Health Benefits of Antioxidant Bioactive Compounds in the Fruits and Leaves of Lonicera caerulea L. and Aronia melanocarpa (Michx.) Elliot
Abstract
1. Introduction
2. Lonicera caerulea Plant
3. Aronia melanocarpa Plant
4. Bioactive Compounds of Lonicera caerulea Fruits and Leaves
4.1. Composition in Bioactive Compounds
4.2. Phenolic Compounds
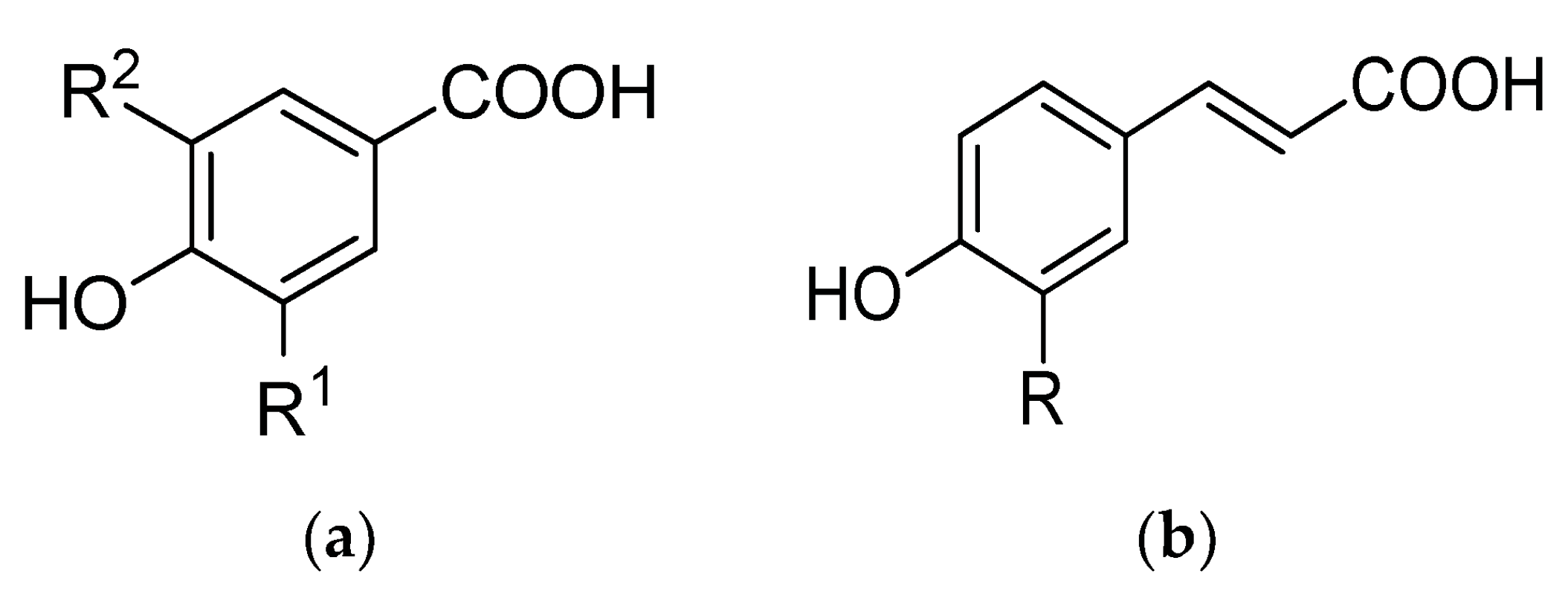
4.2.1. Phenolic Acids and Flavonoids
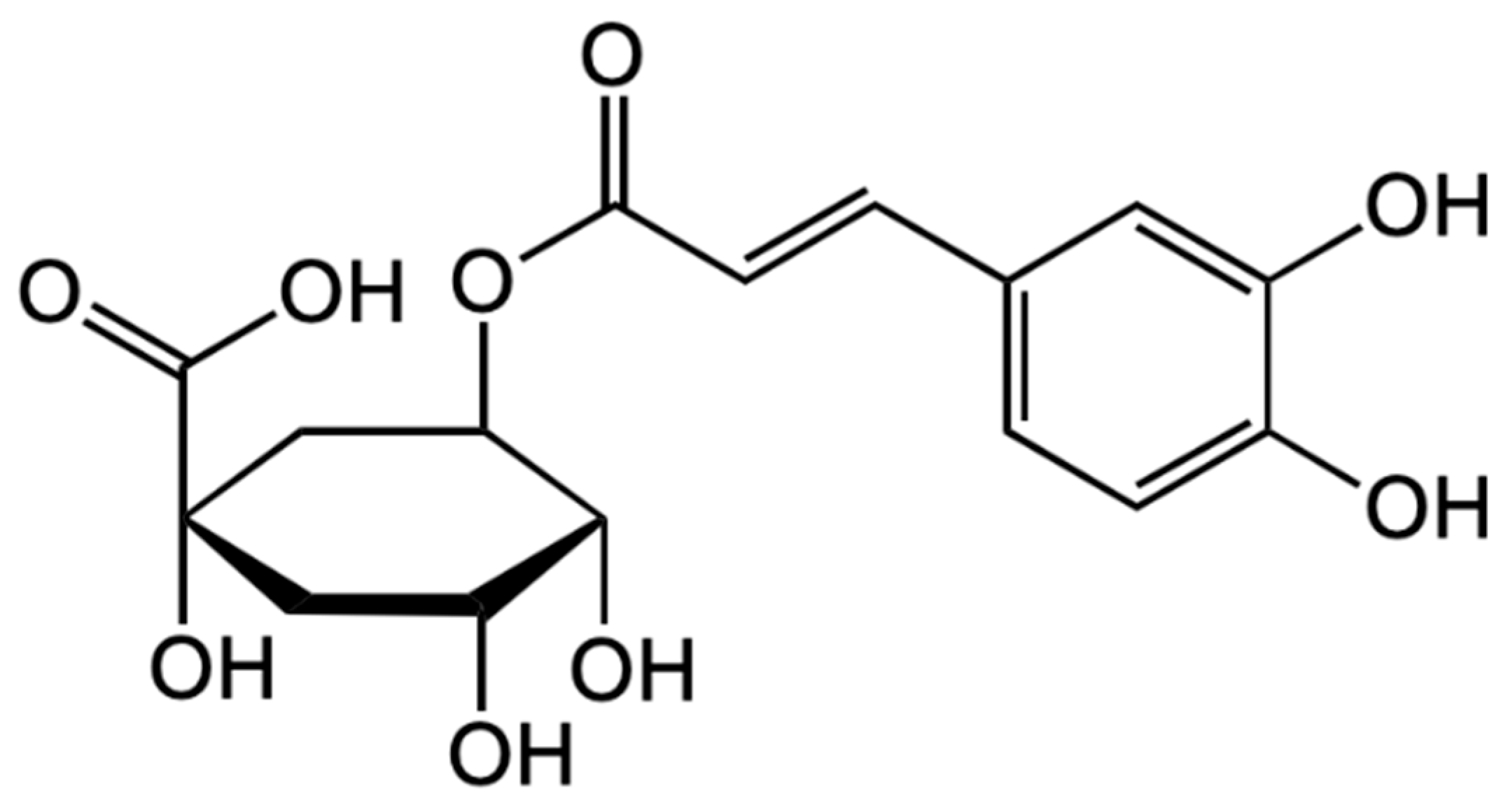
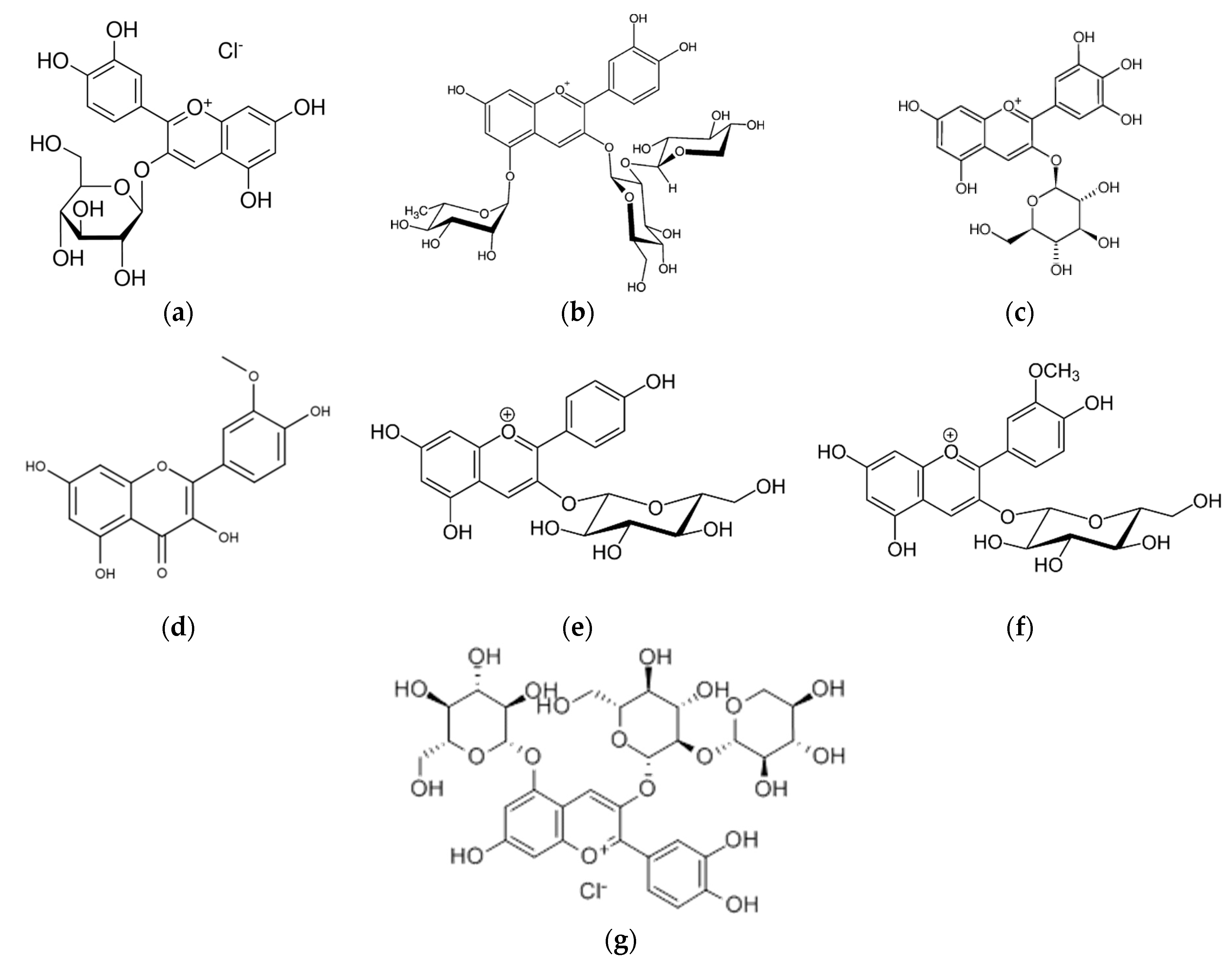
4.2.2. Anthocyanins
4.2.3. Other Compounds
4.2.4. Bioactive Compounds of Lonicera caerulea Leaves
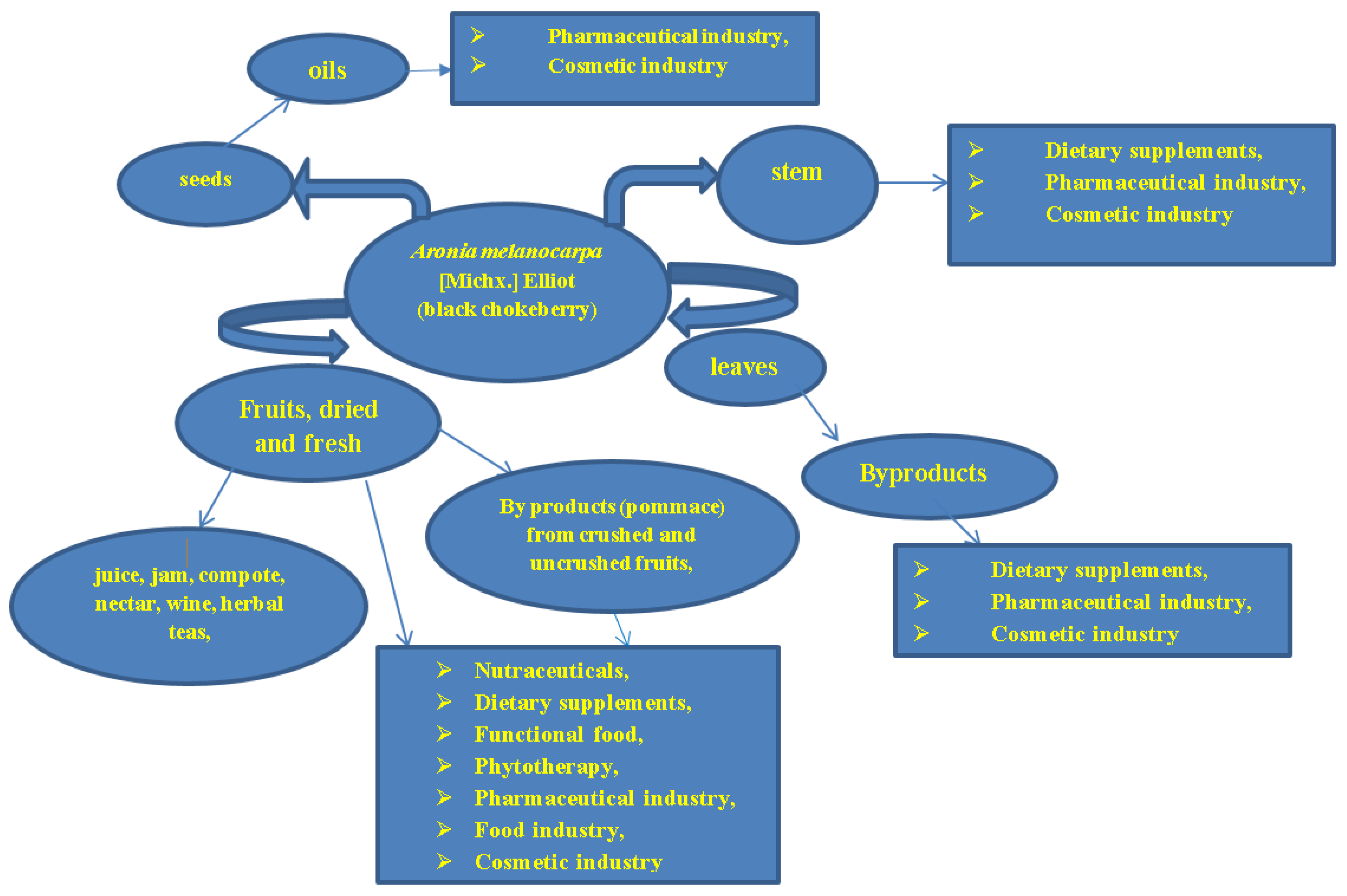
5. Bioactive Compounds of Aronia melanocarpa Fruits and Leaves
5.1. Bioactive Compounds
5.1.1. Phenolic Compounds of Aronia Fruits
5.1.2. Phenolic Compounds of Aronia Leaves
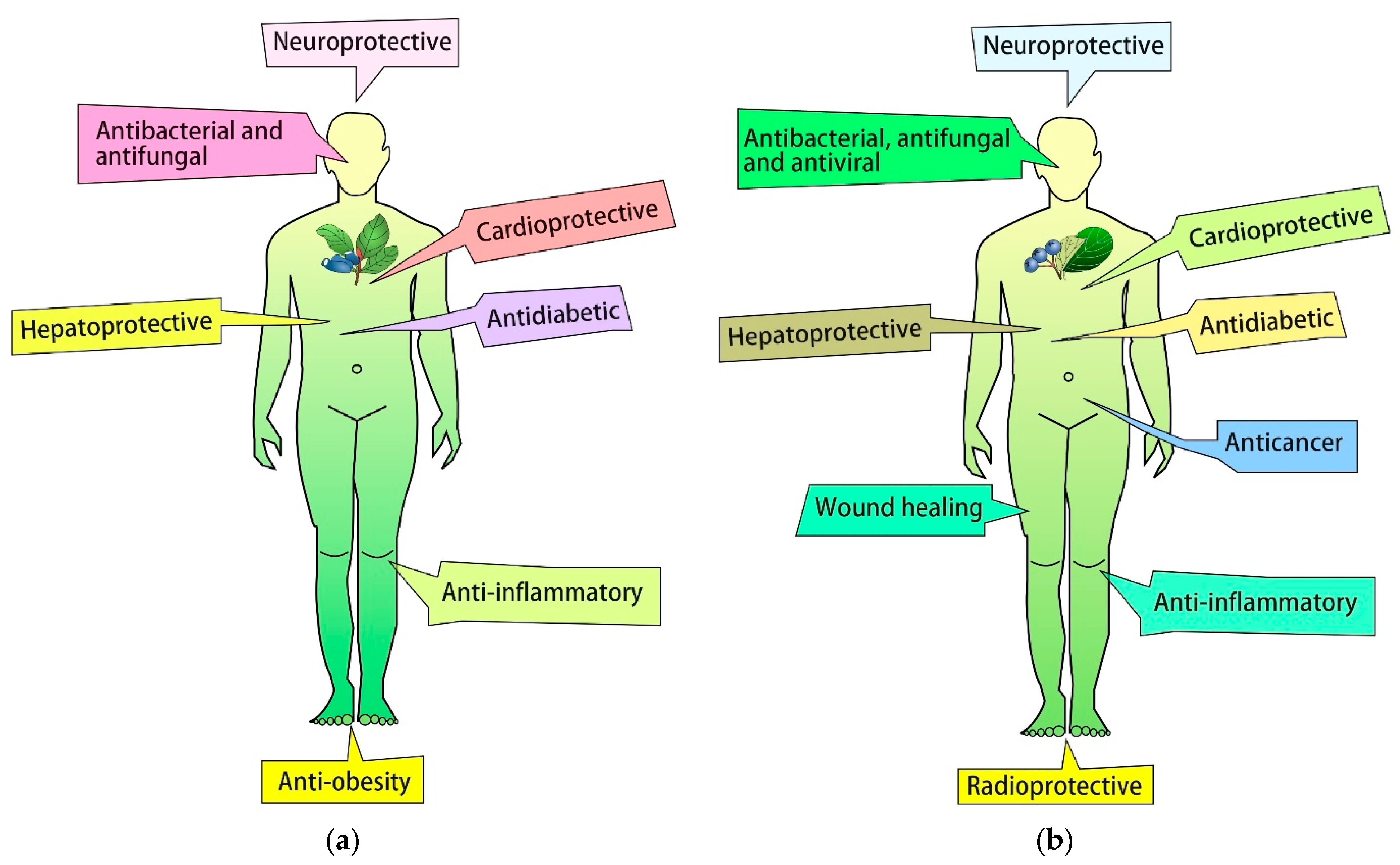
6. Bioactive Properties of L. caerulea and A. melanocarpa Fruits and Leaves
6.1. Antioxidant Activity
6.2. Anti-Inflammatory Activity
6.3. Antitumor Activity
6.4. Antibacterial and Antifungal Activity
6.5. Anti-Diabetic and Anti-Obesity Activity
6.6. Hepatoprotective Activity
6.7. Other Biological Activities
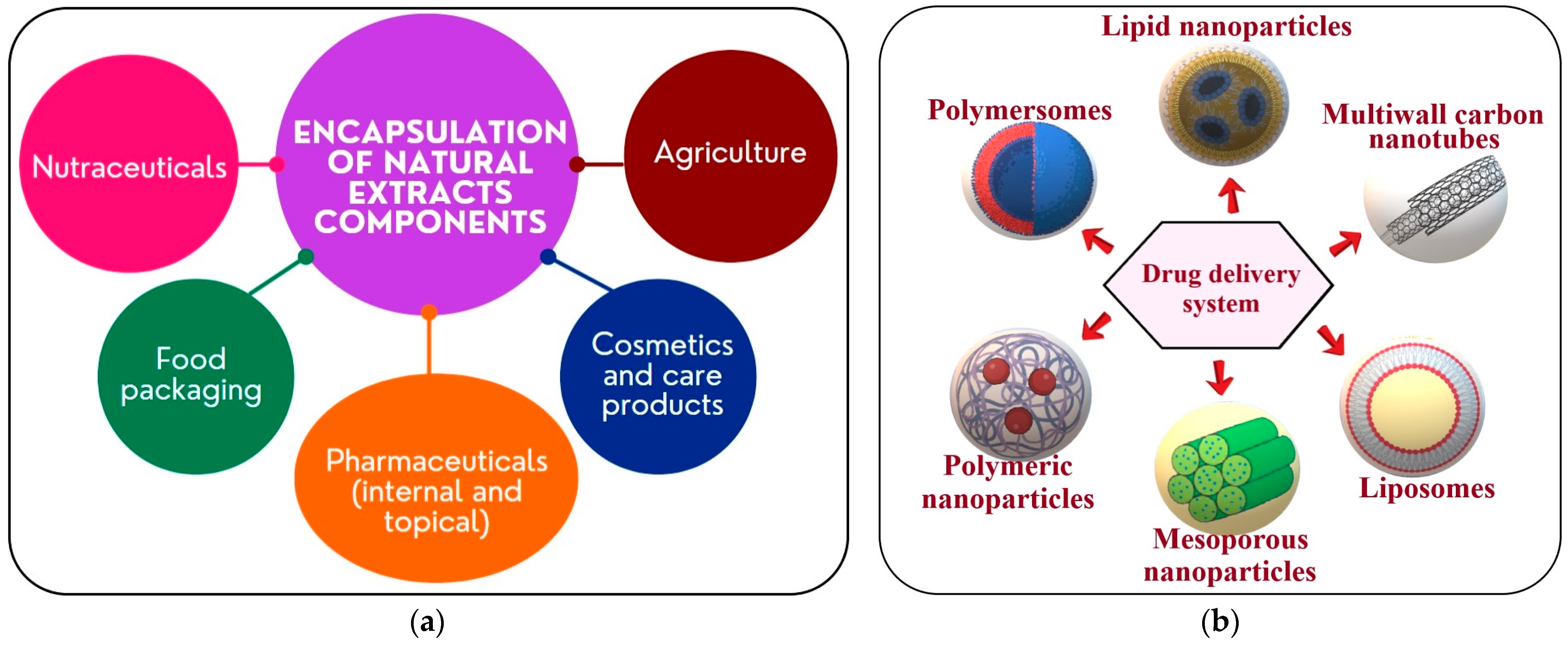
7. Encapsulation and Delivery Systems of the Bioactive Compounds
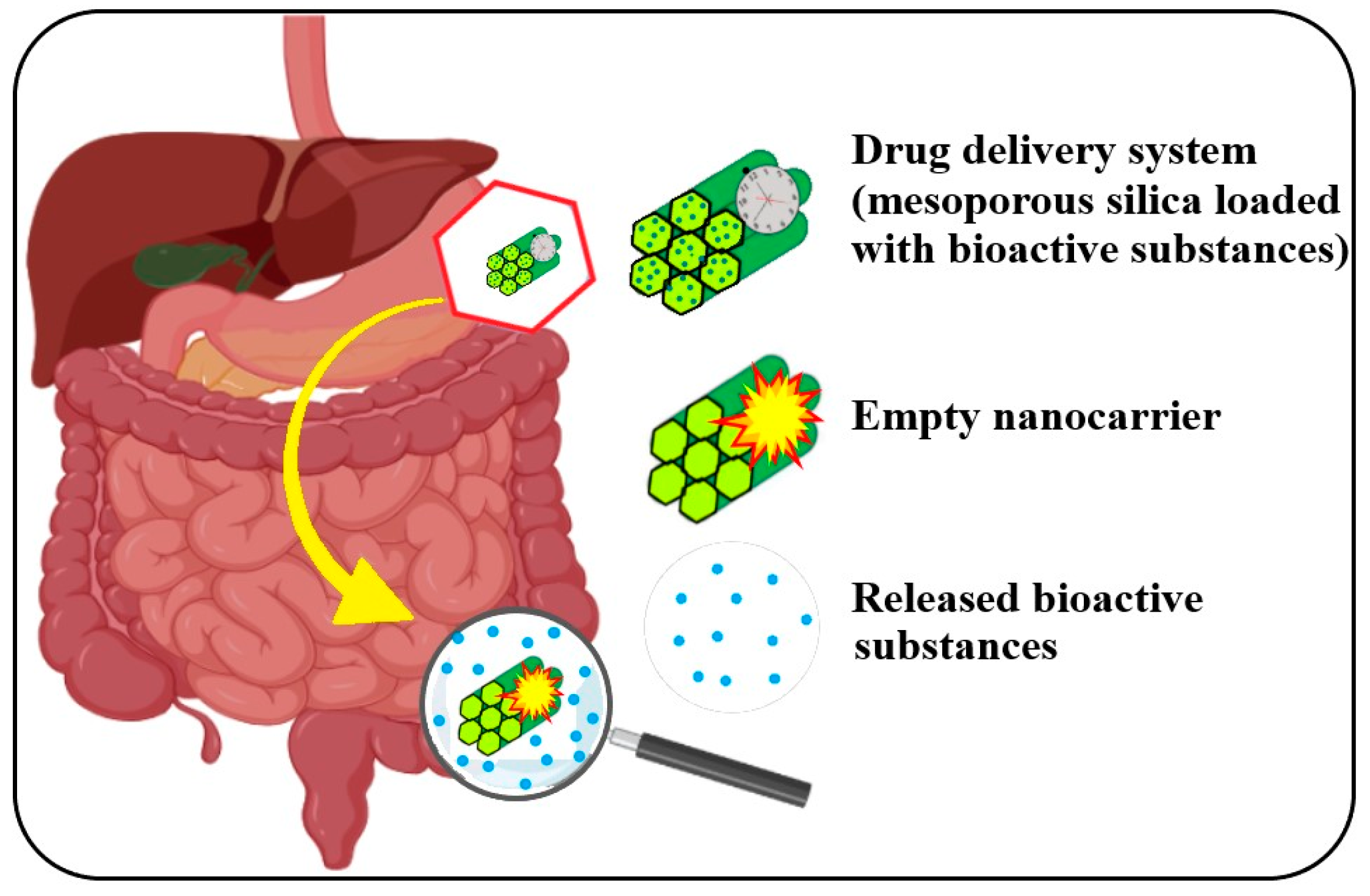
7.1. Encapsulation of A. melanocarpa or L. caerulea Bioactive Compounds for Oral Administration
7.2. Encapsulation of L. caerulea and A. melanocarpa Extracts and Their Advantages
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Komarnicka, U.K.; Lesiow, M.K.; Witwicki, M.; Bienko, A. The bright and dark sides of reactive oxygen species generated by copper-peptide complexes. Separations 2022, 9, 73. [Google Scholar] [CrossRef]
- Djacbou, D.S.; Pieme, C.A.; Biapa, P.C.; Penlap, B.V. Comparison of in vitro antioxidant properties of extracts from three plants used for medical purpose in Cameroon: Acalypha racemosa, Garcinia lucida and Hymenocardia lyrata. Asian Pac. J. Trop. Bio. 2014, 4, S625–S632. [Google Scholar]
- Aguilar, T.A.F.; Navarro, B.C.H.; Pérez, J.A.M. Endogenous Antioxidants: A Review of Their Role in Oxidative Stress; IntechOpen: London, UK, 2016; Volume The Transcription Factor Nrf2. [Google Scholar]
- Spoiala, A.; Ilie, C.I.; Ficai, D.; Ficai, A.; Andronescu, E. Synergic effect of honey with other natural agents in developing efficient wound dressings. Antioxidants 2023, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Bratu, M.M.; Negreanu-Pirjol, T. Elderberries Extracts: Biologic Effects, Application for Therapy: A Review; Nova Science Publishers Inc.: New York, NY, USA, 2015; Chapter 12. [Google Scholar]
- Negreanu-Pirjol, T.; Negreanu-Pirjol, B.S.; Popescu, A.; Bratu, M.M.; Udrea, M.; Busuricu, F. Comparative antioxidant properties of some Romanian food fruits extracts. J. Environ. Prot. Ecol. 2014, 15, 1139–1148. [Google Scholar]
- Brezoiu, A.M.; Bajenaru, L.; Berger, D.; Mitran, R.A.; Deaconu, M.; Lincu, D.; Guzun, A.S.; Matei, C.; Moisescu, M.G.; Negreanu-Pirjol, T. Effect of nanoconfinement of polyphenolic extract from grape pomace into functionalized mesoporous silica on its biocompatibility and radical scavenging activity. Antioxidants 2020, 9, 696. [Google Scholar] [CrossRef]
- Alhazmi, H.A.; Najmi, A.; Javed, S.A.; Sultana, S.; Al Bratty, M.; Makeen, H.A.; Meraya, A.M.; Ahsan, W.; Mohan, S.; Taha, M.M.E.; et al. Medicinal plants and isolated molecules demonstrating immunomodulation activity as potential alternative therapies for viral diseases including COVID-19. Front. Immunol. 2021, 12, 637553. [Google Scholar] [CrossRef]
- Raza, S.A.; Rashid, A.; William, J.; Arshed, S.F.; Arshad, M. Comparison of antioxidant activity of some medicinally important plants from Pakistan. Acta Sci. Pol. Technol. Aliment. 2013, 4, 403–410. [Google Scholar]
- Negreanu-Pirjol, B.-S.; Negreanu-Pirjol, T.; Jurja, S.; Moise, I.; Lepadatu, A.C. Comparative Antimicrobial Activity of Some Indigenous Berries Fruits Extracts. In Proceedings of the 17th International Multidisciplinary Scientific GeoConferences—SGEM 2017—Nano, Bio and Green–Technologies for a Sustainable Future, Albena, Bulgaria, 29 June–5 July 2017; pp. 569–576. [Google Scholar]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, antioxidant efficacies, and health effects—A comprehensive review. Crit. Rev. Food Sci. 2019, 59, 1580–1604. [Google Scholar] [CrossRef]
- Grosso, G. Effects of polyphenol-rich foods on human health. Nutrients 2018, 10, 1089. [Google Scholar] [CrossRef]
- Negreanu-Pirjol, T.; Sirbu, R.; Negreanu-Pirjol, B.S. Antioxidant activity of some nutraceuticals based on Romanian black and red fruits mixed extracts. Acad. J. Interdiscip. Stud. 2015, 4, 199–206. [Google Scholar] [CrossRef]
- Negreanu-Pîrjol, B.S.; Cadar, E.; Sirbu, R.; Negreanu-Pirjol, T. Antioxidant activity of some fluids extracts of indigenous wild cherry fruits. Eur. J. Nat. Sci. Med. 2021, 4, 48–58. [Google Scholar] [CrossRef]
- Negreanu-Pirjol, B.-S.; Negreanu-Pirjol, T.; Popoviciu, D.R.; Artem, V.; Ranca, A.; Craciunescu, O.; Prelipcean, A.-M.; Motelica, L.; Vasile, M. Preliminary data regarding bioactive compounds and total antioxidant capacity of some fluid extracts of lonicera caerulea l. Berries. U.P.B. Sci. Bull. Ser. B 2023, 85, 101–116. [Google Scholar]
