Effect of Brewing Conditions on Antioxidant Properties of Ginkgo biloba Leaves Infusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Basic Composition of Dried Leaves
2.3. Infusions
2.4. Determination of pH
2.5. Determination of Apparent and Real Extract
2.6. Determination of Acidity
2.7. Chemical and Physical Analyses
2.8. Determination of Active Compounds by Liquid Chromatography (HPLC)
2.9. Sensory Assessment
2.10. Analysis of Micro- and Macroelements
2.11. Statistical Analysis
3. Results and Discussion
3.1. Basic Characteristics of Dried Ginkgo biloba Leaves
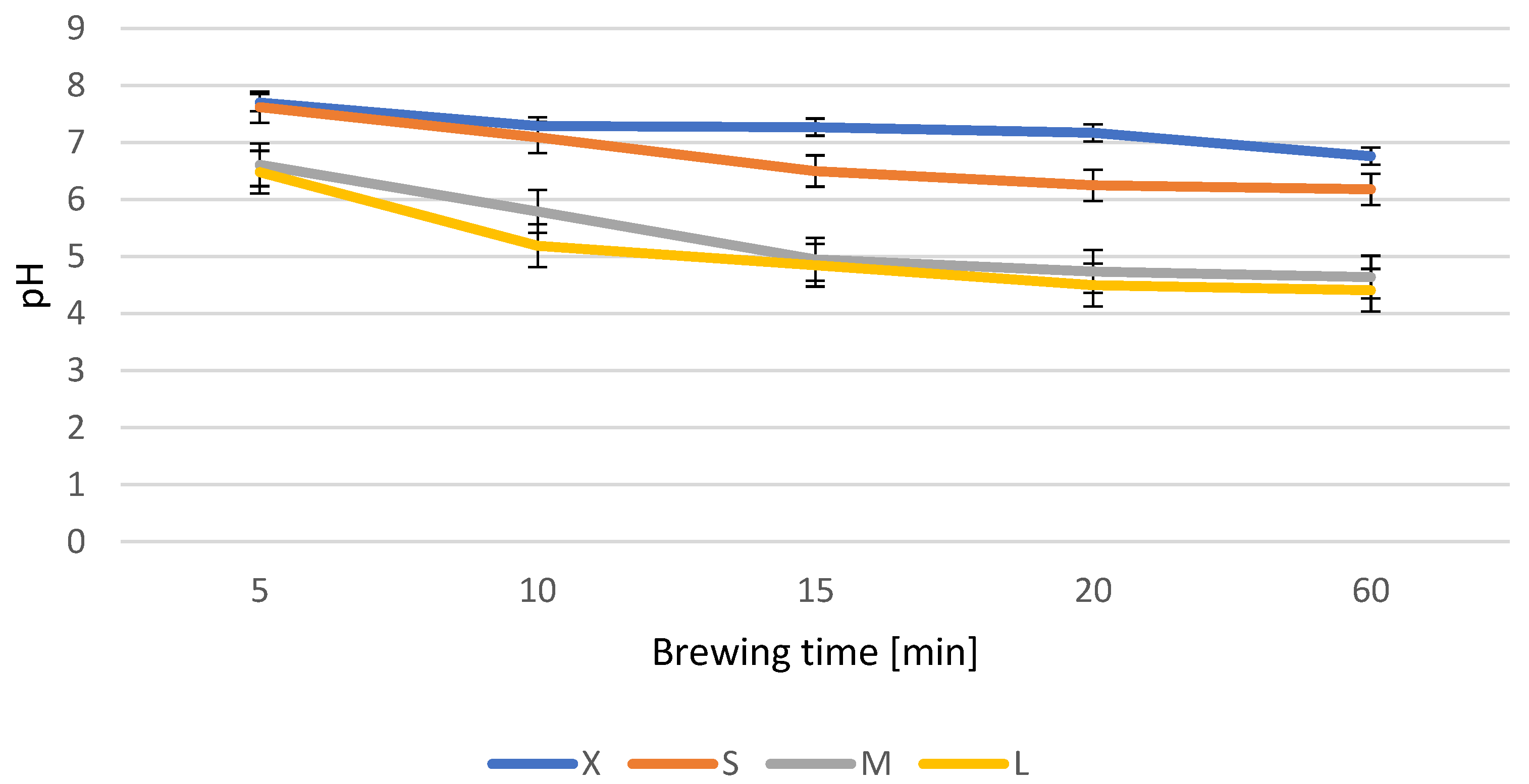
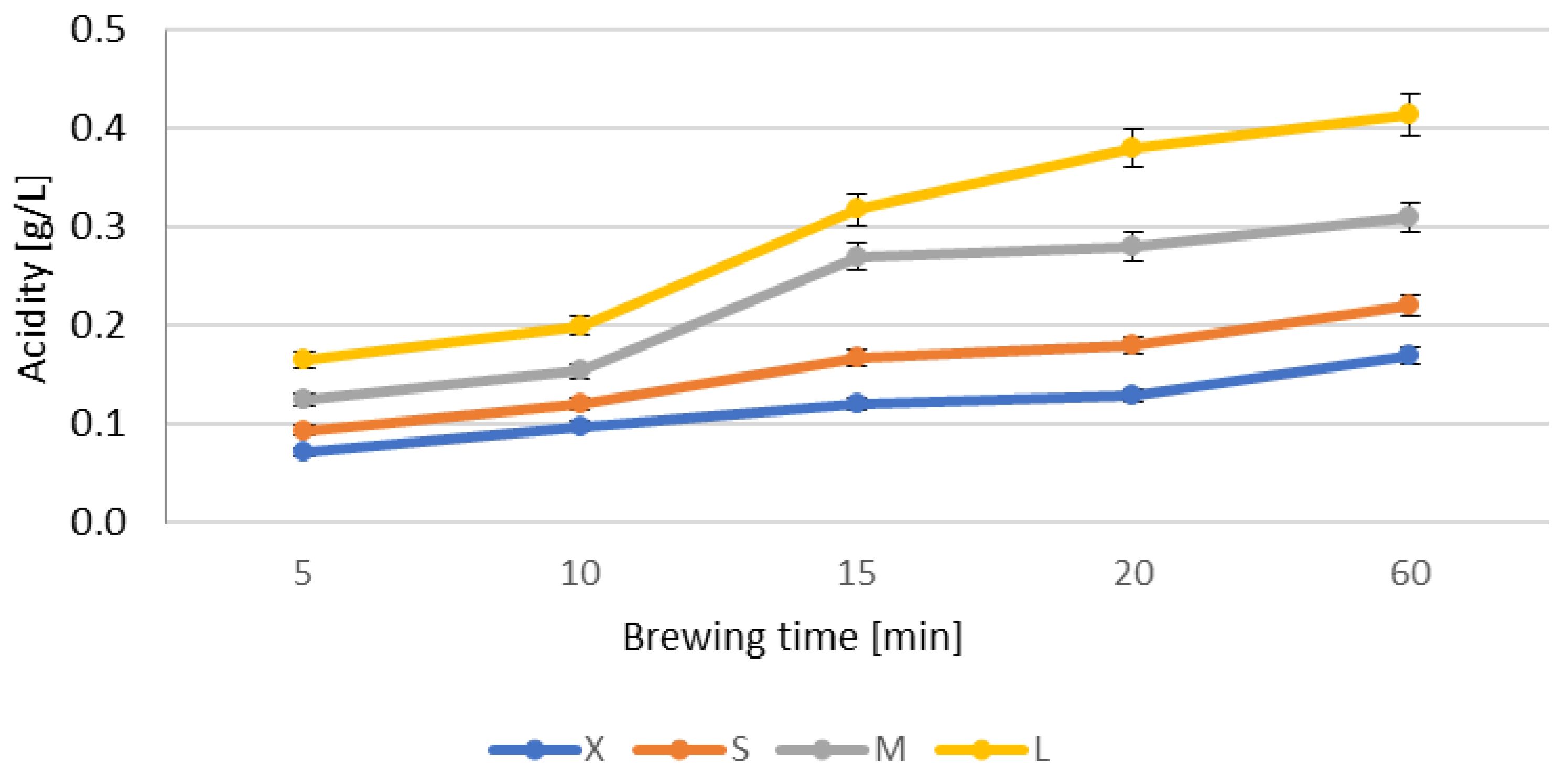
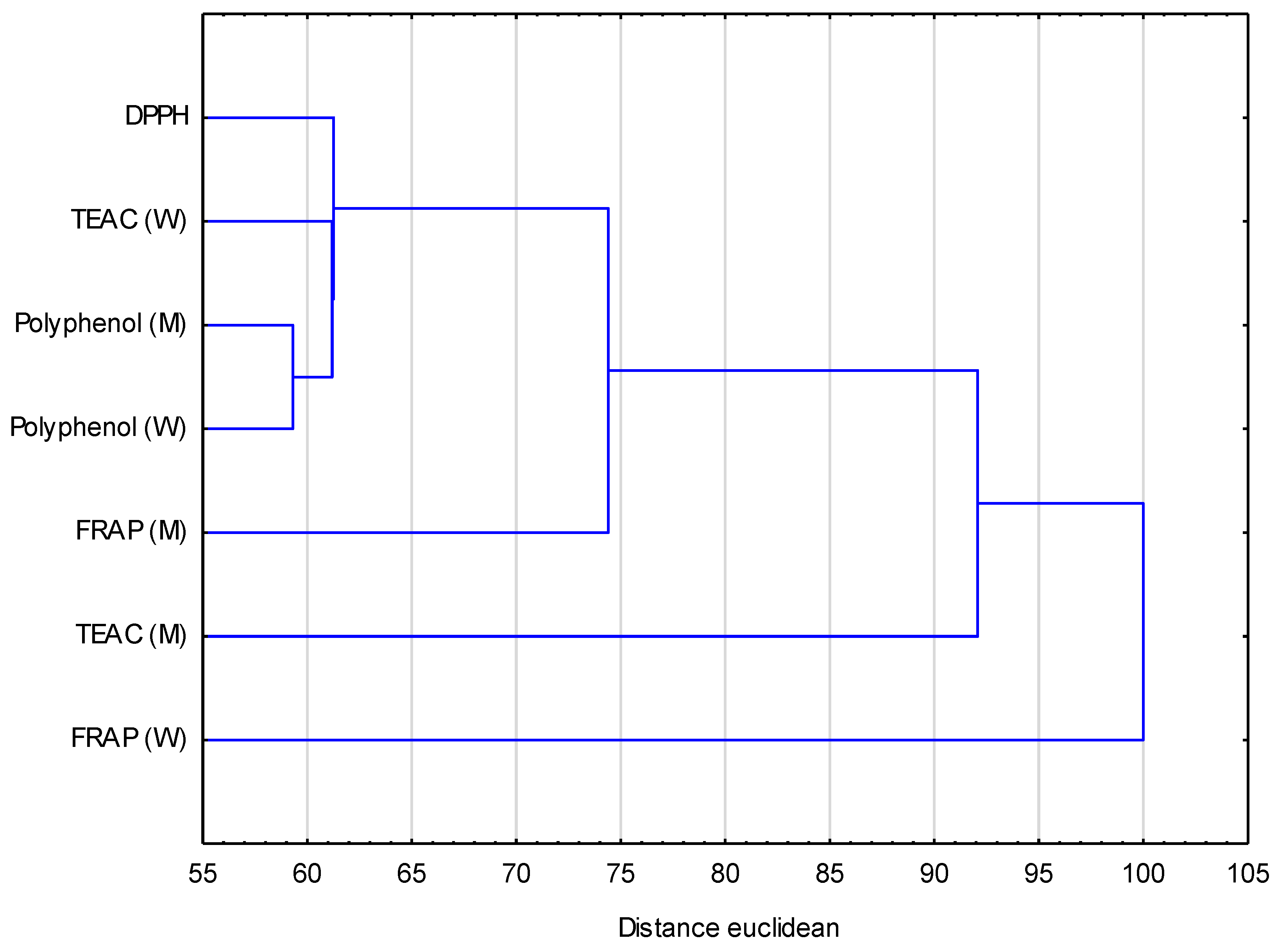
3.2. Basic Composition and Antioxidant Activity of Infusions from Dried Ginkgo biloba Leaves
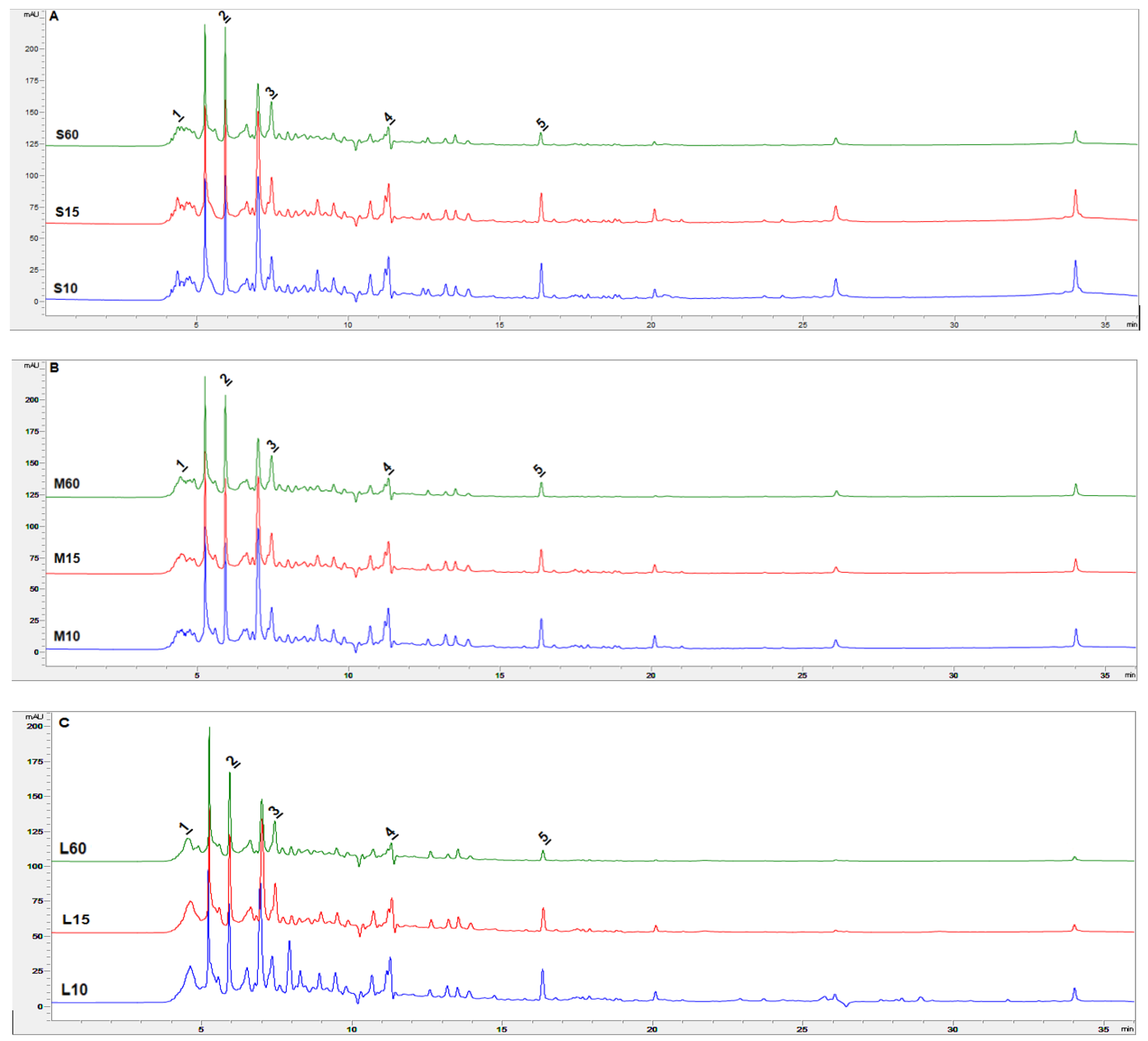
3.3. Determination of Active Compounds by Liquid Chromatography (HPLC) and Total Flavonoid Content
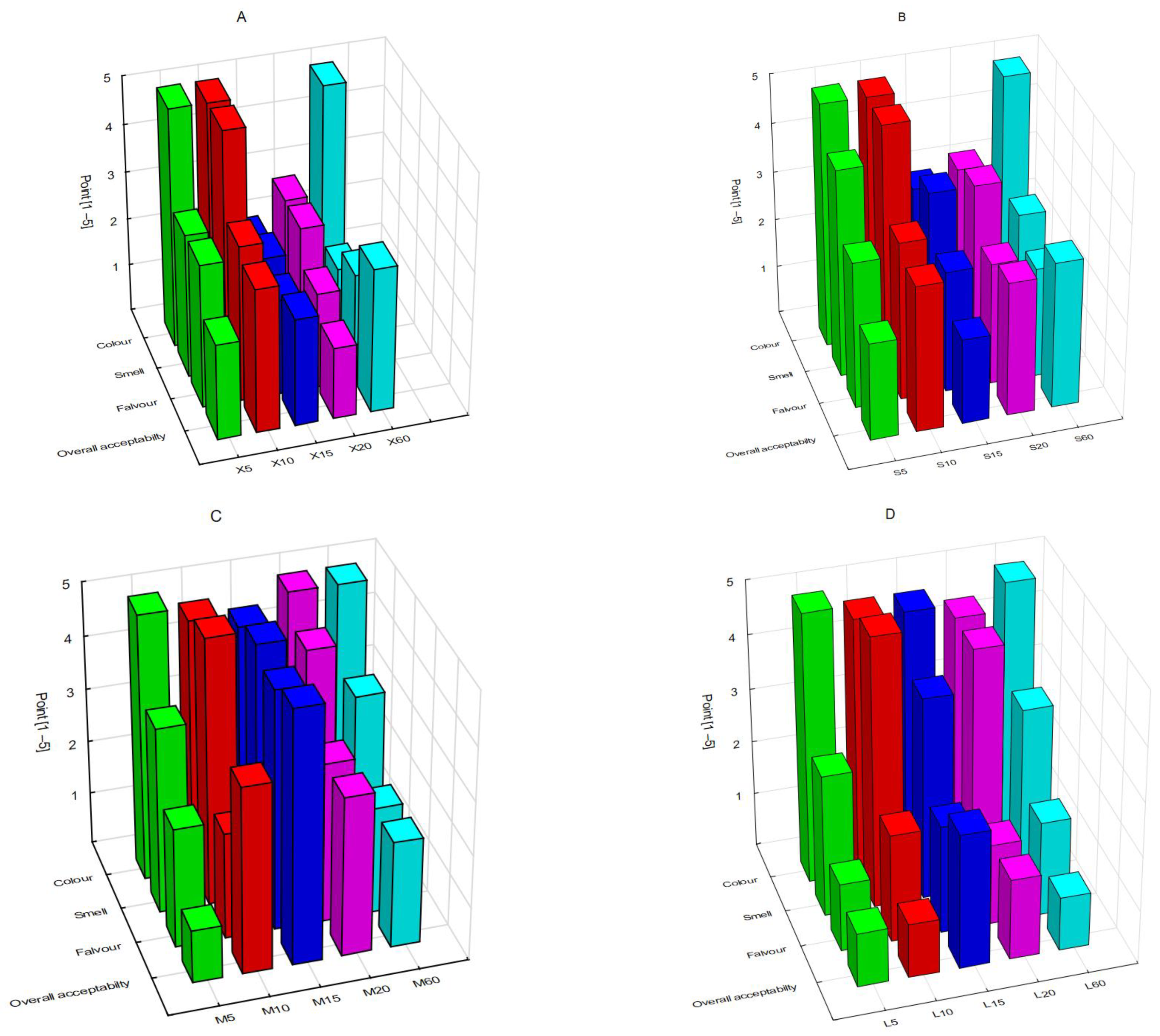
3.4. Sensory Assessment
3.5. Analyses of Micro- and Macroelements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DPPH | Free DPPH radical scavenging ability; 2,2-diphenyl-1-picrylhydrazyl free radical |
| FRAP | Ferric ion reducing antioxidant power |
| LM | Methanol extract from dried Ginkgo biloba leaves |
| LWC | Cold water extract from dried Ginkgo biloba leaves |
| LWH | Hot water extract from dried Ginkgo biloba leaves |
| M | Methanol extract |
| TEAC | Trolox Equivalent Antioxidant Capacity |
| W | Water extract |
References
- Kumari, T. A study on knowledge and attitude towards digital health of rural population of india-Innovations in practice to improve healthcare in the rural population. Acad. Soc. Converg. Sci. 2019, 3, 13–21. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, C.; Wu, Z.; Xu, H.; Liu, H.; Schneider, H.; Lin, J. Ginkgo biloba. Trends Genet. 2021, 37, 488–489. [Google Scholar] [CrossRef] [PubMed]
- Eisvand, F.; Razavi, M.; Hosseinzadeh, H. The effects of Ginkgo biloba on metabolic syndrome: A review. Phytother. Res. 2020, 34, 1798–1811. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Jin, X.; Man, C.; Gong, D. Does Adjuvant Treatment with Ginkgo Biloba to Statins Have Additional Benefits in Patients with Dyslipidemia? Front. Pharmacol 2018, 9, 659. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Yang, X.; Zhang, L.; Ansari, I.A.; Khan, M.S.; Han, S.; Feng, Y. Ginkgolide B ameliorates oxidized low-density lipoprotein-induced endothelial dysfunction via modulating Lectin-like ox-LDL-receptor-1 and NADPH oxidase 4 expression and inflammatory cascades. Phytother. Res. 2018, 32, 2417–2427. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.A.; Assad, H.C.; Rabeea, I.S. Antihyperlipidemic, Antioxidant and Anti-Inflammatory Effects of Ginkgo Biloba in High Cholesterol Fed Rabbits. J. Pharm. Sci. Res. 2017, 9, 2163–2167. [Google Scholar]
- Jang-Soon, P. A convergence study on the properties of hair coated with Ginkgo biloba extract. J. Korea Converg. Soc. 2020, 11, 223–228. [Google Scholar]
- Klimowicz, A.; Zielonka, J.; Turek, M.; Nowak, A. Substancje pochodzenia naturalnego stasowane w terapii cellulitu. Postępy Fitoter. 2015, 16, 96–101. [Google Scholar]
- Tabassum, N.-E.; Das, R.; Lami, M.S.; Chakraborty, A.J.; Mitra, S.; Tallei, T.E.; Idroes, R.; Mohamed, A.A.-R.; Hossain, J.; Dhama, K.; et al. Ginkgo biloba: A Treasure of Functional Phytochemicals with Multimedicinal Applications. Evid. Based Complement Altern. Med. 2022, 2022, 8288818. [Google Scholar] [CrossRef]
- Shukla, S.; Gupta, S.; Nishad, R.K.; Yadav, Y.K. Treating Sensorineural Tinnitus- Oral forms of 5-MTHF (folate), Vitamin B12 and Gingko Biloba Vs oral forms of Caroverine and Gingko biloba. Eur. J. Mol. Clin. Med. 2022, 09, 8525–8534. [Google Scholar]
- Tomova, T.; Slavova, I.; Tomov, D.; Kirova, G.; Argirova, M.D. Ginkgo biloba Seeds—An Environmental Pollutant or a Functional Food. Horticulturae 2021, 7, 218. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis, 18th ed.; Association of Officiating Analytical Chemists: Washington, DC, USA, 2015; Method 935.14 and 992.24. [Google Scholar]
- Krełowska-Kułas, M. Badanie Jakości Produktów Spożywczych [Analyses of Food Products Quality]; PWE: Warsaw, Poland, 1993; pp. 271–272. [Google Scholar]
- Turkmen, N.; Dari, F.; Velioglu, Y.S. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem. 2005, 93, 713–718. [Google Scholar] [CrossRef]
- Tang, S.Z.; Kerry, J.P.; Shehan, D.; Buckley, D.J. Antioxidative mechanism of tea catechins in chicken meat system. Food Chem. 2002, 76, 45–51. [Google Scholar] [CrossRef]
