Abstract
Inflammatory bowel disease (IBD), characterized by an abnormal immune response, includes two distinct types: Crohn’s disease (CD) and ulcerative colitis (UC). Extensive research has revealed that the pathogeny of IBD encompasses genetic factors, environmental factors, immune dysfunction, dysbiosis, and lifestyle choices. Furthermore, patients with IBD exhibit both local and systemic oxidative damage caused by the excessive presence of reactive oxygen species. This oxidative damage exacerbates immune response imbalances, intestinal mucosal damage, and dysbiosis in IBD patients. Meanwhile, the weaning period represents a crucial phase for pigs, during which they experience pronounced intestinal immune and inflammatory responses, leading to severe diarrhea and increased mortality rates. Pigs are highly similar to humans in terms of physiology and anatomy, making them a potential choice for simulating human IBD. Although the exact mechanism behind IBD and post-weaning diarrhea remains unclear, the oxidative damage, in its progression and pathogenesis, is well acknowledged. Besides conventional anti-inflammatory drugs, certain probiotics, particularly Lactobacillus and Bifidobacteria strains, have been found to possess antioxidant properties. These include the scavenging of reactive oxygen species, chelating metal ions to inhibit the Fenton reaction, and the regulation of host antioxidant enzymes. Consequently, numerous studies in the last two decades have committed to exploring the role of probiotics in alleviating IBD. Here, we sequentially discuss the oxidative damage in IBD and post-weaning diarrhea pathogenesis, the negative consequences of oxidative stress on IBD, the effectiveness of probiotics in IBD treatment, the application of probiotics in weaned piglets, and the potential antioxidant mechanisms of probiotics.
1. Introduce
Inflammatory bowel disease (IBD) is an incurable chronic inflammatory gastrointestinal disease that primarily includes Crohn’s disease (CD) and ulcerative colitis (UC). The global prevalence of IBD has rapidly increased in recent years, with approximately 6.8 million people suffering from IBD in 2022 [1]. IBD types are typically distinguished by the location of inflammation and the histopathological characteristics of the gastrointestinal tract. Clinically, CD can occur in any region of the gastrointestinal tract, including the ileum and colon, characterized by transmural inflammation. On the other hand, UC specifically appears in the colon and rectum, with inflammation limited to the mucosa [2]. As an inappropriate immune response, the causes of IBD are considered multifaceted, involving genetic predisposition, environmental factors (Western diet, poor sanitation, and smoking), damage to intestinal epithelial integrity, and dysbiosis in the gut microbiome [3]. The exact underlying mechanisms of IBD remain unknown; however, accumulated data from animal experimental models and clinical studies suggest that oxidative stress (OS) signaling occupies a dominant position in the pathogeny of IBD. In brief, OS leads to damage to the gastrointestinal mucosal layer and dysbiosis, which are important features of IBD patients. This, in turn, stimulates immune responses and triggers IBD [4]. Therefore, relieving systemic oxidative stress becomes a crucial goal in treating IBD. Currently, the supplementation of reactive oxygen species (ROS) production inhibitors, corticosteroids, aminosalicylates, and substances that stimulate endogenous antioxidant enzymes have gradually emerged as complementary and alternative therapies for IBD treatment [5]. In addition, probiotics are being developed as therapeutic strategies for IBD. Studies have shown that probiotics, particularly Lactobacilli and Bifidobacteria, offer benefits for IBD patients by improving intestinal microecology, protecting intestinal mucosal barrier integrity, and modulating immune responses [6]. These effects are associated with their ability to scavenge ROS, chelate metals, and regulate the levels of host antioxidant enzymes [7]. Moreover, evidence from clinical studies suggests that the transplantation of healthy donor-derived microbiota to IBD patients promotes the recovery of their gut microbiota and the resolution of inflammation [8]. Therefore, approaches such as gut microbiota transplantation or the oral administration of probiotics hold potential as therapeutic interventions with which to alleviate clinical symptoms in IBD patients.
In pork production, weaning and feed transition often lead to intestinal barrier damage, intestinal villus atrophy, and an overload of proinflammatory factors (TNF-α, IL-6), resulting in diarrhea, decreased feed intake, and compromised growth [9]. Post-weaning diarrhea (PWD), caused by intestinal inflammation and oxidative stress, contributes to significant economic losses. Traditionally, antibiotics have been used as a means to alleviate diarrhea and promote growth [10]; however, due to the rise in antibiotic resistance among intestinal pathogens and concerns about drug residues, antibiotics are gradually being banned in many countries. Currently, certain probiotics have been suggested as alternatives to antibiotics in weaned piglets, exerting anti-inflammatory and antioxidant effects, modulating the microbiome, enhancing intestinal epithelial barrier function, and alleviating diarrhea [11,12].
In clinical research, difficulty in sampling, environmental limitations, and ethics often hinder direct research on human diseases. For a long time, small rodents have been important model animals for basic medical research, making significant contributions to the understanding of the pathogenesis and treatment of human diseases; however, pigs are more similar to humans in terms of physiology and anatomy than rodents, making them a potential choice for simulating human diseases [13]. At present, there are pig models for cardiovascular diseases, metabolic disorders, and neurological diseases, which provide considerable support for the analysis and treatment of human diseases [14]. Therefore, we take the intestinal inflammation of weaned piglets as an example with which to discuss the following points sequentially: the relationship between oxidative stress and IBD, the potential of probiotics in IBD treatment, the application of probiotics in weaned piglets, and the possible mechanisms of probiotics in IBD treatment.
We conducted a thorough search using PubMed, Medline, and Web of Science databases from 2010 to 2023, and found a total of 41 papers to include in this review. These papers included in vitro cell tests, small rodents (mainly induced with dextran sodium sulphate or 2,4,6-trinitrobenzenesulfonic acid), and piglets (induced with weaning stress) as IBD models. In addition, we also searched for double-blind, placebo-controlled trials of adults and children with active or quiescent CD or UC within the past 20 years. This review provides valuable insights into the potential of probiotics, particularly lactobacillus, in alleviating IBD symptoms.
2. Oxidative Stress in Inflammatory Bowel Disease
OS is an imbalance between oxidants and antioxidants, with reactive oxygen species (ROS) being the most common highly reactive molecules in organisms. ROS, including superoxide (O2·−), peroxy radical (RO2·), hydroxyl radical (HO·), and hydroperoxy radical (HO2·), are natural byproducts of metabolism [15]. They are primarily produced by organelles such as the endoplasmic reticulum, mitochondria, and peroxisome, as well as by enzymes, such as peroxidase, NADPH oxidase, xanthine oxidase, lipoxygenase, glucose oxidase, and epoxidase [16]. Antioxidant systems, on the other hand, consist of enzymatic and nonenzymatic defenses. Enzymatic defenses, including catalase, superoxide dismutase (SOD), and glutathione peroxidase (GP-x), are present in all cells. Nonenzymatic defenses typically involve substances such as glutathione, ascorbic acid, vitamin E, C, A, and metal elements (zinc, copper, manganese, and iron) [2]. At homeostatic levels, ROS have numerous physiological functions, such as cell signal transmission, growth, differentiation, apoptosis, and inflammation; however, under OS, overloaded ROS would damage cell biomacromolecules, especially membrane lipids, DNA, and proteins [2].
2.1. Oxidative Stress Is the Trigger of IBD
Currently, OS is receiving increasing attention as a potential etiology or trigger of IBD. Numerous studies have compared the levels of OS markers between healthy individuals and IBD patients. The results have shown that the levels of antioxidant enzymes (including PON1, SOD, CAT, and GP-x) and nonenzymatic antioxidant substances (vitamins A, C, E, and β-carotene) are higher in healthy individuals compared to IBD patients [4,17]. Additionally, the most commonly evaluated index is the total antioxidant status (TAS) or total antioxidant capacity (TAC), which reflects the overall antioxidant capacity of an individual [18]. The study found that the TAS/TAC in the serum or plasma of adult patients with IBD (including CD and UC) uniformly decreased [3]. Conversely, pro-oxidases/agents, such as MPO, NO, spermine oxidase, COX2, NOX2, and NOS2, were all raised in the intestinal mucosa and serum of IBD patients [19]. Moreover, the concentrations of lipid peroxidation products (4-hydroxynonenal and malondialdehyde), DNA oxidation products (8-OHdG), and oxidative protein products (hydroxylated or carbonylated proteins) with proinflammatory properties have been shown to be positively correlated with the severity of IBD [20,21,22]. Additionally, oxidative-damage-induced DNA strand breakage, pyrimidine/purine loss, or abnormal pyrimidine and purine modification are considered key factors in the occurrence of IBD [3,23]. Similarly, another index, the “Oxidative Stress Index”, obtained by dividing the total oxidative capacity by the total antioxidant status, is significantly higher in IBD patients compared to the healthy population [3].
