Effect of Sprouting on the Phenolic Compounds, Glucosinolates, and Antioxidant Activity of Five Camelina sativa (L.) Crantz Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Chemicals
2.3. Extraction of Free and Bound Forms of Phenolic Compounds and Glucosinolates
2.4. Total Phenolic Compounds
2.5. Determination of Phenolic Acids
2.6. Antioxidant Activity
2.7. Glucosinolates
2.8. UHPLC Method Validation
2.9. Statistical Analysis
3. Results and Discussions
3.1. Germination and Sprout Growth
3.2. Phytochemical Content and Antioxidant Activity
3.2.1. Total Phenolic Compounds
3.2.2. Phenolic Acids
3.2.3. Glucosinolates
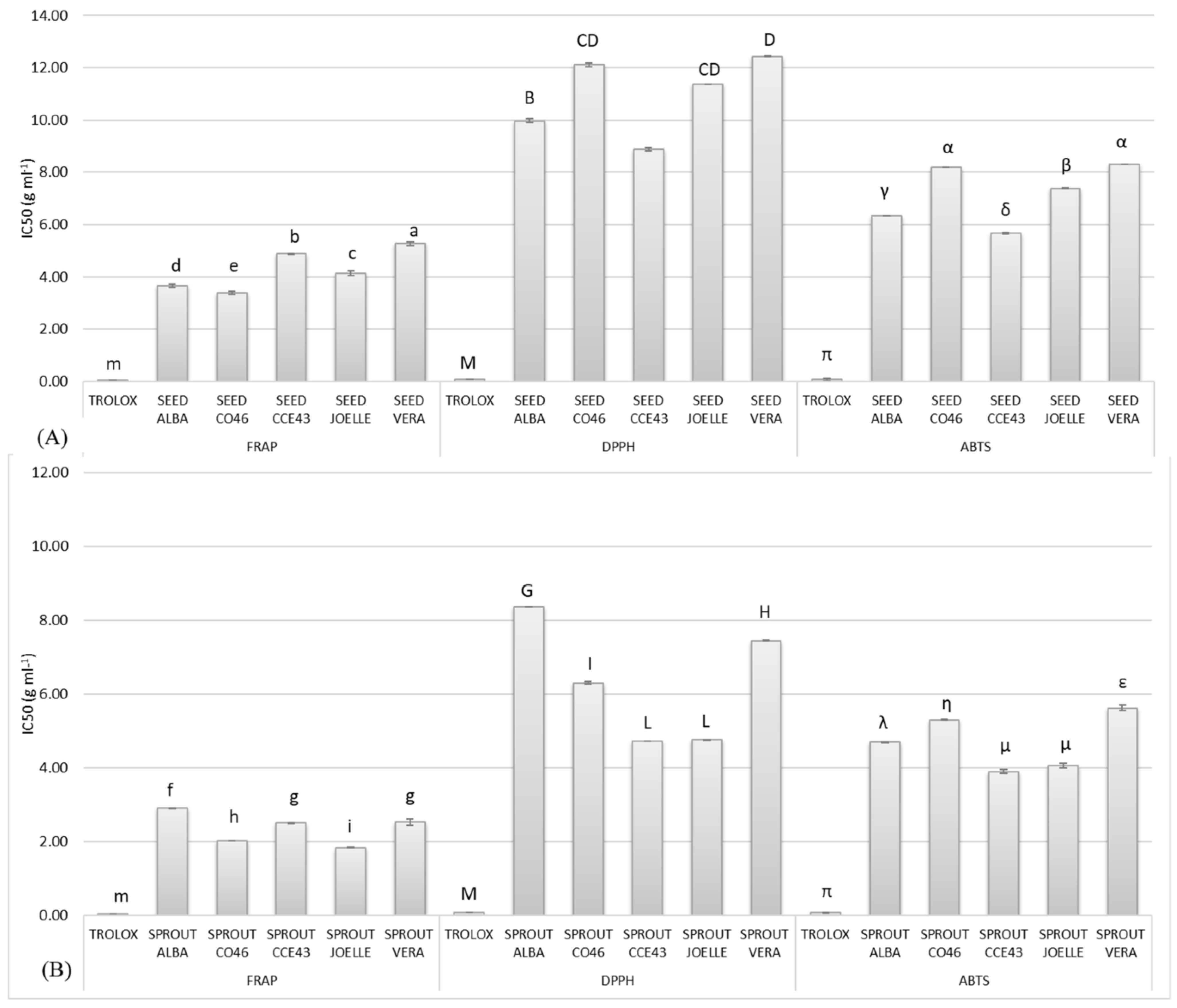
3.2.4. Antioxidant Activity
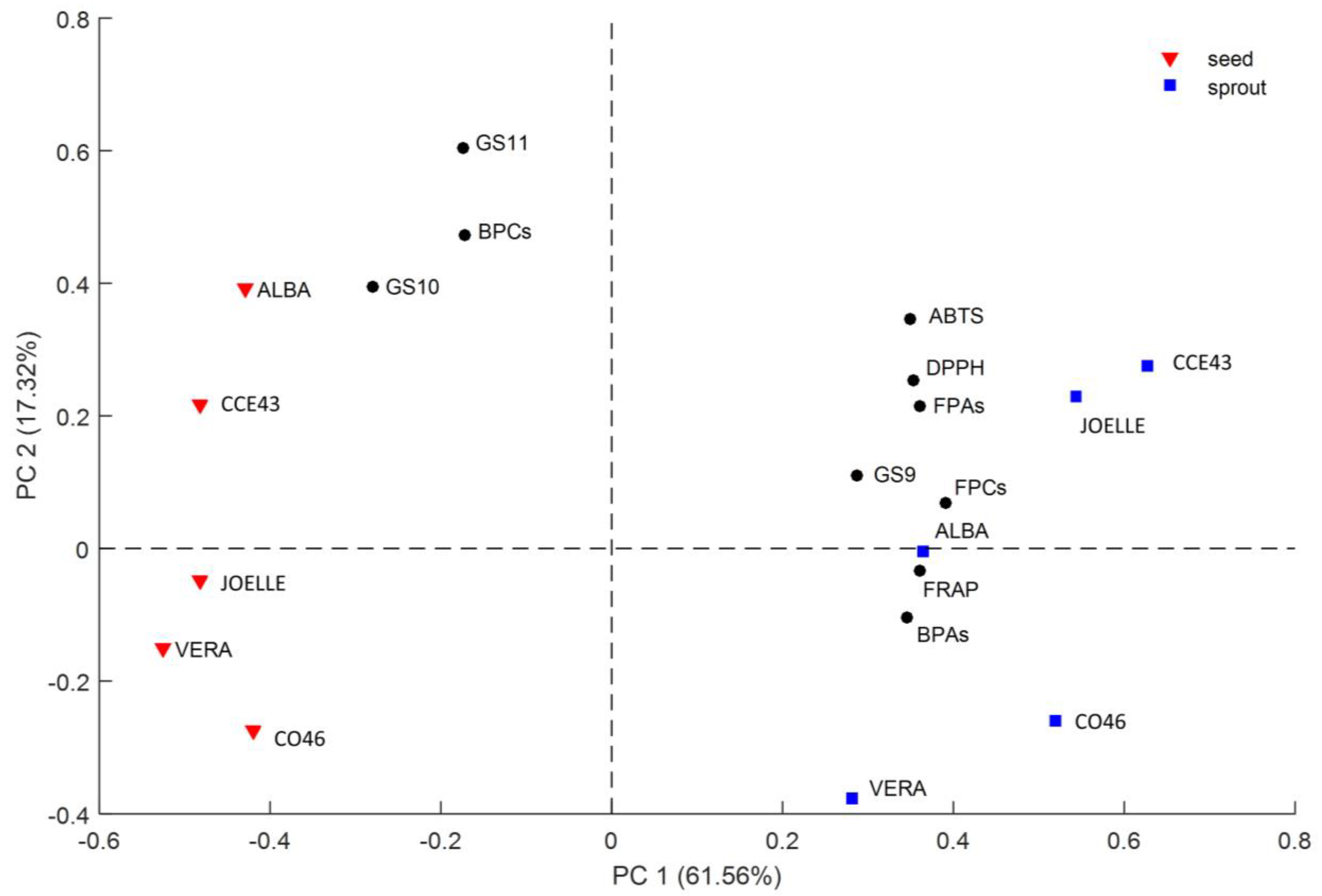
3.2.5. PCA Analysis
3.2.6. Method Validation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sydor, M.; Kurasiak-Popowska, D.; Stuper-Szablewska, K.; Rogoziński, T. Camelina sativa. Status quo and future perspectives. Ind. Crops Prod. 2022, 187, 115531. [Google Scholar] [CrossRef]
- Walia, M.K.; Zanetti, F.; Gesch, R.W.; Krzyżaniak, M.; Eynck, C.; Puttick, D.; Monti, A. Winter camelina seed quality in different growing environments across Northern America and Europe. Ind. Crops Prod. 2021, 169, 113639. [Google Scholar] [CrossRef]
- Royo-Esnal, A.; Edo-Tena, E.; Torra, J.; Recasens, J.; Gesch, R.W. Using fitness parameters to evaluate three oilseed Brassicaceae species as potential oil crops in two contrasting environments. Ind. Crops Prod. 2017, 95, 148–155. [Google Scholar] [CrossRef][Green Version]
- Royo-Esnal, A.; Valencia-Gredilla, F. Camelina as a rotation crop for weed control in organic farming in a semiarid Mediterranean climate. Agriculture 2018, 8, 156. [Google Scholar] [CrossRef]
- Mondor, M.; Hernández-Álvarez, A.J. Camelina sativa composition, attributes, and applications: A review. Eur. J. Lipid Sci. Technol. 2022, 124, 2100035. [Google Scholar] [CrossRef]
- Huang, P.; He, L.; Abbas, A.; Hussain, S.; Hussain, S.; Du, D.; Hafeez, M.B.; Balooch, S.; Zahra, N.; Ren, X.; et al. Seed Priming with Sorghum Water Extract Improves the Performance of Camelina (Camelina sativa (L.) Crantz.) under Salt Stress. Plants 2021, 10, 749. [Google Scholar] [CrossRef]
- Luo, Z.; Szczepanek, A.; Abdel-Haleem, H. Genome-Wide Association Study (GWAS) Analysis of Camelina Seedling Germination under Salt Stress Condition. Agronomy 2020, 10, 1444. [Google Scholar] [CrossRef]
