Voluntary Exercise-Mediated Protection in TNBS-Induced Rat Colitis: The Involvement of NETosis and Prdx Antioxidants
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and TNBS Instillation
2.2. Macroscopic Evaluation of the Lesions and Damage Score
2.3. Western Blotting of TGF-β, citH3, PAD4, and MPO
2.4. Prdx ELISAs
2.5. Protein Concentration Determination
2.6. Statistical Analysis
3. Results
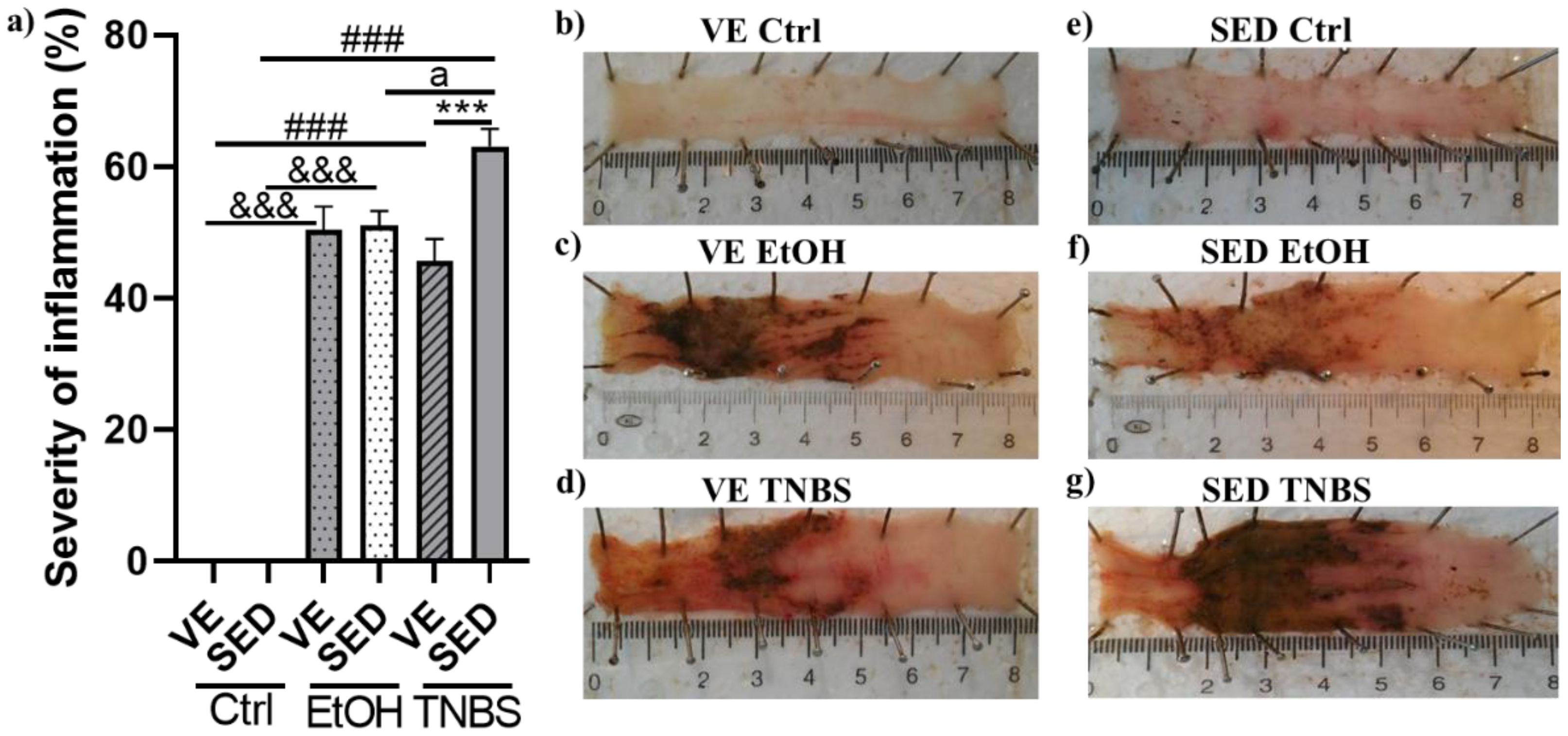
3.1. Voluntary Exercise-Induced Protection on the Severity of Inflammation in TNBS-Induced Colitis
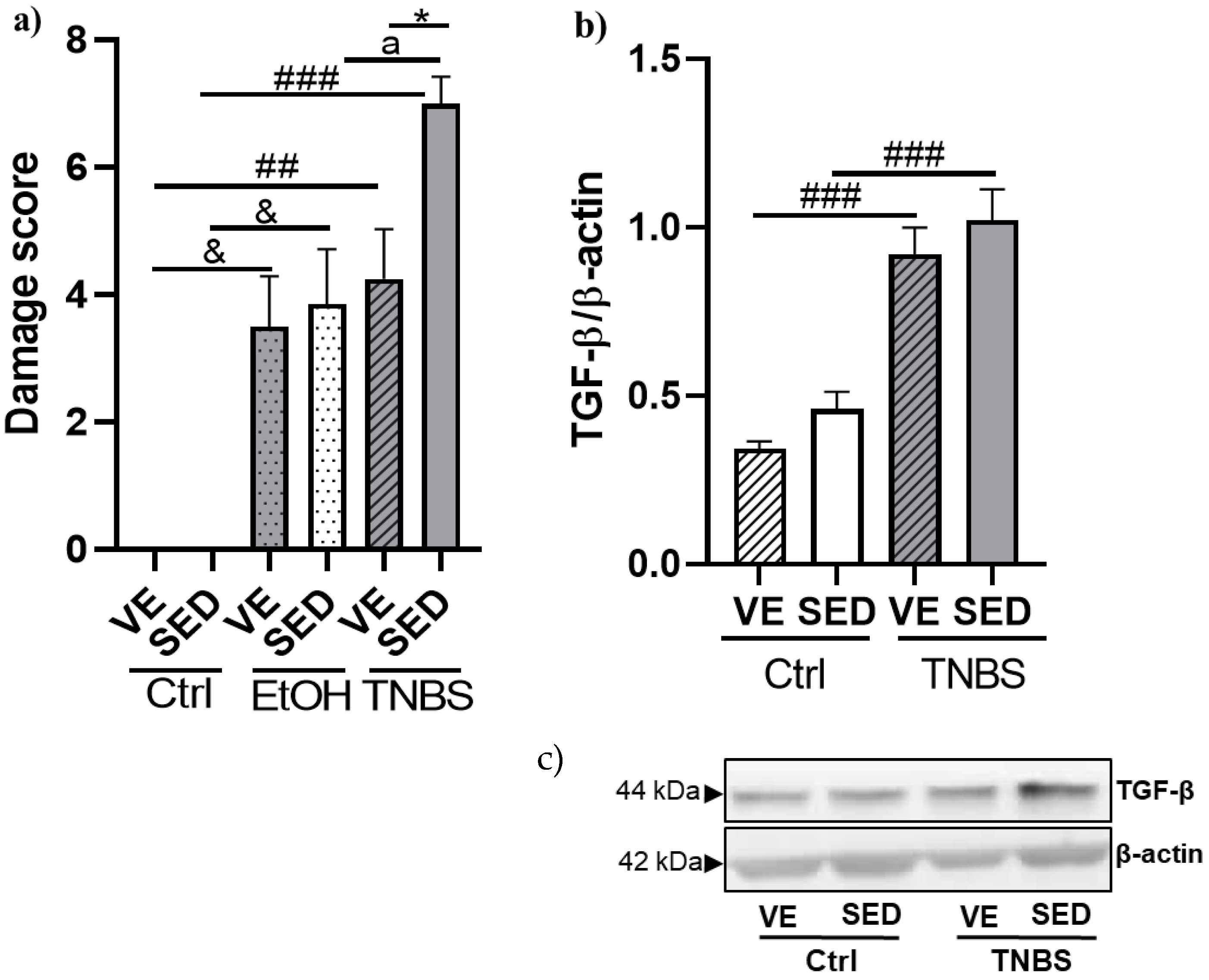
3.2. Effects of Voluntary Exercise on the Damage Score of the Colonic Inflammation and Expression of TGF-β in TNBS Rat Colitis
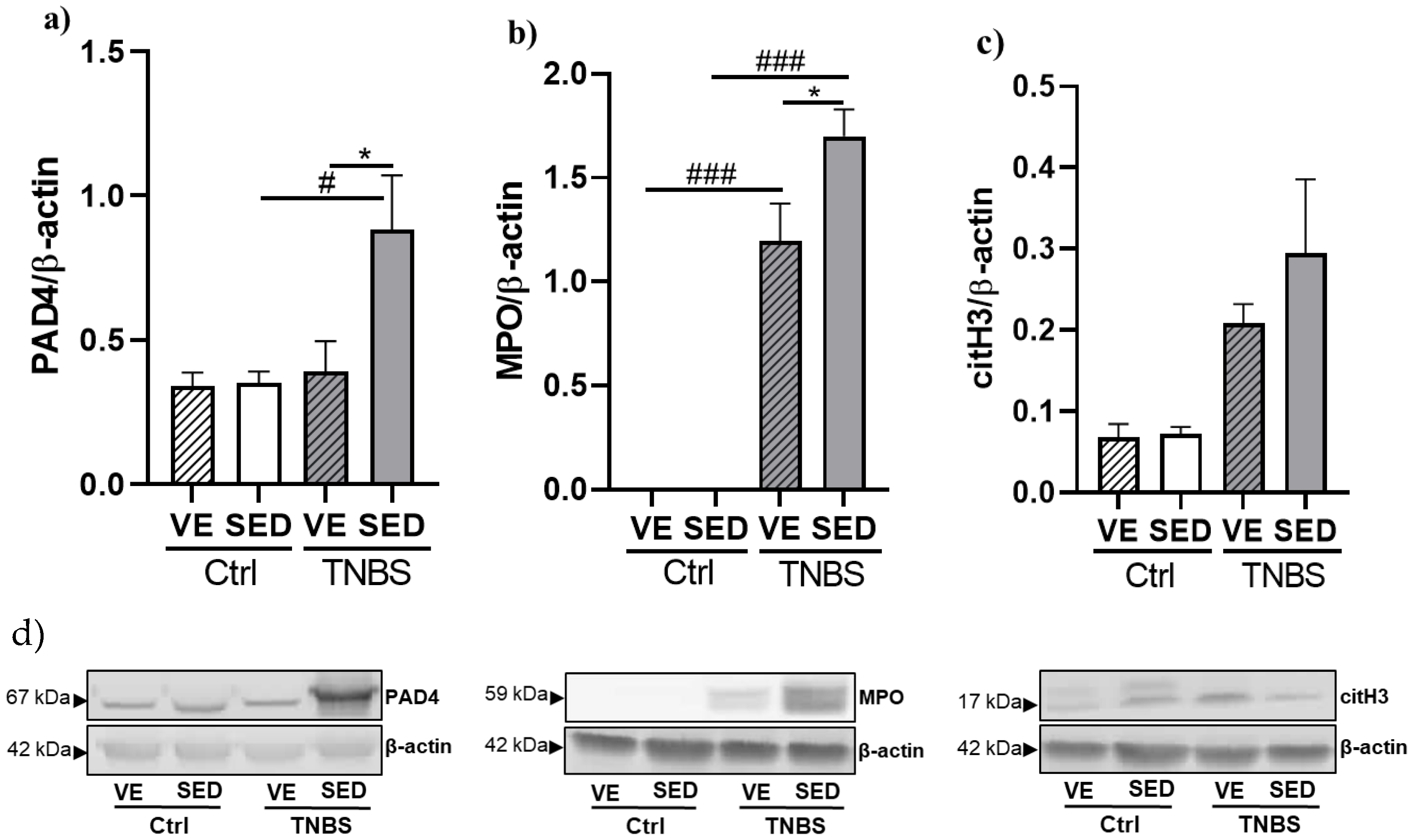
3.3. The Effects of Voluntary Exercise on NETosis Marker Expressions in TNBS-Induced Colitis
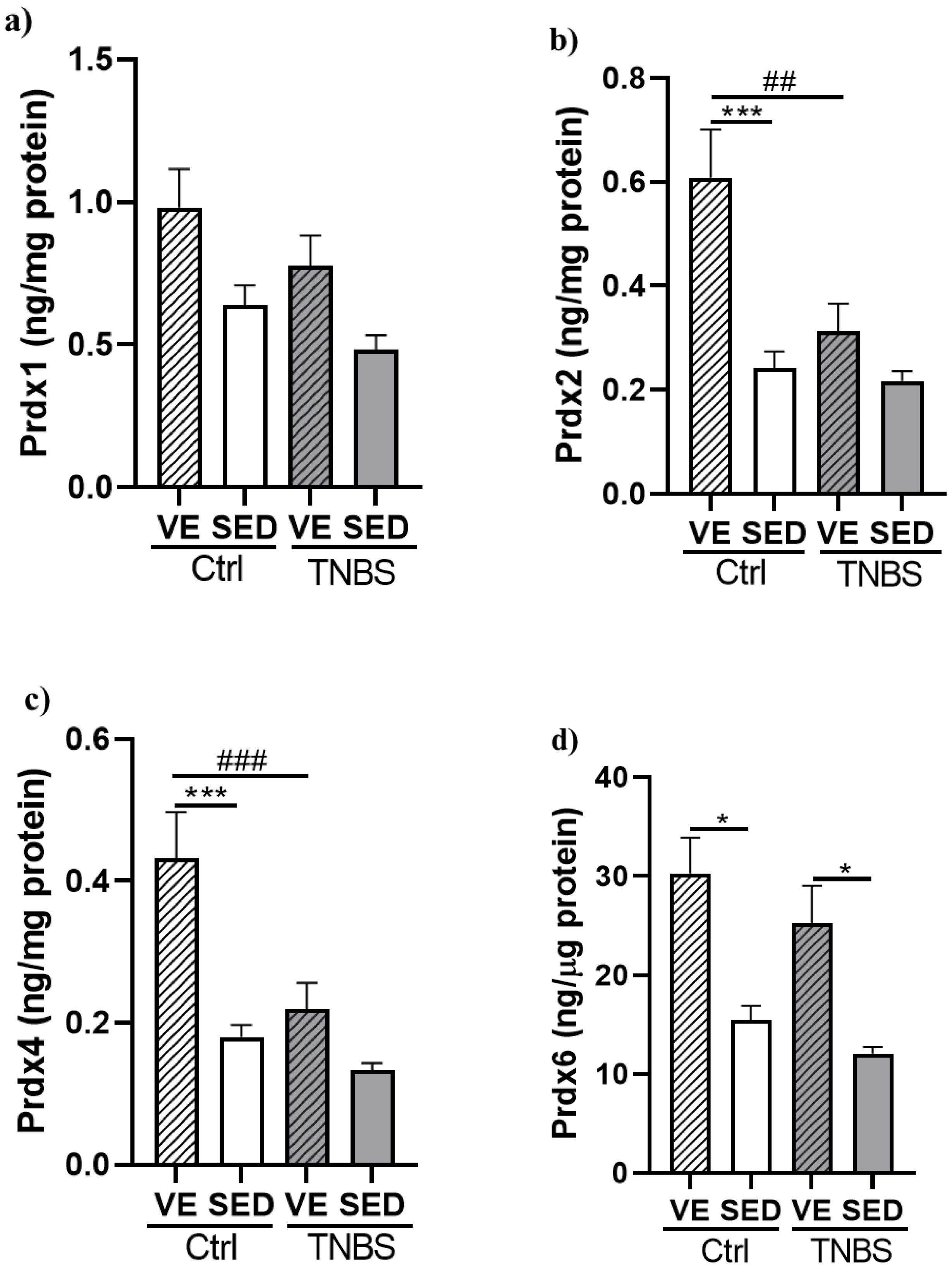
3.4. Effects of Voluntary Exercise on the Levels of Prdx Enzyme Family in TNBS Rat Colitis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef] [PubMed]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef]
- Loddo, I.; Romano, C. Inflammatory Bowel Disease: Genetics, Epigenetics, and Pathogenesis. Front. Immunol. 2015, 6, 551. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ruan, G.; Cheng, Y.; Yi, A.; Chen, D.; Wei, Y. The role of Th17 cells in inflammatory bowel disease and the research progress. Front. Immunol. 2022, 13, 1055914. [Google Scholar] [CrossRef] [PubMed]
- Xavier, R.J.; Podolsky, D.K. Faculty Opinions recommendation of Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef]
- Adolph, T.E.; Zhang, J. Diet fuelling inflammatory bowel diseases: Preclinical and clinical concepts. Gut 2022, 71, 2574–2586. [Google Scholar] [CrossRef]
