Microbiome–Metabolomic Analysis Reveals Beneficial Effects of Dietary Kelp Resistant Starch on Intestinal Functions of Hybrid Snakeheads (Channa maculata ♀ × Channa argus ♂)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Experimental Fish and Design
2.3. Serum Biochemical Analysis
2.4. Intestinal Histopathological Analysis
2.5. Intestinal Biochemical Analysis
2.6. Intestinal Microbiome Analysis
2.7. Metabolomic Analysis
2.8. Statistical Analysis
3. Results
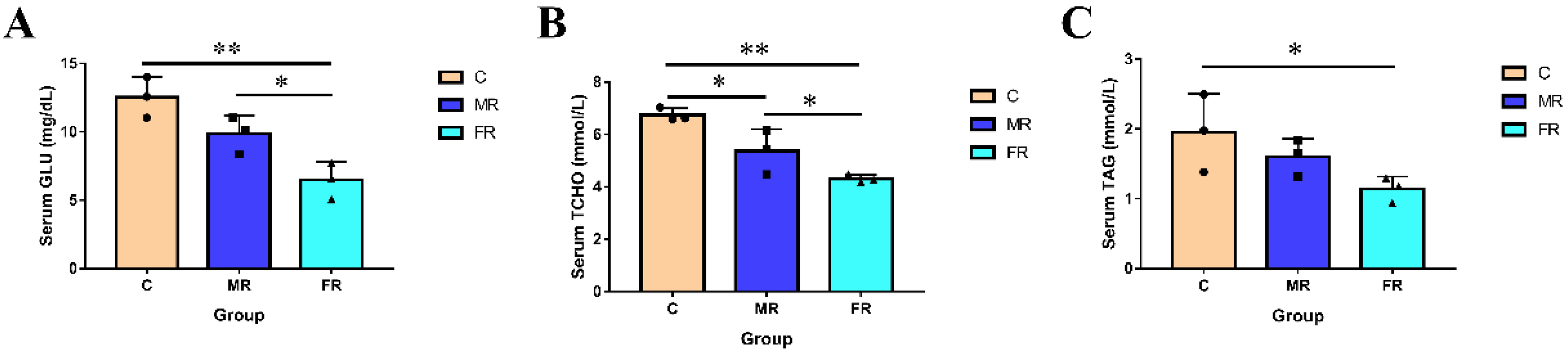
3.1. Regulation of KRS on Blood Glucose and Blood Lipids
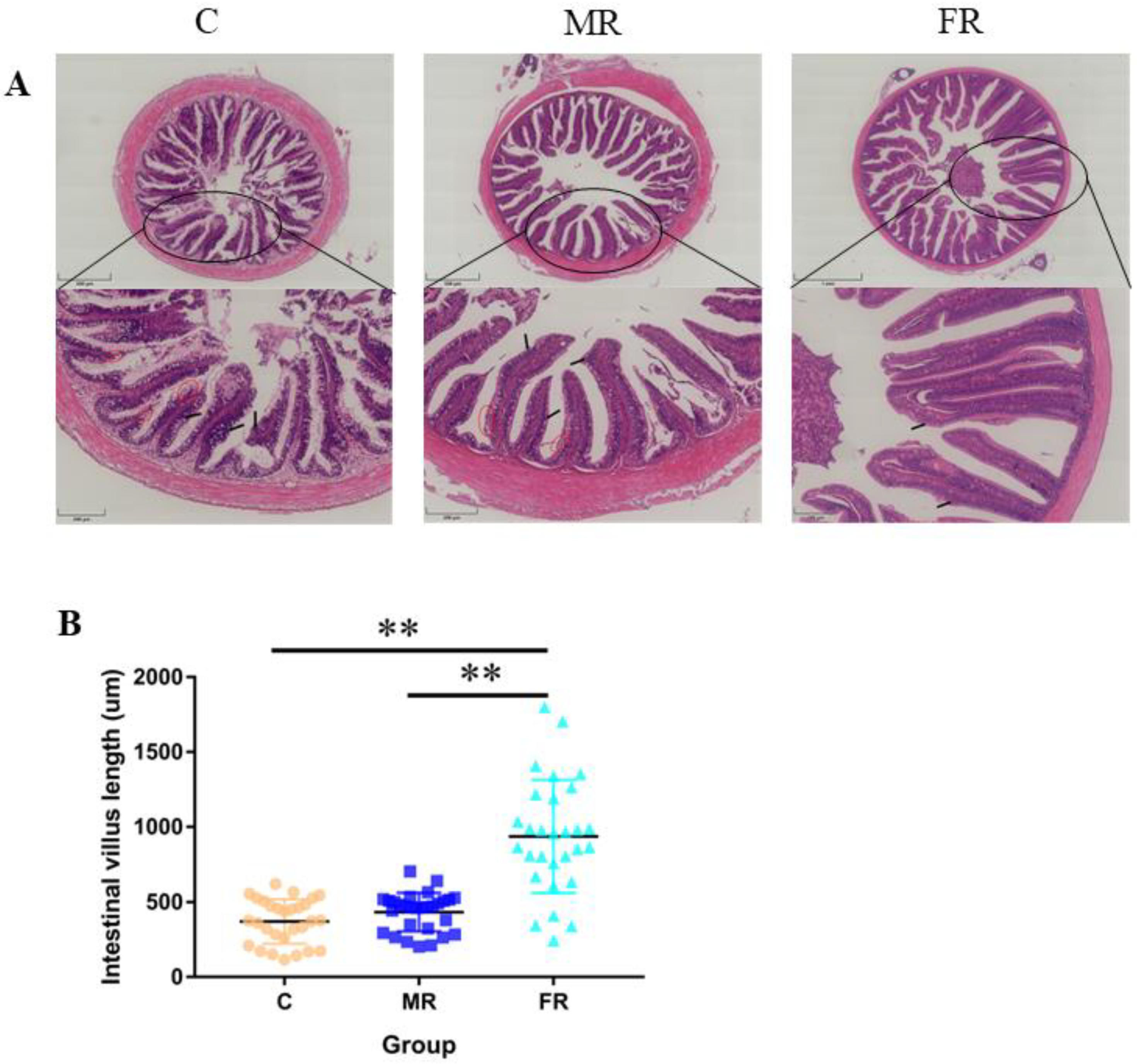
3.2. KRS Favors Intestinal Villi Growth and Reduces Villi Damage
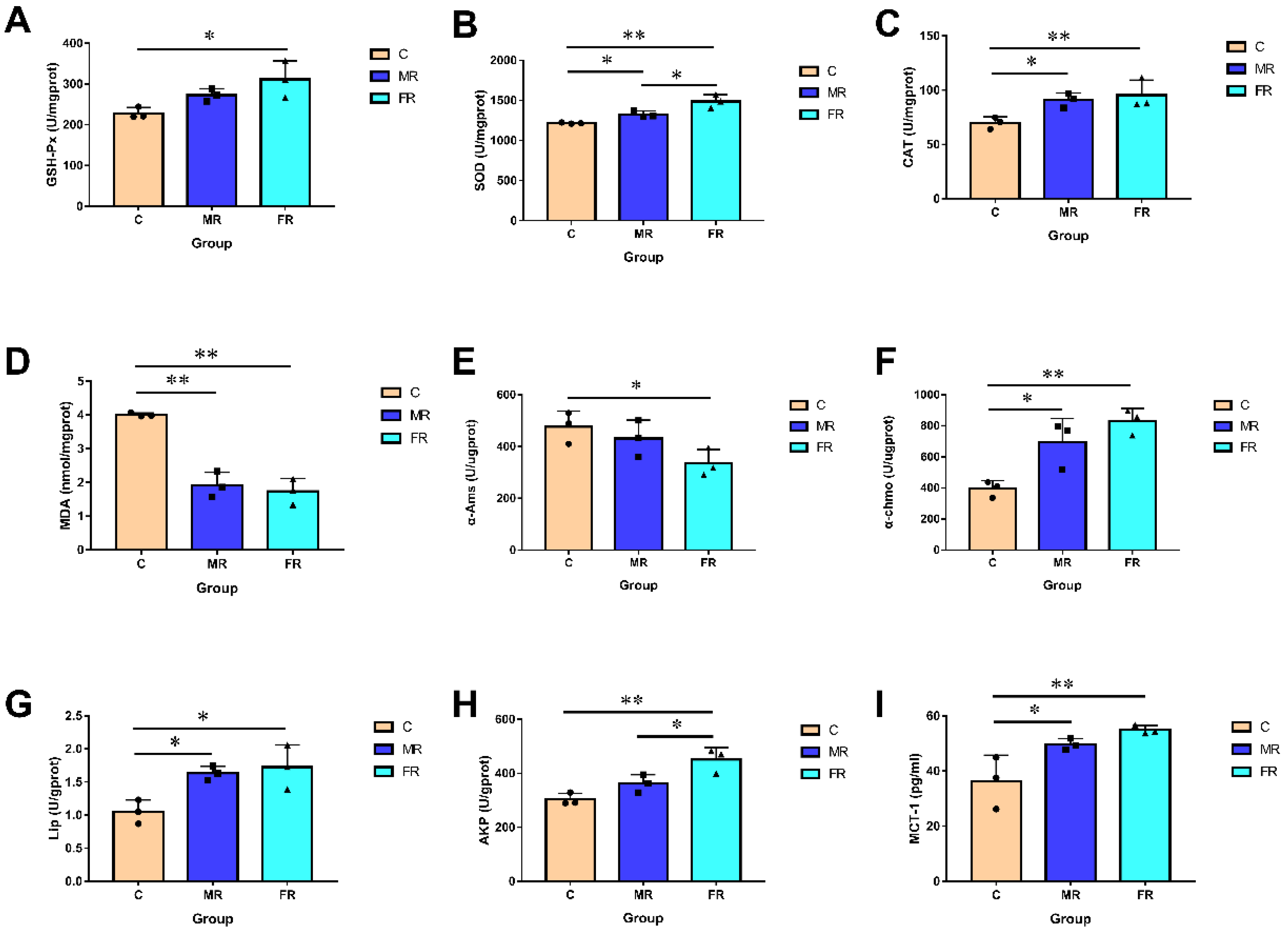
3.3. KRS Has the Potential to Improve Intestine Antioxidant Function, Digestion and Absorption, and Mucosal Barrier Function
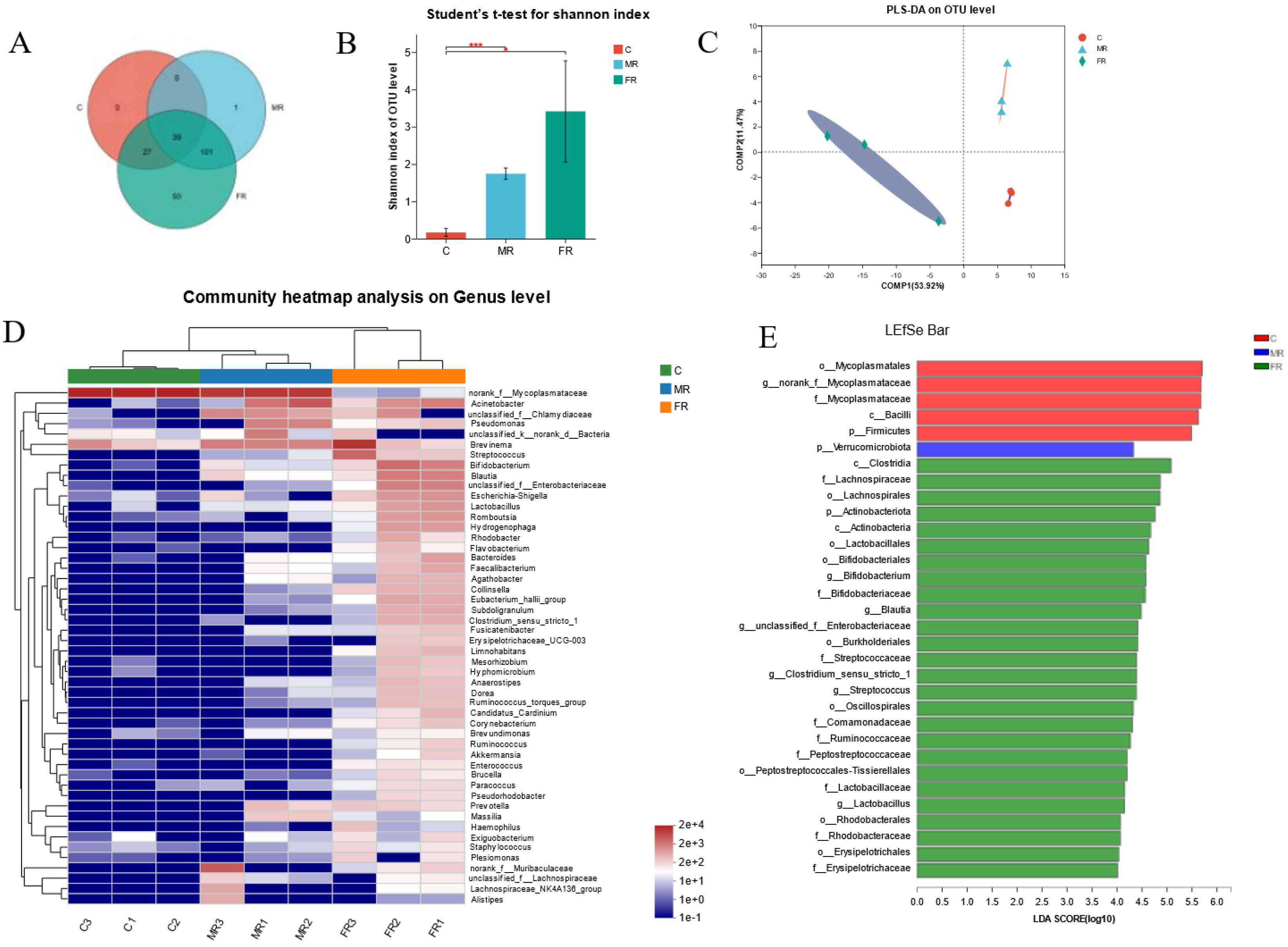
3.4. Modulation of Intestinal Microbial Composition via KRS at Different Dietary Carbohydrate Levels
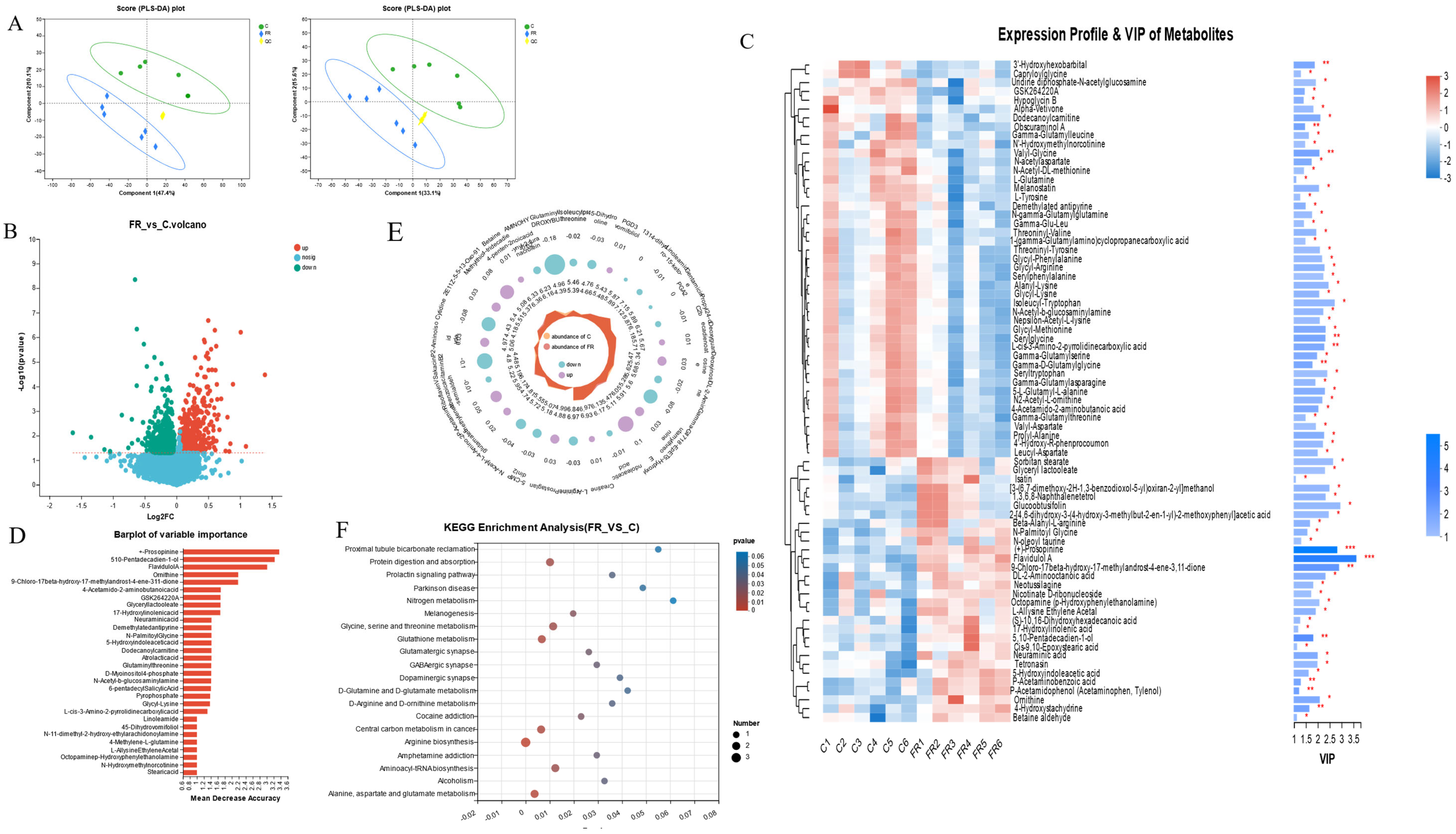
3.5. Effects of Dietary KRS on Intestinal Metabolite Profiles
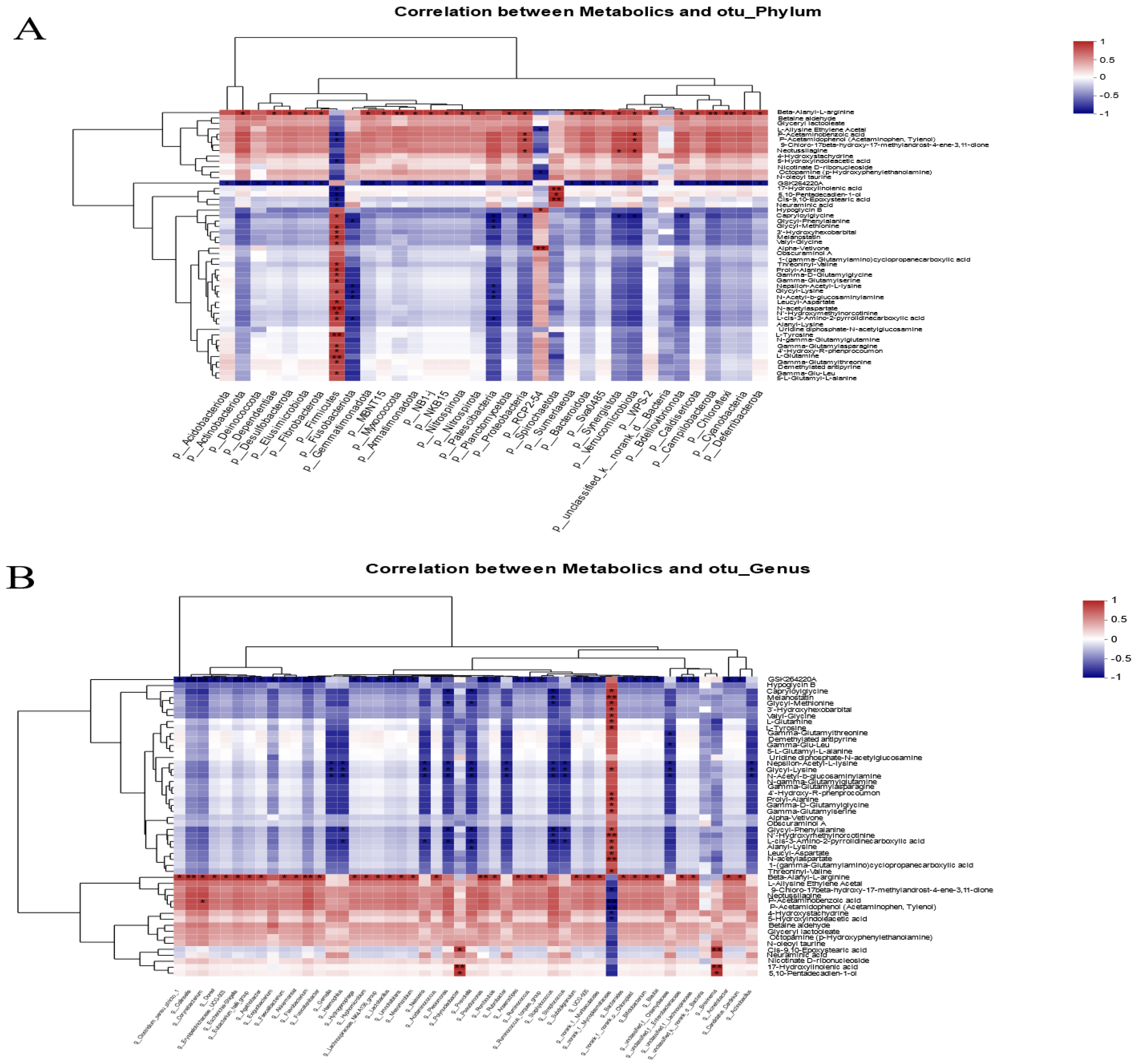
3.6. Correlation Analysis of Intestinal Microbial Communities and Metabolite Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ebrahimi, A.; Akrami, R.; Najdegerami, E.H.; Ghiasvand, Z.; Koohsari, H. Effects of different protein levels and carbon sources on water quality, antioxidant status and performance of common carp (Cyprinus carpio) juveniles raised in biofloc based system. Aquaculture 2020, 516, 734639. [Google Scholar] [CrossRef]
- Balasubramanian, M.N.; Panserat, S.; Dupont-Nivet, M.; Quillet, E.; Montfort, J.; Le Cam, A.; Medale, F.; Kaushik, S.J.; Geurden, I. Molecular pathways associated with the nutritional programming of plant-based diet acceptance in rainbow trout following an early feeding exposure. BMC Genom. 2016, 17, 449. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, X.; He, S.; Wang, J.; Li, L.; Zhang, Z.; Li, J.; Chen, X.; Li, L.; Alam, M.S. Metabolic responses of Chinese perch (Siniperca chuatsi) to different levels of dietary carbohydrate. Fish Physiol. Biochem. 