Investigating the Interplay between Tomato Leaf Curl New Delhi Virus Infection, Starch Metabolism and Antioxidant Defence System in Potato (Solanum tuberosum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Pure Virus Culture and Planting Material
2.2. Virus Inoculation Studies in Potato
2.3. Estimation of Photosynthetic Pigments
2.4. Estimation of Proline, MDA, LWC, and REC
2.5. Determination of Carbohydrates in Potato Leaves
2.6. Estimation of Starch Hydrolyzing Enzymes
2.7. Estimation of Antioxidant Enzymes
2.8. Statistical Analysis
3. Results
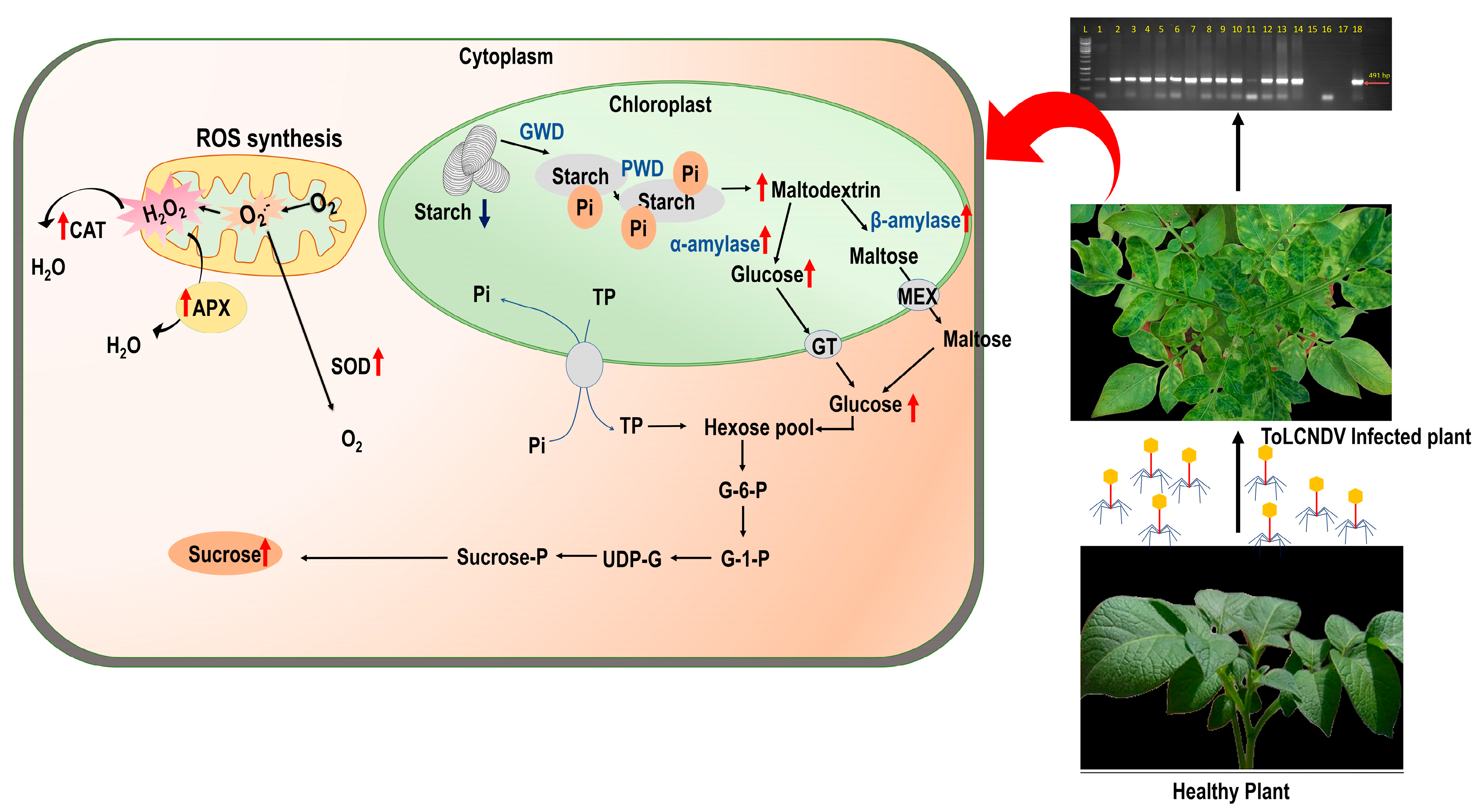
3.1. Symptom Expressions and Molecular Detection of the Virus
3.2. Modification of Potato Morphological Parameters by PALCV
3.3. Effect of ToLCNDV-Potato on the Photosynthetic Parameters of Potato Plant
3.4. Effect of ToLCNDV-Potato on Proline, MDA and Relative Electrical Conductivity of Potato Plant
3.5. Effect of ToLCNDV-Potato on Carbohydrate Profile of Potato Plant
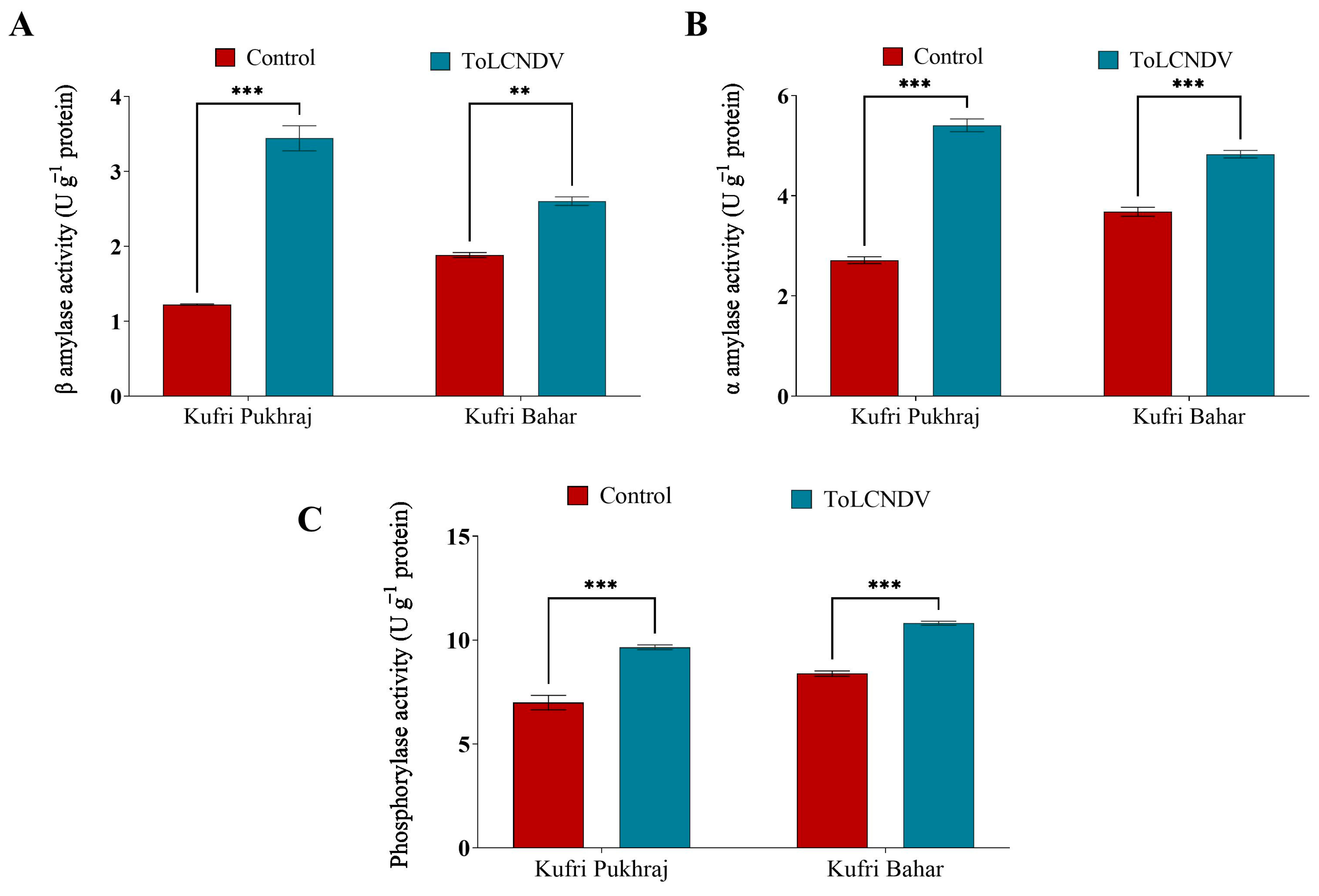
3.6. Effect of ToLCNDV-Potato on Carbohydrate Metabolic Enzymes of Potato Plant
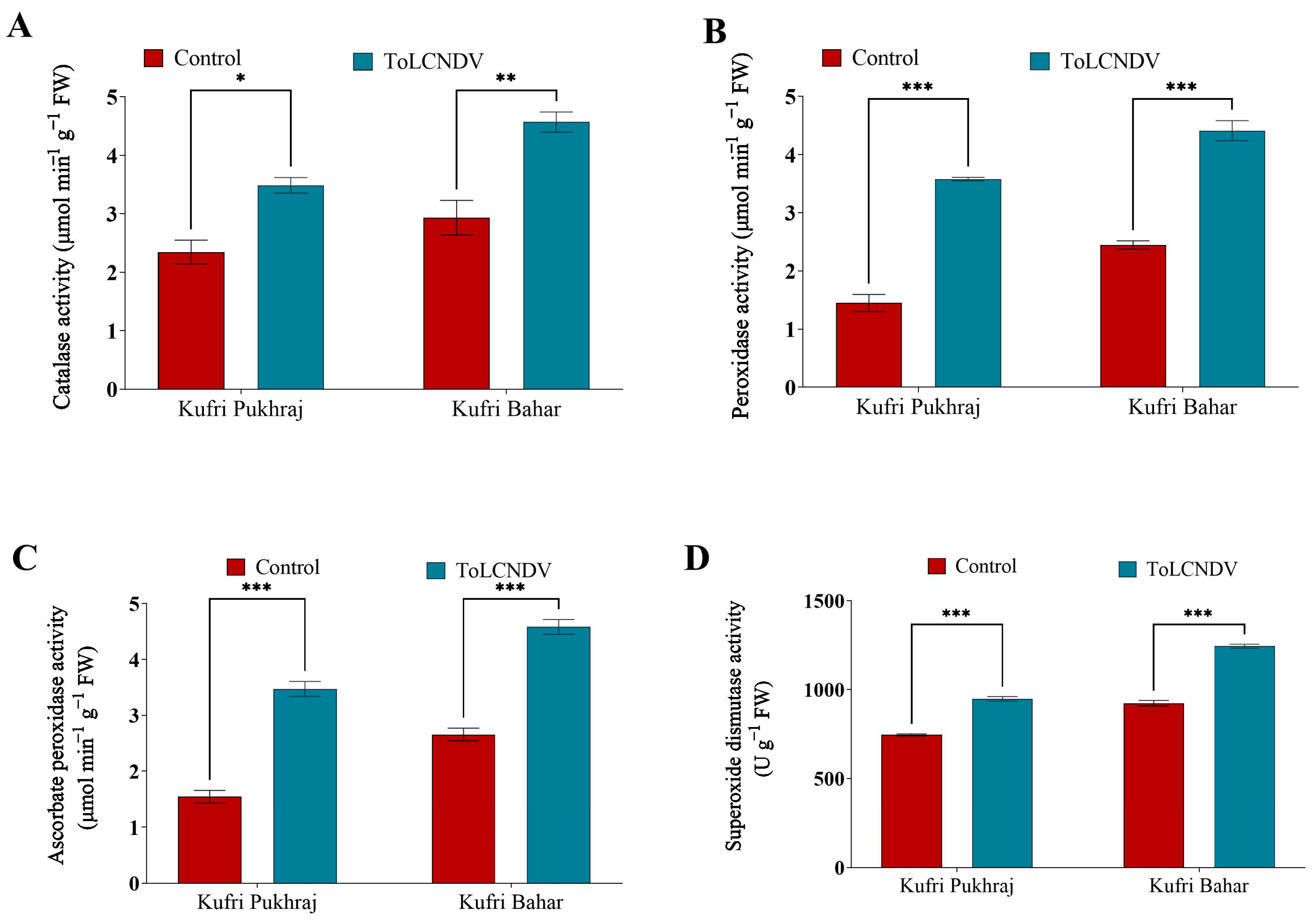
3.7. Effect of ToLCNDV-Potato on Antioxidant Enzyme Activity of Potato Plant
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raymundo, R.; Asseng, S.; Robertson, R.; Petsakos, A.; Hoogenboom, G.; Quiroz, R.; Hareau, G.; Wolf, J. Climate Change Impact on Global Potato Production. Eur. J. Agron. 2018, 100, 87–98. [Google Scholar] [CrossRef]
- Lal, M.K.; Tiwari, R.K.; Kumar, R.; Naga, K.C.; Kumar, A.; Singh, B.; Raigond, P.; Dutt, S.; Chourasia, K.N.; Kumar, D.; et al. Effect of Potato Apical Leaf Curl Disease on Glycemic Index and Resistant Starch of Potato (Solanum tuberosum L.) Tubers. Food Chem. 2021, 359, 129939. [Google Scholar] [CrossRef]