- Rozich, J.J.; Holmer, A.; Singh, S. Effect of Lifestyle Factors on Outcomes in Patients with Inflammatory Bowel Diseases. Am. J. Gastroenterol. 2020, 115, 832–840. [Google Scholar] [CrossRef]
- Lavie, C.J.; Ozemek, C.; Carbone, S.; Katzmarzyk, P.T.; Blair, S.N. Sedentary Behavior, Exercise, and Cardiovascular Health. Circ. Res. 2019, 124, 799–815. [Google Scholar] [CrossRef]
- Qin, L.; Knol, M.J.; Corpeleijn, E.; Stolk, R.P. Does physical activity modify the risk of obesity for type 2 diabetes: A review of epidemiological data. Eur. J. Epidemiol. 2009, 25, 5–12. [Google Scholar] [CrossRef]
- Demarzo, M.M.P.; Martins, L.V.; Fernandes, C.R.; Herrero, F.A.; Perez, S.E.D.A.; Turatti, A.; Garcia, S.B. Exercise Reduces Inflammation and Cell Proliferation in Rat Colon Carcinogenesis. Med. Sci. Sports Exerc. 2008, 40, 618–621. [Google Scholar] [CrossRef]
- Pedersen, B.K. Anti-inflammatory effects of exercise: Role in diabetes and cardiovascular disease. Eur. J. Clin. Investig. 2017, 47, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Exercise-induced myokines and their role in chronic diseases. Brain Behav. Immun. 2011, 25, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y. The role of exercise-induced myokines in regulating metabolism. Arch. Pharmacal Res. 2018, 41, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Leal, L.G.; Lopes, M.A.; Batista, M.L., Jr. Physical Exercise-Induced Myokines and Muscle-Adipose Tissue Crosstalk: A Review of Current Knowledge and the Implications for Health and Metabolic Diseases. Front. Physiol. 2018, 9, 1307. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.P. Interleukin-6 in acute exercise and training: What is the biological relevance? Exerc. Immunol. Rev. 2006, 12, 6–33. [Google Scholar]