- Kurasiak-Popowska, D.; Stuper-Szablewska, K. The phytochemical quality of Camelina sativa seed and oil. Acta Agric. Scand. Sect. B Soil Plant Sci. 2020, 70, 39–47. [Google Scholar] [CrossRef]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted grains: A comprehensive review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef]
- Mattioli, S.; Cartoni Mancinelli, A.; Bravi, E.; Angelucci, E.; Falcinelli, B.; Benincasa, P.; Castellini, C.; Sileoni, V.; Marconi, O.; Dal Bosco, A. Dietary Freeze-Dried Flaxseed and Alfalfa Sprouts as Additional Ingredients to Improve the Bioactive Compounds and Reduce the Cholesterol Content of Hen Eggs. Antioxidants 2023, 12, 103. [Google Scholar] [CrossRef]
- Galieni, A.; Falcinelli, B.; Stagnari, F.; Datti, A.; Benincasa, P. Sprouts and microgreens: Trends, opportunities, and horizons for novel research. Agronomy 2020, 10, 1424. [Google Scholar] [CrossRef]
- Baenas, N.; Moreno, D.A.; García-Viguera, C. Selecting sprouts of Brassicaceae for optimum phytochemical composition. J. Agric. Food Chem. 2012, 60, 11409–11420. [Google Scholar] [CrossRef]
- Kumar, S.; Andy, A. Health promoting bioactive phytochemicals from Brassica. Int. Food Res. J. 2012, 19, 141. [Google Scholar]
- Francisco, M.; Moreno, D.A.; Cartea, M.E.; Ferreres, F.; García-Viguera, C.; Velasco, P. Simultaneous identification of glucosinolates and phenolic compounds in a representative collection of vegetable Brassica rapa. J. Chromatogr. A 2009, 1216, 6611–6619. [Google Scholar] [CrossRef]
- Hassan, M.A.; Xu, T.; Tian, Y.; Zhong, Y.; Ali, F.Z.; Yang, X.; Lu, B. Health benefits and phenolic compounds of Moringa oleifera leaves: A comprehensive review. Phytomedicine 2021, 93, 153771. [Google Scholar] [CrossRef]
- Park, C.H.; Park, Y.E.; Yeo, H.J.; Kim, J.K.; Park, S.U. Effects of Light-Emitting Diodes on the Accumulation of Phenolic Compounds and Glucosinolates in Brassica juncea Sprouts. Horticulturae 2020, 6, 77. [Google Scholar] [CrossRef]
- Basli, A.; Belkacem, N.; Amrani, I. Health Benefits of Phenolic Compounds Against Cancers; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Oliviero, T.; Lamers, S.; Capuano, E.; Dekker, M.; Verkerk, R. Bioavailability of Isothiocyanates from Broccoli Sprouts in Protein, Lipid, and Fiber Gels. Mol. Nutr. Food Res. 2018, 62, e1700837. [Google Scholar] [CrossRef]
- Felker, P.; Bunch, R.; Leung, A.M. Concentrations of thiocyanate and goitrin in human plasma, their precursor concentrations in brassica vegetables, and associated potential risk for hypothyroidism. Nutr. Rev. 2016, 74, 248–258. [Google Scholar] [CrossRef]
- Torres, A.V.; Tish, N.; Rodov, V. Enhancement of Glucosinolate Formation in Broccoli Sprouts by Hydrogen Peroxide Treatment. Foods 2022, 11, 655. [Google Scholar] [CrossRef]