2021, 47, 1449–1465. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.M.; Shi, C.M.; Mu, M.M.; Chen, Y.J.; Luo, L. Effect of high dietary starch levels on growth, hepatic glucose metabolism, oxidative status and immune response of juvenile largemouth bass, Micropterus salmoides. Fish Shellfish Immunol. 2018, 78, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Borges, P.; Valente, L.; Veron, V.; Dias, K.; Panserat, S.; Medale, F. High Dietary Lipid Level Is Associated with Persistent Hyperglycaemia and Downregulation of Muscle Akt-mTOR Pathway in Senegalese Sole (Solea senegalensis). PLoS ONE 2014, 9, e102196. [Google Scholar] [CrossRef]
- Polakof, S.; Panserat, S.; Soengas, J.L.; Moon, T.W. Glucose metabolism in fish: A review. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2012, 182, 1015–1045. [Google Scholar] [CrossRef]
- Heo, J.M.; Opapeju, F.O.; Pluske, J.R.; Kim, J.C.; Hampson, D.J.; Nyachoti, C.M. Gastrointestinal health and function in weaned pigs: A review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. 2013, 97, 207–237. [Google Scholar] [CrossRef]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M.; et al. Resistant Starch: Promise for Improving Human Health. Adv. Nutr. 2013, 4, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Maier, T.V.; Lucio, M.; Lee, L.H.; VerBerkmoes, N.C.; Brislawn, C.J.; Bernhardt, J.; Lamendella, R.; McDermott, J.E.; Bergeron, N.; Heinzmann, S.S.; et al. Impact of Dietary Resistant Starch on the Human Gut Microbiome, Metaproteome, and Metabolome. mBio 2017, 8, e01343-17. [Google Scholar] [CrossRef]
- Trachsel, J.; Briggs, C.; Gabler, N.K.; Allen, H.K.; Loving, C.L. Dietary Resistant Potato Starch Alters Intestinal Microbial Communities and Their Metabolites, and Markers of Immune Regulation and Barrier Function in Swine. Front. Immunol. 2019, 10, 1381. [Google Scholar] [CrossRef]