- Belviranlı, M.; Okudan, N. Voluntary, involuntary and forced exercises almost equally reverse behavioral impairment by regulating hippocampal neurotrophic factors and oxidative stress in experimental Alzheimer’s disease model. Behav. Brain Res. 2019, 364, 245–255. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Greenwood, B.N.; Fleshner, M. Voluntary Wheel Running: A Useful Rodent Model for Investigating the Mechanisms of Stress Robustness and Neural Circuits of Exercise Motivation. Curr. Opin. Behav. Sci. 2019, 28, 78–84. [Google Scholar] [CrossRef]
- Allen, J.M.; Miller, M.E.B.; Pence, B.D.; Whitlock, K.; Nehra, V.; Gaskins, H.R.; White, B.A.; Fryer, J.D.; Woods, J.A. Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. J. Appl. Physiol. 2015, 118, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Szalai, Z.; Szász, A.; Nagy, I.; Puskás, L.G.; Kupai, K.; Király, A.; Berkó, A.M.; Pósa, A.; Strifler, G.; Baráth, Z.; et al. Anti-Inflammatory Effect of Recreational Exercise in TNBS-Induced Colitis in Rats: Role of NOS/HO/MPO System. Oxidative Med. Cell. Longev. 2014, 2014, 925981. [Google Scholar] [CrossRef]
- Herrero-Cervera, A.; Soehnlein, O.; Kenne, E. Neutrophils in chronic inflammatory diseases. Cell. Mol. Immunol. 2022, 19, 177–191. [Google Scholar] [CrossRef]
- Delgado-Rizo, V.; Martínez-Guzmán, M.A.; Iñiguez-Gutierrez, L.; García-Orozco, A.; Alvarado-Navarro, A.; Fafutis-Morris, M. Neutrophil Extracellular Traps and Its Implications in Inflammation: An Overview. Front. Immunol. 2017, 8, 81. [Google Scholar] [CrossRef]
- Huang, J.; Hong, W.; Wan, M.; Zheng, L. Molecular mechanisms and therapeutic target of NETosis in diseases. Medcomm 2022, 3, e162. [Google Scholar] [CrossRef]