- Biondi, F.; Balducci, F.; Capocasa, F.; Visciglio, M.; Mei, E.; Vagnoni, M.; Mezzetti, B.; Mazzoni, L. Environmental Conditions and Agronomical Factors Influencing the Levels of Phytochemicals in Brassica Vegetables Responsible for Nutritional and Sensorial Properties. Appl. Sci. 2021, 11, 1927. [Google Scholar] [CrossRef]
- Raiola, A.; Errico, A.; Petruk, G.; Monti, D.M.; Barone, A.; Rigano, M.M. Bioactive Compounds in Brassicaceae Vegetables with a Role in the Prevention of Chronic Diseases. Molecules 2017, 23, 15. [Google Scholar] [CrossRef] [PubMed]
- Borges, C.V.; Junior, S.S.; Ponce, F.S.; Lima, G.P.P. Agronomic Factors Influencing Brassica Productivity and Phytochemical Quality. In Brassica Germplasm—Characterization, Breeding and Utilization; El-Esawi, M.A., Ed.; InTech: London, UK, 2018. [Google Scholar] [CrossRef][Green Version]
- Liu, H.Y.; Liu, Y.; Li, M.Y.; Ge, Y.Y.; Geng, F.; He, X.Q.; Xia, Y.; Guo, B.L.; Gan, R.Y. Antioxidant capacity, phytochemical profiles, and phenolic metabolomics of selected edible seeds and their sprouts. Front. Nutr. 2022, 9, 1067597. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Lamy, E.; Schreiner, M.; Rohn, S. Reactivity and stability of glucosinolates and their breakdown products in foods. Angew. Chem. Int. Ed. 2014, 53, 11430–11450. [Google Scholar] [CrossRef] [PubMed]
- Sarikamiş, G.; Yildirim, A.; Alkan, D. Glucosinolates in seeds, sprouts and seedlings of cabbage and black radish as sources of bioactive compounds. Can. J. Plant Sci. 2015, 95, 681–687. [Google Scholar] [CrossRef]
- Gu, Z.; Guo, Q.; Gu, Y. Factors Influencing Glucoraphanin and Sulforaphane Formation in Brassica Plants: A Review. J. Integr. Agric. 2012, 11, 1804–1816. [Google Scholar] [CrossRef]
- Le, T.N.; Chiu, C.H.; Hsieh, P.C. Bioactive Compounds and Bioactivities of Brassica oleracea L. var. Italica Sprouts and Microgreens: An Updated Overview from a Nutraceutical Perspective. Plants 2020, 9, 946. [Google Scholar] [CrossRef]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Genotypic effects on the phytochemical quality of seeds and sprouts from commercial broccoli cultivars. Food Chem. 2011, 125, 348–354. [Google Scholar] [CrossRef]
- Bellostas Muguerza, N.; Kachlicki, P.; Sørensen, J.C.; Sørensen, H. Glucosinolate profiling of seeds and sprouts of B. oleracea varieties used for food. Sci. Hortic. 2007, 114, 234–242. [Google Scholar] [CrossRef]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Glucosinolates in broccoli sprouts (Brassica oleracea var. italica) as conditioned by sulphate supply during germination. J. Food Sci. 2010, 75, C673–C677. [Google Scholar] [CrossRef]
