Vitamin E Ameliorates Impaired Ovarian Development, Oxidative Stress, and Disrupted Lipid Metabolism in Oreochromis niloticus Fed with a Diet Containing Olive Oil Instead of Fish Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Experimental Diet Preparation
2.3. Feeding Trial and Sample Collection
2.4. Proximate Composition and VE Content Analysis
2.5. Histological Analysis
2.6. Serum Hormone Contents and Inflammatory Parameters
2.7. Antioxidant Parameters
2.8. Lipid Extraction and Analysis
2.9. RNA Extraction and qRT-PCR Analysis
2.10. Calculations and Statistical Analysis
3. Results
3.1. Growth Performance and Whole-Body Composition
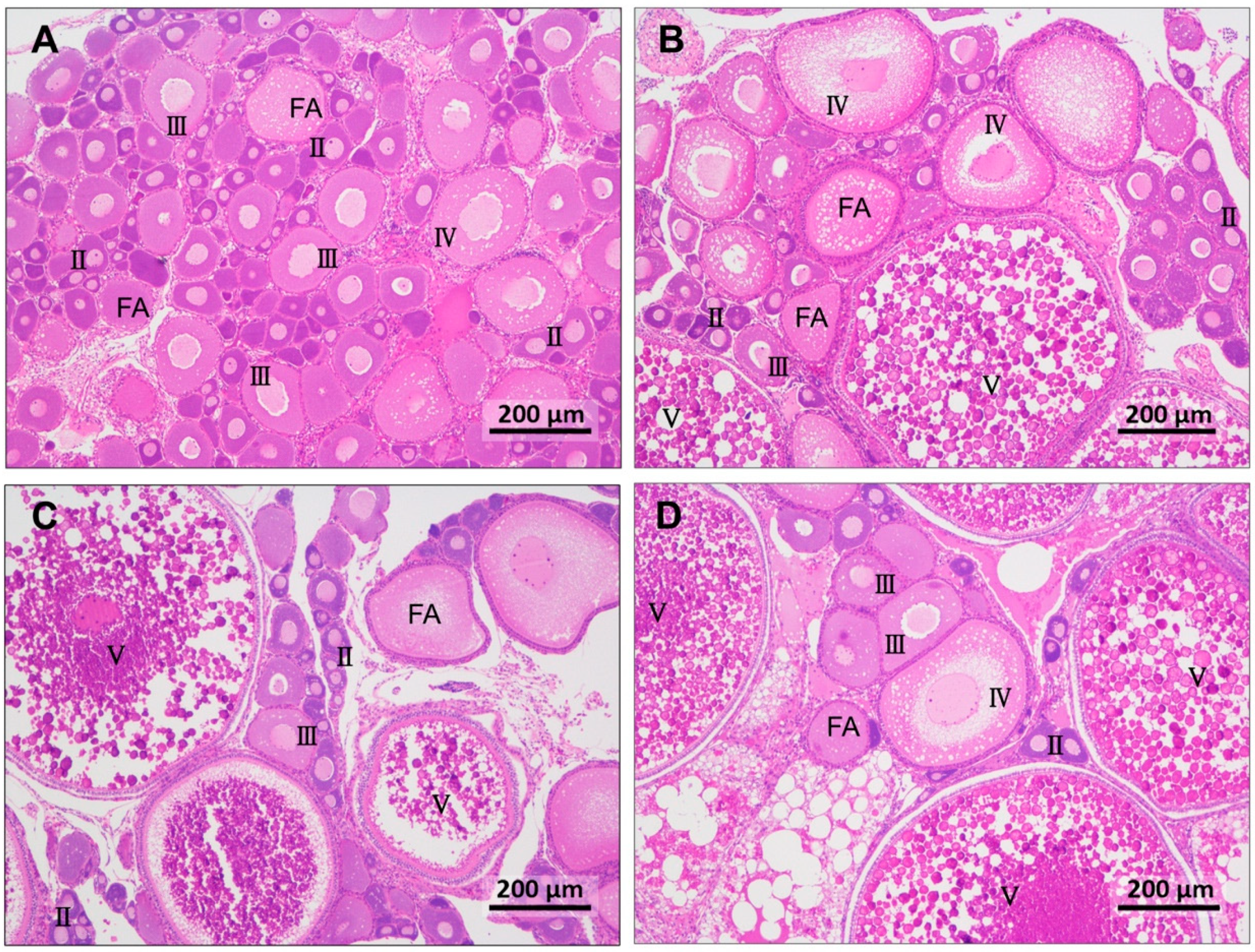
3.2. Ovary Morphology
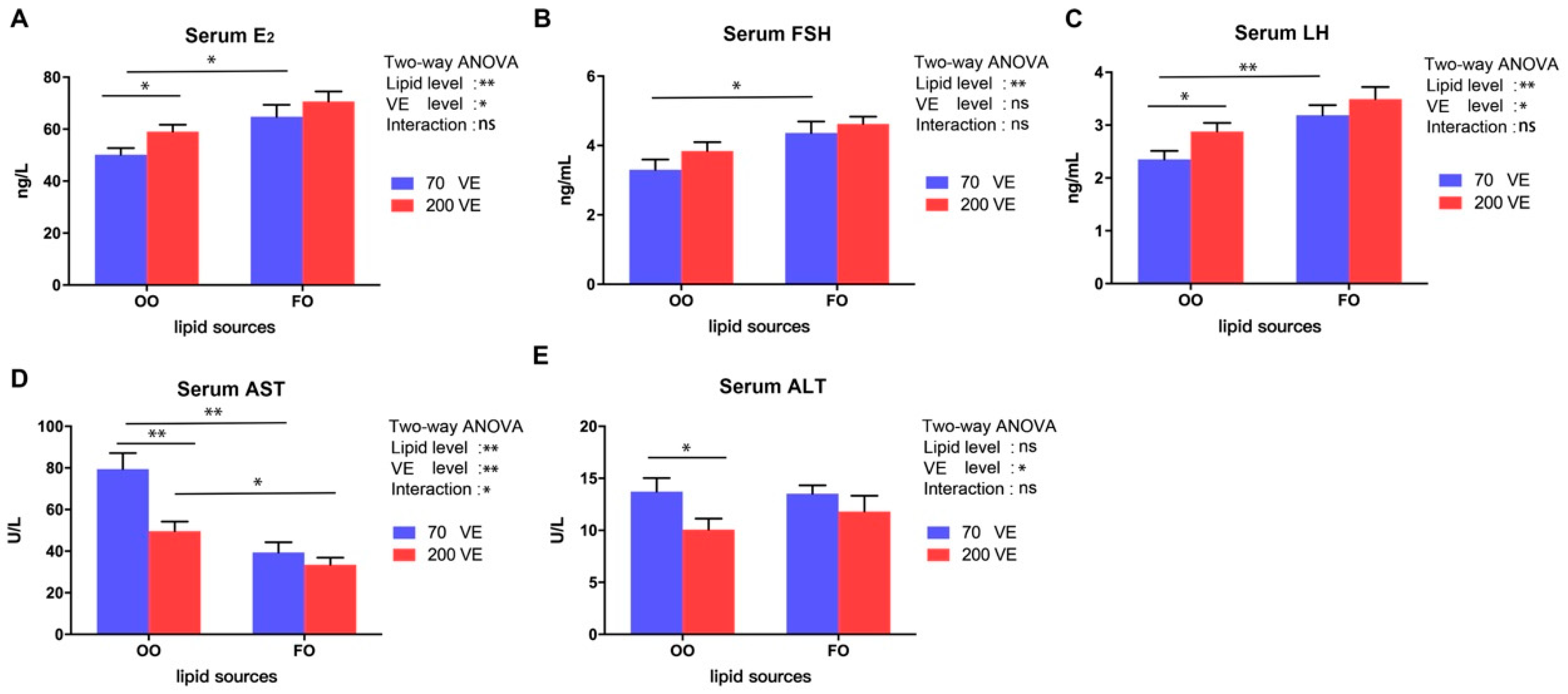
3.3. Serum Hormone Contents and Inflammatory Parameters
3.4. Serum and Ovary Antioxidant Parameters
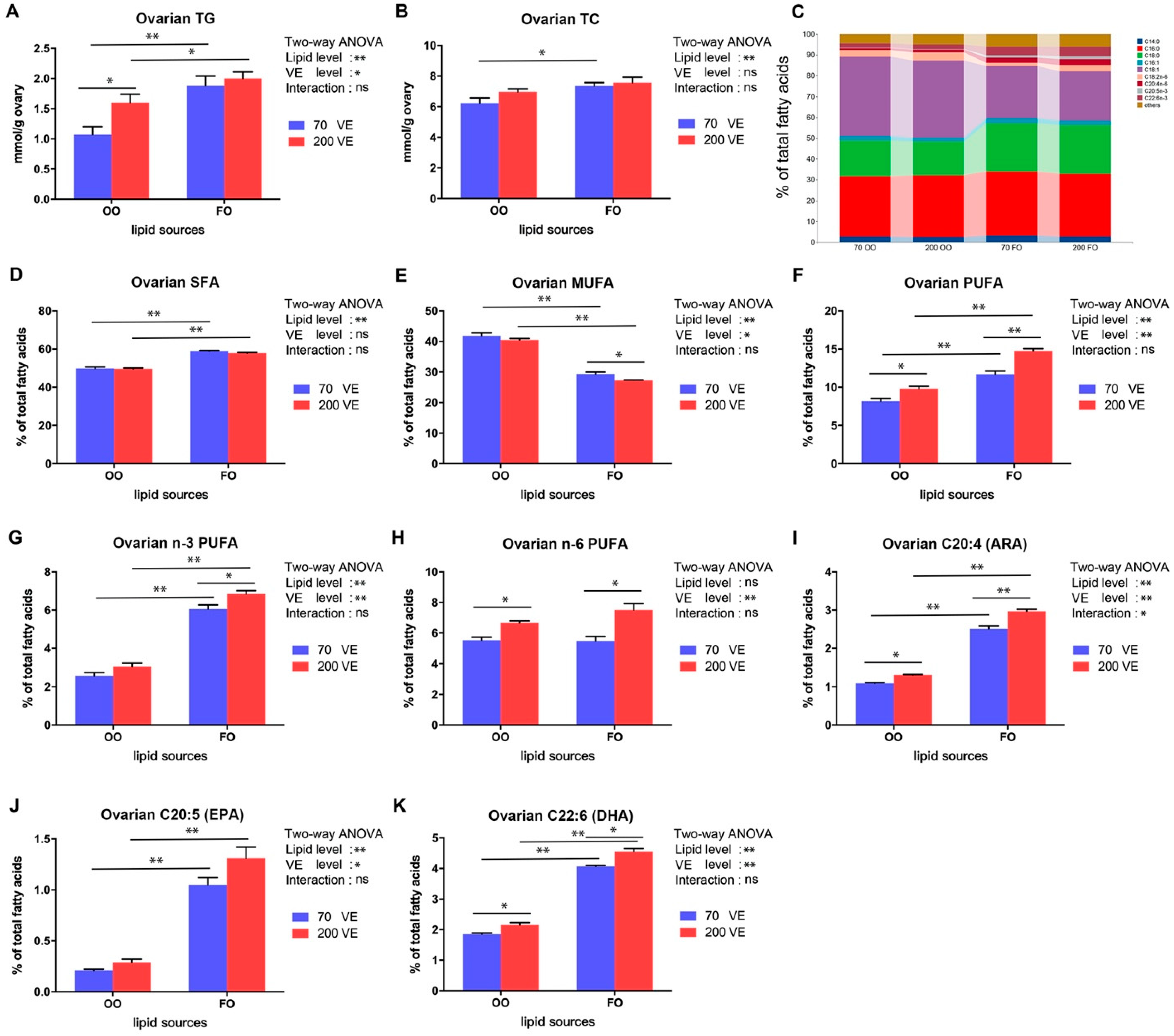
3.5. Lipid Content and Fatty Acid Composition in the Ovary
3.6. Expression Levels of Genes Encoding Hormone Receptors in the Ovary
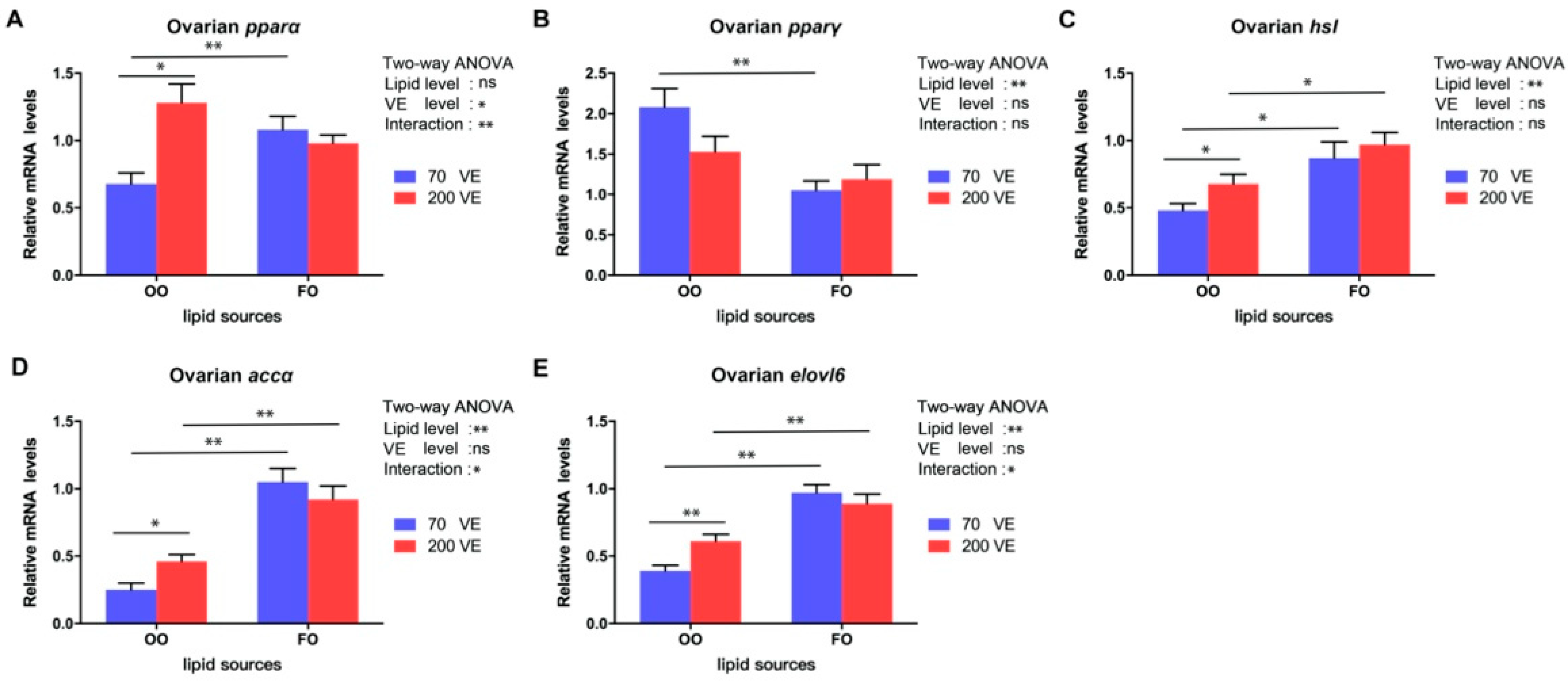
3.7. Expression Levels of Genes Related to Lipid Metabolism in the Ovary
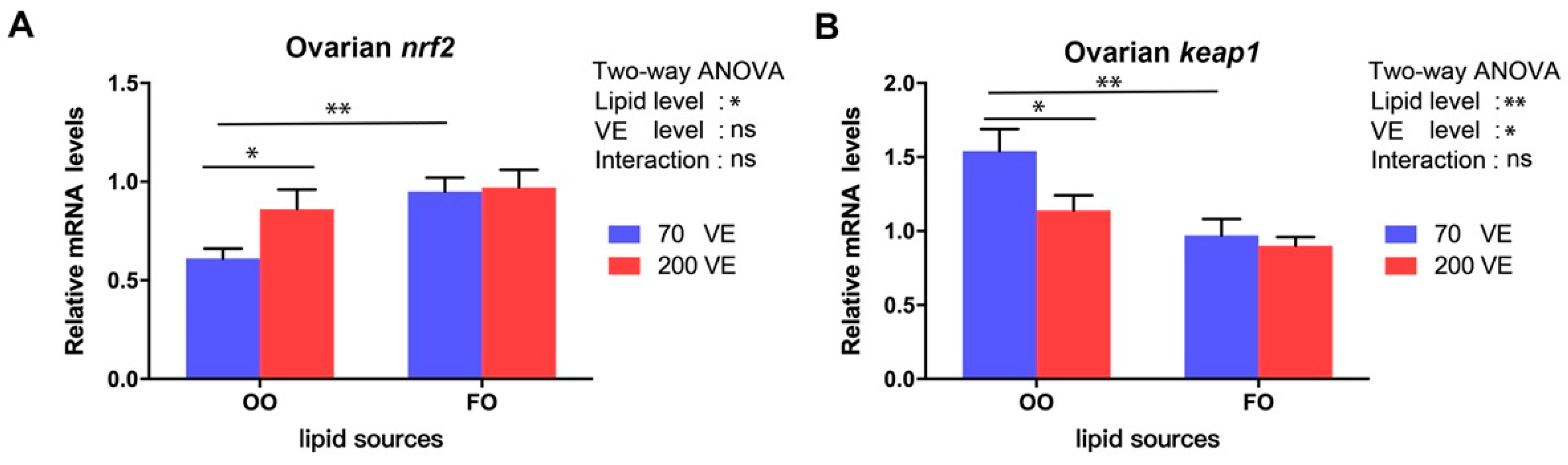
3.8. Transcript Levels of Genes Related to the nrf2 Signaling Pathway in the Ovary
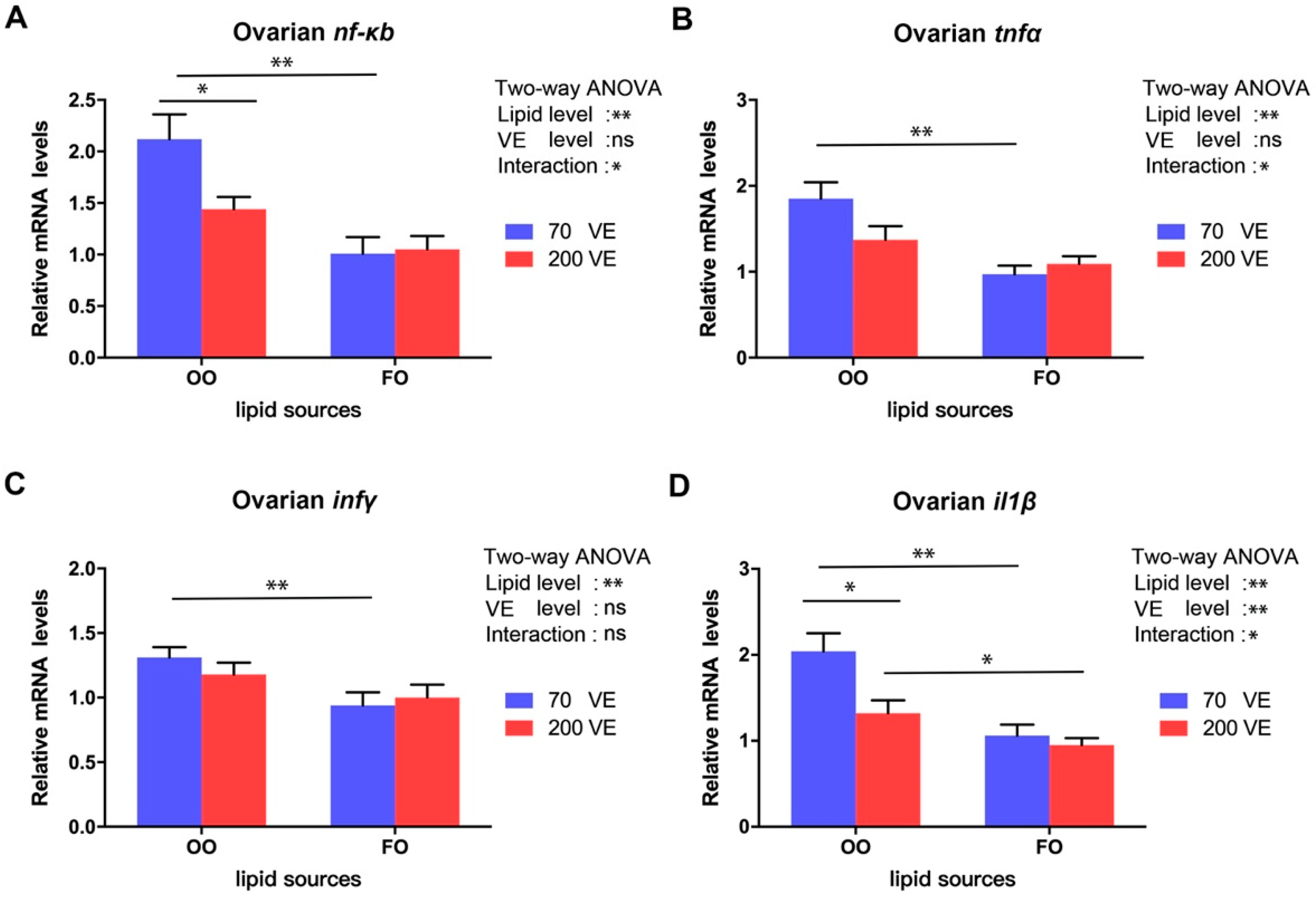
3.9. Expression Levels of Genes Related to nf-κb Signaling Pathway in the Ovary
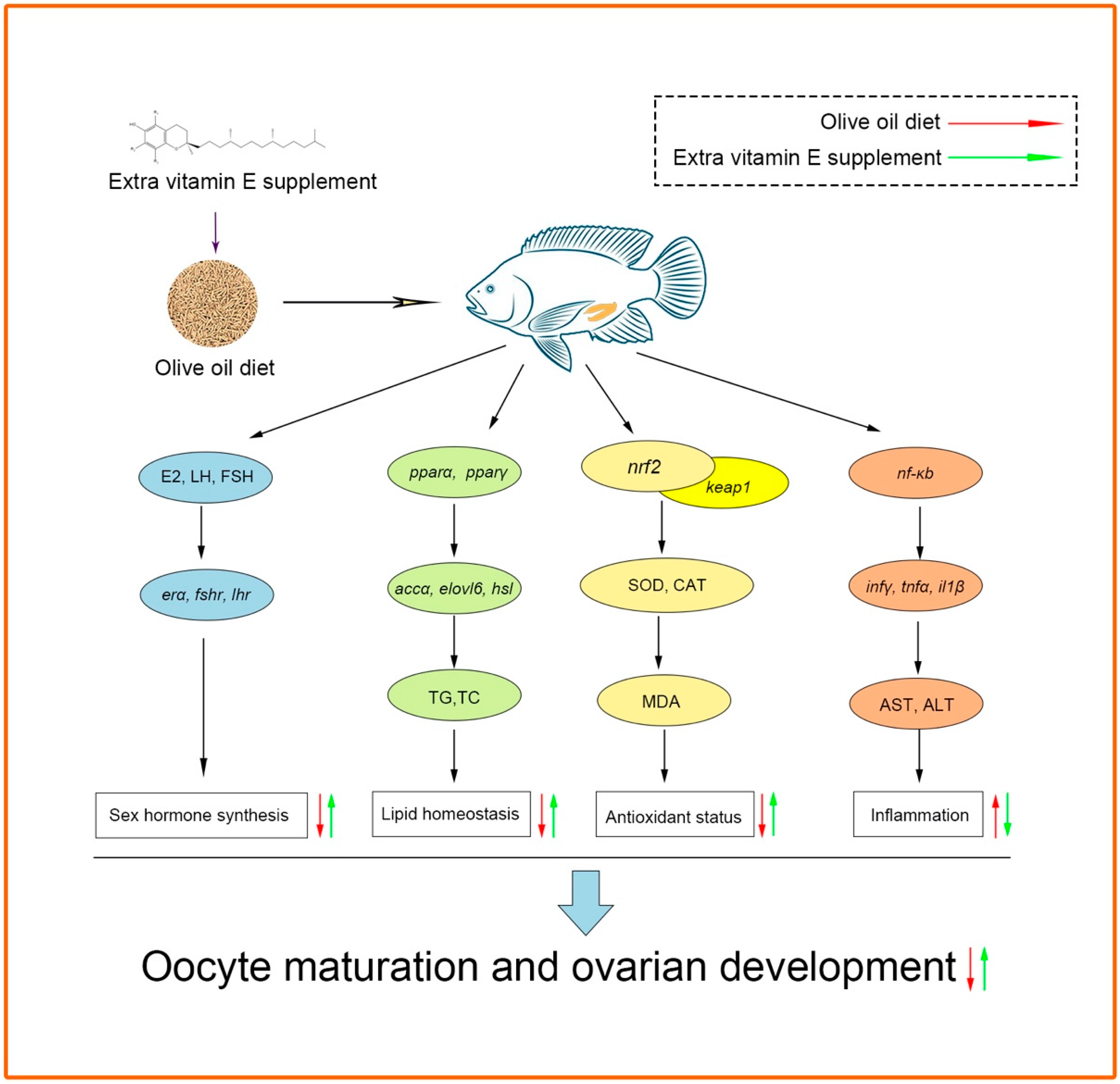
