Abstract
Hepatocellular carcinoma (HCC) represents a worryingly increasing cause of malignancy-related mortality, while Metabolic Associated Fatty Liver Disease (MAFLD) is going to become its most common cause in the next decade. Understanding the complex underlying pathophysiology of MAFLD-related HCC can provide opportunities for successful targeted therapies. Of particular interest in this sequela of hepatopathology is cellular senescence, a complex process characterised by cellular cycle arrest initiated by a variety of endogenous and exogenous cell stressors. A key biological process in establishing and maintaining senescence is oxidative stress, which is present in multiple cellular compartments of steatotic hepatocytes. Oxidative stress-induced cellular senescence can change hepatocyte function and metabolism, and alter, in a paracrine manner, the hepatic microenvironment, enabling disease progression from simple steatosis to inflammation and fibrosis, as well as HCC. The duration of senescence and the cell types it affects can tilt the scale from a tumour-protective self-restricting phenotype to the creator of an oncogenic hepatic milieu. A deeper understanding of the mechanism of the disease can guide the selection of the most appropriate senotherapeutic agent, as well as the optimal timing and cell type targeting for effectively combating HCC.
1. Introduction
According to the latest GLOBOCAN report, primary liver malignancies comprised 4.7% of cases of cancer in 2020, but they were also the fourth-leading cause of cancer-related deaths (responsible for 8.3% of total cancer deaths). This suggests that primary liver malignancies are “silent” killers [1]. It is well-established that hepatocellular carcinoma (HCC) is the most frequent primary hepatic carcinoma, representing up to 80% of hepatic malignancies [2]. In comparison to other types of cancer that show slowly declining rates, HCC is steadily on the rise for multiple reasons. While sufficient effort has been put towards establishing effective measures to tackle the rising incidence of HCC, such as rigorous Hepatitis B Virus (HBV) vaccination programmes and wide adoption of Direct-Antiviral Agents (DAA) for Hepatitis C Virus (HCV), much can still be achieved in the primary disease prevention section [2].
As evident, the rising numbers in HCC can be partly attributed to an increase in fatty liver disease linked with Metabolic Syndrome (MS) [2]. The gradual adoption of a sedentary lifestyle with a high-fat, high-sugar, low-fibre diet in the last few decades has led to a marked increase in Metabolic Syndrome (MS), an umbrella diagnosis indicative of metabolic dysregulation and insulin resistance, linked with Cardiovascular Disease (CVD), Diabetes Mellitus (DM), and carcinogenesis [3].
Fatty infiltration and fibrosis of the liver were observed for the first time by Ludwig et al. in a cohort of patients with a high prevalence of obesity, DM, and normal alcohol consumption, leading to the creation of the term Non-Alcoholic Steatohepatitis (NASH) [4]. This histopathological phenotype evolved into a more easily recognisable clinicopathological entity, Non-Alcoholic Fatty Liver Disease (NAFLD), which was defined by clinical exclusion criteria and solid pathological evidence [5], linked to MS and, subsequently, the sequence of liver steatosis–fibrosis–hepatocarcinogenesis [6]. A recent expert consensus has come to emphasise the pathophysiological importance of metabolic dysregulation and highlight the interaction between MS and hepatic steatogenesis, the initial event in a sequela leading to HCC [7]. In addition to that, the new term, Metabolic-Associated Fatty Liver Disease (MAFLD), offers a definition of the disease that takes into account new diagnostic modalities and facilitates research as well as patient identification [7,8]. The increasing burden of chronic liver disease and HCC attributable to NAFLD/MAFLD is evident in multiple cohorts, especially after the introduction of DAA [9,10,11].
The pathophysiology of hepatocarcinogenesis in the context of MAFLD is not yet fully understood and remains a field of intense research with significant progress over the last few years. Understanding the clinical characteristics of these patients, as well as the pathological features of these tumours, potentially holds the key to deepening our understanding of the intricate mechanisms of disease. A recent meta-analysis showed that when compared to other aetiology HCC, MAFLD–HCC patients are significantly older, have higher Body Mass Index, are more likely to have cardiovascular comorbidities, and are less likely to be cirrhotic [6,12]. When examining tumour characteristics, MALFD patients had higher chances of presenting with uninodular lesions and larger tumour diameter [6,12].
In some high-incidence countries, rigorous surveillance programmes have been introduced and have been proven successful in detecting early disease, amenable to curative modalities of treatment, leading to a notable reduction in disease burden and increased survival rates for patients [2]. However, this is not the case for MAFLD-related HCC, where lower rates of surveillance were reported in comparison to other causes of HCC [12]; nevertheless, that did not result in any significant differences in allocation to curative modalities of treatment versus palliative care [12]. Interestingly, MAFLD-derived HCC patients were less likely to receive a liver transplant because of their multiple medical comorbidities and better-preserved liver function, compared to their hepatitis counterparts [12]. All the above-mentioned facts draw a picture of the natural history of the disease and generate questions about the mechanisms implicated in disease progression.
There is a rapidly growing body of literature regarding the factors that trigger and the mechanisms involved in MAFLD to HCC progression. Genetic [13,14] and epigenetic factors initiate a constellation of inter- and intracellular mechanisms, amongst which studies have identified autophagy [15,16], ferroptosis [17], gut microbiota [18], lipotoxicity [19], oxidative stress (OS) [20], and cellular senescence (CS) [21]. The last two biological processes, as well as their interplay, seem to hold a key role in the initiation and propagation of fatty liver disease and its sequelae, by harbouring a hostile carcinogenic hepatic microenvironment mediated by different types of CS in the non-linear MAFLD–MASH–HCC sequence of events. The aim of this review is to investigate in depth the role of oxidative stress and cellular senescence, as well as their interaction, in the broad spectrum of MAFLD and its related hepatocarcinogenesis.
2. Oxidative Stress-Induced Cellular Senescence in the MAFLD Spectrum: Connected?
2.1. Oxidative Stress and Liver Metabolic Disease
2.1.1. The Oxygen Paradox
The appearance of molecular oxygen (O2) in the earth’s atmosphere approximately 2.1–2.4 billion years ago led to a change in biodiversity and evolutionary adaptations in living organisms’ metabolism, switching from anaerobic to aerobic [22]. For all aerobic organisms, O2 is a key molecule in oxidative energy production, acting as the terminal acceptor of electrons in the final step of the mitochondrial electron transport chain [23]. However, its chemical properties allow for the generation of intermediate by-products of O2 metabolism with oxidizing capacity themselves, which can negatively affect cells and, by extension, organisms [23]. Thus, an inconvenient paradox is revealed; O2 is indispensable for the survival and growth of aerobic organisms while, in parallel, it has deleterious effects on them [24].
2.1.2. ROS in Various Biological Roles
The constellation of molecules that are produced via the incomplete reduction of O2 are collectively known as reactive oxygen species (ROS). ROS is a collective term that includes free radicals (species that contain at least one unpaired valency electron), but also some non-radical derivatives of O2. “Reactive“ is a relative term, as these molecules display varying oxidative capacity [25]. On one side of the spectrum, molecules such as the superoxide anion (O2•−) and hydrogen peroxide (H2O2) are relatively unreactive. On the other side of the spectrum, species such as the hydroxyl radical (•OH), which is generated via the non-enzymatic oxidation of H2O2, are extremely reactive and can oxidise in a non-discrete manner any biological macromolecule in its close vicinity [23,25].
Mitochondria are a major source of intracellular ROS. Within mitochondria, O2•− which is produced via the one-electron reduction of O2, is usually the first ROS that is formed. Even though not a strong oxidant, O2•− can subsequently produce other downstream ROS. Important sources of endogenous ROS generation are the transmembrane NADPH oxidases. This family of NADPH oxidases includes multiple transmembrane proteins that transport electrons across membranes, using NADPH to allow for the reduction of oxygen to O2•−. The best-described family member is NADPH oxidase 2 (Nox2), abundant in neutrophils and macrophages, yielding high amounts of ROS that serve for chemotaxis of other phagocytes and dramatic increases in local oxygen consumption, known as the “respiratory” burst [23,26]. The rest Nox enzymes produce less significant amounts of ROS that serve for signalling purposes. Other sites that contribute significantly to the cellular ROS pool are the peroxisomes and the endoplasmic reticulum [23,27].
Relative unreactive ROS, with H2O2 being a prominent example, has been shown to play key roles, directly or indirectly, in redox sensing (a specific molecule sensing the reductive-oxidative state within a cellular component) or redox signalling [25]. A key property of H2O2 that renders it a suitable molecule for redox signalling is its selective increase of reactivity when encountered with cysteine residues in specific proteins. This means that it can diffuse within a certain distance in a cell without reacting with its surrounding molecules, and then when encountered with these specific cysteine residues, it leads to structural changes of intermediate molecules and activation of signalling transduction pathways [25].
A characteristic example of cysteine-based structural changes linked to redox sensing is the Nrf2/KEAP1 system. Nrf2 (Nuclear factor E2 related factor 2) is a transcription factor that enables the expression of multiple antioxidant enzymes. Keap 1 (Kelch-like ECH-associated protein 1) mediated ubiquitination of Nrf2 under unstressed conditions controls the expression of Nrf2 target genes. However, OS-mediated cysteine residue modifications in Keap1 render it unable to mediate Nrf2 ubiquitination, leading to antioxidant enzymes gene expression [25].
