Dietary Supplementation of Chlorella vulgaris Effectively Enhanced the Intestinal Antioxidant Capacity and Immune Status of Micropterus salmoides
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Experimental Diets Preparation
2.3. Fish Sample Collection
2.4. Analysis of the Serum Biochemical Indices and Intestinal Antioxidant Parameters
2.5. The Process of High-Throughput Sequencing
2.6. Quality Inspection of Sequencing Data
2.7. Quantitative Real-Time PCR Detection
2.8. Statistical Analysis
3. Results
3.1. Effects of Dietary Supplementation of C. vulgaris on the Serum Biochemical Indices
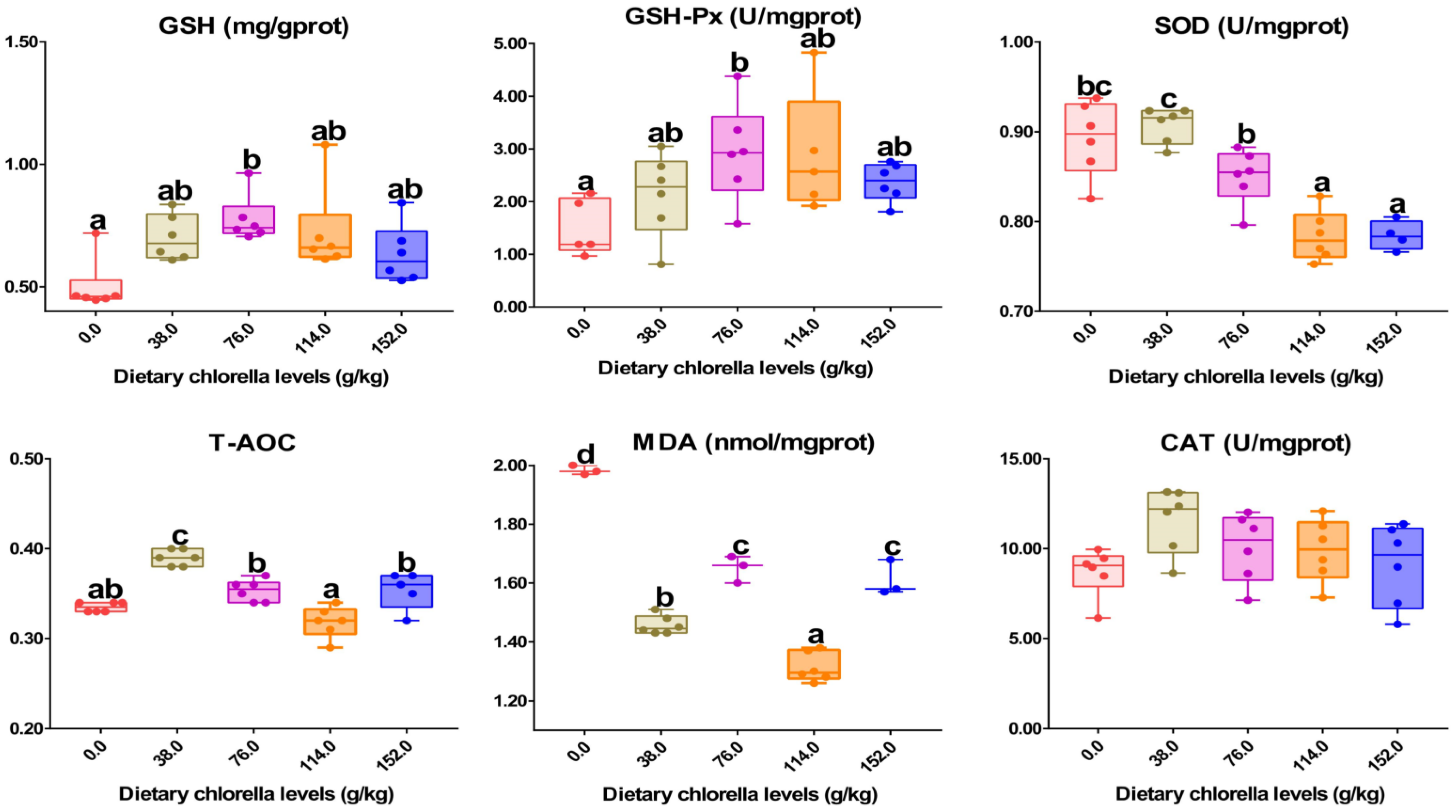
3.2. Effects of Dietary Supplementation of C. vulgaris on the Intestinal Antioxidant Parameters
3.3. Optimal Supplementation Level of C. vulgaris in M. salmoides Diet
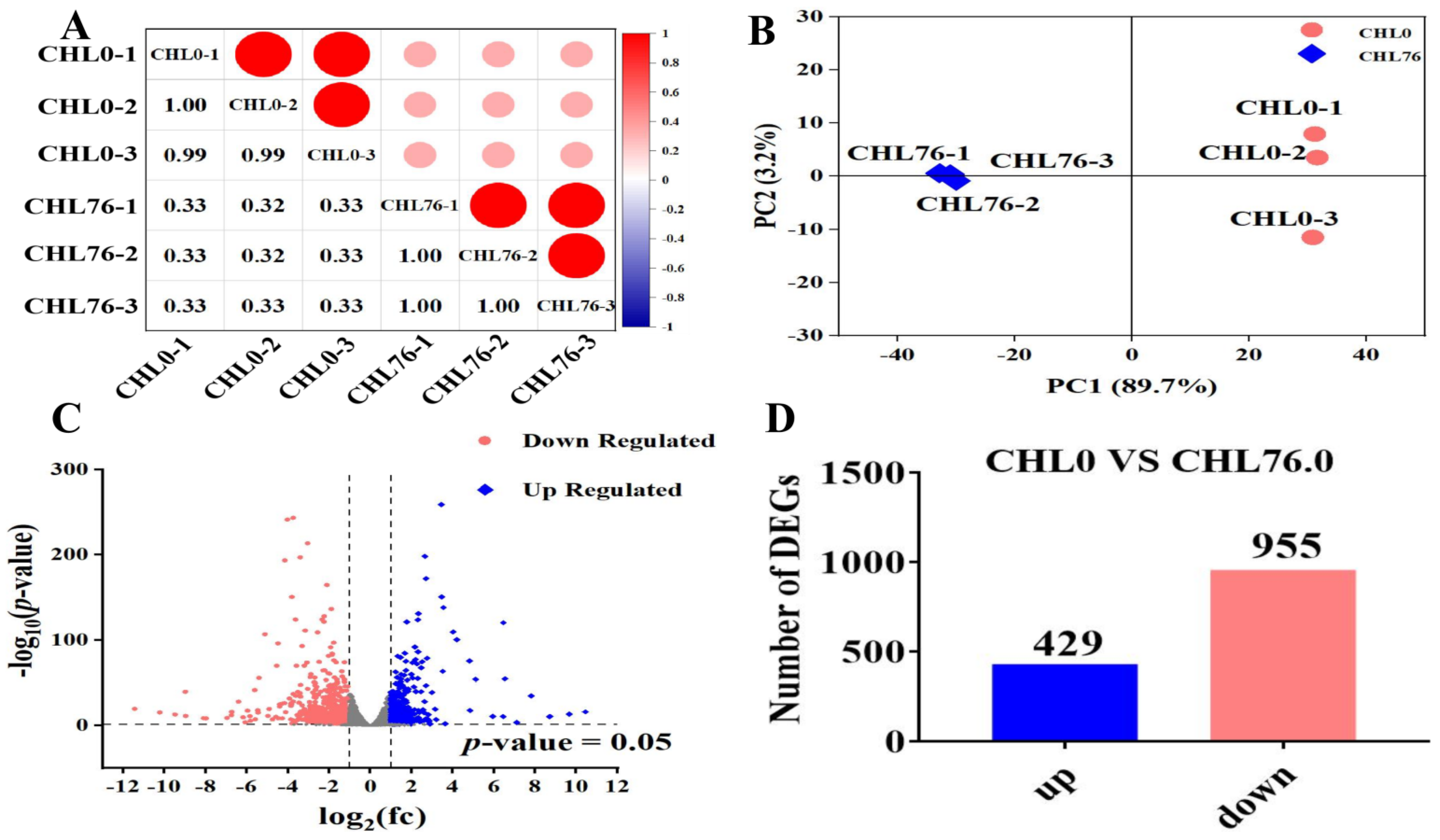
3.4. Library Sequencing Quality
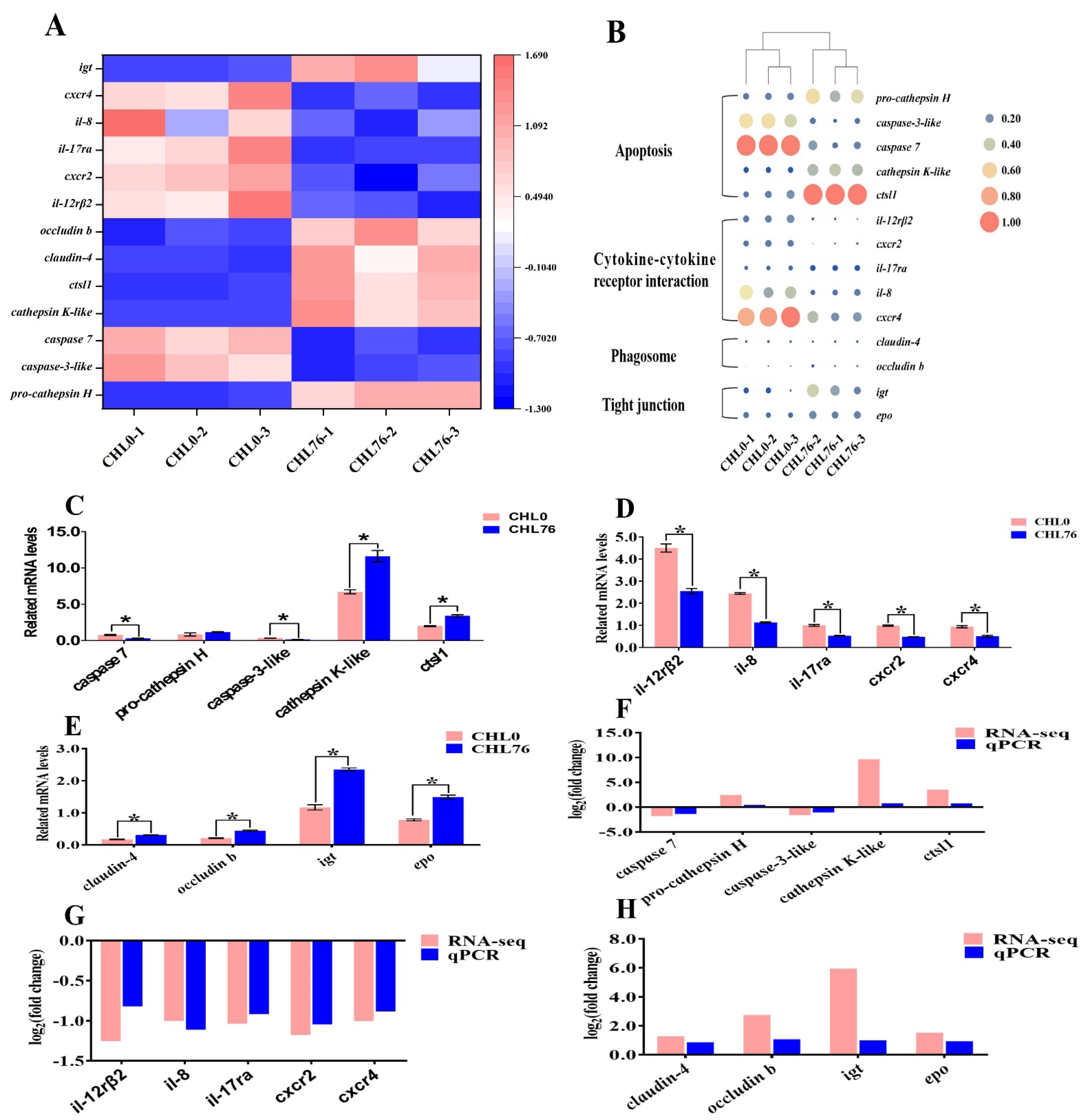
3.5. DEG Analysis
3.6. qPCR Assay
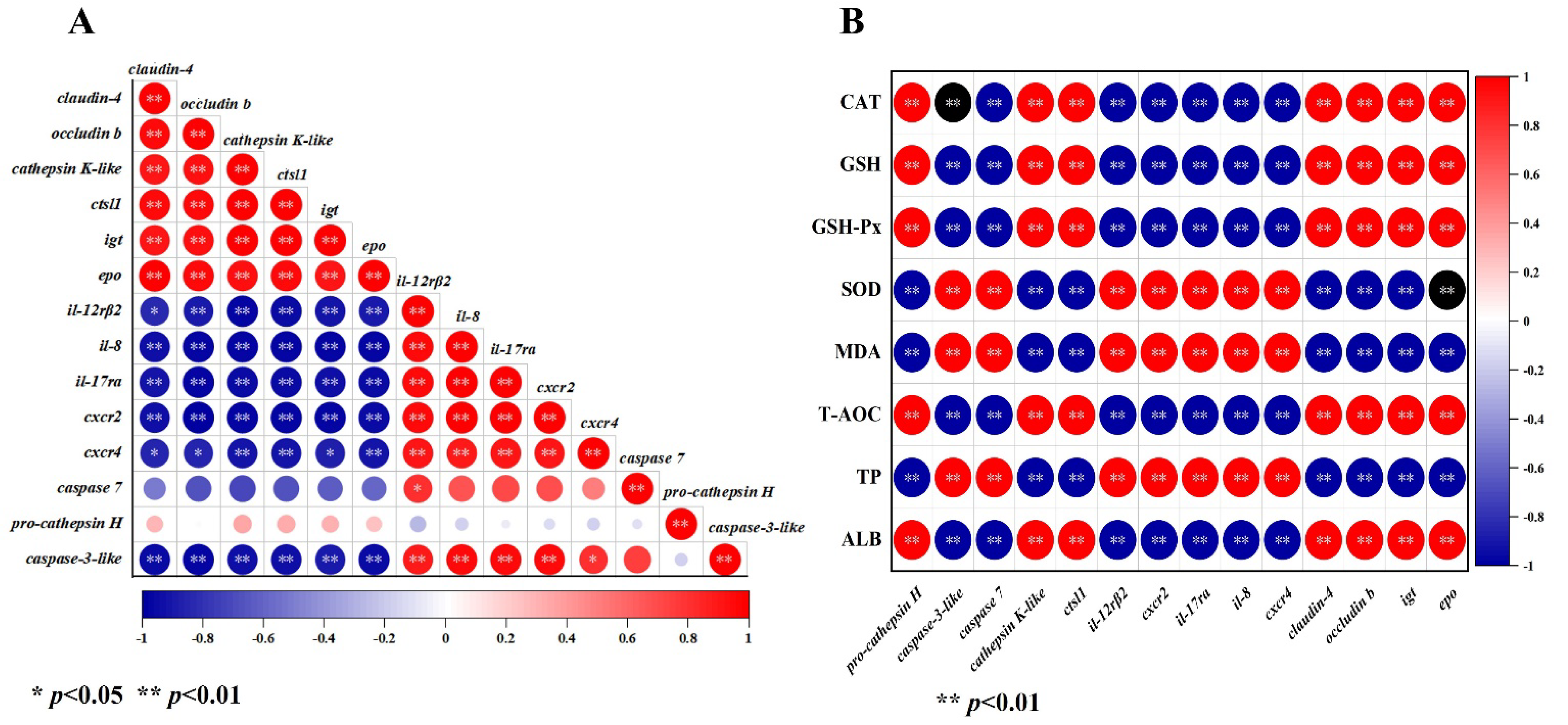
3.7. Correlation Analysis between DEGs, Serum Biochemical Indices, and Antioxidant Parameters
4. Discussion
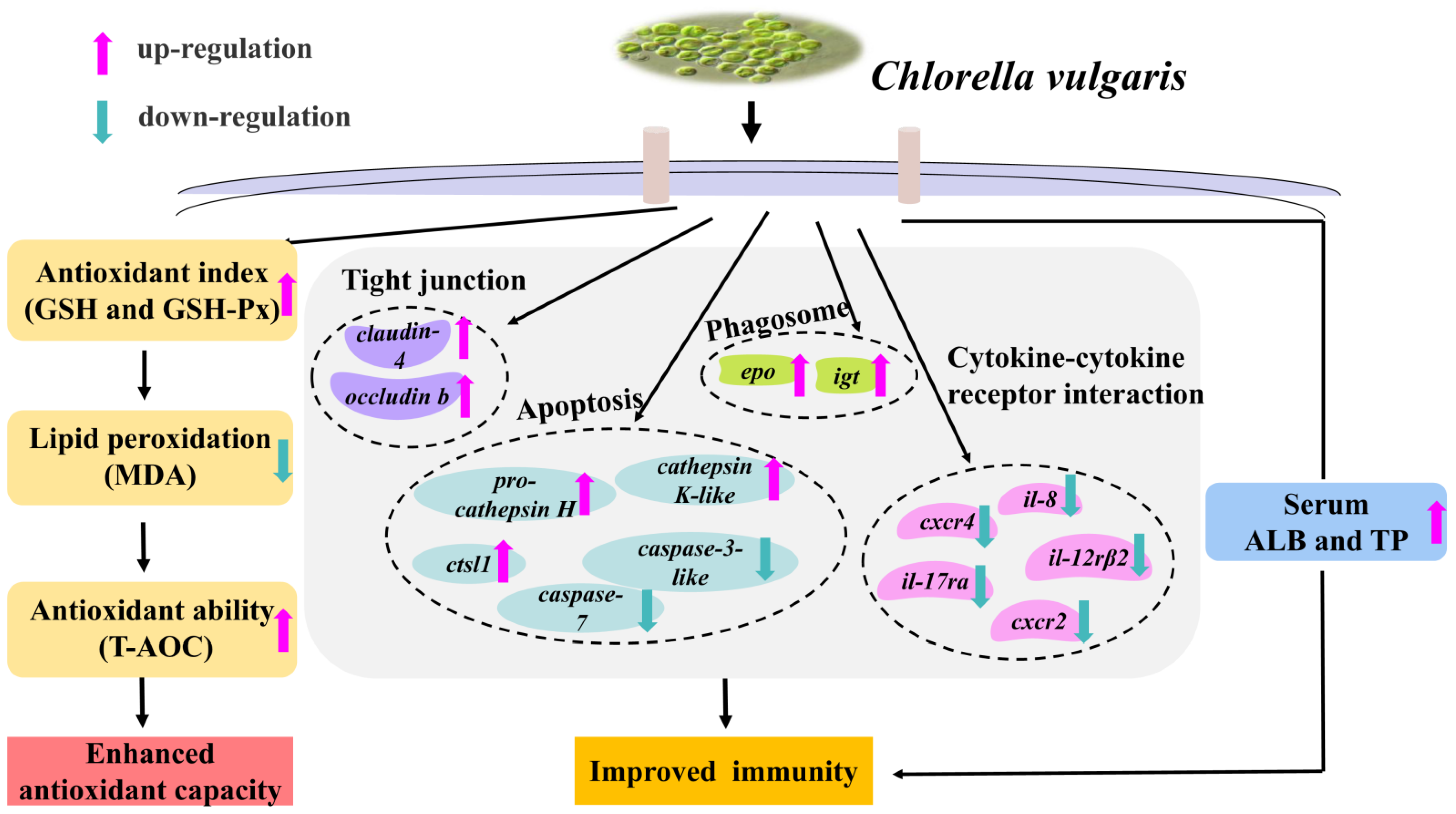
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyd, C.E.; McNevin, A.A.; Davis, R.P. The contribution of fisheries and aquaculture to the global protein supply. Food Secur. 2022, 14, 805–827. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture; Food and Agricultural Organization: Rome, Italy, 2020. Available online: http://www.fao.org/documents/card/en/c/ca9229en (accessed on 2 August 2023).
- FAO. The State of World Fisheries and Aquaculture—Towards Blue Transformation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022.
- Hussein, G.H.G.; Chen, M.; Qi, P.P.; Cui, Q.K.; Yu, Y.; Hu, W.H.; Tian, Y.; Fan, Q.X.; Gao, Z.X.; Feng, M.W.; et al. Aquaculture industry development, annual price analysis and out-of-season spawning in largemouth bass Micropterus salmoides. Aquaculture 2020, 220, 725–735. [Google Scholar] [CrossRef]
- Gatlin, D.; Barrows, F.; Brown, P.; Dabrowski, K.; Gaylord, T.; Hardy, R.; Wurtele, E. Expanding the utilization of sustainable plant products in aquafeeds: A review. Aquac. Res. 2007, 38, 551–579. [Google Scholar] [CrossRef]
- Huang, D.; Wu, Y.B.; Lin, Y.Y.; Chen, J.M.; Karrow, N.; Ren, X.; Wang, Y. Dietary protein and lipid requirements for juvenile Largemouth Bass, Micropterus salmoides. J. World Aquac. Soc. 2017, 48, 782–790. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture; Food and Agricultural Organization: Rome, Italy, 2020. Available online: http://www.fao.org/3/ca9229en/ca9229en.pdf (accessed on 2 August 2023).
- Yan, N.; Fan, C.; Chen, Y.; Hu, Z. The potential for microalgae as bioreactors to produce pharmaceuticals. Int. J. Mol. Sci. 2016, 17, 962. [Google Scholar] [CrossRef]
- Buono, S.; Langellotti, A.L.; Martello, A.; Rinna, F.; Fogliano, V. Functional ingredients from microalgae. Food Funct. 2014, 5, 1669–1685. [Google Scholar] [CrossRef]