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, W.K.; Chong, C.Y.; Wang, Y.; Romano, N. Effects of dietary fish and vegetable oils on the growth, tissue fatty acid composition, oxidative stability and vitamin E content of red hybrid tilapia and efficacy of using fish oil finishing diets. Aquaculture 2013, 372–375, 97–110. [Google Scholar] [CrossRef]
- Turchini, G.M.; Torstensen, B.E.; Ng, W.K. Fish oil replacement in finfish nutrition. Rev. Aquac. 2010, 1, 10–57. [Google Scholar] [CrossRef]
- Li, X.S.; Cui, K.; Fang, W.; Chen, Q.; Xu, D.; Mai, K.S.; Zhang, Y.J.; Ai, Q.H. High level of dietary olive oil decreased growth, increased liver lipid deposition and induced inflammation by activating the p38 MAPK and JNK Cheek for pathways in large yellow croaker (Larimichthys crocea). Fish Shellfish. Immun. 2019, 94, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.J.; Chen, C.Y.; Yan, X.; Li, Y.Y.; Wen, X.B.; You, C.H.; Monroig, O.; Tocher, D.R.; Wang, S.Q. Effects of different dietary oil sources on growth performance, antioxidant capacity and lipid deposition of juvenile golden pompano Trachinotus ovatus. Aquaculture 2021, 530, 735923. [Google Scholar] [CrossRef]
- Alhazzaa, R.; Bridle, A.R.; Mori, T.A.; Barden, A.E.; Nichols, P.D.; Carter, C.G. Echium oil is better than rapeseed oil in improving the response of barramundi to a disease challenge. Food Chem. 2013, 141, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Mobaraki, M.; Kenari, A.A.; Gorji, S.B.; Esmaeili, M. Effect