Application of a Cold-Pressing Treatment to Improve Virgin Olive Oil Production and the Antioxidant Phenolic Profile of Its by-Products
Abstract
1. Introduction
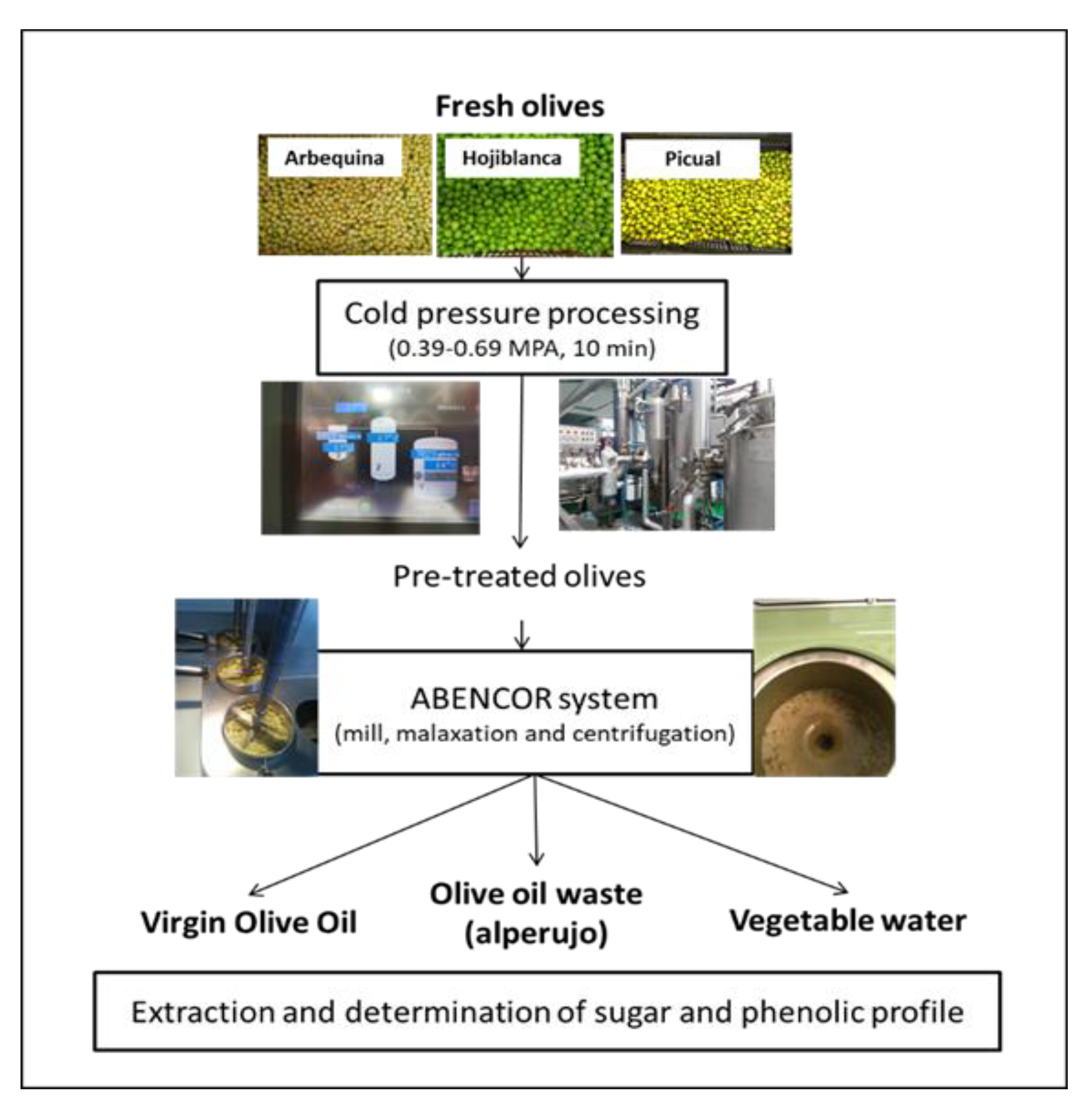
2. Materials and Methods
2.1. Samples
2.2. Standard Compounds
2.3. Cold-Pressure Treatment
2.4. Olive Oil Extraction
2.5. Determination of Moisture Content
2.6. Determination of the Maturity Index
2.7. Extraction and Determination of Total Phenolics and Sugars
2.8. Analysis of Individual Phenolics with High-Performance Liquid Chromatography (HPLC)
2.9. Statistical Analysis
3. Results
3.1. Oil and Water Extractability and Moisture
3.2. Alperujo Characterization
3.3. Characterization of the Vegetation Water
3.4. Phenolic Characterization of the Extracted Olive Oil
4. Discussion
4.1. Effect of the Treatment on Oil Extractability and Water Separation
4.2. Effect of Treatment on Alperujo
4.3. Effect of Treatment on the Water Fraction
4.4. Effect on the Phenolic Profile of the Oil
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández-Rodríguez, M.J.; Cubero-Cardoso, J.; de la Lama-Calvente, D.; Fernández-Prior, A.; Rodríguez-Gutiérrez, G.; Borja, R. Performance and kinetic evaluation of the anaerobic digestion of olive pomace derived from a novel manufacturing process based on an olive cold-pressing system: Influence of the fruit ripening level. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Rubio-Senent, F.; Bermúdez-Oria, A.; Fernández-Bolaños, J.; Fernández Prior, A.; Rodríguez-Gutiérrez, G. The use of industrial thermal techniques to improve the bioactive compounds extraction and the olive oil solid waste utilization. Innov. Food Sci. Emerg. Technol. 2019, 55, 11–17. [Google Scholar] [CrossRef]
- Fernández-Bolaños, J.G.; López, O.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Hydroxytyrosol and derivatives: Isolation, synthesis, and biological properties. Curr. Org. Chem. 2008, 12, 442–463. [Google Scholar] [CrossRef]
- Fernández-Prior, A.; Bermúdez-Oria, A.; Millán-Linares, M.C.; Fernández-Bolaños, J.; Espejo-Calvo, J.A.; Rodríguez-Gutiérrez, G. Anti-Inflammatory and Antioxidant Activity of Hydroxytyrosol and 3,4-Dihydroxyphenyglycol Purified from Table Olive Effluents. Foods 2021, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Kurek, M.; Benaida-Debbache, N.; Elez Garofuli´c, I.; Gali´c, K.; Avallone, S.; Voilley, A.; Waché, Y. Antioxidants and Bioactive Compounds in Food: Critical Review of Issues and Prospects. Antioxidants 2022, 11, 742. