Influence of Increased Radiation Background on Antioxidative Responses of Helianthus tuberosus L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of Experimental Areas and Plant Material
2.2. Soil and Plant Sample Collection in Experimental Area
2.3. Determination of 137Cs in Soils and Plants
2.4. Determination of Antioxidant Enzymes Activity
2.4.1. Antioxidant Enzyme Extraction from Tubers
2.4.2. Determination of Catalase Activity
2.4.3. Determination of Ascorbate Peroxidase Activity
2.4.4. Determination of Peroxidase Activity
2.5. Determination of Low Molecular Weight Antioxidants Concentrations
2.5.1. Low Molecular Weight Antioxidant Extraction from Tubers
2.5.2. Determination of Total Antioxidant Activity
2.5.3. Determination of Total Water-Soluble and Alcohol-Soluble Phenolics Content
2.5.4. Determination of Total Flavonoid Content
2.5.5. Determination of Proline Concentration
2.5.6. Determination of Ascorbic Acid Content
2.5.7. Determination of Reducing Sugars
2.6. Study of Climatic Conditions
2.7. Statistical Analysis
3. Results
3.1. Determination of 137Cs in Soils and Plants
3.2. Determination of Antioxidant Enzymes Activity
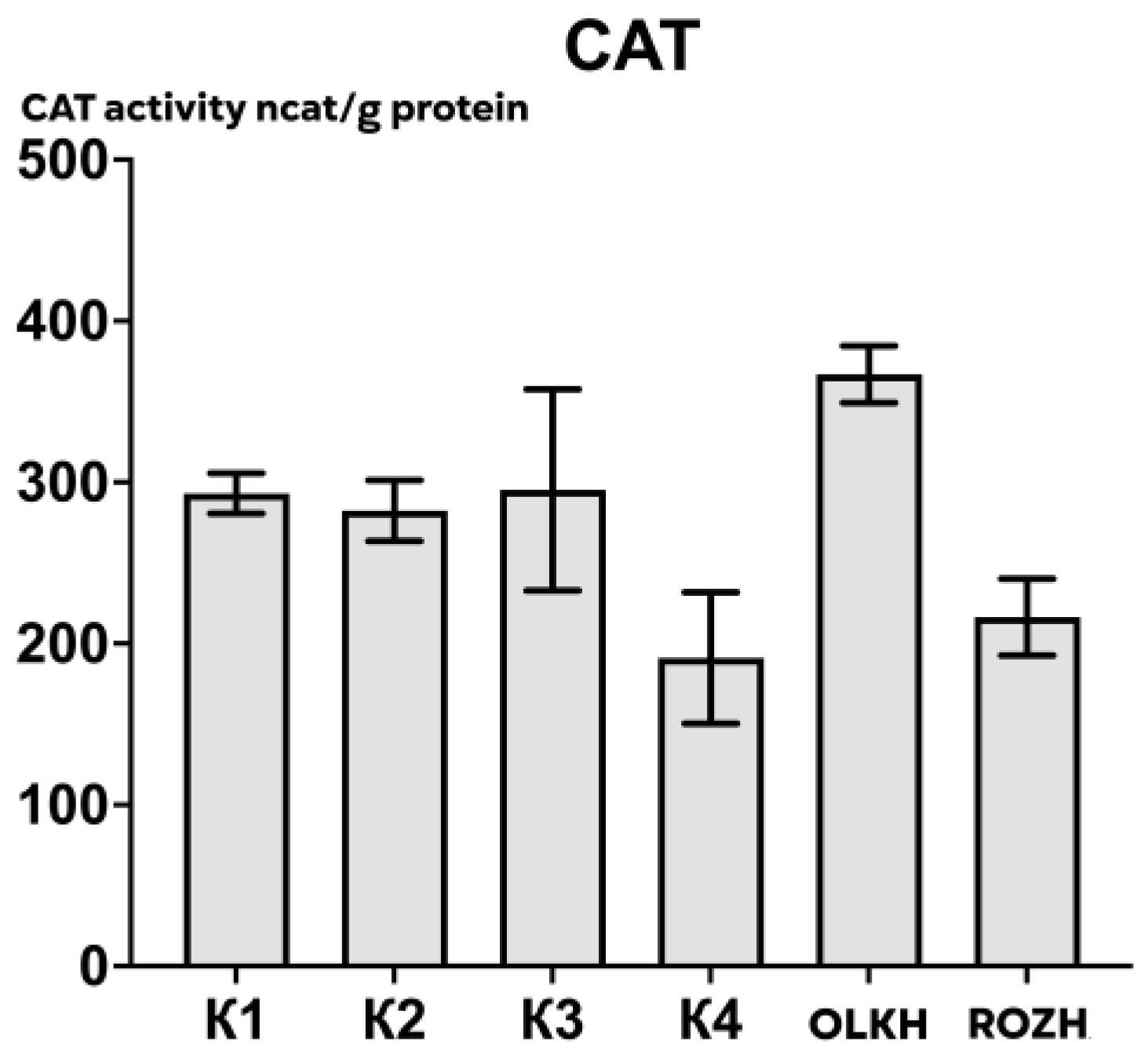
3.2.1. Determination of Catalase Activity
3.2.2. Determination of Ascorbate Peroxidase Activity
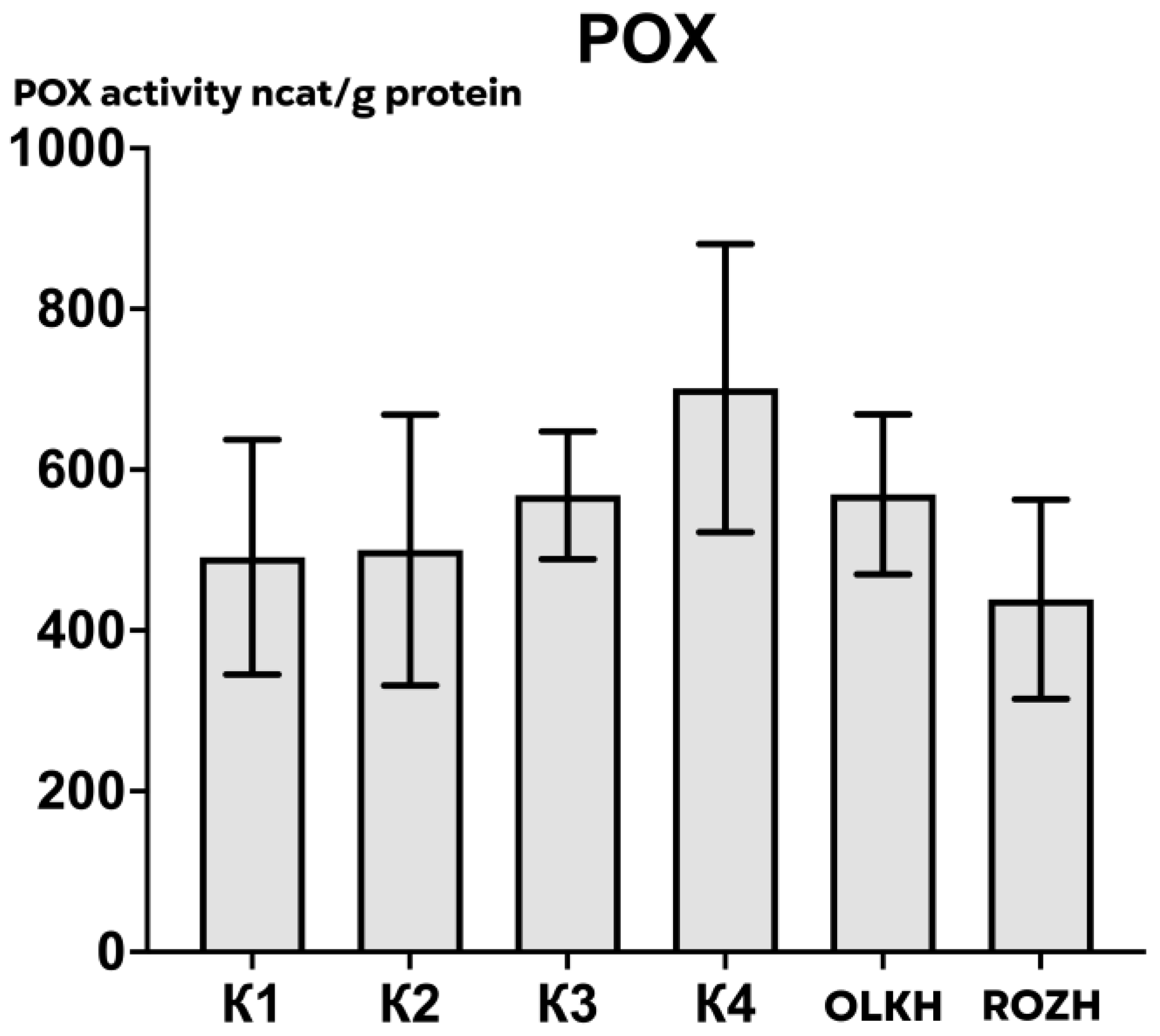
3.2.3. Determination of Peroxidase Activity
3.3. Determination of Low Molecular Weight Antioxidants Concentrations
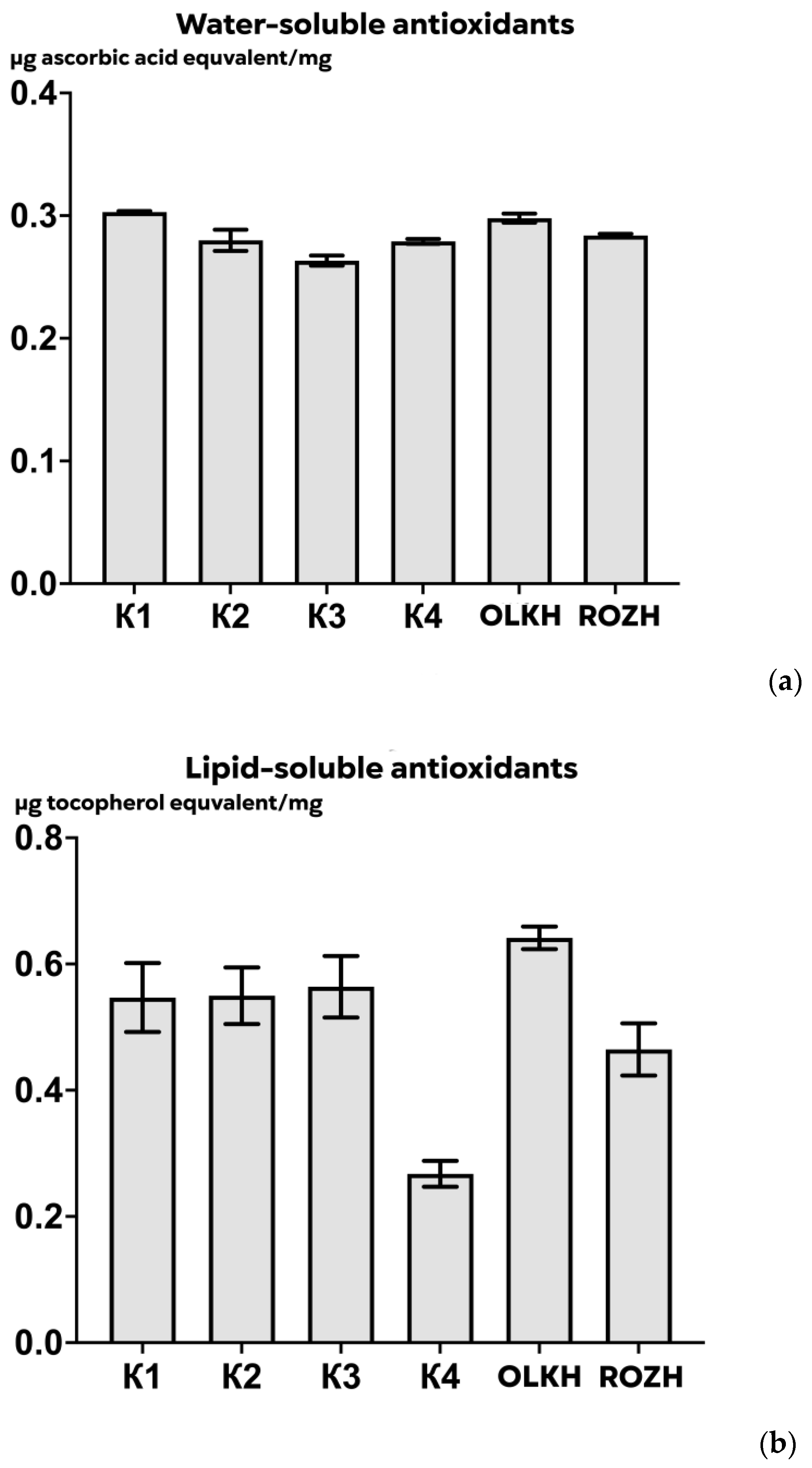
3.3.1. Determination of Total Antioxidant Activity
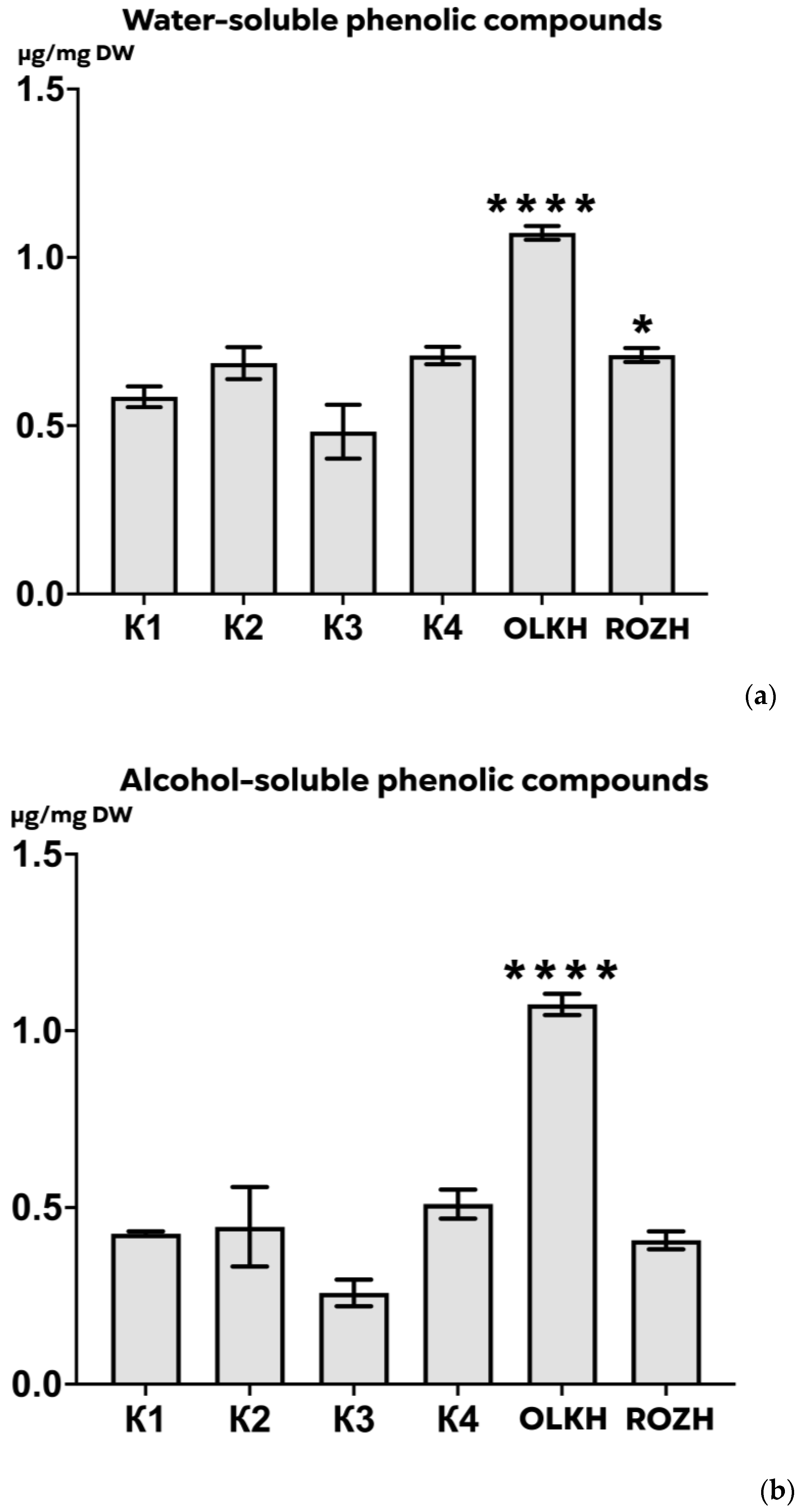