- Józefiak, D.; Rutkowski, A.; Martin, S.A. Carbohydrate fermentation in the avian ceca: A review. Anim. Feed Sci. Technol. 2004, 113, 1–15. [Google Scholar] [CrossRef]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, B.; McCormack, L.; Fardin-Kia, A.R.; Juenemann, R.; Nichenametla, S.; Clapper, J.; Specker, B.; Dey, M. Impact of dietary resistant starch type 4 on human gut microbiota and immunometabolic functions. Sci. Rep. 2016, 6, 28797. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, F.; Gao, Y.; Xue, C.; Li, R.W.; Tang, Q. Transcriptome analysis revealed anti-obesity effects of the Sodium Alginate in high-fat diet-induced obese mice. Int. J. Biol. Macromol. 2018, 115, 861–870. [Google Scholar] [CrossRef]
- Qiang, T.; Wang, J.; Jiang, L.; Xiong, K. Modulation of hyperglycemia by sodium alginate is associated with changes of serum metabolite and gut microbiota in mice. Carbohydr. Polym. 2022, 291, 119359. [Google Scholar] [CrossRef]
- Gao, J.; Lin, L.; Chen, Z.; Cai, Y.; Xiao, C.; Zhou, F.; Sun, B.; Zhao, M. In vitro digestion and fermentation of three polysaccharide fractions from Laminaria japonica and their impact on lipid metabolism-associated human gut microbiota. J. Agric. Food Chem. 2019, 67, 7496–7505. [Google Scholar] [CrossRef]
- Fang, F.; Xiao, C.; Wan, C.; Li, Y.; Lu, X.; Lin, Y.; Gao, J. Two Laminaria japonica polysaccharides with distinct structure characterization affect gut microbiota and metabolites in hyperlipidemic mice differently. Food Res. Int. 2022, 159, 111615. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Liu, B.; He, N. Unsaturated alginate oligosaccharides attenuated obesity-related metabolic abnormalities by modulating gut microbiota in high-fat-diet mice. Food Funct. 2020, 11, 4773–4784. [Google Scholar] [CrossRef]