- Re, T.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Ecans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzi, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT–Food Sci. Technol. 2021, 150, 111932. [Google Scholar] [CrossRef]
- Hrebień-Filisińska, A.M.; Bartkowiak, A. Antioxidative Effect of Sage (Salvia officinalis L.) Macerate as “Green Extract” in Inhibiting the Oxidation of Fish Oil. Antioxidant 2022, 11, 100. [Google Scholar] [CrossRef]
- PN-ISO 3972; Analiza Sensoryczna—Metodologia–Metoda Sprawdzania Wrażliwości Smakowej (pol.). Polish Committee for Standardization: Warsaw, Poland, 1998.
- ISO 3103:2019; Tea—Preparation of Liquor for Use in Sensory Tests. International Standards: Switzerland, Geneva, 2019; 8p.
- StatSoft Polska. Available online: https://www.statsoft.pl/statistica_13/ (accessed on 16 July 2023).
- Nwosu, O.; Ubaoji, K.I.; Okaka, A.N.C. Evaluation of Nutritional and Anti-nutritional Compositions of Leaves of Ginkgo biloba (Maiden Hair) Tree Found in Nigeria. J. Exp. Res. 2018, 6, 66–72. [Google Scholar]
- Lin, Y.; Lou, K.; Wu, G.; We, X.; Zhou, Y.; Feng, H.; Zhang, H.; Yu, P. Bioactive metabolites in of Ginkgo biloba leaves: Variations by seasonal, meteorological and soil. Braz. J. Biology. 2020, 80, 790–797. [Google Scholar] [CrossRef]
- Sati, P.; Pandey, A.; Rawat, S.; Rani, A. Phytochemicals and antioxidants in leaf extracts of Ginkgo biloba with reference to location, seasonal variation and solvent system. J. Pharm. Res. 2013, 7, 804–809. [Google Scholar] [CrossRef]
- Nazliniwaty; Hanum, I.; Laila, L. Antioxidant Activity Test of Green Tea (Camellia sinensis L. Kuntze) Ethanolic Extract using DPPH Method. Proc. Int. Conf. Sci. Technol. Eng. Environ. Ramif. Res. 2018, 1, 752–754. [Google Scholar]
- Anesini, C.; Ferraro, G.E.; Filip, R. Total Polyphenol Content and Antioxidant Capacity of Commercially Available Tea (Camellia sinensis) in Argentina. J. Agric. Food Chem. 2008, 56, 9225–9229. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.; Barros, L.; Ferreira, I.C.F.R. Chemical characterization of Ginkgo biloba L. and antioxidant properties of its extracts and dietary supplements. Ind. Crop. Prod. 2013, 51, 244–248. [Google Scholar] [CrossRef]
- Kurleto, K.; Kurowski, G.; Laskowska, B.; Malinowska, M.; Sikora, E.; Vogt, O. Wpływ warunków parzenia na zawartość antyoksydantów w naparach różnych rodzajów herbat. Wiadomości Chem. 2013, 67, 11–12. [Google Scholar]