3.3.2. Determination of Total Water-Soluble and Alcohol-Soluble Phenolics Content
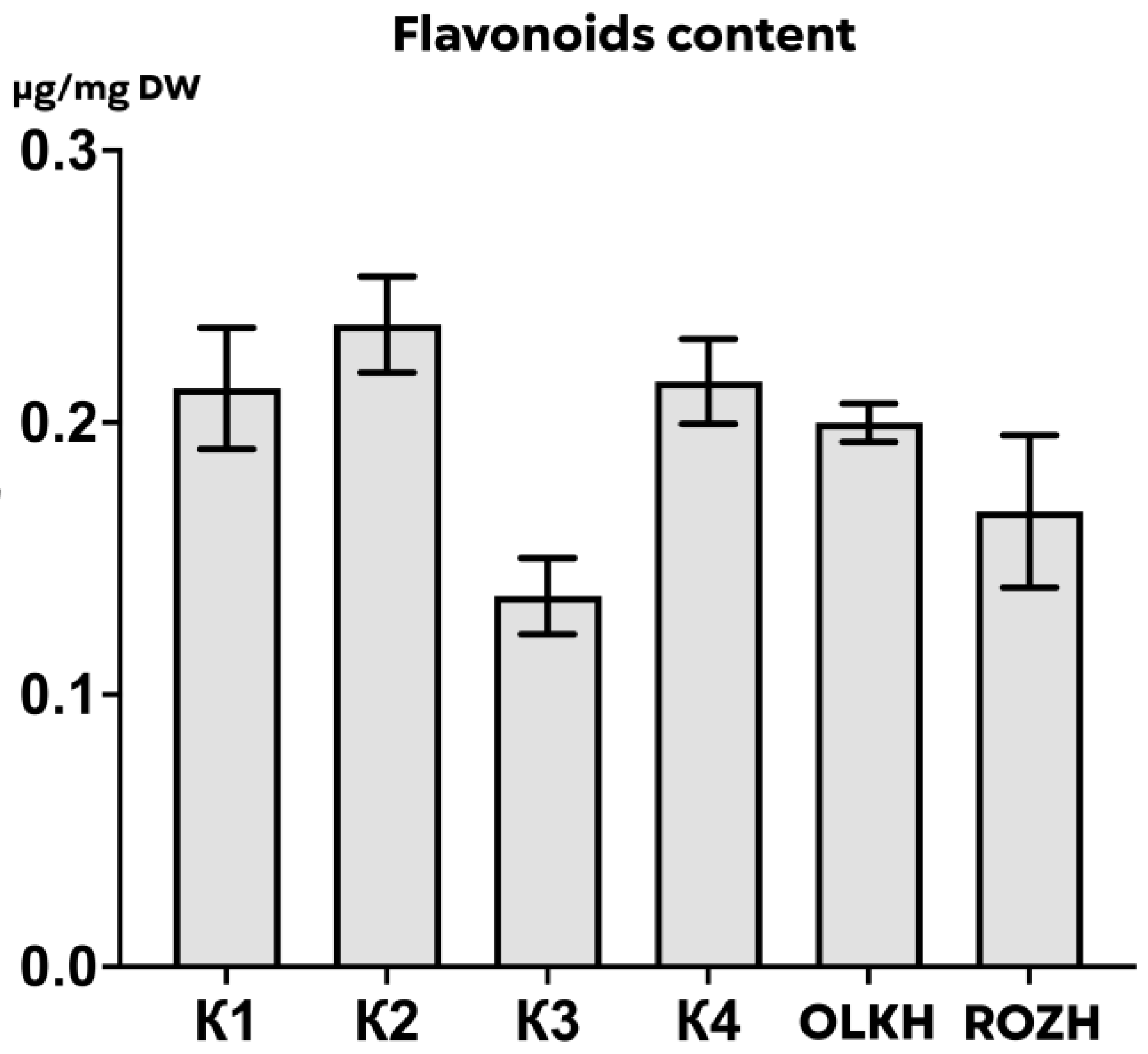
3.3.3. Determination of Total Flavonoid Content
3.3.4. Determination of Proline Concentration
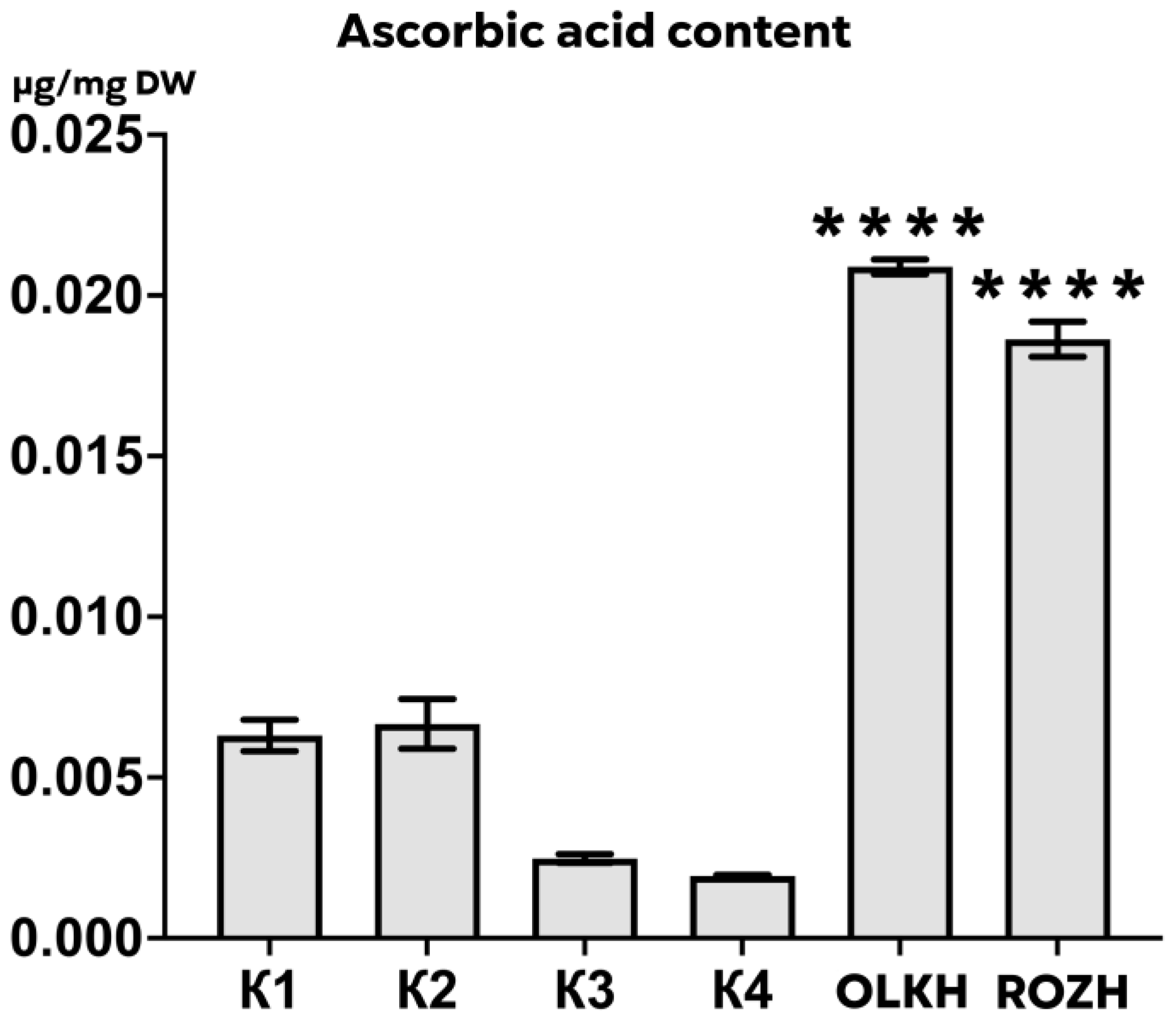
3.3.5. Determination of Ascorbic Acid Content
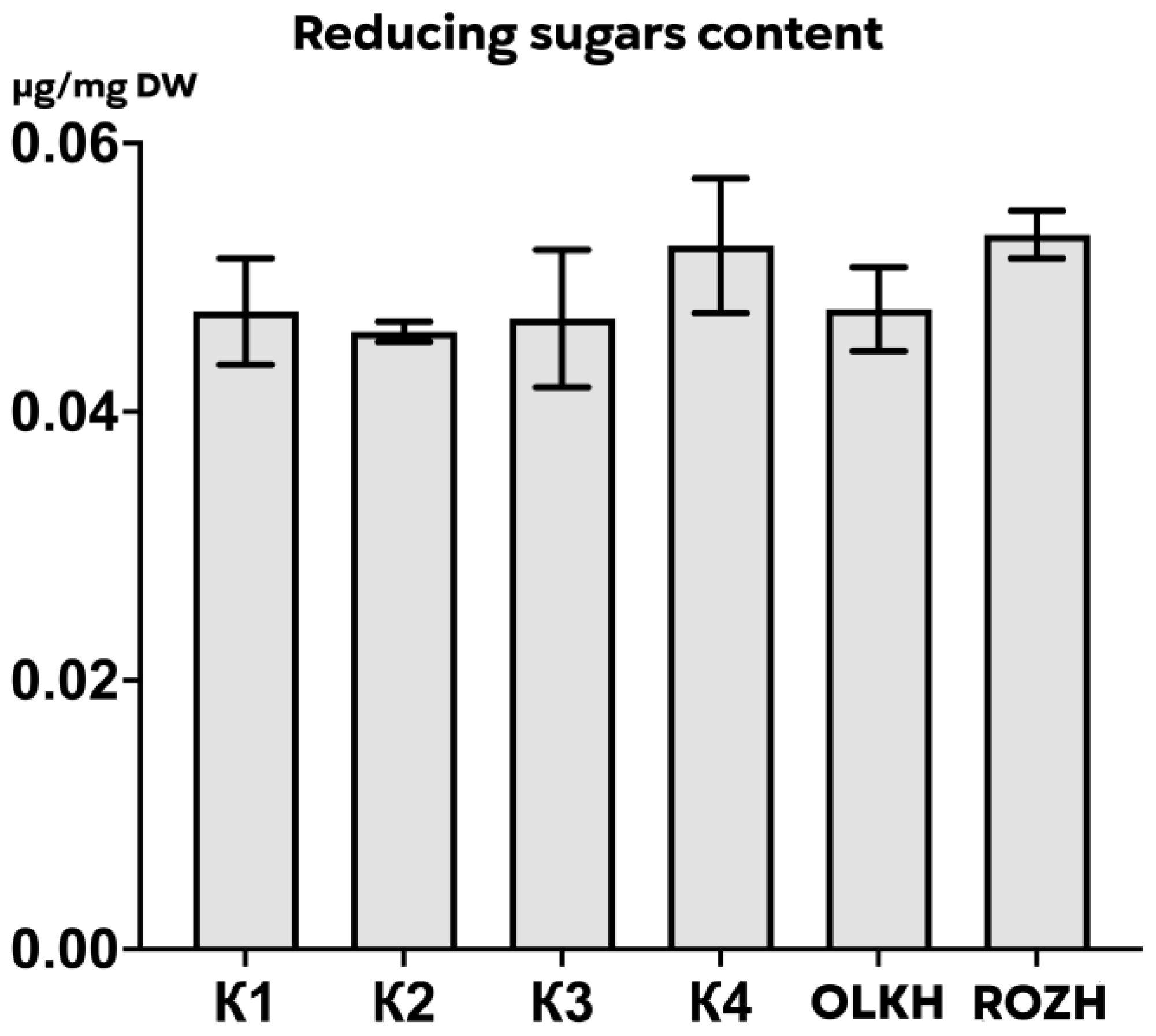
3.3.6. Determination of Reducing Sugars
3.4. Changes in Climatic Conditions
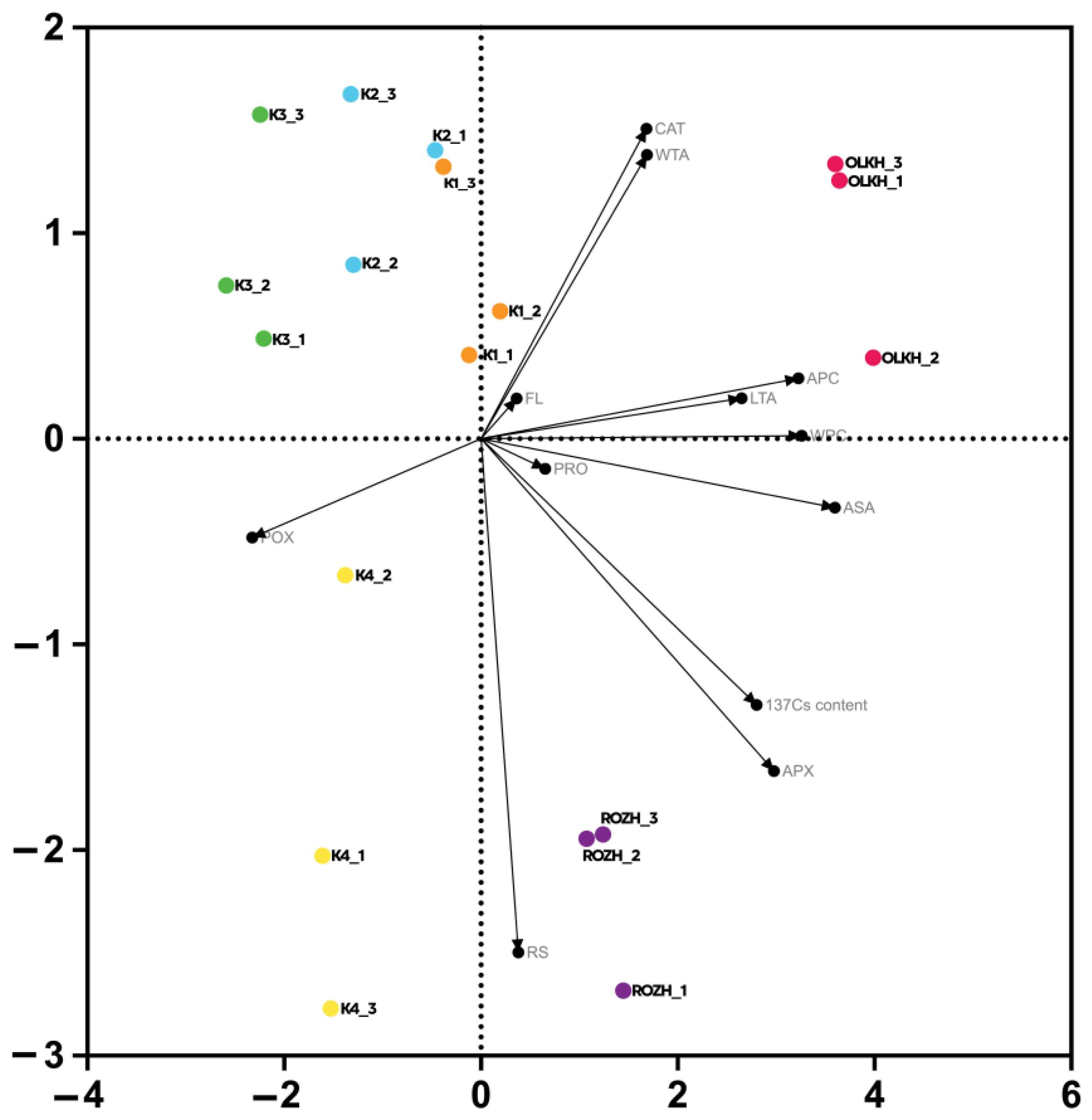
3.5. Statistical Processing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mousseau, T.A.; Møller, A.P. Plants in the Light of Ionizing Radiation: What Have We Learned from Chernobyl, Fukushima, and Other “Hot” Places? Front. Plant Sci. 2020, 11, 552. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Anjum, N.A. Book Review: Oxidative Damage to Plants-Antioxidant Networks and Signaling. Front. Plant Sci. 2015, 6, 452. [Google Scholar] [CrossRef]
- Blokhina, O. Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: A Review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- United Nation Scientific Committee on the Effects of Atomic Radiation. Report to the General Assembly with Scientific An-Nexes Effect of Ionizing Radiation on Non-Human Biota, 2008; United Nations: New York, NY, USA, 2010; Volume 2, ISBN 978-92-1-142280-1. [Google Scholar]
- Chakravarty, B.; Sen, S. Enhancement of Regeneration Potential and Variability by γ-Irradiation in Cultured Cells of Scilla Indica. Biol. Plant. 2001, 44, 189–193. [Google Scholar] [CrossRef]
