Abstract
The tripeptide glutathione plays important roles in many cell processes, including differentiation, proliferation, and apoptosis; in fact, disorders in glutathione homeostasis are involved both in the etiology and in the progression of several human diseases, including cancer. Natural compounds have been found to modulate glutathione levels and function beyond their role as mere antioxidants. For example, certain compounds can upregulate the expression of glutathione-related enzymes, increase the availability of cysteine, the limiting amino acid for glutathione synthesis, or directly interact with glutathione and modulate its function. These compounds may have therapeutic potential in a variety of disease states where glutathione dysregulation is a contributing factor. On the other hand, flavonoids’ potential to deplete glutathione levels could be significant for cancer treatment. Overall, while natural compounds may have potential therapeutic and/or preventive properties and may be able to increase glutathione levels, more research is needed to fully understand their mechanisms of action and their potential benefits for the prevention and treatment of several diseases. In this review, particular emphasis will be placed on phytochemical compounds belonging to the class of polyphenols, terpenoids, and glucosinolates that have an impact on glutathione-related processes, both in physiological and pathological conditions. These classes of secondary metabolites represent the most food-derived bioactive compounds that have been intensively explored and studied in the last few decades.
1. Introduction
Glutathione (GSH) is a tripeptide composed of cysteine, glycine, and glutamate. A French chemist, J. de Rey-Pailhade, first isolated and characterized GSH in 1888, but its composition and biosynthesis were established later [1]. Despite being discovered more than a century ago, interest in this biological molecule continues to grow, and new biological activities have been attributed to this tripeptide [2]. The reduced form, known as GSH, contains a thiol (-SH) group on the cysteine residue, which enables it to function as a reducing agent. Then, in its reduced form, GSH can donate an electron to reactive oxygen species (ROS) or other oxidizing agents, effectively neutralizing them and preventing oxidative damage to cells and tissues. GSH also plays a crucial role in enzymatic reactions that involve the reduction and detoxification of reactive compounds, such as peroxides and xenobiotics. Through its thiol group, GSH can directly react with and detoxify harmful substances, protecting cells from oxidative stress and maintaining their normal functions [3].
Furthermore, the thiol group of GSH is involved in thiol-disulfide exchange reactions, allowing GSH to regulate the redox status of proteins in the cell. This redox regulation is essential for maintaining proper protein structure and function [3]. Numerous studies have shown that GSH levels decline with age and in various diseases, including cancer, cardiovascular disease, and neurodegenerative disorders [2,3,4,5,6]. Therefore, the importance of GSH in various physiological and pathological processes has driven significant interest in understanding its role and exploring interventions to modulate GSH levels for therapeutic benefits. So, researchers have investigated strategies to replenish GSH levels in these conditions as a potential therapeutic approach [5,7,8,9,10,11]. One approach is the direct administration of GSH or its precursor molecules, such as Cysteine or N-acetylcysteine (NAC), to enhance GSH synthesis [12]. In addition to direct supplementation, research has focused on identifying natural compounds and interventions that can indirectly increase GSH levels by stimulating GSH synthesis or preventing GSH depletion. This includes the exploration of dietary interventions, such as consuming foods rich in GSH precursors or phytochemicals that enhance GSH synthesis or limit its depletion [13,14,15].
By restoring or enhancing GSH levels, it is possible to promote antioxidant defense, support detoxification processes, reduce oxidative stress, and potentially mitigate disease progression or alleviate symptoms. However, it is important to highlight that additional research is still being conducted on the therapeutic effectiveness and best methods for modifying GSH levels in particular disorders.
To completely comprehend the methods, doses, and potential side effects of GSH modulation therapies in various therapeutic scenarios, more research is required. In general, the attention paid to GSH levels in therapeutic research emphasizes the importance of this molecule in preserving cellular health and the possibility that therapies that might raise GSH levels will have a favorable effect on managing human health and disease [6]. It has been investigated whether there are any natural compounds that can enhance GSH synthesis or function. These include vitamins, minerals, and phytochemicals including polyphenols and flavonoids. These compounds have been shown to prevent oxidative stress in several cell and animal models [2,16,17,18,19]. On the other hand, over the past 15 to 20 years, evidence has emerged that GSH plays a crucial role in cell proliferation, promoting the idea that GSH depletion may enhance the efficacy of cancer treatments [8,10,20], so the role of GSH must be analyzed in light of the pathophysiology and desired outcome. In this review, particular emphasis will be placed on phytochemical compounds belonging to the class of polyphenols, terpenoids, and glucosinolates, that have an impact on glutathione-related processes, both in physiological and pathological conditions. These classes of secondary metabolites represent the most food-derived bioactive compounds that have been intensively explored and studied in the last decades.
2. Materials and Methods
To find literature pertinent to the study topic, a search was conducted on the PubMed, Web of Science, Scopus, and Google Scholar databases, with the publication window set to 31 May 2023. The search technique applied the following keywords: “glutathione” AND “natural compounds”; “polyphenols” AND “glutathione”; “flavonoids” AND “glutathione,” etc., in combination with terms that were relevant to the study’s goal.
The following inclusion criteria were used by the authors to determine which studies were pertinent: (1) in vitro, in vivo, and human studies; (2) original research papers and reviews published in the English language; and (3) studies focusing on the beneficial effects of natural substances on GSH homeostasis. Studies that did not fit these criteria were not considered.
3. Glutathione Biosynthesis and Functions
GSH biosynthesis is a two-step process catalyzed by γ-glutamyl-l-cysteine ligase (or γ-glutamylcysteine synthase) (γGCL, EC 6.3.2.2), and by glutathione synthase (GLS, EC 6.3.2.3). Both enzymes require ATP hydrolysis; the first enzyme (γGCL) requires ATP hydrolysis for the formation of the bond between the γ-carboxyl group of glutamate and the amino group of cysteine; the second enzyme catalyzes the addition of glycine to the dipeptide, producing GSH [1]. In contrast to the α-peptide bond, normally present in biological peptides, in the GSH molecule, glutamic acid and cysteine are bound by a γ-peptide bond. This ensures selectivity for interactions with GSH-dependent enzymes and makes the peptide resistant to hydrolysis catalyzed by typical intracellular peptidases [21,22]. The cleavage of GSH can therefore occur only by the action of γ-glutamyltranspeptidase (GGT), an enzyme exclusively found on the outer face of the membrane of some cells [23]; consequently, intracellular GSH is relatively stable, and its levels are the result of a balance with its oxidized form (GSSG) and is also related to intracellular distribution and efflux rate (Figure 1). Numerous processes, including oxidation, conjugation, and hydrolysis, deplete GSH [24].
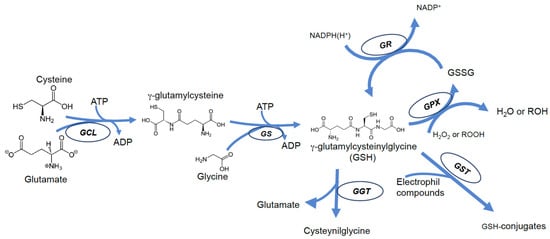
Figure 1.
Scheme of most important pathways of GSH biosynthesis, recycling, and degradation in mammalian cells.
GSSG typically accounts for 15 percent of cytoplasmic glutathione and is often considered only a byproduct of GSH metabolism. However, oxidation of GSH to GSSG may contribute significantly to its depletion under conditions of excessive exposure to ROS or RNS and may be an important event during cytotoxic agent-induced apoptosis [25,26]; if glutathione reductase (GR, EC 1.8.1.7) activity or NADPH levels are not enough to restore GSH levels by reducing GSSG back to GSH, GSH depletion occurs [27]. On the other hand, during apoptosis, mechanisms of GSH efflux from cells are activated, which further contributes to GSH depletion [28,29,30]. Thus, in this light, it is likely that all substances that can interfere in some way with one of the above mechanisms are also likely to cause disturbances in GSH homeostasis. Cells may lose GSH due to the export of its reduced, oxidized, or conjugated forms.
In addition to acting as a substrate in reactions catalyzed by glutathione peroxidase (GPX), GSH can directly react with radicals and other oxidizing chemical species [21,31,32] forming thiyl radicals that self-extinguish by reacting with each other to give GSSG [33,34,35]. In fact, oxidants such as peroxynitrite (ONOO) or hydroxyl radical (OH•) can directly oxidize GSH, leading to the production of thiyl radicals and then, of GSSG [31,36]. The reduced form of glutathione is restored by a reaction catalyzed by the enzyme glutathione reductase (GR), which uses NADPH(H+) as a reducing coenzyme [37] (Figure 1).
GSH cellular content is also dependent on mechanisms of transport; the extracellular membrane-bound enzyme GGT catalyzes the cleavage of the γ-peptide bond of both GSH and GSSG, generating cysteinyl-glycine and transferring the γ-glutamyl group onto amino acid acceptors. This event may represent a loss in glutathione.
Although all these products can be recycled and brought back in, forming GSH again according to cellular needs [23], the efflux of GSH may represent a relevant event, which can cause both an imbalance in cellular redox equilibrium and ultimately can result in oxidative stress-independent cell death [28,38]. It has been reported that GSH depletion is promoted by apoptosis produced via various stimuli (especially death receptors) [38], by activating a plasma membrane efflux transporter [27,39]. Several proteins are proposed as GSH transporters, including organic anion-transporting polypeptides (OATPs), and multidrug resistance proteins (MRPs) [27,39,40]. Some of these are ATP-dependent cotransporters and are members of the ATP-Binding Cassette (ABC) family [39,40], implicated in the sensitization of cells to apoptosis [27,28].
The potential roles of GSH are determined by its chemical structure, and its widespread presence in every living organism is evidence of its relevant biological significance.