- Drury, B.; Hardisty, G.; Gray, R.D.; Ho, G.-T. Neutrophil Extracellular Traps in Inflammatory Bowel Disease: Pathogenic Mechanisms and Clinical Translation. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Chamardani, T.M.; Amiritavassoli, S. Inhibition of NETosis for treatment purposes: Friend or foe? Mol. Cell. Biochem. 2022, 477, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, D.; Zhou, Z.; Liu, F.; Shen, Y.; You, Q.; Lu, S.; Wu, J. The role of protein arginine deiminase 4-dependent neutrophil extracellular traps formation in ulcerative colitis. Front. Immunol. 2023, 14, 1144976. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxidative Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Lan, S. Implications of Antioxidant Systems in Inflammatory Bowel Disease. BioMed Res. Int. 2018, 2018, 1290179. [Google Scholar] [CrossRef]
- Horie, K.; Mikami, T.; Yoshida, T.; Sato, Y.; Okayasu, I. Peroxiredoxin 1 expression in active ulcerative colitis mucosa identified by proteome analysis and involvement of thioredoxin based on immunohistochemistry. Oncol. Lett. 2018, 15, 2364–2372. [Google Scholar] [CrossRef] [PubMed]
- Senhaji, N.; Zaid, Y.; Khalfi, B.E.; Fahimi, M.; Martin, J.; Badre, W.; Nadifi, S.; Soukri, A. Peroxiredoxin-2 up-regulation in inflammatory bowel disease: Friend or foe? J. Gastroenterol. Hepatol. 2017, 32, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Homma, T.; Fujii, J.; Shirasawa, N.; Yoriki, H.; Hotta, Y.; Higashimura, Y.; Mizushima, K.; Hirai, Y.; Katada, K.; et al. Elevated ER stress exacerbates dextran sulfate sodium-induced colitis in PRDX4-knockout mice. Free. Radic. Biol. Med. 2019, 134, 153–164. [Google Scholar] [CrossRef]
- Melhem, H.; Spalinger, M.R.; Cosin-Roger, J.; Atrott, K.; Lang, S.; Wojtal, K.A.; Vavricka, S.R.; Rogler, G.; Frey-Wagner, I. Prdx6 Deficiency Ameliorates DSS Colitis: Relevance of Compensatory Antioxidant Mechanisms. J. Crohn’s Colitis 2017, 11, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Wadley, A.J.; Aldred, S.; Coles, S.J. An unexplored role for Peroxiredoxin in exercise-induced redox signalling? Redox Biol. 2016, 8, 51–58. [Google Scholar] [CrossRef]