- Lee, H.Y.; Kim, J.-S.; Baek, M.H.; Yoo, J.C.; Kwon, S.T. Effects of Low Dose 7 Irradiation on Physiological Activities of Radish (Raphanussativus) during Early Growth and Reduction of Ultraviolet-B Stress. Kor. Soc. Hort. Sci. 2003, 44, 314–320. [Google Scholar]
- Kim, J.-H.; Chung, B.Y.; Kim, J.-S.; Wi, S.G. Effects of in Planta Gamma-Irradiation on Growth, Photosynthesis, and Antioxidative Capacity of Red Pepper (Capsicum Annuum L.) Plants. J. Plant Biol. 2005, 48, 47–56. [Google Scholar] [CrossRef]
- Amirikhah, R.; Etemadi, N.; Sabzalian, M.R.; Nikbakht, A.; Eskandari, A. Gamma Radiation Negatively Impacted Seed Germination, Seedling Growth and Antioxidant Enzymes Activities in Tall Fescue Infected with Epichloë Endophyte. Ecotoxicol. Environ. Saf. 2021, 216, 112169. [Google Scholar] [CrossRef]
- Hong, M.J.; Kim, D.Y.; Jo, Y.D.; Choi, H.-I.; Ahn, J.-W.; Kwon, S.-J.; Kim, S.H.; Seo, Y.W.; Kim, J.-B. Biological Effect of Gamma Rays According to Exposure Time on Germination and Plant Growth in Wheat. Appl. Sci. 2022, 12, 3208. [Google Scholar] [CrossRef]
- Maity, J.P.; Mishra, D.; Chakraborty, A.; Saha, A.; Santra, S.C.; Chanda, S. Modulation of Some Quantitative and Qualitative Characteristics in Rice (Oryza Sativa L.) and Mung (Phaseolus Mungo L.) by Ionizing Radiation. Radiat. Phys. Chem. 2005, 74, 391–394. [Google Scholar] [CrossRef]
- Kovalchuk, I.; Molinier, J.; Yao, Y.; Arkhipov, A.; Kovalchuk, O. Transcriptome Analysis Reveals Fundamental Differences in Plant Response to Acute and Chronic Exposure to Ionizing Radiation. Mutat. Res. Mol. Mech. Mutagen. 2007, 624, 101–113. [Google Scholar] [CrossRef]