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Clodoveo, M.L.; Durante, V.; La Notte, D.; Punzi, R.; Gambacorta, G. Ultrasound-assisted extraction of virgin olive oil to improve the process efficiency. Eur. J. Lipid Sci. Technol. 2013, 115, 1062–1069. [Google Scholar] [CrossRef]
- Beltrán, G.; Del Río, C.; Sánchez, S.; Martínez, L. Seasonal changes in olive fruit characteristics and oil accumulation during ripening process. J. Sci. Food Agric. 2004, 84, 1783–1790. [Google Scholar] [CrossRef]
- Perez, M.; Lopez-Yerena, A.; Lozano-Castellon, J.; Olmo-Cunillera, A.; Lamuela-Raventos, R.M.; Martin-Belloso, O.; Vallverdu-Queralt, A. Impact of Emerging Technologies on Virgin Olive Oil Processing, Consumer Acceptance, and the Valorization of Olive Mill Wastes. Antioxidants 2021, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Uceda, M.; Frías, L. Épocas de recolección. Evolución del contenido graso del fruto y de la composición y calidad del aceite. In Proceedings of the II Seminario Oleícola International, Córdoba, Spain, 6–17 October 1975; pp. 25–46. [Google Scholar]
- Vázquez Roncero, A.; Janer Del Valle, C.; Janer Del Valle, M.L. Determinación de polifenoles totales en el aceite de oliva. Grasas Aceites 1973, 24, 350–357. [Google Scholar]
- Obied, H.K.; Bedgood, D.R.; Prenzler, P.D.; Robards, K. Chemical screening of olive biophenol extracts by hyphenated liquid chromatography. Anal. Chim. Acta 2007, 603, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Dische, Z. Color Reactions of Carbohydrates. En: Methods in Carbohydrate Chemistry, Págs; Whistler, R.L., Wolfrom, M.L., Eds.; Academic Press: New York, NY, USA, 1962; pp. 477–512. [Google Scholar]
- Serrano, A.; Fermoso, F.G.; Rodríguez-Gutiérrez, G.; Fernandez-Bolaños, J.; Borja, R. Biomethanization of olive mill solid waste after phenols recovery through low-temperature thermal pre-treatment. Waste Manag. 2017, 61, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Granados-Principal, S.; Quiles, J.L.; Ramírez-Tortosa, C.L.; Sanchez-Rovira, P.; Ramírez-Tortosa, M.C. Hydroxytyrosol: From laboratory investigations to future clinical trials. Nutr. Rev. 2010, 68, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Prior, M.A.; Charfi, A.; Bermúdez-Oria, A.; Rodríguez-Juan, E.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Deep eutectic solvents improve the biorefinery of alperujo by extraction of bioactive molecules in combination with industrial thermal treatments. Food Bioprod. Process. 2020, 121, 131–142. [Google Scholar] [CrossRef]
