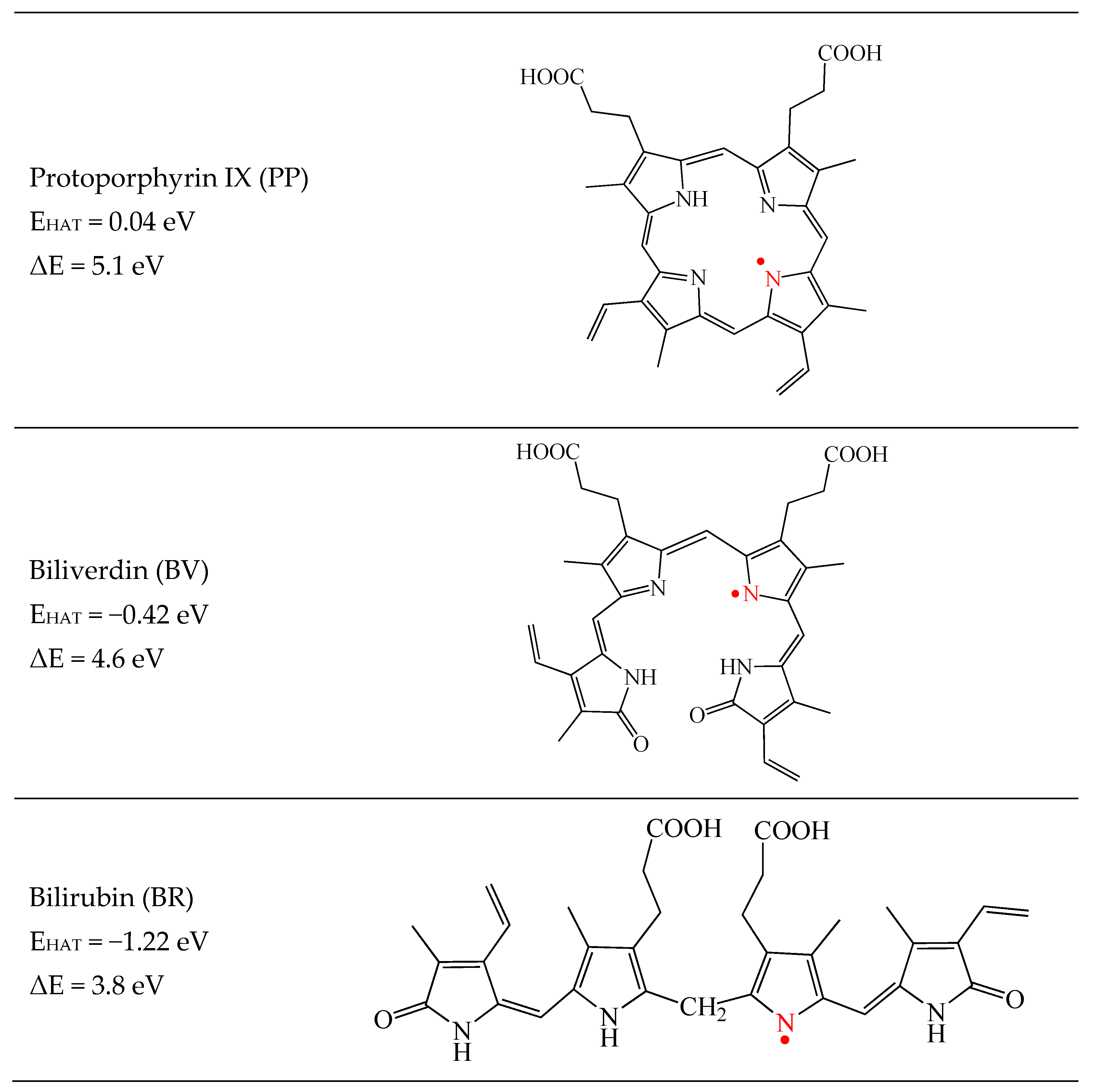

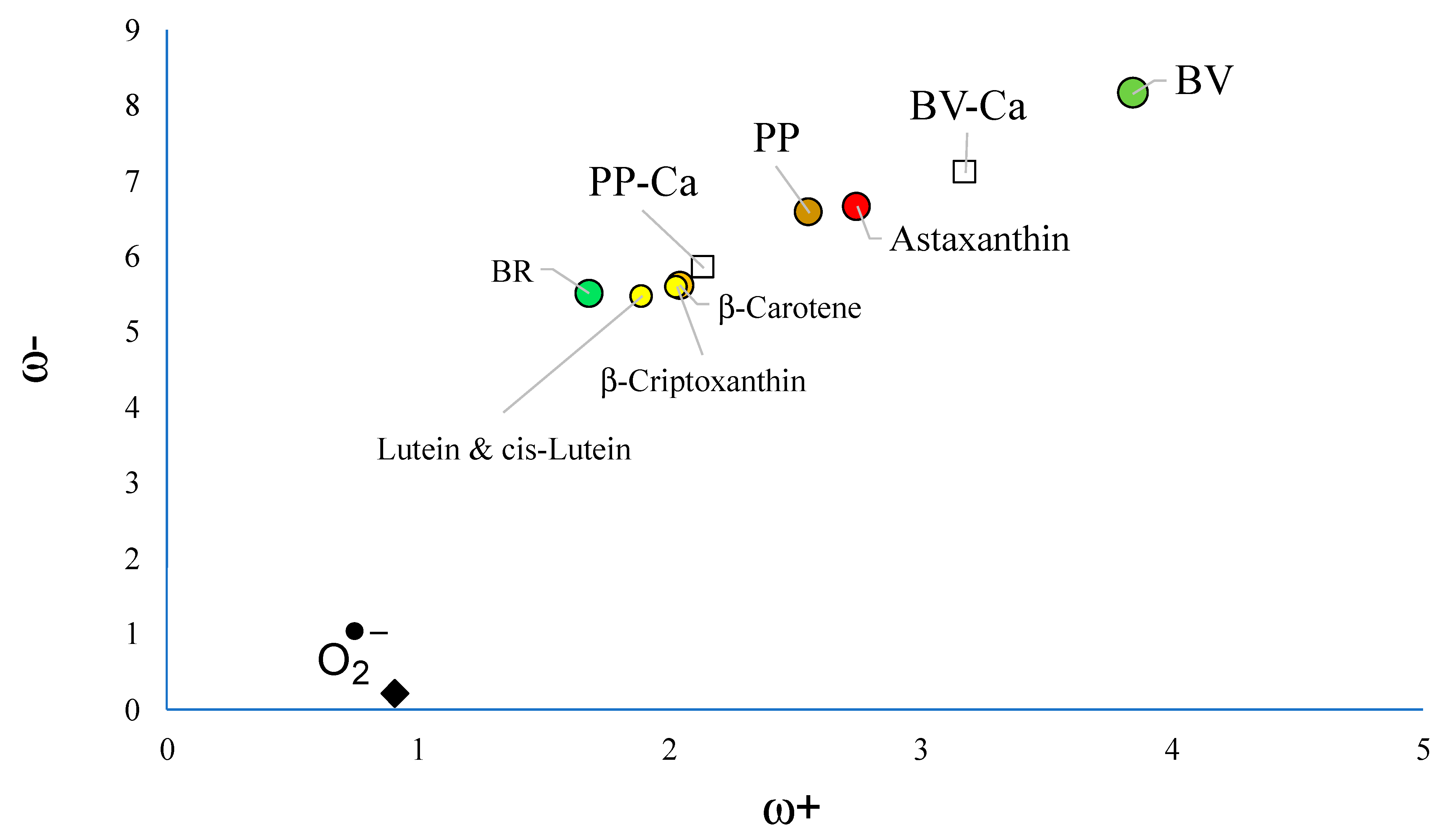
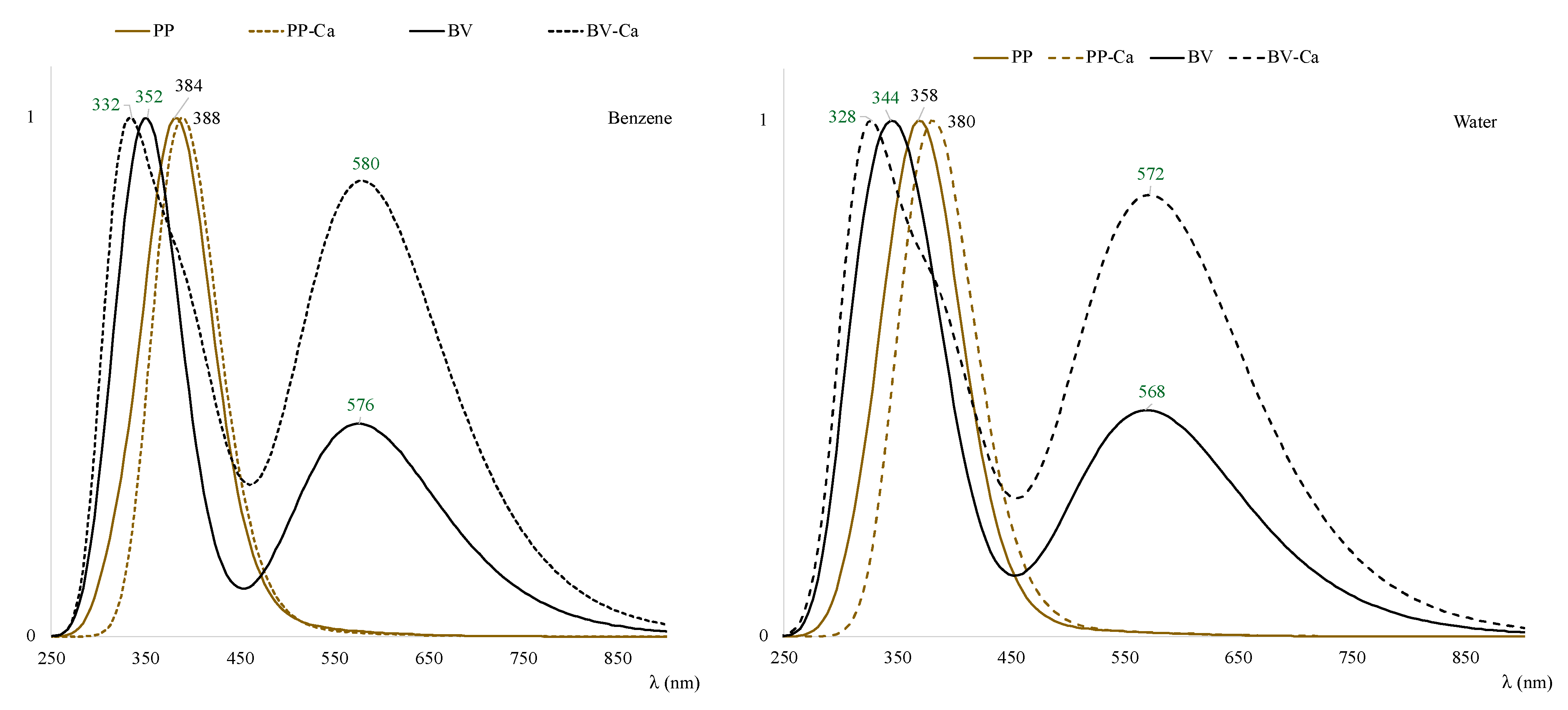
| System | E (Triplet) | Singlet | ΔE (t-s) |
|---|---|---|---|
| PP | −1835.725452 | −1835.791581 | 1.80 eV |
| BV | −1948.107976 | −1948.158904 | 1.39 eV |
| PP-Ca | −2512.278101 | −2512.345637 | 1.84 eV |
| BV-Ca | −2624.627534 | −2624.677715 | 1.37 eV |
| O2 | −150.313875 | −150.291324 | −0.61 eV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, A.; López-Rull, I.; Fargallo, J.A. To Prevent Oxidative Stress, What about Protoporphyrin IX, Biliverdin, and Bilirubin? Antioxidants 2023, 12, 1662. https://doi.org/10.3390/antiox12091662
Martínez A, López-Rull I, Fargallo JA. To Prevent Oxidative Stress, What about Protoporphyrin IX, Biliverdin, and Bilirubin? Antioxidants. 2023; 12(9):1662. https://doi.org/10.3390/antiox12091662
Chicago/Turabian StyleMartínez, Ana, Isabel López-Rull, and Juan A. Fargallo. 2023. "To Prevent Oxidative Stress, What about Protoporphyrin IX, Biliverdin, and Bilirubin?" Antioxidants 12, no. 9: 1662. https://doi.org/10.3390/antiox12091662
APA StyleMartínez, A., López-Rull, I., & Fargallo, J. A. (2023). To Prevent Oxidative Stress, What about Protoporphyrin IX, Biliverdin, and Bilirubin? Antioxidants, 12(9), 1662. https://doi.org/10.3390/antiox12091662