- Condrea, E.; Mirea, M.; Aivaz, K.A.; Nitu, O. Risk analysis—Important activity in the food safety management. Hazards and risks identified in the milk processing industry. J. Environ. Prot. Ecol. 2012, 13, 2151–2160. [Google Scholar]
- Vancea, D.P.C.; Aivaz, K.A.; Simion, L.; Vanghele, D. Export expansion policies. An analysis of romanian exports between 2005-2020 using the principal component analysis method and short recommandations for increasing this activity. Transform. Bus. Econ. 2021, 20, 614–634. [Google Scholar]
- Shahin, L.; Phaal, S.S.; Vaidya, B.N.; Brown, J.E.; Joshee, N. Aronia (Chokeberry): An underutilized, highly nutraceutical plant. J. Med. Act. Plants 2019, 8, 46–63. [Google Scholar]
- Kalwij, J.M. Review of the plant list, a working list of all plant species’. J. Veg. Sci. 2012, 23, 998–1002. [Google Scholar] [CrossRef]
- North American Cornucopia: Top 100 Indigenous Food Plants; CRC Press: Boca Raton, FL, USA, 2013.
- USDA, NRCS (n.d.). “Lonicera caerulea”. The plants database. Available online: plants.usda.gov (accessed on 6 January 2023).
- Lahring, H. Water and Wetland Plants of the Prairie Provinces; Canadian Plains Research Center, University of Regina Press: Regina, SK, Canada, 2003. [Google Scholar]
- Aguilera, J.M.; Toledo, T. Wild berries and related wild small fruits as traditional healthy foods. Crit. Rev. Food Sci. 2022, 14, 1–5. [Google Scholar] [CrossRef]
- Raudone, L.; Liaudanskas, M.; Vilkickyte, G.; Kviklys, D.; Zvikas, V.; Viskelis, J.; Viskelis, P. Phenolic profiles, antioxidant activity and phenotypic characterization of Lonicera caerulea L. Berries, cultivated in Lithuania. Antioxidants 2021, 10, 115. [Google Scholar] [CrossRef]
- Dayar, E.; Cebova, M.; Lietava, J.; Panghyova, E.; Pechanova, O. Antioxidant effect of Lonicera caerulea L In the cardiovascular system of obese zucker rats. Antioxidants 2021, 10, 1199. [Google Scholar] [CrossRef]
- Plekhanova, M.N. Blue honeysuckle (Lonicera caerulea L.)—A new commercial berry crop for temperate climate: Genetic resources and breeding. Eucarpia Symp. Fruit Breed. Genet. 2000, 538, 159–164. [Google Scholar] [CrossRef]
- Svarcova, I.; Heinrich, J.; Valentova, K. Berry fruıts as a source of biologically active compounds: The case of Lonicera caerulea. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2007, 151, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Celli, G.B.; Ghanem, A.; Brooks, M.S.L. Haskap berries (Lonicera caerulea L.)—A critical review of antioxidant capacity and health-related studies for potential value-added products. Food Bioprocess. Technol. 2014, 7, 1541–1554. [Google Scholar] [CrossRef]
- Luo, J.Y.; Fan, Z.L.; Yang, X.; Bao, Y.H.; Liang, M.; Guo, Y. Anthocyanins and antioxidant activity of Lonicera caerulea berry wine during different processes. Food Sci. Tech.-Braz. 2022, 42, e25121. [Google Scholar] [CrossRef]
- Snebergrova, J.; Cizkova, H.; Neradova, E.; Kapci, B.; Rajchl, A.; Voldrich, M. Variability of characteristic components of aronia. Czech J. Food Sci. 2014, 32, 25–30. [Google Scholar] [CrossRef]
- Oszmianski, J.; Lachowicz, S. Effect of the production of dried fruits and juice from chokeberry (Aronia melanocarpa L.) on the content and antioxidative activity of bioactive compounds. Molecules 2016, 21, 1098. [Google Scholar] [CrossRef]
- Mladin, G.; Mladin, P.; Oprea, E.; Isac, V.; Ancu, I. Bluehoneysuckle (Lonicera caerulea var. Kamtschatica (sevast.Pojark.) a valuable specıes for fruıts growıng and human health. Lucr. Științifice Ser. Hortic. 2010, 53, 347–352. [Google Scholar]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Soja, J.; Gancarz, M.; Wojtunik-Kulesza, K.; Markut-Miotla, E.; Oniszczuk, A. The efficacy of black chokeberry fruits against cardiovascular diseases. Int. J. Mol. Sci. 2021, 22, 6541. [Google Scholar] [CrossRef]
- Piasecka, I.; Gorska, A.; Ostrowska-Ligeza, E.; Kalisz, S. The study of thermal properties of blackberry, chokeberry and raspberry seeds and oils. Appl. Sci. 2021, 11, 7704. [Google Scholar] [CrossRef]
- Piasecka, I.; Wiktor, A.; Gorska, A. Alternative methods of bioactive compounds and oils extraction from berry fruit by-products-a review. Appl. Sci. 2022, 12, 1734. [Google Scholar] [CrossRef]
- Zlatanov, M.D. Lipid composition of bulgarian chokeberry, black currant and rose hip seed oils. J. Sci. Food Agric. 1999, 79, 1620–1624. [Google Scholar] [CrossRef]
- Zlabur, J.S.; Dobricevic, N.; Pliestic, S.; Galic, A.; Bilic, D.P.; Voca, S. Antioxidant potential of fruit juice with added chokeberry powder (Aronia melanocarpa). Molecules 2017, 22, 2158. [Google Scholar]
- Jara-Palacios, M.J.; Santisteban, A.; Gordillo, B.; Hernanz, D.; Heredia, F.J.; Escudero-Gilete, M.L. Comparative study of red berry pomaces (blueberry, red raspberry, red currant and blackberry) as source of antioxidants and pigments. Eur. Food Res. Technol. 2019, 245, 1–9. [Google Scholar] [CrossRef]
- Halasz, K.; Csoka, L. Black chokeberry (Aronia melanocarpa) pomace extract immobilized in chitosan for colorimetric ph indicator film application. Food Packag. Shelf 2018, 16, 185–193. [Google Scholar] [CrossRef]