OS is closely associated with the main pathological feature of IBD, which is inflammation. Studies have found a positive correlation between indices of OS and levels of C-reactive protein, an inflammatory marker in CD patients [24]. Similarly, clinical evidence suggests that plasma free thiols, the main substrates of ROS, are inversely correlated with inflammatory biomarkers [24,25]. Mechanistically, redox signaling stimulates NF-κB signaling, which is intimately involved in the upregulation of inflammatory cytokines (IL-1, IL-8) and inflammatory cell infiltration. During the immune response, polymorphonuclear leukocytes and monocytes infiltrate massively into the injured intestinal mucosa, stimulating the ROS/RN-generating system to increase oxidative stress [24] (Figure 1).
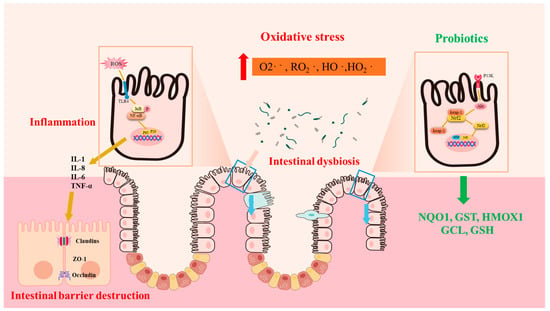
Figure 1.
Oxidative stress is associated with the pathogenesis of IBD. Oxidative stress leads to the overexpression of inflammatory factors, which destroy the intestinal barrier and cause opportunistic pathogens to invade the mucosa, exacerbating the vicious cycle. Probiotics stimulate the expression of antioxidant enzymes and relieve IBD by activating the Nrf2 signaling pathway. Superoxide (O2·−), peroxy radical (RO2·), hydroxyl radical (HO·), hydroperoxy radical (HO2·), NAD(P)H dehydrogenase quinone 1 (NQO1), glutathione S-transferase (GST), heme oxygenase 1 (HMOX1), glutamate cysteine ligase (GCL), and glutathione (GSH).
2.2. Oxidative Stress Leads to Intestinal Dysbiosis
Notably, intestinal dysbiosis, marked by microbial diversity and a decrease in beneficial bacteria alongside an increase in pathogenic bacteria, is another prominent feature of IBD patients [26]. Accumulating evidence suggests that disharmony between the intestinal flora and the immune response of the intestinal mucosa occupies a central role in IBD pathogenesis [27]. Some opportunistic pathogenic bacteria (E. coli and Helicobacter pylor) have been identified as the main sources of intestinal redox signals, which directly or indirectly (stimulate neutrophils) produce ROS, leading to the development of IBD exacerbations [28,29]. Additionally, the differential structure of the gut microbiota in patients with IBD compared to healthy individuals has been extensively studied [30]. The abundance of beneficial bacteria normally present in the gut of healthy individuals, such as Bacteroidetes and Firmicutes, is significantly reduced in patients with IBD, while harmful populations, such as Proteobacteria and Actinobacteria, are increased [31]. Among the beneficial bacteria, Faecalibacterium, producing butyrate, with anti-inflammatory effects, is one of the most abundant species in the human gut [26]; however, the abundance of Faecalibacterium prausnitzii is decreased in the gut of IBD patients [32]. Similarly, levels of Roseburia spp., another butyrate-producing bacteria, are remarkably lower in populations at a high genetic risk for IBD [26]. Additionally, the abundance of Bifidobacterium is also decreased. In terms of pathogenic bacteria, the relative abundance of Proteobacteria (mainly Escherichia coli) and proinflammatory properties (Escherichia and Fusobacterium) is higher in IBD patients [33]. Similarly, a TNBS-induced murine model found raised populations of E. coli as well as Clostridium spp. and reduced populations of Bifidobacterium and Lactobacillus [34]. Dysbiosis leads to damage of intestinal mucosal integrity, which causes opportunistic bacteria to invade the mucosa, leading to inflammatory cascades [35].
Additionally, oxidative stress and inflammation caused by ROS overload are tightly intertwined, leading to intestinal mucosal barrier damage in IBD patients. This, in turn, increases mucosal permeability, allowing pathogen invasion, which further stimulates proinflammatory factor and ROS production, creating a vicious cycle [36].
Currently, the focus of IBD therapy is on reducing inflammation, and mainstream drugs include combinations of immunosuppressive and anti-inflammatory agents, such as anti-TNF-α antibodies and corticosteroids [37]. Furthermore, many antioxidant therapies, such as ROS production inhibitors, dietary interventions, and antioxidants, are being investigated as auxiliary therapies for IBD, exhibiting promising results [5]. Since gut microbiota interfere with both local and systemic immune responses, and their dynamic changes markedly depending on environmental factors and IBD treatment [38], supplementing IBD patients with probiotics that have antioxidant capacity might be a potential new therapy.
3. Probiotics in the Treatment of IBD
The advantages of probiotics in the treatment of IBD have been extensively studied in recent decades. The use of probiotics in patients with IBD has increased by 50% in recent years. Accumulating evidence suggests that certain probiotic strains are beneficial for the treatment and prevention of IBD, both in animal models and humans. The most commonly used probiotics are Lactobacilli and Bifidobacteria, which have been reported to improve the total antioxidant status of IBD patients. This improvement may be attributed to their ability to scavenge reactive oxygen species (ROS), chelate metals, stimulate host antioxidant enzyme levels (SOD, CAT, and GP-X), and modulate the gut flora [39,40,41].
3.1. Effect of Probiotics on Alleviating UC
The colon harbors the highest concentration of microbes in the human body. Several probiotics that normalize the composition of the colonic microbiome have shown benefits for patients with ulcerative colitis (UC). Currently, dextran sulfate sodium (DSS) and 2,4,6-trinitrobenzene sulfonic acid (TNBS) are commonly used to induce experimental models of UC and CD, respectively. In mouse model of DSS-induced colitis, Bifidobacterium lactis A6, Bifidobacterium longum. infantis BB-02, and Bifidobacterium animalis lactis BB12 have been shown to inhibit OS, reduce colonic inflammation, and improve intestinal permeability [42]. Similarly, Lactobacillus plantarum 2142 inhibited oxidative-stress-induced proinflammatory cytokine overexpression in the IPEC-J2 cell line [43]. In human studies, Bifidobacterium and Lactobacillus acidophilus have demonstrated benefits for UC patients, including reduced rectal bleeding symptoms, improved endoscopic scores, and better redox statuses [44,45,46]. The administration of Lactobacillus reuteri enemas to children with ulcerative proctitis effectively improved their clinical scores [47]; however, some studies have not observed significant differences with the supplementation of the same species, suggesting that the combined use of multiple strains may be more effective. The De Simone formulation, consisting of eight lactic-acid-producing species, has been extensively studied and shown to provide relief for pediatric and adult UC patients [35,48]. Another effective probiotic mixture is VSL#3, which includes Lactobacillus, Bifidobacterium, and Streptococcus thermophilus [49]. It has demonstrated efficacy in mouse models of UC and mild to moderate UC patients, reducing rectal bleeding, inflammatory markers, and improving mucosal antioxidant capacity. The alleviating effect of probiotics on IBD has been summarized in Supplementary Table S1. It is important to note that most patients in clinical settings receive anti-inflammatory drugs as part of their routine care, and investigating the synergistic effects between conventional drugs and probiotics is necessary. Combining VSL#3 with probiotics has shown reduced rectal bleeding frequency [50]. Dual treatment with probiotic mixtures and mesalazine has resulted in shorter recovery times and improved endoscopic images in UC patients [51], whereas some studies have shown that probiotics (E. coli Nissle 1917 and VSL#3) have no significant therapeutic effect on UC patients [48,50,52]. The controversial results might be due to the differences in trial design, evaluation criteria, treatment duration, research scale, and patient characteristics (such as age, disease development stage, geographical location, and intervention type) [53]. Therefore, it is necessary to carry out a unified design experiment on a larger patient group to correctly evaluate the beneficial effects of probiotics on UC; however, it is generally believed that a longer treatment with the combination of VSL#3 and lactobacillus after surgery may have a better therapeutic effect on UC.
3.2. Effect of Probiotics on Alleviating CD
The effectiveness of probiotics in Crohn’s disease (CD) treatment remains disputed. Some studies have shown positive outcomes, such as reduced colonic edema and improved histological scores in CD mice treated with Bifidobacterium bifidum in addition to the relief of symptoms in children with CD treated with Lactobacillus rhamnosus and Saccharomyces boulardii [54,55]; however, other studies have not found significant benefits with the use of probiotics in CD patients, including the effectiveness of Lactobacillus rhamnosus and VSL#3 [34,56,57]. Overall, probiotics are not recommended for the treatment of CD patients based on current evidence [47].