- Huseynova, I.M.; Mirzayeva, S.M.; Sultanova, N.F.; Aliyeva, D.R.; Mustafayev, N.S.; Aliyev, J.A. Virus-Induced Changes in Photosynthetic Parameters and Peroxidase Isoenzyme Contents in Tomato (Solanum lycopersicum L.) Plants. Photosynthetica 2018, 56, 841–850. [Google Scholar] [CrossRef]
- Kumar, R.; Tiwari, R.K.; Sundaresha, S.; Kaundal, P.; Raigond, B. Potato Viruses and Their Management. In Sustainable Management of Potato Pests and Diseases; Springer: Singapore, 2022; pp. 309–335. [Google Scholar]
- Kumar, R.; Tiwari, R.K.; Jeevalatha, A.; Siddappa, S.; Shah, M.A.; Sharma, S.; Sagar, V.; Kumar, M.; Chakrabarti, S.K. Potato Apical Leaf Curl Disease: Current Status and Perspectives on a Disease Caused by Tomato Leaf Curl New Delhi Virus. J. Plant Dis. Prot. 2021, 128, 897–911. [Google Scholar] [CrossRef]
- Usharani, K.S.; Surendranath, B.; Paul-Khurana, S.M.; Garg, I.D.; Malathi, V.G. Potato Leaf Curl—A New Disease of Potato in Northern India Caused by a Strain of Tomato Leaf Curl New Delhi Virus. Plant Pathol. 2004, 53, 235. [Google Scholar] [CrossRef]
- Jeevalatha, A.; Siddappa, S.; Kumar, A.; Kaundal, P.; Guleria, A.; Sharma, S.; Nagesh, M.; Singh, B.P. An Insight into Differentially Regulated Genes in Resistant and Susceptible Genotypes of Potato in Response to Tomato Leaf Curl New Delhi Virus-[Potato] Infection. Virus Res. 2017, 232, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health-Promoting Components of Fruits and Vegetables in the Diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef]
- Kapinga, R.; Ndunguru, J.; Mulokozi, G.; Tumwegamire, S. Impact of Common Sweetpotato Viruses on Total Carotenoids and Root Yields of an Orange-Fleshed Sweetpotato in Tanzania. Sci. Hortic. 2009, 122, 1–5. [Google Scholar] [CrossRef]