2.1.3. ROS Regulation and Removal—Oxidative Eustress and Distress
During their evolutionary course, eukaryotic cells have developed fine-tuned defence mechanisms that can rapidly eliminate the continuously generated O2-derived species [28]. A complex and heterogenous assortment of antioxidant enzymes, synthesized by all known aerobic organisms, provide a first line of oxidative defence. As such, superoxide dismutase (SOD) catalyses the dismutation of O2•− into O2 and H2O2, while the latter can be safely reduced to H2O by catalases (Cat), glutathione peroxidases (Gpx), and peroxiredoxins (Prx) [25,29].
Thus, for normal cellular function to be preserved, a fine equilibrium between the production and elimination of oxidative species has to be maintained. In modern literature, a shift is observed from the classical term “oxidative stress” to two newly described, dynamically evolving states, oxidative “eustress” and “distress” [30]. While oxidative stress reflects an excess of ROS and its consequent macromolecular damage, it overlooks that low steady-state ROS levels regulate several normal physiological functions. The introduction of the terms eustress and distress serves this exact purpose; to clarify that ROS may have both beneficial and harmful effects. Thus, at low physiological levels, ROS plays various physiological roles, and this state is defined as oxidative eustress. Yet, an abundance of ROS is linked with macromolecular damage and cellular dysfunction, and this state is defined as oxidative distress [25,30].
The ability of certain ROS to alter macromolecular structures within a cell or cellular compartment, causing structural damage and subsequent dysfunction, was described by Harman in 1956 [31]. In 1972 he expanded his theory: free radicals that are mainly generated in mitochondria as by-products of aerobic metabolism cause oxidative damage. The accumulated oxidative damage to essential macromolecules contributes to aging; the mitochondrial free radical theory of ageing was later renamed oxidative stress theory (OST) [30,32]. The long-term accumulation of faulty macromolecules that cells cannot dispose of leads to disruptions in signalling pathways, as well as a self-perpetuating cycle of metabolic dysregulation and OS, resulting inadvertently in cellular ageing [33,34].
2.1.4. The Role of Iron in OS
An element that is often implicated in redox reactions and oxidative stress regulation is iron. In the human organism, ferrous (Fe2+) or ferric (Fe3+) iron, either by participating in macromolecular configurations or freely available in small quantities, facilitates a diverse set of cellular functions such as oxygen transport in haemoglobin and myoglobin, cellular respiration, enzymatic reactions, and nucleotide metabolism. The involvement of iron in oxidative stress is classically illustrated in the Fenton reaction, where labile iron, which is normally freely available in the cell in minuscule amounts, catalyses the conversion of H2O2, a relatively weak ROS, to the •OH. As previously mentioned, the •OH is extremely reactive and can oxidise any macromolecule in its vicinity, leading to temporary—dependent on the available repair mechanisms—or permanent structural and functional damage [22,35].
Furthermore, iron is indirectly implicated in H2O2-mediated signalling. This link is particularly demonstrated when iron chelation or depletion impacts adhesion molecule expression or transcriptional pathways modulation of lipopolysaccharide exposure response, linking iron with OS and inflammatory response [22]. Labile iron modulation, either by iron-chelating drugs or by diet-derived iron-chelating compounds, was shown to play a key role in preventing H2O2-mediated apoptosis, and OS and iron metabolism are interconnected and tightly regulated [36,37]. In MAFLD, the dysmetabolic iron overload syndrome, a deranged iron metabolism phenotype, mostly evidenced as hepatocellular or reticuloendothelial iron overload, creates one of the prerequisites for redox imbalance and OS in the context of the disease [6].
2.1.5. OS in Liver Metabolic Disease—The Concept of Lipotoxicity
For a deeper understanding of the role of OS in MS, one must consider the fact that some types of non-adipose cells, such as hepatocytes, β-cells, myocytes, and podocytes, display a certain capacity of storing lipids in their cytoplasm. Oversaturation of these cell types with lipids and their derivatives leads to a generalised metabolic dysfunction with multiple sequelae, termed lipotoxicity [19]. In the case of MAFLD, the hepatocyte is the main site of lipotoxicity, and while the lipotoxic effects of fatty acids are mediated by multiple biological processes, of particular interest is OS.
The connection between OS and lipotoxicity can be traced to lipid peroxidation. Under OS conditions, polyunsaturated fatty acids can undergo peroxidation. The enzymatic pathway of lipid peroxidation is mediated by different enzymes, amongst which prominent is the role of iron-containing lipoxygenases (LOX), which produce lipid peroxides (LOOH) [38]. Glutathione peroxidase 4 (Gxp4) is the key antioxidant enzyme in neutralising these lipid peroxides [39].
However, polyunsaturated fatty acids can also undergo non-enzymatic iron-mediated oxidation, the highly reactive end-products of which are lipid alkoxy free radicals (LO•) [38,39]. Their oxidative potential is such that they can create a local self-sustaining milieu of lipid peroxidation, which in its turn, when interacting with other molecules, can lead to neo-epitopes, known as oxidation-specific epitopes, that activate the innate immune system, propagating OS and inflammation and perpetuating chronic degenerative diseases, such as atheromatosis [40].
An interesting biological process that could be involved in MAFLD pathogenesis is ferroptosis. Ferroptosis, a recently recognised type of regulated cell death, is elicited by the iron-dependent uncontrolled lipid peroxidation of polyunsaturated fatty acids to lethal levels when Gxp4 is disabled secondary to glutathione depletion. As MAFLD is characterised by antioxidant depletion, iron metabolism dysregulation, and a rich free fatty acid cellular environment, it comes as no surprise that ferroptosis has been shown to contribute to the progression of simple steatosis to steatohepatitis both in vitro and in vivo [41,42,43].
When looking outside the hepatocyte, another aspect of MAFLD that implicates OS in its pathogenesis and disease progression is the altered gut microbiota. The changes in gut microbiota in MAFLD patients are attributed to the Western-type diet and insulin resistance. The resultant qualitative and quantitative changes in gut flora create an environment of dysbiosis that leads to an increased baseline inflammatory response of the resident immune cells, such as the hepatic Kupffer cells, which in turn show phenotypes of increased ROS release [44].
2.2. Cellular Senescence
2.2.1. Overview of CS
CS, an irreversible state during which cells lose their ability to proliferate, is triggered by several endogenous and exogenous stimuli, such as aging, ROS, oncogene expression, and radiation [45]. CS is a true example of the principle of antagonistic pleiotropy; when in younger individuals and precancerous or early-stage cancer, it serves as a tumour suppressive mechanism, whereas in older individuals or established cancer, its unique properties favour cancer cell proliferation.
There are two main mechanisms of CS, the replicative CS, which is induced normally under aging conditions, where shortening of telomeres leads inevitably to cell cycle arrest, and the stress-induced premature CS, where external or internal factors cause DNA damage, thereby inducing CS pathways independently of telomere length [46]. Both mechanisms activate the same pathway, known as DNA damage response (DDR), which recruits Ataxia Telangiectasia Mutated (ATM) protein kinase to activate its target protein Checkpoint Kinase 2 (CHK2) [47,48]. Subsequently, ATM and CHK2 stabilise p53 resulting in the upregulation of its target protein p21 [48,49]. Both p53 and p21 inhibit the phosphorylation of the Retinoblastoma 1 factor (RB1), which can now bind to the E2F transcription factor, subsequently preventing cell cycle progression [48].
Another well-described molecular pathway leading to CS is the one mediated by p16, encoded by the CKDN2A locus. p16 serves as another proliferation inhibitor, which interferes with the action of Cyclin-dependent kinase (CDK)-4/6 on the Rb proteins [45].
These two major CS pathways can be simultaneously activated depending on the cause of DNA damage and the cell type. The belated activation of p16 after the p53/p21 pathway may signify the transition from an early, potentially reversible cell cycle arrest status to an irreversible one [50]. Despite the above two being the best-described mechanisms of CS, the list of molecular mechanisms that participate in the initiation and propagation of CS is still expanding.
Apart from activating p53 and p16 to promote cell cycle arrest, DDR is also a well-known activator of senescence-associated secretory phenotype (SASP), thus senescent cells, despite entering a cell proliferation arrest, remain metabolically active and are able to communicate with their microenvironment [48]. SASP is composed of several cytokines secreted from senescent cells, such as interleukins (IL-1b, IL-6, IL-8, ROS, growth factors, such as hepatocyte growth factor (HGF), and proteases, such as Matrix Metalloproteinases (MMPs) [51,52]. SASP serves as a regulator of the cellular microenvironment, with autocrine and paracrine actions, by promoting immunological removal of senescent cells, reinforcing senescence in neighbouring cells, and paradoxically acquiring pro-tumorigenic properties [53] depending on the stimulus that initially triggered CS [54].
2.2.2. CS and Molecular Players
Although the detection and study of senescent cells are based on several morphological and biochemical features, none of them is completely indicative of CS; thus, a combination of characteristics is typically used to identify senescent cells [55].
Despite the fact that several phenotypical changes have been observed in senescent cells, such as an enlarged and flattened shape, reorganisation of chromatin, and changes in the nuclear membrane [56], these morphological features are not sufficient to detect CS. To that end, numerous studies have underlined specific biomarkers for the identification of senescent cells.