- Tlusty, G.M.; Rhyne, A.; Szczebak, J.T.; Bourque, B.; Bowen, J.L.; Burr, G.; Marx, C.J.; Feinberg, L. A transdisciplinary approach to the initial validation of a single cell protein as an alternative protein source for use in aquafeeds. PeerJ 2017, 5, 3170. [Google Scholar] [CrossRef]
- An, B.K.; Kim, K.E.; Jeon, J.Y.; Lee, K.W. Effect of dried Chlorella vulgaris and Chlorella growth factor on growth performance, meat qualities and humoral immune responses in broiler chickens. SpringerPlus 2016, 5, 718. [Google Scholar] [CrossRef]
- Pakravan, S.; Akbarzadeh, A.; Sajjadi, M.M.; Hajimoradloo, A.; Noori, F. Chlorella vulgaris meal improved growth performance, digestive enzyme activities, fatty acid composition and tolerance of hypoxia and ammonia stress in juvenile Pacific white shrimp Litopenaeus vannamei. Aquac. Nutr. 2018, 24, 594–604. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Mousa, M.A.A.; Mamoon, A.; Abdelghany, M.F.; Abdel-Hamid, E.A.A.; Abdel-Razek, N.; Ali, F.S.; Shady, S.H.H.; Gewida, A.G.A. Dietary Chlorella vulgaris modulates the performance, antioxidant capacity, innate immunity, and disease resistance capability of Nile tilapia fingerlings fed on plant-based diets. Anim. Feed. Sci. Technol. 2022, 283, 115181. [Google Scholar] [CrossRef]
- Karapanagiotidis, I.T.; Metsoviti, M.N.; Gkalogianni, E.Z.; Psofakis, P.; Asimaki, A.; Katsoulas, N.; Papapolymerou, G.; Zarkadas, I. The effects of replacing fishmeal by Chlorella vulgaris and fish oil by Schizochytrium sp. and Microchloropsis gaditana blend on growth performance, feed efficiency, muscle fatty acid composition and liver histology of gilthead seabream (Sparus aurata). Aquaculture 2022, 561, 738709. [Google Scholar] [CrossRef]
- Safari, O.; Paolucci, M.; Motlagh, H.A. Dietary supplementation of Chlorella vulgaris improved growth performance, immunity, intestinal microbiota and stress resistance of juvenile narrow clawed crayfish, Pontastacus leptodactylus Eschscholtz, 1823. Aquaculture 2022, 554, 738138. [Google Scholar] [CrossRef]
- Yu, H.; Liang, H.L.; Ge, X.P.; Zhu, J.; Wang, Y.L.; Ren, M.C.; Chen, X.R. Dietary chlorella (Chlorella vulgaris) supplementation effectively improves body color, alleviates muscle inflammation and inhibits apoptosis in largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2022, 127, 140–147. [Google Scholar] [CrossRef]
- Dawood, M.A.O. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquac. 2021, 13, 642–663. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Xi, L.W.; Liu, H.K.; Jin, J.Y.; Yang, Y.X.; Zhu, X.M.; Han, D.; Xie, S.Q. High replacement of fishmeal by Chlorella meal affects intestinal microbiota and the potential metabolic function in largemouth bass (Micropterus salmoides). Front. Microbiol. 2022, 13, 3772. [Google Scholar] [CrossRef] [PubMed]