- Rubio-Senent, F.; Rodríguez-Gutiérrez, G.; Lama-Muñoz, A.; Fernández-Bolaños, J. New Phenolic Compounds Hydrothermally Extracted from the Olive Oil By-Product Alperujo and their Antioxidative Activities. J. Agric. Food Chem. 2012, 60, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Safarzadeh Markhali, F. Effect of Processing on Phenolic Composition of Olive Oil Products and Olive Mill By-Products and Possibilities for Enhancement of Sustainable Processes. Processes 2021, 9, 953. [Google Scholar] [CrossRef]
- Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Lama-Muñoz, A.; García, A.; Fernández-Bolaños, J. Properties of lignin, cellulose and hemicelluloses isolated from olive cake and olive stones- binding of water, oil, bile acids and glucose. J. Agric. Food Chem. 2014, 62, 8973–8981. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, C.S.; Lyri, P.; Xintaropoulou, I.; Diamantopoulos, I.; Zagklis, D.P.; Paraskeva, C.A. High-Yield Production of a Rich-in-Hydroxytyrosol Extract from Olive (Olea europaea) Leaves. Antioxidants 2022, 11, 1042. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Senent, F.; Martos, S.; García, A.; Lama-Muñoz, A.; Fernández-Bolaños, J.G.; Rodríguez-Gutiérrez, G.; Fernández-Bolaños, J. Isolation and characterization of a secoiridoid derivative from two-phase olive waste (alperujo). J. Agric. Food Chem. 2015, 63, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Lama-Muñoz, A.; Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Palacios-Díaz, R.; Fernández-Bolaños, J. A study of the precursors of the natural antioxidant phenol 3,4-dihydroxyphenylglycol in olive oil waste. Food Chem. 2013, 140, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Oleocanthal: A naturally occurring anti-inflammatory agent in virgin olive oil. In Olive Oil-Constituents, Quality, Health Properties and Bioconversions; Boskou, D., Ed.; InTech: Rijeka, Croatia, 2012; pp. 357–374. [Google Scholar] [CrossRef]

| Variety/MI | Treat | Total Sugars | Total Phenolics | Individual Phenolics (mg/kg Dry Matter) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DHPG | HT-Glu | HT | Ty | Sy A | Ve | Lu-Glu | Cg | |||||