Proteins playing a crucial role in the induction of cell cycle arrest, such as p53/p21 and p16, are common senescence biomarkers [55]. Furthermore, CS is often characterised by increased lysosomal activity, which can be detected by specific enzymes, such as senescence-associated β-galactosidase (SA-β-Gal), which constitutes a widely known senescence biomarker [57]. Moreover, another established CS biomarker is lipofuscin, a yellowish-brown residue known as “age pigment”, consisting of incompletely degraded oxidized molecules due to increased lysosomal content [58]. Interestingly, it has been demonstrated that SA-β-Gal co-localizes with lipofuscin; thus, these two molecules can be used alternatively to detect CS [59]. An additional CS biomarker is the senescence-associated heterochromatin foci (SAHF), a group of heterochromatin and proteins that suppress the expression of proliferation genes [60]. Some studies have also investigated the detection of SASP compounds, mainly associated with pro-inflammatory features of senescent cells; however, confirming CS based on these factors is still a matter of debate [55].
2.3. MAFLD Specific Considerations
2.3.1. CS in MAFLD
There is substantial evidence indicating that CS, especially of the hepatocytes, may foster fat accumulation and inflammation in different stages of MAFLD [58]. In this context, numerous studies have indulged in the role of CS in MAFLD progression, with major CS phenotypical characteristics, such as shorter telomeres and elevated CS biomarkers, such as p53 and SAHF, being observed in patients with MAFLD [61,62].
An increased prevalence of MAFLD in older patients has set the context for further investigation of the role of CS in liver fat accumulation and propagation of the disease [63]. However, as mentioned above, induction of CS is not only mediated by aging and telomere shortening, but also under stressful stimuli provoking DNA damage.
Preclinical studies in animal models have pointed out a strong correlation between CS and mitochondrial dysfunction, a key parameter of metabolic dysregulation at the early stages of liver steatosis [64]. Moreover, mitochondrial dysfunction results in excessive production of ROS, thereby suggesting a relation between oxidative stress and induction of CS [65]. In animal models of diet-induced MAFLD, propagation of the disease has been shown to progress slower in p53-deficient mice, thus pointing out that the induction of CS favours MAFLD development [60,66]. Intriguingly, elevated mRNA levels of p21 and p16, as well as decreased levels of p53, have been observed in animal models of MAFLD [60]. Furthermore, one of the key characteristics of the transition from early to advanced stages of MAFLD is the inflammation mediated by the recruitment of macrophages in the liver [66]. In this context, CS has been proposed as a crucial regulator through the secretion of chemokines and cytokines of the SASP secretome, such as IL-6, transforming growth factor-beta (TGF-β), which have been found elevated in mice models [67].
Results from human clinical studies further confirm the hypothesis that CS is strongly associated with MAFLD. The length of telomeres has been found to be shorter in patients with MAFLD compared to healthy ones [68]. Furthermore, MAFLD hepatocytes have been found to express high levels of major CS biomarkers, such as p21, and present senescent-associated morphological features, such as larger nuclei. Interestingly, the increased expression of p21 in hepatocytes has been with the degree of fibrosis, irrespective of telomere length, suggesting a premature rather than replicative type of CS [68]. In addition, mutations in the genes coding for the p21 protein have been shown to affect the initial stages of steatosis, but they did not seem to alter the progression of the disease after the establishment of fibrosis, suggesting potentially different pathophysiological mechanisms underlying each stage [69].
Given the complexity of hepatic architecture and function, as well as the broad phenotypic spectrum of MAFLD, one should consider how CS of other cell types helps establish MAFLD. The main regulator of CS in the hepatic microenvironment is hepatocyte-derived SASP, which depending on the duration of the pathological stimulus, can display a vast array of paracrine functions, activating, recruiting, or rendering senescent, adjacent, and distant cells.
The first cellular type to look into are hepatic stellate cells (HSCs), composite myofibroblast precursors, which during normal liver function, remain in a quiescent state. When a liver injury of any aetiology, including hepatocyte lipotoxicity, is sustained, HSCs enter an activated state, causing liver fibrosis [70]. A recent “proof of concept” study by Wijayasiri et al. showed that hepatocyte CS in MAFLD, evidenced by p16 expression in human MAFLD biopsies with various degrees of fibrosis, is correlated with HSC activation, shown with α-Smooth Muscle Actin (α-SMA), in a positive manner [71]. Furthermore, HSC cultivated in media where senescent HepG2 cells had previously developed showed markers of activation. Interestingly, the only SASP component identified to play a role in HSC activation was the Platelet-Derived Growth Factor (PDGF), both in cultures and cirrhotic patient serum samples [71].
When entering a senescent state, HSCs cease their fibrotic activity and generate a SASP characterised by matrix metalloproteinases, leading to fibrosis restriction and resolution [70,72]. Such an example is stellate cell CS mediated by Insulin Growth Factor–I (IGF–I). Reversal of insulin resistance is linked with higher IGF–I, which induces activated HSC senescence using the p53 pathway both in vivo and in vitro [73,74]. In p53 knockout mice, activated HSC cannot enter CS, with a continuous liver fibrotic phenotype. Interestingly, apart from attenuating fibrosis, another crucial element of CS was observed in this study, immunosurveillance, as senescent HSCs were cleared by NK cells [75]. Last, IL-22, via STAT3, was shown to activate p53/p21 CS in HSC, leading to fibrosis attenuation [76].
Another significant player in this biological process is hepatic macrophages, consisting of two broad categories: Kupffer cells, which are tissue-resident macrophages, and monocyte-derived macrophages [70]. As shown by Irvine et al., OS-induced hepatocyte CS triggers the generation of a pro-inflammatory SASP, including amongst many IL-6 and IL-8, and when macrophages were cultured in a senescent HepG2 medium, inflammatory-type macrophages showed migration activity [77]. Activated macrophages create the inflammatory milieu of NASH. One of the inflammatory cytokines secreted by these activated macrophages, IL-17, has been shown to activate HSCs in a TGF-β1 dependent manner, promoting hepatic fibrosis [78].
2.3.2. CS and OS in MAFLD
The intertwined roles of OS and CS in extrahepatic MAFLD pathogenesis could not be better demonstrated than in the murine model of Sriram et al. Visceral adipose tissue-derived stem cells display high OS characteristics, with intense Nox activity linked to early CS phenotype, attenuated by ascorbic acid treatment, a potent antioxidant [79]. Knowing the well-established connection between visceral adipose tissue and fatty liver disease, the above study shows the importance of extrahepatic CS in MAFLD [80].
In another murine mode developed by Keshavjee et al., reduced protein intake in pregnant mice led to higher amounts of visceral adiposity, MAFLD, and insulin resistance in adult male mice offspring [81]. This MS phenotype was only observed in male individuals and was linked with a variety of premature senescence markers, such as p21 and p16, in the context of a high OS environment (high superoxide anion levels, DNA damages, decreased Cu/Zn SOD, increased catalase protein expression, increased nfr2 and decreased keap1 mRNA expression) [81]. Furthermore, Kondo et al. developed another mouse model that shows SMP-30/SOD1 double knockout individuals developed OS-related MAFLD, possibly secondary to OS-impaired VLDL (very low-density lipoprotein) transport [82]. Ogrodnik and colleagues [83] showed that hepatocyte CS induction is a key event in initiating fat accumulation and correlated it with mitochondrial-derived OS, demonstrating a known observation of Passos et al. [84] in fibroblasts, to be confirmed in hepatocytes. Their key difference is that while Passos showed a p21-dependent CS pathway activation linked to OS, Ogrodnik’s team deployed senolytics to demonstrate a p16 related CS pathway.
Lohr and colleagues also used a C57BL/6J mouse model to study the effects of a high-fat diet and age on MAFLD development. They analysed hepatic mitochondria and concluded that susceptibility to diet-induced obesity and fatty liver increased with age. This also correlated with an age-related reduction in mitochondrial mass and was aggravated by impaired fatty acid oxidation in high-fat diet mice [85].
Kondo et al. examined Senescence Marker Protein–30 (SMP-30)—an antioxidant and anti-apoptotic protein that declines with increasing age—and observed that when comparing standard fat diet-fed mice with SMP-30 knockout to normal counterparts, the former developed fatty liver with inflammatory cells infiltrates and increased oxidative stress linked with impaired fatty acid oxidation and lipoprotein uptake [86]. However, human hepatic biopsy studies of SMP-30 failed to draw conclusive results on the exact role of this ageing-related protein in MAFLD, despite the clear correlation between its expression and disease occurrence [87].
2.3.3. Mitochondrial Dysfunction as a Link between OS and CS in MAFLD
Given the intense oxidative activity that normally takes place within the mitochondria, when the hepatocyte is oversaturated with fatty acids, as in the case of MAFLD, initially, β-oxidation is accelerated [88,89]. When β-oxidation is intensified, mitochondrial ROS production increases dramatically. Fatty acids in the inner mitochondrial membrane are rapidly turned into lipid peroxides, intensifying the OS milieu and leading to macromolecular impairment (mitochondrial DNA, lipids, and proteins) [90]. This functional impairment, in turn, deteriorates the already oversaturated β-oxidation system, leading to further OS deterioration, oxidative phosphorylation deficiency, and mitochondrial dysfunction-driven CS [83]. A key player in mitochondrial dysfunction appears to be acyl-CoA:lysocardiolipin acyltransferase 1 (ALCAT1), an enzyme responsible for cardiolipin—a key structural mitochondrial membrane phospholipid–remodelling, activated under conditions of lipotoxicity and OS, whose detrimental impacts to the mitochondrial structure are mediated by mitofusin 2 (MFN2) downregulation [90,91].