- Milek, M.; Grabek-Lejko, D.; Stepien, K.; Sidor, E.; Molon, M.; Dzugan, M. The enrichment of honey with Aronia melanocarpa fruits enhances its in vitro and in vivo antioxidant potential and intensifies its antibacterial and antiviral properties. Food Funct. 2021, 12, 8920–8931. [Google Scholar] [CrossRef]
- Raudsepp, P.; Koskar, J.; Anton, D.; Meremae, K.; Kapp, K.; Laurson, P.; Bleive, U.; Kaldmae, H.; Roasto, M.; Pussa, T. Antibacterial and antioxidative properties of different parts of garden rhubarb, blackcurrant, chokeberry and blue honeysuckle. J. Sci. Food Agric. 2019, 99, 2311–2320. [Google Scholar] [CrossRef]
- Rupasinghe, H.; Boehm, M.; Sekhon-Loodu, S.; Parmar, I.; Bors, B.; Jamieson, A. Anti-inflammatory activity of haskap cultivars is polyphenols-dependent. Biomolecules 2015, 5, 1079–1098. [Google Scholar] [CrossRef]
- Gorzelany, J.; Basara, O.; Kapusta, I.; Pawel, K.; Belcar, J. Evaluation of the chemical composition of selected varieties of L. caerulea var. Kamtschatica and L. caerulea var. Emphyllocalyx. Molecules 2023, 28, 2525. [Google Scholar] [CrossRef]
- Senica, M.; Stampar, F.; Mikulic-Petkovsek, M. Blue honeysuckle (Lonicera cearulea L. Subs. Edulis) berry; a rich source of check for some nutrients and their differences among four different cultivars. Sci. Hortic. 2018, 238, 215–221. [Google Scholar] [CrossRef]
- Jurikova, T.; Rop, O.; Mlcek, J.; Sochor, J.; Balla, S.; Szekeres, L.; Hegedusova, A.; Hubalek, J.; Adam, V.; Kizek, R. Phenolic profile of edible honeysuckle berries (genus Lonicera) and their biological effects. Molecules 2012, 17, 61–79. [Google Scholar] [CrossRef]
- Wojdylo, A.; Jauregui, P.N.N.; Carbonell-Barrachina, A.A.; Oszmianski, J.; Golis, T. Variability of phytochemical properties and content of bioactive compounds in Lonicera caerulea L. Var. Kamtschatica berries. J. Agric. Food Chem. 2013, 61, 12072–12084. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Jurikova, T.; Sochor, J.; Rop, O.; Mlcek, J.; Balla, S.; Szekeres, L.; Zitny, R.; Zitka, O.; Adam, V.; Kizek, R. Evaluation of polyphenolic profile and nutritional value of non-traditional fruit species in the czech republic—A comparative study. Molecules 2012, 17, 8968–8981. [Google Scholar] [CrossRef] [PubMed]
- Khattab, R.; Brooks, M.S.L.; Ghanem, A. Phenolic analyses of haskap berries (Lonicera caerulea L.): Spectrophotometry versus high performance liquid chromatography. Int. J. Food Prop. 2016, 19, 1708–1725. [Google Scholar] [CrossRef]
- Ochmian, I.; Skupien, K.; Grajkowski, J.; Smolik, M.; Ostrowska, K. Chemical composition and physical characteristics of fruits of two cultivars of blue honeysuckle (Lonicera caerulea L.) in relation to their degree of maturity and harvest date. Not. Bot. Horti Agrobo 2012, 40, 155–162. [Google Scholar] [CrossRef]
- Becker, R.; Szakiel, A. Phytochemical characteristics and potential therapeutic properties of blue honeysuckle Lonicera caerulea L. (caprifoliaceae). J. Herb. Med. 2019, 16, 100237. [Google Scholar] [CrossRef]
- Furia, E.; Beneduci, A.; Malacaria, L.; Fazio, A.; La Torre, C.; Plastina, P. Modeling the solubility of phenolic acids in aqueous media at 37 degrees c. Molecules 2021, 26, 6500. [Google Scholar] [CrossRef]
- Zadernowski, R.; Naczk, M.; Nesterowicz, J. Phenolic acid profiles in some small berries. J. Agric. Food Chem. 2005, 53, 2118–2124. [Google Scholar] [CrossRef]
- Kucharska, A.Z.; Sokol-Letowska, A.; Oszmianski, J.; Piorecki, N.; Fecka, I. Iridoids, phenolic compounds and antioxidant activity of edible honeysuckle berries (Lonicera caerulea var. Kamtschatica sevast.). Molecules 2017, 22, 405. [Google Scholar] [CrossRef]
- Khattab, R.; Celli, G.B.; Ghanem, A.; Brooks, M.S.L. Effect of frozen storage on polyphenol content and antioxidant activity of haskap berries (Lonicera caerulea L.). J. Berry Res. 2015, 5, 231–242. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef]
- Jiang, Y.; Kusama, K.; Satoh, K.; Takayama, F.; Watanabe, S.; Sakagami, H. Induction of cytotoxicity by chlorogenic acid in human oral tumor cell lines. Phytomedicine 2000, 7, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Yagasaki, K.; Miura, Y.; Okauchi, R.; Furuse, T. Inhibitory effects of chlorogenic acid and its related compounds on the invasion of hepatoma cells in culture. Cytotechnology 2000, 33, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, A.; Currie, J.C.; Desgagnes, J.; Annabi, B. The chemopreventive properties of chlorogenic acid reveal a potential new role for the microsomal glucose-6-phosphate translocase in brain tumor progression. Cancer Cell. Int. 2006, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Noratto, G.; Porter, W.; Byrne, D.; Cisneros-Zevallos, L. Identifying peach and plum polyphenols with chemopreventive potential against estrogen-independent breast cancer cells. J. Agric. Food Chem. 2009, 57, 5219–5226. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Zhou, C.Y.; Qiu, C.H.; Lu, X.M.; Wang, Y.T. Chlorogenic acid induced apoptosis and inhibition of proliferation in human acute promyelocytic leukemia hl-60 cells. Mol. Med. Rep. 2013, 8, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Onakpoya, I.J.; Spencer, E.A.; Thompson, M.J.; Heneghan, C.J. The effect of chlorogenic acid on blood pressure: A systematic review and meta-analysis of randomized clinical trials. J. Hum. Hypertens. 2015, 29, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Wang, J.K.; Ballevre, O.; Luo, H.L.; Zhang, W.G. Antihypertensive effects and mechanisms of chlorogenic acids. Hypertens. Res. 2012, 35, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Lee, H.J. Lonicera caerulea: An updated account of its phytoconstituents and health-promoting activities. Trends Food Sci. Tech. 2021, 107, 130–149. [Google Scholar] [CrossRef]