- Li, L.K.; Liu, X.J.; Wang, Y.; Huang, Y.Q.; Wang, C.F. Effects of alternate feeding between fish meal and novel protein diets on the intestinal health of juvenile largemouth bass (Micropterus salmoides). Aquac. Rep. 2022, 23, 101023. [Google Scholar] [CrossRef]
- Li, L.K.; Wang, Y.; Zhang, Z.; Wang, C.F. Microbiomic and metabonomic analysis provide new insights into the enhanced intestinal health in large-size largemouth bass (Micropterus salmoides) when fed novel proteins: Novel proteins are promising foods for future aquaculture. Aquaculture 2023, 563, 739019. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine. Free Radic. Biol. Med. 1985, 1, 331–332. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Sheiha, A.M.; Taha, A.E.; Swelum, A.A.; Alarifi, S.; Alkahtani, S.; Ali, D.; AlBasher, G.; Almeer, R.; Falodah, F.; et al. Impacts of enriching growing rabbit diets with Chlorella vulgaris microalgae on growth, blood variables, carcass traits, immunological and antioxidant indices. Animals 2019, 9, 788. [Google Scholar] [CrossRef] [PubMed]
- Akbary, P.; Aminikhoei, Z. Impact of dietary supplementation of Chlorella vulgaris (Beijerinck, 1890) on the growth, antioxidant defense and immune status of the grey mullet, Mugil cephalus (Linnaeus, 1758). Sustain. Aquac. Health Manag. J. 2019, 5, 57–70. [Google Scholar] [CrossRef][Green Version]
- Parvez, S.; Raisuddin, S. Protein carbonyls: Novel biomarkers of exposure to oxidative stress-inducing pesticides in freshwater fish Channa punctata (Bloch). Environ. Toxicol. Pharmacol. 2005, 20, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Bengwayan, P.T.; Laygo, J.C.; Pacio, A.E.; Poyaoan, J.L.Z.; Rebugio, J.F.; Yuson, A.L.L. A comparative study on the antioxidant property of Chlorella (Chlorella sp.) tablet and glutathione tablet. E-Int. Sci. Res. J. 2010, 2, 12–25. [Google Scholar]
- Vijayavel, K.; Anbuselvam, C.; Balasubramanian, M.P. Antioxidant effect of the marine algae Chlorella vulgaris against naphthalene-induced oxidative stress in the albino rats. Mol. Cell. Biochem. 2007, 303, 39–44. [Google Scholar] [CrossRef]
- Yaakob, Z.; Ali, E.; Zainal, A.; Mohamad, M.; Takriff, M.S. An overview: Biomolecules from microalgae for animal feed and aquaculture. J. Biol. Res. Thessalon. 2014, 21, 6. [Google Scholar] [CrossRef]
- Řehulka, J. Influence of astaxanthin on growth rate, condition, and some blood indices of rainbow trout, Oncorhynchus mykiss. Aquaculture 2000, 190, 27–47. [Google Scholar] [CrossRef]
- Ghwenm, S.S.; Kata, F.S.; Athbi, A.M. Hypoglycemicandantioxidant effect of the ethanol extract of Chlorella vulgaris inalloxan-induced diabetes mice. Arch. Biochem. Biophys. 2020, 20, 3535–3542. [Google Scholar]
- Congleton, J.L.; Wagner, T. Blood-chemistry indicators of nutritional status in juvenile salmonids. J. Fish Biol. 2010, 69, 473–490. [Google Scholar] [CrossRef]