- Vale, A.P.; Santos, J.; Melia, N.; Peixoto, V.; Brito, N.V.; Oliveira, M.B.P.P. Phytochemical composition and antimicrobial properties of four varieties of Brassica oleracea sprouts. Food Control 2015, 55, 248–256. [Google Scholar] [CrossRef]
- Falcinelli, B.; Sileoni, V.; Marconi, O.; Perretti, G.; Quinet, M.; Lutts, S.; Benincasa, P. Germination under moderate salinity increases phenolic content and antioxidant activity in rapeseed (Brassica napus var oleifera Del.) sprouts. Molecules 2017, 22, 1377. [Google Scholar] [CrossRef]
- Benincasa, P.; Galieni, A.; Manetta, A.C.; Pace, R.; Guiducci, M.; Pisante, M.; Stagnari, F. Phenolic compounds in grains, sprouts and wheatgrass of hulled and non-hulled wheat species. J. Sci. Food Agric. 2015, 95, 1795–1803. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists (AOAC): Arlington, VA, USA, 1995. [Google Scholar]
- Bravi, E.; Francesco, G.D.; Sileoni, V.; Perretti, G.; Galgano, F.; Marconi, O. Brewing by-product upcycling potential: Nutritionally valuable compounds and antioxidant activity evaluation. Antioxidants 2021, 10, 165. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Benincasa, P.; Tosti, G.; Farneselli, M.; Maranghi, S.; Bravi, E.; Marconi, O.; Falcinelli, B.; Guiducci, M. Phenolic content and antioxidant activity of einkorn and emmer sprouts and wheatgrass obtained under different radiation wavelengths. Ann. Agric. Sci. 2020, 65, 68–76. [Google Scholar] [CrossRef]
- Ravisankar, P.; Naga Navya, C.; Pravallika, D.; Navya Sri, D.A. Review on Step-by-Step Analytical Method Validation. IOSR J. Pharm. 2015, 5, 7–19. [Google Scholar]
- Eurachem Working Group. Eurachem Guide. The Fitness for Purpose of Analytical Methods. A Laboratory Guide to Method Validation and Related Topics; Magnusson, B., Ornemark, U., Eds.; Eurachem: Middlesex, UK, 2014; ISBN 979187461590. Available online: www.eurachem.org (accessed on 1 September 2022).
- Definitions and Calculations of Horrat Values from Interlaboratory Data, AOAC International. 2013. Available online: www.aoac.org (accessed on 24 May 2023).
- Dos Santos Mathias, T.R.; Alexandre, V.M.F.; Cammarota, M.C.; de Mello, P.P.M.; Sérvulo, E.F.C. Characterization and determination of brewer’s solid wastes composition. J. Inst. Brew. 2015, 121, 400–404. [Google Scholar] [CrossRef]
- R Core Team 2017. R: A Language and Environment of Statistical Computing. R Foundation. Available online: https://www.rproject.org (accessed on 1 April 2023).