- Grobelna, A.; Kalisz, S.; Kieliszek, M.; Giurgiulescu, L. Blue honeysuckle berry (Lonicera caerulea L.), as raw material, is particularly predisposed to the production of functional foods. Carpathian J. Food Sci. Technol. 2020, 12, 144–155. [Google Scholar]
- Zhao, H.T.; Wang, Z.Y.; Cheng, C.L.; Yao, L.; Wang, L.; Lu, W.H.; Yang, X.; Ma, F.M. In-vitro free radical scavenging activities of anthocyanins from three berries. J. Med. Plants Res. 2011, 5, 7036–7042. [Google Scholar]
- Fujita, R.; Hayasaka, T.; Jin, S.; Hui, S.P.; Hoshino, Y. Comparison of anthocyanin distribution in berries of haskap (Lonicera caerulea subsp. edulis (Turcz. Ex. Herder) Hulten), Miyamauguisukagura (Lonicera gracilipes Miq.), and their interspecific hybrid using imaging mass spectrometry. Plant Sci. 2020, 300, 110633. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.P.V.; Arumuggam, N.; Amararathna, M.; De Silva, A.B.K.H. The potential health benefits of haskap (Lonicera caerulea L.): Role of cyanidin-3-o-glucoside. J. Funct. Foods 2018, 44, 24–39. [Google Scholar] [CrossRef]
- Gruia, M.I.; Oprea, E.; Gruia, I.; Negoita, V.; Farcasanu, I.C. The antioxidant response induced by Lonicera caerulaea berry extracts in animals bearing experimental solid tumors. Molecules 2008, 13, 1195–1206. [Google Scholar] [CrossRef]
- Caprioli, G.; Iannarelli, R.; Innocenti, M.; Bellumori, M.; Fiorini, D.; Sagratini, G.; Vittori, S.; Buccioni, M.; Santinelli, C.; Bramucci, M.; et al. Blue honeysuckle fruit (Lonicera caerulea L.) from eastern Russia: Phenolic composition, nutritional value and biological activities of its polar extracts. Food Funct. 2016, 7, 1892–1903. [Google Scholar] [CrossRef] [PubMed]
- Khattab, R.; Ghanem, A.; Brooks, M.S. Stability of haskap berry (Lonicera caerulea L.) anthocyanins at different storage and processing conditions. J. Food Res. 2016, 5, 67–79. [Google Scholar] [CrossRef]
- Cesoniene, L.; Labokas, J.; Jasutiene, I.; Sarkinas, A.; Kaskoniene, V.; Kaskonas, P.; Kazernaviciute, R.; Pazereckaite, A.; Daubaras, R. Bioactive compounds, antioxidant, and antibacterial properties of Lonicera caerulea berries: Evaluation of 11 cultivars. Plants 2021, 10, 624. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhu, J.Y.; Meng, X.J.; Liu, S.W.; Mu, J.J.; Ning, C. Comparison of polyphenol, anthocyanin and antioxidant capacity in four varieties of Lonicera caerulea berry extracts. Food Chem. 2016, 197, 522–529. [Google Scholar] [CrossRef]
- Liu, S.W.; You, L.; Zhao, Y.H.; Chang, X.D. Wild Lonicera caerulea berry polyphenol extract reduces cholesterol accumulation and enhances antioxidant capacity in vitro and in vivo. Food Res. Int. 2018, 107, 73–83. [Google Scholar] [CrossRef]
- Senica, M.; Bavec, M.; Stampar, F.; Mikulic-Petkovsek, M. Blue honeysuckle (Lonicera caerulea subsp edulis (Turcz. Ex Herder) Hulten.) berries and changes in their ingredients across different locations. J. Sci. Food Agric. 2018, 98, 3333–3342. [Google Scholar] [CrossRef]
- Wang, Y.H.; Xie, X.; Ran, X.L.; Chou, S.R.; Jiao, X.Y.; Li, E.H.; Zhang, Q.; Meng, X.J.; Li, B. Comparative analysis of the polyphenols profiles and the antioxidant and cytotoxicity properties of various blue honeysuckle varieties. Open. Chem. 2018, 16, 637–646. [Google Scholar] [CrossRef]
- Palikova, I.; Heinrich, J.; Bednar, P.; Marhol, P.; Kren, V.; Cvak, L.; Valentova, K.; Ruzicka, F.; Hola, V.; Kolar, M.; et al. Constituents and antimicrobial properties of blue honeysuckle: A novel source for phenolic antioxidants. J. Agric. Food Chem. 2008, 56, 11883–11889. [Google Scholar] [CrossRef] [PubMed]
- Myjavcova, R.; Marhol, P.; Kren, V.; Simanek, V.; Ulrichova, J.; Palikova, I.; Papouskova, B.; Lemr, K.; Bednar, P. Analysis of anthocyanin pigments in Lonicera (caerulea) extracts using chromatographic fractionation followed by microcolumn liquid chromatography-mass spectrometry. J. Chromatogr. A 2010, 1217, 7932–7941. [Google Scholar] [CrossRef] [PubMed]
- Ponder, A.; Najman, K.; Aninowski, M.; Leszczynska, J.; Glowacka, A.; Bielarska, A.M.; Lasinskas, M.; Hallmann, E. Polyphenols content, antioxidant properties and allergenic potency of organic and conventional blue honeysuckle berries. Molecules 2022, 27, 6083. [Google Scholar] [CrossRef] [PubMed]
- Bieniek, A.; Grygorieva, O.; Bielska, N. Biological properties of honeysuckle (Lonicera caerulea L.): A review. Agrobiodiversity Improv. Nutr. Health Life Qual. 2021, 2, 287–295. [Google Scholar]
- Gawronski, J.; Zebrowska, J.; Pabich, M.; Jackowska, I.; Kowalczyk, K.; Dyduch-Sieminska, M. Phytochemical characterization of blue honeysuckle in relation to the genotypic diversity oflonicerasp. Appl. Sci. 2020, 10, 6545. [Google Scholar] [CrossRef]
