The Dose-Related Efficacy of Human Placenta-Derived Mesenchymal Stem Cell Transplantation on Antioxidant Effects in a Rat Model with Ovariectomy
Abstract
1. Introduction
2. Methods
2.1. Cell Culture of PD-MSCs
2.2. Establishment of a 1/2 OVX Rat Model
2.3. Ovarian Explant Culture (Ex Vivo)
2.4. RNA Isolation and Quantitative Polymerase Reaction
2.5. Protein Isolation and Western Blotting Analysis
2.6. Enzyme-Linked Immunosorbent Assay
2.7. Immunofluorescence Staining for the Accumulation of Superoxide in Mitochondria
2.8. Histological Analysis of the Number of Follicles
2.9. Histological Analysis for Immunohistochemistry and TUNEL Assay
2.10. Statistical Analysis
3. Results
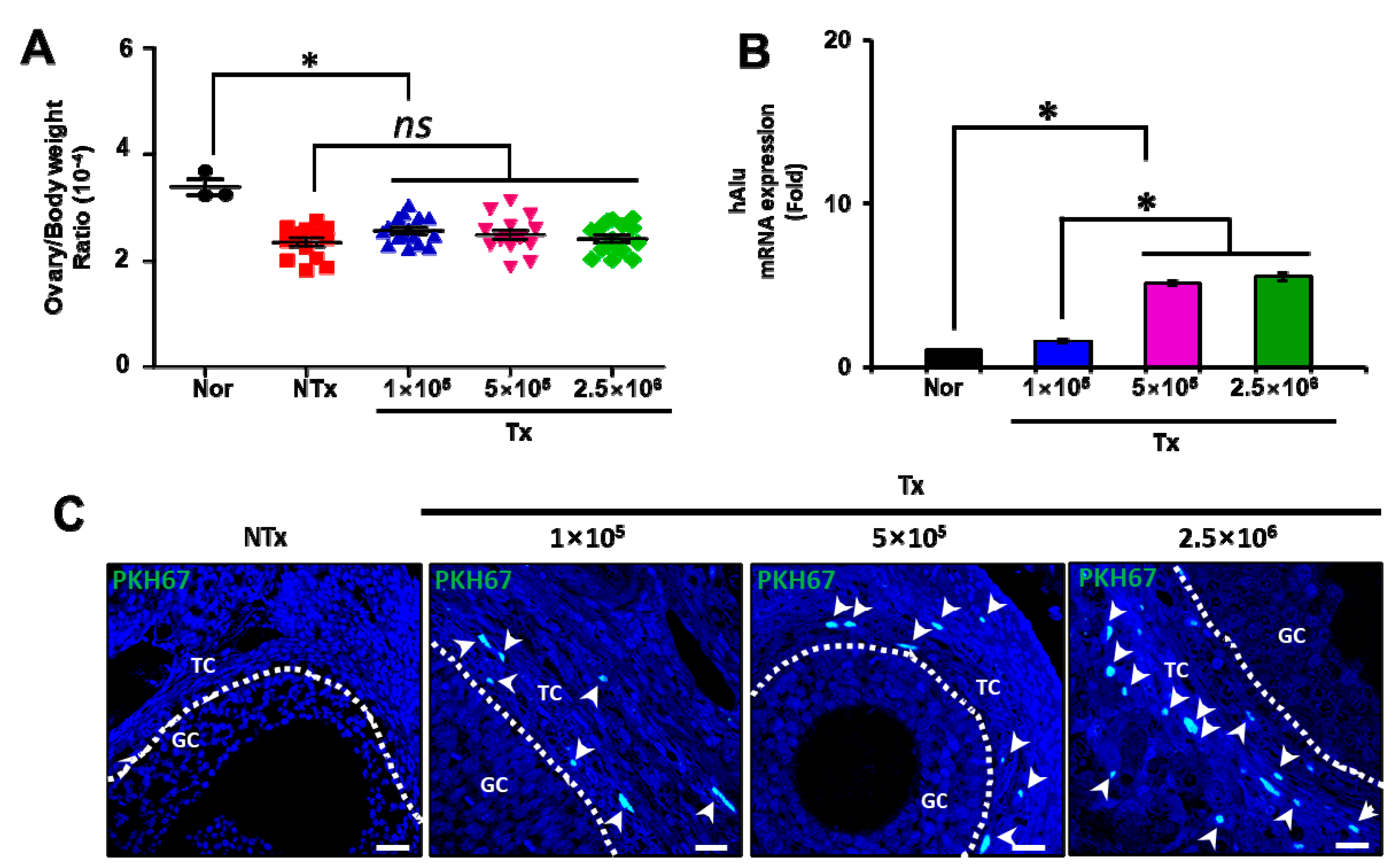
3.1. Transplanted PD-MSCs Can Engraft into Injured Ovarian Tissues in a Half-Ovariectomized Model
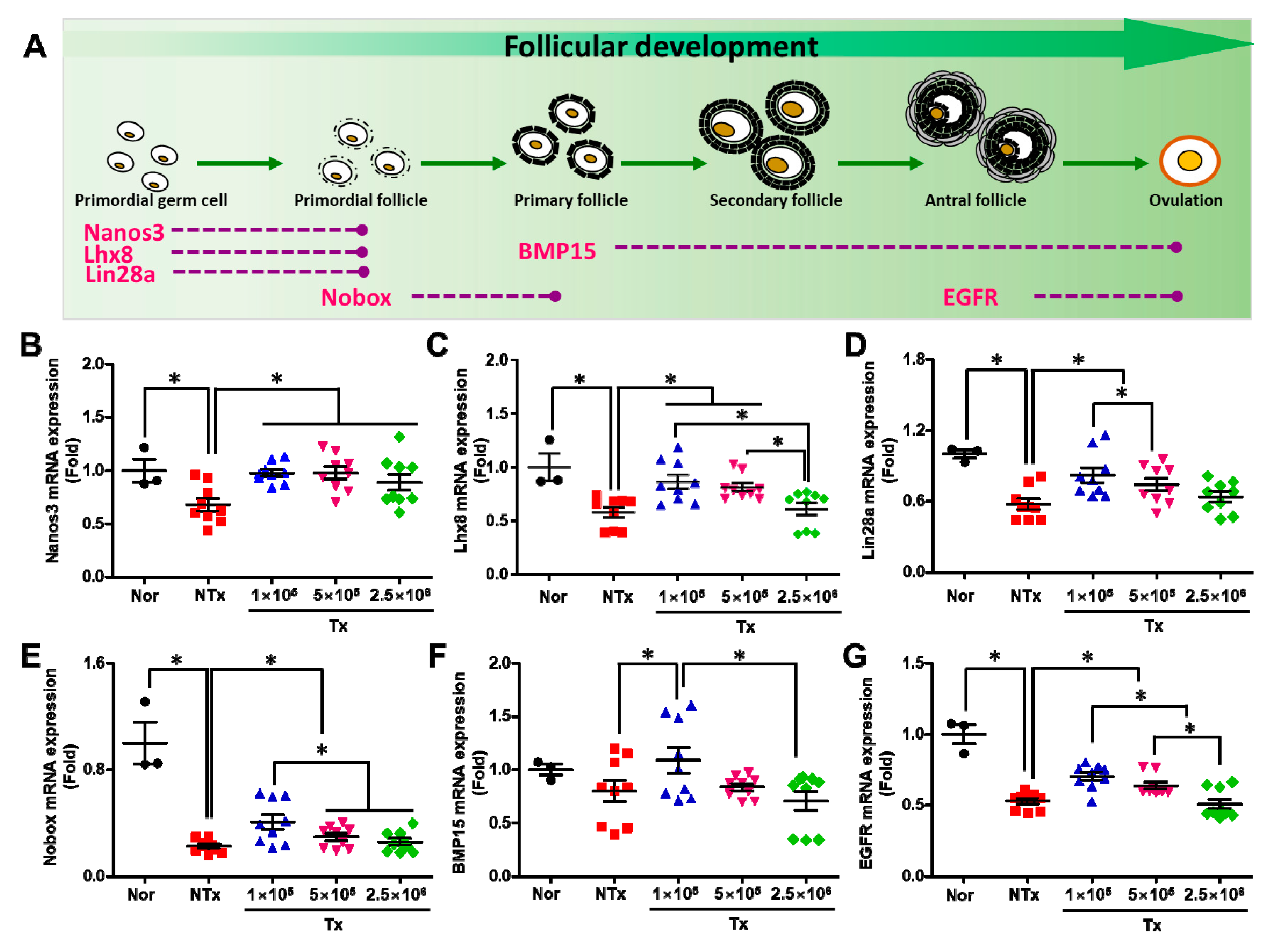
3.2. Transplanted PD-MSCs Improve Ovarian Function with Follicular Development in 1/2 OVX Model
3.3. Transplanted PD-MSCs Regulate Ovarian Function, Increasing the Reproductive Hormone and Numbers of Follicles in the 1/2 OVX Model
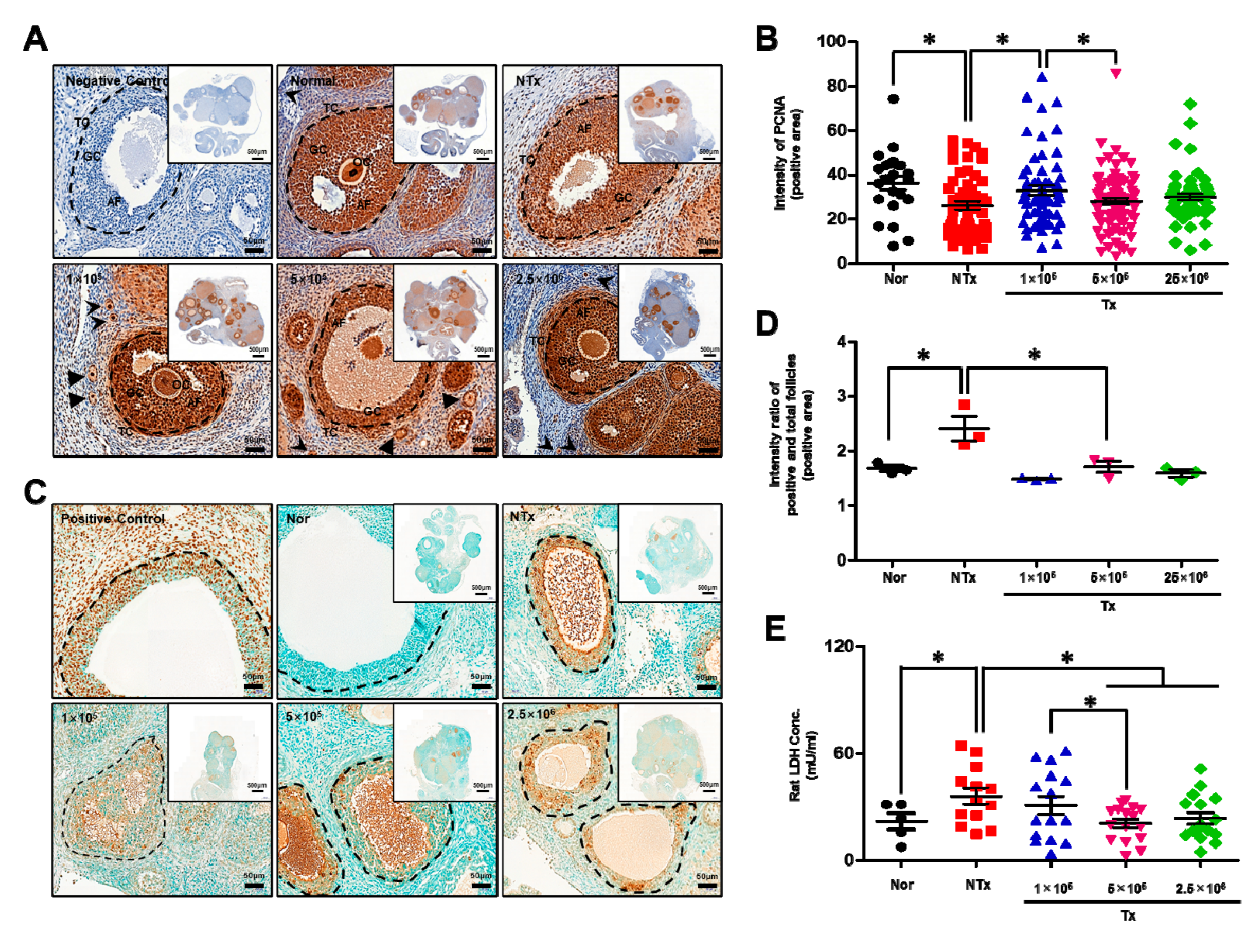
3.4. Transplanted PD-MSCs Stimulate Proliferation by Inhibiting Cell Death in a 1/2 OVX Rat Model
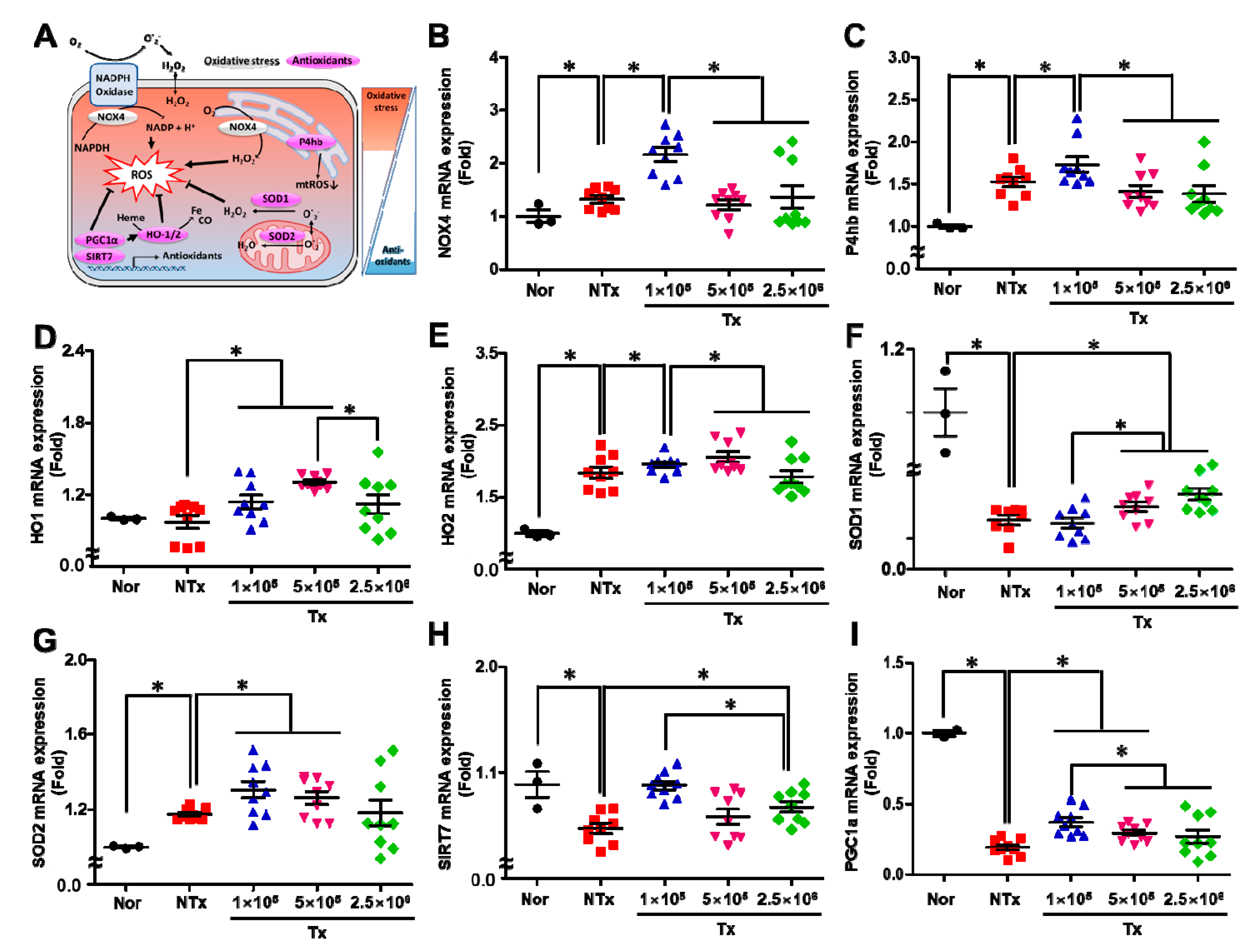
3.5. Transplanted PD-MSCs Enhance Antioxidant Activity to Reduce Oxidative Stress in the 1/2 OVX Model
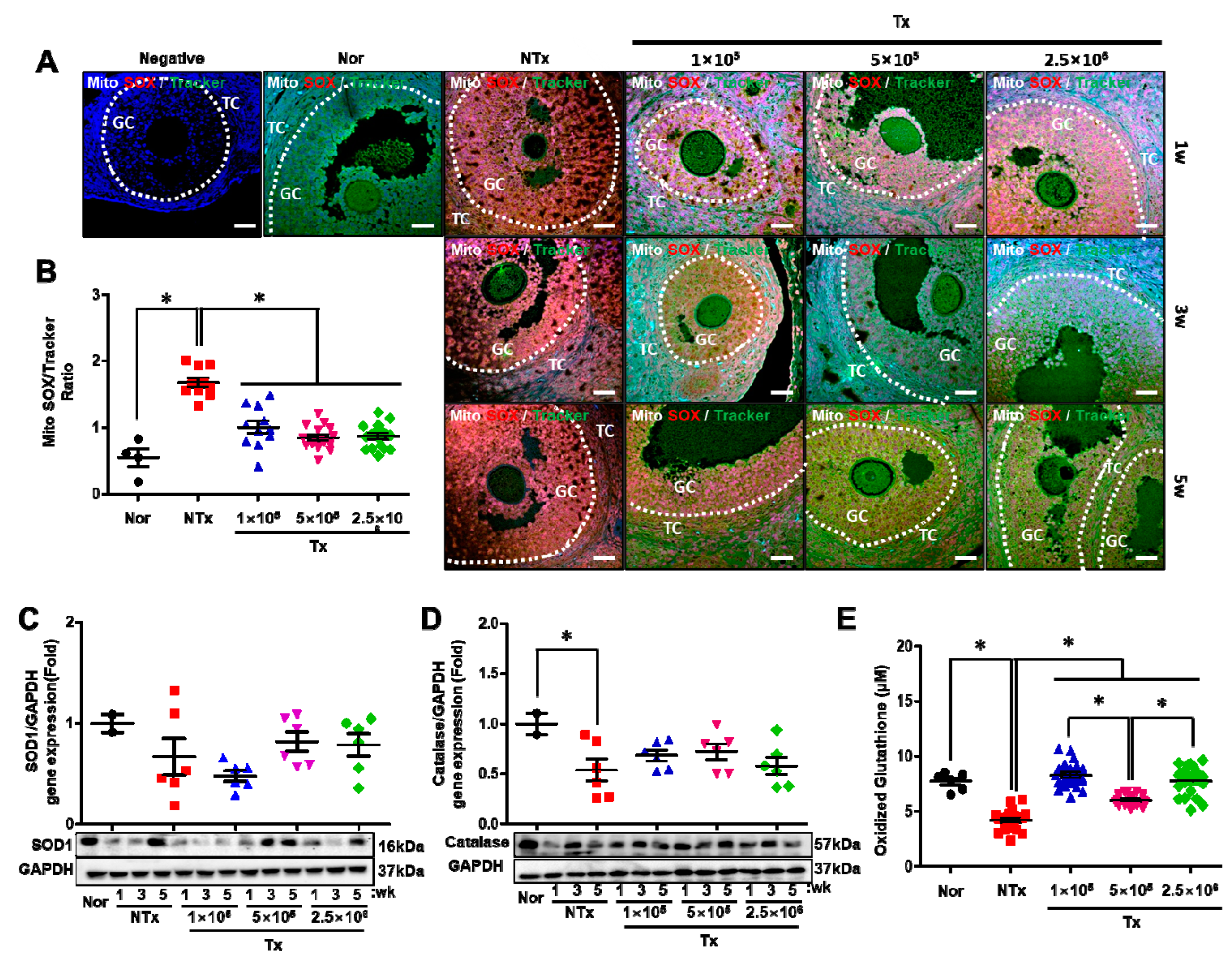
3.6. Transplanted PD-MSCs Enhance Antioxidant Activity and Reduce Cell Death in a 1/2 OVX Model