- Amaral, J.S.; Valentão, P.; Andrade, P.B.; Martins, R.C.; Seabra, R.M. Phenolic composition of hazelnut leaves: Influence of cultivar, geographical origin and ripening stage. Sci. Horticulturae 2010, 126, 306–313. [Google Scholar] [CrossRef]
- Wang, X.; Gong, X.; Zhang, H.; Zhu, W.; Jiang, Z.; Shi, Y.; Li, L. In vitro anti-aging activities of Ginkgo biloba leaf extract and its chemical constituents. Food Sci. Technol. 2020, 40, 476–482. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Birasuren, B.; Kim, N.Y.; Jeon, H.L.; Kim, M.R. Evaluation of the antioxidant capacity and phenolic content of Agriophyllum pungens seed extracts from Mongolia. Prev Nutr Food Sci. 2013, 18, 188–195. [Google Scholar] [CrossRef]
- Biernacka, P.; Adamska, I.; Felisiak, K. The Potential of Ginkgo biloba as a Source of Biologically Active Compounds—A Review of the Recent Literature and Patents. Molecules 2023, 28, 3993. [Google Scholar] [CrossRef]
- Prakash, M.; Basavaraj, B.V.; Murthy, K.N.C. Biological functions of epicatechin: Plant cell to human cell health. J. Funct. Foods 2018, 52, 14–24. [Google Scholar] [CrossRef]
- Idemura, M. Catechin in Human Health and Disease. Molecules 2019, 24, 528. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef] [PubMed]
- Narukawa, M.; Noga, C.; Ueno, Y.; Sato, T.; Misaka, T.; Watanabe, T. Evaluation of the bitterness of green tea catechins by a cell-based assay with the human bitter taste receptor hTAS2R39. Biochem. Biophys. Res. Commun. 2011, 405, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Torre, C.L.; Fazio, A.; Caputo, P.; Plastina, P.; Caroleo, M.C.; Cannataro, R.; Cione, E. Effects of Long-Term Storage on Radical Scavenging Properties and Phenolic Content of Kombucha from Black Tea. Molecules 2021, 26, 5474. [Google Scholar] [CrossRef]
- Narukawa, M.; Misaki, T. Tas2r125 functions as the main receptor for detecting bitterness of tea catechins in the oral cavity of mice. Biochem. Biophys. Res. Communications. 2018, 503, 2301–2305. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Q.; Granato, D.; Xu, Y.; Ho, C. Association between chemistry and taste of tea: A review. Trends Food Sci. Technol. 2020, 101, 139–149. [Google Scholar] [CrossRef]