Given that IBD is a multifactorial disease, it is not reasonable to use the same probiotic species for all patients to achieve the same efficacy. Personalized medicine should be considered in future studies, taking into account factors such as IBD subtype, the location of the pathology, disease severity, the composition of the patient’s microbiota, and environmental as well as genetic background, to determine the appropriate bacterial strains and doses for individual patients.
4. Probiotics Inhibit Intestinal Oxidative Damage in Weaning Piglets
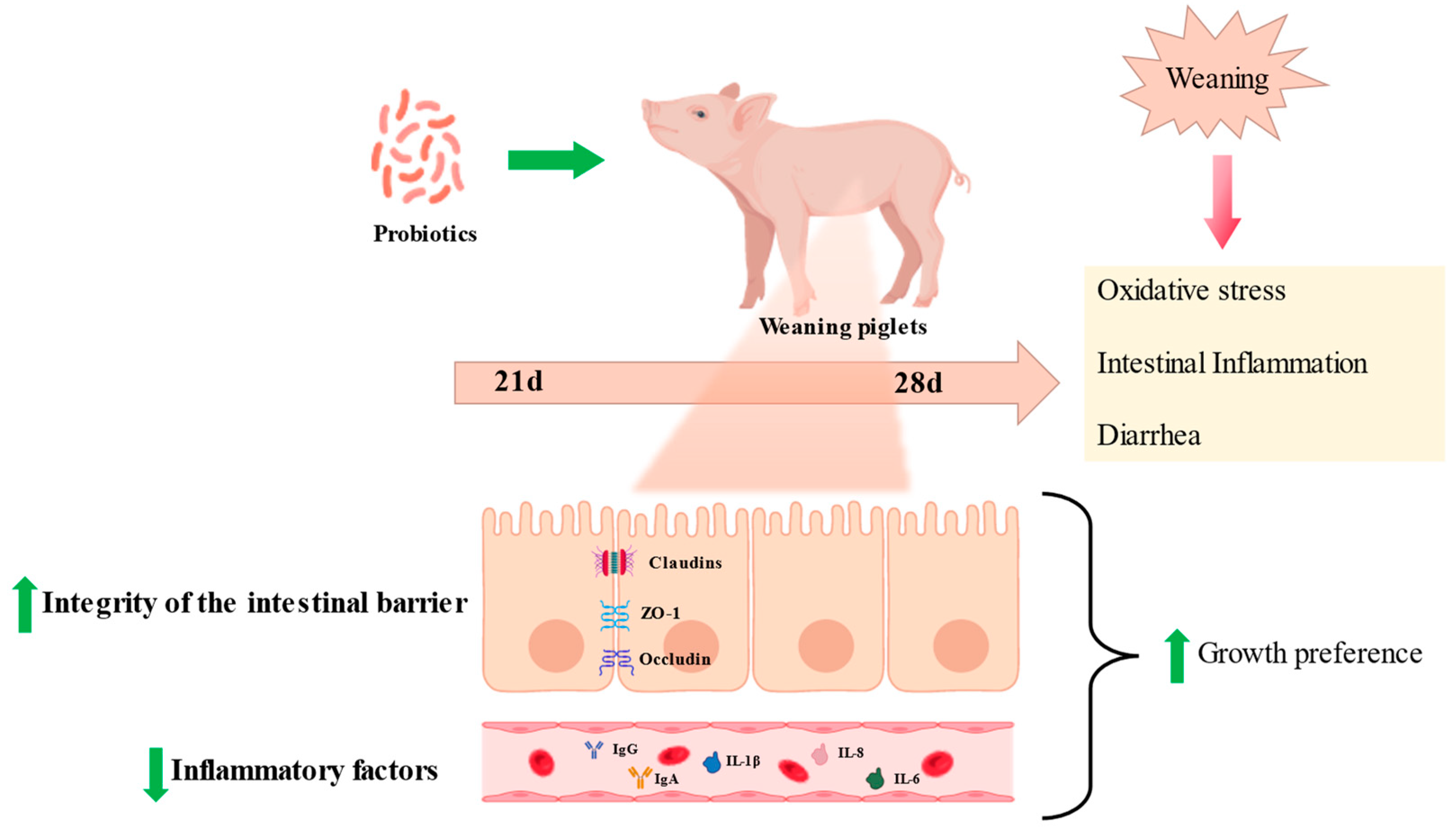
In pig farming, oxidative stress is one of the major causes of disease. The intestine is a main target of ROS attack, which easily leads to intestinal inflammation, barrier disruption, diarrhea, and microbial disorders, ultimately resulting in reduced feed intake and slow weight gain, severely compromising farming benefits. Studies have shown that MDA, protein hydroxyl, and ROS are significantly increased in the liver, intestine, and blood of weaned piglets [58], whereas the activities of GSH-PX and SOD are significantly inhibited [59]. Intestinal oxidative stress induces microbiome dysregulation, leading to post-weaning diarrhea (PWD), and intestinal infections are a major problem facing pig production [9].
Given its antioxidant properties, various strains of Lactobacillus have been reported as potentially being able to alleviate piglets’ post-weaning diarrhea. Research shows that an increased mean daily weight gain and a significant decrease in the rate of diarrhea were observed when LPS-challenged piglets were fed Lactobacillus salivarius, and also accompanied by decreased levels of proinflammatory mediators (IL-6 β, TNF- α, IL-2, and IFN- γ) in serum and mesenteric lymph nodes [9]. Similarly, the alleviating effect of Lactobacillus gasseri, Lactobacillus reuteri, and Lactobacillus acidophilus on diarrhea in piglets was demonstrated in several reports, which was associated with a reduction in enterotoxigenic Escherichia coli (ETEC) adhesion [11,60]. In addition, Lactobacillus acidophilus and Lactobacillus casei alleviated the severity of PWD by decreasing systemic immune responses and intestinal oxidative stress [11].
The intestine is the crucial place of nutrient digestion and absorption; thus, villus health remarkably affects the growth conditions of livestock, and is usually evaluated through villi height (VH), crypt depth (CD), and the villus-height-to-crypt-depth ratio (VCR) [49], whereas the shortening of intestinal villi and increased crypt depth via oxidative stress impede nutrient absorption [61]. On the other hand, intestinal nutrient absorption is mainly performed in a transmembrane or paracellular manner, closely related to tight junction proteins [62]. Tight junctions mainly include occludin, claudin-1, and ZO-1 proteins, and their dynamic changes have a key role in regulating the intestinal barrier and cell survival [63]. In addition to the function of digesting and absorbing nutrients, the intestinal epithelium also serves as a barrier against harmful antigens and pathogens. Intestinal barrier damage caused by oxidative stress is usually manifested by the disruption of tight junction proteins and the release of diamine oxidase (DAO) into the serum [64].
Interestingly, research showed piglets fed with Lactobacillus plantarum have higher VH as well as VCR and lower CD [65]. Additionally, dietary supplementation with Lactobacillus delbrueckii successfully reversed the LPS-induced increase in serum DAO and intestinal CD, as well as raised occludin, ZO-1, and Claudin-1 levels in the ileum of piglets [66]. Pretreatment with L. salivarius could stimulate the expression levels of SOD, GSH-PX4, and CAT in the intestine of weaned piglets, but inhibited the immune response and oxidative stress caused by LPS infection, thus restoring the intestinal integrity of the weaned piglets [9]. Furthermore, Lactobacillus delbrueckii supplementation alleviated an LPS-induced increase in MDA in serum but decreased jejunal mucosa 8-hydroxy-2-deoxyguanosine levels of piglets [67]. These results suggest that Lactobacillus maintains the intestinal epithelial barrier integrity by reducing oxidative stress. Other similar reports are summarized in Supplementary Table S2.
5. Potential Signaling Pathways Underlying the Antioxidant Actions of Probiotics
Nuclear factor erythroid 2–related factor 2 (Nrf2) is a member of the cap‘n’collar transcription factor family and consists of seven NEH domains. Currently, Nrf2 has emerged as a well established ubiquitin-dependent signaling system in response to OS [68]. High levels of ROS stimulate the separation of Nrf2 from its constitutive inhibitor: Keap1. Subsequently, Nrf2 enters the nucleus and binds to antioxidant response element (ARE) sequences, initiating the transcription of antioxidant genes such as NQO1, GST, HMOX1, GCL, and GSH (Figure 2). A large body of investigation indicates that Nrf2 activation could inhibit OS and inflammation, thereby preventing UC [69]. The activation of the Nrf2 system by probiotics in a host is believed to be one of the important mechanisms through which they exert antioxidant properties. In an in vivo UC rat model, Lactobacillus delbrueckii and Lactobacillus fermentum play a protective role by upregulating the Nrf2/Ho-1 pathway [70]. Similarly, Lactobacillus helveticus has also been shown to activate the Nrf2 pathway, relieving intestinal oxidative stress in mouse models [71]. Furthermore, the remission of LPS-induced intestinal injury by Bacillus coagulans TL3 is also related to Nrf2 signaling activation [72]. In vitro, the activation of the Nrf2 pathway has also been shown to mediate the antioxidant effect of Bifidobacterium infantis, Clostridium butyricum, and Lactobacillus casei Shirota in intestinal injury [73,74,75]. The activation of toll-like receptors (TLRs) has been reported to stimulate Nrf2-ARE signaling and HO-1, both in vivo and in vitro [76]. Additionally, numerous studies have reported that the stimulation of TLR-Nrf2 signaling by Lactobacilli might also be responsible for their antioxidant benefits in the piglets’ guts [77,78].