- Terpinc, P.; Polak, T.; Makuc, D.; Ulrih, N.P.; Abramovič, H. The occurrence and characterisation of phenolic compounds in Camelina sativa seed, cake and oil. Food Chem. 2012, 131, 580–589. [Google Scholar] [CrossRef]
- Quezada, N.; Cherian, G. Lipid characterization and antioxidant status of the seeds and meals of Camelina sativa and flax. Eur. J. Lipid Sci. Technol. 2012, 114, 974–982. [Google Scholar] [CrossRef]
- Karamac, M.; Gai, F.; Peiretti, P.G. Effect of the growth stage of false flax (Camelina sativa L.) on the phenolic compound content and antioxidant potential of the aerial part of the plant. Pol. J. Food Nutr. Sci. 2020, 70, 189–198. [Google Scholar] [CrossRef]
- Kaur, J.; Gulati, M.; Singh, S.K.; Kuppusamy, G.; Kapoor, B.; Mishra, V.; Corrie, L. Discovering multifaceted role of vanillic acid beyond flavours: Nutraceutical and therapeutic potential. Trends Food Sci. 2022, 122, 187–200. [Google Scholar] [CrossRef]
- Aldaba-Muruato, L.R.; Ventura-Juárez, J.; Perez-Hernandez, A.M.; Hernández-Morales, A.; Muñoz-Ortega, M.H.; Martínez-Hernández, S.L.; Macías-Pérez, J.R. Therapeutic perspectives of p-coumaric acid: Anti-necrotic, anti-cholestatic and anti-amoebic activities. World Acad. Sci. 2021, 3, 1–8. [Google Scholar] [CrossRef]
- Pei, J.; Velu, P.; Zareian, M.; Feng, Z.; Vijayalakshmi, A. Effects of Syringic Acid on Apoptosis, Inflammation, and AKT/mTOR Signaling Pathway in Gastric Cancer Cells. Front. Nutr. 2021, 8, 788929. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef]
- Traka, M.H. Health benefits of glucosinolates. Adv. Bot. Res. 2016, 80, 247–279. [Google Scholar]
- Miękus, N.; Marszałek, K.; Podlacha, M.; Iqbal, A.; Puchalski, C.; Świergiel, A.H. Health Benefits of Plant-Derived Sulfur Compounds, Glucosinolates, and Organosulfur Compounds. Molecules 2020, 25, 3804. [Google Scholar] [CrossRef]
- Russo, R.; Reggiani, R. Antinutritive compounds in twelve Camelina sativa genotypes. Am. J. Plant Sci. 2012, 3, 1408–1412. [Google Scholar] [CrossRef]
- Schuster, A.; Friedt, W. Glucosinolate content and composition as parameters of quality of Camelina seed. Ind. Crops Prod. 1998, 7, 297–302. [Google Scholar] [CrossRef]
- Berhow, M.A.; Polat, U.; Glinski, J.A.; Glensk, M.; Vaughn, S.F.; Isbell, T.; Gardner, C. Optimized analysis and quantification of glucosinolates from Camelina sativa seeds by reverse-phase liquid chromatography. Ind. Crops Prod. 2013, 43, 119–125. [Google Scholar] [CrossRef]
- Punetha, H.N.; Saif, S.; Dinesh, P. Glucosinolates in oilseed Brassica: Neutraceuticals with tremendous health benefits. Biotech Today 2019, 9, 26–32. [Google Scholar] [CrossRef]
- Yang, H.; Qina, J.; Wanga, X.; EI-Shorac, H.M.; Yang, B.Y. Production of plant-derived anticancer precursor glucoraphanin in chromosomally engineered Escherichia coli. Microbiol. Res. 2020, 238, 126484. [Google Scholar] [CrossRef]
- Tavarini, S.; De Leo, M.; Matteo, R.; Lazzeri, L.; Braca, A.; Angelini, L.G. Flaxseed and camelina meals as potential sources of health-beneficial compounds. Plants 2021, 10, 156. [Google Scholar] [CrossRef]
- Lockhart, J. Methionine-Derived Glucosinolates: The Compounds That Give Brassicas Their Bite. Plant Cell 2019, 31, 1429. [Google Scholar] [CrossRef]
- Mattosinhos, P.D.S.; Sarandy, M.M.; Novaes, R.D.; Esposito, D.; Gonçalves, R.V. Anti-inflammatory, antioxidant, and skin regenerative potential of secondary metabolites from plants of the Brassicaceae family: A systematic review of in vitro and in vivo preclinical evidence (biological activities Brassicaceae skin diseases). Antioxidants 2022, 11, 1346. [Google Scholar] [CrossRef]
- Shankar, S.; Segaran, G.; Sundar, R.D.V.; Settu, S.; Sathiavelu, M. Brassicaceae-A classical review on its pharmacological activities. Int. J. Pharm. Sci. Rev. Res. 2019, 55, 107–113. [Google Scholar]
- Falcinelli, B.; Marconi, O.; Maranghi, S.; Lutts, S.; Rosati, A.; Famiani, F.; Benincasa, P. Effect of genotype on the sprouting of pomegranate (Punica granatum L.) seeds as a source of phenolic compounds from juice industry by-products. Plant Foods Hum. Nutr. 2017, 72, 432–438. [Google Scholar] [CrossRef]
- Falcinelli, B.; Famiani, F.; Paoletti, A.; D’Egidio, S.; Stagnari, F.; Galieni, A.; Benincasa, P. Phenolic compounds and antioxidant activity of sprouts from seeds of Citrus species. Agriculture 2020, 10, 33. [Google Scholar] [CrossRef]
- Rahman, M.J.; Costa de Camargo, A.; Shahidi, F. Phenolic profiles and antioxidant activity of defatted camelina and sophia seeds. Food Chem. 2018, 240, 917–925. [Google Scholar] [CrossRef]
- Mieriņa, I.; Adere, L.; Krasauska, K.; Zoltnere, E.; Dārta, Z.S.; Jure, M. Antioxidant properties of Camelina sativa oil and press-cakes. In Proceedings of the Latvian Academy of Sciences; De Gruyter: Warsaw, Poland, 2017; Volume 71, p. 515. [Google Scholar]
- AOAC. Guidelines for Single Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals; AOAC International: Gaithersburg, MD, USA, 2002. [Google Scholar]
- Methods, method verification and validation. In Ora Laboratory Procedure; Food and Drug Administration Document No.: Version No.: 1.1 ORALAB.5.4.5; Food and Drug Administration: Silver Spring, MD, USA, 2020; pp. 1–12.