- Kimura, S.; Shibato, J.; Agrawal, G.K.; Kim, Y.K.; Nahm, B.H.; Jwa, H.S.; Iwahashi, H.; Rakwal, R. Microarray Analysis of Rice Leaf Response to Radioactivity from Contaminated Chernobyl Soil. Rice Gen. Newsl. 2008, 24, 52–54. [Google Scholar]
- Hayashi, G.; Moro, C.F.; Rohila, J.S.; Shibato, J.; Kubo, A.; Imanaka, T.; Kimura, S.; Ozawa, S.; Fukutani, S.; Endo, S.; et al. 2D-DIGE-Based Proteome Expression Changes in Leaves of Rice Seedlings Exposed to Low-Level Gamma Radiation at Iitate Village, Fukushima. Plant Signal. Behav. 2015, 10, e1103406. [Google Scholar] [CrossRef]
- Boratyński, Z.; Arias, J.M.; Garcia, C.; Mappes, T.; Mousseau, T.A.; Møller, A.P.; Pajares, A.J.M.; Piwczyński, M.; Tukalenko, E. Ionizing Radiation from Chernobyl Affects Development of Wild Carrot Plants. Sci. Rep. 2016, 6, 39282. [Google Scholar] [CrossRef] [PubMed]
- Ludovici, G.M.; Chierici, A.; de Souza, S.O.; d’Errico, F.; Iannotti, A.; Malizia, A. Effects of Ionizing Radiation on Flora Ten Years after the Fukushima Dai-Ichi Disaster. Plants 2022, 11, 222. [Google Scholar] [CrossRef]
- Dias, N.S.; Ferreira, J.F.S.; Liu, X.; Suarez, D.L. Jerusalem Artichoke (Helianthus Tuberosus L.) Maintains High Inulin, Tuber Yield, and Antioxidant Capacity under Moderately-Saline Irrigation Waters. Ind. Crops Prod. 2016, 94, 1009–1024. [Google Scholar] [CrossRef]
- Zhong, Q.; Yang, S.; Sun, X.; Wang, L.; Li, Y. The Complete Chloroplast Genome of the Jerusalem Artichoke (Helianthus Tuberosus L.) and an Adaptive Evolutionary Analysis of the Ycf2 Gene. PeerJ 2019, 7, e7596. [Google Scholar] [CrossRef] [PubMed]