- Scharbert, S.; Hofmann, T. Molecular Definition of Black Tea Taste by Means of Quantitative Studies, Taste Reconstitution, and Omission Experiments. J. Agric. Food Chem. 2005, 53, 5377–5384. [Google Scholar] [CrossRef]
- Pawlak, M.; Drzeżdżon, J.; Jacewicz, D. Zastosowanie związków kompleksowych rutenu, złota, wanadu, chromu, bizmutu, technetu w medycynie—część II. Widomości Chem. 2020, 74, 11–12. [Google Scholar]
- Liang, J.; Fang, H.L.; Zhang, T.L.; Wang, X.X.; Liu, Y.D. Heavy metal in leaves of twelve plant species from seven different areas in Shanghai, China. Urban For. Urban Greening. 2017, 27, 390–398. [Google Scholar] [CrossRef]
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Normy Żywienia dla Populacji Polski i ich Zastosowanie; Narodowy Instytut Zdrowia Publicznego–Państwowy Zakład Higieny: Warszawa, Poland, 2020; ISBN 978-83-65870-28-5.
- Zhao, F.; Pan, D.; Wang, N.; Xia, H.; Zhang, H.; Wang, S.; Sun, G. Effect of Chromium Supplementation on Blood Glucose and Lipid Levels in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis. Biol. Trace Elem. Res. 2022, 200, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Goh, L.M.; Barlow, P.J. Antioxidant capacity in Ginkgo biloba. Food Res. Int. 2002, 35, 815–820. [Google Scholar] [CrossRef]


| Brewing Time [min] | ||||||
|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 20 | 60 | ||
| Weight [g] and Symbols | 1.25 (X) | X5 | X10 | X15 | X20 | X60 |
| 2.50 (S) | S5 | S10 | S15 | S20 | S60 | |
| 5.00 (M) | M5 | M10 | M15 | M20 | M60 | |
| 7.50 (L) | L5 | L10 | L15 | L20 | L60 | |
| Parameters | Value |
|---|---|
| pH | 4.09 ± 0.00 |
| Dry weight [%] | 92.31 ± 0.13 |
| Ash [%] | 0.09 ± 0.01 |
| Fat [%] | 5.04 ± 0.36 |
| Protein [%] | 11.48 ± 0.17 |
| Carbohydrates [%] | 75.70 |
| Total Polyphenol Content [mg GAE/g] | FRAP [µg TE/g] | DPPH [µg TE/g] | TEAC [µg TE/g] | |
|---|---|---|---|---|
| LWC | 85.786 a ± 0.521 | 0.322 a ± 0.004 | - | 10.751 a ± 0.209 |
| LWH | 77.968 a ± 0.489 | 0.277 b ± 0.008 | - | 11.416 b ± 0.104 |
| LM | 169.492 b ± 0.489 | 6.439 c ± 0.010 | 10.852 ± 0.000 | 17.786 c ± 0.225 |
| DPPH [µg TE/mL] | TEAC (M) [µg TE/mL] | TEAC (W) [µg TE/mL] | FRAP (M) [µg TE/mL] | FRAP (W) [µg TE/mL] | Total Polyphenol Content (M) [mg GAE/mL] | Total Polyphenol Content (W) [mg GAE/mL] | |
|---|---|---|---|---|---|---|---|
| X5 | 0.020 a ± 0.009 | 0.408 e ± 0.003 | 0.701 d ± 0.002 | 0.063 b ± 0.003 | 0.045 a ± 0.002 | 0.315 c ± 0.018 | 0.169 a ± 0.012 |