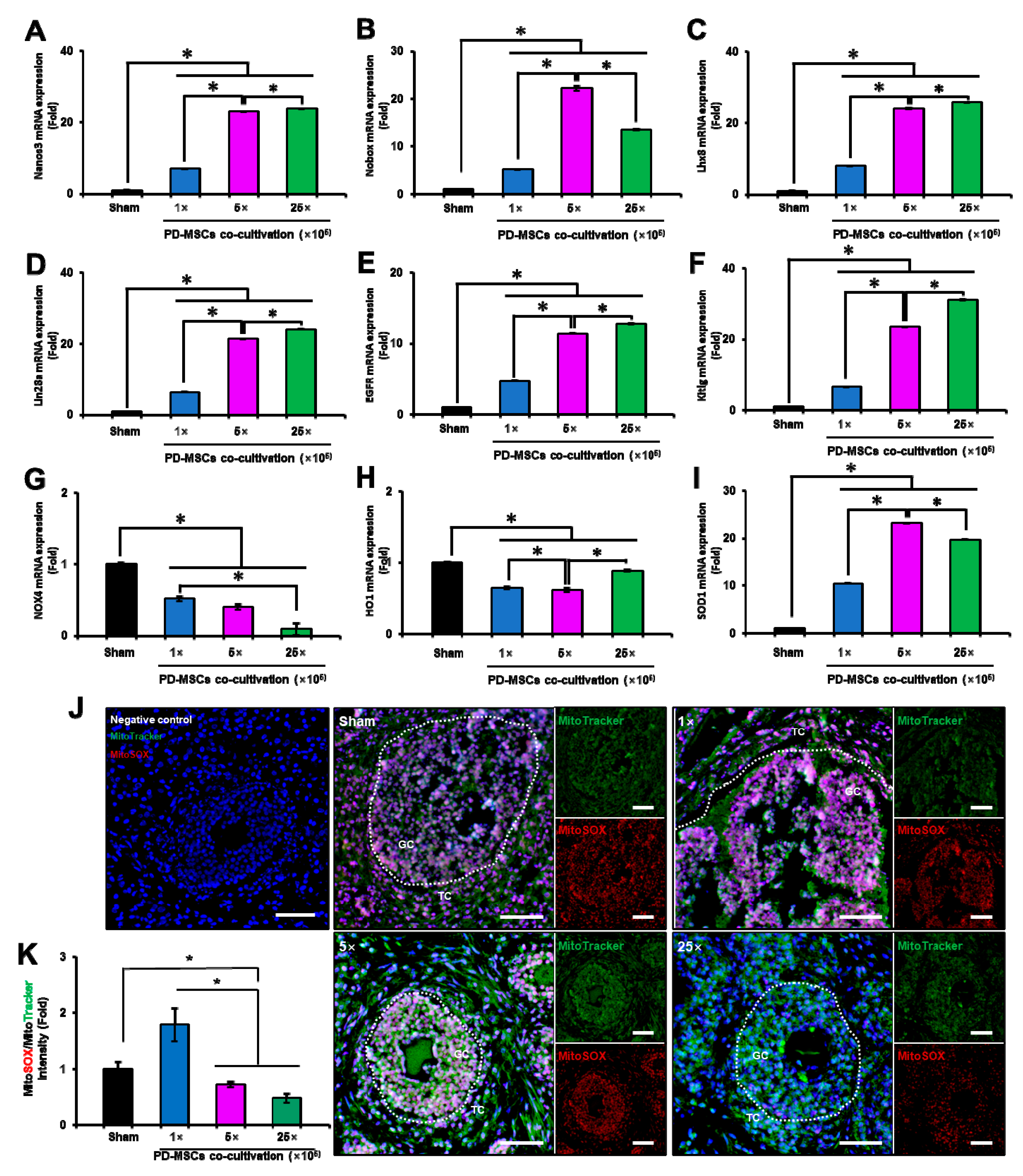
3.7. PD-MSC Cocultivation Improves Antioxidant Activity and Reduces Cell Death in a 1/2 OVX Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Luderer, U. Ovarian toxicity from reactive oxygen species. Vitam. Horm. 2014, 94, 99–127. [Google Scholar]
- Pandey, A.N.; Tripathi, A.; Premkumar, K.V.; Shrivastav, T.G.; Chaube, S.K. Reactive oxygen and nitrogen species during meiotic resumption from diplotene arrest in mammalian oocytes. J. Cell Biochem. 2010, 111, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.N.; Chaube, S.K. A moderate increase of hydrogen peroxide level is beneficial for spontaneous resumption of meiosis from diplotene arrest in rat oocytes cultured in vitro. Biores Open Access. 2014, 3, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Shrivastav, T.G.; Chaube, S.K. An increase of granulosa cell apoptosis mediates aqueous neem (Azadirachta indica) leaf extract-induced oocyte apoptosis in rat. Int. J. Appl. Basic Med. Res. 2013, 3, 27–36. [Google Scholar] [PubMed]
- Prasad, S.; Tiwari, M.; Tripathi, A.; Pandey, A.N.; Chaube, S.K. Changes in signal molecules and maturation promoting factor levels associate with spontaneous resumption of meiosis in rat oocytes. Cell Biol. Int. 2015, 39, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Spees, J.L.; Lee, R.H.; Gregory, C.A. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016, 7, 125. [Google Scholar] [CrossRef]