- Orsavova, J.; Sytarova, I.; Mlcek, J.; Misurcova, L. Phenolic compounds, vitamins c and e and antioxidant activity of edible honeysuckle berries (Lonicera caerulea L. Var. Kamtschatica pojark) in relation to their origin. Antioxidants 2022, 11, 433. [Google Scholar] [CrossRef]
- Sip, S.; Sip, A.; Szulc, P.; Cielecka-Piontek, J. Haskap berry leaves (Lonicera caerulea L.)-the favorable potential of medical use. Nutrients 2022, 14, 3898. [Google Scholar] [CrossRef]
- Chong, K.Y.; Stefanova, R.; Zhang, J.Z.; Brooks, M.S.L. Extraction of bioactive compounds from haskap leaves (Lonicera caerulea) using salt/ethanol aqueous two-phase flotation. Food Bioprocess. Technol. 2020, 13, 2131–2144. [Google Scholar] [CrossRef]
- Chong, K.Y.; Stefanova, R.; Zhang, J.Z.; Brooks, M.S.L. Aqueous two-phase extraction of bioactive compounds from haskap leaves (Lonicera caerulea): Comparison of salt/ethanol and sugar/propanol systems. Sep. Purif. Technol. 2020, 252, 117399. [Google Scholar] [CrossRef]
- Krotova, I.V.; Demina, L.N.; Zhdanova, P.A.; Nikolaeva, A.U. Extraction products from kind Lonicera vegetative parts usage possibility. IOP Conf. Ser. Earth Environ. Sci. 2021, 839, 042040. [Google Scholar] [CrossRef]
- Cvetanovic, A.; Zengin, G.; Zekovic, Z.; Svarc-Gajic, J.; Razic, S.; Damjanovic, A.; Magkovic, P.; Mitic, M. Comparative in vitro studies of the biological potential and chemical composition of stems, leaves and berries Aronia melanocarpa’s extracts obtained by subcritical water extraction. Food Chem. Toxicol. 2018, 121, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Jurendic, T.; Scetar, M. Aronia melanocarpa products and by-products for health and nutrition: A review. Antioxidants 2021, 10, 1052. [Google Scholar] [CrossRef] [PubMed]
- Zdunic, G.; Aradski, A.A.; Godevac, D.; Zivkovic, J.; Lausevic, S.D.; Milosevic, D.K.; Savikin, K. In vitro hypoglycemic, antioxidant and antineurodegenerative activity of chokeberry (Aronia melanocarpa) leaves. Ind. Crop. Prod. 2020, 148, 112328. [Google Scholar] [CrossRef]
- Pirvu, L.; Panteli, M.; Rasit, I.; Grigore, A.; Bubueanu, C. The leaves of Aronia melanocarpa L. and Hippophae rhamnoides L. as source of active ingredients for biopharmaceutical engineering. Agric. Agric. Sci. Proc. 2015, 6, 593–600. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Kubica, P.; Banaszczak, P.; Wojtanowska-Krosniak, A.; Krosniak, M.; Marzec-Wroblewska, U.; Badura, A.; Zagrodzki, P.; Bucinski, A.; et al. Comparative analysis of different groups of phenolic compounds in fruit and leaf extracts of Aronia sp.: A-melanocarpa, A. arbutifolia, and A. xprunifolia and their antioxidant activities. Eur. Food Res. Technol. 2017, 243, 1645–1657. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, G.S.; Park, S.; Kim, Y.H.; Kim, M.B.; Lee, W.S.; Jeong, S.W.; Lee, S.J.; Jin, J.S.; Shin, S.C. Determination of chokeberry (Aronia melanocarpa) polyphenol components using liquid chromatography-tandem mass spectrometry: Overall contribution to antioxidant activity. Food Chem. 2014, 146, 1–5. [Google Scholar] [CrossRef]
- Teleszko, M.; Wojdylo, A. Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves. J. Funct. Foods 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Thi, N.D.; Hwang, E.S. Bioactive compound contents and antioxidant activity in Aronia (Aronia melanocarpa) leaves collected at different growth stages. Prev. Nutr. Food Sci. 2014, 19, 204–212. [Google Scholar] [CrossRef]
- Zielinska, A.; Bryk, D.; Paradowska, K.; Wawer, I. Aronia melanocarpa leaves as a source of chlorogenic acids, anthocyanins, and sorbitol, and their anti-inflamtory activity. Pol. J. Food Nutr. Sci. 2020, 70, 409–418. [Google Scholar]
- Biel, W.; Jaroszewska, A. The nutritional value of leaves of selected berry species. Sci. Agric. 2017, 74, 405–410. [Google Scholar] [CrossRef]
- Munteanu, A.; Enache, A.; Neagu, G.; Bubueanu, B.; Grigore, G.; Rusu, N.; Pirvu, L. Aronia melanocarpa fruit and leaves hot-assisted ethanolic extracts antioxidant activity. Proceedings 2020, 57, 57. [Google Scholar]
- Brand, M. Aronia: Native shrubs with untapped potential. Arnoldia 2010, 67, 14–25. [Google Scholar]
- Banach, M.; Wiloch, M.; Zawada, K.; Cyplik, W.; Kujawski, W. Evaluation of antioxidant and anti-inflammatory activity of anthocyanin-rich water-soluble Aronia dry extracts. Molecules 2020, 25, 4055. [Google Scholar] [CrossRef]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Lupascu, N.; Sirbu, R. Studies concerning the stability if antioxidant compounds in aronia melanocarpa fruits. Eur. J. Nat. Sci. Med. 2020, 3, 78–83. [Google Scholar] [CrossRef]
- Lazar, M.A.; Catana, M.; Burnete, A.G.; Teodorescu, R.I.; Asănica, A.C.; Belc, N. Valoriation of Aronia melanocarpa pomace for development of functional ingredients with high nutritional value and antioxidant capacity. Sci. Pap. Ser. B Hortic. 2020, 64, 403–410. [Google Scholar]
- Tolić, M.T.; Krbavcic, I.P.; Vujevic, P.; Milinovic, B.; Jurcevic, I.L.; Vahcic, N. Effects of weather conditions on phenolic contentand antioxidant capacity in juice of chokeberries (Aronia melanocarpa L.). Pol. J. Food Nutr. Sci. 2016, 67, 67–74. [Google Scholar] [CrossRef]
- Oszmianski, J.; Wojdylo, A. Aronia melanocarpa phenolics and their antioxidant activity. Eur. Food Res. Technol. 2005, 221, 809–813. [Google Scholar] [CrossRef]
