Comparative Analysis of Hot and Cold Brews from Single-Estate Teas (Camellia sinensis) Grown across Europe: An Emerging Specialty Product
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Equipment
2.2. Tea Samples
2.3. Preparation of Tea Brews
2.4. Determination of Total Phenolic Content (TPC)
2.5. Total Flavonoid Content (TFC)
2.6. Determination of In Vitro Antioxidant Capacity (ABTS, FRAP, ORAC)
2.6.1. ORAC Assay
2.6.2. ABTS Assay
2.6.3. FRAP Assay
2.7. Metal Chelating Assay
2.8. UV-Vis Spectrophotometric Measurements
2.9. Metabolomic Profiling
2.10. Data Analysis
3. Results
3.1. Total Polyphenol and Flavonoid Contents in Brews from Tea Grown in Europe
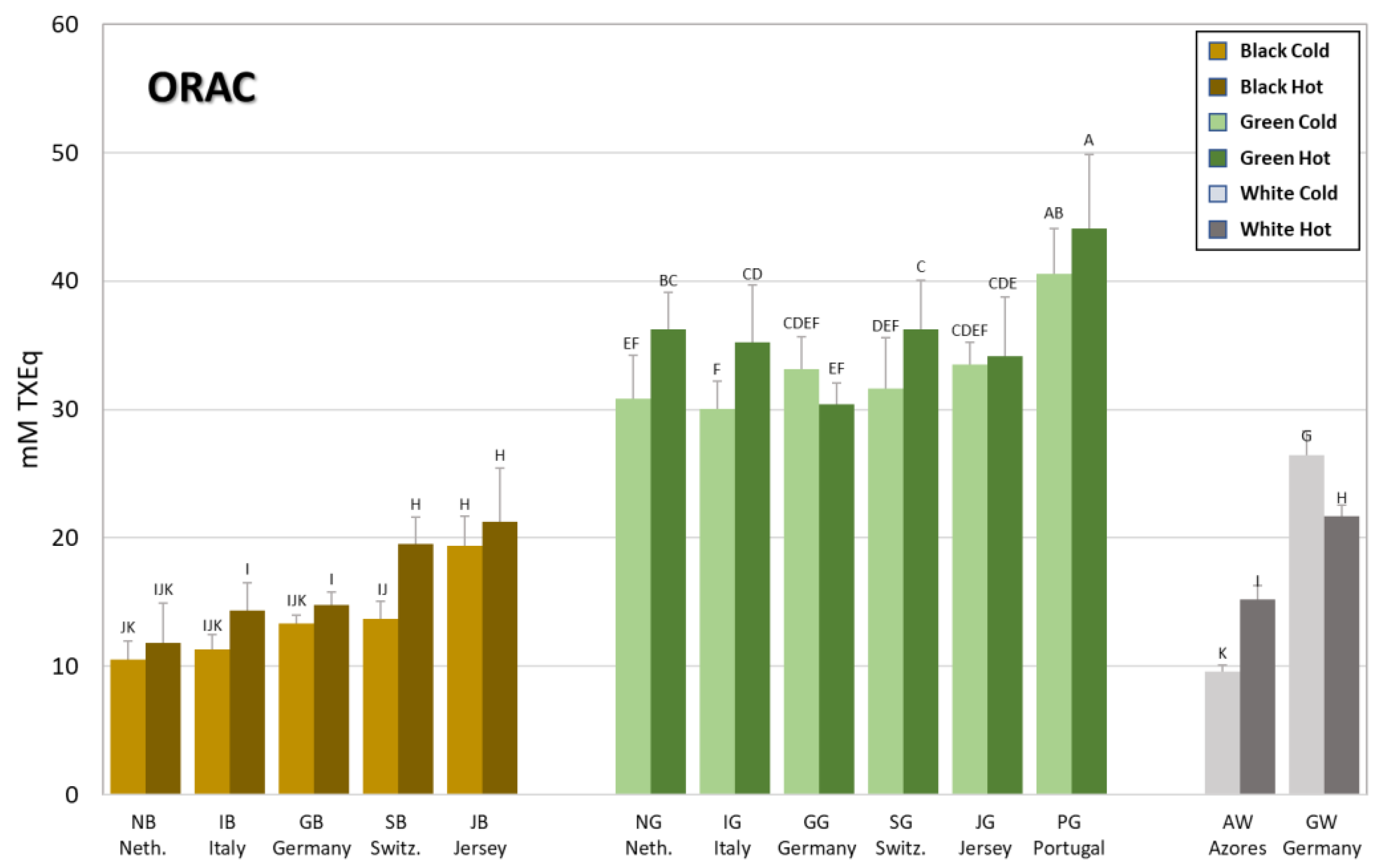
3.2. Antioxidant Capacity of Brews from Tea Grown in Europe
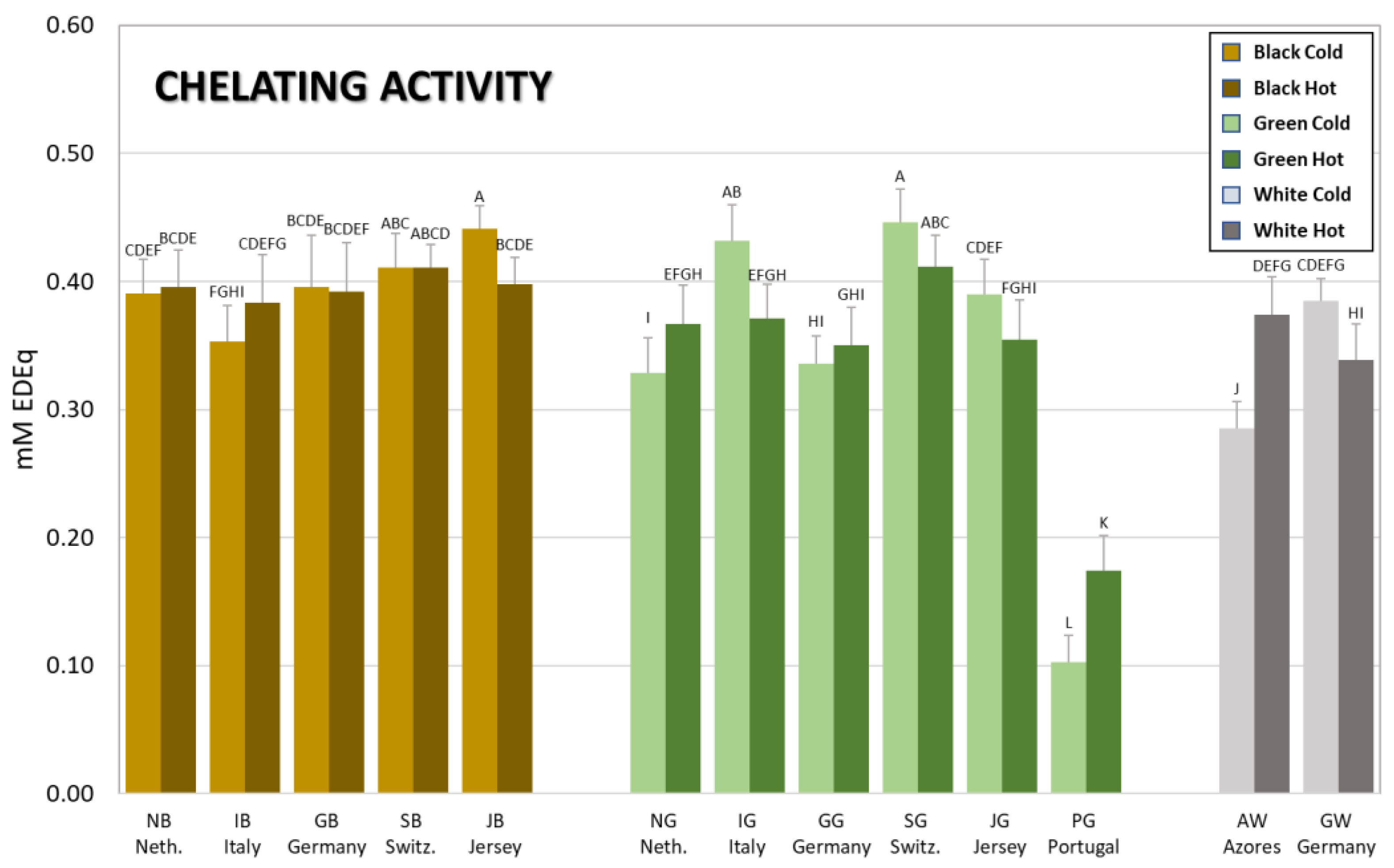
3.3. Metal Chelating Activity of Brews from Tea Grown in Europe
3.4. UV-Visible Spectral Characteristics of Brews from Tea Grown in Europe
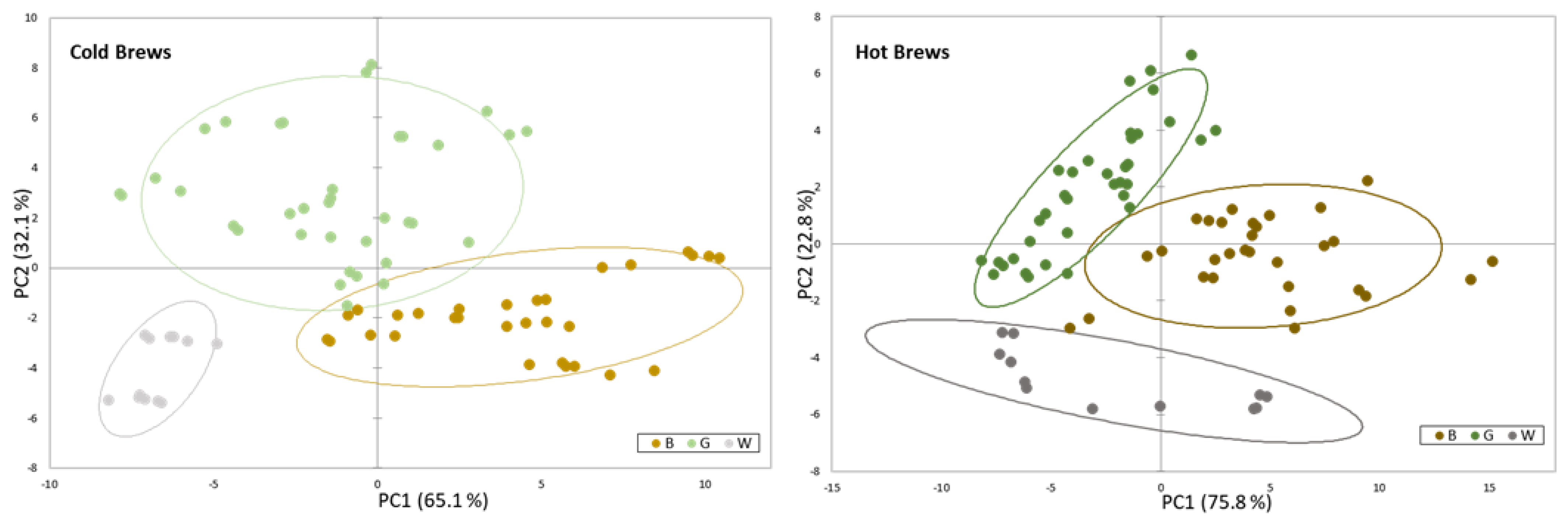
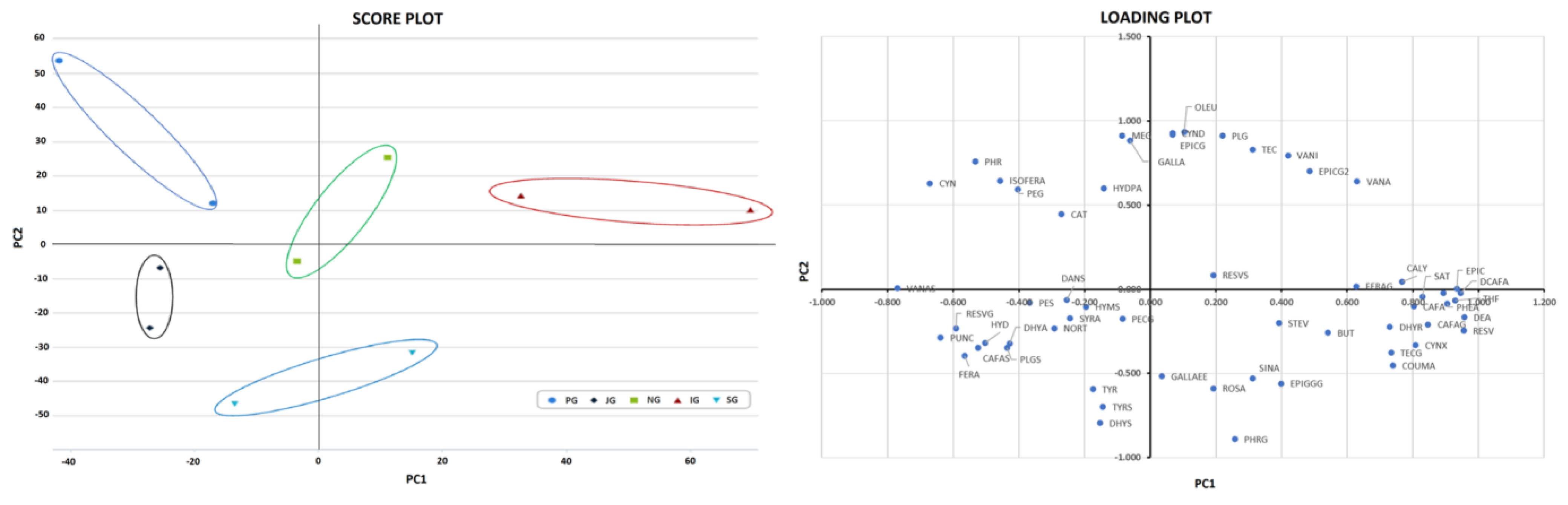
3.5. Metabolomics Analysis of Brews from Tea Grown in Europe
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saptadip, S. Potential bioactive components and health promotional benefits of tea (Camellia sinensis). J. Am. Coll. Nutr. 2020, 41, 65–93. [Google Scholar] [CrossRef]
- MordorIntelligence, Europe Tea Market Size & Share Analysis-Growth Trends & Forecasts (2023–2028). Available online: https://www.mordorintelligence.com/industry-reports/europe-tea-market (accessed on 25 May 2023).