- Garg, I.D.; Khurana, S.M.P.; Kumar, S.; Lakra, B.S. Association of a Geminivirus with Potato Apical Leaf Curl in India and Its Immuno-Electron Microscopic Detection. J. Indian Potato Assoc. 2001, 28, 227–232. [Google Scholar]
- Alvarez, V.H.; Cahyadi, J.; Xu, D.; Saldaña, M.D.A. Optimization of Phytochemicals Production from Potato Peel Using Subcritical Water: Experimental and Dynamic Modeling. J. Supercrit. Fluids 2014, 90, 8–17. [Google Scholar] [CrossRef]
- Sun, H.; Fan, J.; Tian, Z.; Ma, L.; Meng, Y.; Yang, Z.; Zeng, X.; Liu, X.; Kang, L.; Nan, X. Effects of Treatment Methods on the Formation of Resistant Starch in Purple Sweet Potato. Food Chem. 2022, 367, 130580. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Wang, G.X. Doubled CO2 could improve the drought tolerance better in sensitive cultivars than in tolerant cultivars in spring wheat. Plant Sci. 2002, 163, 627–637. [Google Scholar] [CrossRef]
- Jeevalatha, A.; Singh, B.P.; Kaundal, P.; Kumar, R.; Raigond, B. RCA-PCR: A Robust Technique for the Detection of Tomato Leaf Curl New Delhi Virus-Potato at Ultra Low Virus Titre. Potato J. 2014, 41, 76–80. [Google Scholar]
- Kumar, R.; Kaundal, P.; Arjunan, J.; Sharma, S.; Chakrabarti, S.K. Development of a Visual Detection Method for Potato Virus S by Reverse Transcription Loop-Mediated Isothermal Amplification. 3 Biotech 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, Q.; Wang, B.; Yuan, F. Roles of Phytohormones and Their Signaling Pathways in Leaf Development and Stress Responses. J. Agric. Food Chem. 2021, 69, 3566–3584. [Google Scholar] [CrossRef]
- Ben Rejeb, I.; Pastor, V.; Mauch-Mani, B. Plant Responses to Simultaneous Biotic and Abiotic Stress: Molecular Mechanisms. Plants 2014, 3, 458–475. [Google Scholar] [CrossRef] [PubMed]
- Deja-Sikora, E.; Werner, K.; Hrynkiewicz, K. AMF Species Do Matter: Rhizophagus Irregularis and Funneliformis Mosseae Affect Healthy and PVY-Infected Solanum tuberosum L. in a Different Way. Front. Microbiol. 2023, 14, 1127278. [Google Scholar] [CrossRef] [PubMed]