- Liava, V.; Karkanis, A.; Danalatos, N.; Tsiropoulos, N. Cultivation Practices, Adaptability and Phytochemical Composition of Jerusalem Artichoke (Helianthus Tuberosus L.): A Weed with Economic Value. Agronomy 2021, 11, 914. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Sousa, L.; Cabanas, J.E.; Arrobas, M. Tuber Yield and Leaf Mineral Composition of Jerusalem Artichoke (Helianthus Tuberosus L.) Grown under Different Cropping Practices. Span. J. Agric. Res. 2007, 5, 545. [Google Scholar] [CrossRef]
- Puangbut, D.; Jogloy, S.; Vorasoot, N.; Patanothai, A. Responses of Growth, Physiological Traits and Tuber Yield in Helianthus Tuberosus to Seasonal Variations under Tropical Area. Sci. Hortic. 2015, 195, 108–115. [Google Scholar] [CrossRef]
- Shen, S.; Xu, G.; Li, D.; Yang, S.; Jin, G.; Liu, S.; Clements, D.R.; Chen, A.; Rao, J.; Wen, L.; et al. Potential Use of Helianthus Tuberosus to Suppress the Invasive Alien Plant Ageratina Adenophora under Different Shade Levels. BMC Ecol. Evol. 2021, 21, 85. [Google Scholar] [CrossRef] [PubMed]
- Chernobyl’s Legacy: Health, Environmental and Socio-Economic Impacts and Recommendations to the Governments of Belarus, the Russian Federation and Ukraine. In Proceedings of the The Chernobyl Forum 2003–2005; International Atomic Energy Agency (IAEA): Vienna, Austria, 2006; Volume 3.
- Góth, L. A Simple Method for Determination of Serum Catalase Activity and Revision of Reference Range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Kruger, N.J. The Bradford Method for Protein Quantitation. In The Protein Protocols Handbook; Walker, J.M., Ed.; Springer Protocols Handbooks; Humana Press: Totowa, NJ, USA, 2009; pp. 17–24. ISBN 978-1-60327-474-6. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Gibson, D.M.; Liu, E.H. Substrate Specificities of Peroxidase Isozymes in the Developing Pea Seedling. Ann. Bot. 1978, 42, 1075–1083. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R.; Cwalina-Ambroziak, B.; Janiak, M.A.; Bogucka, B. Effect of N Fertilization on the Content of Phenolic Compounds in Jerusalem Artichoke (Helianthus Tuberosus L.) Tubers and Their Antioxidant Capacity. Agronomy 2020, 10, 1215. [Google Scholar] [CrossRef]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of Total Flavonoid Content by Aluminum Chloride Assay: A Critical Evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Šrobárová, A.; Pavlová, A. Toxicity of Secondary Metabolites of the Fungus F. Culmorum in Relation to Resistance of Winter Wheat Cultivars. Cereal Res. Commun. 2001, 29, 101–108. [Google Scholar] [CrossRef]
- Zhou, C.Y.; Qu, W.; Cai, L.L. Combined Determination of Phosphorus and Arsenic in Molybdenum Concentrate by Molybdenum Blue Spectrophotometric Method. Adv. Mater. Res. 2014, 1033, 521–525. [Google Scholar] [CrossRef]
- Hernández-López, A.; Sánchez Félix, D.A.; Zuñiga Sierra, Z.; García Bravo, I.; Dinkova, T.D.; Avila-Alejandre, A.X. Quantification of Reducing Sugars Based on the Qualitative Technique of Benedict. ACS Omega 2020, 5, 32403–32410. [Google Scholar] [CrossRef]
- All-Russian Research Institute of Hydrometeorological Information. Available online: http://meteo.ru (accessed on 20 December 2022).