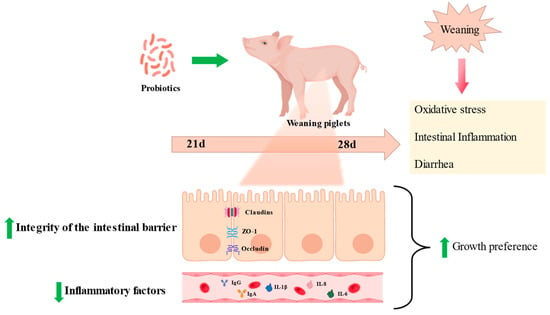
Figure 2.
Probiotics relieve weaning stress in piglets. Probiotics can relieve intestinal inflammation, protect intestinal morphology, and reduce the diarrhea rate caused by oxidative stress in weaned piglets.
Nuclear factor kappa-B (NF-κB) is another transcription factor that accounts for the redox mechanism of probiotics. It has been found that NF-κB has a close relationship with Nrf2. Specifically, the loss of Nrf2 can increase NF-κB activity, leading to more serious inflammation, and NF-κB activation can also mediate the transcription of Nrf2 [50]. Lactobacillus species, such as Lactobacillus johnsonii L531, Lactobacillus reuteri, Lactobacillus brevis, and Lactobacillus fermentans, have been shown to activate the NF-κB pathway, relieving intestinal OS and inflammation in rats [50,79,80]. Additionally, the artificial modification of Lactobacillus expressing SOD showed similar results [81]. Moreover, Bifidobacterium downregulates ROS and inhibits NF-κB pathways to modulate the intestinal immune system and protect the intestinal epithelium, as has been addressed in detail [82].
Silent information regulator factor 2-related enzymes (SIRTs) are highly conserved NAD+-dependent class III histone deacetylases. Currently, there are seven recognized members in the human SIRT family: SIRT1 to SIRT7.
The crosstalk between SIRT1 and Nrf2/ARE plays a key role in antioxidant defense. In brief, SIRT1 prompts the nuclear translocation of Nrf2, thereby upregulating the expression of antioxidant proteins and phase II detoxification enzymes [83]. In rats with aging-induced colitis, Lactobacillus C29 treatment decreased the plasma levels of ROS, malondialdehyde (MDA), and C-reactive protein, while increasing SIRT1 expression [84]. Furthermore, the alleviation of high-fat-diet-induced UC by B. longum and L. plantarum is also associated with the activation of SIRT1 [85]. It has also been shown that activated SIRT2 can deacetylate the forkhead box proteins (FOXO1a and FOXO3a) to increase the expression of FoxO-dependent antioxidant enzymes [86]. The activation of manganese superoxide dismutase (Mn-SOD)/SOD2 by B. longum and L. acidophilus to reduce cellular ROS levels mediated by SIRT2 has been reported [87].
Mitogen-activated protein kinases (MAPKs) pertain to the serine/threonine kinase family and participate in numerous biological processes, including cell inflammation, antioxidation, and cell death [88]. As of now, three MAPK types have been discovered: ERK, JNK, and p38 MAPK. It is generally accepted that Nrf2 activation mediated by ERK1/2, JNK, and p38 accounts for the expression of phase II detoxifying enzymes [52,89]. Lactobacillus rhamnosus GG prevents the H2O2-induced disruption of tight junctions in the human intestinal epithelium, which may be mediated through ERK1/2 [90]. Additionally, both heat-killed and active Lactobacillus brevis effectively ameliorated subtotal duodenal and colonic injury caused by mercury poisoning or DSS by blocking oxidative stress and inflammation through a p38-MAPK-mediated pathway [91,92].
6. Summary and Outlook
Taken together, IBD and post-weaning diarrhea are complex and multifactorial diseases with unclear direct causes and pathological mechanisms; however, the significant role of oxidative stress in their pathogenesis has been widely recognized. Currently, anti-inflammation and anti-oxidation are important treatment targets for IBD and post-weaning diarrhea.
Studies have shown that probiotics, particularly Lactobacillus and Bifidobacteria, possess antioxidant properties in mammals. Therefore, providing probiotics appears to be a promising strategy for IBD treatment. Probiotics may improve various pathological aspects of IBD, with mixed-species formulations being more effective than single-species ones. VSL#3 and the De Simone formulation have emerged as the most effective microbial agents for treating UC; however, probiotic formulations seem to be less effective in treating CD compared to UC, potentially due to differences in inflammation location, disease severity, and duration. Similarly, incorporating probiotics into the feed of weaned piglets can significantly reduce diarrhea, intestinal inflammation, and barrier disruption. The antioxidant properties of probiotics mainly involve scavenging free radicals, chelating metal ions, modulating antioxidant enzyme expression, and influencing gut microbiota. At the molecular level, probiotics can impact signaling pathways, such as Nrf-2, NF-κB, MAPK, and SIRTs, to exert antioxidant effects.
It should be noted that animal IBD models do not fully replicate the human immunological profile, particularly in multifactorial diseases. Therefore, the efficacy of probiotics should be tested in various models. Additionally, the lack of standardized evaluation criteria for antioxidant capacity hampers the comparison of results between studies. Moreover, the appropriate dosage, species type, mixing ratio, and treatment duration of probiotic preparations have yet to be determined. Furthermore, chemical drugs and surgical treatments remain preferred in current clinical practices, so the combination therapy of conventional treatment approaches with probiotics should be further investigated. These aforementioned issues should be the focus of future studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox12071342/s1. Table S1: The alleviating effect of probiotics on IBD; Table S2: The therapeutic effect of probiotics on intestinal inflammation in weaned piglets. References [93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120] are cited in the supplementary materials.