- Wang, S.; Xu, G.; Zou, J. Soluble non-starch polysaccharides in fish feed: Implications for fish metabolism. Fish Physiol. Biochem. 2022, 1–22. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. eBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Cantone, E.; Cassarano, S.; Tuccinardi, D.; Barrea, L.; Savastano, S.; Colao, A. Gut microbiota: A new path to treat obesity. Int. J. Obes. Suppl. 2019, 9, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Keenan, M.J.; Zhou, J.; Hegsted, M.; Pelkman, C.; Durham, H.A.; Coulon, D.B.; Martin, R.J. Role of resistant starch in improving gut health, adiposity, and insulin resistance. Adv. Nutr. 2015, 6, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Zhang, K.; Ding, X.; Bai, S.; Wang, J.; Tian, G.; Xuan, Y.; Su, Z.; Zeng, Q. Microbiome-metabolomics analysis insight into the effects of dietary resistant starch on intestinal integrity. Food Chem. 2023, 401, 134148. [Google Scholar] [CrossRef]
- Sun, Y.; Su, Y.; Zhu, W. Microbiome-Metabolome Responses in the Cecum and Colon of Pig to a High Resistant Starch Diet. Front. Microbiol. 2016, 7, 779. [Google Scholar] [CrossRef]
- Prisingkorn, W.; Prathomya, P.; Jakovlić, I.; Liu, H.; Zhao, Y.H.; Wang, W.M. Transcriptomics, metabolomics and histology indicate that high-carbohydrate diet negatively affects the liver health of blunt snout bream (Megalobrama amblycephala). BMC Genom. 2017, 18, 856. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Meng, Q.; Jiang, B.; Chen, J.; Zhang, T.; Zhou, L. Structure characterization and in vitro hypoglycemic effect of partially degraded alginate. Food Chem. 2021, 356, 129728. [Google Scholar] [CrossRef]
- Bang, S.; Lee, E.; Song, E.; Nam, Y.; Seo, M.; Kim, H.; Park, C.; Lim, M.Y.; Seo, D. Effect of raw potato starch on the gut microbiome and metabolome in mice. Int. J. Biol. Macromol. 2019, 133, 37–43. [Google Scholar] [CrossRef]
- Rosado, C.P.; Rosa, V.H.C.; Martins, B.C.; Soares, A.C.; Santos, I.B.; Monteiro, E.B.; Moura-Nunes, N.; Da Costa, C.A.; Mulder, A.D.R.P.; Daleprane, J.B. Resistant starch from green banana (Musa sp.) attenuates non-alcoholic fat liver accumulation and increases short-chain fatty acids production in high-fat diet-induced obesity in mice. Int. J. Biol. Macromol. 2020, 145, 1066–1072. [Google Scholar] [CrossRef]