- Wang, T.; Li, Q.; Bi, K. Bioactive Flavonoids in Medicinal Plants: Structure, Activity and Biological Fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Rossini, F.; Provenzano, M.E.; Kuzmanović, L.; Ruggeri, R. Jerusalem Artichoke (Helianthus Tuberosus L.): A Versatile and Sustainable Crop for Renewable Energy Production in Europe. Agronomy 2019, 9, 528. [Google Scholar] [CrossRef]
- Kenyon, J.A.; Buesseler, K.O.; Casacuberta, N.; Castrillejo, M.; Otosaka, S.; Masqué, P.; Drysdale, J.A.; Pike, S.M.; Sanial, V. Distribution and Evolution of Fukushima Dai-Ichi Derived 137 Cs, 90 Sr, and 129 I in Surface Seawater off the Coast of Japan. Environ. Sci. Technol. 2020, 54, 15066–15075. [Google Scholar] [CrossRef] [PubMed]
- Arutyunyan, R.V.; Bol’shov, L.A.; Vasil’ev, S.K.; Evdokimov, I.V.; Petrov, B.F.; Pleskachevskii, L.A. Statistical Characteristics of the Spatial Distribution of Territorial Contamination by Radionuclides from the Chernobyl Accident. At. Energy 1993, 75, 940–945. [Google Scholar] [CrossRef]
- Dighton, J.; Tugay, T.; Zhdanova, N. Fungi and Ionizing Radiation from Radionuclides: Fungi and Ionizing Radiation. FEMS Microbiol. Lett. 2008, 281, 109–120. [Google Scholar] [CrossRef]
- Esnault, M.-A.; Legue, F.; Chenal, C. Ionizing Radiation: Advances in Plant Response. Environ. Exp. Bot. 2010, 68, 231–237. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Goh, E.J.; Kim, J.-B.; Kim, W.-J.; Ha, B.-K.; Kim, S.H.; Kang, S.; Seo, Y.W.; Kim, D.S. Physiological Changes and Anti-Oxidative Responses of Arabidopsis Plants after Acute and Chronic γ-Irradiation. Radiat. Environ. Biophys. 2014, 53, 677–693. [Google Scholar] [CrossRef]
- Ramabulana, T.; Mavunda, R.D.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A.; Madala, N.E. Secondary Metabolite Perturbations in Phaseolus Vulgaris Leaves Due to Gamma Radiation. Plant Physiol. Biochem. 2015, 97, 287–295. [Google Scholar] [CrossRef]
- Volkova, P.Y.; Geras’kin, S.A.; Kazakova, E.A. Radiation Exposure in the Remote Period after the Chernobyl Accident Caused Oxidative Stress and Genetic Effects in Scots Pine Populations. Sci. Rep. 2017, 7, 43009. [Google Scholar] [CrossRef]
- Hanafy, R.S.; Akladious, S.A. Physiological and Molecular Studies on the Effect of Gamma Radiation in Fenugreek (Trigonella Foenum-Graecum L.) Plants. J. Genet. Eng. Biotechnol. 2018, 16, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, S.S.; Jaafar, H.; Ibrahim, R.; Rahmat, A.; Aziz, M.A.; Philip, E. Effects of Acute Gamma Irradiation on Physiological Traits and Flavonoid Accumulation of Centella Asiatica. Molecules 2011, 16, 4994–5007. [Google Scholar] [CrossRef]
- Taheri, S.; Abdullah, T.; Karimi, E.; Oskoueian, E.; Ebrahimi, M. Antioxidant Capacities and Total Phenolic Contents Enhancement with Acute Gamma Irradiation in Curcuma Alismatifolia (Zingiberaceae) Leaves. Int. J. Mol. Sci. 2014, 15, 13077–13090. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.; Kumar, M.; Kumar, P.; Gupta, V.K.; Singhal, R.K.; Yadav, A.; Singh, B. Metabolic and Biochemical Changes Caused by Gamma Irradiation in Plants. J. Radioanal. Nucl. Chem. 2014, 300, 199–212. [Google Scholar] [CrossRef]
- Archanachai, K.; Teepoo, S.; Sansenya, S. Effect of Gamma Irradiation on Growth, Proline Content, Bioactive Compound Changes, and Biological Activity of 5 Popular Thai Rice Cultivars. J. Biosci. Bioeng. 2021, 132, 372–380. [Google Scholar] [CrossRef]
- Falahati, A.; Kazemitabar, S.K.; Bahrami, A.R.; Lahouti, M.; Rahimi, M.F. The Study of Gamma Irradiation Effects on Drought Tolerance in Rice (Oryza Sativa L.). Indian J. Crop Sci. 2007, 2, 155–158. [Google Scholar]
- Hussein, H.-A.A. Influence of Radio-Grain Priming on Growth, Antioxidant Capacity, and Yield of Barley Plants. Biotechnol. Rep. 2022, 34, e00724. [Google Scholar] [CrossRef]
- Duarte, G.T.; Volkova, P.Y.; Geras’kin, S.A. The Response Profile to Chronic Radiation Exposure Based on the Transcriptome Analysis of Scots Pine from Chernobyl Affected Zone. Environ. Pollut. 2019, 250, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Mounir, A.M.; El-Hefny, A.M.; Mahmoud, S.H.; El-Tanahy, A.M.M. Effect of Low Gamma Irradiation Doses on Growth, Productivity and Chemical Constituents of Jerusalem Artichoke (Helianthus Tuberosus) Tubers. Bull. Natl. Res. Cent. 2022, 46, 146. [Google Scholar] [CrossRef]