Author Contributions
Q.L.: investigation, writing—original draft, and visualization. T.Z.: investigation and visualization. H.D.: investigation and writing—original draft. J.C.: investigation and writing—original draft. B.L.: investigation and visualization. Q.Z.: investigation and visualization. S.Y.: writing—original draft. S.Z.: supervision, project administration, and writing—review and editing. W.G.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the National Key R&D Program of China (2021YFD1300700), the Guangdong Basic and Applied Basic Research Foundation (2021A1515010440 and 2023A1515012098), and the Science and Technology Program of Guangzhou (202102020056).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef] [PubMed]
- Storz, G.; Imlayt, J.A. Oxidative stress. Curr. Opin. Microbiol. 1999, 2, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, M.; Ates, I.; Kaplan, M.; Arikan, M.F.; Ozin, Y.O.; Kilic, Z.M.Y.; Topcuoglu, C.; Kayacetin, E. Is oxidative stress associated with activation and pathogenesis of inflammatory bowel disease? J. Med Biochem. 2017, 36, 341. [Google Scholar] [CrossRef]
- Balmus, I.M.; Ciobica, A.; Trifan, A.; Stanciu, C. The implications of oxidative stress and antioxidant therapies in Inflammatory Bowel Disease: Clinical aspects and animal models. Saudi J. Gastroenterol. 2016, 22, 3. [Google Scholar] [PubMed]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid. Med. Cell Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, R.D. The role of probiotic cultures in the control of gastrointestinal health. J. Nutr. 2000, 130, 396S–402S. [Google Scholar] [CrossRef]
- Mishra, V.; Shah, C.; Mokashe, N.; Chavan, R.; Yadav, H.; Prajapati, J. Probiotics as potential antioxidants: A systematic review. J. Agric. Food Chem. 2015, 63, 3615–3626. [Google Scholar] [CrossRef]
- Anderson, J.; Edney, R.; Whelan, K. Systematic review: Faecal microbiota transplantation in the management of inflammatory bowel disease. Aliment. Pharmacol. Ther. 2012, 36, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, H.; Li, Y.; Qiao, J. Lactobacillus salivarius, a potential probiotic to improve the health of LPS-challenged piglet intestine by alleviating inflammation as well as oxidative stress in a dose-dependent manner during weaning transition. Front. Veter.-Sci. 2020, 7, 547425. [Google Scholar] [CrossRef]
- Hao, Y.; Xing, M.; Gu, X. Research progress on oxidative stress and its nutritional regulation strategies in pigs. Animals 2021, 11, 1384. [Google Scholar] [CrossRef]
- Sun, Y.; Duarte, M.E.; Kim, S.W. Dietary inclusion of multispecies probiotics to reduce the severity of post-weaning diarrhea caused by Escherichia coli F18+ in pigs. Anim. Nutr. 2021, 7, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wu, M.; Xiao, H.; Ren, W.; Duan, J.; Yang, G.; Li, T.; Yin, Y.L. Development of an antioxidant system after early weaning in piglets. J. Anim. Sci. 2014, 92, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Prather, R.S.; Lorson, M.; Ross, J.W.; Whyte, J.J.; Walters, E. Genetically engineered pig models for human diseases. Annu. Rev. Anim. Biosci. 2013, 1, 203–219. [Google Scholar] [CrossRef]
- Hou, N.; Du, X.; Wu, S. Advances in pig models of human diseases. Anim. Model. Exp. Med. 2022, 5, 141–152. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.-H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Swindle, E.J.; Metcalfe, D.D. The role of reactive oxygen species and nitric oxide in mast cell-dependent inflammatory processes. Immunol. Rev. 2007, 217, 186–205. [Google Scholar] [CrossRef]
- Alzoghaibi, M.A. Concepts of oxidative stress and antioxidant defense in Crohn’s disease. World J. Gastroenterol. 2013, 19, 6540. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Aslan, M.; Nazligul, Y.; Bolukbas, C.; Bolukbas, F.F.; Horoz, M.; Dulger, A.C.; Erdur, F.M.; Celik, H.; Kocyigit, A. Peripheral lymphocyte DNA damage and oxidative stress in patients with ulcerative colitis. Pol. Arch. Intern. Med. 2011, 121, 223–229. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Yang, H.; Shao, D.; Zhao, X.; Zhang, G. Intraperitoneal injection of 4-hydroxynonenal (4-HNE), a lipid peroxidation product, exacerbates colonic inflammation through activation of Toll-like receptor 4 signaling. Free. Radic. Biol. Med. 2019, 131, 237–242. [Google Scholar] [CrossRef]
- Krzystek-Korpacka, M.; Neubauer, K.; Berdowska, I.; Boehm, D.; Zielinski, B.; Petryszyn, P.; Terlecki, G.; Paradowski, L.; Gamian, A. Enhanced formation of advanced oxidation protein products in IBD. Inflamm. Bowel Dis. 2008, 14, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Szczeklik, K.; Krzyściak, W.; Cibor, D.; Domagała-Rodacka, R.; Pytko-Polończyk, J.; Mach, T.; Owczarek, D. Markers of lipid peroxidation and antioxidant status in the serum and saliva of patients with active Crohn disease. Pol. Arch. Intern. Med. 2018, 128, 362–370. [Google Scholar]
- Wiseman, H.; Halliwell, B. Damage to DNA by reactive oxygen and nitrogen species: Role in inflammatory disease and progression to cancer. Biochem. J. 1996, 313, 17. [Google Scholar] [CrossRef] [PubMed]
- Beltran, B.; Nos, P.; DasÃ, F.; Iborra, M.; Bastida, G.; Martínez, M.; O’Connor, J.-E.; Saez, G.; Moret, I.; Ponce, J. Mitochondrial dysfunction, persistent oxidative damage, and catalase inhibition in immune cells of naive and treated Crohn’s disease. Inflamm. Bowel Dis. 2010, 16, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; von Martels, J.Z.; Bulthuis, M.L.; van Londen, M.; Faber, K.N.; Dijkstra, G.; van Goor, H. Crohn’s disease in clinical remission is marked by systemic oxidative stress. Front. Physiol. 2019, 10, 499. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- MacDonald, T.T.; Monteleone, G. Immunity, inflammation, and allergy in the gut. Science 2005, 307, 1920–1925. [Google Scholar] [CrossRef]
- Giaffer, M.; Holdsworth, C.; Duerden, B. Virulence properties of Escherichia coli strains isolated from patients with inflammatory bowel disease. Gut 1992, 33, 646–650. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Shanahan, F. Disorders of a modern lifestyle: Reconciling the epidemiology of inflammatory bowel diseases. Gut 2008, 57, 1185–1191. [Google Scholar] [CrossRef]
- Sokol, H.; Seksik, P.; Rigottier-Gois, L.; Lay, C.; Lepage, P.; Podglajen, I.; Marteau, P.; Doré, D. Specificities of the fecal microbiota in inflammatory bowel disease. Inflamm. Bowel Dis. 2006, 12, 106–111. [Google Scholar] [CrossRef]
- Alemany-Cosme, E.; Sáez-González, E.; Moret, I.; Mateos, B.; Iborra, M.; Nos, P.; Sandoval, J.; Beltrán, B. Oxidative Stress in the Pathogenesis of Crohn’s Disease and the Interconnection with Immunological Response, Microbiota, External Environmental Factors, and Epigenetics. Antioxidants 2021, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Toumi, R.; Samer, A.; Soufli, I.; Rafa, H.; Touil-Boukoffa, C. Role of probiotics and their metabolites in inflammatory bowel diseases (IBDs). Gastroenterol. Insights 2021, 12, 56–66. [Google Scholar]
- Rizzatti, G.; Lopetuso, L.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A common factor in human diseases. BioMed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef] [PubMed]
- Huynh, H.Q.; deBruyn, J.; Guan, L.; Diaz, H.; Li, M.; Girgis, S.; Turner, J.; Fedorak, R.; Madsen, K. Probiotic preparation VSL#3 induces remission in children with mild to moderate acute ulcerative colitis: A pilot study. Inflamm. Bowel Dis. 2009, 15, 760–768. [Google Scholar] [PubMed]
- Tomasello, G.; Mazzola, M.; Leone, A.; Sinagra, E.; Zummo, G.; Farina, F.; Damiani, P.; Cappello, F.; Gerges Geagea, A.; Jurjus, A.; et al. Nutrition, oxidative stress and intestinal dysbiosis: Influence of diet on gut microbiota in inflammatory bowel diseases. Biomed. Pap. 2016, 160, 461–466. [Google Scholar] [CrossRef]