of dietary fish and vegetable oil on the growth performance, body composition, fatty acids profile, reproductive performance and larval resistance in pearl gourami (Trichogaster leeri). Aquac. Nutr. 2020, 26, 894–907. [Google Scholar] [CrossRef]
- Wassef, E.A.; Shalaby, W.S.H. Effects of dietary vegetable oils on liver and gonad fatty acid metabolism and gonad maturation in gilthead seabream (Sparus aurata) males and females. Aquac. Int. 2012, 20, 255–281. [Google Scholar] [CrossRef]
- Xu, H.G.; Lu, Q.K.; Liang, M.Q.; Zheng, K.K. Effects of Different Dietary Lipid Sources on Spawning Performance, Egg and Larval Quality, and Egg Fatty Acid Composition in Tongue Sole Cynoglossus semilaevis. Isr. J. Aquac.-Bamid. 2015, 67, 1091. [Google Scholar] [CrossRef]
- Rennie, S.; Huntingford, F.A.; Loeland, A.L.; Rimbach, M. Long term partial replacement of dietary fish oil with rapeseed oil; effects on egg quality of Atlantic salmon Salmo salar. Aquaculture 2005, 248, 135–146. [Google Scholar] [CrossRef]
- Sarameh, S.P.; Bahri, A.H.; Falahatkar, B.; Yarmohammadi, M.; Salarzadeh, A. The effect of fish and rapeseed oils on growth performance, egg fatty acid composition and offspring quality of sterlet sturgeon (Acipenser ruthenus). Aquac. Nutr. 2019, 25, 543–554. [Google Scholar] [CrossRef]
- Hamre, K. Metabolism, interactions, requirements and functions of vitamin E in fish. Aquac. Nutr. 2011, 17, 98–115. [Google Scholar] [CrossRef]