As the disease progresses from simple steatosis to NASH, the repetition of this vicious lipotoxic circle leads to structural mitochondrial defects, with mitochondrial oedema and loss of the typical inner membrane morphology [92]. NASH deterioration is directly linked to impaired mitophagy, the process of removal of defective mitochondria [93,94]. An important link has been observed between impaired mitophagy and CS, as p53 blocks Parkin from translocating defective mitochondria, resulting in their accumulation and intensifying OS [95]. A shared mechanism potentially linking mitochondrial dysfunction and CS is that of the cyclic GMP-AMP synthase and stimulator of interferon genes (cGAS-STING) pathway, as it serves as a SASP activator in CS [96], and can be activated by free mitochondrial DNA leading to an inflammatory phenotype [93].
Furthermore, mitochondrial damage and increased cell-free mitochondrial DNA have been shown to act as damage associate molecular patterns, activating several inflammatory pathways, amongst which is the NLRP3 inflammasome, leading to IL-1β, a critical SASP component [93]. On the other hand, Wiley et al. showed a distinct mitochondrial dysfunction associated with CS phenotype that is activated through a NAD–AMPK–p53 pathway and results in SASP production without IL-1, implicating mitochondrial sirtuins as culprits in this event sequelae [97].
In total, all the above show a clear connection between OS-induced CS and the initiation and progression of MAFLD to NASH and fibrosis. High-fat hepatocyte concentrations, both dietary and from de novo lipogenesis—propagated by insulin resistance—impair hepatic lipid metabolism and generate high amounts of cytoplasmic and mitochondrial ROS, which in turn cause nuclear and mitochondrial DNA damage, triggering CS and installing an elaborate SASP. This SASP displays both autocrine functions, condemning the cell in a vicious circle of metabolic dysregulation secondary to deteriorating OS, and paracrine functions, remodelling the hepatic microenvironment, giving the microscopic characteristics of the MAFLD spectrum.
2.4. Does CS Protect from or Induce Hepatocarcinogenesis in the Context of MAFLD?
The role of CS in the progression of hepatocarcinogenesis is considered pivotal, as it has been shown to have both protective and tumorigenic properties.
CS is widely considered a potent tumour suppressive mechanism since the induction of senescence in premalignant hepatocytes has been shown to limit cancer development. One of the main features of senescent cells that allow for the early restriction of hepatic tumorigenesis is their capacity to control their surroundings via SASP secretion. Premalignant or malignant hepatocytes entering the CS programme secrete SASP factors that activate innate immunity and recruit local and distant macrophages to mediate their own clearance, thus halting the progression to an organized HCC tumour. This mechanism of immune-mediated clearance of senescent cells is widely known as immunosurveillance and is of paramount importance as a barrier to hepatocarcinogenesis [98,99,100]. Several SASP components have been studied, considering their suppressive role in HCC development. More specifically, TGF-β1 has been demonstrated to act in an autocrine manner by inducing ROS generation and perpetuating senescent phenotype in HCC mice cells, thereby leading to a restriction in tumour growth by 75% [101,102].
Despite its repeatedly proven cancer-protective properties, when the hepatic micro-environment is changed, and tumour conditions are developed, CS can, under specific circumstances, usher HCC development. This has been explicitly shown in a series of experiments by Eggert et al., who demonstrated that hepatocyte senescence in tumour-free mice led to the recruitment of immune cells, a process mediated by SASP components, thus resulting in clearance of senescent hepatocytes, a well-known tumour repressing effect [103]. However, when HCC cells were infused in mice with senescent hepatocytes, tumour growth was observed, and further analysis showed that the SASP-mediated immune cells inhibited NK and CD8+ T cells from clearing the cancerous hepatocytes [103]. Another clear example of how the shifts in immunosurveillance can herald hepato-carcinogenesis was shown in the experimental and clinical work of Huang et al. Hepato-cellular CS induced a SASP characterized by IL-6 and IL-8 molecules, with macrophage activation. These macrophages displayed M2 polarization, a characteristic of advanced hepatocarcinogenesis, with pro-inflammatory and fibrotic properties, whereas they did not demonstrate any phagocytotic activity, as M1 macrophages show in early HCC, where they clear away premalignant senescent hepatocytes [104].
A progressive shift to a quantitative increase in hepatocyte senescent phenotype driven by telomeres was observed by Donati et al. [105] when comparing cohorts of MAFLD cirrhotic to HCC patients. They showed a higher prevalence of germline mutations of the telomerase reverse transcriptase (TERT), associated with reduced telomere length in HCC patients versus their cirrhotic counterparts [105]. The role of telomere attrition secondary to TERT dysfunction in early MAFLD versus other aetiologies of chronic liver disease has also been highlighted in other studies [62]. Despite these two studies showing a linear relationship between replicative senescence and the natural history of MAFLD, spontaneous somatic mutations of any of the telomerase components due to oxidative stress can trigger a CS-phenotype and act as a possible mechanism of MAFLD-associated hepatocarcinogenesis. Last, TERT promoter somatic mutations along with 8p loss were shown to be characteristic genomic changes of HCC secondary to MAFLD, frequently accompanied by TP53 as well as SWI/SNF complex constituents (ARID1A and ARID2) mutations, clarifying the landscape of genomic instability in these patients [106]. Thus, it is evident that hepatocellular senescence, when maintained in an advanced HCC milieu, can harbour hepatocarcinogenesis by altering SASP and immune cell signalling.
In addition to the role of senescent hepatocytes in HCC development, it is also worth considering the impact of CS in other cellular populations and its effects on tumour biology. Intriguingly, it is well established that the development of HCC secondary to MAFLD often occurs in the absence of liver cirrhosis or in livers with a low degree of fibrosis [6]. In this context, studies have shown that MAFLD-associated factors, such as obesity, have been linked with hepatic stellate cells (HSCs) senescence and expression of tumour-promoting SASP [21]. Although senescence of HSCs has been considered as a protective mechanism against fibrosis and subsequently to HCC, this seems to be reversed in the case of steatohepatic HCC, as depletion of senescent HSCs prevented HCC development in obese mice [107].
Considering the above-mentioned pivotal role of CS regarding hepatocarcinogenesis, studies have focused on the factors that trigger this alteration of the CS profile. Interestingly, chronic moderate liver injury, similar to the one MAFLD causes to the liver, led to the development of HCC, while the induction of acute injury in a chronic injury environment activated hepatocellular senescence and subsequently caused HCC reduction [108], showing the detrimental impact of chronic low-intensity stressors in hepatic tumorigenesis. In this context, we suppose that hepatocytes sense initial premalignant stimuli as an acute liver injury during the early stages of HCC development; thus, induction of senescence in premalignant hepatocytes acts as a protective mechanism, leading to the clearance of premalignant cells and tumour restriction [109]. On the other hand, when tumour conditions are established following chronic moderate MAFLD-associated tumorigenic stimuli, senescence is not further activated; thus, premalignant hepatocytes do not enter this cell-cycle arrest process, thereby leading to HCC development and expansion. Furthermore, there is substantial evidence supporting that the existing senescent hepatocytes, instead of promoting immunosurveillance, seem to induce aberrant proliferation of the adjacent hepatocytes via SASP secretion; thus, abnormal proliferation of hepatocytes favours hepatic carcinogenesis [108] (Figure 1).
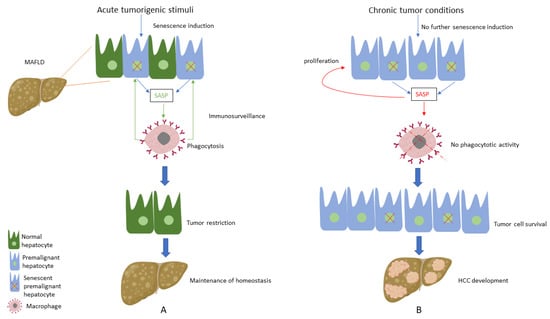
Figure 1.
(A) Under acute tumorigenic conditions, activation of oncogenes and subsequent DNA damage response results in senescence induction, with senescent hepatocytes secreting multiple types of soluble factors, known as senescence-associated secretory phenotype (SASP). SASP results in the recruitment of immune cells, which eliminate premalignant senescent hepatocytes, a process known as immunosurveillance. Thus, under these conditions, senescence plays a protective role by restricting tumour development and contributing to the maintenance of homeostasis. (B) When tumorigenic stimuli become chronic, there is no further senescence induction in hepatocytes. SASP secreted from the existing senescent hepatocytes presents an altered profile by recruiting immune cells, which present no phagocytotic activity, while concomitantly contributing to the proliferation of premalignant cells. Thus, these conditions result in tumour cell survival and the development of hepatocellular carcinoma (HCC).