- Rapozo, D.C.; Bernardazzi, C.; de Souza, H.S.P. Diet and microbiota in inflammatory bowel disease: The gut in disharmony. World J. Gastroenterol. 2017, 23, 2124. [Google Scholar] [CrossRef]
- Piechota-Polanczyk, A.; Fichna, J. The role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 605–620. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Amaretti, A.; Di Nunzio, M.; Pompei, A.; Raimondi, S.; Rossi, M.; Bordoni, A. Antioxidant properties of potentially probiotic bacteria: In vitro and in vivo activities. Appl. Microbiol. Biotechnol. 2013, 97, 809–817. [Google Scholar] [CrossRef]
- Sengül, N.; Işık, S.; Aslım, B.; Uçar, G.; Demirbağ, A.E. The effect of exopolysaccharide-producing probiotic strains on gut oxidative damage in experimental colitis. Dig. Dis. Sci. 2011, 56, 707–714. [Google Scholar] [CrossRef]
- Ballal, S.A.; Veiga, P.; Fenn, K.; Michaud, M.; Kim, J.H.; Gallini, C.A.; Glickman, J.N.; Quéré, G.; Garault, P.; Béal, C.; et al. Host lysozyme-mediated lysis of Lactococcus lactis facilitates delivery of colitis-attenuating superoxide dismutase to inflamed colons. Proc. Natl. Acad. Sci. USA 2015, 112, 7803–7808. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.M.; Heo, W.; Cho, H.T.; Lee, D.H.; Kim, J.H.; Rhee, M.S.; Park, T.-S.; Kim, Y.K.; Lee, J.H.; Kim, Y.J. Effects of orally-administered Bifidobacterium animalis subsp. lactis strain BB12 on dextran sodium sulfate-induced colitis in mice. J. Microbiol. Biotechnol. 2018, 28, 1800–1805. [Google Scholar] [CrossRef] [PubMed]
- Paszti-Gere, E.; Szeker, K.; Csibrik-Nemeth, E.; Csizinszky, R.; Marosi, A.; Palocz, O.; Farkas, O.; Galfi, P. Metabolites of Lactobacillus plantarum 2142 prevent oxidative stress-induced overexpression of proinflammatory cytokines in IPEC-J2 cell line. Inflammation 2012, 35, 1487–1499. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, S.K.; El-Bedewy, M.M. Effect of probiotics on pro-inflammatory cytokines and NF-N:B activation in ulcerative colitis. World J. Gastroenterol. 2010, 16, 4145. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, H.; Nakase, H.; Inoue, S.; Kawanami, C.; Itani, T.; Ohana, M.; Kusaka, T.; Uose, S.; Hisatsune, H.; Tojo, M.; et al. Efficacy of probiotic treatment with Bifidobacterium longum 536 for induction of remission in active ulcerative colitis: A randomized, double-blinded, placebo-controlled multicenter trial. Dig. Endosc. 2016, 28, 67–74. [Google Scholar] [CrossRef]
- Ishikawa, H.; Matsumoto, S.; Ohashi, Y.; Imaoka, A.; Setoyama, H.; Umesaki, Y.; Tanaka, R.; Otani, T. Beneficial effects of probiotic bifidobacterium and galacto-oligosaccharide in patients with ulcerative colitis: A randomized controlled study. Digestion 1955, 84, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Oliva, S.; Di Nardo, G.; Ferrari, F.; Mallardo, S.; Rossi, P.; Patrizi, G.; Cucchiara, S.; Stronati, L. Randomised clinical trial: The effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis. Aliment. Pharmacol. Ther. 2012, 35, 327–334. [Google Scholar] [CrossRef]
- Miele, E.; Pascarella, F.; Giannetti, E.; Quaglietta, L.; Baldassano, R.N.; Staiano, A. Effect of a probiotic preparation (VSL# 3) on induction and maintenance of remission in children with ulcerative colitis. Am. J. Gastroenterol. 2009, 104, 437–443. [Google Scholar]
- Hu, J.; Ma, L.; Nie, Y.; Chen, J.; Zheng, W.; Wang, X.; Xie, C.; Zheng, Z.; Wang, Z.; Yang, T.; et al. A microbiota-derived bacteriocin targets the host to confer diarrhea resistance in early-weaned piglets. Cell Host Microbe 2018, 24, 817–832.e818. [Google Scholar] [CrossRef]
- Tursi, A.; Brandimarte, G.; Papa, A.; Giglio, A.; Elisei, W.; Giorgetti, G.M.; Forti, G.; Morini, S.; Hassan, C.; Pistoia, M.A.; et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL# 3 as adjunctive to a standard pharmaceutical treatment: A double-blind, randomized, placebo-controlled study. Am. J. Gastroenterol. 2010, 105, 2218. [Google Scholar]
- Palumbo, V.D.; Romeo, M.; Marino Gammazza, A.; Carini, F.; Damiani, P.; Damiano, G.; Buscemi, S.; Ignazio, A.; Monte, L.; Gerges-Geagea, A. The long-term effects of probiotics in the therapy of ulcerative colitis: A clinical study. Biomed. Pap. Med. 2016, 160, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.; Mirsepasi, H.; Halkjær, S.I.; Mortensen, E.M.; Nordgaard-Lassen, I.; Krogfelt, K.A. Ciprofloxacin and probiotic Escherichia coli Nissle add-on treatment in active ulcerative colitis: A double-blind randomized placebo controlled clinical trial. J. Crohn’s Colitis 2014, 8, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Ganji-Arjenaki, M.; Rafieian-Kopaei, W. Probiotics are a good choice in remission of inflammatory bowel diseases: A meta analysis and systematic review. J. Cell Physiol. 2018, 233, 2091–2103. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Ran, H.Z.; Yin, M.H.; Zhou, T.X.; Xiao, D.S. Meta-analysis: The effect and adverse events of Lactobacilli versus placebo in maintenance therapy for Crohn disease. Intern. Med. J. 2009, 39, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Garcia Vilela, E.; De Lourdes De Abreu Ferrari, M.; Oswaldo Da Gama Torres, H.; Guerra Pinto, A.; Carolina Carneiro Aguirre, A.; Paiva Martins, F.; Marcos Andrade Goulart, E.n.; Sales Da Cunha, A.s. Influence of Saccharomyces boulardii on the intestinal permeability of patients with Crohn’s disease in remission. Scand. J. Gastroenterol. 2008, 43, 842–848. [Google Scholar] [CrossRef]
- McIlroy, J.; Ianiro, G.; Mukhopadhya, I.; Hansen, R.; Hold, G.L. The gut microbiome in inflammatory bowel disease—Avenues for microbial management. Aliment. Pharmacol. Ther. 2018, 47, 26–42. [Google Scholar] [CrossRef]
- Miele, E.; Shamir, R.; Aloi, M.; Assa, A.; Braegger, C.; Bronsky, J.; De Ridder, L.; Escher, J.C.; Hojsak, I.; Kolaček, S.; et al. Nutrition in pediatric inflammatory bowel disease: A position paper on behalf of the Porto Inflammatory Bowel Disease Group of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 687–708. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, L.; Zhang, W.; Yang, Z.; Ding, B.; Zhu, H.; Liu, Y.; Qiu, Y.; Yin, Y.; Wu, G. Protective effects of N-acetylcysteine on intestinal functions of piglets challenged with lipopolysaccharide. Amino Acids 2012, 43, 1233–1242. [Google Scholar] [CrossRef]
- Novais, A.K.; Deschêne, K.; Martel-Kennes, Y.; Roy, C.; Laforest, J.-P.; Lessard, M.; Matte, J.J.; Lapointe, J. Weaning differentially affects mitochondrial function, oxidative stress, inflammation and apoptosis in normal and low birth weight piglets. PLoS ONE 2021, 16, e0247188. [Google Scholar] [CrossRef]
- Hou, C.; Zeng, X.; Yang, F.; Liu, H.; Qiao, S. Study and use of the probiotic Lactobacillus reuteri in pigs: A review. J. Anim. Sci. Biotechnol. 2015, 6, 14. [Google Scholar] [CrossRef]
- Nabuurs, M.; Hoogendoorn, A.; Van Der Molen, E.; Van Osta, A. Villus height and crypt depth in weaned and unweaned pigs, reared under various circumstances in the Netherlands. Res. Veter- Sci. 1993, 55, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Madara, J.L. Loosening tight junctions. Lessons from the intestine. J. Clin. Investig. 1989, 83, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef] [PubMed]
- Wolvekamp, M.; De Bruin, R. Diamine oxidase: An overview of historical, biochemical and functional aspects. Dig Dis. 1994, 12, 2–14. [Google Scholar] [CrossRef]
- Wang, T.; Teng, K.; Liu, Y.; Shi, W.; Zhang, J.; Dong, E.; Zhang, X.; Tao, Y.; Zhong, J. Lactobacillus plantarum PFM 105 promotes intestinal development through modulation of gut microbiota in weaning piglets. Front. Microbiol. 2019, 10, 90. [Google Scholar] [CrossRef]
- Li, Y.; Hou, S.; Peng, W.; Lin, Q.; Chen, F.; Yang, L.; Li, F.; Huang, X. Oral administration of Lactobacillus delbrueckii during the suckling phase improves antioxidant activities and immune responses after the weaning event in a piglet model. Oxidative Med. Cell. Longev. 2019, 2019, 6919803. [Google Scholar] [CrossRef]