GSH serves more purposes than just acting as an antioxidant, because it is a crucial detoxification system that plays a role in the modification of xenobiotics and2constitutes part of the process used to eliminate potentially hazardous compounds [41,42,43]. In addition, a mutual influence between estrogen and glutathione has been described [43,44,45], and involvement in the metabolism of some mediators of the inflammatory response, such as prostaglandins and leukotrienes [46,47] was reported.
Another, often overlooked, role of GSH is its involvement in the homeostasis of some metals. GSH, in fact, can interact with some metals for which the SH group shows high affinity; among these, the most studied for their impact on health are chromium, cadmium, lead, zinc, copper, and iron [21,48,49,50,51,52].
In fact, GSH plays an essential role in iron homeostasis and metabolism. The synthesis of heme, iron-sulfur (FeS) clusters and many enzymes depends on the availability of iron supplied by the labile iron pool, and iron(II)glutathione complex (FeIIGSH) is proposed to be one of the major components of it [53]. Furthermore, as heme and FeS cluster synthesis mainly occurs in mitochondria, it has been suggested that FeIIGSH can enter this organelle via di-anionic exchangers, providing a source of iron for its biosynthetic activities [54]. A connection between GSH and iron metabolism was demonstrated by the findings of Kumar et al. [55], which showed that GSH depletion induces an iron starvation-like response in yeast cells. Interestingly, a similar effect was found also with a toxic accumulation of GSH induced by overexpression of the GSH transporter Hgt1.
GSH is directly involved in FeS cluster biosynthesis, as it is necessary to transport an unidentified FeS cluster precursor from the mitochondria into the cytosol, and it is an essential cofactor for the activity of glutaredoxins [56]. What was found by Wang et al. [57] further corroborates the critical role of GSH in FeS cluster biosynthesis. In fact, they showed that as a mitochondrial carrier, namely SLC25A39, is involved in GSH import in mitochondria, depletion of this protein was directly connected with a reduction in the activity and stability of proteins containing FeS clusters.
Due to its antioxidant activity and its role in iron homeostasis, GSH can also be a potential target for cancer therapy. In fact, elevated GSH levels may be found in cancer cells as a response to the high amount of ROS produced due to their accelerated metabolism, thus protecting them from the consequent oxidative damage. Hence, strategies that lead to GSH depletion can be used to increase the efficacy of ROS-based therapies, reduce the GSH-dependent detoxification of chemotherapeutics, and induce ferroptosis in cancer cells [58]. Ferroptosis is a mechanism of cell death that occurs because of an imbalance in the intracellular redox state due to three key factors: free iron accumulation, glutathione depletion, and lipid peroxidation. GSH plays a central role in orchestrating this event since low GSH levels are linked with labile iron overload and deficiency in GPx4 activity [9]. Additionally, depletion of GSH is also able to increase the expression of heme oxygenase 1, further increasing the amount of labile iron and the consequent oxidative stress [59].
Another important feature is the intracellular distribution of GSH; although it is synthesized exclusively in the cytoplasm, it is also present in the nucleus, endoplasmic reticulum, and mitochondria, where it plays specific roles that are not merely limited to protective or antioxidant activity [60,61,62,63]. Given the prominent role played by mitochondria in oxygen consumption, ROS production, and apoptosis, mitochondrial GSH (mGSH) is of particular interest. Although the exact role played by mitochondrial GSH in apoptotic death is not yet fully understood, the control of oxidative stress in the mitochondrion involves its thiol pool, and mitochondrial GSH depletion has been shown to be closely related to the promotion of cell death [63].
Due to its dual role as a vital antioxidant and its relationship with GSH-related enzymes, GSH plays a critical role in the regulation of redox homeostasis; thus, changes in GSH levels or redox state dysregulation contribute to several illnesses and aging.
This has prompted researchers’ interest in finding therapeutic strategies to restore GSH levels by controlling the activity of GSH-related enzymes or enhancing the availability of its precursors [12,13,21].
Beyond the scope of this review, the numerous recognized antioxidant, and preventive functions of GSH are thoroughly discussed in other comprehensive papers [3,21,22].
Actually, it appears that, for the purposes of chemotherapy, the process known as glutathionylation, which results in the creation of mixed disulfides between GSH and protein thiols, is more significant than the capacity of GSH to counteract oxidative stress. In fact, oxidized protein thiol groups can undergo reactions with GSH, resulting in S-glutathionylation, a process that can be spontaneous or enzyme-driven (by glutathione S-transferases, GSTs). So, cancer cells can regulate the amount of GSH by changing the expression of enzymes involved in glutathione synthesis and metabolism [64].
4. GSH and Tumor Cells
Although GSH plays a prominent role in protecting against oxidative stress and, therefore, in the prevention of carcinogenesis, a growing number of studies show that tumor cells differ from normal, non-cancerous cells also in their abnormal ROS homeostatic properties. Compared to nearby non-cancerous cells, most malignant cells have a higher ROS concentration, which seems to promote growth, proliferation, metastasis, and survival in many different types of tumor cells [11,65].
To avoid ROS anticancer effects, tumor cells can alter their own antioxidant network, including pathways such as nuclear factor erythroid 2/Kelch-like ECH-associated protein 1 (NRF2/KEAP1), GSH and thioredoxin [7,8,11,64,65,66].
The primary mechanism that is triggered upon ROS generation is the NRF2 pathway. Remarkable is the frequent activation of the NRF2 pathway in many cancers, highlighting its dual function in carcinogenesis. Under normal conditions, KEAP1 collaborates with NRF2 to function. In fact, NRF2 is controlled by KEAP1, a cytoplasmic adaptor protein containing E3 ubiquitin ligases that, in non-stressed cells, binds to NRF2 motifs, causing ubiquitination and subsequently degrading NRF2 [67]. During oxidative stress, KEAP1 cysteine residues are altered, changing its conformation and interfering with its connection with NRF2. The subsequent stability of NRF2 allows it to migrate into the nucleus, where it binds to antioxidant response elements (ARE) of the genome, which activate downstream effector genes [67,68]. ARE genes activated by NFR2 include those controlling the synthesis and metabolism of GSH, antioxidant proteins such as GPX, and other drug and xenobiotic metabolizing enzymes and transporters [64].
On the other hand, NRF2’s novel function as an oncogene was demonstrated by the identification of its hyperactivation in a significant number of cancers, which gave tumor cells an advantage and led to the encouragement of growth and therapeutic resistance. The constitutive activation of NRF2 in this situation leads to the growth of the tumor as well as the progression and chemoresistance of the already-established tumor cells [11].
Increased GSH is produced as a result of the activation of the NRF2 pathway, and increased GSH levels have actually been observed in many types of cancer [69,70].
These observations seem to suggest that the hypothetical beneficial effects of antioxidant supplementation are limited or even counterproductive. In addition, since GSH plays a significant role in chemotherapy resistance, inhibiting GSH synthesis or its depletion may represent successful strategies for increasing the effectiveness of chemotherapy in light of the glutathionylation process, which, together with GST activity, contributes to the detoxification and inactivation of anticancer drugs [7,58,66,71].
5. Natural Compounds and GSH
5.1. Polyphenols and GSH
Polyphenols, a group of naturally occurring compounds widely distributed in the plant kingdom, are classified into various subclasses, including flavonoids, phenolic acids, stilbenes, and lignans. These secondary metabolites have undergone significant research to determine their mechanisms of action and potential health benefits. For instance, flavonoids, including quercetin, kaempferol, and catechins, have been linked to increased cognitive performance, a lowered risk of heart disease, and cancer prevention. Researchers have investigated the potential anti-aging and anti-inflammatory properties of resveratrol, a stilbene found in grapes and red wine [19].
Together with GSH, polyphenolic compounds play a significant role in maintaining overall health and protecting the body from oxidative stress. Interestingly, these substances have potent antioxidant effects that are comparable to GSH. They improve overall health by lowering oxidative stress and defending cells from free radical damage. According to some research, several polyphenols, including resveratrol, quercetin, and epigallocatechin gallate (EGCG), can increase the production of GSH and boost its activity in cells [30,72].
Additionally, polyphenols can help GSH levels by promoting the expression and activity of GPX, GR, and GLS enzymes, which are involved in GSH synthesis and regeneration [73]. It is crucial to remember that depending on the type of polyphenol, dosage, and individual variances in metabolism, the effects of polyphenols on GSH levels and activity may differ. A study reported that exposure of H9C2 cells to resveratrol results in an increase in GSH concentrations, GR, and GLS activities without affecting GPx activity [74]. On the other hand, treating PC12 cells with epicatechin and EGCG prevents the drop in GSH levels brought on by Pb++ treatment. This result can be attributed to the polyphenolic chemicals’ capacity to sustain GR activity [73].
Also, oleuropein, protocatechuic acid, and isoflavones significantly increase GR and GPx activities in J774A.1, LNCap, and PC3 cells [75].
By aiding the GSH recycling process, the various subclasses of polyphenolic compounds can contribute to the balance of GSH within cells. For instance, certain polyphenols can activate the enzyme glutamate-cysteine ligase, which oversees the initial stage in the synthesis of GSH. Polyphenols enhance body GSH levels by increasing the availability of cysteine, a crucial precursor for GSH synthesis.
Multiple investigations revealed that γGCL, a crucial gene for GSH synthesis in cells, was stimulated by relatively low quantities of flavonoids [76]. Quercetin is the most effective flavonoid, and both onion extracts, and pure flavonoids, transactivated γGCL through antioxidant response elements at the promoter level in COS-1 and HepG2 cells [76]. Structurally similar flavonoids were not as powerful; myricetin, which has just one more hydroxyl group than quercetin, was inactive, highlighting the apparent specificity of γGCL activation [77].