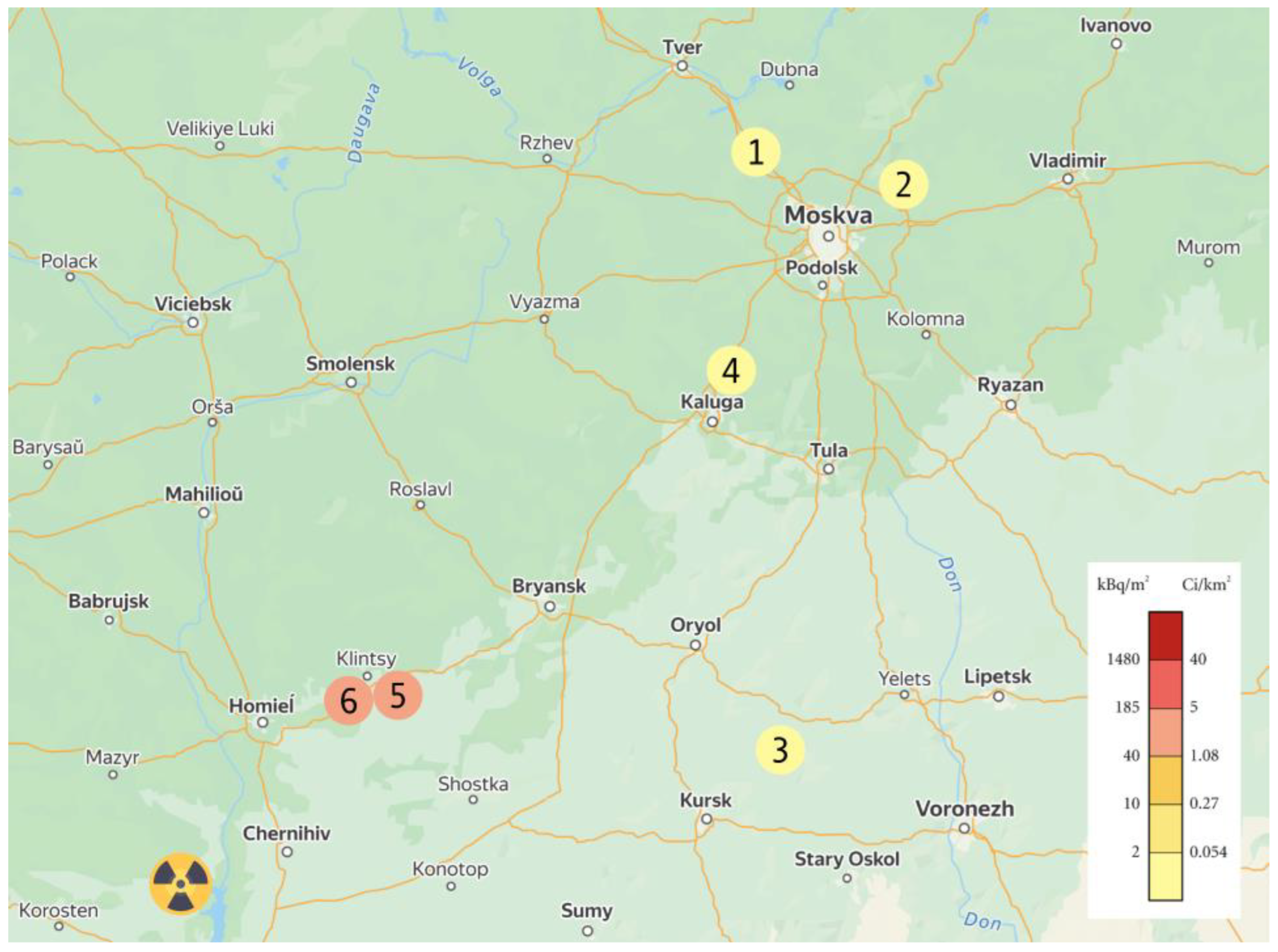


| № | Areas | Coordinates | Description |
|---|---|---|---|
| 1 | K1 | Discreet city Solnechnogorsk, 56.121170° N 36.889667° W | Field, 15 m from the road sod-podzolic soil |
| 2 | K2 | Discreet city Chernogolovka, 56.050380° N 38.423595° W | Field, 50 m from the road sod-podzolic soil |
| 3 | K3 | Orel region, 52.220666° N 37.020390° W | Field, 20 m from the road chernozemic soil |
| 4 | K4 | Discreet city Zhukov, 51.709466° N 36.123476° W | Field, 30 m from the road sod-podzolic soil |
| 5 | OLKH | Discreet city Klintsy, 52.742743° N 32.130348° W | Field, 50 m from the road sod-podzolic soil |
| 6 | ROZH | Discreet city Klintsy, 52.740622° N 32.083456° W | Field, 40 m from the road sod-podzolic soil |
| № | Areas | Weather Station | Coordinates |
|---|---|---|---|
| 1 | K1 | “Moscow (VDNH)” | Moscow, 55.830286° N 37.623578° W |
| 2 | K2 | ||
| 3 | K3 | “Ponyri” | Orel region, 52.320755° N 36.311341° W |
| 4 | K4 | “Kolomna” | Kolomna, 55.137384° N 38.730738° W |
| 5 | OLKH | “Krasnya gora” | Discreet city Klintsy, 52.999033° N 31.602508° W |
| 6 | ROZH |
| Areas | A 137Cs, Bq·kg−1 Soil | Density of Surface Contamination, Cu/km2 | Level of Contamination | 137Cs Activity in Tubers | TC |
|---|---|---|---|---|---|
| K1 | 23.92 ± 0.91 | 0.19 ± 0.01 | - | Not detriment | - |
| K2 | 18.14 ± 1.62 | 0.15 ± 0.01 | - | Not detriment | - |
| K3 | 52.23 ± 1.73 | 0.42 ± 0.01 | - | Not detriment | - |
| K4 | 21.41 ± 0.72 | 0.17 ± 0.01 | - | Not detriment | - |
| OLKH | 570.78 ± 13.66 | 4.64 ± 0.03 | low | 31.96 ± 2.43 | 0.056 |
| ROZH | 350.95 ± 3.40 | 2.85 ± 0.03 | low | 18.51 ± 1.13 | 0.053 |
| Areas | APX Activity, ncat/g Protein | CAT Activity, ncat/g Protein | POX Activity, ncat/g Protein |
|---|---|---|---|
| K1 | 505.12 ± 65.90 | 293.11 ± 14.18 | 491.20 ± 165.38 |
| K2 | 198.51 ± 109.67 | 282.45 ± 21.32 | 500.03 ± 190.54 |
| K3 | 234.34 ± 52.14 | 295.22 ± 70.53 | 568.09 ± 89.86 |
| K4 | 500.49 ± 62.79 | 191.12 ± 46.04 | 701.44 ± 202.94 |
| OLKH | 733.87 ± 62.34 * | 366.96 ± 19.94 | 569.34 ± 112.58 |
| ROZH | 821.20 ± 119.17 * | 216.37 ± 26.91 | 438.61 ± 140.20 |
| Areas | WPC, μg/mg DW | APC, μg/mg DW | WTA, μg/mg DW | LTA, μg/mg DW | PRO, μg/mg DW | AsA, μg/mg DW | RS, μg/mg DW | FL, μg/mg DW |
|---|---|---|---|---|---|---|---|---|
| K1 | 0.59 ± 0.03 | 0.426 ± 0.008 | 0.30 ± 0.001 | 0.55 ± 0.06 | 0.1605 ± 0.0111 | 0.00631 ± 0.00055 | 0.047 ± 0.004 | 0.21 ± 0.03 |
| K2 | 0.69 ± 0.05 | 0.445 ± 0.127 | 0.28 ± 0.010 | 0.55 ± 0.05 | 0.1220 ± 0.0125 | 0.00667 ± 0.00087 | 0.046 ± 0.001 | 0.24 ± 0.02 |
| K3 | 0.48 ± 0.09 | 0.258 ± 0.043 | 0.26 ± 0.005 | 0.56 ± 0.06 | 0.1464 ± 0.0075 | 0.00248 ± 0.00015 | 0.047 ± 0.006 | 0.14 ± 0.02 |
| K4 | 0.71 ± 0.03 | 0.510 ± 0.046 | 0.28 ± 0.002 | 0.27 ± 0.02 | 0.1480 ± 0.0203 | 0.00193 ± 0.00004 | 0.052 ± 0.006 | 0.22 ± 0.02 |
| OLKH | 1.07 ± 0.02 **** | 1.074 ± 0.034 **** | 0.30 ± 0.004 | 0.64 ± 0.02 | 0.1522 ± 0.0102 | 0.02089 ± 0.00026 **** | 0.048 ± 0.004 | 0.20 ± 0.01 |
| ROZH | 0.71 ± 0.02 * | 0.407 ± 0.029 | 0.28 ± 0.002 | 0.46 ± 0.05 | 0.1343 ± 0.0005 | 0.01864 ± 0.00061 **** | 0.053 ± 0.002 | 0.17 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polivanova, O.B.; Tiurin, K.N.; Sivolapova, A.B.; Goryunova, S.V.; Zhevora, S.V. Influence of Increased Radiation Background on Antioxidative Responses of Helianthus tuberosus L. Antioxidants 2023, 12, 956. https://doi.org/10.3390/antiox12040956
Polivanova OB, Tiurin KN, Sivolapova AB, Goryunova SV, Zhevora SV. Influence of Increased Radiation Background on Antioxidative Responses of Helianthus tuberosus L. Antioxidants. 2023; 12(4):956. https://doi.org/10.3390/antiox12040956
Chicago/Turabian StylePolivanova, Oksana B., Kirill N. Tiurin, Anastasia B. Sivolapova, Svetlana V. Goryunova, and Sergey V. Zhevora. 2023. "Influence of Increased Radiation Background on Antioxidative Responses of Helianthus tuberosus L." Antioxidants 12, no. 4: 956. https://doi.org/10.3390/antiox12040956
APA StylePolivanova, O. B., Tiurin, K. N., Sivolapova, A. B., Goryunova, S. V., & Zhevora, S. V. (2023). Influence of Increased Radiation Background on Antioxidative Responses of Helianthus tuberosus L. Antioxidants, 12(4), 956. https://doi.org/10.3390/antiox12040956