- Xin, J.; Zeng, D.; Wang, H.; Sun, N.; Zhao, Y.; Dan, Y.; Pan, K.; Jing, B.; Ni, X. Probiotic Lactobacillus johnsonii BS15 promotes growth performance, intestinal immunity, and gut microbiota in piglets. Probiotics Antimicrob. Proteins 2020, 12, 184–193. [Google Scholar] [CrossRef]
- Jones, R.M.; Mercante, J.W.; Neish, A.S. Reactive oxygen production induced by the gut microbiota: Pharmacotherapeutic implications. Curr. Med. Chem. 2012, 19, 1519–1529. [Google Scholar] [CrossRef]
- Trivedi, P.; Jena, G.; Tikoo, K.B.; Kumar, V. Melatonin modulated autophagy and Nrf2 signaling pathways in mice with colitis-associated colon carcinogenesis. Mol. Carcinog 2016, 55, 255–267. [Google Scholar] [CrossRef]
- El-Baz, A.M.; Khodir, A.E.; El-Sokkary, M.M.A.; Shata, A. The protective effect of Lactobacillus versus 5-aminosalicylic acid in ulcerative colitis model by modulation of gut microbiota and Nrf2/Ho-1 pathway. Life Sci. 2020, 256, 117927. [Google Scholar] [CrossRef]
- Li, B.; Evivie, S.E.; Lu, J.; Jiao, Y.; Wang, C.; Li, Z.; Liu, F.; Huo, G. Lactobacillus helveticus KLDS1. 8701 alleviates d-galactose-induced aging by regulating Nrf-2 and gut microbiota in mice. Food Funct. 2018, 9, 6586–6598. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, J.; Cheng, Z.; Wang, T.; Chen, J.; Long, M. Bacillus coagulans TL3 inhibits LPS-induced caecum damage in rat by regulating the TLR4/MyD88/NF- κ B and Nrf2 signal pathways and modulating intestinal microflora. Oxid. Med. Cell Longev. 2022, 2022, 5463290. [Google Scholar] [CrossRef]
- Dou, C.; Shang, Z.; Qiao, J.; Wang, Y.; Li, H. Clostridium butyricum Protects IPEC-J2 Cells from ETEC K88-Induced Oxidative Damage by Activating the Nrf2/ARE Signaling Pathway. Oxid. Med. Cell Longev. 2021, 2021, 4464002. [Google Scholar] [CrossRef]
- Finamore, A.; Ambra, R.; Nobili, F.; Garaguso, I.; Raguzzini, A.; Serafini, M. Redox role of Lactobacillus casei shirota against the cellular damage induced by 2,2′-Azobis (2-Amidinopropane) dihydrochloride-induced oxidative and inflammatory stress in enterocytes-like epithelial cells. Front. Immunol. 2018, 9, 1131. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, A.M.; Pacheco, A.R.; Henrick, B.M.; Taft, D.; Xu, G.; Huda, M.N.; Mishchuk, D.; Goodson, M.L.; Slupsky, C.; Barile, D.; et al. Indole-3-lactic acid associated with Bifidobacterium-dominated microbiota significantly decreases inflammation in intestinal epithelial cells. BMC Microbiol. 2020, 20, 357. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.; Siddiqui, N.; Al-Harbi, N.O.; Al-Harbi, M.M.; Ahmad, S.F. TLR-7 agonist attenuates airway reactivity and inflammation through Nrf2-mediated antioxidant protection in a murine model of allergic asthma. Int. J. Biochem. Cell Biol. 2016, 73, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C.; Ulluwishewa, D.; Young, W.; Ryan, L.J.; Henderson, G.; Meijerink, M.; Maier, E.; Wells, J.M.; Roy, N.C. Human oral isolate Lactobacillus fermentum AGR1487 induces a pro-inflammatory response in germ-free rat colons. Sci. Rep. 2016, 6, 20318. [Google Scholar] [CrossRef]
- Castillo, N.A.; PerdigC3n, G.; de Moreno de LeBlanc, A. Oral administration of a probiotic Lactobacillus modulates cytokine production and TLR expression improving the immune response against Salmonella enterica serovar Typhimurium infection in mice. BMC Microbiol. 2011, 11, 177. [Google Scholar] [CrossRef]
- Liu, Y.; Fatheree, N.Y.; Mangalat, N.; Rhoads, J.M. Lactobacillus reuteri strains reduce incidence and severity of experimental necrotizing enterocolitis via modulation of TLR4 and NF-N:B signaling in the intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G608–G617. [Google Scholar] [CrossRef]
- Pan, N.; Wang, K.; Qi, Y.; Yi, S.; Hu, G. Effects of Lactobacillus on the levels of the NF-N:B and inflammatory mediators in the mouse inflammation model. Chin. J. Prev. Vet. Med. 2016, 38, 686–689. [Google Scholar]
- Wu, T.; Zhang, Y.; Lv, Y.; Li, P.; Yi, D.; Wang, L.; Zhao, D.; Chen, H.; Gong, J.; Hou, Y. Beneficial Impact and Molecular Mechanism of Bacillus coagulans on Piglets’ Intestine. Int. J. Mol Sci. 2018, 19, 2084. [Google Scholar] [CrossRef]
- Yao, S.; Zhao, Z.; Wang, W.; Liu, X. Bifidobacterium longum: Protection against inflammatory bowel disease. J. Immunol. Res. 2021, 2021, 8030297. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.-W.; Zhao, G.-J.; Li, X.-L.; Hong, G.-L.; Li, M.-F.; Qiu, Q.-M.; Wu, B.; Lu, Z.-Q. SIRT1 exerts protective effects against paraquat-induced injury in mouse type II alveolar epithelial cells by deacetylating NRF2 in vitro. Int. J. Mol. Med. 2016, 37, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-J.; Kim, K.-A.; Jang, S.-E.; Woo, J.-Y.; Han, M.J.; Kim, D.-H. Orally administrated Lactobacillus pentosus var. plantarum C29 ameliorates age-dependent colitis by inhibiting the nuclear factor-kappa B signaling pathway via the regulation of lipopolysaccharide production by gut microbiota. PLoS ONE 2015, 10, e0116533. [Google Scholar]
- Kim, H.I.; Kim, J.-K.; Kim, J.-Y.; Jang, S.-E.; Han, M.J.; Kim, D.-H. Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 simultaneously alleviate high-fat diet-induced colitis, endotoxemia, liver steatosis, and obesity in mice. Nutr. Res. 2019, 67, 78–89. [Google Scholar] [CrossRef]
- Kitada, M.; Ogura, Y.; Monno, I.; Koya, D. Sirtuins and type 2 diabetes: Role in inflammation, oxidative stress, and mitochondrial function. Front. Endocrinol. 2019, 10, 187. [Google Scholar] [CrossRef]
- Guo, Q.; Li, S.; Xie, Y.; Zhang, Q.; Liu, M.; Xu, Z.; Sun, H.; Yang, Y. The NAD+-dependent deacetylase, Bifidobacterium longum Sir2 in response to oxidative stress by deacetylating SigH (O H) and FOXO3a in Bifidobacterium longum and HEK293T cell respectively. Free Radic. Biol. Med. 2017, 108, 929–939. [Google Scholar] [CrossRef]
- Yu, R.; Chen, C.; Mo, Y.-Y.; Hebbar, V.; Owuor, E.D.; Tan, T.-H.; Kong, A.-N.T. Activation of mitogen-activated protein kinase pathways induces antioxidant response element-mediated gene expression via a Nrf2-dependent mechanism. J. Biol. Chem. 2000, 275, 39907–39913. [Google Scholar] [CrossRef]
- Varì, R.; D’Archivio, M.; Filesi, C.; Carotenuto, S.; Scazzocchio, B.; Santangelo, C.; Giovannini, C.; Masella, R. Protocatechuic acid induces antioxidant/detoxifying enzyme expression through JNK-mediated Nrf2 activation in murine macrophages. J. Nutr. Biochem. 2011, 22, 409–417. [Google Scholar] [CrossRef]
- Seth, A.; Yan, F.; Polk, D.B.; Rao, R.K.; Bluemel, S.; Williams, B.; Knight, R.; Schnabl, B.; Dunagan, M.; Chaudhry, K.; et al. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC-and MAP kinase-dependent mechanism. Am. J. Physiol. Liver Physiol. 2008, 294, G1060–G1069. [Google Scholar] [CrossRef]
- Jiang, X.; Gu, S.; Liu, D.; Zhao, L.; Xia, S.; He, X.; Chen, H.; Ge, J. Lactobacillus brevis 23017 relieves mercury toxicity in the colon by modulation of oxidative stress and inflammation through the interplay of MAPK and NF-N:B signaling cascades. Front. Microbiol. 2018, 9, 2425. [Google Scholar] [CrossRef] [PubMed]
- Ueno, N.; Fujiya, M.; Segawa, S.; Nata, T.; Moriichi, K.; Tanabe, H.; Mizukami, Y.; Kobayashi, N.; Ito, K.; Kohgo, Y. Heat-killed body of Lactobacillus brevis SBC8803 ameliorates intestinal injury in a murine model of colitis by enhancing the intestinal barrier function. Inflamm. Bowel Dis. 2011, 17, 2235–2250. [Google Scholar] [CrossRef] [PubMed]