- Parolini, O.; Alviano, F.; Bagnara, G.P.; Bilic, G.; Buhring, H.J.; Evangelista, M.; Hennerbichler, S.; Liu, B.; Magatti, M.; Mao, N. Concise review: Isolation and characterization of cells from human term placenta: Outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells 2008, 26, 300–311. [Google Scholar] [CrossRef]
- Choi, J.H.; Seok, J.; Lim, S.M.; Kim, T.H.; Kim, G.J. Microenvironmental changes induced by placenta-derived mesenchymal stem cells restore ovarian function in ovariectomized rats via activation of the PI3K-FOXO3 pathway. Stem Cell Res. Ther. 2020, 11, 486. [Google Scholar] [CrossRef]
- Abd-Allah, S.H.; El-Shal, A.S.; Shalaby, S.M.; Abd-Elbary, E.; Mazen, N.F.; Abdel Kader, R.R. The role of placenta-derived mesenchymal stem cells in healing of induced full-thickness skin wound in a mouse model. IUBMB Life 2015, 67, 701–709. [Google Scholar] [CrossRef]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.F.; Lian, Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspectives. Cell Transpl. 2014, 23, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Kim, T.H.; Seok, J.; Jun, J.H.; Park, H.; Kweon, M.; Lim, J.-Y.; Kim, G.J. Vascular remodeling by placenta-derived mesenchymal stem cells restores ovarian function in ovariectomized rat model via the VEGF pathway. Lab Invest. 