| Arb | 0.8 | Control | 6.51 ± 0.30 bc * | 3.14 ± 0.13 b | 40.75 ± 2.04 a | 593.24 ± 18.97 e | 113.51 ± 8.79 a | 169.97 ± 12.32 g | 8.72 ± 0.84 a | 345.21 ± 17.81 e | Traces | 290.96 ± 10.21 c |
| 0.39 MPa | 7.37 ± 0.13 d | 3.57 ± 0.11 b | 35.04 ± 1.98 a | 730.41 ± 32.47 f | 216.58 ± 23.41 c | 166.89 ± 11.00 g | 36.68 ± 2.57 c | 413.13 ± 10.51 fg | Traces | 403.78 ± 32.82 | ||
| 0.69 MPa | 7.09 ± 0.26 cd | 3.47 ± 0.14 b | 36.75 ± 1.87 a | 755.27 ± 44.08 fg | 282.09 ± 25.12 c | 29.93 ± 3.80 a | 7.00 ± 1.05 a | 365.01 ± 19.52 e | 231.35 ± 8.69 c | 348.45 ± 4.90 d | ||
| Ho | 1.1 | Control | 5.79 ± 0.32 b | 2.76 ± 0.14 a | 269.20 ± 11.70 h | 870.91 ± 33.75 g | 141.02 ± 5.64 a | 89.34 ± 4.46 e | 31.08 ± 3.44 c | 446.60 ± 15.50 g | 42.92 ± 7.78 b | 188.51 ± 15.30 a |
| 0.39 MPa | 6.69 ± 0.21 c | 3.31 ± 0.15 b | 190.38 ± 9.51 f | 1585.50 ± 45.60 h | 386.80 ± 30.01 d | 127.04 ± 12.27 | 28.87 ± 1.20 c | 795.60 ± 20.67 h | 477.77 ± 23.15 d | 179.52 ± 7.84 a | ||
| 0.69 MPa | 6.75 ± 0.29 c | 3.32 ± 0.16 b | 56.24 ± 4.22 b | 1669.91 ± 52.67 h | 469.14 ± 26.80 de | 112.38 ± 17.12 f | 48.00 ± 2.77 d | 902.52 ± 32.60 i | 657.76 ± 30.50 e | 228.23 ± 6.23 b | ||
| 1.5 | Control | 3.67 ± 0.06 a | 12.24 ± 0.07 e | 87.72 ± 7.24 c | 208.7 ± 10.12 b | 308.69 ± 9.04 c | 73.18 ± 5.60 e | Traces | Traces | 1124.78 ± 33.81 f | 841.26 ± 27.85 e | |
| 0.69 MPa | 3.47 ± 0.05 a | 11.77 ± 0.03 de | 89.86 ± 5.50 c | 204.27 ± 12.29 b | 186.48 ± 14.77 b | 99.72 ± 3.42 e | Traces | Traces | 1084.01 ± 42.55 f | 934.92 ± 32.40 e | ||
| Pi | 0.9 | Control | 5.08 ± 0.02 b | 2.40 ± 0.08 a | 70.75 ± 2.87 c | 364.42 ± 12.03 cd | 130.30 ± 9.68 a | 60.66 ± 3.30 c | Traces | Traces | Traces | 259.43 ± 10.45 bc |
| 0.39 MPa | 4.40 ± 0.42 ab | 2.30 ± 0.09 a | 61.16 ± 2.11 b | 348.13 ± 7.42 c | 150.21 ± 18.87 ab | 61.74 ± 4.02 c | Traces | Traces | Traces | 166.79 ± 4.35 a | ||
| 0.69 MPa | 5.04 ± 0.04 b | 2.38 ± 0.05 a | 102.41 ± 1.20 d | 450.61 ± 21.80 d | 138.13 ± 12.43 a | 67.48 ± 7.60 cd | Traces | Traces | Traces | 324.54 ± 15.60 d | ||
| 1.2 | Control | 20.05 ± 0.24 f | 9.88 ± 0.12 d | 176.85 ± 11.87 ef | 85.98 ± 16.12 a | 256.53 ± 27.58 c | 43.65 ± 2.32 b | 9.36 ± 1.29 a | 101.14 ± 3.20 d | 12.18 ± 1.10 a | 284.37 ± 16.27 c | |