Collectively, the above demonstrates the importance of cellular senescence in hepatocarcinogenesis. Chronic lipotoxicity and OS induce CS in hepatocytes, triggering SASP secretion, with induction of CS in adjacent cells, such as HSC, halting hepatic fibrosis. Inflammatory phagocytotic cell infiltrates make sure that senescent malignant and premalignant cells are removed. However, continuous chronic exposure to OS lipotoxicity leads to the establishment of a senescent hepatocarcinogenic environment. Local immunomodulatory cells protect malignant hepatocytes, and SASP helps spread CS in neighbouring hepatocytes and HSC. Finally, a chronically ill surrounding hepatic parenchyma helps conserve this carcinogenic niche.
3. Future Directions and Conclusions
Overall, there seems to be a connection between oxidative stress and hepatocarcinogenesis. High-fat, high-sugar, and low-fibre diets, in conjunction with a sedentary lifestyle, induce insulin resistance, leading to hepatocyte fatty acid accumulation. This lipid overload, when combined with the iron imbalances observed in MAFLD, creates a lipotoxic OS environment that depletes the antioxidant mechanisms of defence of the cell and propagates macromolecular damage that contributes to a premature CS phenotype. The combination of OS and CS inevitably leads to the progression of plain steatosis to steatohepatitis, and chronicity of these injuries leads to hepatocarcinogenesis, with multiple other mechanisms contributing to that complex pathway.
Given the above-described role of CS in MAFLD-related hepatocarcinogenesis, one could sensibly assume that we could harness the therapeutic potential of CS induction in order to halt the natural history of this spectrum of disease. However, the dual effect of CS on HCC raises several questions on the relevance of senotherapeutics, an umbrella term for all the agents that modify senescent cells in a tissue. First, one needs to consider what intervention would be the most appropriate. The field of senotherapeutics is going through explosive development, and there are several options, from senolytics—agents that selectively facilitate the clearance of senescent cells—to senomorphics—drugs that can inhibit SASP—and senoptotics—that induce apoptosis of CS cells and using cells or extracellular vesicles to induce or reverse a senescent phenotype. The options are endless, and the list is ever-growing [110,111].
However, to yield the desired results, we must clarify the appropriate timing and cell type to target for any of the above categories of senotherapeutics. That is clearly illustrated in the study by Raffaele et al., where the combined use of dasatinib and quercetin, two well-studied senolytics, failed to halt the progression of MAFLD in a high-fat fed and diethylnitrosamine mouse model. Not only that, but the senolytics-treated group of mice displayed a higher ratio of larger HCC foci. The above show that non-targeted senotherapeutic interventions might yield undesired results. Understanding deeply the mechanisms of actions of these agents, as well as the cellular populations we target with them is critical to senotherapeutics success. This could be the impact of senescent macrophages, and impaired immune clearance of cancerous cells [112].
Another question is whether these medications can be used as neoadjuvant therapies before hepatic resection, as bridging agents before liver transplantation, post-resection as adjuvant therapies, or as a treatment for recurrent or advanced disease. As suggested by Giannakoulis et al., senotherapeutics might actually play a key role in tackling sorafenib resistance, and a combination of senescence induction of malignant cells and subsequent clearance with senolytics can be a feasible strategy in treating advanced HCC. However, there is no clinical evidence focusing on MAFLD-related HCC, and most of the current data are drawn from experimental studies [111].
A different pathway for translating our current knowledge of the above-described mechanisms in HCC therapeutics is to tap into iron depletion. HCC patients with low storage of iron showed improved outcomes when treated with sorafenib, while in vitro, the iron-depleting agent deferasirox enhanced the anti-proliferative properties of sorafenib [113,114].
MAFLD is a composite term engulfing pathologic entities ranging from simple steatosis to inflammation and fibrosis that does not follow a linear temporal evolution and can progress or regress, ultimately leading to hepatocarcinogenesis in a substantial number of patients. Interestingly, CS of different resident hepatic cell types as a dynamic cellular process comes as a consequence of chronic exposure to the multiple stressors of metabolic dysregulation, ranging from lipotoxicity to microbial dysbiosis. The alterations that CS causes to the hepatic microenvironment reflect the different pathological stages of MAFLD, as well as the clinical features of these patients.
However, despite the recent emergence of the term MAFLD, giving a clearer and more mechanistic definition of disease, all the above-included studies were designed in the era of NAFLD, carrying its inherent definition limitations. Not only will MAFLD help better identify patients that need therapy or surveillance, but it will help increase sample size in human studies, providing a deeper understanding of the unique role of CS in human HCC. There are still multiple burning questions in the field of metabolic-associated hepatocarcinogenesis. Intensive research in both disease mechanisms and senotherapeutics is still needed in order to draw concrete conclusions as to what the role of CS is during this complex aspect of disease and how it can be harnessed appropriately.
Author Contributions
N.-A.A., A.B., A.V.C., E.M.M. and A.C.G. wrote the manuscript, E.M.M. designed the figure, N.-A.A. designed the visual abstract, A.V.C. and A.C.G. edited and reviewed the manuscript, reviewed the figure, A.D.K. and D.C. provided substantial corrections to the manuscript, G.K.G., edited and reviewed the manuscript, overall supervision, and team coordination. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A Global View of Hepatocellular Carcinoma: Trends, Risk, Prevention and Management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tomás, T.; Rueda-Robles, A.; Plaza-Díaz, J.; Álvarez-Mercado, A.I. Nutrition and Cellular Senescence in Obesity-Related Disorders. J. Nutr. Biochem. 2022, 99, 108861. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, J.; Viggiano, T.R.; McGill, D.B.; Oh, B.J. Non-alcoholic Steatohepatitis: Mayo Clinic Experiences with a Hitherto Unnamed Disease. Mayo Clin. Proc. 1980, 55, 434–438. [Google Scholar]
- Byrne, C.D.; Targher, G. EASL–EASD–EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Anastasopoulos, N.-A.T.; Lianos, G.D.; Tatsi, V.; Karampa, A.; Goussia, A.; Glantzounis, G.K. Clinical Heterogeneity in Patients with Non-Alcoholic Fatty Liver Disease-Associated Hepatocellular Carcinoma. Expert. Rev. Gastroenterol. Hepatol. 2020, 14, 1025–1033. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A New Definition for Metabolic Dysfunction-Associated Fatty Liver Disease: An International Expert Consensus Statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Tilg, H.; Effenberger, M. From NAFLD to MAFLD: When Pathophysiology Succeeds. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 387–388. [Google Scholar] [CrossRef]
- Shingina, A.; Dewitt, P.E.; Dodge, J.L.; Biggins, S.W.; Gralla, J.; Sprague, D.; Bambha, K. Future Trends in Demand for Liver Transplant: Birth Cohort Effects among Patients with NASH and HCC. Transplantation 2019, 103, 140–148. [Google Scholar] [CrossRef]