- Király, L.; Albert, R.; Zsemberi, O.; Schwarczinger, I.; Hafez, Y.M.; Künstler, A. Reactive Oxygen Species Contribute to Symptomless, Extreme Resistance to Potato Virus X in Tobacco. Phytopathology 2021, 111, 1870–1884. [Google Scholar] [CrossRef]
- Kolychikhina, M.S.; Beloshapkina, O.O.; Phiri, C. Change in Potato Productivity under the Impact of Viral Diseases. IOP Conf. Ser. Earth Environ. Sci. 2021, 663, 012035. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts: I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Steup, M.; Latzko, E. Intracellular Localization of Phosphorylases in Spinach and Pea Leaves. Planta 1979, 145, 69–75. [Google Scholar] [CrossRef]
- Spychalla, J.P.; Desborough, S.L. Superoxide dismutase, catalase, and α-tocopherol content of stored potato tubers. Plant Physiol. 1990, 94, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Moriones, E.; Praveen, S.; Chakraborty, S. Tomato Leaf Curl New Delhi Virus: An Emerging Virus Complex Threatening Vegetable and Fiber Crops. Viruses 2017, 9, 264. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, J.; Liu, J.; Zhang, J.; Song, B.; Xie, C. The Roles of Starch Metabolic Pathways in the Cold-Induced Sweetening Process in Potatoes. Starch-Stärke 2017, 69, 1600194. [Google Scholar] [CrossRef]
- Flores-Castellanos, J.; Fettke, J. The Plastidial Glucan Phosphorylase Affects the Maltooligosaccharide Metabolism in Parenchyma Cells of Potato (Solanum tuberosum L.) Tuber Discs. Plant Cell Physiol. 2023, 64, 422–432. [Google Scholar] [CrossRef]