- Miyazawa, T.; Burdeos, G.C.; Itaya, M.; Nakagawa, K.; Miyazawa, T. Vitamin E: Regulatory Redox Interactions. IUBMB Life 2019, 71, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.Z.; Zheng, Q.Z.; Yang, Q.H.; Tan, B.P.; Dong, X.H.; Chi, S.Y.; Liu, H.Y.; Zhang, S. Alterations on growth performance, antioxidant responses and lipid metabolism in liver for juvenile hybrid grouper (♀ Epinephelus fuscoguttatus × ♂ Epinephelus lanceolatus) fed dietary vitamin E. Aquac. Rep. 2021, 21, 100862. [Google Scholar] [CrossRef]
- Saheli, M.; Rajabi Islami, H.; Mohseni, M.; Soltani, M. Effects of dietary vitamin E on growth performance, body composition, antioxidant capacity, and some immune responses in Caspian trout (Salmo caspius). Aquac. Rep. 2021, 21, 100857. [Google Scholar] [CrossRef]
- Pan, J.H.; Feng, L.; Jiang, W.D.; Wu, P.; Kuang, S.Y.; Tang, L.; Zhang, Y.A.; Zhou, X.Q.; Liu, Y. Vitamin E deficiency depressed fish growth, disease resistance, and the immunity and structural integrity of immune organs in grass carp (Ctenopharyngodon idella): Referring to NF-κB, TOR and Nrf2 signaling. Fish Shellfish Immun. 2017, 60, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, R.; James, R.; Indira, S.P. Effect of Dietary Vitamin E on Growth, Gonad Weight and Embryo Development in Female Red Swordtail, Xiphophorus helleri (Poeciliidae). Isr. J. Aquac.-Bamid. 2011, 63, 640. [Google Scholar]