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavova, J. Fruits of black chokeberry Aronia melanocarpa in the prevention of chronic diseases. Molecules 2017, 22, 944. [Google Scholar] [CrossRef]
- Deineka, V.I.; Tretyakov, M.Y.; Oleiniz, E.Y.; Pavlov, A.A.; Deineka, L.A.; Blinova, I.P.; Manokhina, L.A. Determination of anthocyanins and chlorogenic acids in fruits of aronia genus: The experience of chemosystematics. Russ. J. Bioorg. Chem. 2020, 46, 1390–1395. [Google Scholar] [CrossRef]
- Denev, P.; Ciz, M.; Kratchanova, M.; Blazheva, D. Black chokeberry (Aronia melanocarpa) polyphenols reveal different antioxidant, antimicrobial and neutrophil-modulating activities. Food Chem. 2019, 284, 108–117. [Google Scholar] [CrossRef]
- Gralec, M.; Wawer, I.; Zawada, K. Aronia melanocarpa berries: Phenolics composition and antioxidant properties changes during fruit development and ripening. Emir. J. Food Agric. 2019, 31, 214–221. [Google Scholar]
- Miguel, M.G. Anthocyanins: Antioxidant and/or anti-inflammatory activities J. Appl. Pharm. Sci. 2011, 6, 7–15. [Google Scholar]
- Riaz, M.; Zia-Ul-Haq, M.; Saad, B. The role of anthocyanins in health as antioxidant, in bone health and as heart protecting agents. Springerbrief Food 2016, 87–107. [Google Scholar] [CrossRef]
- Wilkes, K.; Howard, L.R.; Brownmiller, C.; Prior, R.L. Changes in chokeberry (Aronia melanocarpa L.) polyphenols during juice processing and storage. J. Agric. Food Chem. 2014, 62, 4018–4025. [Google Scholar] [CrossRef]
- Liang, Z.X.; Liang, H.R.; Guo, Y.Z.; Yang, D. Cyanidin 3-o-galactoside: A natural compound with multiple health benefits. Int. J. Mol. Sci. 2021, 22, 2261. [Google Scholar] [CrossRef] [PubMed]
- Sidor, A.; Gramza-Michalowska, A. Black chokeberry Aronia melanocarpa L.—A qualitative composition, phenolic profile and antioxidant potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef] [PubMed]
- Shikov, A.N.; Narkevich, I.A.; Flisyuk, E.V.; Luzhanin, V.G.; Pozharitskaya, O.N. Medicinal plants from the 14th edition of the russian pharmacopoeia, recent updates. J. Ethnopharmacol. 2021, 268, 113685. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Nair, S.; Robinson, R. Studies in Natural Products Chemistry; Elsevier Science Publishers: Amsterdam, The Netherlands, 2014; Volume 42. [Google Scholar]
- Golba, M.; Sokol-Letowska, A.; Kucharska, A.Z. Health properties and composition of honeysuckle berry Lonicera caerulea L. An update on recent studies. Molecules 2020, 25, 749. [Google Scholar] [CrossRef]
- Zhao, H.T.; Wang, Z.Y.; Ma, F.M.; Yang, X.; Cheng, C.L.; Yao, L. Protective effect of anthocyanin from Lonicera caerulea var. Edulis on radiation-induced damage in mice. Int. J. Mol. Sci. 2012, 13, 11773–11782. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.S.; Yano, S.; Chen, J.H.; Hisanaga, A.; Sakao, K.; He, X.; He, J.H.; Hou, D.X. Polyphenols from Lonicera caerulea L. Berry inhibit lps-induced inflammation through dual modulation of inflammatory and antioxidant mediators. J. Agric. Food Chem. 2017, 65, 5133–5141. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.W.; Meng, F.N.; Zhang, D.; Shi, D.L.; Zhou, J.Y.; Guo, S.; Chang, X.D. Lonicera caerulea berry polyphenols extract alleviates exercise fatigue in mice by reducing oxidative stress, inflammation, skeletal muscle cell apoptosis, and by increasing cell proliferation. Front. Nutr. 2022, 9, 853225. [Google Scholar] [CrossRef] [PubMed]
- Piekarska, J.; Szczypka, M.; Gorczykowski, M.; Sokol-Letowska, A.; Kucharska, A.Z. Evaluation of immunotropic activity of iridoid-anthocyanin extract of honeysuckle berries (Lonicera caerulea L.) in the course of experimental trichinellosis in mice. Molecules 2022, 27, 1949. [Google Scholar] [CrossRef]
- An, M.Y.; Eo, H.J.; Son, H.J.; Geum, N.G.; Park, G.H.; Jeong, J.B. Anti-inflammatory effects of leaf and branch extracts of honeyberry (Lonicera caerulea) on lipopolysaccharide-stimulated raw264.7 cells through atf3 and nrf2/ho-1 activation. Mol. Med. Rep. 2020, 22, 5219–5230. [Google Scholar] [CrossRef]
- Minami, M.; Nakamura, M.; Makino, T. Effect of lonicera caerulea var. Emphyllocalyx extracts on murine streptococcus pyogenes infection by modulating immune system. Biomed. Res. Int. 2019, 2019, 1797930. [Google Scholar] [CrossRef]
- Kang, S.H.; Jeon, Y.D.; Moon, K.H.; Lee, J.H.; Kim, D.G.; Kim, W.; Myung, H.; Kim, J.S.; Kim, H.J.; Bang, K.S.; et al. Aronia berry extract ameliorates the severity of dextran sodium sulfate-induced ulcerative colitis in mice. J. Med. Food 2017, 20, 667–675. [Google Scholar] [CrossRef]
- Borissova, P.; Valcheva, S.; Belcheva, A. Antiinflammatory effect of flavonoids in the natural juice from Aronia melanocarpa, rutin and rutin-magnesium complex on an experimental model of in flammation induced by histamine and serotonin. Acta Physiol. Pharm. Bulg. 1994, 20, 25–30. [Google Scholar]
- Martin, D.A.; Taheri, R.; Brand, M.H.; Draghi, A.; Sylvester, F.A.; Bolling, B.W. Anti-inflammatory activity of Aronia berry extracts in murine splenocytes. J. Funct. Foods 2014, 8, 68–75. [Google Scholar] [CrossRef]