- Yang, X.; Song, Y.; Zhang, C.; Pang, Y.; Song, X.; Wu, M.; Cheng, Y. Effects of the glyphosate-based herbicide roundup on the survival, immune response, digestive activities and gut microbiota of the Chinese mitten crab, Eriocheir sinensis. Aquat. Toxicol. 2019, 214, 105243. [Google Scholar] [CrossRef]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Oxidative Stress and Stress-Activated Signaling Pathways: A Unifying Hypothesis of Type 2 Diabetes. Endocr. Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wu, Y.; Chen, X.; Yang, X.; Xiao, H. Water extract of shepherd’s purse prevents high-fructose induced-liver injury by regulating glucolipid metabolism and gut microbiota. Food Chem. 2021, 342, 128536. [Google Scholar] [CrossRef]
- Liang, D.; Zhang, L.; Chen, H.; Zhang, H.; Hu, H.; Dai, X. Potato resistant starch inhibits diet-induced obesity by modifying the composition of intestinal microbiota and their metabolites in obese mice. Int. J. Biol. Macromol. 2021, 180, 458–469. [Google Scholar] [CrossRef]
- Malcomson, F.C.; Willis, N.D.; McCallum, I.; Xie, L.; Ouwehand, A.C.; Stowell, J.D.; Kelly, S.; Bradburn, D.M.; Belshaw, N.J.; Johnson, I.T. Resistant starch supplementation increases crypt cell proliferative state in the rectal mucosa of older healthy participants. Br. J. Nutr. 2020, 124, 374–385. [Google Scholar] [CrossRef]
- Yan, Y.; Peng, Y.; Tang, J.; Mi, J.; Lu, L.; Li, X.; Ran, L.; Zeng, X.; Cao, Y. Effects of anthocyanins from the fruit of Lycium ruthenicum Murray on intestinal microbiota. J. Funct. Foods 2018, 48, 533–541. [Google Scholar] [CrossRef]
- Parshukov, A.N.; Kashinskaya, E.N.; Simonov, E.P.; Hlunov, O.V.; Izvekova, G.I.; Andree, K.B.; Solovyev, M.M. Variations of the intestinal gut microbiota of farmed rainbow trout, Oncorhynchus mykiss (Walbaum), depending on the infection status of the fish. J. Appl. Microbiol. 