| X10 | 0.038 b ± 0.008 | 0.408 e ± 0.005 | 0.845 g ± 0.025 | 0.313 a ± 0.003 | 0.061 b ± 0.001 | 0.350 d ± 0.012 | 0.214 b ± 0.000 |
| X15 | 0.237 j ± 0.005 | 0.471 f ± 0.008 | 1.309 l ± 0.002 | 0.261 a ± 0.006 | 0.262 k ± 0.002 | 0.716 l ± 0.005 | 0.596 j ± 0.010 |
| X20 | 0.268 k ± 0.007 | 0.521 g ± 0.002 | 1.356 n ± 0.015 | 0.314 a ± 0.008 | 0.349 n ± 0.006 | 0.883 p ± 0.009 | 0.759 n ± 0.008 |
| X60 | 0.056 c ± 0.007 | 0.561 i ± 0.010 | 0.814 f ± 0.010 | 0.120 a ± 0.006 | 0.076 d ± 0.002 | 0.379 e ± 0.006 | 0.222 d ± 0.007 |
| S5 | 0.103 e ± 0.001 | 0.544 h ± 0.005 | 1.033 i ± 0.020 | 0.141 a ± 0.003 | 0.117 g ± 0.006 | 0.494 h ± 0.010 | 0.366 g ± 0.010 |
| S10 | 0.234 j ± 0.005 | 0.696 k ± 0.020 | 1.511 p ± 0.010 | 0.277 a ± 0.010 | 0.289 l ± 0.001 | 0.832 n ± 0.016 | 0.671 k ± 0.007 |
| S15 | 0.300 m ± 0.005 | 0.724 l ± 0.023 | 1.822 r ± 0.030 | 0.392 a ± 0.015 | 0.406 o ± 0.001 | 1.033 q ± 0.000 | 0.907 o ± 0.013 |
| S20 | 0.033 b ± 0.006 | 0.406 d ± 0.050 | 0.272 b ± 0.022 | 0.092 a ± 0.004 | 0.088 e ± 0.008 | 0.316 b ± 0.000 | 0.216 c ± 0.010 |
| S60 | 0.128 f ± 0.002 | 0.471 f ± 0.080 | 0.739 e ± 0.010 | 0.188 a ± 0.002 | 0.198 i ± 0.005 | 0.543 i ± 0.000 | 0.216 c ± 0.007 |
| M5 | 0.098 e ± 0.002 | 0.941 m ± 0.041 | 1.091 k ± 0.030 | 0.270 a ± 0.000 | 0.444 r ± 0.004 | 0.727 m ± 0.005 | 0.745 m ± 0.008 |
| M10 | 0.023 a ± 0.003 | 0.329 b ± 0.044 | 0.179 a ± 0.017 | 0.077 a ± 0.009 | 0.068 c ± 0.007 | 0.291 a ± 0.026 | 0.302 f ± 0.004 |
| M15 | 0.170 h ± 0.004 | 1.334 p ± 0.025 | 1.441 o ± 0.024 | 0.355 a ± 0.004 | 0.532 t ± 0.003 | 0.877 o ± 0.019 | 0.933 p ± 0.016 |
| M20 | 0.075 d ± 0.004 | 0.574 j ± 0.025 | 0.491 c ± 0.016 | 0.152 a ± 0.002 | 0.505 s ± 0.011 | 0.457 g ± 0.008 | 0.495 h ± 0.005 |
| M60 | 0.137 g ± 0.003 | 0.986 n ± 0.053 | 1.083 j ± 0.016 | 0.265 a ± 0.006 | 0.261 j ± 0.003 | 0.709 k ± 0.011 | 0.725 l ± 0.009 |
| L5 | 0.214 i ± 0.001 | 1.573 r ± 0.051 | 1.755 q ± 0.040 | 0.448 a ± 0.005 | 0.418 p ± 0.003 | 1.039 r ± 0.028 | 0.991 q ± 0.014 |
| L10 | 0.078 d ± 0.008 | 0.236 a ± 0.007 | 0.963 h ± 0.070 | 0.120 a ± 0.003 | 0.091 f ± 0.000 | 0.427 f ± 0.002 | 0.262 e ± 0.010 |
| L15 | 0.176 h ± 0.003 | 0.354 c ± 0.007 | 1.333 m ± 0.015 | 0.224 a ± 0.007 | 0.187 h ± 0.002 | 0.648 j ± 0.016 | 0.505 i ± 0.013 |