- Serezli, R.; Akhan, S.; Sonay, F.D. The Effect of vitamin E on Black Sea trout (Salmo labrax) broodstock performance. Kafkas. Univ. Vet. Fak. 2010, 16, 219–222. [Google Scholar]
- Qiang, J.; Wasipe, A.; He, J.; Tao, Y.F.; Xu, P.; Bao, J.W.; Chen, D.J.; Zhu, J.H. Dietary vitamin E deficiency inhibits fat metabolism, antioxidant capacity, and immune regulation of inflammatory response in genetically improved farmed tilapia (GIFT, Oreochromis niloticus) fingerlings following Streptococcus iniae infection. Fish Shellfish Immun. 2019, 92, 395–404. [Google Scholar] [CrossRef]
- Salehi, I.; Karamian, R.; Komaki, A.; Tahmasebi, L.; Taheri, M.; Nazari, M.; Shahidi, S.; Sarihi, A. Effects of vitamin E on lead-induced impairments in hippocampal synaptic plasticity. Brain Res. 2015, 1629, 270–281. [Google Scholar] [CrossRef]
- Mourente, G.; Diaz-Salvago, E.; Bell, J.G.; Tocher, D.R. Increased activities of hepatic antioxidant defence enzymes in juvenile gilthead sea bream (Sparus aurata L.) fed dietary oxidised oil: Attenuation by dietary vitamin E. Aquaculture 2002, 214, 343–361. [Google Scholar] [CrossRef]
- Sun, C.X.; Shan, F.; Liu, M.Y.; Liu, B.; Zhou, Q.L.; Zheng, X.C.; Xu, X.D. High-Fat-Diet-Induced Oxidative Stress in Giant Freshwater Prawn (Macrobrachium rosenbergii) via NF-κB/NO Signal Pathway and the Amelioration of Vitamin E. Antioxidants 2022, 11, 228. [Google Scholar] [CrossRef]
- National Bureau of Statistics in China. China Fishery Statistical Yearbook; China AgriculturePress: Beijing, China, 2022; pp. 1–180.