- Zhang, K.; Lu, H.; Wan, C.; Tang, D.; Zhao, Y.; Luo, K.; Li, S.; Wang, J. The Spread and Transmission of Sweet Potato Virus Disease (SPVD) and Its Effect on the Gene Expression Profile in Sweet Potato. Plants 2020, 9, 492. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Spetz, C.; Li, L.; Wang, X. Comparative Transcriptome Analysis in Triticum aestivum Infecting Wheat Dwarf Virus Reveals the Effects of Viral Infection on Phytohormone and Photosynthesis Metabolism Pathways. Phytopathol. Res. 2020, 2, 1–13. [Google Scholar] [CrossRef]
- Ratnadass, A.; Fernandes, P.; Avelino, J.; Habib, R. Plant Species Diversity for Sustainable Management of Crop Pests and Diseases in Agroecosystems: A Review. Agron. Sustain. Dev. 2012, 32, 273–303. [Google Scholar] [CrossRef]
- Li, J.W.; Chen, H.Y.; Li, J.; Zhang, Z.; Blystad, D.R.; Wang, Q.C. Growth, Microtuber Production and Physiological Metabolism in Virus-Free and Virus-Infected Potato In Vitro Plantlets Grown under NaCl-Induced Salt Stress. Eur. J. Plant Pathol. 2018, 152, 417–432. [Google Scholar] [CrossRef]
- Zhang, S.-h.; Xu, X.-f.; Sun, Y.-m.; Zhang, J.-l.; Li, C.-z. Influence of Drought Hardening on the Resistance Physiology of Potato Seedlings under Drought Stress. J. Integr. Agric. 2018, 17, 336–347. [Google Scholar] [CrossRef]
- Baebler, Š.; Stare, K.; Kovač, M.; Blejec, A.; Prezelj, N.; Stare, T.; Kogovšek, P.; Pompe-Novak, M.; Rosahl, S.; Ravnikar, M.; et al. Dynamics of Responses in Compatible Potato—Potato Virus y Interaction Are Modulated by Salicylic Acid. PLoS ONE 2011, 6, e29009. [Google Scholar] [CrossRef] [PubMed]
- Jyothsna, P.; Haq, Q.M.I.; Singh, P.; Sumiya, K.V.; Praveen, S.; Rawat, R.; Briddon, R.W.; Malathi, V.G. Infection of Tomato Leaf Curl New Delhi Virus (ToLCNDV), a Bipartite Begomovirus with Betasatellites, Results in Enhanced Level of Helper Virus Components and Antagonistic Interaction between DNA B and Betasatellites. Appl. Microbiol. Biotechnol. 2013, 97, 5457–5471. [Google Scholar] [CrossRef]