| 0.69 MPa | 20.35 ± 0.05 f | 10.03 ± 0.02 d | 152.79 ± 9.65 e | 237.99 ± 30.09 b | 532.20 ± 18.00 e | 71.00 ± 4.33 d | 12.79 ± 2.00 b | 30.14 ± 1.58 a | 201.87 ± 22.37 c | 284.24 ± 12.77 c | ||
| 2.4 | Control | 10.51 ± 0.10 e | 5.14 ± 0.25 c | 340.12 ± 12.77 i | 342.80 ± 29.36 bc | 1040.91 ± 48.10 f | 117.38 ± 9.23 f | 11.72 ± 1.78 ab | 47.17 ± 3.24 c | 191.26 ± 18.20 c | 324.33 ± 16.80 d | |
| 0.69 MPa | 9.16 ± 0.38 e | 4.47 ± 0.19 c | 200.20 ± 15.03 fg | 401.57 ± 26.48 d | 1047.75 ± 52.20 f | 111.15 ± 7.74 f | 12.23 ± 1.92 b | 37.18 ± 1.01 b | 222.21 ± 20.13 c | 290.22 ± 17.62 cd | ||
| Variety/MI | Treat | Total Sugars | Total Phenolics | Individual Phenolics (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DHPG | HT-Glu | HT | Ty | Sy A | Ve | p-Co | Cg | |||||
| Arb | 0.8 | Control | 28.12 ± 0.34 b * | 5.22 ± 0.19 d | 18.23 ± 1.8 a | 300.31 ± 17.08 c | 184.26 ± 14.21 b | 123.25 ± 2.99 d | 15.79 ± 1.39 a | 192.89 ± 11.07 d | 6.51 ± 0.38 a | 219.10 ± 4.73 c |
| 0.39 MPa | 31.11 ± 0.61 b | 5.15 ± 0.24 d | 18.99 ± 1.92 a | 271.29 ± 13.20 c | 174.19 ± 15.85 b | 122.74 ± 6.06 d | 15.97 ± 1.54 a | 212.02 ± 17.58 d | 6.25 ± 0.08 a | 224.20 ± 9.77 c | ||
| 0.69 MPa | 26.96 ± 0.48 b | 5.42 ± 0.25 d | 16.74 ± 1.80 a | 295.74 ± 25.53 c | 175.45 ± 19.23 b | 43.46 ± 2.42 a | 17.53 ± 2.89 ab | 190.67 ± 17.77 d | 4.91 ± 0.64 a | 212.15 ± 0.54 c | ||
| Ho | 1.1 | Control | 35.27 ± 0.33 c | 4.97 ± 0.23 cd | 95.28 ± 13.12 c | 552.89 ± 20.01 d | 196.57 ± 22.48 bc | 118.58 ± 6.97 d | 12.71 ± 2.76 a | 263.95 ± 16.24 e | 17.26 ± 1.09 c | 95.01 ± 5.73 a |
| 0.39 MPa | 36.29 ± 1.14 c | 5.22 ± 0.15 d | 120.29 ± 16.39 d | 550.66 ± 7.00 d | 218.76 ± 13.91 c | 121.27 ± 5.99 d | 15.20 ± 2.96 a | 277.84 ± 18.96 e | 17.71 ± 1.46 c | 102.86 ± 7.10 a | ||
| 0.69 MPa | 36.28 ± 0.67 c | 5.29 ± 0.20 d | 137.91 ± 9.99 de | 559.17 ± 17.84 d | 202.86 ± 2.61 c | 123.85 ± 9.93 d | 20.79 ± 1.58 b | 247.80 ± 13.69 e | 18.20 ± 1.51 c | 100.75 ± 6.16 a | ||
| 1.5 | Control | 17.80 ± 1.22 a | 6.79 ± 0.66 e | 34.21 ± 3.90 b | 155.82 ± 7.42 b | 558.40 e ±12.24 | 62.15 ±3.08 b | Traces | Traces | 12.87 ± 0.79 b | 799.05 ± 22.75 d | |
| 0.69 MPa | 15.42 ± 0.55 a | 5.95 ± 0.78 de | 32.02 ± 4.14 b | 148.24 ± 8.80 b | 520.63 ± 15.61 e | 44.08 ± 2.20 a | Traces | Traces | 11.92 ± 1.05 b | 805.12 ± 27.40 d | ||
| Pi | 0.9 | Control | 33.12 ± 0.92 bc | 4.72 ± 0.08 c | 198.62 ± 6.10 f | 126.41 ± 3.47 a | 141.34 ± 2.60 a | 76.51 ± 1.11 b | 18.14 ± 1.65 b | 79.02 ± 9.88 bc | 13.67 ± 0.25 b | 190.01 ± 3.01 b |