| Cultivar | G (%) | MGT (Days) | Shoot Length (mm) | Root Length (mm) | Individual Sprout Fresh Weight (mg) | Moisture (%) |
|---|---|---|---|---|---|---|
| ALBA | 88 ± 0.9 | 1.9 ± 0.05 | 6.0 ± 0.23 | 16.2 ± 0.69 | 9.9 ± 0.09 | 85.2 ± 1.10 |
| CO46 | 96 ± 0.9 | 2.0 ± 0.04 | 6.13 ± 0.23 | 38.5 ± 0.65 | 14.8 ± 0.81 | 91.3 ± 1.29 |
| CCE43 | 98 ± 0.9 | 1.1 ± 0.04 | 7.84 ± 0.61 | 24.4 ± 1.33 | 11.6 ± 0.49 | 93.2 ± 0.41 |
| JOELLE | 98 ± 0.3 | 2.1 ± 0.04 | 7.26 ± 0.31 | 22.6 ± 0.49 | 11.0 ± 0.84 | 90.3 ± 1.27 |
| VERA | 93 ± 0.6 | 1.3 ± 0.03 | 7.32 ± 0.22 | 32.1 ± 1.05 | 9.6 ± 0.43 | 90.7 ± 1.44 |
| Significance of ANOVA | - | ** | * | ** | ** | - |
| LSD0.05 | - | 0.124 | 1.272 | 3.249 | 2.171 | - |
| Seeds | Sprouts | Significance | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic Compounds | ALBA | CO46 | CCE43 | JOELLE | VERA | ALBA | CO46 | CCE43 | JOELLE | VERA | G | GS | G × GS | LSD0.05 |
| FPC (mg GAEg−1 dm) | 9.23 ± 0.16 d | 8.39 ± 0.293 de | 7.95 ± 0.23 ef | 7.95 ± 0.46 ef | 7.45 ± 0.05 f | 13.76 ± 0.14 c | 18.87 ± 0.15 b | 18.58 ± 0.38 b | 19.87 ± 0.15 a | 13.67 ± 0.48 c | ** | ** | ** | 0.900 |
| BPC | 2.18 ± 0.22 bc | 1.29 ± 0.16 efg | 3.36 ± 0.15 a | 1.71 ± 0.16 cde | 2.06 ± 0.27 bcd | 1.05 ± 0.07 g | 1.20 ± 0.05 fg | 2.23 ± 0.24 b | 1.66 ± 0.08 def | 1.29 ± 0.05 efg | ** | ** | * | 0.512 |
| TPC | 11.41 ± 0.46 d | 9.69 ± 0.60 e | 11.31 ± 0.50 d | 9.67 ± 0.85 e | 9.51 ± 0.35 e | 14.81 ± 0.24 c | 20.06 ± 0.12 b | 20.81 ± 0.32 ab | 21.53 ± 0.16 ab | 14.96 ± 0.19 c | ** | ** | ** | 1.138 |
| FPA (µg g−1 dm) | ||||||||||||||
| Homovanillic | 1116.31 ± 1.30 a | 330.95 ± 3.77 d | 471.49 ± 3.2.8 c | 279.31 ± 1.17 e | 544.86 ± 2.66 b | - | - | - | - | - | ** | ** | ** | 5.90 |
| 3- hydroxybenzoic | - | - | - | - | - | 48.66 ± 0.16 b | - | 143.65 ± 0.33 a | - | - | ** | ** | ** | 0.36 |
| Vanillic | - | - | - | - | - | 24.63 ± 0.42 d | 27.37 ± 0.96 c | 61.10 ± 1.11 a | - | 42.54 ± 0.18 b | ** | ** | ** | 1.53 |
| Syringic | 2.07 ± 0.02 f | 2.27 ± 0.03 f | 1.12 ± 0.03 f | 3.30 ± 0.13 f | 2.01 ± 0.01 f | 291.47 ± 2.20 c | 530.75 ± 0.61 a | 178.43 ± 1.21 e | 210.67 ± 4.08 c | 466.71 ± 3.62 b | ** | ** | ** | 6.01 |
| Salicylic | - | - | - | - | - | 292.22 ± 11.45 b | 258.08 ± 7.92 c | 291.89 ± 6.52 b | 365.60 ± 0.85 a | 195.51 ± 3.03 d | ** | ** | ** | 21.91 |
| Chlorogenic | - | - | - | - | - | 1275.62 ± 2.21 b | 1059.88 ± 9.56 c | 2161.59 ± 17.61 a | 945.09 ± 1.06 d | 423.87 ± 8.72 e | ** | ** | ** | 3.83 |
| p-coumaric | - | - | - | - | - | 65.54 ± 1.32 b | 52.27 ± 1.57 c | 30.87 ± 2.01 d | 80.92 ± 0.78 a | 31.17 ± 2.43 d | ** | ** | ** | 15.64 |
| Ferulic | 2.75 ± 0.10 d | 3.48 ± 0.09 d | 1.95 ± 0.15 d | 1.89 ± 0.22 de | 2.63 ± 0.02 d | 23.87 ± 1.09 b | 21.99 ± 0.36 b | 26.40 ± 1.44 a | - | 18.43 ± 0.73 c | ** | ** | ** | 2.00 |