- FAO. Intergovernmental Group on Tea (24th Session) 23 February 2022. Available online: https://www.fao.org/markets-and-trade/commodities/tea/teaigg24/en/ (accessed on 25 May 2023).
- de Melo, P.P.F. Breve historia de cultura do chà na Ilha de Sao Miguel. Acores Rev. Oriente 2012, 21, 40–49. [Google Scholar]
- Tea Grown in Europe Association. Available online: https://tea-grown-in-europe.eu (accessed on 25 May 2023).
- Wei, K.; Wang, L.; Zhou, J.; He, W.; Zeng, J.; Jiang, Y.; Cheng, H. Catechin contents in tea (Camellia sinensis L.) as affected by cultivar and environment and their relation to chlorophyll contents. Food Chem 2011, 125, 44–48. [Google Scholar] [CrossRef]
- Wong, M.; Sirisena, S.; Ng, K. Phytochemical profile of differently processed tea: A review. J. Food Sci. 2022, 87, 1925–1942. [Google Scholar] [CrossRef]
- Tea and Herbal Infusions Europe. Compendium of Guidelines for Tea (Camellia sinensis). Available online: https://thie-online.eu/files/thie/docs/2018-08-20_Compendium_of_Guidelines_for_Tea_ISSUE_5.pdf (accessed on 25 May 2023).
- ISO 3103:1980; Tea-Preparation of Liquor for Use in Sensory Tests. International Organization for Standardization: Geneva, Switzerland, 1980. Available online: https://iso.org/standard/8250.html (accessed on 25 May 2023).
- Damiani, E.; Carloni, P.; Rocchetti, G.; Senizza, B.; Tiano, L.; Joubert, E.; de Beer, D.; Lucini, L. Impact of Cold versus Hot Brewing on the Phenolic Profile and Antioxidant Capacity of Rooibos (Aspalathus linearis) Herbal Tea. Antioxidants 2019, 8, 499. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and anti-oxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Kim, D.-O.; Chun, O.K.; Kim, Y.J.; Moon, H.-Y.; Lee, C.Y. Quantification of Polyphenolics and Their Antioxidant Capacity in Fresh Plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Devaki, M. The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: Concepts, procedures, limitations and applications. In Measurement of Antioxidant Activity and Capacity: Trends and Applications; Apak, R., Capanoglu, E., Shahidi, F., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2018; pp. 77–106. [Google Scholar]
- Gillespie, K.M.; Ainsworth, E.A. Measurement of reduced, oxidized and total ascorbate content in plants. Nat. Protoc. 2007, 2, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Chua, M.-T.; Tung, Y.-T.; Chang, S.-T. Antioxidant activities of ethanolic extracts from the twigs of Cinnamomum osmophloeum. Bioresour. Technol. 2008, 99, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Stefano, M.; Astolfi, P.; Carloni, P. Chemometric approach to the analysis of antioxidant properties and colour of typical Italian monofloral honeys. Int. J. Food Sci. Technol. 2017, 52, 1138–1146. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Das, T.K.; Wati, M.R.; Fatima-Shad, K. Oxidative Stress Gated by Fenton and Haber Weiss Reactions and Its Association with Alzheimer’s Disease. Arch. Neurosci. 2014, 2, e20078. [Google Scholar] [CrossRef]
- Lakey-Beitia, J.; Burillo, A.M.; La Penna, G.; Hegde, M.L.; Rao, K. Polyphenols as Potential Metal Chelation Compounds Against Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 82, S335–S357. [Google Scholar] [CrossRef]
- Zhao, T.; Li, C.; Wang, S.; Song, X. Green Tea (Camellia sinensis): A Review of Its Phytochemistry, Pharmacology, and Toxicology. Molecules 2022, 27, 3909. [Google Scholar] [CrossRef] [PubMed]