- Qiang, J.; Duan, X.J.; Zhu, H.J.; He, J.; Tao, Y.F.; Bao, J.W.; Zhu, X.W.; Xu, P. Some ‘white’ oocytes undergo atresia and fail to mature during the reproductive cycle in female genetically improved farmed tilapia (Oreochromis niloticus). Aquaculture 2021, 534, 736278. [Google Scholar] [CrossRef]
- Peng, X.; Li, F.; Lin, S.; Chen, Y. Effects of total replacement of fish oil on growth performance, lipid metabolism and antioxidant capacity in tilapia (Oreochromis niloticus). Aquac. Int. 2016, 24, 145–156. [Google Scholar] [CrossRef]
- Corrêa, C.F.; Nobrega, R.O.; Mattioni, B.; Block, J.M.; Fracalossi, D.M. Dietary lipid sources affect the performance of Nile tilapia at optimal and cold, suboptimal temperatures. Aquac. Nutr. 2017, 23, 1016–1026. [Google Scholar] [CrossRef]
- Satoh, S.; Takeuchi, T.; Watanabe, T. Requirement of Tilapia for α-Tocopherol. Nsugaf 1987, 53, 119–124. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.Q.; Xiao, J.; Zhong, H.; Guo, Z.B.; Zhou, C.; Li, M.Y.; Tang, Z.Y.; Huang, K.; Liu, T. Effects of vitamin E on the reproductive performance of female and male Nile tilapia (Oreochromis niloticus) at the physiological and molecular levels. Aquac. Res. 2021, 52, 3518–3531. [Google Scholar] [CrossRef]
- Nascimento, T.S.R.; De Stéfani, M.V.; Malheiros, E.B.; Koberstein, T.C.R.D. High levels of dietary vitamin E improve the reproductive performance of female Oreochromis niloticus. Acta Sci. Biol. Sci. 2014, 36, 19–26. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemist: Washington, DC, USA, 2003. [Google Scholar]
- Qiang, J.; Tao, Y.F.; Zhu, J.H.; Lu, S.Q.; Cao, Z.M.; Ma, J.L.; He, J.; Xu, P. Effects of heat stress on follicular development and atresia in Nile tilapia (Oreochromis niloticus) during one reproductive cycle and its potential regulation by autophagy and apoptosis. Aquaculture 2022, 555, 738171. [Google Scholar] [CrossRef]
- Zhang, X.D.; Zhu, Y.F.; Cai, L.S.; Wu, T.X. Effects of fasting on the meat quality and antioxidant defenses of market-size farmed large yellow croaker (Pseudosciaena crocea). Aquaculture 2008, 280, 136–139. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Method. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Qiang, J.; Yang, H.; Xu, P.; Zhu, Z.X.; Yang, R.Q. Changes in the fatty acid composition and regulation of antioxidant enzymes and physiology of juvenile genetically improved farmed tilapia Oreochromis niloticus (L.), subjected to short-term low temperature stress. J. Therm. Biol. 2015, 53, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Mourente, G.; Good, J.E.; Bell, J.G. Partial substitution of fish oil with rapeseed, linseed and olive oils in diets for European sea bass (Dicentrarchus labrax L.): Effects on flesh fatty acid composition, plasma prostaglandins E2 and F2α, immune function and effectiveness of a fish oil finishing diet. Aquac. Nutr. 2005, 11, 25–40. [Google Scholar]
- Li, X.S.; Sun, J.C.; Wang, L.; Song, K.; Lu, K.L.; Zhang, L.; Ma, X.K.; Zhang, C.X. Effects of dietary vitamin E levels on growth, antioxidant capacity and immune response of spotted seabass (Lateolabrax maculatus) reared at different water temperatures. Aquaculture 2023, 565, 739141. [Google Scholar] [CrossRef]
- Zhang, G.R.; Xu, C.; You, C.H.; Wang, S.Q.; Xie, D.Z.; Hasan, A.K.M.M.; Ma, Y.C.; Li, Y.Y. Effects of dietary vitamin E on growth performance, antioxidant capacity and lipid metabolism of juvenile golden pompano Trachinotus ovatus. Aquac. Nutr. 2021, 27, 2205–2217. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Liang, X.; Gao, J. Effects of dietary vitamin E supplementation on growth performance, fatty acid composition, lipid peroxidation and peroxisome proliferator-activated receptors (PPAR) expressions in juvenile blunt snout bream Megalobrama amblycephala. Fish Physiol. Biochem. 2017, 43, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Vassallo-Agius, R. Broodstock nutrition research on marine finfish in Japan. Aquaculture 2003, 227, 35–61. [Google Scholar] [CrossRef]
- Næve, I.; Mommens, M.; Arukwe, A.; Virtanen, J.; Hoque, M.E.; Kjørsvik, E. Ultrasound as a noninvasive tool for monitoring reproductive physiology in male Atlantic salmon (Salmo salar). Physiol. Rep. 2019, 7, e14167. [Google Scholar] [CrossRef] [Green Version]
- Taghizadeh, V.; Imanpoor, M.R.; Mehdinejad, N. Study the seasonal steroid hormones of common carp in Caspian Sea, Iran. Springerplus 2013, 2, 193. [Google Scholar] [CrossRef] [Green Version]
- Orn, S.; Holbech, H.; Madsen, T.H.; Norrgren, L.; Petersen, G.I. Gonad development and vitellogenin production in zebrafish (Danio rerio) exposed to ethinylestradiol and methyltestosterone. Aquat. Toxicol. 2003, 65, 397–411. [Google Scholar] [CrossRef]
- Mackenzie, D.S.; Thomas, P.; Farrar, S.M. Seasonal-changes in thyroid and reproductive steroid-hormones in female channel catfish (Ictalurus punctatus) in pond culture. Aquaculture 1989, 78, 63–80. [Google Scholar] [CrossRef]
- Hollander-Cohen, L.; Golan, M.; Levavi-Sivan, B. Differential Regulation of Gonadotropins as Revealed by Transcriptomes of Distinct LH and FSH Cells of Fish Pituitary. Int. J. Mol. Sci. 2021, 22, 6478. [Google Scholar] [CrossRef] [PubMed]