- Johnson, P.T.J.; Rohr, J.R.; Hoverman, J.T.; Kellermanns, E.; Bowerman, J.; Lunde, K.B. Living fast and dying of infection: Host life history drives interspecific variation in infection and disease risk. Ecol. Lett. 2012, 15, 235–242. [Google Scholar] [CrossRef]
- Mao, M.G.; Jiang, J.L.; Jiang, Z.Q.; Liu, R.T.; Zhang, Q.Y.; Gui, J.F. Molecular characterization of caspase members and expression response to nervous necrosis virus outbreak in Pacific cod. Fish Shellfish Immunol. 2018, 74, 559–566. [Google Scholar] [CrossRef]
- Yabu, T.; Todoriki, S.; Yamashita, M. Stress-induced apoptosis by heat shock, UV and γ-ray irradiation in zebrafish embryos detected by increased caspase activity and whole-mount TUNEL staining. Fish. Sci. 2001, 67, 333–340. [Google Scholar] [CrossRef]
- Kim, Y.M.; Talanian, R.V.; Billiar, T.R. Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J. Biol. Chem. 1997, 272, 31138–31148. [Google Scholar] [CrossRef] [PubMed]
- Eykelbosh, A.J.; Kraak, G.V.D. A role for the lysosomal protease cathepsin B in zebrafish follicular apoptosis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 156, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Saha, D.R.; Ghosh, D.; Majumdar, T.; Bhattacharya, S.; Mazumder, S. Sub-lethal concentration of arsenic interferes with the proliferation of hepatocytes and induces in vivo apoptosis in Clarias batrachus L. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 145, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.L.; Ding, M.M.; Liu, H.S.; Wu, L.T.; Li, B.X.; Wang, A.L.; Liao, S.A.; Ye, J.M. Identification and characterization of caspase-7 in pufferfish (Takifugu obscurus) in response to bacterial infection and cell apoptosis. Aquaculture 2019, 512, 734268. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Liang, H.L.; Longshaw, M.; Wang, J.; Ge, X.P.; Zhu, J.; Li, S.L.; Ren, M.C. Effects of replacing fishmeal with methanotroph (Methylococcus capsulatus, Bath) bacteria meal (FeedKind®) on growth and intestinal health status of juvenile largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2022, 122, 298–305. [Google Scholar] [CrossRef]
- Ge, C.Y.; Liang, X.F.; Wu, X.L.; Wang, J.; Wang, H.; Qin, Y.C.; Xue, M. Yellow mealworm (Tenebrio Molitor) enhances intestinal immunity in largemouth bass (Micropterus salmoides) via the NFκB/survivin signaling pathway. Fish Shellfish Immunol. 2023, 136, 108736. [Google Scholar] [CrossRef]
- Müller, S.; Faulhaber, A.; Sieber, C.; Pfeifer, D.; Hochberg, T.; Gansz, M.; Deshmukh, S.D.; Dauth, S.; Brix, K.; Saftig, P.; et al. The endolysosomal cysteine cathepsins L and K are involved in macrophage-mediated clearance of Staphylococcus aureus and the concomitant cytokine induction. FASEB J. 2014, 28, 162–175. [Google Scholar] [CrossRef]