| 0.39 MPa | 28.90 ± 1.23 b | 4.48 ± 0.21 c | 164.81 ± 24.30 e | 160.40 ± 4.30 b | 180.31 ± 18.07 b | 86.62 ± 4.66 c | 16.91 ± 1.19 ab | 85.15 ± 5.58 c | 14.10 ± 0.94 b | 185.93 ± 9.59 b | ||
| 0.69 MPa | 32.00 ± 0.36 bc | 4.65 ± 0.22 cd | 207.21 ± 18.62 f | 147.11 ± 5.71 b | 160.19 ± 17.41 ab | 90.32 ± 4.32 c | 19.09 ± 0.74 b | 90.61 ± 5.16 c | 13.83 ± 0.62 b | 188.66 ± 8.38 b | ||
| 1.2 | Control | 32.63 ± 0.22 bc | 3.42 ± 0.04 b | 204.21 ± 12.35 f | 590.01 ± 24.09 d | 438.11 ± 19.18 d | 126.71 ± 6.81 d | 25.76 ± 1.12 c | 72.50 ± 2.07 b | 34.78 ± 0.87 d | 597.66 ± 24.50d | |
| 0.69 MPa | 34.38 ± 0.91 c | 3.50 ± 0.08 b | 99.70 ± 11.47 b | 302.07 ± 21.20 c | 798.12 ± 17.50 f | 112.33 ± 9.80 cd | 25.84 ± 2.55 c | 56.42 ± 1.08 a | 31.72 ± 1.29 d | 602.12 ± 32.00 d | ||
| 2.4 | Control | 32.05 ± 0.06 c | 3.00 ± 0.06 a | 113.50 ± 7.11 bc | 304.92 ± 15.29 c | 842.51 ± 20.03 f | 148.77 ± 7.88 de | 27.05 ± 3.42 c | 54.01 ± 2.22 a | 30.87 ± 2.07 d | 603.50 ± 27.09 d | |
| 0.69 MPa | 33.85 ± 0.06 c | 2.88 ± 0.11 a | 171.86 ± 5.42 d | 267.97 ± 9.88 c | 520.97 ± 23.77 e | 153.22 ± 5.43 e | 28.97 ± 2.20 c | 70.41 ± 3.91 b | 35.86 ± 2.57 d | 641.07 ± 37.84 d | ||
| Variety/MI | Treat | Total Phenolics | Individual Phenols (mg/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DHPG | HT | Ty | Vanillic Acid | Oleocanthal | Oleacein | ||||
| Arb | 0.8 | Control | 0.8 ± 0.0 | 1.99 ± 0.37 ef* | 11.01 ± 1.16 c | 4.92 ± 0.81 ab | 6,46 ± 1.03 g | 311,23 ± 12.98 f | 177,53 ± 11.09 bc |
| 0.39 MPa | 0.6 ± 0.0 | 0.68 ± 0.26 c | 8.11 ± 0.67 bc | 3.50 ± 0.04 a | 4,73 ± 0.86 f | 243,95 ± 11.23 e | 140,96 ± 12.08 a | ||
| 0.69 MPa | 0.6 ± 0.0 | 1.46 ± 0.27 e | 8.82 ± 0.45 c | 4.93 ± 0.07 b | 5,41 ± 0.71 fg | 233,47 ± 15.12 e | 141,98 ± 6.66 a | ||
| Ho | 1.1 | Control | 0.9 ± 0.0 | 0.36 ± 0.07 ab | 10.27 ± 0.11 c | 7.01 ± 0.06 d | 4,78 ± 0.70 f | 222,73 ± 9.88 e | 193,77 ± 7.01 c |
| 0.39 MPa | 0.6 ± 0.0 | 0.60 ± 0.11 c | 8.31 ± 1.60 bc | 5.24 ± 0.09 b | 3,58 ± 0.63 ef | 189,84 ± 10.42 d | 163,06 ± 9.89 ab | ||
| 0.69 MPa | 1.1 ± 0.0 | 0.30 ± 0.07 a | 9.80 ± 1.42 c | 6.36 ± 0.10 c | 1,05 ± 0.08 a | 215,20 ± 15.18 de | 170,67 ± 7.09 b | ||
| 1.5 | Control | 1.3 ± 0.0 | nd | 12.5 ± 2.31 c | 4.3 ± 1.05 a | nd | 222.9 ± 17.18 e | 270.9 ±8.50 d | |
| 0.69 MPa | 1.2 ± 0.0 | nd | 11.9 ± 1.99 c | 3.8 ± 1.11 a | nd | 145.6 ± 12.33 b | 252.8 ± 13.2 d | ||