- Carloni, P.; Tiano, L.; Padella, L.; Bacchetti, T.; Customu, C.; Kay, A.; Damiani, E. Antioxidant activity of white, green and black tea obtained from the same tea cultivar. Food Res. Int. 2013, 53, 900–908. [Google Scholar] [CrossRef]
- Chan, E.; Lim, Y.; Chew, Y. Antioxidant activity of Camellia sinensis leaves and tea from a lowland plantation in Malaysia. Food Chem. 2007, 102, 1214–1222. [Google Scholar] [CrossRef]
- Lin, S.-D.; Udompornmongkol, P.; Yang, J.-H.; Chen, S.Y. Quality and antioxidant properties of three types of tea infusions. J. Food Process. Preserv. 2014, 38, 1401–1408. [Google Scholar] [CrossRef]
- Nhu-Trang, T.-T.; Nguyen, Q.-D.; Cong-Hau, N.; Anh-Dao, L.-T.; Behra, P. Characteristics and Relationships between Total Polyphenol and Flavonoid Contents, Antioxidant Capacities, and the Content of Caffeine, Gallic Acid, and Major Catechins in Wild/Ancient and Cultivated Teas in Vietnam. Molecules 2023, 28, 3470. [Google Scholar] [CrossRef]
- Saito, S.T.; Gosmann, G.; Saffi, J.; Presser, M.; Richter, M.F.; Bergold, A.M. Characterization of the Constituents and Antioxidant Activity of Brazilian Green Tea (Camellia sinensis var. assamica IAC-259 Cultivar) Extracts. J. Agric. Food Chem. 2007, 55, 9409–9414. [Google Scholar] [CrossRef]
- Yao, L.; Caffin, N.; D’Arcy, B.; Jiang, Y.; Shi, J.; Singanusong, R.; Liu, X.; Datta, N.; Kakuda, Y.; Xu, Y. Seasonal Variations of Phenolic Compounds in Australia-Grown Tea (Camellia sinensis). J. Agric. Food Chem. 2005, 53, 6477–6483. [Google Scholar] [CrossRef]
- Zhao, C.-N.; Tang, G.-Y.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Liu, Q.; Mao, Q.-Q.; Shang, A.; Li, H.-B. Phenolic Profiles and Antioxidant Activities of 30 Tea Infusions from Green, Black, Oolong, White, Yellow and Dark Teas. Antioxidants 2019, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Baptista, J.; Lima, E.; Paiva, L.; Andrade, A.L.; Alves, M.G. Comparison of Azorean tea theanine to teas from other origins by HPLC/DAD/FD. Effect of fermentation, drying temperature, drying time and shoot maturity. Food Chem. 2012, 132, 2181–2187. [Google Scholar] [CrossRef]
- Baptista, J.A.B.; da P Tavares, J.F.; Carvalho, R.C.B. Comparative study and partial characterization of Azorean green tea polyphenols. J. Food Compos. Anal. 1999, 12, 273–287. [Google Scholar] [CrossRef]
- Barreira, S.; Moutinho, C.; Silva, A.M.N.; Neves, J.; Seo, E.-J.; Hegazy, M.-E.F.; Efferth, T.; Gomes, L.R. Phytochemical charac-terization and biological activities of green tea (Camellia sinensis) produced in the Azores, Portugal. Phytomed. Plus 2021, 1, 100001. [Google Scholar] [CrossRef]
- Paiva, L.; Rego, C.; Lima, E.; Marcone, M.; Baptista, J. Comparative Analysis of the Polyphenols, Caffeine, and Antioxidant Activities of Green Tea, White Tea, and Flowers from Azorean Camellia sinensis Varieties Affected by Different Harvested and Processing Conditions. Antioxidants 2021, 10, 183. [Google Scholar] [CrossRef]
- Robalo, J.; Lopes, M.; Cardoso, O.; Silva, A.S.; Ramos, F. Efficacy of Whey Protein Film Incorporated with Portuguese Green Tea (Camellia sinensis L.) Extract for the Preservation of Latin-Style Fresh Cheese. Foods 2022, 11, 1158. [Google Scholar] [CrossRef]
- Falla, N.M.; Demasi, S.; Caser, M.; Scariot, V. Phytochemical Profile and Antioxidant Properties of Italian Green Tea, a New High Quality Niche Product. Horticulturae 2021, 7, 91. [Google Scholar] [CrossRef]