- Morris, G.P.; Beck, P.L.; Herridge, M.S.; Depew, W.T.; Szewczuk, M.R.; Wallace, J.L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 1989, 96, 795–803. [Google Scholar] [CrossRef]
- Yu, X.; Yang, G.; Jiang, H.; Lin, S.; Liu, Y.; Zhang, X.; Zeng, H.; Su, Z.; Huang, S.; Shen, L.; et al. Patchouli oil ameliorates acute colitis: A targeted metabolite analysis of 2,4,6-trinitrobenzenesulfonic acid-induced rats. Exp. Ther. Med. 2017, 14, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.; Pinto, R.; Mateus, V. Preclinical Study in Vivo for New Pharmacological Approaches in Inflammatory Bowel Disease: A Systematic Review of Chronic Model of TNBS-Induced Colitis. J. Clin. Med. 2019, 8, 1574. [Google Scholar] [CrossRef]
- Woods, J.; Cook, M.; Martin, S.; Williams, C.; Whitlock, K.; Wallig, M.; Pence, B. Forced treadmill exercise training exacerbates inflammation and causes mortality while voluntary wheel training is protective in a mouse model of colitis. Brain Behav. Immun. 2013, 33, 46–56. [Google Scholar] [CrossRef][Green Version]
- Qin, L.; Yao, Z.-Q.; Chang, Q.; Zhao, Y.-L.; Liu, N.-N.; Zhu, X.-S.; Liu, Q.-Q.; Wang, L.-F.; Yang, A.-G.; Gao, C.-F.; et al. Swimming attenuates inflammation, oxidative stress, and apoptosis in a rat model of dextran sulfate sodium-induced chronic colitis. Oncotarget 2017, 8, 7391–7404. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Wu, C.; Gao, Q.; Li, Q.; Li, S.; Li, Y. Effect of fecal microbiota transplantation on the TGF-beta1/Smad signaling pathway in rats with TNBS-induced colitis. Ann. Transl. Med. 2022, 10, 825. [Google Scholar] [CrossRef] [PubMed]