| Sinapic | 46.49 ± 0.24 de | 22.25 ± 0.63 fg | 20.75 ± 0.53 g | 69.42 ± 0.09 b | 28.11 ± 0.70 f | 99.55 ± 0.64 a | 48.70 ± 2.77 d | 62.13 ± 4.10 c | 99.47 ± 3.83 a | 41.17 ± 0.99 e | ** | ** | ** | 6.44 |
| Total FPA | 1067.63 ± 1.66 f | 358.95 ± 3.08 i | 495.31 ± 3.63 h | 353.92 ± 0.98 i | 577.61 ± 0.98 g | 2121.55 ± 5.18 b | 1999.04 ± 7.07 c | 2956.06 ± 34.81 a | 1701.75 ± 5.10 d | 1219.39 ± 8.18 e | ** | ** | ** | 22.91 |
| BPA (µg g−1 dm) | ||||||||||||||
| 3.5-dihydroxybenzoic | 52.37 ± 0.64 d | 23.11 ± 0.13 f | 24.83 ± 1.72 f | 54.66 ± 1.43 d | 31.08 ± 0.62 e | 81.97 ± 0.96 b | 25.05 ± 0.11 f | 156.81 ± 3.33 a | 60.54 ± 1.61 c | 85.42 ± 0.76 b | ** | ** | ** | 4.57 |
| 3- hydroxybenzoic | - | - | - | - | - | 196.55 ± 2.65 d | 217.51 ± 3.60 c | 466.56 ± 3.40 a | 191.62 ± 1.48 d | 450.32 ± 2.40 b | ** | ** | ** | 6.26 |
| Syringic | - | - | - | - | - | 10.48 ± 0.50 c | - | 41.93 ± 0.96 a | - | 20.04 ± 2.09 b | ** | ** | ** | 2.35 |
| m-coumaric | - | - | - | - | - | 27.70 ± 1.50 c | 16.59 ± 0.37 d | 61.73 ± 0.78 a | - | 35.48 ± 0.62 e | ** | ** | ** | 1.84 |
| Caffeic | - | - | - | - | - | 12.57 ± 1.39 a | - | - | - | - | ** | ** | ** | 1.39 |
| p-coumaric | 13.00 ± 0.62 f | 9.97 ± 0.20 fg | 6.26 ± 1.13 g | 21.70 ± 0.35 e | 19.96 ± 0.21 e | 31.51 ± 1.64 d | 38.56 ± 1.36 c | 54.48 ± 1.67 b | 30.65 ± 1.51 d | 65.49 ± 1.35 a | ** | ** | ** | 3.63 |
| Salicylic | 29.81 ± 1.27 g | 28.20 ± 0.01 g | 44.01 ± 2.10 f | 42.85 ± 1.03 f | 25.07 ± 0.13 g | 133.07 ± 0.08 d | 250.37 ± 2.77 a | 83.08 ± 2.12 e | 141.03 ± 2.08 c | 217.50 ± 1.67 b | ** | ** | ** | 5.12 |
| Ferulic | 18.87 ± 1.23 e | 12.36 ± 0.75 f | 10.49 ± 1.09 f | 26.26 ± 0.78 d | 18.43 ± 0.98 e | 33.58 ± 0.41 c | 29.65 ± 0.53 cd | 78.30 ± 0.42 a | 29.16 ± 1.40 cd | 44.94 ± 1.24 b | ** | ** | ** | 4.77 |
| Sinapic | 91.84 ± 2.75 b | 63.06 ± 0.57 e | 40.95 ± 1.92 g | 111.67 ± 2.38 a | 72.04 ± 0.84 d | 86.98 ± 1.08 c | 54.29 ± 0.10 f | 60.50 ± 0.69 e | 51.98 ± 2.04 f | 64.46 ± 0.16 e | ** | ** | ** | 4.86 |
| Total BPA | 205.89 ± 6.51 e | 136.70 ± 1.26 g | 126.53 ± 5.79 g | 257.14 ± 5.98 d | 166.58 ± 0.83 e | 624.07 ± 3.48 b | 632.02 ± 4.95 b | 1003.39 ± 12.19 a | 504.99 ± 3.21 c | 1000.48 ± 1.23 a | ** | ** | ** | 15.33 |
| TPA | 1273.52 ± 4.85 e | 495.65 ± 1.92 h | 621.84 ± 2.16 g | 611.06 ± 6.96 g | 744.19 ± 2.50 f | 2745.62 ± 4.64 b | 2631.06 ± 2.83 c | 3959.45 ± 25.56 a | 2206.74 ± 17.03 d | 2219.88 ± 5.02 d | ** | ** | ** | 32.81 |
| GLSs (mg g−1 dm) | Seeds | Sprouts | Significance | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALBA | CO46 | CCE43 | JOELLE | VERA | ALBA | CO46 | CCE43 | JOELLE | VERA | G | GS | G × GS | LSD0.05 | |
| GS9 | 3.00 ± 0.02 d | 1.45 ± 0.02 f | 0.84 ± 0.02 h | 1.40 ± 0.01 f | 1.20 ± 0.01 g | 6.12 ± 0.02 a | 3.68 ± 0.02 c | 2.98 ± 0.06 d | 3.91 ± 0.02 b | 2.37 ± 0.03 e | ** | ** | ** | 0.080 |