- Hu, X.; Hu, X.; Hu, B.; Wen, C.; Xie, Y.; Wu, D.; Tao, Z.; Li, A.; Gao, Q. Molecular cloning and characterization of cathepsin L from freshwater mussel, Cristaria plicata. Fish Shellfish Immunol. 2014, 40, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.D.; Cai, Q.F.; Yan, L.J.; Du, C.H.; Liu, G.M.; Su, W.J.; Ke, C.; Cao, M.J. Cathepsin L is an immune-related protein in Pacific abalone (Haliotis discus hannai)-Purification and characterization. Fish Shellfish Immunol. 2015, 47, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Ma, Z.T.; Zheng, T.R.; Sun, W.J.; Zhang, Y.J.; Jin, W.Q.; Zhan, J.Y.; Cai, Y.T.; Tang, Y.J.; Wu, Q.; et al. Insights into the correlation between physiological changes in and seed development of tartary buckwheat (Fagopyrum tataricum Gaertn.). BMC Genomics 2018, 19, 648. [Google Scholar] [CrossRef] [PubMed]
- Harikrishnan, R.; Kim, M.-C.; Kim, J.-S.; Han, Y.-J.; Jang, I.-S.; Balasundaram, C.; Heo, M.-S. Immune response and expression analysis of cathepsin K in goldfish during Aeromonas hydrophila infection. Fish Shellfish Immunol. 2010, 28, 511–516. [Google Scholar] [CrossRef]
- Lv, Z.; Qiu, L.M.; Liu, Z.Q.; Wang, W.L.; Chen, H.; Jia, Y.K.; Jia, Z.H.; Jiang, S.; Wang, L.L.; Song, L.S. Molecular characterization of a cathepsin L1 highly expressed in phagocytes of pacific oyster Crassostrea gigas. Dev. Comp. Immunol. 2018, 89, 152–162. [Google Scholar] [CrossRef]
- Schweiger, A.; Christensen, I.J.; Nielsen, H.J.; Sørensen, S.; Brunner, N.; Kos, J. Serum cathepsin H as a potential prognostic marker in patients with colorectal cancer. Int. J. Biol. Markers 2004, 19, 289–294. [Google Scholar] [CrossRef]
- Fan, J.; Yang, Y.; Ma, C.; Liu, X.; Wang, Y.; Chen, F.; Wang, B.; Bian, X.; Yang, C.; Zhang, N. The effects and cell barrier mechanism of main dietary nutrients on intestinal barrier. Curr. Opin. Food Sci. 2022, 48, 100942. [Google Scholar] [CrossRef]
- Rymuszka, A.; Adaszek, Ł. Pro-and anti-inflammatory cytokine expression in carp blood and head kidney leukocytes exposed to cyanotoxin stresse-An in vitro study. Fish Shellfish Immunol. 2012, 33, 382–388. [Google Scholar] [CrossRef]
- Zuo, Z.H.; Wang, S.D.; Wang, Q.J.; Wang, D.J.; Wu, Q.P.; Xie, S.L.; Zou, J.X. Effects of partial replacement of dietary flour meal with seaweed polysaccharides on the resistance to ammonia stress in the intestine of hybrid snakehead (Channa maculatus♀× Channa argus♂). Fish Shellfish Immunol. 2022, 127, 271–279. [Google Scholar] [CrossRef]
- Qiu, X.Y.; Wang, D.; Lv, M.Y.; Sun, H.; Ren, J.Q.; Wang, X.Y.; Zhou, H. Identification and functional characterization of interleukin-12 receptor beta 1 and 2 in grass carp (Ctenopharyngodon idella). Mol. Immunol. 2022, 143, 58–67. [Google Scholar] [CrossRef]
- Song, X.H.; Tang, J.; Gao, T.T.; Xu, X.F.; Yang, H.X.; Wu, K.; Yang, C.G.; Cheng, Z.Q.; Sun, B.Y. Interleukin-12 receptor β2 from grass carp: Molecular characterization and its involvement in Aeromonas hydrophila-induced intestinal inflammation. Fish Shellfish Immunol. 2019, 87, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Le, Y.; Zhou, Y.; Iribarren, P.; Wang, J. Chemokines and chemokine receptors: Their manifold roles in homeostasis and disease. Cell. Mol. Immunol. 2004, 1, 95–104. [Google Scholar] [PubMed]