- Ohgami, K.; Ilieva, I.; Shiratori, K.; Koyama, Y.; Jin, X.H.; Yoshida, K.; Kase, S.; Kitaichi, N.; Suzuki, Y.; Tanaka, T.; et al. Anti-inflammatory effects of Aronia extract on rat endotoxin-induced uveitis. Investig. Ophth. Vis. Sci. 2005, 46, 275–281. [Google Scholar] [CrossRef]
- Skupien, K.; Kostrzewa-Nowak, D.; Oszmianski, J.; Tarasiuk, J. In vitro antileukaemic activity of extracts from chokeberry (Aronia melanocarpa [michx] Elliott) and mulberry (Morus alba L.) leaves against sensitive and multidrug resistant hl60 cells. Phytother. Res. 2008, 22, 689–694. [Google Scholar] [CrossRef]
- Catana, L.; Catana, M.; Iorga, E.; Asanica, A.C.; Lazar, A.G.; Lazar, M.A.; Belc, N. Vitamin c and total polyphenol content and antioxidant capacity of fresh and processed fruits of Aronia melanocarpa. Sci. Pap.-Ser. B-Hortic. 2017, 61, 433–440. [Google Scholar]
- Malik, M.; Zhao, C.W.; Schoene, N.; Guisti, M.M.; Moyer, M.P.; Magnuson, B.A. Anthocyanin-rich extract from Aronia meloncarpa E. Induces a cell cycle block in colon cancer but not normal colonic cells. Nutr. Cancer 2003, 46, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.C.; Gao, J.; Hao, R.B.; Zhang, C.J.; Liu, H.W.; Fan, J.G.; Wei, J. Aronia melanocarpa Elliot anthocyanins inhibit colon cancer by regulating glutamine metabolism. Food Biosci. 2021, 40, 100910. [Google Scholar] [CrossRef]
- Thi, N.D.; Hwang, E.S. Anti-cancer and anti-inflammatory activities of Aronia (Aronia melanocarpa) leaves. Asian Pac. J. Trop. Bio. 2018, 8, 586–592. [Google Scholar]
- Raudsepp, P.; Anton, D.; Roasto, M.; Meremae, K.; Pedastsaar, P.; Maesaar, M.; Raal, A.; Laikoja, K.; Pussa, T. The antioxidative and antimicrobial properties of the blue honeysuckle (Lonicera caerulea L.), siberian rhubarb (Rheum. rhaponticum L.) and some other plants, compared to ascorbic acid and sodium nitrite. Food Control 2013, 31, 129–135. [Google Scholar] [CrossRef]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A.; Sniadowska, M.; Otlewska, A.; Zyzelewicz, D. Antibacterial mechanisms of Aronia melanocarpa (michx.), chaenomeles Superba lind L. and Cornus mas L. Leaf extracts. Food Chem. 2021, 350, 129218. [Google Scholar] [CrossRef]
- Deng, H.T.; Zhu, J.Y.; Tong, Y.Q.; Kong, Y.W.; Tan, C.; Wang, M.Y.; Wan, M.Z.; Meng, X.J. Antibacterial characteristics and mechanisms of action of Aronia melanocarpa anthocyanins against Escherichia coli. Lwt-Food Sci. Technol. 2021, 150, 112018. [Google Scholar] [CrossRef]
- Ren, Y.L.; Frank, T.; Meyer, G.; Lei, J.Z.; Grebenc, J.R.; Slaughter, R.; Gao, Y.G.; Kinghorn, A.D. Potential benefits of black chokeberry (Aronia melanocarpa) fruits and their constituents in improving human health. Molecules 2022, 27, 7823. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.S.; Ku, S.K.; Kim, J.K.; Park, S.; Cho, I.H.; Lee, N.J. Hepatoprotective and anti-obesity effects of korean blue honeysuckle extracts in high fat diet-fed mice. J. Exerc. Nutr. Biochem. 2018, 22, 39–54. [Google Scholar] [CrossRef]
- Valcheva-Kuzmanova, S.; Kuzmanov, K.; Tancheva, S.; Belcheva, A. Hypoglycemic and hypolipidemic effects of Aronia melanocarpa fruit juice in streptozotocin-induced diabetic rats. Method Find Exp. Clin. 2007, 29, 101–105. [Google Scholar] [CrossRef]
- Jeon, Y.D.; Kang, S.H.; Moon, K.H.; Lee, J.H.; Kim, D.G.; Kim, W.; Kim, J.S.; Ahn, B.Y.; Jin, J.S. The effect of Aronia berry on type 1 diabetes in vivo and in vitro. J. Med. Food 2018, 21, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Yoo, J.H.; Lee, Y.S.; Lee, H.J. Lonicera caerulea extract attenuates non-alcoholic fatty liver disease in free fatty acid-induced hepg2 hepatocytes and in high fat diet-fed mice. Nutrients 2019, 11, 494. [Google Scholar] [CrossRef]
- Lee, D.; Ham, J.; Kang, K.S.; Lee, H.J. Cyanidin 3-o-glucoside isolated from Lonicera caerulea fruit improves glucose response in ins-1 cells by improving insulin secretion and signaling. B Korean Chem. Soc. 2016, 37, 2015–2018. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, Y.S.; Seol, D.J.; Cho, I.J.; Ku, S.K.; Choi, J.S.; Lee, H.J. Anti-obesity and fatty liver-preventing activities of Lonicera caerulea in high-fat diet-fed mice. Int. J. Mol. Med. 2018, 42, 3047–3064. [Google Scholar] [CrossRef]
- Kong, Y.W.; Yan, T.C.; Tong, Y.Q.; Deng, H.T.; Tan, C.; Wan, M.Z.; Wang, M.Y.; Meng, X.J.; Wang, Y.H. Gut microbiota modulation by polyphenols from Aronia melanocarpa of lps-induced liver diseases in rats. J. Agric. Food Chem. 2021, 69, 3312–3325. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, E.; Kopff, A.; Fijalkowski, P.; Kopff, M.; Niedworok, J.; Blaszczyk, J.; Kedziora, J.; Tyslerowicz, P. Effect of anthocyanins on selected biochemical parameters in rats exposed to cadmium. Acta Biochim. Pol. 2003, 50, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Broncel, M.; Kozirog, M.; Duchnowicz, P.; Koter-Michalak, M.; Sikora, J.; Chojnowska-Jezierska, J. Aronia melanocarpa extract reduces blood pressure, serum endothelin, lipid, and oxidative stress marker levels in patients with metabolic syndrome. Med. Sci. Monit. 2010, 16, Cr28–Cr34. [Google Scholar]
- Daskalova, E.; Delchev, S.; Peeva, Y.; Vladimirova-Kitova, L.; Kratchanova, M.; Kratchanov, C.; Denev, P. Antiatherogenic and cardioprotective effects of black chokeberry (Aronia melanocarpa) juice in aging rats. Evidence-Based Complement. Altern. Med. 2015, 2015, 717439. [Google Scholar] [CrossRef]