- Wong, R.J.; Aguilar, M.; Cheung, R.; Perumpail, R.B.; Harrison, S.A.; Younossi, Z.M.; Ahmed, A. Non-alcoholic Steatohepatitis Is the Second Leading Etiology of Liver Disease among Adults Awaiting Liver Transplantation in the United States. Gastroenterology 2015, 148, 547–555. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Ong, J.; Trimble, G.; Alqahtani, S.; Younossi, I.; Ahmed, A.; Racila, A.; Henry, L. Non-alcoholic Steatohepatitis Is the Most Rapidly Increasing Indication for Liver Transplantation in the United States. Clin. Gastroenterol. Hepatol. 2021, 19, 580–589.e5. [Google Scholar] [CrossRef]
- Tan, D.J.H.; Ng, C.H.; Lin, S.Y.; Pan, X.H.; Tay, P.; Lim, W.H.; Teng, M.; Syn, N.; Lim, G.; Yong, J.N.; et al. Clinical Characteristics, Surveillance, Treatment Allocation, and Outcomes of Non-Alcoholic Fatty Liver Disease-Related Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Lancet Oncol. 2022, 23, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Meroni, M.; Longo, M.; Fargion, S.; Fracanzani, A.L. Genetics, Immunity and Nutrition Boost the Switching from Nash to Hcc. Biomedicines 2021, 9, 1524. [Google Scholar] [CrossRef] [PubMed]
- Miyaaki, H.; Nakao, K. Significance of Genetic Polymorphisms in Patients with Non-alcoholic Fatty Liver Disease. Clin. J. Gastroenterol. 2017, 10, 201–207. [Google Scholar] [CrossRef]
- Schulze, R.J.; McNiven, M.A. Lipid Droplet Formation and Lipophagy in Fatty Liver Disease. Semin. Liver Dis. 2019, 39, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Udoh, U.S.; Rajan, P.K.; Nakafuku, Y.; Finley, R.; Sanabria, J.R. Cell Autophagy in NASH and NASH-Related Hepatocellular Carcinoma. Int. J. Mol. Sci. 2022, 23, 7734. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, Y.; Jiang, R.; Xue, R.; Yin, X.; Wu, M.; Meng, Q. Ferroptosis in Liver Disease: New Insights into Disease Mechanisms. Cell Death Discov. 2021, 7, 276. [Google Scholar] [CrossRef]
- Ni, Y.; Lu, M.; Xu, Y.; Wang, Q.; Gu, X.; Li, Y. The Role of Gut Microbiota-Bile Acids Axis in the Progression of Non-Alcoholic Fatty Liver Disease. Front. Microbiol. 2022, 13, 908011. [Google Scholar] [CrossRef]
- Lipke, K.; Kubis-Kubiak, A.; Piwowar, A. Molecular Mechanism of Lipotoxicity as an Interesting Aspect in the Development of Pathological States—Current View of Knowledge. Cells 2022, 11, 844. [Google Scholar] [CrossRef]
- Agosti, P.; Sabbà, C.; Mazzocca, A. Emerging Metabolic Risk Factors in Hepatocellular Carcinoma and Their Influence on the Liver Microenvironment. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 607–617. [Google Scholar] [CrossRef]
- Papatheodoridi, A.M.; Chrysavgis, L.; Koutsilieris, M.; Chatzigeorgiou, A. The Role of Senescence in the Development of Non-alcoholic Fatty Liver Disease and Progression to Non-alcoholic Steatohepatitis. Hepatology 2020, 71, 363–374. [Google Scholar] [CrossRef]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron Homeostasis and Oxidative Stress: An Intimate Relationship. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef]
- Barbouti, A.; Lagopati, N.; Veroutis, D.; Goulas, V.; Evangelou, K.; Kanavaros, P.; Gorgoulis, V.G.; Galaris, D. Implication of Dietary Iron-Chelating Bioactive Compounds in Molecular Mechanisms of Oxidative Stress-Induced Cell Ageing. Antioxidants 2021, 10, 491. [Google Scholar] [CrossRef]
- Davies, K.J. Oxidative Stress: The Paradox of Aerobic Life. Biochem. Soc. Symp. 1995, 61, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Gonzalez, A.; Huerta-Salgado, C.; Orozco-Aguilar, J.; Aguirre, F.; Tacchi, F.; Simon, F.; Cabello-Verrugio, C. Role of Oxidative Stress in Hepatic and Extrahepatic Dysfunctions during Non-alcoholic Fatty Liver Disease (NAFLD). Oxid. Med. Cell Longev. 2020, 2020, 1617805. [Google Scholar] [CrossRef] [PubMed]
- Arauz, J.; Ramos-Tovar, E.; Muriel, P. Redox State and Methods to Evaluate Oxidative Stress in Liver Damage: From Bench to Bedside. Ann. Hepatol. 2016, 15, 160–173. [Google Scholar] [PubMed]
- Varesi, A.; Chirumbolo, S.; Campagnoli, L.I.M.; Pierella, E.; Piccini, G.B.; Carrara, A.; Ricevuti, G.; Scassellati, C.; Bonvicini, C.; Pascale, A. The Role of Antioxidants in the Interplay between Oxidative Stress and Senescence. Antioxidants 2022, 11, 1224. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 716–748. [Google Scholar] [CrossRef]
- Harman, D. Aging: A Theory Based on Free Radical and Radiation Chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Harman, D. The Biologic Clock: The Mitochondria? J. Am. Geriatr. Soc. 1972, 20, 145–147. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Sosa, P.; Melendez, J.A. Redox Control of Senescence and Age-Related Disease. Redox Biol. 2017, 11, 91–102. [Google Scholar] [CrossRef]
- Bonomini, F.; Rodella, L.F.; Rezzani, R. Metabolic Syndrome, Aging and Involvement of Oxidative Stress. Aging Dis. 2015, 6, 109–120. [Google Scholar] [CrossRef]
- Yan, F.; Li, K.; Xing, W.; Dong, M.; Yi, M.; Zhang, H. Role of Iron-Related Oxidative Stress and Mitochondrial Dysfunction in Cardiovascular Diseases. Oxid. Med. Cell Longev. 2022, 2022, 5124553. [Google Scholar] [CrossRef]
- Mantzaris, M.D.; Bellou, S.; Skiada, V.; Kitsati, N.; Fotsis, T.; Galaris, D. Intracellular Labile Iron Determines H2O2-Induced Apoptotic Signaling via Sustained Activation of ASK1/JNK-P38 Axis. Free Radic. Biol. Med. 2016, 97, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Kitsati, N.; Mantzaris, M.D.; Galaris, D. Hydroxytyrosol Inhibits Hydrogen Peroxide-Induced Apoptotic Signaling via Labile Iron Chelation. Redox Biol. 2016, 10, 233–242. [Google Scholar] [CrossRef]
- Salekeen, R.; Haider, A.N.; Akhter, F.; Billah, M.M.; Islam, M.E.; Didarul Islam, K.M. Lipid Oxidation in Pathophysiology of Atherosclerosis: Current Understanding and Therapeutic Strategies. Int. J. Cardiol. Cardiovasc. Risk Prev. 2022, 14, 200143. [Google Scholar] [CrossRef] [PubMed]
- Maiorino, M.; Conrad, M.; Ursini, F. GPx4, Lipid Peroxidation, and Cell Death: Discoveries, Rediscoveries, and Open Issues. Antioxid. Redox Signal. 2017, 29, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Binder, C.J.; Papac-Milicevic, N.; Witztum, J.L. Innate Sensing of Oxidation-Specific Epitopes in Health and Disease. Nat. Rev. Immunol. 2016, 16, 485–497. [Google Scholar] [CrossRef]
- Qi, J.; Kim, J.W.; Zhou, Z.; Lim, C.W.; Kim, B. Ferroptosis Affects the Progression of Non-alcoholic Steatohepatitis via the Modulation of Lipid Peroxidation–Mediated Cell Death in Mice. Am. J. Pathol. 2020, 190, 68–81. [Google Scholar] [CrossRef]
- Ajoolabady, A. Ferroptosis in Hepatocellular Carcinoma: Mechanisms and Targeted Therapy. Br. J. Cancer 2023, 128, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Delli Bovi, A.P.; Marciano, F.; Mandato, C.; Siano, M.A.; Savoia, M.; Vajro, P. Oxidative Stress in Non-Alcoholic Fatty Liver Disease. An Updated Mini Review. Front. Med. 2021, 8, 595371. [Google Scholar] [CrossRef]
- Mittermeier, C.; Konopa, A.; Muehlich, S. Molecular Mechanisms to Target Cellular Senescence in Hepatocellular Carcinoma. Cells 2020, 9, 2540. [Google Scholar] [CrossRef]
- Aravinthan, A. Cellular Senescence: A Hitchhiker’s Guide. Hum. Cell 2015, 28, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Mun Tho, L.; Xu, N.; Gillespie, D.A. The ATM-Chk2 and ATR-Chk1 Pathways in DNA Damage Signaling and Cancer, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2010; Volume 108. [Google Scholar]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef] [PubMed]
- D’Adda Di Fagagna, F.; Reaper, P.M.; Clay-Farrace, L.; Fiegler, H.; Carr, P.; Von Zglinicki, T.; Saretzki, G.; Carter, N.P.; Jackson, S.P. A DNA Damage Checkpoint Response in Telomere-Initiated Senescence. Nature 2003, 426, 194–198. [Google Scholar] [CrossRef]
- Childs, B.G.; Durik, M.; Baker, D.J.; Van Deursen, J.M. Cellular Senescence in Aging and Age-Related Disease: From Mechanisms to Therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef]
- Coppé, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Tchkonia, T.; Zhu, Y.; van Deursen, J.; Campisi, J.; Kirkland, J.L. Cellular Senescence and the Senescent Secretory Phenotype: Therapeutic Opportunities. J. Clin. Investig. 2013, 123, 966–972. [Google Scholar] [CrossRef]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.-W.; Lasitschka, F.; Andrulis, M.; et al. A Complex Secretory Program Orchestrated by the Inflammasome Controls Paracrine Senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef]