- Del Zotto, B.; Mumolo, G.; Pronio, A.M.; Montesani, C.; Tersigni, R.; Boirivant, M. TGF-beta1 production in inflammatory bowel disease: Differing production patterns in Crohn’s disease and ulcerative colitis. Clin. Exp. Immunol. 2003, 134, 120–126. [Google Scholar] [CrossRef]
- Silva, V.R.R.; Katashima, C.K.; Lenhare, L.; Silva, C.G.B.; Morari, J.; Camargo, R.L.; Velloso, L.A.; Saad, M.A.; da Silva, A.S.R.; Pauli, J.R. Chronic exercise reduces hypothalamic transforming growth factor-beta1 in middle-aged obese mice. Aging 2017, 9, 1926–1940. [Google Scholar] [CrossRef]
- Zhang, T.; Mei, Y.; Dong, W.; Wang, J.; Huang, F.; Wu, J. Evaluation of protein arginine deiminase-4 inhibitor in TNBS- induced colitis in mice. Int. Immunopharmacol. 2020, 84, 106583. [Google Scholar] [CrossRef] [PubMed]
- Török, S.; Almási, N.; Valkusz, Z.; Pósa, A.; Varga, C.; Kupai, K. Investigation of H2S Donor Treatment on Neutrophil Extracellular Traps in Experimental Colitis. Int. J. Mol. Sci. 2021, 22, 12729. [Google Scholar] [CrossRef]
- Orysiak, J.; Tripathi, J.K.; Brodaczewska, K.K.; Sharma, A.; Witek, K.; Sitkowski, D.; Malczewska-Lenczowska, J. The impact of physical training on neutrophil extracellular traps in young male athletes—A pilot study. Biol. Sport 2021, 38, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, T.; Nieman, D.C.; Cui, Y.; Li, F.; Yang, L.; Shi, H.; Chen, P. Aerobic Exercise Attenuates Acute Lung Injury Through NET Inhibition. Front. Immunol. 2020, 11, 409. [Google Scholar] [CrossRef]
- Krzystek-Korpacka, M.; Kempiński, R.; Bromke, M.A.; Neubauer, K. Oxidative Stress Markers in Inflammatory Bowel Diseases: Systematic Review. Diagnostics 2020, 10, 601. [Google Scholar] [CrossRef]