Although it has been suggested that one or more NRF2 binding sites (i.e., AREs/EpREs) mediate the effects of polyphenols, several flavonoid activities could be responsible for the results on the γGCL promoter. Accordingly, oxidative stress, thiol-reactive substances, and antioxidants are presumably sensitive to the release and subsequent translocation of NRF2 to the nucleus [78], which implies that the regulation of transcriptional γGCL depends on other flavonoid properties.
Various studies in vitro, in vivo, and clinical trials have documented that the protective activity of flavonoids is not only attributed to their antioxidant capacity but also to their pro-oxidant property and ability to exert modulatory effects in cells through the alteration of different signaling pathways [77,79,80,81]. Several flavonoids exhibit pro-oxidant behavior by preventing complexes I and II of the mitochondrial respiratory chain from functioning [82].
Additionally, certain flavonoids cause GSH depletion by activating the ABC transporter of the multidrug resistance protein 1 (MRP1) [83].
Since GSH depletion is the primary cause of cytotoxicity, it has long been recognized as a potential method for sensitizing cancer cells [29]. In fact, cancer cells display high levels of intracellular GSH due to an adaptive reaction to increased metabolism and consequently higher levels of ROS [84].
GSH depletion can be induced by using inhibitors of GSH synthesis such as l-buthionine sulfoximine (BSO) or through the activation of MRP1 [85,86].
In various types of neoplastic cells, including A549, HL-60, and PC-3, flavonoids can decrease intracellular levels of GSH with different effects depending on both, the structure of the compounds and the tumor cell line. The flavone chrysin induced more GSH depletion in A549 cells than in PC-3 and HL-60 at the same concentration and in a shorter treatment time. Apigenin was most effective in PC-3 cells, while hydroxychalcones and dihydroxychalcones are more active in A549 cells. The significance of a hydroxyl group at the 2′ position on chalcones was highlighted by these data, whereas a hydroxyl group at the 4′ position lessens the effect of chalcones in A549 cells and raises GSH levels in HL-60 and PC-3 cells [87]. Flavonoids′ chemopreventive abilities may be connected to their pro-oxidant behaviors. The cytotoxicity of hydroxy chalcones, apigenin, genistein, and chrysin may be caused by both interference with the mitochondrial respiratory chain and MRP-mediated GSH depletion [82,88].
Several studies in vitro highlighted that polyphenols, such as quercetin, apigenin, rhein, and resveratrol, may act in conjunction with chemotherapeutic drugs in order to increase cell cycle arrest and trigger apoptosis [72,89,90].
These synergistic effects were shown to be at least partly regulated by a decrease in GSH levels, and an increase in DNA damage was observed when polyphenols were combined with etoposide and doxorubicin [89,91,92].
Due to the broad pharmacological properties of polyphenols, it is possible to consider this large class of plant secondary metabolites as a source of complementary nutritional/pharmacological biomolecules for disease treatment and prevention strictly linked to GSH metabolism (Table 1). Although, more in-depth studies are needed on bioavailability, toxicity, and drug interactions in humans.

Table 1.
Polyphenols’ effects on GSH metabolism.
5.2. Terpenoids and GSH
Terpenoids are a diverse class of naturally occurring organic compounds widely distributed in the plant kingdom. They are responsible for the characteristic flavors and aromas of many fruits, flowers, and herbs. Terpenoids play crucial roles in plant defense mechanisms, attracting pollinators, and regulating growth and development. Terpenoids are derived from a precursor called isoprene and are classified based on the number of isoprene units they contain. Monoterpenoids, sesquiterpenoids, diterpenoids, and triterpenoids are some of the major subclasses within this chemical family. Each subclass exhibits unique chemical structures and biological activities, leading to diverse health effects [93].
Some terpenoids possess potent antioxidant and anti-inflammatory properties that can help combat oxidative stress and reduce chronic inflammation, both of which are implicated in the development of numerous diseases, including cancer [94,95,96]. Terpenoids have been found to modulate GSH homeostasis in various ways [97,98].
According to the literature, terpenoids have been reported to exhibit various anticancer effects on each stage of tumor development, acting as antiproliferative, pro-apoptotic, anti-angiogenetic, antimetastatic, and sensitizer molecules [99].
Recently, a novel form of cell death, named ferroptosis, was described in multiple diseases [100], including cancer [101]. Intracellular iron accumulation is one of the metabolic hallmarks of ferroptosis and depends on the regulation of iron trafficking across the membrane, mediated by various transporters and proteins [102,103]. Another metabolic hallmark is represented by dysregulation of the thiol redox system, composed of GSH, GR, and GPX, which, along with iron overload, resulted in the overproduction of ROS and lipid hydroperoxide (LOOH), leading to ferroptotic cell death. Notably, increasing evidence demonstrates that terpenoids induce ferroptosis in cancer [104].
Oridonin, a tetracyclic diterpenoid from Isodon rubescens Hemls, promotes ferroptosis through multiple effects on the thiol redox system via inhibition of GGT1 activity, GSH synthesis, and GPX4 expression in an esophageal cancer cell line [105,106]. Similarly, 18--glycyrrhetinic acid, a triterpene glycoside from medicinal herbal licorice, induces ROS/RNS production, increases lipid peroxidation, and triggers ferroptosis in MDA-MB-231 triple-negative breast cancer through downregulation of SLC7A11 expression, which negatively impacts GSH content and GPx activity [107]. Furthermore, Betula etnensis Raf. (Betulaceae) extract, titled betulinic acid (a pentacyclic triterpenoid), induces HO-1-mediated ferropototic cell death by reducing the GSH pool and subsequently enhancing ROS and LOOH levels [101].
Given the high levels of GSH present in most cancer cells [65], its depletion can be deleterious to cancer cells, which are thus deprived of one of the means by which they become chemoresistant, improving the therapeutic efficacy not only of ferroptotic compounds but also of ROS-based therapies (photodynamic, sonodynamic, and chemodynamic therapies) and chemotherapy [58]. Elemene, a sesquiterpene extracted from Curcuma wenyujin Y.H. Chen and C. Ling with β-elemene and trace amounts of β-caryophyllene, γ-elemene and δ-elemene isomers, is commonly used in clinical treatments of lung adenocarcinoma in combination with conventional therapy [108,109]. Its anticancer effects are linked to glutathione metabolism, which results in a decrease in the GSH/GSSG ratio and a significant reduction of SLC7A11 protein and glutaminase expression, leading to a decrease in GSH synthesis. Moreover, the low intracellular GSH concentration is also maintained by the upregulation of the glutamate-cysteine ligase modifier subunit (GCLM), the regulatory subunit of GCL, and the downregulation of GS, leading to cell apoptosis [110].
The influence of terpenoids on the glutathione network has also been studied in other pathological contexts. Recently, the protective effect of total terpenoids of Inula japonica Thunb (TTIJ) on lipopolysaccharide (LPS)-induced acute lung injury in mice was reported. TTIJs exert their beneficial effects by reducing inflammation and oxidative stress via NRF2-mediated upregulation of various genes, including glutamate-cysteine ligase catalytic subunit (GCLC) and GCLM, triggering GSH synthesis [111]. The antimigraine effects of carvacrol, a monoterpene phenol found in many aromatic plants, such as some species of Origanum [112], result from multiple mechanisms that also restore redox imbalance through an increase in GSH and GST levels [113]. In addition, glycyrrhetinic acid, in combination with paeoniflorin, a monoterpene glucoside isolated from the root of Paeonia lactiflora L., has shown antiparkinsonism activity in vitro and in vivo, also upregulating GCLC and GCLM [114]. The anti-diabetic properties of terpenoids have been established [115,116], and modulation of GSH metabolism seems to play a crucial role in the treatment of diabetes and its complications. For instance, the monoterpene D-limonene ameliorates diabetes in streptozotocin (STZ)-induced diabetic rats by also affecting the thiol redox system. In particular, an increase in GSH levels and GPX enzyme activity was found along with a reduction in GR activity [117]. In the same in vivo diabetic model, Betula etnensis Raf. ethanolic extract exerted protective activity, restoring both plasma and tissue levels of some metabolites, including GSH and lipid LOOH [118]. Intriguingly, the dysregulation of the GSH system and the induction of ferroptosis are also associated with the development of cardiovascular disease [119]. In this recent review, many strategies are proposed to activate the GSH system and alleviate the progression of myocardial injury, including the use of nutraceuticals such as terpenoids. The authors reported that britanin extracted from Inula linearifolia L. increased intracellular GSH levels and inhibited GPX in ferroptosis-induced myocardial I/R injury [120]. Furthermore, a water-soluble derivative of tanshinone IIA (Tan IIA), a diterpene extracted from Salvia miltiorrhiza Bunge, showed protective effects in an I/R-mediated myocardial injury model by triggering the GSH system via the NRF2 pathway [121].
Conversely, some terpenoids are toxic precisely because they disrupt glutathione metabolism. This is the case with the sesquiterpene lactones hymenoxon and helenalin, which showed severe hepatic toxicity in mice by causing a rapid and marked reduction in glutathione to the point of being lethal. Administration of substances that increase the intracellular glutathione pool, such as N-acetylcysteine or l-2-oxothiazolidine 4-carboxylate, protected against hepatic glutathione depletion and the lethal toxicity of both toxins [98]. Another example is the bioactivation of obacunone, which is first metabolized into a BDA intermediate by CYP3A4 and then reacts with GSH through 1,2, or 1,4 additions to form S-conjugates. The formation of these conjugates has been implicated in obacunone-induced liver injury following bioactivation reactions, as demonstrated by the presence of GSH-conjugates in the bile and urine of rats [122].
The wide range of beneficial effects that terpenoids exert on the glutathione thiol system (Table 2), coupled with some toxic effects of certain classes of these molecules, suggests that the mechanisms of action of these nutraceuticals should continue to be explored in various pathophysiological contexts.

Table 2.
Terpenoids’ effects on GSH metabolism.