- Girolametti, F.; Annibaldi, A.; Illuminati, S.; Damiani, E.; Carloni, P.; Truzzi, C. Essential and Potentially Toxic Elements (PTEs) Content in European Tea (Camellia sinensis) Leaves: Risk Assessment for Consumers. Molecules 2023, 28, 3802. [Google Scholar] [CrossRef]
- Liu, S.-L.; Jaw, Y.-M.; Wang, L.-F.; Chuang, G.C.-C.; Zhuang, Z.-Y.; Chen, Y.-S.; Liou, B.-K. Evaluation of Sensory Quality for Taiwanese Specialty Teas with Cold Infusion Using CATA and Temporal CATA by Taiwanese Consumers. Foods 2021, 10, 2344. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Damiani, E.; Astolfi, P.; Carloni, P. Influence of steeping conditions (time, temperature, and particle size) on antioxidant properties and sensory attributes of some white and green teas. Int. J. Food Sci. Nutr. 2015, 66, 491–497. [Google Scholar] [CrossRef]
- Lin, S.-D.; Yang, J.-H.; Hsieh, Y.-J.; Liu, E.-H.; Mau, J.-L. Effect of Different Brewing Methods on Quality of Green Tea. J. Food Process. Preserv. 2014, 38, 1234–1243. [Google Scholar] [CrossRef]
- Lin, S.-D.; Liu, E.-H.; Mau, J.-L. Effect of different brewing methods on antioxidant properties of steaming green tea. LWT Food Sci. Technol. 2008, 41, 1616–1623. [Google Scholar] [CrossRef]
- Pan, Y.; Qin, R.; Hou, M.; Xue, J.; Zhou, M.; Xu, L.; Zhang, Y. The interactions of polyphenols with Fe and their application in Fenton/Fenton-like reactions. Sep. Purif. Technol. 2022, 300, 121831. [Google Scholar] [CrossRef]
- Venditti, E.; Bacchetti, T.; Tiano, L.; Carloni, P.; Greci, L.; Damiani, E. Hot vs. cold water steeping of different teas: Do they affect antioxidant activity? Food Chem. 2010, 119, 1597–1604. [Google Scholar] [CrossRef]
- Damiani, E.; Bacchetti, T.; Padella, L.; Tiano, L.; Carloni, P. Antioxidant activity of different white teas: Comparison of hot and cold tea infusions. J. Food Compos. Anal. 2014, 33, 59–66. [Google Scholar] [CrossRef]
- Komes, D.; Belščak-Cvitanović, A.; Horžić, D.; Rusak, G.; Likić, S.; Berendika, M. Phenolic composition and antioxidant properties of some traditionally used medicinal plants affected by the extraction time and hydrolysis. Phytochem. Anal. 2010, 22, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Perez-Burillo, S.; Giménez, R.; Rufián-Henares, J.; Pastoriza, S. Effect of brewing time and temperature on antioxidant capacity and phenols of white tea: Relationship with sensory properties. Food Chem. 2018, 248, 111–118. [Google Scholar] [CrossRef]
- Coe, S.; Fraser, A.; Ryan, L. Polyphenol Bioaccessibility and Sugar Reducing Capacity of Black, Green, and White Teas. Int. J. Food Sci. 2013, 2013, 238216. [Google Scholar] [CrossRef]
- Rechner, A.; Wagner, E.; Van Buren, L.; Van De Put, F.; Wiseman, S.; Rice-Evans, C. Black tea represents a major source of dietary phenolics among regular tea drinkers. Free. Radic. Res. 2002, 36, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Morillo, A.; Alcázar, A.; de Pablos, F.; Jurado, J.M. Differentiation of tea varieties using UV–Vis spectra and pattern recognition techniques. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 103, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Lai, G.; Wen, M.; Han, Z.; Zhang, L. Identification of low-molecular-weight color contributors of black tea infusion by metabolomics analysis based on UV–visible spectroscopy and mass spectrometry. Food Chem. 2022, 386, 132788. [Google Scholar] [CrossRef]