| Pi | 0.9 | Control | 0.5 ± 0.0 | 1.33 ± 0.11 de | 6.18 ± 0.01 a | 3.46 ± 0.21 a | 2,98 ± 0.08 e | 140,62 ± 7.02 b | 166,52 ± 7.77 ab |
| 0.39 MPa | 0.7 ± 0.0 | 0.40 ± 0.01 b | 28.30 ± 1.03 d | 8.30 ± 0.41 d | 2,90 ± 0.07 e | 51,47 ± 5.63 a | 167,08 ± 9.02 ab | ||
| 0.69 MPa | 0.5 ± 0.0 | 1.20 ± 0.21 d | 7.91 ± 0.52 bc | 9.37 ± 0.56 de | 2,53 ± 0.11 d | 162,24 ± 4.32 c | 169,60 ± 10.10 ab | ||
| 1.2 | Control | 1.2 ± 0.0 | 2.14 ± 0.04 f | 33.14 ± 2.40 d | 10,87 ± 1.03 e | 1.73 ± 0.02 c | 146,04 ± 7.77 b | 392,76 ± 15.50 e | |
| 0.69 MPa | 1.0 ± 0.0 | 0.40 ± 0.01 b | 8.70 ± 0.22 bc | 13,90 ±1.00 f | 1.23 ± 0.01 a | 134,07 ± 6.99 b | 230,77 ± 10.9 d | ||
| 2.4 | Control | 1.2 ± 0.0 | 1.90 ± 0.02 ef | 73.15 ± 2.47 f | 17,88 ± 2.06 g | 1.55 ± 0.01 b | 146,66 ± 11.70 b | 491,54 ± 22.70 f | |
| 0.69 MPa | 1.5 ± 0.0 | 4.30 ± 0.65 g | 57.60 ± 4.84 e | 16,61 ± 1.93 a | 1.23 ± 0.01 a | 140,02 ± 10.02 b | 434,80 ± 17.95 ef | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Prior, Á.; Cardoso, J.C.; Bermúdez-Oria, A.; Reyes, Á.T.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Application of a Cold-Pressing Treatment to Improve Virgin Olive Oil Production and the Antioxidant Phenolic Profile of Its by-Products. Antioxidants 2023, 12, 1162. https://doi.org/10.3390/antiox12061162
Fernández-Prior Á, Cardoso JC, Bermúdez-Oria A, Reyes ÁT, Fernández-Bolaños J, Rodríguez-Gutiérrez G. Application of a Cold-Pressing Treatment to Improve Virgin Olive Oil Production and the Antioxidant Phenolic Profile of Its by-Products. Antioxidants. 2023; 12(6):1162. https://doi.org/10.3390/antiox12061162
Chicago/Turabian StyleFernández-Prior, África, Juan Cubero Cardoso, Alejandra Bermúdez-Oria, Ángeles Trujillo Reyes, Juan Fernández-Bolaños, and Guillermo Rodríguez-Gutiérrez. 2023. "Application of a Cold-Pressing Treatment to Improve Virgin Olive Oil Production and the Antioxidant Phenolic Profile of Its by-Products" Antioxidants 12, no. 6: 1162. https://doi.org/10.3390/antiox12061162
APA StyleFernández-Prior, Á., Cardoso, J. C., Bermúdez-Oria, A., Reyes, Á. T., Fernández-Bolaños, J., & Rodríguez-Gutiérrez, G. (2023). Application of a Cold-Pressing Treatment to Improve Virgin Olive Oil Production and the Antioxidant Phenolic Profile of Its by-Products. Antioxidants, 12(6), 1162. https://doi.org/10.3390/antiox12061162