5.3. Glucosinolates and GSH
In the plant kingdom, the order of Capparales includes the families of Tovariaceae, Resedaceae, Capparaceae, Moringaceae, and Brassicaceae, to which well-known crops such as broccoli, cabbage, mustard greens, etc., belong. Plants from these families are the primary source of a complex group of secondary metabolites known as glucosinolates (GSLs). These sulfur-rich molecules are essential for the plant’s defense against pathogens and pests, and recently, their potential medicinal uses and health benefits have attracted research attention [123].
GSLs (ß-thioglucoside-N-hydroxysulfates) are formed from an amino acid derivative, a sulfate group, and a β-d-thioglucose moiety. The chemical distinctive features of these compounds make them stable in whole plant tissues; however, when plant cells are broken or disrupted, for example, by mastication, the myrosinase enzyme, contained in the vacuole of specialized cells called myrosin cells, comes into contact with glucosinolates, causing their hydrolysis [124].
Depending on the peculiar chemical structure of the parent glucosinolate, hydrolysis of glucosinolates results in the generation of numerous breakdown products, collectively known as isothiocyanates, nitriles, and epithionitriles. Isothiocyanates (ITCs) are among those that show the most marked biological activity [123].
The potential health benefits of ITCs have been well investigated. Inhibiting the growth of cancer cells, warding off chronic illnesses such as cardiovascular problems and neurological issues, and controlling the body’s detoxification pathways are all potential benefits of these substances [125,126].
The antioxidant properties of ITCs can neutralize free radicals and prevent oxidative damage to DNA, proteins, and lipids in cells by activating the body’s natural antioxidant defense mechanisms [127]. Phase II detoxification enzymes, including GSH S-transferases and quinone reductases, which are essential for reducing reactive oxygen species and boosting cellular antioxidant capacity, have been demonstrated to be induced by glucosinolate hydrolysis products. The maintenance of cellular redox balance and defense against oxidative stress-related damage are both aided by the activation of detoxification mechanisms [128].
Following GSL hydrolysis by the myrosinase enzyme, the ITC derivatives, absorbed in the intestine, are metabolized through the mercapturic acid pathway, primarily through the conjugation with GSH by GST, with the formation of a dithiocarbamate GSH-conjugate. Some authors suggested that the ITCs’ distribution in vivo is strictly based on GSH content in the organism’s tissues [123]. Afterward, subsequent cleavage reactions catalyzed by different enzymes, such as GGT, result in the formation of the mercapturic acid derivatives, which are more water-soluble and easily eliminated in the urine and can be used as biomarkers of GSL intake [126,127,128,129]. The mercapturic acid derivatives are also found in human plasma in appreciable concentrations and seem to maintain biological activity, most likely via dissociating from the ITC derivative [128].
It is plausible that prolonged exposure to high levels of ITCs could result in a large reduction in cellular GSH since GSH conjugation is the main pathway in the metabolism of isothiocyanates. ITCs derivatives also interact with GSH by regulating its levels and activity within cells and by boosting the expression of critical enzymes involved in biosynthesis, such as γGLCL and GS [13,128,129]. This enhanced GSH synthesis and the induction of phase II detoxification enzymes aid in strengthening cellular antioxidant defenses, reducing oxidative stress, and getting rid of potentially hazardous substances [128].
The beneficial effects of ITC on GSH homeostasis were also evidenced in different animal models of metabolic disorder, where the ITC derivative of glucoraphanin, sulforaphane (SFN), boosted GSH activities and restored its production imbalance by modulating the NRF2/GPx4 pathway, which presumably is the primary mechanism, including the epigenetic, through which ITCs exert modulatory effects on GSH homeostasis [130,131,132].
ITCs have been demonstrated to possess hormetic effects on tumor growth both in vitro in different cancer cell lines such as HepG2, MDA-MB-231, and MCF-7 and in vivo. SFN at high doses suppressed tumor growth and cell proliferation, while at low concentrations, both were increased [133]. The same hormetic trend was shown in the modulation of GSH amount, suggesting that its close interaction with ITCs is strictly correlated with its antitumor and antiproliferative activities [134,135]. Such an effect can be explained since tumor cells show a high GSH content that readily interacts with elevated concentrations of ITCs, causing intracellular accumulation of GSH conjugate derivatives accountable for the antitumor activities [136].
Despite their well-documented anticancer activities (Table 3), ITCs have been found to exhibit hormetic activities in tumor cells, which involve GSH amount in a biphasic dose-response relationship, wherein low doses of these compounds can induce beneficial effects while high doses may be detrimental in different cancer models in vitro and in vivo [133,134,135].

Table 3.
Glucosinolates effects on GSH metabolism.
The modulatory effects of ITCs on GSH homeostasis and its hormetic activities are significant and hold great potential for therapeutic applications. Numerous studies have demonstrated that ITCs interact directly with GSH, resulting in bioactive derivatives, and enhance its production and activity by activating the NRF2-ARE pathway, a fundamental signaling pathway involved in cellular defense against oxidative stress [130,131,132]. This activation leads to an upregulation of various enzymes involved in GSH synthesis, thereby increasing the intracellular levels of GSH [13,128].
However, further research is needed to fully understand the mechanisms underlying the modulatory effects of ITCs on GSH homeostasis.
6. Conclusions
A Janus-like role for GSH is emerging, whereby it may both be protective and also contribute to the resistance of cancer cells to chemotherapy. To optimize therapeutic targeting, it will be crucial to better comprehend GSH’s antioxidant-independent roles in cancer cells and to look beyond its basic antioxidant capabilities. Research into natural substances and their interactions with glutathione uncovered a fascinating and intricate interplay that goes beyond their traditional functions as simple antioxidants. Then, through its intricate interactions with various plant secondary metabolites, glutathione exhibits a broad range of cellular effects.
The studies reviewed have demonstrated that natural compounds can modulate glutathione homeostasis by stimulating key enzymes involved in its metabolism or regulating gene expression through the modulation of signal transduction pathways, affecting various cellular processes.
Although the present literature offers insightful information on the interaction between natural substances and glutathione, further study is still required to completely clarify the underlying mechanisms and maximize their potential applications.
The intricate relationships between these substances and glutathione point to the possibility of therapeutic interventions and approaches to treat many disorders, including cancer.
Moreover, it is noteworthy the importance of the emerging field of miRNAs as crucial regulatory factors of GSH metabolism, which can aggravate pathological states or favor the resolution of specific disease settings. Recently, microRNA was proposed as a novel regulation mechanism for the GSH cycle. The intracellular levels of GSH are mainly affected by miRNAs’ regulation at the level of GSH synthesis by targeting any of the proteins involved in its production. Particularly, γGLCL is regulated by various miRNAs (miR-1, miR-433) in several pathologies and experimental models [137,138]. Other studies demonstrated that miRNAs also act on GSH levels or GSH-related metabolic pathways beyond the specific targeting of the GSH enzymatic system involved in GSH synthesis [139,140,141,142]. Several groups have also examined miRNAs targeting GSH homeostasis in pathological contexts, including cancer [143], obesity and related morbidities [144], and multiple system atrophy [145].
Continued study in this field may lead to the discovery of new therapeutic strategies and an improvement in general human health.