- Shalitin, D.; Wolf, S. Cucumber Mosaic Virus Infection Affects Sugar Transport in Melon Plants. Plant Physiol. 2000, 123, 597. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.L.; Lehrer, A.T.; Hajirezaei, M.R.; Springer, A.; Komor, E. Modulation of Carbohydrate Metabolism and Chloroplast Structure in Sugarcane Leaves Which Were Infected by Sugarcane Yellow Leaf Virus (SCYLV). Physiol. Mol. Plant Pathol. 2008, 73, 78–87. [Google Scholar] [CrossRef]
- Herbers, K.; Takahata, Y.; Melzer, M.; Mock, H.P.; Hajirezaei, M.; Sonnewald, U. Regulation of Carbohydrate Partitioning during the Interaction of Potato Virus Y with Tobacco. Mol. Plant Pathol. 2000, 1, 51–59. [Google Scholar] [CrossRef]
- Gupta, U.P.; Srivastava, M.; Gupta, U. Influence of Soybean Mosaic Virus Infection on Carbohydrate Content in Nodule of Soybean (Glycine max L. Merr.). Int. J. Virol. 2010, 6, 240–245. [Google Scholar] [CrossRef]
- Kulakova, A.V.; Efremov, G.I.; Shchennikova, A.V.; Kochieva, E.Z. Dependence of the Content of Starch and Reducing sugars on the Level of Expression of the Genes of β-Amylases and StBAM9 and the Amylase Inhibitor StAI during Long-Term Low-Temperature Storage of Potato Tubers. Vavilov J. Genet. Breed. 2022, 26, 507. [Google Scholar] [CrossRef]
- Orzechowski, S.; Sitnicka, D.; Grabowska, A.; Compart, J.; Fettke, J.; Zdunek-Zastocka, E. Effect of Short-Term Cold Treatment on Carbohydrate Metabolism in Potato Leaves. Int. J. Mol. Sci. 2021, 22, 7203. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Sharma, N.; Muthamilarasan, M.; Dulani, P.; Prasad, M. Genomic Dissection of ROS Detoxifying Enzyme Encoding Genes for Their Role in Antioxidative Defense Mechanism against Tomato Leaf Curl New Delhi Virus Infection in Tomato. Genomics 2021, 113, 889–899. [Google Scholar] [CrossRef] [PubMed]




| Kufri Pukhraj | Kufri Bahar | ||||