- Yenuganti, V.R.; Viergutz, T.; Vanselow, J. Oleic acid induces specific alterations in the morphology, gene expression and steroid hormone production of cultured bovine granulosa cells. Gen. Comp. Endocrinol. 2016, 232, 134–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amevor, F.K.; Cui, Z.F.; Du, X.X.; Ning, Z.F.; Shu, G.; Jin, N.N.; Deng, X.; Tian, Y.F.; Zhang, Z.C.; Kang, X.C.; et al. Combination of Quercetin and Vitamin E Supplementation Promotes Yolk Precursor Synthesis and Follicle Development in Aging Breeder Hens via Liver–Blood–Ovary Signal Axis. Animals 2021, 11, 1915. [Google Scholar] [CrossRef]
- Johnson, R.B.; Kroeger, E.L.; Reichert, W.L.; Carter, C.S.; Rust, M.B. Uptake and selective partitioning of dietary lipids to ovarian and muscle tissue of maturing female coho salmon, Oncorhynchus kisutch, during secondary oocyte growth. Comp. Biochem. Phys. B 2017, 208, 7–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.Q.; Fei, F.; Liu, Z.F.; Guan, C.T.; Huang, B.; Liu, B.L.; Jia, Y.D.; Guo, Z.L.; Wang, Y.H.; Hong, L. Lipid content and fatty acid composition in female American shad, Alosa sapidissima, at different stages of ovarian development under reared conditions. Aquac. Res. 2019, 50, 439–448. [Google Scholar] [CrossRef]
- Shirai, N.; Suzuki, H.; Toukairin, S.; Wada, S. Spawning and season affect lipid content and fatty acid composition of ovary and liver in Japanese catfish (Silurus asotus). Comp. Biochem. Phys. B 2001, 129, 185–195. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Z.L.; Li, G.L.; Mustapha, U.F.; Ndandala, C.B.; Shi, H.J.; Zhu, C.H.; Chen, H.P.; Huang, Y.; Jiang, D.N. Ovary transcriptomic analysis reveals regulation effects of dietary fish oil on hormone, lipid, and glucose metabolism in female adult spotted scat (Scatophagus argus). Front. Mar. Sci. 2022, 9, 935968. [Google Scholar] [CrossRef]
- Furuita, H.; Tanaka, H.; Yamamoto, T.; Shiraishi, M.; Takeuchi, T. Effects of n-3 HUFA levels in broodstock diet on the reproductive performance and egg and larval quality of the Japanese flounder, Paralichthys olivaceus. Aquaculture 2000, 187, 387–398. [Google Scholar] [CrossRef]
- Luo, L.; Ai, L.C.; Liang, X.F.; Hu, H.X.; Xue, M.; Wu, X.F. n-3 Long-chain polyunsaturated fatty acids improve the sperm, egg, and offspring quality of Siberian sturgeon (Acipenser baerii). Aquaculture 2017, 473, 266–271. [Google Scholar] [CrossRef]
- Qin, J.Y.; Ru, S.G.; Wang, W.W.; Hao, L.P.; Wei, S.H.; Zhang, J.; Xiong, J.Q.; Wang, J.; Zhang, X.N. Unraveling the mechanism of long-term bisphenol S exposure disrupted ovarian lipids metabolism, oocytes maturation, and offspring development of zebrafish. Chemosphere 2021, 277, 130304. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, M.Q.; Luo, Z.; Xu, Y.H.; Li, D.D.; Pan, Y.X.; Wu, K. Functional Analysis of Promoters from Three Subtypes of the PI3K Family and Their Roles in the Regulation of Lipid Metabolism by Insulin in Yellow Catfish Pelteobagrus fulvidraco. Int. J. Mol. Sci. 2018, 19, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef]
- Han, S.L.; Liu, Y.; Limbu, S.M.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y. The reduction of lipid-sourced energy production caused by ATGL inhibition cannot be compensated by activation of HSL, autophagy, and utilization of other nutrients in fish. Fish Physiol. Biochem. 2021, 47, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.Y.; Zhou, Y.F.; Liu, H.; Peng, Y.X.; Huang, Y.Q.; Deng, S.P.; Huang, Y.; Shi, G.; Zhu, C.H.; Li, G.L.; et al. Transcriptomic analysis provides new insights into the secondary follicle growth in spotted scat (Scatophagus argus). Front. Mar. Sci. 2023, 10, 1114872. [Google Scholar] [CrossRef]
- Lin, Z.D.; Qi, C.L.; Han, F.L.; Chen, X.F.; Qin, C.J.; Wang, C.L.; Wang, X.D.; Qin, J.G.; Chen, L.Q. Selecting suitable phospholipid source for female Eriocheir sinensis in pre-reproductive phase. Aquaculture 2020, 528, 735610. [Google Scholar] [CrossRef]
- Jia, R.; Cao, L.P.; Du, J.L.; He, Q.; Gu, Z.Y.; Jeney, G.; Xu, P.; Yin, G.J. Effects of High-Fat Diet on Steatosis, Endoplasmic Reticulum Stress and Autophagy in Liver of Tilapia (Oreochromis niloticus). Front. Mar. Sci. 2020, 7, 363. [Google Scholar] [CrossRef]
- Yang, S.P.; Liu, H.L.; Wang, C.G.; Yang, P.; Sun, C.B.; Chan, S.M. Effect of oxidized fish oil on growth performance and oxidative stress of Litopenaeus vannamei. Aquac. Nutr. 2015, 21, 121–127. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yamamoto, M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid. Redox Sign. 2005, 7, 385–394. [Google Scholar] [CrossRef]
- Jaiswal, A.K. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Bio. Med. 2004, 36, 1199–1207. [Google Scholar] [CrossRef]
- Narasimhan, M.; Riar, A.K.; Rathinam, M.L.; Vedpathak, D.; Henderson, G.; Mahimainathan, L. Hydrogen peroxide responsive miR153 targets Nrf2/ARE cytoprotection in paraquat induced dopaminergic neurotoxicity. Toxicol. Lett. 2014, 228, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Tan, P.; Dong, X.J.; Xu, H.L.; Mai, K.S.; Ai, Q.H. Dietary vegetable oil suppressed non-specific immunity and liver antioxidant capacity but induced inflammatory response in Japanese sea bass (Lateolabrax japonicus). Fish Shellfish Immun. 2017, 63, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Cao, L.P.; Du, J.L.; He, Q.; Gu, Z.Y.; Jeney, G.; Xu, P.; Yin, G.J. Effects of high-fat diet on antioxidative status, apoptosis and inflammation in liver of tilapia (Oreochromis niloticus) via Nrf2, TLRs and JNK pathways. Fish Shellfish Immun. 2020, 104, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Guo, Z.X.; Wang, A.L. Growth performance and protective effect of vitamin E on oxidative stress pufferfish (Takifugu obscurus) following by ammonia stress. Fish Physiol. Biochem. 2018, 44, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef] [Green Version]