- Borthakur, A.; Anbazhagan, A.N.; Kumar, A.; Raheja, G.; Singh, V.; Ramaswamy, K.; Dudeja, P.K. The probiotic Lactobacillus plantarum counteracts TNF-N1-induced downregulation of SMCT1 expression and function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G928–G934. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Lee, S.Y.; Kim, N.R.; Lee, H.Y.; Ko, M.Y.; Jung, B.J.; Kim, C.M.; Lee, J.M.; Park, J.H.; Han, S.H. Lactobacillus plantarum lipoteichoic acid down-regulated Shigella flexneri peptidoglycan-induced inflammation. Mol. Immunol. 2011, 48, 382–391. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Lu, Y.-C.; Ou, C.-C.; Lin, S.-L.; Tsai, C.-C.; Huang, C.-T.; Lin, M.-Y. Lactobacillus plantarum MYL26 induces endotoxin tolerance phenotype in Caco-2 cells. BMC Microbiol. 2013, 13, 190. [Google Scholar] [CrossRef]
- Wang, B.; Li, J.; Chen, J.; Huang, Q.; Li, N.; Li, J. Effect of live Lactobacillus plantarum L2 on TNF-N1-induced MCP-1 production in Caco-2 cells. Int. J. Food Microbiol. 2010, 142, 237–241. [Google Scholar] [CrossRef]
- Ferreira dos Santos, T.; Alves Melo, T.; Almeida, M.E.; Passos Rezende, R.; Romano, C.C. Immunomodulatory effects of Lactobacillus plantarum Lp62 on intestinal epithelial and mononuclear cells. BioMed Res. Int. 2016, 2016, 8404156. [Google Scholar] [CrossRef]
- Llopis, M.; Antolin, M.; Carol, M.; Borruel, N.; Casellas, F.; Martinez, C.; EspC-n-Basany, E.; Guarner, F.; Malagelada, J.R. Lactobacillus casei downregulates commensals’ inflammatory signals in Crohn’s disease mucosa. Inflamm. Bowel Dis. 2009, 15, 275–283. [Google Scholar] [CrossRef]
- Matsumoto, S.; Hara, T.; Hori, T.; Mitsuyama, K.; Nagaoka, M.; Tomiyasu, N.; Suzuki, A.; Sata, M. Probiotic Lactobacillus-induced improvement in murine chronic inflammatory bowel disease is associated with the down-regulation of pro-inflammatory cytokines in lamina propria mononuclear cells. Clin. Exp. Immunol. 2005, 140, 417–426. [Google Scholar] [CrossRef]
- Nemoto, M.; Kuda, T.; Eda, M.; Yamakawa, H.; Takahashi, H.; Kimura, B. Protective effects of mekabu aqueous solution fermented by Lactobacillus plantarum Sanriku-SU7 on human enterocyte-like HT-29-luc cells and DSS-induced murine IBD model. Proteins 2017, 9, 48–55. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Wang, X.; Wang, S.; Bi, D. The impact of Lactobacillus plantarum on the gut microbiota of mice with DSS-induced colitis. Biomed. Res. Int. 2019, 2019, 3921315. [Google Scholar] [PubMed]
- Peran, L.; Camuesco, D.; Comalada, M.; Bailon, E.; Henriksson, A.; Xaus, J.; Zarzuelo, A.; Galvez, J. A comparative study of the preventative effects exerted by three probiotics, Bifidobacterium lactis, Lactobacillus casei and Lactobacillus acidophilus, in the TNBS model of rat colitis. J. Appl. Microbiol. 2007, 103, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Duary, R.K.; Bhausaheb, M.A.; Batish, V.K.; Grover, S. Anti-inflammatory and immunomodulatory efficacy of indigenous probiotic Lactobacillus plantarum Lp91 in colitis mouse model. Mol. Biol. Rep. 2012, 39, 4765–4775. [Google Scholar] [CrossRef]
- Dieleman, L.A.; Goerres, M.; Arends, A.; Sprengers, D.; Torrice, C.; Hoentjen, F.; Grenther, W.; Sartor, R.B. Lactobacillus GG prevents recurrence of colitis in HLA-B27 transgenic rats after antibiotic treatment. Gut 2003, 52, 370–376. [Google Scholar] [CrossRef]
- Schultz, M.; Veltkamp, C.; Dieleman, L.A.; Grenther, W.B.; Wyrick, P.B.; Tonkonogy, S.L.; Sartor, R. Lactobacillus plantarum 299V in the treatment and prevention of spontaneous colitis in interleukin-10-deficient mice. Bowel Dis. 2002, 8, 71–80. [Google Scholar] [CrossRef]
- Peran, L.; Sierra, S.; Comalada, M.; Lara-Villoslada, F.; Bailón, E.; Nieto, A.; Concha, A.; Olivares, M.; Zarzuelo, A.; Xaus, J.; et al. A comparative study of the preventative effects exerted by two probiotics, Lactobacillus reuteri and Lactobacillus fermentum, in the trinitrobenzenesulfonic acid model of rat colitis. Br. J. Nutr. 2007, 97, 96–103. [Google Scholar] [CrossRef] [PubMed]
- van der Waal, M.B.; Flach, J.; Browne, P.D.; Besseling-van der Vaart, I.; Claassen, E.; van de Burgwal, L.H.M. Probiotics for improving quality of life in ulcerative colitis: Exploring the patient perspective. Pharma Nutr. 2019, 7, 100139. [Google Scholar] [CrossRef]
- Zocco, M.; Dal Verme, L.Z.; Cremonini, F.; Piscaglia, A.; Nista, E.; Candelli, M.; Novi, M.; Rigante, D.; Cazzato, I.; Ojetti, V.; et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment. Pharmacol. Ther. 2006, 23, 1567–1574. [Google Scholar] [CrossRef]
- Henker, J.; Müller, S.; Laass, M.W.; Schreiner, A.; Schulze, J. Probiotic Escherichia coli Nissle 1917 (EcN) for successful remission maintenance of ulcerative colitis in children and adolescents: An open-label pilot study. Z Gastroenterol. 2008, 46, 874–875. [Google Scholar] [CrossRef]
- Mao, X.; Gu, C.; Hu, H.; Tang, J.; Chen, D.; Yu, B.; He, J.; Yu, J.; Luo, J.; Tian, G. Dietary Lactobacillus rhamnosus GG supplementation improves the mucosal barrier function in the intestine of weaned piglets challenged by porcine rotavirus. PLoS ONE 2016, 11, e0146312. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Zhuo, Y.; Fang, Z.; Che, L.; Xu, S.; Feng, B.; Lin, Y.; Jiang, X.; Zhao, X.; et al. Effects of Multi-Strain Probiotics and Perilla frutescens Seed Extract Supplementation Alone or Combined on Growth Performance, Antioxidant Indices, and Intestinal Health of Weaned Piglets. Animals 2022, 12, 2246. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Su, W.; Li, W.; Wen, C.; Du, S.; He, H.; Zhang, Y.; Gong, T.; Wang, X.; Wang, Y. Bacillus amyloliquefaciens 40 regulates piglet performance, antioxidant capacity, immune status and gut microbiota. Anim. Nutr. 2023, 12, 116–127. [Google Scholar] [CrossRef]
- Yu, X.; Cui, Z.; Qin, S.; Zhang, R.; Wu, Y.; Liu, J.; Yang, C. Effects of Bacillus licheniformis on Growth Performance, Diarrhea Incidence, Antioxidant Capacity, Immune Function, and Fecal Microflora in Weaned Piglets. Front. Immunol. 2022, 12, 1609. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hou, S.; Chen, J.; Peng, W.; Wen, W.; Chen, F.; Huang, X. Oral administration of Lactobacillus delbrueckii during the suckling period improves intestinal integrity after weaning in piglets. J. Funct. Foods 2019, 63, 103591. [Google Scholar] [CrossRef]
- Yu, X.; Dai, Z.; Cao, G.; Cui, Z.; Zhang, R.; Xu, Y.; Wu, Y.; Yang, C. Protective effects of Bacillus licheniformis on growth performance, gut barrier functions, immunity and serum metabolome in lipopolysaccharide-challenged weaned piglets. Front. Immunol. 2023, 14, 1140564. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yao, J.; Xu, Q.; Zheng, Y.; Dong, X. Clostridium butyricum relieves diarrhea by enhancing digestive function, maintaining intestinal barrier integrity, and relieving intestinal inflammation in weaned piglets. Livest. Sci. 2020, 239, 104112. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Wang, S.; Zhang, D.; Liu, H.; Shan, D.; Wang, Y. Lactobacillus plantarum ZLP001: In vitro assessment of antioxidant capacity and effect on growth performance and antioxidant status in weaning piglets. Asian-Australas. J. Anim. Sci. 2012, 25, 1153. [Google Scholar] [CrossRef]
- Sarkar, V.; De, U.; Kala, A.; Chauhan, A.; Verma, A.; Paul, B.; Soni, S.; Chaudhuri, P.; Patra, M.; Gaur, G.K. Effects of oral probiotic and lactoferrin interventions on iron-zinc homeostasis, oxidant/antioxidant equilibrium and diarrhoea incidence of neonatal piglets. Benef. Microbes 2023, 7, 1–12. [Google Scholar] [CrossRef]
- Sun, W.; Chen, W.; Meng, K.; Cai, L.; Li, G.; Li, X.; Jiang, X. Dietary Supplementation with Probiotic Bacillus licheniformis S6 Improves Intestinal Integrity via Modulating Intestinal Barrier Function and Microbial Diversity in Weaned Piglets. Biology 2023, 12, 238. [Google Scholar] [CrossRef]
- Chen, F.; Chen, J.; Chen, Q.; Yang, L.; Yin, J.; Li, Y.; Huang, X. Lactobacillus delbrueckii Protected Intestinal Integrity, Alleviated Intestinal Oxidative Damage, and Activated Toll-Like Receptor-Bruton’s Tyrosine Kinase-Nuclear Factor Erythroid 2-Related Factor 2 Pathway in Weaned Piglets Challenged with Lipopolysaccharide. Antioxidants 2021, 10, 468. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).