Author Contributions
Conceptualization, C.D.G. and R.A.; writing—original draft preparation, C.D.G., G.A.M., B.T., S.B. and R.A.; writing—review and editing, C.D.G., G.A.M. and R.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Emilia Giacco, a native English-speaking, for proofreading the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meister, A. On the Discovery of Glutathione. Trends Biochem. Sci. 1988, 13, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Hristov, B.D. The Role of Glutathione Metabolism in Chronic Illness Development and Its Potential Use as a Novel Therapeutic Target. Cureus 2022, 14, e29696. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of Its Protective Roles, Measurement, and Biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Averill-Bates, D.A. The Antioxidant Glutathione. Vitam. Horm. 2023, 121, 109–141. [Google Scholar] [CrossRef]
- Diaz-Vivancos, P.; De Simone, A.; Kiddle, G.; Foyer, C.H. Glutathione—Linking Cell Proliferation to Oxidative Stress. Free Radic. Biol. Med. 2015, 89, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.P.; Rahman, H.S. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef]
- Cheng, X.; Xu, H.D.; Ran, H.H.; Liang, G.; Wu, F.G. Glutathione-Depleting Nanomedicines for Synergistic Cancer Therapy. ACS Nano 2021, 15, 8039–8068. [Google Scholar] [CrossRef]
- Pallardó, F.V.; Markovic, J.; García, J.L.; Viña, J. Role of Nuclear Glutathione as a Key Regulator of Cell Proliferation. Mol. Asp. Med. 2009, 30, 77–85. [Google Scholar] [CrossRef]
- Bertrand, R.L. Iron Accumulation, Glutathione Depletion, and Lipid Peroxidation Must Occur Simultaneously during Ferroptosis and Are Mutually Amplifying Events. Med. Hypotheses 2017, 101, 69–74. [Google Scholar] [CrossRef]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of Glutathione in Cancer Progression and Chemoresistance. Oxid. Med. Cell. Longev. 2023, 2013, 972913. [Google Scholar] [CrossRef]
- Jaganjac, M.; Milkovic, L.; Sunjic, S.B.; Zarkovic, N. The NRF2, Thioredoxin, and Glutathione System in Tumorigenesis and Anticancer Therapies. Antioxidants 2020, 9, 1151. [Google Scholar] [CrossRef] [PubMed]
- Teskey, G.; Cao, R.; Islamoglu, H.; Medina, A.; Prasad, C.; Prasad, R.; Sathananthan, A.; Fraix, M.; Subbian, S.; Zhong, L.; et al. The Synergistic Effects of the Glutathione Precursor, NAC and First-Line Antibiotics in the Granulomatous Response against Mycobacterium Tuberculosis. Front. Immunol. 2018, 9, 2069. [Google Scholar] [CrossRef] [PubMed]
- Scharf, G.; Prustomersky, S.; Knasmüller, S.; Schulte-Hermann, R.; Huber, W.W. Enhancement of Glutathione and γ-Glutamylcysteine Synthetase, the Rate Limiting Enzyme of Glutathione Synthesis, by Chemoprotective Plant-Derived Food and Beverage Components in the Human Hepatoma Cell Line HepG2. Nutr. Cancer 2003, 45, 74–83. [Google Scholar] [CrossRef]
- Luceri, C.; Caderni, G.; Sanna, A.; Dolara, P. Nutrition and Cancer-Research Communication Red Wine and Black Tea Polyphenols Modulate the Expression of Cycloxygenase-2, Inducible Nitric Oxide Synthase and Glutathione-Related Enzymes in Azoxymethane-Induced F344 Rat Colon Tumors. J. Nutr. 2002, 132, 1376–1379. [Google Scholar] [CrossRef]
- Havsteen, B.H. The Biochemistry and Medical Significance of the Flavonoids. Pharmacol. Therapeut. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Hodnick, W.F.; Ahmad, S.; Pardini, R.S. Induction of oxidative stress by redox active flavonoids in vitro production of reactive oxygen species. In Flavonoids in the Living System; Manthey, J.A., Buslig, B.S., Eds.; Plenum Press: New York, NY, USA, 1998; p. 131. [Google Scholar]
- van Zanden, J.J.; Geraets, L.; Wortelboer, H.M.; van Bladeren, P.J.; Rietjens, I.M.; Cnubben, N.H. Structural requirements for the flavonoid-mediated modulation of glutathione S-transferase P1-1 and GS-X pump activity in MCF7 breast cancer cells. Biochem. Pharmacol. 2004, 67, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Engwa, G.A. Free Radicals and the Role of Plant Phytochemicals as Antioxidants Against Oxidative Stress-Related Diseases. In Phytochemicals—Source of Antioxidants and Role in Disease Prevention; InTechOpen: London, UK, 2018. [Google Scholar]
- Zhou, D.-D.; Luo, M.; Huang, S.-Y.; Saimaiti, A.; Shang, A.; Gan, R.-Y.; Li, H.-B. Effects and Mechanisms of Resveratrol on Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2021, 2021, 9932218. [Google Scholar] [CrossRef] [PubMed]
- Valenti, G.E.; Tasso, B.; Traverso, N.; Domenicotti, C.; Marengo, B. Glutathione in Cancer Progression and Chemoresistance: An Update. Redox Exp. Med. 2023, 2023, e220023. [Google Scholar] [CrossRef]
- Lushchak, V.I. Glutathione Homeostasis and Functions: Potential Targets for Medical Interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione Synthesis. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Antioxidant Defences Synthesized in Vivo. In Free Radicals in Biology and Medicine; Clarendon Press, Ed.; Oxford University Press: Oxford, UK, 2015; pp. 77–152. [Google Scholar]
- Oda, T.; Sadakata, N.; Komatsu, N.; Muramatsu, T. Specific Efflux of Glutathione from the Basolateral Membrance Domain in Polarized MDCK Cells during Ricin-Induced Apoptosis. J. Biochem. 1999, 126, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Mattson, D.M.; Ahmad, I.M.; Dayal, D.; Parsons, A.D.; Aykin-Burns, N.; Li, L.; Orcutt, K.P.; Spitz, D.R.; Dornfeld, K.J.; Simons, A.L. Cisplatin Combined with Zidovudine Enhances Cytotoxicity and Oxidative Stress in Human Head and Neck Cancer Cells via a Thiol-Dependent Mechanism. Free Radic. Biol. Med. 2009, 46, 232–237. [Google Scholar] [CrossRef]
- Franco, R.; Cidlowski, J.A. Glutathione Efflux and Cell Death. Antioxid. Redox. Signal. 2012, 17, 1694–1713. [Google Scholar] [CrossRef]
- Franco, R.; Cidlowski, J.A. Apoptosis and Glutathione: Beyond an Antioxidant. Cell. Death Differ. 2009, 16, 1303–1314. [Google Scholar] [CrossRef]
- Trompier, D.; Chang, X.-B.; Barattin, R.; d’Hardemare, A.d.M.; Di Pietro, A.; Baubichon-Cortay, H. Verapamil and Its Derivative Trigger Apoptosis through Glutathione Extrusion by Multidrug Resistance Protein MRP1. Cancer Res. 2004, 64, 4950–4956. [Google Scholar] [CrossRef] [PubMed]
- Guha, P.; Dey, A.; Sen, R.; Chatterjee, M.; Chattopadhyay, S.; Bandyopadhyay, S.K. Intracellular GSH Depletion Triggered Mitochondrial Bax Translocation to Accomplish Resveratrol-Induced Apoptosis in the U937 Cell Line. J. Pharmacol. Exp. Ther. 2011, 336, 206–214. [Google Scholar] [CrossRef]
- Lim, C.H.; Dedon, P.C.; Deen, W.M. Kinetic Analysis of Intracellular Concentrations of Reactive Nitrogen Species. Chem. Res. Toxicol. 2008, 21, 2134–2147. [Google Scholar] [CrossRef]
- Sagone, A.J.; Husney, R.; O’Dorisio, M.; Metz, E. Mechanisms for the Oxidation of Reduced Gluthathione by Stimulated Granulocytes. Blood 1984, 63, 96–104. [Google Scholar] [CrossRef]
- Abedinzadeh, Z.; Gardes-Albert, M.; Ferradini, C. Kinetic Study of the Oxidation Mechanism of Glutathione by Hydrogen Peroxide in Neutral Aqueous Medium. Can. J. Chem. 1989, 67, 1247–1255. [Google Scholar] [CrossRef]
- Zinatullina, K.M.; Kasaikina, O.T.; Kuz’min, V.A.; Khrameeva, N.P. Interaction of Glutathione with Hydrogen Peroxide: A Kinetic Model. Kinet. Catal. 2019, 60, 266–272. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Radical Scavenging by Thiols and the Fate of Thiyl Radicals. In Oxidative Stress and Redox Regulation; Springer Netherlands: Dordrecht, The Netherlands, 2013; pp. 43–58. [Google Scholar]
- Kalyanaraman, B.; Karoui, H.; Jit Singh, R.; Felix, C.C. Detection of Thiyl Radical Adducts Formed during Hydroxyl Radical- and Peroxynitrite-Mediated Oxidation of Thiols--A High Resolution ESR Spin-Trapping Study at Q-Band (35 GHz). Anal. Biochem. 1996, 241, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Regulation of Glutathione Synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Di Monte, D.; Sandy, M.S.; Smith, M.T. Increased Efflux Rather than Oxidation Is the Mechanism of Glutathione Depletion by 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP). Biochem. Biophys. Res. Commun. 1987, 148, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Brechbuhl, H.M.; Gould, N.; Kachadourian, R.; Riekhof, W.R.; Voelker, D.R.; Day, B.J. Glutathione Transport Is a Unique Function of the ATP-Binding Cassette Protein ABCG2. J. Biol. Chem. 2010, 285, 16582–16587. [Google Scholar] [CrossRef]