- Waugh, D.J.; Wilson, C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Manegold, P.C.; Hong, Y.K.; Zhang, W.; Pohl, A.; Lurje, G.; Winder, T.; Yang, D.Y.; LaBonte, M.J.; Wilson, P.M.; et al. Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int. J. Cancer 2011, 128, 2038–2049. [Google Scholar] [CrossRef]
- Liu, K.W.; Wu, L.J.; Yuan, S.G.; Wu, M.; Xu, Y.M.; Sun, Q.Q.; Li, S.; Zhao, S.W.; Hua, T.; Liu, Z.J. Structural basis of CXC chemokine receptor 2 activation and signalling. Nature 2020, 585, 135–140. [Google Scholar] [CrossRef]
- Koelink, P.J.; Overbeek, S.A.; Braber, S.; Kruijf, P.; Folkerts, G.; Smit, M.J.; Kraneveld, A.D. Targeting chemokine receptors in chronic inflammatory diseases: An extensive review. Pharmacol. Ther. 2012, 133, 1–18. [Google Scholar] [CrossRef]
- Balkwill, F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin. Cancer Biol. 2004, 14, 171–179. [Google Scholar] [CrossRef]
- Dey, R.; Ji, K.; Liu, Z.; Chen, L. A Cytokine-Cytokine Interaction in the assembly of Higher-Order structure and activation of the Interleukine-3: Receptor Complex. PLoS ONE 2009, 4, e5188. [Google Scholar] [CrossRef]
- Lasry, A.; Zinger, A.; Benneriah, Y. Inflammatory networks underlying colorectal cancer. Nat. Immunol. 2016, 17, 230–240. [Google Scholar] [CrossRef]
- Ma, T.Y.; Iwamoto, G.K.; Hoa, N.T.; Akotia, V.; Said, H.M.J.A.G.; Physiology, L. TNF-α-induced increase in intestinal epithelial tight junction permeability requires NF-κB activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G367–G376. [Google Scholar] [CrossRef]
- Xie, M.; Xie, Y.; Li, Y.; Zhou, W.; Zhang, Z.; Yang, Y.; Zhou, Z. The effects of fish meal replacement with ultra-micro ground mixed plant proteins (uPP) in practical diet on growth, gut and liver health of common carp (Cyprinus carpio). Aquac. Rep. 2001, 19, 100558. [Google Scholar] [CrossRef]
- Xie, X.Z.; Wang, J.; Guan, Y.; Xing, S.J.; Liang, X.F.; Xue, M.; Wang, J.J.; Chang, Y.; Leclercq, E. Cottonseed protein concentrate as fishmeal alternative for largemouth bass (Micropterus salmoides) supplemented a yeast-based paraprobiotic: Effects on growth performance, gut health and microbiome. Aquaculture 2022, 551, 737898. [Google Scholar] [CrossRef]
- Pinton, P.; Braicu, C.; Nougayrede, J.P.; Laffitte, J.; Taranu, I.; Oswald, I.P. Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of claudin-4 through a mitogen-activated protein kinase-dependent mechanism. J. Nutr. 2010, 140, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.P.; Waddell, A.; Fulkerson, P.C. Eosinophils in infection and intestinal immunity. Curr. Opin. Gastroen. 2013, 29, 7. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Ming, Y.; Ma, Y.X.; Zhang, X.T.; Qian, S.C.; Fei, H. Molecular cloning, characterization, and expression analyses of immunoglobulin tau heavy chain (IgT) in largemouth bass (Micropterus salmoides). Aquac. Rep. 2022, 22, 100989. [Google Scholar] [CrossRef]