| GS10 | 2.51 ± 0.01 a | 1.16 ± 0.01 c | 1.10 ± 0.01 d | 1.34 ± 0.01 b | 1.10 ± 0.03 d | 0.22 ± 0.01 h | 0.31 ± 0.02 g | 0.55 ± 0.02 f | 0.91 ± 0.02 e | 0.26 ± 0.01 gh | ** | ** | * | 0.050 |
| GS11 | 0.66 ± 0.01 a | 0.27 ± 0.01 g | 0.41 ± 0.01 d | 0.47 ± 0.01 b | 0.30 ± 0.01 g | 0.43 ± 0.01 c | 0.04 ± 0.01 h | 0.34 ± 0.01 f | 0.38 ± 0.02 e | 0.05 ± 0.01 h | ** | ** | ** | 0.025 |
| Total GLSs | 6.16 ± 0.04 b | 2.88 ± 0.04 g | 2.34 ± 0.03 i | 3.21 ± 0.01 f | 2.59 ± 0.04 h | 6.77 ± 0.05 a | 4.02 ± 0.01 d | 3.87 ± 0.13 e | 5.19 ± 0.03 c | 2.68 ± 0.04 h | ** | ** | ** | 0.110 |
| GLSs | Cc | R | Linear Equation | tR (Min) | A | N | T | LOD | LOQ | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | RSD% | Mean | RSD% | Mean | RSD% | Mean | RSD% | ||||||
| GS9 | 0.251 | 0.9984 | y = 357.723 × x − 15.66 | 1.837 | 0.574 | 545.213 | 1.279 | 5396.532 | 1.148 | 1.298 | 1.356 | 0.025 | 0.083 |
| GS10 | 0.230 | 0.9984 | y = 357.723 × x − 15.66 | 2.969 | 0.313 | 483.704 | 1.389 | 13,668.939 | 1.014 | 1.448 | 1.335 | 0.052 | 0.174 |
| GS11 | 0.250 | 0.9980 | y = 357.723 × x − 15.66 | 4.302 | 0.224 | 1167.362 | 1.423 | 25,080.563 | 1.231 | 1.164 | 1.374 | 0.039 | 0.129 |
| GLSs | Concentration Level (mg g−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 15 | |||||||
| R′% | RDSr% | HorRat | R′% | RDSr% | HorRat | R′% | RDSr% | HorRat | |
| (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | |||||||
| GS9 | 100 ± 2 | 1.8 | 0.2 | 102 ± 2 | 2.3 | 0.3 | 102 ± 3 | 2.5 | 0.4 |
| GS10 | 92 ± 2 | 2.4 | 0.2 | 98 ± 2 | 2.4 | 0.2 | 102 ± 2 | 2.1 | 0.2 |
| GS11 | 110 ± 2 | 1.4 | 0.3 | 102 ± 3 | 2.6 | 0.5 | 94 ± 2 | 2.2 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravi, E.; Falcinelli, B.; Mallia, G.; Marconi, O.; Royo-Esnal, A.; Benincasa, P. Effect of Sprouting on the Phenolic Compounds, Glucosinolates, and Antioxidant Activity of Five Camelina sativa (L.) Crantz Cultivars. Antioxidants 2023, 12, 1495. https://doi.org/10.3390/antiox12081495
Bravi E, Falcinelli B, Mallia G, Marconi O, Royo-Esnal A, Benincasa P. Effect of Sprouting on the Phenolic Compounds, Glucosinolates, and Antioxidant Activity of Five Camelina sativa (L.) Crantz Cultivars. Antioxidants. 2023; 12(8):1495. https://doi.org/10.3390/antiox12081495
Chicago/Turabian StyleBravi, Elisabetta, Beatrice Falcinelli, Giorgia Mallia, Ombretta Marconi, Aritz Royo-Esnal, and Paolo Benincasa. 2023. "Effect of Sprouting on the Phenolic Compounds, Glucosinolates, and Antioxidant Activity of Five Camelina sativa (L.) Crantz Cultivars" Antioxidants 12, no. 8: 1495. https://doi.org/10.3390/antiox12081495
APA StyleBravi, E., Falcinelli, B., Mallia, G., Marconi, O., Royo-Esnal, A., & Benincasa, P. (2023). Effect of Sprouting on the Phenolic Compounds, Glucosinolates, and Antioxidant Activity of Five Camelina sativa (L.) Crantz Cultivars. Antioxidants, 12(8), 1495. https://doi.org/10.3390/antiox12081495