- Nasr, R.; Lorendeau, D.; Khonkarn, R.; Dury, L.; Pérès, B.; Boumendjel, A.; Cortay, J.-C.; Falson, P.; Chaptal, V.; Baubuchon-Cortay, H. Molecular Analysis of the Massive GSH Transport Mechanism Mediated by The Human Multidrug Resistant Protein 1/ABCC1. Sci. Rep. 2020, 10, 7616. [Google Scholar] [CrossRef]
- Dickinson, D.A.; Forman, H.J. Cellular Glutathione and Thiols Metabolism. Biochem. Pharmacol. 2002, 64, 1019–1026. [Google Scholar] [CrossRef]
- Ketterer, B. The Role of Nonenzymatic Reactions of Glutathione in Xenobiotic Metabolism. Drug Metab. Rev. 1982, 13, 161–187. [Google Scholar] [CrossRef]
- Sheng-Huang, C.; Chieh-Hsin, C.; Mu-Chun, Y.; Wen-Tung, H.; Chia-Ying, H.; Ya-Ting, H.; Wan-Ling, S.; Jiuan-Jen, S.; Chih-Yang, H.; Jer-Yuh, L. Effects of Estrogen on Glutathione and Catalase Levels in Human Erythrocyte during Menstrual Cycle. Biomed. Rep. 2015, 3, 266–268. [Google Scholar] [CrossRef]
- Suojanen, J.N.; Gay, R.J.; Hilf, R. Influence of Estrogen on Glutathione Levels and Glutathione-Metabolizing Enzymes in Uteri and R3230AC Mammary Tumors of Rats. Biochim. Biophys. Acta General. Subj. 1980, 630, 485–496. [Google Scholar] [CrossRef]
- Almeida, M.; Soares, M.; Fonseca-Moutinho, J.; Ramalhinho, A.C.; Breitenfeld, L. Influence of Estrogenic Metabolic Pathway Genes Polymorphisms on Postmenopausal Breast Cancer Risk. Pharmaceuticals 2021, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Kotsos, D.; Tziomalos, K. Microsomal Prostaglandin E Synthase-1 and -2: Emerging Targets in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2023, 24, 3049. [Google Scholar] [CrossRef]
- Thorén, S.; Jakobsson, P.-J. Coordinate Up- and down-Regulation of Glutathione-Dependent Prostaglandin E Synthase and Cyclooxygenase-2 in A549 Cells. Eur. J. Biochem. 2000, 267, 6428–6434. [Google Scholar] [CrossRef]
- Maryon, E.B.; Molloy, S.A.; Kaplan, J.H. Cellular Glutathione Plays a Key Role in Copper Uptake Mediated by Human Copper Transporter 1. Am. J. Physiol. Cell Physiol. 2013, 304, C768–C779. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.H.; Ciriolo, M.R.; Peisach, J. The Role of Glutathione in Copper Metabolism and Toxicity. J. Biol. Chem. 1989, 264, 5598–5605. [Google Scholar] [CrossRef]
- Yilmaz, A.; Çomakli, V. Investigation of Effects of Some Metal Ions and Some Pesticides on Glutathione S-Transferase (GST) Enzyme Purified from Van Lake Fish (Chalcalburnus Tarichi) Kidney. Iğdır Üniversitesi Fen. Bilim. Enstitüsü Derg. 2023, 13, 1101–1109. [Google Scholar] [CrossRef]
- Park, M.K.; Choi, B.Y.; Kho, A.R.; Lee, S.H.; Hong, D.K.; Kang, B.S.; Lee, S.H.; Suh, S.W. The Protective Role of Glutathione on Zinc-Induced Neuron Death after Brain Injuries. Int. J. Mol. Sci. 2023, 24, 2950. [Google Scholar] [CrossRef] [PubMed]
- de Paula Arrifano, G.; Crespo-Lopez, M.E.; Lopes-Araújo, A.; Santos-Sacramento, L.; Barthelemy, J.L.; de Nazaré, C.G.L.; Freitas, L.G.R.; Augusto-Oliveira, M. Neurotoxicity and the Global Worst Pollutants: Astroglial Involvement in Arsenic, Lead, and Mercury Intoxication. Neurochem. Res. 2023, 48, 1047–1065. [Google Scholar] [CrossRef]
- Hider, R.C.; Kong, X.L. Glutathione: A Key Component of the Cytoplasmic Labile Iron Pool. BioMetals 2011, 24, 1179–1187. [Google Scholar] [CrossRef]
- Hider, R.; Aviles, M.V.; Chen, Y.-L.; Latunde-Dada, G.O. The Role of GSH in Intracellular Iron Trafficking. Int. J. Mol. Sci. 2021, 22, 1278. [Google Scholar] [CrossRef]
- Kumar, C.; Igbaria, A.; D’Autreaux, B.; Planson, A.-G.; Junot, C.; Godat, E.; Bachhawat, A.K.; Delaunay-Moisan, A.; Toledano, M.B. Glutathione Revisited: A Vital Function in Iron Metabolism and Ancillary Role in Thiol-Redox Control. EMBO J. 2011, 30, 2044–2056. [Google Scholar] [CrossRef] [PubMed]
- Daniel, T.; Faruq, H.M.; Laura Magdalena, J.; Manuela, G.; Christopher Horst, L. Role of GSH and Iron-Sulfur Glutaredoxins in Iron Metabolism—Review. Molecules 2020, 25, 3860. [Google Scholar] [CrossRef]
- Wang, Y.; Yen, F.S.; Zhu, X.G.; Timson, R.C.; Weber, R.; Xing, C.; Liu, Y.; Allwein, B.; Luo, H.; Yeh, H.-W.; et al. SLC25A39 Is Necessary for Mitochondrial Glutathione Import in Mammalian Cells. Nature 2021, 599, 136–140. [Google Scholar] [CrossRef]
- Niu, B.; Liao, K.; Zhou, Y.; Wen, T.; Quan, G.; Pan, X.; Wu, C. Application of Glutathione Depletion in Cancer Therapy: Enhanced ROS-Based Therapy, Ferroptosis, and Chemotherapy. Biomaterials 2021, 277, 121110. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.-K.; Chen, S.-E.; Chang, L.-C. A Dual Role of Heme Oxygenase-1 in Cancer Cells. Int. J. Mol. Sci. 2018, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Appenzeller-Herzog, C. Glutathione- and Non-Glutathione-Based Oxidant Control in the Endoplasmic Reticulum. J. Cell. Sci. 2011, 124, 847–855. [Google Scholar] [CrossRef]
- Holmgren, A.; Sengupta, R. The Use of Thiols by Ribonucleotide Reductase. Free. Radic. Biol. Med. 2010, 49, 1617–1628. [Google Scholar] [CrossRef]
- Marí, M.; De Gregorio, E.; De Dios, C.; Roca-Agujetas, V.; Cucarull, B.; Tutusaus, A.; Morales, A.; Colell, A. Mitochondrial Glutathione: Recent Insights and Role in Disease. Antioxidants. 2020, 9, 909. [Google Scholar] [CrossRef]
- Oestreicher, J.; Morgan, B. Glutathione: Subcellular Distribution and Membrane Transport. Biochem. Cell Biol. 2019, 97, 270–289. [Google Scholar] [CrossRef]
- Bansal, A.; Simon, M.C. Glutathione Metabolism in Cancer Progression and Treatment Resistance. J. Cell Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef]
- Kennedy, L.; Sandhu, J.K.; Harper, M.E.; Cuperlovic-culf, M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef]
- Pal, D.; Rai, A.; Checker, R.; Patwardhan, R.S.; Singh, B.; Sharma, D.; Sandur, S.K. Role of Protein S-Glutathionylation in Cancer Progression and Development of Resistance to Anti-Cancer Drugs. Arch. Biochem. Biophys. 2021, 704, 108890. [Google Scholar] [CrossRef]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.-L. The Keap1-NRF2 Pathway: Mechanisms of Activation and Dysregulation in Cancer. Redox. Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef]
- Gupta, R.K.; Patel, A.K.; Shah, N.; Choudhary, A.K.; Jha, U.K.; Yadav, U.C.; Gupta, P.K.; Pakuwal, U. Oxidative Stress and Antioxidants in Disease and Cancer: A Review. Asian Pac. J. Cancer Prev. 2014, 15, 4405–4409. [Google Scholar] [CrossRef]
- Kitano, Y.; Baba, Y.; Nakagawa, S.; Miyake, K.; Iwatsuki, M.; Ishimoto, T.; Yamashita, Y.; Yoshida, N.; Watanabe, M.; Nakao, M.; et al. NRF2 Promotes Oesophageal Cancer Cell Proliferation via Metabolic Reprogramming and Detoxification of Reactive Oxygen Species. J. Pathol. 2018, 244, 346–357. [Google Scholar] [CrossRef]
- Gamcsik, M.P.; Kasibhatla, M.S.; Teeter, S.D.; Colvin, O.M. Glutathione Levels in Human Tumors. Biomarkers 2012, 17, 671–691. [Google Scholar] [CrossRef]
- Potęga, A. Glutathione-Mediated Conjugation of Anticancer Drugs: An Overview of Reaction Mechanisms and Biological Significance for Drug Detoxification and Bioactivation. Molecules 2022, 27, 5252. [Google Scholar] [CrossRef]
- Alaswad, H.A.; Mahbub, A.A.; Le Maitre, C.L.; Jordan-Mahy, N. Molecular Action of Polyphenols in Leukaemia and Their Therapeutic Potential. Int. J. Mol. Sci. 2021, 22, 3085. [Google Scholar] [CrossRef]
- Jeon, S.E.; Choi-Kwon, S.; Park, K.A.; Lee, H.J.; Park, M.S.; Lee, J.H.; Kwon, S.B.; Park, K.C. Dietary Supplementation of (+)-Catechin Protects against UVB-Induced Skin Damage by Modulating Antioxidant Enzyme Activities. Photodermatol. Photoimmunol. Photomed. 2003, 19, 235–241. [Google Scholar] [CrossRef]
- Cao, Z.; Li, Y. Potent Induction of Cellular Antioxidants and Phase 2 Enzymes by Resveratrol in Cardiomyocytes: Protection against Oxidative and Electrophilic Injury. Eur. J. Pharmacol. 2004, 489, 39–48. [Google Scholar] [CrossRef]
- Acquaviva, R.; Di Giacomo, C.; Sorrenti, V.; Galvano, F.; Santangelo, R.; Cardile, V.; Gangia, S.; D’Orazio, N.; Abraham, N.G.; Vanella, L. Antiproliferative Effect of Oleuropein in Prostate Cell Lines. Int. J. Oncol. 2012, 41, 31–38. [Google Scholar] [CrossRef]