- Diniz, P.H.G.D.; Barbosa, M.F.; De Melo Milanez, K.D.T.; Pistonesi, M.F.; de Araújo, M.C.U. Using UV–Vis spectroscopy for simultaneous geographical and varietal classification of tea infusions simulating a home-made tea cup. Food Chem. 2016, 192, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Qiu, Q.; Wang, Y.; Wang, Y.; Lu, Y.; Fan, D.; Wang, X. Rapid Identification of Different Grades of Huangshan Maofeng Tea Using Ultraviolet Spectrum and Color Difference. Molecules 2020, 25, 4665. [Google Scholar] [CrossRef]
- Farag, M.A.; Elmetwally, F.; Elghanam, R.; Kamal, N.; Hellal, K.; Hamezah, H.S.; Zhao, C.; Mediani, A. Metabolomics in tea products; a compile of applications for enhancing agricultural traits and quality control analysis of Camellia sinensis. Food Chem. 2023, 404, 134628. [Google Scholar] [CrossRef]



| European Garden | Country | Type | Label |
|---|---|---|---|
| Het Zuyderblad | The Netherlands | Black | NB |
| Green | NG | ||
| Compagnia del Lago Maggiore | Italy | Black | IB |
| Green | IG | ||
| Tschanara Teagarden | Germany | Black | GB |
| Green | GG | ||
| White | GW | ||
| Casa del Tè Monte Verità | Switzerland | Black | SB |
| Green | SG | ||
| Jersey Fine Tea | Jersey (UK) | Black | JB |
| Green | JG | ||
| Chà Camèlia | Portugal | Green | PG |
| Agrarian Devt. Services Sao Miguel | Azores (Portugal) | White | AW |
| Code | mM GAEq | mM CEq | mM TXEq | mM TXEq | mM AAEq | mM EDEq |
|---|---|---|---|---|---|---|
| Cold | ||||||
| NBC | 1.3 ± 0.1 M | 0.21 ± 0.02 N | 10.5 ± 1.4 JK | 5.3 ± 0.7 O | 2.4 ± 0.4 K | 0.39 ± 0.03 CDEF |
| IBC | 2.0 ± 0.2 LM | 0.32 ± 0.02 M | 11.3 ± 1.2 IJK | 7.1 ± 0.9 NO | 3.9 ± 0.6 J | 0.35 ± 0.03 FGHI |
| GBC | 2.4 ± 0.1 KL | 0.36 ± 0.02 LM | 13.3 ± 0.6 IJK | 7.7 ± 1.3 MN | 3.5 ± 0.6 JK | 0.40 ± 0.04 BCDE |
| SBC | 2.8 ± 0.3 JK | 0.40 ± 0.02 L | 13.7 ± 1.4 IJ | 9.4 ± 1.4 KLM | 5.3 ± 0.7 HI | 0.41 ± 0.03 ABC |
| JBC | 3.8 ± 0.4 HI | 0.59 ± 0.06 JK | 19.4 ± 2.3 H | 10.5 ± 1.1 KL | 6.3 ± 0.7 GH | 0.44 ± 0.02 A |
| black | 2.5 ± 0.9 z | 0.4 ± 0.1 z | 13.6 ± 3.5 y | 8.0 ± 2.0 z | 4.3 ± 1.5 z | 0.40 ± 0.03 x |
| NGC | 6.9 ± 0.4 F | 1.01 ± 0.09 H | 30.8 ± 3.4 EF | 18.4 ± 2.4 HI | 13.7 ± 1.7 E | 0.33 ± 0.03 I |
| IGC | 7.1 ± 0.5 F | 1.14 ± 0.07 FG | 30.0 ± 2.2 F | 19.0 ± 1.8 GHI | 14.0 ± 1.5 DE | 0.43 ± 0.03 AB |
| GGC | 8.9 ± 0.3 DE | 1.42 ± 0.12 C | 33.2 ± 2.5 CDEF | 21.8 ± 3.1 EF | 17.5 ± 1.8 B | 0.34 ± 0.02 HI |
| SGC | 8.9 ± 0.9 DE | 1.22 ± 0.11 EF | 31.6 ± 4.0 DEF | 22.4 ± 1.7 DEF | 18.0 ± 2.9 B | 0.45 ± 0.03 A |
| JGC | 8.7 ± 0.5 DE | 1.29 ± 0.09 DE | 33.5 ± 1.7 CDEF | 22.1 ± 2.4 EF | 16.1 ± 1.9 C | 0.39 ± 0.03 CDEF |
| PGC | 10.5 ± 0.8 B | 1.61 ± 0.09 B | 40.5 ± 3.6 AB | 26.2 ± 1.8 BC | 21.8 ± 2.4 A | 0.10 ± 0.02 L |
| green | 8.5 ± 1.3 xy | 1.3 ± 0.2 xy | 33.3 ± 3.8 x | 21.7 ± 2.8 xy | 16.9 ± 3.0 x | 0.34 ± 0.13 x |
| AWC | 1.6 ± 0.1 LM | 0.30 ± 0.02 M | 9.6 ± 0.5 K | 6.9 ± 0.7 NO | 4.0 ± 0.5 IJ | 0.29 ± 0.02 J |
| GWC | 7.1 ± 0.4 F | 1.10 ± 0.05 G | 26.5 ± 1.8 G | 20.6 ± 2.2 FG | 15.4 ± 2.0 CD | 0.38 ± 0.02 CDEFG |
| white | 4.4 ± 3.9 yz | 0.70 ± 0.56 yz | 18.03 ± 11.9 y | 13.74 ± 9.7 yz | 9.7 ± 8.1 xyz | 0.34 ± 0.07 x |
| Hot | ||||||
| NBH | 2.4 ± 0.6 KL | 0.32 ± 0.06 M | 11.8 ± 3.1 IJK | 7.2 ± 2.4 NO | 3.1 ± 0.6 JK | 0.40 ± 0.03 BCDE |
| IBH | 3.5 ± 0.6 IJ | 0.43 ± 0.07 L | 14.3 ± 2.2 I | 10.2 ± 1.1 KL | 4.3 ± 0.4 IJ | 0.38 ± 0.04 CDEFG |
| GBH | 3.7 ± 0.2 HI | 0.52 ± 0.04 K | 14.7 ± 1.1 I | 9.2 ± 0.7 LM | 4.4 ± 0.3 IJ | 0.39 ± 0.04 BCDEF |
| SBH | 4.8 ± 0.9 G | 0.63 ± 0.08 J | 19.5 ± 2.1 H | 13.6 ± 1.8 J | 6.7 ± 0.9 G | 0.41 ± 0.02 ABCD |