2019, 127, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Kent, M.L.; Wall, E.S.; Sichel, S.; Watral, V.; Stagaman, K.; Sharpton, T.J.; Guillemin, K. Pseudocapillaria tomentosa, Mycoplasma spp., and Intestinal Lesions in Experimentally Infected Zebrafish Danio rerio. Zebrafish 2021, 18, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Udayappan, S.; Manneras-Holm, L.; Chaplin-Scott, A.; Belzer, C.; Herrema, H.; Dallinga-Thie, G.M.; Duncan, S.H.; Stroes, E.; Groen, A.K.; Flint, H.J.; et al. Oral treatment with Eubacterium hallii improves insulin sensitivity in db/db mice. npj Biofilms Microbiomes 2016, 2, 16009. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhai, R.; Xie, X.; Chen, T.; Zhang, Z.; Liu, H.; Nie, C.; Yuan, X.; Tu, A.; Tian, B.; et al. Hypoglycemic Effects of Lycium barbarum Polysaccharide in Type 2 Diabetes Mellitus Mice via Modulating Gut Microbiota. Front. Nutr. 2022, 9, 916271. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, S.; Xu, W.; Zang, C.; Zhang, F.; Xie, Y.; Wu, C. Dual modulation of gut bacteria and fungi manifests the gut-based anti-hyperlipidemic effect of Coptidis Rhizoma. Biomed. Pharmacother. 2022, 153, 113542. [Google Scholar] [CrossRef]
- Kim, G.; Yoon, Y.; Park, J.H.; Park, J.W.; Noh, M.G.; Kim, H.; Park, C.; Kwon, H.; Park, J.H.; Kim, Y.; et al. Bifidobacterial carbohydrate/nucleoside metabolism enhances oxidative phosphorylation in white adipose tissue to protect against diet-induced obesity. Microbiome 2022, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.; Lannoy, V.; Decobecq, M.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome–brain–β-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhou, L.; Guo, H.; Xu, Y.; Xu, Y. The role of short-chain fatty acids in kidney injury induced by gut-derived inflammatory response. Metabolism 2017, 68, 20–30. [Google Scholar] [CrossRef]
- Zeng, H.; Chi, H. Metabolic control of regulatory T cell development and function. Trends Immunol. 2015, 36, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, Z.; Chen, W.; Rong, T.; Wang, G.; Ma, X. Microbiome-Metabolomics Analysis Investigating the Impacts of Dietary Starch Types on the Composition and Metabolism of Colonic Microbiota in Finishing Pigs. Front. Microbiol. 2019, 10, 1143. [Google Scholar] [CrossRef]