|---|---|---|---|---|---|
| S.N. | Parameters | Control | To LCNDV | Control | ToLCNDV |
| 1. | Plant height (cm) | 81.90 ± 0.88 a | 70.92 ± 1.19 b | 67.23 ± 0.34 a | 61.24 ± 0.58 b |
| 2. | Dry matter of potato tuber(g) | 14.93 ± 0.35 a | 11.69 ± 0.32 b | 22.93 ± 0.36 a | 18.67 ± 0.33 b |
| 3. | Stem diameter (cm) | 0.75 ± 0.01 a | 0.62 ± 0.01 b | 0.62 ± 0.01 b | 0.74 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Lal, M.K.; Tiwari, R.K.; Chourasia, K.N.; Kumar, A.; Kumar, R.; Sharma, S.; Singh, B. Investigating the Interplay between Tomato Leaf Curl New Delhi Virus Infection, Starch Metabolism and Antioxidant Defence System in Potato (Solanum tuberosum L.). Antioxidants 2023, 12, 1447. https://doi.org/10.3390/antiox12071447
Kumar R, Lal MK, Tiwari RK, Chourasia KN, Kumar A, Kumar R, Sharma S, Singh B. Investigating the Interplay between Tomato Leaf Curl New Delhi Virus Infection, Starch Metabolism and Antioxidant Defence System in Potato (Solanum tuberosum L.). Antioxidants. 2023; 12(7):1447. https://doi.org/10.3390/antiox12071447
Chicago/Turabian StyleKumar, Ravinder, Milan Kumar Lal, Rahul Kumar Tiwari, Kumar Nishant Chourasia, Awadhesh Kumar, Rakesh Kumar, Shivangi Sharma, and Brajesh Singh. 2023. "Investigating the Interplay between Tomato Leaf Curl New Delhi Virus Infection, Starch Metabolism and Antioxidant Defence System in Potato (Solanum tuberosum L.)" Antioxidants 12, no. 7: 1447. https://doi.org/10.3390/antiox12071447
APA StyleKumar, R., Lal, M. K., Tiwari, R. K., Chourasia, K. N., Kumar, A., Kumar, R., Sharma, S., & Singh, B. (2023). Investigating the Interplay between Tomato Leaf Curl New Delhi Virus Infection, Starch Metabolism and Antioxidant Defence System in Potato (Solanum tuberosum L.). Antioxidants, 12(7), 1447. https://doi.org/10.3390/antiox12071447