2021, 101, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Na, J.; Song, J.; Kim, H.H.; Seok, J.; Kim, J.Y.; Jun, J.H.; Kim, G.J. Human placenta-derived mesenchymal stem cells trigger repair system in TAA-injured rat model via antioxidant effect. Aging 2020, 13, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Halabian, R.; Imani Fooladi, A.A. A revealing review of mesenchymal stem cells therapy, clinical perspectives and Modification strategies. Stem Cell Investig. 2019, 6, 34. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, Q.; Wang, H.; Han, L.; Dai, H.; Qian, X.; Yu, H.; Yin, M.; Shi, F.; Qi, N. Mesenchymal Stem Cell Therapy Using Human Umbilical Cord in a Rat Model of Autoimmune-Induced Premature Ovarian Failure. Stem Cells Int. 2020, 2020, 3249495. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, J.; Zhang, X.; Liu, Y.; Chen, J.; Hu, B.; Song, J.; Zhang, Y. Strategies to Optimize Adult Stem Cell Therapy for Tissue Regeneration. Int. J. Mol. Sci. 2016, 17, 982. [Google Scholar] [CrossRef]
- Mousaei Ghasroldasht, M.; Seok, J.; Park, H.S.; Liakath Ali, F.B.; Al-Hendy, A. Stem Cell Therapy: From Idea to Clinical Practice. Int. J. Mol Sci. 2022, 23, 2850. [Google Scholar] [CrossRef]
- Kohen, R.; Nyska, A. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef]
- Zitka, O.; Skalickova, S.; Gumulec, J.; Masarik, M.; Adam, V.; Hubalek, J.; Trnkova, L.; Kruseova, J.; Eckschlager, T.; Kizek, R. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol Lett. 2012, 4, 1247–1253. [Google Scholar] [CrossRef]