- Khedr, N.F. Fish oil and wheat-germ oil supplementation restores ovarian function in streptozotocin-diabetic rats. Reprod. Fertil. Dev. 2017, 29, 1689–1698. [Google Scholar] [CrossRef]
- Hatzirodos, N.; Irving-Rodgers, H.F.; Hummitzsch, K.; Rodgers, R.J. Transcriptome Profiling of the Theca Interna from Bovine Ovarian Follicles during Atresia. PLoS ONE 2014, 9, e99706. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Hur, D.H.; Lee, S.M.; Jeoung, E.; Jeong, H.D.; Hong, S. Effects of Vitamin E with Different Levels or Sources of Dietary Lipid on the Growth and Expression of Inflammatory, Oxidative Stress, and Apoptotic Genes in the Head Kidney of Olive Flounder, Paralichthys olivaceus. J. World Aquac. Soc. 2017, 48, 518–530. [Google Scholar] [CrossRef]



| 70 OO | 200 OO | 70 FO | 200 FO | |
|---|---|---|---|---|
| Fish meal | 60.00 | 60.00 | 60.00 | 60.00 |
| Wheat middling | 80.00 | 80.00 | 80.00 | 80.00 |
| Wheat flour | 200.00 | 200.00 | 200.00 | 200.00 |
| Olive oil | 50.00 | 50.00 | ||
| Fish oil | 50.00 | 50.00 | ||
| Soybean meal | 200.00 | 200.00 | 200.00 | 200.00 |
| Cottonseed meal | 180.00 | 180.00 | 180.00 | 180.00 |
| Rapeseed meal | 165.00 | 165.00 | 165.00 | 165.00 |
| Ca(H2PO4)2 | 15.00 | 15.00 | 15.00 | 15.00 |
| α-cellulose | 32.93 | 32.8 | 32.93 | 32.8 |
| Vitamin Premix (vitamin E free) ‡ | 5.00 | 5.00 | 5.00 | 5.00 |
| Mineral Premix § | 5.00 | 5.00 | 5.00 | 5.00 |
| Vitamin C phosphate ester | 2.00 | 2.00 | 2.00 | 2.00 |
| Choline chloride | 5.00 | 5.00 | 5.00 | 5.00 |
| Vitamin E | 0.07 | 0.20 | 0.07 | 0.20 |
| Total | 1000 | 1000 | 1000 | 1000 |
| Proximate composition (% dry matter) | ||||
| Dry matter | 94.79 | 94.81 | 94.72 | 94.93 |
| Crude protein | 33.82 | 33.92 | 33.94 | 33.89 |
| Crude lipid | 6.64 | 6.83 | 6.75 | 6.73 |
| Ash | 4.55 | 4.51 | 4.57 | 4.49 |
| VE (mg/kg) | 70.75 | 199.87 | 70.32 | 200.22 |
| Fatty Acid | 70 OO | 200 OO | 70 FO | 200 FO |
|---|---|---|---|---|
| C12:0 | 0.61 | 0.57 | 0.12 | 0.11 |
| C14:0 | 0.65 | 0.57 | 2.99 | 2.87 |
| C15:0 | 0.06 | 0.06 | 0.36 | 0.35 |
| C16:0 | 14.17 | 14.10 | 16.74 | 16.71 |
| C17:0 | 0.12 | 0.15 | 0.27 | 0.27 |
| C18:0 | 3.76 | 3.70 | 3.66 | 3.66 |
| C20:0 | 0.42 | 0.42 | 0.71 | 0.71 |
| C22:0 | 0.21 | 0.21 | 0.44 | 0.45 |
| C16:1 | 0.81 | 0.84 | 2.70 | 2.69 |
| C18:1 | 56.07 | 56.11 | 18.16 | 18.53 |
| C20:1 | 0.33 | 0.35 | 3.00 | 3.02 |
| C22:1 | 0.19 | 0.28 | 10.77 | 11.03 |
| C18:2n-6 | 16.82 | 16.68 | 20.36 | 20.40 |
| C18:3n-3 | 1.38 | 1.38 | 3.49 | 3.52 |
| C18:3n-6 | 0.70 | 0.71 | 1.68 | 1.47 |
| C20:2n-6 | 0.08 | 0.09 | 0.22 | 0.22 |
| C20:3n-6 | 0.03 | 0.03 | 0.05 | 0.05 |
| C20:3n-3 | 0.05 | 0.06 | 0.12 | 0.12 |
| C20:4n-6 (ARA) | 0.08 | 0.10 | 2.63 | 2.58 |
| C20:5n-3 (EPA) | 0.54 | 0.56 | 4.09 | 3.97 |
| C22:3 | 1.67 | 1.77 | 0.19 | 0.20 |
| C22:4n-6 | 0.21 | 0.20 | 0.29 | 0.30 |
| C22:5n-3 | 0.14 | 0.13 | 0.65 | 0.65 |
| C22:6n-3(DHA) | 0.90 | 0.92 | 6.31 | 6.13 |
| ΣSFA | 20.00 | 19.78 | 25.29 | 25.13 |
| ΣMUFA | 57.40 | 57.58 | 34.63 | 35.27 |
| ΣPUFA | 22.60 | 22.63 | 40.08 | 39.61 |
| Σn-3 PUFA | 3.01 | 3.05 | 14.66 | 14.39 |
| Σn-6 PUFA | 17.92 | 17.81 | 25.23 | 25.02 |
| Moisture (%) | Crude Protein (%) | Crude Lipid (%) | Ash (%) | Ovarian Vitamin E (μg/g Wet Tissue) | |
|---|---|---|---|---|---|
| 70 OO | 69.17 ± 0.39 | 15.57 ± 0.41 | 8.97 ± 0.39 | 3.19 ± 0.10 | 25.47 ± 1.64a |
| 200 OO | 68.92 ± 0.38 | 15.89 ± 0.36 | 8.85 ± 0.31 | 3.16 ± 0.06 | 41.90 ± 1.53 b |
| 70 FO | 69.74 ± 0.51 | 16.53 ± 0.44 | 8.60 ± 0.32 | 3.31 ± 0.12 | 23.78 ± 1.15 a |
| 200 FO | 69.92 ± 0.46 | 16.27 ± 0.33 | 8.21 ± 0.27 | 3.25 ± 0.07 | 44.31 ± 2.08 b |
| Two-way ANOVA: | |||||
| lipid level | ns | ns | ns | ns | ns |
| VE level | ns | ns | ns | ns | ** |
| Interactions | ns | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, Y.; Pan, Y.; Wang, Q.; Lu, S.; Li, Y.; Liu, W.; Zheng, T.; Wang, B.; Qiang, J.; Xu, P. Vitamin E Ameliorates Impaired Ovarian Development, Oxidative Stress, and Disrupted Lipid Metabolism in Oreochromis niloticus Fed with a Diet Containing Olive Oil Instead of Fish Oil. Antioxidants 2023, 12, 1524. https://doi.org/10.3390/antiox12081524
Tao Y, Pan Y, Wang Q, Lu S, Li Y, Liu W, Zheng T, Wang B, Qiang J, Xu P. Vitamin E Ameliorates Impaired Ovarian Development, Oxidative Stress, and Disrupted Lipid Metabolism in Oreochromis niloticus Fed with a Diet Containing Olive Oil Instead of Fish Oil. Antioxidants. 2023; 12(8):1524. https://doi.org/10.3390/antiox12081524
Chicago/Turabian StyleTao, Yifan, Yifan Pan, Qingchun Wang, Siqi Lu, Yan Li, Wenting Liu, Tao Zheng, Bei Wang, Jun Qiang, and Pao Xu. 2023. "Vitamin E Ameliorates Impaired Ovarian Development, Oxidative Stress, and Disrupted Lipid Metabolism in Oreochromis niloticus Fed with a Diet Containing Olive Oil Instead of Fish Oil" Antioxidants 12, no. 8: 1524. https://doi.org/10.3390/antiox12081524
APA StyleTao, Y., Pan, Y., Wang, Q., Lu, S., Li, Y., Liu, W., Zheng, T., Wang, B., Qiang, J., & Xu, P. (2023). Vitamin E Ameliorates Impaired Ovarian Development, Oxidative Stress, and Disrupted Lipid Metabolism in Oreochromis niloticus Fed with a Diet Containing Olive Oil Instead of Fish Oil. Antioxidants, 12(8), 1524. https://doi.org/10.3390/antiox12081524