| L20 | 0.282 l ± 0.002 | 1.198 o ± 0.007 | 1.990 s ± 0.012 | 0.411 a ± 0.024 | 0.339 m ± 0.000 | 1.044 t ± 0.027 | 0.992 r ± 0.014 |
| L60 | 0.302 m ± 0.001 | 1.374 q ± 0.005 | 2.162 t ± 0.022 | 0.530 a ± 0.015 | 0.433 q ± 0.021 | 1.098 s ± 0.013 | 1.317 s ± 0.001 |
| X | S | M | L | |
|---|---|---|---|---|
| 5 | 0.000 a ± 0.000 | 0.000 a ± 0.000 | 0.100 ab ± 0.000 | 0.100 a ± 0.000 |
| 10 | 0.030 ab ± 0.001 | 0.000 a ± 0.000 | 0.170 b ± 0.001 | 0.130 a ± 0.002 |
| 15 | 0.370 d ± 0.000 | 0.300 b ± 0.004 | 0.300 c ± 0.003 | 0.370 b ± 0.000 |
| 20 | 0.570 c ± 0.002 | 0.570 c ± 0.002 | 0.570 d ± 0.000 | 0.570 c ± 0.000 |
| 60 | 0.000 a ± 0.000 | 0.000 a ± 0.000 | 0.020 a ± 0.000 | 0.500 c ± 0.001 |
| DPPH | TEAC (M) | TEAC (W) | FRAP (M) | FRAP (W) | Polyphenol (M) | Polyphenol (W) | Acidity | Soluble Dry Matter | |
|---|---|---|---|---|---|---|---|---|---|
| DPPH | 0.54 | 0.90 | 0.82 | 0.63 | 0.94 | 0.84 | −0.27 | 0.05 | |
| TEAC (M) | 0.54 | 0.69 | 0.79 | 0.72 | 0.77 | 0.85 | −0.39 | 0.59 | |
| TEAC (W) | 0.90 | 0.69 | 0.89 | 0.59 | 0.93 | 0.86 | −0.15 | 0.06 | |
| FRAP (M) | 0.82 | 0.79 | 0.89 | 0.69 | 0.90 | 0.90 | −0.31 | 0.28 | |
| FRAP (W) | 0.63 | 0.72 | 0.59 | 0.69 | 0.76 | 0.82 | −0.32 | 0.53 | |
| Polyphenol (M) | 0.94 | 0.77 | 0.93 | 0.90 | 0.76 | 0.94 | −0.37 | 0.25 | |
| Polyphenol (W) | 0.84 | 0.85 | 0.86 | 0.90 | 0.82 | 0.94 | −0.41 | 0.44 | |
| Acidity | −0.27 | −0.39 | −0.15 | −0.31 | −0.32 | −0.37 | −0.41 | −0.58 | |
| Soluble dry matter | 0.05 | 0.59 | 0.06 | 0.28 | 0.53 | 0.25 | 0.44 | −0.58 |
| Compounds | S10 | S15 | S60 | M10 | M15 | M60 | L10 | L15 | L60 |
|---|---|---|---|---|---|---|---|---|---|
| Chlorogenic acid | 4.1 | 4.4 | 3.4 | 6.7 | 5.2 | 4.5 | 8.9 | 8.2 | 7.6 |
| Epicatechin | 8.1 | 8.9 | 11.9 | 10.0 | 8.8 | 11.3 | 8.3 | 9.4 | 12.6 |
| Catechin | 4.8 | 7.5 | 8.3 | 7.8 | 7.4 | 7.9 | 5.6 | 6.2 | 8.8 |
| Coumarin | 9.2 | 9.3 | 7.8 | 9.9 | 8.0 | 7.1 | 7.8 | 7.7 | 7.4 |
| Apigenin | 4.3 | 3.6 | 2.3 | 4.5 | 3.5 | 2.7 | 3.5 | 2.8 | 2.1 |
| S10 | S15 | S60 | M10 | M15 | M60 | L10 | L15 | L60 | |
|---|---|---|---|---|---|---|---|---|---|
| TFC (µg QE/mL) | 1.66 b ± 0.05 | 2.29 c ± 0.05 | 2.48 d ± 0.05 | 3.46 e ± 0.02 | 4.30 f ± 0.03 | 5.02 a ± 0.02 | 5.05 a ± 0.02 | 5.73 g ± 0.03 | 5.97 h ± 0.03 |
| (a) | |||||||
|---|---|---|---|---|---|---|---|