- Liang, J.; Kou, S.; Chen, C.; Raza, S.H.A.; Wang, S.; Ma, X.; Zhang, W.; Nie, C. Effects of Clostridium butyricum on growth performance, metabonomics and intestinal microbial differences of weaned piglets. BMC Microbiol. 2021, 21, 85. [Google Scholar] [CrossRef]
- Khalaf, D.; Krüger, M.; Wehland, M.; Infanger, M.; Grimm, D. The effects of oral l-arginine and l-citrulline supplementation on blood pressure. Nutrients 2019, 11, 1679. [Google Scholar] [CrossRef]
- Wang, H.; Dong, P.; Liu, X.; Zhang, Z.; Li, H.; Li, Y.; Zhang, J.; Dai, L.; Wang, S. Active Peptide AR-9 From Eupolyphaga sinensis Reduces Blood Lipid and Hepatic Lipid Accumulation by Restoring Gut Flora and Its Metabolites in a High Fat Diet-Induced Hyperlipidemia Rat. Front. Pharmacol. 2022, 13, 918505. [Google Scholar] [CrossRef]
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Günther, J. Kynurenic acid: The Janus-faced role of an immunomodulatory tryptophan metabolite and its link to pathological conditions. Front. Immunol. 2018, 8, 1957. [Google Scholar] [CrossRef]
- Wassie, T.; Cheng, B.; Zhou, T.; Gao, L.; Lu, Z.; Xie, C.; Wu, X. Microbiome-metabolome analysis reveals alterations in the composition and metabolism of caecal microbiota and metabolites with dietary Enteromorpha polysaccharide and Yeast glycoprotein in chickens. Front. Immunol. 2022, 13, 996897. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Venneti, S.; Nagrath, D. Glutaminolysis: A Hallmark of Cancer Metabolism. Annu. Rev. Biomed. Eng. 2017, 19, 163–194. [Google Scholar] [CrossRef] [PubMed]
- Altman, B.J.; Stine, Z.E.; Dang, C.V. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat. Rev. Cancer 2016, 16, 619–634. [Google Scholar] [CrossRef] [PubMed]
| Ingredients (%) | C | MR | FR |
|---|---|---|---|
| Fish meal (67%) | 35.0 | 35.0 | 35.0 |
| Casein | 10.0 | 10.0 | 10.0 |