- Zhang, Y.A.; Salinas, I.; Li, J.; Parra, D.; Bjork, S.; Xu, Z.; LaPatra, S.E.; Bartholomew, J.; Sunyer, J.O. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 2010, 11, 827–835. [Google Scholar] [CrossRef]
- Xu, Z.; Parra, D.; Gómez, D.; Salinas, I.; Zhang, Y.A.; Von, G.J.L.; Heinecke, R.D.; Buchmann, K.; LaPatra, S.; Sunyer, J.O. Teleost skin, an ancient mucosal surface that elicits gut-like immune responses. Proc. Natl. Acad. Sci. USA 2013, 110, 13097–13102. [Google Scholar] [CrossRef]

| Items | Methods/Assay Kits/Testing Equipment |
|---|---|
| ALT | The serum biochemical indices were measured using an automatic biochemical analyzer, a Mindary BS-400 (Shenzhen, China) and assay kits (Mindray) purchased from Gansu Heyuan Biotechnology Co., Ltd. (Gansu, China). |
| AST | |
| ALB | |
| TP | |
| GSH | The intestinal antioxidant parameters were detected using the kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China), and the samples were prepared according to the manufacturer’s instructions. |
| GSH-Px | |
| SOD | |
| T-AOC | |
| CAT | |
| MDA |
| Gene Name | Sequence | Tm (°C) | GC (%) | Accession No. | |
|---|---|---|---|---|---|
| pro-cathepsin H | F | AAGGCCATGGTTGATGCTGT | 60.25 | 50 | XM_038701870.1 |
| R | CAGACTGTGCCCCAAGAGTT | 59.89 | 55 | ||
| caspase-3-like | F | CCCTCAGCAAACTGGGCTT | 60.23 | 57.89 | XM_038739762.1 |
| R | TGAGGAACACTTTGGCCTTTTTC | 59.87 | 43.48 | ||
| caspase 7 | F | CCTACACCTTCCAGGCCAAA | 59.6 | 55 | XM_038734228.1 |
| R | CGCAGACATCAGTTGCTCAC | 59.56 | 55 | ||
| cathepsin K-like | F | AGGGCCATTTGGGAGAAGAAC | 60.27 | 52.38 | XM_038694428.1 |
| R | CGATTTGGGAAGCTTGGACAC | 59.8 | 52.38 | ||
| ctsl1 | F | AGATCGAGCTGCACAACCTG | 60.39 | 55 | XM_038704447.1 |
| R | CACTGACCCTGGTCCTTCAC | 59.96 | 60 | ||
| il-12rβ2 | F | TCCAGTATCGGACTGAGGCA | 60.03 | 55 | XM_038698864.1 |
| R | TCGAAGCTTGCAGGGAATGT | 59.96 | 50 | ||
| cxcr2 | F | CAGGTTGGACATAGTGCCGT | 60.04 | 55 | XM_038721015.1 |
| R | AAGACCTGCTGCTTCTTGCT | 59.89 | 50 | ||
| il-17ra | F | ATGTGTGGCGACAAAGAGGT | 59.89 | 50 | |
| R | GTGATTCACTCTGCCCGGTT | 60.32 | 55 | ||
| il-8 | F | GAGGGTACATGTCTGGGGGA | 60.33 | 60 | XM_038713529.1 |
| R | CCTTGAAGGTTTGTTCTTCATCGT | 59.72 | 41.67 | ||
| cxcr4 | F | GGTCCAGATGACTGCTGCTT | 60.04 | 55 | XM_038726066.1 |
| R | GCTGGATCACTCGGATGGTT | 59.82 | 55 | ||
| claudin-4 | F | TGAGGTACTCCAAGGCTCGT | 60.25 | 55 | XM_038707645.1 |
| R | GCAACAATGGTGTAGGGGGA | 59.96 | 55 | ||
| occludin b | F | GGTCTGGGAAGTGGAGTTGG | 59.96 | 60 | XM_038703759.1 |
| R | TGGTGAGCGGGCAGTATTTT | 59.96 | 50 | ||
| igt | F | CTTCTGCTGGTTGCTCTCTCT | 59.72 | 52.38 | |