- Rajendran, P.; Nandakumar, N.; Rengarajan, T.; Palaniswami, R.; Gnanadhas, E.N.; Lakshminarasaiah, U.; Gopas, J.; Nishigaki, I. Antioxidants and human diseases. Clin. Chim. Acta. 2014, 436, 332–347. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, S.M.; El-Shal, A.S.; Abd-Allah, S.H.; Selim, A.O.; Selim, S.A.; Gouda, Z.A.; El Motteleb, D.M.A.; Zanfaly, H.E.; El-Assar, H.M.; Abdelazim, S. Mesenchymal stromal cell injection protects against oxidative stress in Escherichia coli-induced acute lung injury in mice. Cytotherapy 2014, 16, 764–775. [Google Scholar] [CrossRef]
- Stavely, R.; Nurgali, K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem Cells Transl. Med. 2020, 9, 985–1006. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, B.; Panahi, M.; Saraygord-Afshari, N.; Taheri, N.; Bilici, M.; Jafari, D.; Alizadeh, E. The secretome of mesenchymal stem cells and oxidative stress: Challenges and opportunities in cell-free regenerative medicine. Mol. Biol. Rep. 2021, 48, 5607–5619. [Google Scholar] [CrossRef] [PubMed]
- Orisaka, M.; Tajima, K.; Tsang, B.K.; Kotsuji, F. Oocyte-granulosa-theca cell interactions during preantral follicular development. J. Ovarian Res. 2009, 2, 9. [Google Scholar] [CrossRef]
- Kaipia, A.; Hsueh, A.J. Regulation of ovarian follicle atresia. Annu. Rev. Physiol. 1997, 59, 349–363. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Chen, Y.; Yuan, L.; Liu, H.; Wang, J.; Liu, Q.; Zhang, Y. Adipose-Derived Stem Cells: Current Applications and Future Directions in the Regeneration of Multiple Tissues. Stem Cells Int. 2020, 2020, 8810813. [Google Scholar] [CrossRef]