- Huda, N.; Khambu, B.; Liu, G.; Nakatsumi, H.; Yan, S.; Chen, X.; Ma, M.; Dong, Z.; Nakayama, K.I.; Yin, X.-M. Senescence Connects Autophagy Deficiency to Inflammation and Tumor Progression in the Liver. Cell Mol. Gastroenterol. Hepatol. 2022, 14, 333–355. [Google Scholar] [CrossRef]
- Kudlova, N.; De Sanctis, J.B.; Hajduch, M. Cellular Senescence: Molecular Targets, Biomarkers, and Senolytic Drugs. Int. J. Mol. Sci. 2022, 23, 4168. [Google Scholar] [CrossRef]
- Adewoye, A.B.; Tampakis, D.; Follenzi, A.; Stolzing, A. Multiparameter Flow Cytometric Detection and Quantification of Senescent Cells In Vitro. Biogerontology 2020, 21, 773–786. [Google Scholar] [CrossRef]
- Itahana, K.; Campisi, J.; Dimri, G.P. Methods to Detect Biomarkers of Cellular Senescence: The Senescence-Associated Beta-Galactosidase Assay. Methods Mol. Biol. 2007, 371, 21–31. [Google Scholar] [CrossRef]
- Ferreira-Gonzalez, S.; Rodrigo-Torres, D.; Gadd, V.L.; Forbes, S.J. Cellular Senescence in Liver Disease and Regeneration. Semin. Liver Dis. 2021, 41, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulou, E.A.; Tsimaratou, K.; Evangelou, K.; Fernandez Marcos, P.J.; Zoumpourlis, V.; Trougakos, I.P.; Kletsas, D.; Bartek, J.; Serrano, M.; Gorgoulis, V.G. Specific Lipofuscin Staining as a Novel Biomarker to Detect Replicative and Stress-Induced Senescence. A Method Applicable in Cryo-Preserved and Archival Tissues. Aging 2013, 5, 37–50. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, D.; Strakovsky, R.; Zhang, Y.; Pan, Y.X. Hepatic Cellular Senescence Pathway Genes Are Induced through Histone Modifications in a Diet-Induced Obese Rat Model. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Ping, F.; Li, Z.-Y.; Lv, K.; Zhou, M.-C.; Dong, Y.-X.; Sun, Q.; Li, Y.-X. Deoxyribonucleic Acid Telomere Length Shortening Can Predict the Incidence of Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus. J. Diabetes Investig. 2017, 8, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Laish, I.; Mannasse-Green, B.; Hadary, R.; Biron-Shental, T.; Konikoff, F.M.; Amiel, A.; Kitay-Cohen, Y. Telomere Dysfunction in Non-alcoholic Fatty Liver Disease and Cryptogenic Cirrhosis. Cytogenet. Genome Res. 2016, 150, 93–99. [Google Scholar] [CrossRef]
- Hardy, T.; Oakley, F.; Anstee, Q.M.; Day, C.P. Non-alcoholic Fatty Liver Disease: Pathogenesis and Disease Spectrum. Annu. Rev. Pathol. 2016, 11, 451–496. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Wu, X.; Chen, H.; Xia, X.; Song, X.; Chen, S.; Lu, X.; Jin, J.; Su, Q.; Cai, D.; et al. Aging-Induced Aberrant RAGE/PPARα Axis Promotes Hepatic Steatosis via Dysfunctional Mitochondrial β Oxidation. Aging Cell 2020, 19, e13238. [Google Scholar] [CrossRef] [PubMed]
- Selen, E.S.; Choi, J.; Wolfgang, M.J. Discordant Hepatic Fatty Acid Oxidation and Triglyceride Hydrolysis Leads to Liver Disease. JCI Insight 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Teratani, T.; Suzuki, T.; Oshikawa, T.; Yokoyama, H.; Shimamura, K.; Nishiyama, K.; Mataki, N.; Irie, R.; Minamino, T.; et al. P53/P66Shc-Mediated Signaling Contributes to the Progression of Non-Alcoholic Steatohepatitis in Humans and Mice. J. Hepatol. 2012, 57, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, C.; Habtesion, A.; Hassan, M.; Kerbert, A.J.; Hammerich, L.; Novelli, S.; Fidaleo, M.; Philips, A.; Davies, N.; Ferreira-Gonzalez, S.; et al. Combination of G-CSF and a TLR4 Inhibitor Reduce Inflammation and Promote Regeneration in a Mouse Model of ACLF. J. Hepatol. 2022, 77, 1325–1338. [Google Scholar] [CrossRef]
- Aravinthan, A.; Scarpini, C.; Tachtatzis, P.; Verma, S.; Penrhyn-Lowe, S.; Harvey, R.; Davies, S.E.; Allison, M.; Coleman, N.; Alexander, G. Hepatocyte Senescence Predicts Progression in Non-Alcohol-Related Fatty Liver Disease. J. Hepatol. 2013, 58, 549–556. [Google Scholar] [CrossRef]
- Aravinthan, A.; Mells, G.; Allison, M.; Leathart, J.; Kotronen, A.; Yki-Jarvinen, H.; Daly, A.K.; Day, C.P.; Anstee, Q.M.; Alexander, G. Gene Polymorphisms of Cellular Senescence Marker P21 and Disease Progression in Non-Alcohol-Related Fatty Liver Disease. Cell Cycle 2014, 13, 1489–1494. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of Hepatic Stellate Cell Activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Wijayasiri, P.; Astbury, S.; Kaye, P.; Oakley, F.; Alexander, G.J.; Kendall, T.J.; Aravinthan, A.D. Role of Hepatocyte Senescence in the Activation of Hepatic Stellate Cells and Liver Fibrosis Progression. Cells 2022, 11, 2221. [Google Scholar] [CrossRef]
- Huda, N.; Liu, G.; Hong, H.; Yan, S.; Khambu, B.; Yin, X.M. Hepatic Senescence, the Good and the Bad. World J. Gastroenterol. 2019, 25, 5069–5081. [Google Scholar] [CrossRef]
- Nishizawa, H.; Iguchi, G.; Fukuoka, H.; Takahashi, M.; Suda, K.; Bando, H.; Matsumoto, R.; Yoshida, K.; Odake, Y.; Ogawa, W.; et al. IGF-I Induces Senescence of Hepatic Stellate Cells and Limits Fibrosis in a P53-Dependent Manner. Sci. Rep. 2016, 6, 34605. [Google Scholar] [CrossRef]
- Hong, H.; Cui, Z.Z.; Zhu, L.; Fu, S.P.; Rossi, M.; Cui, Y.H.; Zhu, B.M. Central IGF1 Improves Glucose Tolerance and Insulin Sensitivity in Mice. Nutr. Diabetes 2017, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Krizhanovsky, V.; Yon, M.; Dickins, R.A.; Hearn, S.; Simon, J.; Miething, C.; Yee, H.; Zender, L.; Lowe, S.W. Senescence of Activated Stellate Cells Limits Liver Fibrosis. Cell 2008, 134, 657–667. [Google Scholar] [CrossRef]
- Xiaoni, K.; Dechun, F.; Hua, W.; Feng, H.; Adeline, B.; Fu-Sheng, W.; Bin, G. Interleukin-22 Induces Hepatic Stellate Cell Senescence and Restricts Liver Fibrosis in Mice. Hepatology 2012, 56, 1150–1159. [Google Scholar]
- Irvine, K.M.; Skoien, R.; Bokil, N.J.; Melino, M.; Thomas, G.P.; Loo, D.; Gabrielli, B.; Hill, M.M.; Sweet, M.J.; Clouston, A.D.; et al. Senescent Human Hepatocytes Express a Unique Secretory Phenotype and Promote Macrophage Migration. World J. Gastroenterol. 2014, 20, 17851–17862. [Google Scholar] [CrossRef]
- Meng, F.; Wang, K.; Aoyama, T.; Grivennikov, S.I.; Paik, Y.; Scholten, D.; Cong, M.; Iwaisako, K.; Liu, X.; Zhang, M.; et al. Interleukin-17 Signaling in Inflammatory, Kupffer Cells, and Hepatic Stellate Cells Exacerbates Liver Fibrosis in Mice. Gastroenterology 2012, 143, 765–776.e3. [Google Scholar] [CrossRef]
- Sriram, S.; Yuan, C.; Chakraborty, S.; Tay, W.; Park, M.; Shabbir, A.; Toh, S.; Han, W.; Sugii, S. Oxidative Stress Mediates Depot-Specific Functional Differences of Human Adipose-Derived Stem Cells. Stem Cell Res. Ther. 2019, 10, 141. [Google Scholar] [CrossRef]
- Ross, R.; Soni, S.; Houle, S. Negative Energy Balance Induced by Exercise or Diet: Effects on Visceral Adipose Tissue and Liver Fat. Nutrients 2020, 12, 891. [Google Scholar] [CrossRef] [PubMed]
- Keshavjee, B.; Lambelet, V.; Coppola, H.; Viertl, D.; Prior, J.O.; Kappeler, L.; Armengaud, J.-B.; Chouraqui, J.-P.; Chehade, H.; Vanderriele, P.-E.; et al. Stress-Induced Premature Senescence Related to Oxidative Stress in the Developmental Programming of Non-alcoholic Fatty Liver Disease in a Rat Model of Intrauterine Growth Restriction. Antioxidants 2022, 11, 1695. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Masutomi, H.; Noda, Y.; Ozawa, Y.; Takahashi, K.; Handa, S.; Maruyama, N.; Shimizu, T.; Ishigami, A. Senescence Marker Protein-30/Superoxide Dismutase 1 Double Knockout Mice Exhibit Increased Oxidative Stress and Hepatic Steatosis. FEBS Open Bio 2014, 4, 522–532. [Google Scholar] [CrossRef]
- Ogrodnik, M.; Miwa, S.; Tchkonia, T.; Tiniakos, D.; Wilson, C.L.; Lahat, A.; Day, C.P.; Burt, A.; Palmer, A.; Anstee, Q.M.; et al. Cellular Senescence Drives Age-Dependent Hepatic Steatosis. Nat. Commun. 2017, 8, 70–72. [Google Scholar] [CrossRef]