| JBH | 4.5 ± 0.3 G | 0.63 ± 0.04 J | 21.2 ± 4.2 H | 11.3 ± 0.7 K | 6.1 ± 0.4 GH | 0.40 ± 0.02 BCDE |
| black | 3.8 ± 1.0 z | 0.5 ± 0.13 z | 16.3 ± 3.9 y | 10.3 ± 2.4 z | 4.9 ± 1.5 z | 0.40 ± 0.01 x |
| NGH | 9.7 ± 0.9 C | 1.27 ± 0.09 DE | 36.2 ± 2.9 BC | 24.4 ± 1.8 CD | 15.6 ± 1.3 C | 0.37 ± 0.03 EFGH |
| IGH | 8.3 ± 0.8 E | 1.12 ± 0.10 G | 35.2 ± 4.5 CD | 20.4 ± 1.2 FGH | 13.4 ± 1.8 E | 0.37 ± 0.03 EFGH |
| GGH | 9.0 ± 0.7 D | 1.30 ± 0.09 D | 30.4 ± 1.7 EF | 22.2 ± 2.7 EF | 15.2 ± 1.1 CD | 0.35 ± 0.03 GHI |
| SGH | 10.9 ± 0.7 B | 1.43 ± 0.09 C | 36.2 ± 3.8 C | 26.9 ± 1.8 B | 18.8 ± 1.3 B | 0.41 ± 0.02 ABC |
| JGH | 9.4 ± 0.7 CD | 1.28 ± 0.10 DE | 34.1 ± 4.6 CDE | 23.7 ± 2.7 DE | 14.2 ± 1.5 DE | 0.35 ± 0.03 FGHI |
| PGH | 13.8 ± 1.1 A | 1.84 ± 0.11 A | 44.1 ± 5.7 A | 31.1 ± 3.7 A | 21.7 ± 2.1 A | 0.17 ± 0.03 K |
| green | 10.2 ± 2.0 x | 1.4 ± 0.25 xy | 36.0 ± 4.5 x | 24.8 ± 3.8 x | 16.5 ± 3.1 xy | 0.34 ± 0.08 x |
| AWH | 4.2 ± 0.2 GH | 0.64 ± 0.05 J | 15.2 ± 1.1 I | 10.9 ± 0.7 KL | 6.0 ± 0.6 GH | 0.37 ± 0.03 DEFG |
| GWH | 6.7 ± 0.5 F | 0.92 ± 0.06 I | 21.7 ± 0.9 H | 17.6 ± 1.2 I | 11.5 ± 1.0 F | 0.34 ± 0.03 HI |
| white | 5.5 ± 1.7 yz | 0.78 ± 0.19 yz | 18.44 ± 4.6 y | 14.27 ± 4.7 yz | 8.7 ± 3.9 xyz | 0.36 ± 0.02 x |
| (a) | TPC | TFC | ORAC | ABTS | FRAP | MCA | |
| TPC | 1 | 0.988 | 0.976 | 0.993 | 0.964 | −0.419 | |
| TFC | 0.988 | 1 | 0.977 | 0.987 | 0.982 | −0.449 | |
| ORAC | 0.976 | 0.977 | 1 | 0.975 | 0.961 | −0.409 | |
| ABTS | 0.993 | 0.987 | 0.975 | 1 | 0.977 | −0.403 | |
| FRAP | 0.964 | 0.982 | 0.961 | 0.977 | 1 | −0.439 | |
| MCA | −0.419 | −0.449 | −0.409 | −0.403 | −0.439 | 1 | |
| (b) | TPC | TFC | ORAC | ABTS | FRAP | MCA | |
| TPC | 0 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.033 | |
| TFC | <0.0001 | 0 | <0.0001 | <0.0001 | <0.0001 | 0.021 | |
| ORAC | <0.0001 | <0.0001 | 0 | <0.0001 | <0.0001 | 0.038 | |
| ABTS | <0.0001 | <0.0001 | <0.0001 | 0 | <0.0001 | 0.041 | |
| FRAP | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0 | 0.025 | |
| MCA | 0.033 | 0.021 | 0.038 | 0.041 | 0.025 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carloni, P.; Albacete, A.; Martínez-Melgarejo, P.A.; Girolametti, F.; Truzzi, C.; Damiani, E. Comparative Analysis of Hot and Cold Brews from Single-Estate Teas (Camellia sinensis) Grown across Europe: An Emerging Specialty Product. Antioxidants 2023, 12, 1306. https://doi.org/10.3390/antiox12061306
Carloni P, Albacete A, Martínez-Melgarejo PA, Girolametti F, Truzzi C, Damiani E. Comparative Analysis of Hot and Cold Brews from Single-Estate Teas (Camellia sinensis) Grown across Europe: An Emerging Specialty Product. Antioxidants. 2023; 12(6):1306. https://doi.org/10.3390/antiox12061306
Chicago/Turabian StyleCarloni, Patricia, Alfonso Albacete, Purificación A. Martínez-Melgarejo, Federico Girolametti, Cristina Truzzi, and Elisabetta Damiani. 2023. "Comparative Analysis of Hot and Cold Brews from Single-Estate Teas (Camellia sinensis) Grown across Europe: An Emerging Specialty Product" Antioxidants 12, no. 6: 1306. https://doi.org/10.3390/antiox12061306
APA StyleCarloni, P., Albacete, A., Martínez-Melgarejo, P. A., Girolametti, F., Truzzi, C., & Damiani, E. (2023). Comparative Analysis of Hot and Cold Brews from Single-Estate Teas (Camellia sinensis) Grown across Europe: An Emerging Specialty Product. Antioxidants, 12(6), 1306. https://doi.org/10.3390/antiox12061306