- Golpanian, S.; Schulman, I.H.; Ebert, R.F.; Heldman, A.W.; DiFede, D.L.; Yang, P.C.; Wu, J.C.; Bolli, R.; Perin, E.C.; Moyé, L.; et al. Concise Review: Review and Perspective of Cell Dosage and Routes of Administration From Preclinical and Clinical Studies of Stem Cell Therapy for Heart Disease. Stem Cells Transl. Med. 2016, 5, 186–191. [Google Scholar] [CrossRef]
- Schuleri, K.H.; Feigenbaum, G.S.; Centola, M.; Weiss, E.S.; Zimmet, J.M.; Turney, J.; Kellner, J.; Zviman, M.M.; Hatzistergos, K.E.; Detrick, B. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur. Heart, J. 2009, 30, 2722–2732. [Google Scholar] [CrossRef]
- Hashemi, S.M.; Ghods, S.; Kolodgie, F.D.; Parcham-Azad, K.; Keane, M.; Hamamdzic, D.; Young, R.; Rippy, M.K.; Virmani, R.; Litt, H. A placebo controlled, dose-ranging, safety study of allogenic mesenchymal stem cells injected by endomyocardial delivery after an acute myocardial infarction. Eur. Heart J. 2008, 29, 251–259. [Google Scholar] [CrossRef]
- Eylert, G.; Dolp, R.; Parousis, A.; Cheng, R.; Auger, C.; Holter, M.; Lang-Olip, I.; Reiner, V.; Kamolz, L.-P.; Jeschke, M.G. Correction to: Skin regeneration is accelerated by a lower dose of multipotent mesenchymal stromal/stem cells-a paradigm change. Stem Cell Res. Ther. 2021, 12, 256. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Choi, J.H.; Jun, Y.; Lim, S.M.; Park, S.; Paek, J.Y.; Lee, S.-H.; Hwang, J.-Y.; Kim, G.J. 3D-cultured human placenta-derived mesenchymal stem cell spheroids enhance ovary function by inducing folliculogenesis. Sci. Rep. 2018, 8, 15313. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Seok, J.; You, J.H.; Lee, D.H.; Lim, J.Y.; Kim, G.J. Can a Large Number of Transplanted Mesenchymal Stem Cells Have an Optimal Therapeutic Effect on Improving Ovarian Function? Int. J. Mol. Sci. 2022, 23, 16009. [Google Scholar] [CrossRef] [PubMed]
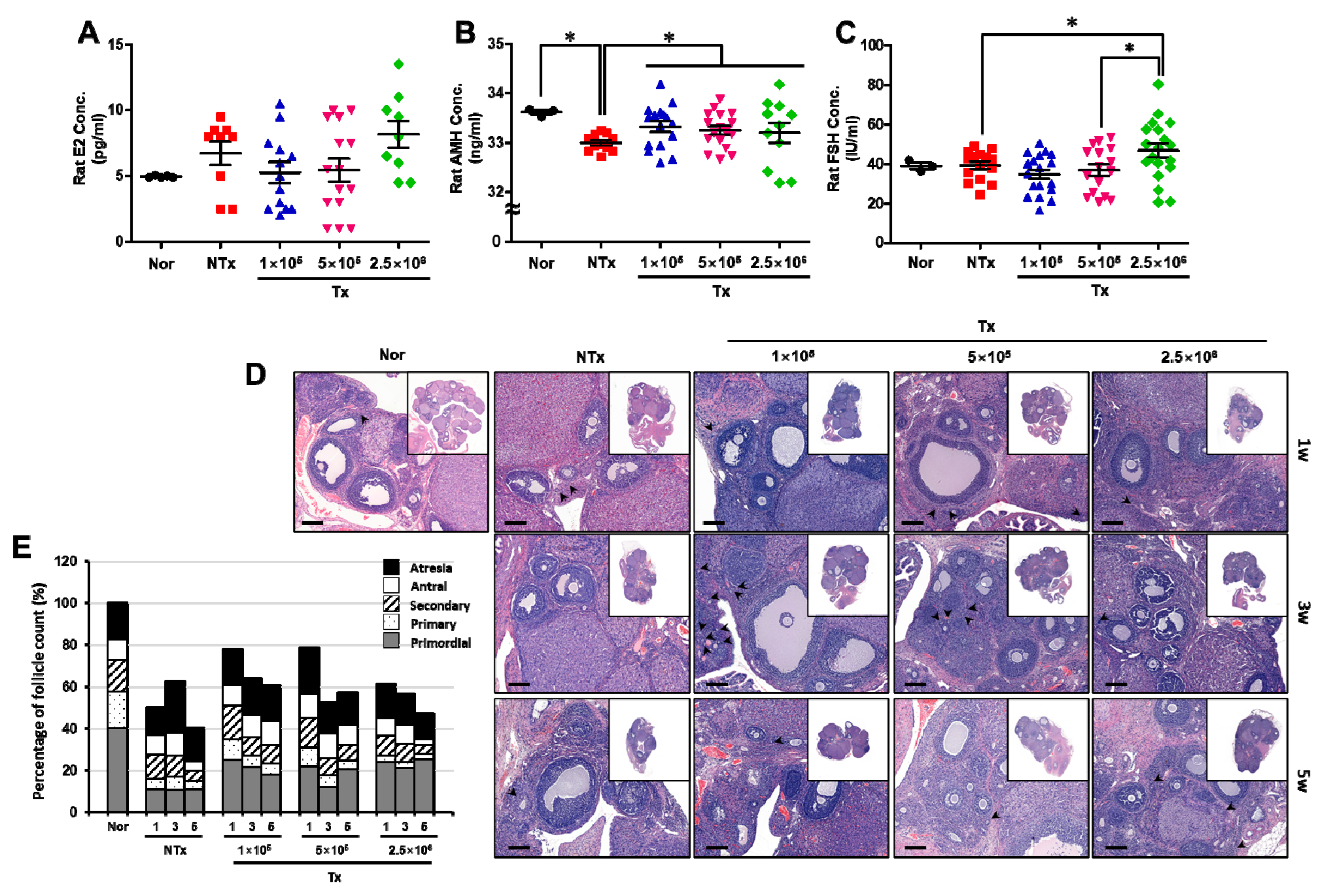
| Group | Primordial (%) | Primary (%) | Secondary (%) | Antral (%) | Atresia (%) | ||
|---|---|---|---|---|---|---|---|
| Nor | 39.92 ± 2.42 | 17.72 ± 0.60 | 14.88 ± 0.70 | 10.02 ± 0.77 | 17.46 ± 0.99 | ||
| 1w | NTx | 22.64 ± 3.54 * | 9.59 ± 0.90 * | 22.93 ± 0.62 * | 19.17 ± 3.73 * | 25.67 ± 5.31 * | |
| Tx | 1 × 105 | 31.56 ± 1.48 ** | 13.02 ± 0.56 ** | 19.84 ± 1.70 ** | 12.21 ± 1.68 | 23.38 ± 4.30 | |
| 5 × 105 | 27.25 ± 5.93 **, # | 11.34 ± 0.80# | 18.08 ± 2.10 | 14.26 ± 1.53 ** | 29.07 ± 6.77 | ||
| 2.5 × 106 | 46.58 ± 7.10 **, ## | 6.57 ± 1.17 **, #, ## | 15.49 ± 1.57 **,# | 11.43 ± 2.47 ** | 19.93 ± 7.46 | ||
| 3w | NTx | 17.12 ± 3.32 * | 10.22 ± 3.32 * | 15.81 ± 0.95 | 17.28 ± 0.57 * | 39.57 ± 0.73 * | |
| Tx | 1 × 105 | 33.67 ± 0.22 ** | 8.62 ± 1.21 | 13.81 ± 1.75 | 16.53 ± 2.25 | 27.37 ± 1.55 ** | |
| 5 × 105 | 26.39 ± 5.60 **, # | 10.57 ± 1.30 | 14.00 ± 1.10 | 20.98 ± 5.32 | 28.05 ± 0.56 ** | ||
| 2.5 × 106 | 37.04 ± 3.25 **, ## | 5.25 ± 1.47 ## | 15.11 ± 3.28 | 15.81 ± 4.77 | 26.78 ± 4.86 ** | ||
| 5w | NTx | 27.64 ± 1.19 * | 9.65 ± 1.70 * | 11.95 ± 1.93 * | 11.32 ± 1.83 | 39.43 ± 6.09 * | |