- Myhrstad, M.C.W.; Carlsen, H.; Nordström, O.; Blomhoff, R.; Moskaug, J.Ø. Flavonoids Increase the Intracellular Glutathione Level by Transactivation of the γ-Glutamylcysteine Synthetase Catalytical Subunit Promoter. Free Radic. Biol. Med. 2002, 32, 386–393. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and Prooxidant Behavior of Flavonoids: Structure-Activity Relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Talalay, P.; Dinkova-Kostova, A.T.; Holtzclaw, W.D. Importance of Phase 2 Gene Regulation in Protection against Electrophile and Reactive Oxygen Toxicity and Carcinogenesis. Adv. Enzym. Regul. 2003, 43, 121–134. [Google Scholar] [CrossRef]
- Li, F.; Li, S.; Li, H.-B.; Deng, G.-F.; Ling, W.-H.; Wu, S.; Xu, X.-R.; Chen, F. Antiproliferative Activity of Peels, Pulps and Seeds of 61 Fruits. J. Funct. Foods 2013, 5, 1298–1309. [Google Scholar] [CrossRef]
- Li, F.; Li, S.; Li, H.-B.; Deng, G.-F.; Ling, W.-H.; Xu, X.-R. Antiproliferative Activities of Tea and Herbal Infusions. Food Funct. 2013, 4, 530–538. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.-P.; Li, S.; Chen, Y.-M.; Li, H.-B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Bohmont, C.; Aaronson, L.M.; Mann, K.; Pardini, R.S. Inhibition of Mitochondrial NADH Oxidase, Succinoxidase, and ATPase by Naturally Occurring Flavonoids. J. Nat. Prod. 1987, 50, 427–433. [Google Scholar] [CrossRef]
- Leslie, E.M.; Deeley, R.G.; Cole, S.P.C. Bioflavonoid Stimulation of Glutathione Transport by the 190-KDa Multidrug Resistance Protein 1 (MRP1). Drug Metab.Dispos. 2003, 31, 11–15. [Google Scholar] [CrossRef]
- Zhang, K.; Mack, P.; Wong, K.P. Glutathione-Related Mechanisms in Cellular Resistance to Anticancer Drugs. Int. J. Oncol. 1998, 12, 871–953. [Google Scholar] [CrossRef]
- Nguyen, H.; Zhang, S.; Morris, M.E. Effect of Flavonoids on MRP1-Mediated Transport in Panc-1 Cells. J. Pharm. Sci. 2003, 92, 250–257. [Google Scholar] [CrossRef]
- Ballatori, N.; Hammond, C.L.; Cunningham, J.B.; Krance, S.M.; Marchan, R. Molecular Mechanisms of Reduced Glutathione Transport: Role of the MRP/CFTR/ABCC and OATP/SLC21A Families of Membrane Proteins. Toxicol. Appl. Pharmacol. 2005, 204, 238–255. [Google Scholar] [CrossRef]
- Kachadourian, R.; Day, B.J. Flavonoid-Induced Glutathione Depletion: Potential Implications for Cancer Treatment. Free Radic. Biol. Med. 2006, 41, 65–76. [Google Scholar] [CrossRef]
- Sabzevari, O.; Galati, G.; Moridani, M.Y.; Siraki, A.; O’Brien, P.J. Molecular Cytotoxic Mechanisms of Anticancer Hydroxychalcones. Chem. Biol. Interact. 2004, 148, 57–67. [Google Scholar] [CrossRef]
- Mahbub, A.; Le Maitre, C.; Haywood-Small, S.; Cross, N.; Jordan-Mahy, N. Polyphenols Act Synergistically with Doxorubicin and Etoposide in Leukaemia Cell Lines. Cell Death Discov. 2015, 1, 15043. [Google Scholar] [CrossRef]
- Mahbub, A.; Le Maitre, C.; Haywood-Small, S.; Cross, N.; Jordan-Mahy, N. Dietary Polyphenols Influence Antimetabolite Agents: Methotrexate, 6-Mercaptopurine and 5-Fluorouracil in Leukemia Cell Lines. Oncotarget 2017, 8, 104877–104893. [Google Scholar] [CrossRef]
- Mahbub, A.A.; Le Maitre, C.L.; Haywood-Small, S.L.; Cross, N.A.; Jordan-Mahy, N. Glutathione Is Key to the Synergistic Enhancement of Doxorubicin and Etoposide by Polyphenols in Leukaemia Cell Lines. Cell. Death Dis. 2015, 6, e2028. [Google Scholar] [CrossRef]
- Li, S.; Qiao, S.; Zhang, J.; Li, K. Quercetin Increase the Chemosensitivity of Breast Cancer Cells to Doxorubicin Via PTEN/Akt Pathway. Anti-Cancer Agent Med. Chem. 2015, 15, 1185–1189. [Google Scholar] [CrossRef]
- Acquaviva, R.; Malfa, G.A.; Loizzo, M.R.; Xiao, J.; Bianchi, S.; Tundis, R. Advances on Natural Abietane, Labdane and Clerodane Diterpenes as Anti-Cancer Agents: Sources and Mechanisms of Action. Molecules 2022, 27, 4791. [Google Scholar] [CrossRef]
- Bailly, C. Atractylenolides, Essential Components of Atractylodes-Based Traditional Herbal Medicines: Antioxidant, Anti-Inflammatory and Anticancer Properties. Eur. J. Pharmacol. 2021, 891, 173735. [Google Scholar] [CrossRef]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Pourbagher-Shahri, A.M.; Samarghandian, S. Anti-Inflammatory Action of Astaxanthin and Its Use in the Treatment of Various Diseases. Biomed. Pharmacother. 2022, 145, 112179. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and Human Health: A Comprehensive Review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- Soethoudt, M.; Peskin, A.V.; Dickerhof, N.; Paton, L.N.; Pace, P.E.; Winterbourn, C.C. Interaction of Adenanthin with Glutathione and Thiol Enzymes: Selectivity for Thioredoxin Reductase and Inhibition of Peroxiredoxin Recycling. Free Radic. Biol. Med. 2014, 77, 331–339. [Google Scholar] [CrossRef]
- Merrill, J.C.; Kim, H.L.; Safe, S.; Murray, C.A.; Hayes, M.A. Role of Glutathione in the Toxicity of the Sesquiterpene Lactones Hymenoxon and Helenalin. J. Toxicol. Environ. Health 1988, 23, 159–169. [Google Scholar] [CrossRef]
- Kamran, S.; Sinniah, A.; Abdulghani, M.A.M.; Alshawsh, M.A. Therapeutic Potential of Certain Terpenoids as Anticancer Agents: A Scoping Review. Cancers 2022, 14, 1100. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Jiang, X.; Gu, W. Emerging Mechanisms and Disease Relevance of Ferroptosis. Trends Cell. Biol. 2020, 30, 478–490. [Google Scholar] [CrossRef]
- Malfa, G.A.; Tomasello, B.; Acquaviva, R.; Genovese, C.; La Mantia, A.; Cammarata, F.P.; Ragusa, M.; Renis, M.; Di Giacomo, C. Betula Etnensis Raf. (Betulaceae) Extract Induced HO-1 Expression and Ferroptosis Cell Death in Human Colon Cancer Cells. Int. J. Mol. Sci. 2019, 20, 2723. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, Biology and Role in Disease. Nat. Rev. Mol. Cell. Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Candido, S.; Tomasello, B.; Lavoro, A.; Falzone, L.; Gattuso, G.; Russo, A.; Paratore, S.; McCubrey, J.A.; Libra, M. Bioinformatic Analysis of the LCN2–SLC22A17–MMP9 Network in Cancer: The Role of DNA Methylation in the Modulation of Tumor Microenvironment. Front. Cell. Dev. Biol. 2022, 10, 945586. [Google Scholar] [CrossRef]
- Zheng, K.; Dong, Y.; Yang, R.; Liang, Y.; Wu, H.; He, Z. Regulation of Ferroptosis by Bioactive Phytochemicals: Implications for Medical Nutritional Therapy. Pharmacol. Res. 2021, 168, 105580. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, N.; Zhou, Y.; Wang, K.; Sun, Y.; Yan, H.; Han, W.; Wang, X.; Wei, B.; Ke, Y.; et al. Oridonin Induces Ferroptosis by Inhibiting Gamma-glutamyl Cycle in TE1 Cells. Phytother. Res. 2021, 35, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Fujii, J.; Homma, T.; Kobayashi, S. Ferroptosis Caused by Cysteine Insufficiency and Oxidative Insult. Free Radic. Res. 2020, 54, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Chen, H.; Zhang, L.; Wu, M.; Zhang, F.; Yang, D.; Shen, J.; Chen, J. Glycyrrhetinic Acid Induces Oxidative/Nitrative Stress and Drives Ferroptosis through Activating NADPH Oxidases and INOS, and Depriving Glutathione in Triple-Negative Breast Cancer Cells. Free Radic. Biol. Med. 2021, 173, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Z.; Sui, X.; Wu, Q.; Wang, J.; Xu, C. Elemene Injection as Adjunctive Treatment to Platinum-Based Chemotherapy in Patients with Stage III/IV Non-Small Cell Lung Cancer: A Meta-Analysis Following the PRISMA Guidelines. Phytomedicine 2019, 59, 152787. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Dong, X.-M.; Song, G.-Q.; Wei, M.-M.; Fang, C.; Zheng, F.-B.; Zhao, Y.-J.; Lu, H.-Q.; Cheng, L.-H.; Zhou, J.-L.; et al. Bioactivity-Guided Discovery of Quality Control Markers in Rhizomes of Curcuma Wenyujin Based on Spectrum-Effect Relationship against Human Lung Cancer Cells. Phytomedicine 2021, 86, 153559. [Google Scholar] [CrossRef] [PubMed]
- Song, G.-Q.; Wu, P.; Dong, X.-M.; Cheng, L.-H.; Lu, H.-Q.; Lin, Y.-Y.; Tang, W.-Y.; Xie, T.; Zhou, J.-L. Elemene Induces Cell Apoptosis via Inhibiting Glutathione Synthesis in Lung Adenocarcinoma. J. Ethnopharmacol. 2023, 311, 116409. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Zhang, W.-H.; Zhu, Q.-M.; Ning, J.; Huo, X.-K.; Xiao, H.-T.; Sun, C.-P. Total Terpenoids of Inula Japonica Activated the NRF2 Receptor to Alleviate the Inflammation and Oxidative Stress in LPS-Induced Acute Lung Injury. Phytomedicine 2022, 107, 154377. [Google Scholar] [CrossRef]