- Moghaddam, D.A.; Heber, A.; Capin, D.; Kreutz, T.; Opitz, D.; Lenzen, E.; Bloch, W.; Brixius, K.; Brinkmann, C. Training increases peroxiredoxin 2 contents in the erythrocytes of overweight/obese men suffering from type 2 diabetes. Wien. Med. Wochenschr. 2011, 161, 511–518. [Google Scholar] [CrossRef]
- Richters, L.; Lange, N.; Renner, R.; Treiber, N.; Ghanem, A.; Tiemann, K.; Scharffetter-Kochanek, K.; Bloch, W.; Brixius, K.; Alleman, R.J.; et al. Exercise-induced adaptations of cardiac redox homeostasis and remodeling in heterozygous SOD2-knockout mice. J. Appl. Physiol. 2011, 111, 1431–1440. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almási, N.; Török, S.; Al-awar, A.; Veszelka, M.; Király, L.; Börzsei, D.; Szabó, R.; Varga, C. Voluntary Exercise-Mediated Protection in TNBS-Induced Rat Colitis: The Involvement of NETosis and Prdx Antioxidants. Antioxidants 2023, 12, 1531. https://doi.org/10.3390/antiox12081531
Almási N, Török S, Al-awar A, Veszelka M, Király L, Börzsei D, Szabó R, Varga C. Voluntary Exercise-Mediated Protection in TNBS-Induced Rat Colitis: The Involvement of NETosis and Prdx Antioxidants. Antioxidants. 2023; 12(8):1531. https://doi.org/10.3390/antiox12081531
Chicago/Turabian StyleAlmási, Nikoletta, Szilvia Török, Amin Al-awar, Médea Veszelka, László Király, Denise Börzsei, Renáta Szabó, and Csaba Varga. 2023. "Voluntary Exercise-Mediated Protection in TNBS-Induced Rat Colitis: The Involvement of NETosis and Prdx Antioxidants" Antioxidants 12, no. 8: 1531. https://doi.org/10.3390/antiox12081531
APA StyleAlmási, N., Török, S., Al-awar, A., Veszelka, M., Király, L., Börzsei, D., Szabó, R., & Varga, C. (2023). Voluntary Exercise-Mediated Protection in TNBS-Induced Rat Colitis: The Involvement of NETosis and Prdx Antioxidants. Antioxidants, 12(8), 1531. https://doi.org/10.3390/antiox12081531