| R | GCTGGCGTAATCTGTTTTGCT | 59.8 | 47.62 | ||
| epo | F | ATCTCGGCAGTCCTCTCCTT | 60.03 | 55 | XM_038733743.1 |
| R | TTGCGTTGAGTGAGCGTTTG | 59.97 | 50 |
| Dietary C. vulgaris Levels (g/kg) | ALT (U/L) | AST (U/L) | ALB (g/L) | TP (g/L) |
|---|---|---|---|---|
| 0 | 3.78 ± 0.67 | 7.75 ± 1.06 | 13.77 ± 0.58 a | 46.56 ± 1.14 a |
| 38 | 3.29 ± 0.75 | 10.71 ± 1.21 | 17.09 ± 0.42 b | 49.52 ± 0.70 ab |
| 76 | 3.63 ± 0.85 | 10.85 ± 2.22 | 17.54 ± 0.34 b | 51.62 ± 1.52 b |
| 114 | 2.79 ± 0.38 | 10.99 ± 1.87 | 15.60 ± 0.81 ab | 45.11 ± 0.97 a |
| 152 | 1.49 ± 0.27 | 8.84 ± 0.60 | 15.18 ± 0.67 ab | 44.78 ± 1.53 a |
| Sample | Total Reads | Valid Ratio (%) | Q30 (%) | GC Content (%) |
|---|---|---|---|---|
| CHL0-1 | 40,723,120 | 95.40% | 94.74 | 47.26 |
| CHL0-2 | 44,880,612 | 96.08% | 95.01 | 47.33 |
| CHL0-3 | 43,214,730 | 96.13% | 94.88 | 47.26 |
| CHL76-1 | 44,842,096 | 96.66% | 95.08 | 49.08 |
| CHL76-2 | 47,363,898 | 95.60% | 94.19 | 47.35 |
| CHL76-3 | 38,524,894 | 95.53% | 93.62 | 47.43 |
| Signaling Pathway Name | Gene Name | q Value | Regulation | Log2FC |
|---|---|---|---|---|
| Apoptosis | pro-cathepsin H | 0.000 | up | 2.46 |
| caspase-3-like | 0.000 | down | −1.62 | |
| caspase-7 | 0.000 | down | −1.80 | |
| cathepsin K-like | 0.000 | up | 9.66 | |
| ctsl1 | 0.000 | up | 3.51 | |
| Cytokine–cytokine receptor interaction | il-12rβ2 | 0.000 | down | −1.25 |
| cxcr2 | 0.001 | down | −1.18 | |
| il-17ra | 0.048 | down | −1.04 | |
| il-8 | 0.032 | down | −1.00 | |
| cxcr4 | 0.002 | down | −1.18 | |
| Tight junction | claudin-4 | 0.001 | up | 1.28 |
| occludin b | 0.000 | up | 2.75 | |
| Phagosome | igt | 0.000 | up | 5.94 |
| epo | 0.000 | up | 1.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Ge, X.; Huang, D.; Xue, C.; Ren, M.; Liang, H. Dietary Supplementation of Chlorella vulgaris Effectively Enhanced the Intestinal Antioxidant Capacity and Immune Status of Micropterus salmoides. Antioxidants 2023, 12, 1565. https://doi.org/10.3390/antiox12081565
Yu H, Ge X, Huang D, Xue C, Ren M, Liang H. Dietary Supplementation of Chlorella vulgaris Effectively Enhanced the Intestinal Antioxidant Capacity and Immune Status of Micropterus salmoides. Antioxidants. 2023; 12(8):1565. https://doi.org/10.3390/antiox12081565
Chicago/Turabian StyleYu, Heng, Xianping Ge, Dongyu Huang, Chunyu Xue, Mingchun Ren, and Hualiang Liang. 2023. "Dietary Supplementation of Chlorella vulgaris Effectively Enhanced the Intestinal Antioxidant Capacity and Immune Status of Micropterus salmoides" Antioxidants 12, no. 8: 1565. https://doi.org/10.3390/antiox12081565
APA StyleYu, H., Ge, X., Huang, D., Xue, C., Ren, M., & Liang, H. (2023). Dietary Supplementation of Chlorella vulgaris Effectively Enhanced the Intestinal Antioxidant Capacity and Immune Status of Micropterus salmoides. Antioxidants, 12(8), 1565. https://doi.org/10.3390/antiox12081565