| Soybean meal | 10.0 | 10.0 | 10.0 |
| Lecithin High Potency | 1.0 | 1.0 | 1.0 |
| Calcium dihydrogen phosphate | 1.0 | 1.0 | 1.0 |
| Premix * | 2.2 | 2.2 | 2.2 |
| Choline chloride (50%) | 0.2 | 0.2 | 0.2 |
| Vitamin C | 0.1 | 0.1 | 0.1 |
| Fish oil | 4.0 | 4.0 | 4.0 |
| Microcrystalline cellulose | 16.5 | 16.5 | 16.5 |
| High-gluten flour | 20.0 | 10.0 | |
| Resistant starch (kelp meal) | 10.0 | 15.0 | |
| Binder (KGM, Glucomannan) | 5.0 | ||
| Total | 100.0 | 100.0 | 100.0 |
| Basic ingredients | |||
| Moisture | 7.53 | 7.43 | 7.78 |
| Crude protein | 39.95 | 40.41 | 40.65 |
| Crude fat | 6.67 | 6.75 | 6.43 |
| Ash | 11.01 | 10.89 | 11.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zuo, Z.; Ye, B.; Zhang, L.; Cheng, Y.; Xie, S.; Zou, J.; Xu, G. Microbiome–Metabolomic Analysis Reveals Beneficial Effects of Dietary Kelp Resistant Starch on Intestinal Functions of Hybrid Snakeheads (Channa maculata ♀ × Channa argus ♂). Antioxidants 2023, 12, 1631. https://doi.org/10.3390/antiox12081631
Wang S, Zuo Z, Ye B, Zhang L, Cheng Y, Xie S, Zou J, Xu G. Microbiome–Metabolomic Analysis Reveals Beneficial Effects of Dietary Kelp Resistant Starch on Intestinal Functions of Hybrid Snakeheads (Channa maculata ♀ × Channa argus ♂). Antioxidants. 2023; 12(8):1631. https://doi.org/10.3390/antiox12081631
Chicago/Turabian StyleWang, Shaodan, Zhiheng Zuo, Bin Ye, Li Zhang, Yanbo Cheng, Shaolin Xie, Jixing Zou, and Guohuan Xu. 2023. "Microbiome–Metabolomic Analysis Reveals Beneficial Effects of Dietary Kelp Resistant Starch on Intestinal Functions of Hybrid Snakeheads (Channa maculata ♀ × Channa argus ♂)" Antioxidants 12, no. 8: 1631. https://doi.org/10.3390/antiox12081631
APA StyleWang, S., Zuo, Z., Ye, B., Zhang, L., Cheng, Y., Xie, S., Zou, J., & Xu, G. (2023). Microbiome–Metabolomic Analysis Reveals Beneficial Effects of Dietary Kelp Resistant Starch on Intestinal Functions of Hybrid Snakeheads (Channa maculata ♀ × Channa argus ♂). Antioxidants, 12(8), 1631. https://doi.org/10.3390/antiox12081631