| Tx | 1 × 105 | 29.86 ± 1.16 | 8.64 ± 0.73 | 14.23 ± 1.15 | 19.38 ± 3.65 ** | 27.89 ± 3.30 ** | |
| 5 × 105 | 28.30 ± 7.97 | 9.47 ± 2.13 | 11.32 ± 1.99 | 16.07 ± 3.22 | 34.84 ± 8.42 | ||
| 2.5 × 106 | 53.74 ± 0.54 **, #, ## | 5.62 ± 0.39 ## | 8.45 ± 0.14 **, # | 5.60 ± 1.13 **, # | 26.59 ± 0.29 ** | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seok, J.; Park, H.; Lee, D.-H.; You, J.H.; Kim, G.J. The Dose-Related Efficacy of Human Placenta-Derived Mesenchymal Stem Cell Transplantation on Antioxidant Effects in a Rat Model with Ovariectomy. Antioxidants 2023, 12, 1575. https://doi.org/10.3390/antiox12081575
Seok J, Park H, Lee D-H, You JH, Kim GJ. The Dose-Related Efficacy of Human Placenta-Derived Mesenchymal Stem Cell Transplantation on Antioxidant Effects in a Rat Model with Ovariectomy. Antioxidants. 2023; 12(8):1575. https://doi.org/10.3390/antiox12081575
Chicago/Turabian StyleSeok, Jin, Hyeri Park, Dae-Hyun Lee, Jun Hyeong You, and Gi Jin Kim. 2023. "The Dose-Related Efficacy of Human Placenta-Derived Mesenchymal Stem Cell Transplantation on Antioxidant Effects in a Rat Model with Ovariectomy" Antioxidants 12, no. 8: 1575. https://doi.org/10.3390/antiox12081575
APA StyleSeok, J., Park, H., Lee, D.-H., You, J. H., & Kim, G. J. (2023). The Dose-Related Efficacy of Human Placenta-Derived Mesenchymal Stem Cell Transplantation on Antioxidant Effects in a Rat Model with Ovariectomy. Antioxidants, 12(8), 1575. https://doi.org/10.3390/antiox12081575