| Pb [mg/kg] | Cd [mg/kg] | Ni [mg/kg] | Cu [mg/kg] | Cr [mg/kg] | Fe [mg/kg] | Mn [mg/kg] | |
| Dry leaves | - | - | - | - | 0.480 ± 0.000 | 5.501 ± 0.024 | 22.631 ± 0.200 |
| S10 | - | - | - | - | 0.051 ± 0.020 | 0.091 ± 0.016 | 11.394 ± 0.090 |
| S15 | - | - | - | - | 0.072 ± 0.000 | 0.103 ± 0.011 | 15.203 ± 0.130 |
| S60 | - | - | - | - | 0.054 ± 0.031 | 0.122 ± 0.040 | 20.727 ± 0.011 |
| M10 | - | - | - | - | 0.056 ± 0.031 | 0.174 ± 0.041 | 10.432 ± 0.028 |
| M15 | - | - | - | - | 0.074 ± 0.012 | 0.160 ± 0.002 | 10.551 ± 0.140 |
| M60 | - | - | - | - | 0.082 ± 0.010 | 0.188 ± 0.020 | 17.039 ± 0.081 |
| (b) | |||||||
| Ca [g/kg] | K [g/kg] | Mg [g/kg] | Na [mg/kg] | N [mg/kg] | |||
| Dry leaves | 13.611 ± 0.020 | 6.521 ± 0.000 | 1.041 ± 0.000 | 113.391 ± 0.020 | 1.812 ± 0.010 | ||
| S10 | 1.158 ± 0.020 | 3.454 ± 0.014 | 2.472 ± 0.031 | 90.612 ± 0.034 | 0.004 ± 0.000 | ||
| S15 | 1.645 ± 0.042 | 4.273 ± 0.061 | 2.217 ± 0.024 | 58.854 ± 0.021 | 0.004 ± 0.000 | ||
| S60 | 1.514 ± 0.051 | 4.834 ± 0.010 | 3.182 ± 0.026 | 59.233 ± 0.010 | 0.004 ± 0.000 | ||
| M10 | 0.974 ± 0.010 | 3.632 ± 0.000 | 2.713 ± 0.010 | 37.132 ± 0.000 | 0.003 ± 0.020 | ||
| M15 | 1.081 ± 0.010 | 3.564 ± 0.021 | 3.181 ± 0.020 | 40.381 ± 0.022 | 0.008 ± 0.000 | ||
| M60 | 1.282 ± 0.000 | 5.677 ± 0.022 | 4.100 ± 0.000 | 46.181 ± 0.010 | 0.004 ± 0.010 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biernacka, P.; Felisiak, K.; Adamska, I.; Śnieg, M.; Podsiadło, C. Effect of Brewing Conditions on Antioxidant Properties of Ginkgo biloba Leaves Infusion. Antioxidants 2023, 12, 1455. https://doi.org/10.3390/antiox12071455
Biernacka P, Felisiak K, Adamska I, Śnieg M, Podsiadło C. Effect of Brewing Conditions on Antioxidant Properties of Ginkgo biloba Leaves Infusion. Antioxidants. 2023; 12(7):1455. https://doi.org/10.3390/antiox12071455
Chicago/Turabian StyleBiernacka, Patrycja, Katarzyna Felisiak, Iwona Adamska, Marek Śnieg, and Cezary Podsiadło. 2023. "Effect of Brewing Conditions on Antioxidant Properties of Ginkgo biloba Leaves Infusion" Antioxidants 12, no. 7: 1455. https://doi.org/10.3390/antiox12071455
APA StyleBiernacka, P., Felisiak, K., Adamska, I., Śnieg, M., & Podsiadło, C. (2023). Effect of Brewing Conditions on Antioxidant Properties of Ginkgo biloba Leaves Infusion. Antioxidants, 12(7), 1455. https://doi.org/10.3390/antiox12071455