- Suntres, Z.E.; Coccimiglio, J.; Alipour, M. The Bioactivity and Toxicological Actions of Carvacrol. Crit. Rev. Food Sci. Nutr. 2015, 55, 304–318. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, A.; Irshad, N. Pharmacological Evaluation of Carvacrol Anti-Migraine Potential. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 1309–1324. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, J.; Rong, H.; Zhang, X.; Dong, M. Paeoniflorin and Plycyrrhetinic Acid Synergistically Alleviate MPP+/MPTP-Induced Oxidative Stress through NRF2-Dependent Glutathione Biosynthesis Mechanisms. ACS Chem. Neurosci. 2021, 12, 1100–1111. [Google Scholar] [CrossRef]
- Castellano, J.M.; Guinda, A.; Delgado, T.; Rada, M.; Cayuela, J.A. Biochemical Basis of the Antidiabetic Activity of Oleanolic Acid and Related Pentacyclic Triterpenes. Diabetes 2013, 62, 1791–1799. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, S.; Vasudeva, N.; Ranga, V. In Vivo Anti-Hyperglycemic and Antioxidant Potentials of Ethanolic Extract from Tecomella Undulata. Diabetol. Metab. Syndr. 2012, 4, 33. [Google Scholar] [CrossRef]
- Bacanlı, M.; Anlar, H.G.; Aydın, S.; Çal, T.; Arı, N.; Ündeğer Bucurgat, Ü.; Başaran, A.A.; Başaran, N. D-Limonene Ameliorates Diabetes and Its Complications in Streptozotocin-Induced Diabetic Rats. Food Chemical. Toxicol. 2017, 110, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Malfa, G.A.; Tomasello, B.; Acquaviva, R.; Mantia, A.L.; Pappalardo, F.; Ragusa, M.; Renis, M.; Di Giacomo, C. The Antioxidant Activities of Betula Etnensis Rafin. Ethanolic Extract Exert Protective and Anti-Diabetic Effects on Streptozotocin-Induced Diabetes in Rats. Antioxidants 2020, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Yin, Y.; Ma, X.; Zhang, J.; Pan, W.; Tan, M.; Zhao, Y.; Yang, T.; Jiang, T.; Li, H. Glutathione System Enhancement for Cardiac Protection: Pharmacological Options against Oxidative Stress and Ferroptosis. Cell. Death Dis. 2023, 14, 131. [Google Scholar] [CrossRef]
- Lu, H.; Xiao, H.; Dai, M.; Xue, Y.; Zhao, R. Britanin Relieves Ferroptosis-Mediated Myocardial Ischaemia/Reperfusion Damage by Upregulating GPX4 through Activation of AMPK/GSK3β/NRF2 Signalling. Pharm. Biol. 2022, 60, 38–45. [Google Scholar] [CrossRef]
- Guo, Z.; Yan, M.; Chen, L.; Fang, P.; Li, Z.; Wan, Z.; Cao, S.; Hou, Z.; Wei, S.; Li, W.; et al. NRF2-dependent Antioxidant Response Mediated the Protective Effect of Tanshinone IIA on Doxorubicin-induced Cardiotoxicity. Exp. Ther. Med. 2018, 16, 3333–3344. [Google Scholar] [CrossRef]
- Lang, X.; Zhang, X.; Wang, D.; Zhou, W. In Vitro and In Vivo Metabolic Activation of Obacunone, A Bioactive and Potentially Hepatotoxic Constituent of Dictamni Cortex. Planta Med. 2020, 86, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.A.; López, C.J.; Simal-Gandara, J. Glucosinolates: Molecular Structure, Breakdown, Genetic, Bioavailability, Properties and Healthy and Adverse Effects. Adv. Food Nutr. Res. 2019, 90, 305–350. [Google Scholar] [PubMed]
- Shirakawa, M.; Tanida, M.; Ito, T. The Cell Differentiation of Idioblast Myrosin Cells: Similarities with Vascular and Guard Cells. Front. Plant Sci. 2022, 12, 829541. [Google Scholar] [CrossRef] [PubMed]
- Kamal, R.M.; Abdull Razis, A.F.; Mohd Sukri, N.S.; Perimal, E.K.; Ahmad, H.; Patrick, R.; Djedaini-Pilard, F.; Mazzon, E.; Rigaud, S. Beneficial Health Effects of Glucosinolates-Derived Isothiocyanates on Cardiovascular and Neurodegenerative Diseases. Molecules 2022, 27, 624. [Google Scholar] [CrossRef]
- Vanduchova, A.; Anzenbacher, P.; Anzenbacherova, E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J. Med. Food 2019, 22, 121–126. [Google Scholar] [CrossRef]
- Figueiredo, S.; Filho, S.; Nogueira-Machado, J.; Caligiorne, R. The Anti-Oxidant Properties of Isothiocyanates: A Review. Recent. Pat. Endocr. Metab. Immune Drug Discov. 2013, 7, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Abdull Razis, A.F.; Konsue, N.; Ioannides, C. Isothiocyanates and Xenobiotic Detoxification. Mol. Nutr. Food Res. 2018, 62, 1700916. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pérez, A.; Núñez-Gómez, V.; Baenas, N.; Di Pede, G.; Achour, M.; Manach, C.; Mena, P.; Del Rio, D.; García-Viguera, C.; Moreno, D.A.; et al. Systematic Review on the Metabolic Interest of Glucosinolates and Their Bioactive Derivatives for Human Health. Nutrients 2023, 15, 1424. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, B.; Di Mauro, M.D.; Malfa, G.A.; Acquaviva, R.; Sinatra, F.; Spampinato, G.; Laudani, S.; Villaggio, G.; Bielak-Zmijewska, A.; Grabowska, W.; et al. Rapha Myr®, a Blend of Sulforaphane and Myrosinase, Exerts Antitumor and Anoikis-Sensitizing Effects on Human Astrocytoma Cells Modulating Sirtuins and DNA Methylation. Int. J. Mol. Sci. 2020, 21, 5328. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Q.; Liu, J.; Zhang, Z.; Ma, X.; Zhang, Y.; Zhu, J.; Thring, R.W.; Wu, M.; Gao, Y.; et al. Sulforaphane Alleviates High Fat Diet-Induced Insulin Resistance via AMPK/NRF2/GPx4 Axis. Biomed. Pharmacother. 2022, 152, 113273. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.Y. Beneficial Effects of Sulforaphane on Diabetes and Its Complications via Both NRF2-Dependent and Independent Mechanisms. Food Suppl. Biomater. Health 2023, 3, e6. [Google Scholar] [CrossRef]
- Na, G.; He, C.; Zhang, S.; Tian, S.; Bao, Y.; Shan, Y. Dietary Isothiocyanates: Novel Insights into the Potential for Cancer Prevention and Therapy. Int. J. Mol. Sci. 2023, 24, 1962. [Google Scholar] [CrossRef]
- Shoaib, S.; Khan, F.B.; Alsharif, M.A.; Malik, M.S.; Ahmed, S.A.; Jamous, Y.F.; Uddin, S.; Tan, C.S.; Ardianto, C.; Tufail, S.; et al. Reviewing the Prospective Pharmacological Potential of Isothiocyanates in Fight against Female-Specific Cancers. Cancers 2023, 15, 2390. [Google Scholar] [CrossRef]
- Patil, P.B.; Patel, J.K. Chemopreventive Aspects, Investigational Anticancer Applications and Current Perspectives on Allyl Isothiocyanate (AITC): A Review. Mol. Cell. Biochem. 2023. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, A.; Gasmi Benahmed, A.; Shanaida, M.; Chirumbolo, S.; Menzel, A.; Anzar, W.; Arshad, M.; Cruz-Martins, N.; Lysiuk, R.; Beley, N.; et al. Anticancer Activity of Broccoli, Its Organosulfur and Polyphenolic Compounds. Crit. Rev. Food Sci. Nutr. 2023, 1–19. [Google Scholar] [CrossRef]
- Espinosa-Diez, C.; Fierro-Fernandez, M.; Sanchez-Gomez, F.; Rodriguez-Pascual, F.; Alique, M.; Ruiz-Ortega, M.; Beraza, N.; Martinez-Chantar, M.L.; Fernandez-Hernando, C.; Lamas, S. Targeting of gamma-glutamyl-cysteine ligase by miR-433 reduces glutathione biosynthesis and promotes TGF-beta-dependent fibrogenesis. Antioxid. Redox Signal. 2015, 23, 1092–1105. [Google Scholar] [CrossRef]
- Li, R.; Chung, A.C.; Dong, Y.; Yang, W.; Zhong, X.; Lan, H.Y. The microRNA miR-433 promotes renal fibrosis by amplifying the TGF-beta/Smad3-Azin1 pathway. Kidney Int. 2013, 84, 1129–1144. [Google Scholar] [CrossRef]
- Kinoshita, C.; Aoyama, K.; Matsumura, N.; Kikuchi-Utsumi, K.; Watabe, M.; Nakaki, T. Rhythmic oscillations of the microRNA miR-96-5p play a neuroprotective role by indirectly regulating glutathione levels. Nat. Commun. 2014, 5, 3823. [Google Scholar] [CrossRef] [PubMed]
- Maes, O.C.; An, J.; Sarojini, H.; Wang, E. Murine microRNAs implicated in liver functions and aging process. Mechan. Ageing Dev. 2008, 129, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jiao, J.W.; Sun, K.X.; Zong, Z.H.; Zhao, Y. microRNA-133b targets glutathione S-transferase pi expression to increase ovarian cancer cell sensitivity to chemotherapy drugs. Drug Des. Dev. Ther. 2015, 9, 5225–5235. [Google Scholar]
- Uchida, Y.; Chiyomaru, T.; Enokida, H.; Kawakami, K.; Tatarano, S.; Kawahara, K.; Nishiyama, K.; Seki, N.; Nakagawa, M. miR-133a induces apoptosis through direct regulation of GSTP1 in bladder cancer cell lines. Urol. Oncol. Semin. Orig. Investig. 2013, 31, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Marengo, B.; Pulliero, A.; Izzotti, A.; Domenicotti, C. miRNA Regulation of Glutathione Homeostasis in Cancer Initiation, Progression and Therapy Resistance. MicroRNA 2020, 9, 187–197. [Google Scholar] [CrossRef]
- Matoušková, P.; Hanousková, B.; Skálová, L. MicroRNAs as Potential Regulators of Glutathione Peroxidases Expression and Their Role in Obesity and Related Pathologies. Int. J. Mol. Sci. 2018, 19, 1199. [Google Scholar] [CrossRef]
- Kinoshita, C.; Kubota, N.; Aoyama, K. Glutathione Depletion and MicroRNA Dysregulation in Multiple System Atrophy: A Review. Int. J. Mol. Sci. 2022, 23, 15076. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).