- Passos, J.F.; Nelson, G.; Wang, C.; Richter, T.; Simillion, C.; Proctor, C.J.; Miwa, S.; Olijslagers, S.; Hallinan, J.; Wipat, A.; et al. Feedback between P21 and Reactive Oxygen Production Is Necessary for Cell Senescence. Mol. Syst. Biol. 2010, 6, 347. [Google Scholar] [CrossRef] [PubMed]
- Lohr, K.; Pachl, F.; Moghaddas Gholami, A.; Geillinger, K.E.; Daniel, H.; Kuster, B.; Klingenspor, M. Reduced Mitochondrial Mass and Function Add to Age-Related Susceptibility toward Diet-Induced Fatty Liver in C57BL/6J Mice. Physiol. Rep. 2016, 4, e12988. [Google Scholar] [CrossRef]
- Kondo, Y.; Hasegawa, G.; Okada, H.; Senmaru, T.; Fukui, M.; Nakamura, N.; Sawada, M.; Kitawaki, J.; Okanoue, T.; Kishimoto, Y.; et al. Leprdb/Db Mice with Senescence Marker Protein-30 Knockout (Leprdb/DbSmp30Y/-) Exhibit Increases in Small Dense-LDL and Severe Fatty Liver Despite Being Fed a Standard Diet. PLoS ONE 2013, 8, e65698. [Google Scholar] [CrossRef]
- Park, H.; Ishigami, A.; Shima, T.; Mizuno, M.; Maruyama, N.; Yamaguchi, K.; Mitsuyoshi, H.; Minami, M.; Yasui, K.; Itoh, Y.; et al. Hepatic Senescence Marker Protein-30 Is Involved in the Progression of Non-alcoholic Fatty Liver Disease. J. Gastroenterol. 2010, 45, 426–434. [Google Scholar] [CrossRef]
- Meijnikman, A.S.; Herrema, H.; Scheithauer, T.P.M.; Kroon, J.; Nieuwdorp, M.; Groen, A.K. Evaluating Causality of Cellular Senescence in Non-Alcoholic Fatty Liver Disease. JHEP Rep. 2021, 3, 100301. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Faber, K.N.; de Meijer, V.E.; Blokzijl, H.; Moshage, H. How Does Hepatic Lipid Accumulation Lead to Lipotoxicity in Non-Alcoholic Fatty Liver Disease? Hepatol. Int. 2021, 15, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Petrosillo, G.; Portincasa, P.; Grattagliano, I.; Casanova, G.; Matera, M.; Ruggiero, F.M.; Ferri, D.; Paradies, G. Mitochondrial Dysfunction in Rat with Nonalcoholic Fatty Liver: Involvement of Complex I, Reactive Oxygen Species and Cardiolipin. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, Y. In Search of the Holy Grail: Toward a Unified Hypothesis on Mitochondrial Dysfunction in Age-Related Diseases. Cells 2022, 11, 1906. [Google Scholar] [CrossRef]
- Noureddin, M.; Yates, K.P.; Vaughn, I.A.; Neuschwander-Tetri, B.A.; Sanyal, A.J.; McCullough, A.; Merriman, R.; Hameed, B.; Doo, E.; Kleiner, D.E.; et al. Clinical and Histological Determinants of Non-alcoholic Steatohepatitis and Advanced Fibrosis in Elderly Patients. Hepatology 2013, 58, 1644–1654. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.; Fielder, E.; Passos, J.F. Mitochondrial Dysfunction and Cell Senescence: Deciphering a Complex Relationship. FEBS Lett. 2019, 593, 1566–1579. [Google Scholar] [CrossRef] [PubMed]
- Middleton, P.; Vergis, N. Mitochondrial Dysfunction and Liver Disease: Role, Relevance, and Potential for Therapeutic Modulation. Therap. Adv. Gastroenterol. 2021, 14, 17562848211031394. [Google Scholar] [CrossRef]
- Ahmad, T.; Sundar, I.K.; Lerner, C.A.; Gerloff, J.; Tormos, A.M.; Yao, H.; Rahman, I. Impaired Mitophagy Leads to Cigarette Smoke Stress-Induced Cellular Senescence: Implications for Chronic Obstructive Pulmonary Disease. FASEB J. 2015, 29, 2912–2929. [Google Scholar] [CrossRef] [PubMed]
- Glück, S.; Guey, B.; Gulen, M.F.; Wolter, K.; Kang, T.W.; Schmacke, N.A.; Bridgeman, A.; Rehwinkel, J.; Zender, L.; Ablasser, A. Innate Immune Sensing of Cytosolic Chromatin Fragments through CGAS Promotes Senescence. Nat. Cell Biol. 2017, 19, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Wiley, C.D.; Velarde, M.C.; Lecot, P.; Liu, S.; Sarnoski, E.A.; Freund, A.; Shirakawa, K.; Lim, H.W.; Davis, S.S.; Ramanathan, A.; et al. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab. 2016, 23, 303–314. [Google Scholar] [CrossRef]
- Greten, T.F.; Eggert, T. Cellular Senescence Associated Immune Responses in Liver Cancer. Hepatic Oncol. 2017, 4, 123–127. [Google Scholar] [CrossRef]
- Liu, P.; Tang, Q.; Chen, M.; Chen, W.; Lu, Y.; Liu, Z.; He, Z. Hepatocellular Senescence: Immunosurveillance and Future Senescence-Induced Therapy in Hepatocellular Carcinoma. Front. Oncol. 2020, 10, 589908. [Google Scholar] [CrossRef]
- Xue, W.; Zender, L.; Miething, C.; Dickins, R.A.; Hernando, E.; Krizhanovsky, V.; Cordon-Cardo, C.; Lowe, S.W. Senescence and Tumour Clearance Is Triggered by P53 Restoration in Murine Liver Carcinomas. Nature 2007, 445, 656–660. [Google Scholar] [CrossRef]
- Gungor, M.Z.; Uysal, M.; Senturk, S. The Bright and the Dark Side of TGF-β Signaling in Hepatocellular Carcinoma: Mechanisms, Dysregulation, and Therapeutic Implications. Cancers 2022, 14, 940. [Google Scholar] [CrossRef]
- Senturk, S.; Mumcuoglu, M.; Gursoy-Yuzugullu, O.; Cingoz, B.; Akcali, K.C.; Ozturk, M. Transforming Growth Factor-Beta Induces Senescence in Hepatocellular Carcinoma Cells and Inhibits Tumor Growth. Hepatology 2010, 52, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Eggert, T.; Wolter, K.; Ji, J.; Ma, C.; Yevsa, T.; Klotz, S.; Medina-Echeverz, J.; Longerich, T.; Forgues, M.; Reisinger, F.; et al. Distinct Functions of Senescence-Associated Immune Responses in Liver Tumor Surveillance and Tumor Progression. Cancer Cell 2016, 30, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, X.; Meng, Y.; Shao, C.; Liao, J.; Li, F.; Li, R.; Jing, Y.; Huang, A. The Hepatic Senescence-Associated Secretory Phenotype Promotes Hepatocarcinogenesis through Bcl3-Dependent Activation of Macrophages. Cell Biosci. 2021, 11, 173. [Google Scholar] [CrossRef] [PubMed]
- Donati, B.; Pietrelli, A.; Pingitore, P.; Dongiovanni, P.; Caddeo, A.; Walker, L.; Baselli, G.; Pelusi, S.; Rosso, C.; Vanni, E.; et al. Telomerase Reverse Transcriptase Germline Mutations and Hepatocellular Carcinoma in Patients with Non-alcoholic Fatty Liver Disease. Cancer Med. 2017, 6, 1930–1940. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Ueda, Y.; Hatano, E.; Kakiuchi, N.; Takeda, H.; Goto, T.; Shimizu, T.; Yoshida, K.; Ikura, Y.; Shiraishi, Y.; et al. TERT Promoter Mutations and Chromosome 8p Loss Are Characteristic of Non-alcoholic Fatty Liver Disease-Related Hepatocellular Carcinoma. Int. J. Cancer 2016, 2518, 2512–2518. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-Induced Gut Microbial Metabolite Promotes Liver Cancer through Senescence Secretome. Nature 2013, 499, 97–101. [Google Scholar] [CrossRef]
- Cai, X.; Guillot, A.; Liu, H. Cellular Senescence in Hepatocellular Carcinoma: The Passenger or the Driver? Cells 2022, 12, 132. [Google Scholar] [CrossRef]
- Wang, C.; Chen, W.J.; Wu, Y.F.; You, P.; Zheng, S.Y.; Liu, C.C.; Xiang, D.; Wang, M.J.; Cai, Y.C.; Zhao, Q.H.; et al. The Extent of Liver Injury Determines Hepatocyte Fate toward Senescence or Cancer Article. Cell Death Dis. 2018, 9, 575. [Google Scholar] [CrossRef]
- Huang, W.; Hickson, L.T.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular Senescence: The Good, the Bad and the Unknown. Nat. Rev. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef]
- Giannakoulis, V.; Dubovan, P.; Papoutsi, E.; Kataki, A.; Koskinas, J. Senescence in HBV-, HCV- and NAFLD—Mediated Hepatocellular Carcinoma and Senotherapeutics: Current Evidence and Future Perspective. Cancers 2021, 13, 4732. [Google Scholar] [CrossRef]
- Raffaele, M.; Kovacovicova, K.; Frohlich, J.; Lo Re, O.; Giallongo, S.; Oben, J.A.; Faldyna, M.; Leva, L.; Giannone, A.G.; Cabibi, D.; et al. Mild Exacerbation of Obesity- and Age-Dependent Liver Disease Progression by Senolytic Cocktail Dasatinib + Quercetin. Cell Commun. Signal. 2021, 19, 44. [Google Scholar] [CrossRef] [PubMed]
- Casini, A.; Leone, S.; Vaccaro, R.; Vivacqua, G.; Ceci, L.; Pannarale, L.; Franchitto, A.; Onori, P.; Gaudio, E.; Mancinelli, R. The emerging role of ferroptosis in liver cancers. Life 2022, 12, 2128. [Google Scholar] [CrossRef]
- Urano, S.; Ohara, T.; Noma, K.; Katsube, R.; Ninomiya, T.; Tomono, Y.; Tazawa, H.; Kagawa, S.; Shirakawa, Y.; Kimura, F.; et al. Iron depletion enhances the effect of sorafenib in hepatocarcinoma. Cancer Biol. Ther. 2016, 6, 648–656. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).