- Meng, L.S.; Xing, G.; Li, B.; Li, D.N.; Sung, X.Y.; Yan, T.C.; Li, L.; Cao, S.; Meng, X.J. Anthocyanins extracted from Aronia melanocarpa protect sh-sy5y cells against amyloid-beta (1-42)-induced apoptosis by regulating ca2+ homeostasis and inhibiting mitochondrial dysfunction. J. Agric. Food Chem. 2018, 66, 12967–12977. [Google Scholar] [CrossRef]
- Cuvorova, I.N.; Davydov, V.V.; Prozorovskiĭ, V.N.; Shvets, V.N. Peculiarity of the antioxidant action of the extract from Aronia melanocarpa leaves antioxidant on the brain. Biomed. Khim. 2005, 51, 66–71. [Google Scholar]
- Kurhajec, S.; Kostelanska, K.; Pavlokova, S.; Vetchy, D.; Wolaschka, T.; Gajdziok, J.; Franc, A. Stabilized antioxidative plant extracts formulated by liquisolid technique. J. Drug Deliv. Sci. Technol. 2020, 60, 102022. [Google Scholar] [CrossRef]
- Niculae, G.; Badea, N.; Meghea, A.; Oprea, O.; Lacatusu, I. Coencapsulation of butyl-methoxydibenzoylmethane and octocrylene into lipid nanocarriers: Uv performance, photostability and in vitro release. Photochem. Photobiol. 2013, 89, 1085–1094. [Google Scholar] [CrossRef]
- Craciunescu, O.; Icriverzi, M.; Florian, P.E.; Roseanu, A.; Trif, M. Mechanisms and pharmaceutical action of lipid nanoformulation of natural bioactive compounds as efficient delivery systems in the therapy of osteoarthritis. Pharmaceutics 2021, 13, 1108. [Google Scholar] [CrossRef]
- Tong, Q.R.; Qiu, N.; Ji, J.B.; Ye, L.; Zhai, G.X. Research progress in bioinspired drug delivery systems. Expert. Opin. Drug. Del. 2020, 17, 1269–1288. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Kumar, S.; Nagpal, M.; Singh, I.; Arora, S. Potential of novel drug delivery systems for herbal drugs. Indian. J. Pharm. Educ. 2011, 45, 225–235. [Google Scholar]
- Motelica, L.; Vasile, B.S.; Ficai, A.; Surdu, A.V.; Ficai, D.; Oprea, O.C.; Andronescu, E.; Jinga, D.C.; Holban, A.M. Influence of the alcohols on the zno synthesis and its properties: The photocatalytic and antimicrobial activities. Pharmaceutics 2022, 14, 2842. [Google Scholar] [CrossRef] [PubMed]
- Istrati, D.; Lacatusu, I.; Bordei, N.; Badea, G.; Oprea, O.; Stefan, L.M.; Stan, R.; Badea, N.; Meghea, A. Phyto-mediated nanostructured carriers based on dual vegetable actives involved in the prevention of cellular damage. Mater. Sci. Eng. C 2016, 64, 249–259. [Google Scholar] [CrossRef]
- Petrisor, G.; Motelica, L.; Ficai, D.; Ilie, C.I.; Trusca, R.D.; Surdu, V.A.; Oprea, O.C.; Mirt, A.L.; Vasilievici, G.; Semenescu, A.; et al. Increasing bioavailability of trans-ferulic acid by encapsulation in functionalized mesoporous silica. Pharmaceutics 2023, 15, 660. [Google Scholar] [CrossRef]
- Croitoru, A.M.; Morosan, A.; Tihauan, B.; Oprea, O.; Motelica, L.; Trusca, R.; Nicoara, A.I.; Popescu, R.C.; Savu, D.; Mihaiescu, D.E.; et al. Novel graphene oxide/quercetin and graphene oxide/juglone nanostructured platforms as effective drug delivery systems with biomedical applications. Nanomaterials 2022, 12, 1943. [Google Scholar] [CrossRef]
- Petrisor, G.; Motelica, L.; Craciun, L.N.; Oprea, O.C.; Ficai, D.; Ficai, A. Melissa officinalis: Composition, pharmacological effects and derived release systems-a review. Int. J. Mol. Sci. 2022, 23, 3591. [Google Scholar] [CrossRef] [PubMed]
- Croitoru, A.M.; Karacelebi, Y.; Saatcioglu, E.; Altan, E.; Ulag, S.; Aydogan, H.K.; Sahin, A.; Motelica, L.; Oprea, O.; Tihauan, B.M.; et al. Electrically triggered drug delivery from novel electrospun poly(lactic acid)/graphene oxide/quercetin fibrous scaffolds for wound dressing applications. Pharmaceutics 2021, 13, 957. [Google Scholar] [CrossRef] [PubMed]
- Petrisor, G.; Motelica, L.; Ficai, D.; Trusca, R.D.; Surdu, V.A.; Voicu, G.; Oprea, O.C.; Ficai, A.; Andronescu, E. New mesoporous silica materials loaded with polyphenols: Caffeic acid, ferulic acid and p-coumaric acid as dietary supplements for oral administration. Materials 2022, 15, 7982. [Google Scholar] [CrossRef] [PubMed]
- Petrisor, G.; Ficai, D.; Motelica, L.; Trusca, R.D.; Birca, A.C.; Vasile, B.S.; Voicu, G.; Oprea, O.C.; Semenescu, A.; Ficai, A.; et al. Mesoporous silica materials loaded with gallic acid with antimicrobial potential. Nanomaterials 2022, 12, 1648. [Google Scholar] [CrossRef]
- Tasinov, O.; Dincheva, I.; Badjakov, I.; Grupcheva, C.; Galunska, B. Comparative phytochemical analysis of Aronia melanocarpa L. Fruit juices on Bulgarian market. Plants 2022, 11, 1655. [Google Scholar] [CrossRef] [PubMed]
- Tache, A.M.; Dinu, L.D.; Vamanu, E. Novel insights on plant extracts to prevent and treat recurrent urinary tract infections. Appl. Sci. 2022, 12, 2635. [Google Scholar] [CrossRef]
- Raczkowska, E.; Nowicka, P.; Wojdylo, A.; Styczynska, M.; Lazar, Z. Chokeberry pomace as a component shaping the content of bioactive compounds and nutritional, health-promoting (anti-diabetic and antioxidant) and sensory properties of shortcrust pastries sweetened with sucrose and erythritol. Antioxidants 2022, 11, 190. [Google Scholar] [CrossRef]
- Olechno, E.; Puscion-Jakubik, A.; Zujko, M.E. Chokeberry (A. melanocarpa (michx.) Elliott)—A natural product for metabolic disorders? Nutrients 2022, 14, 2688. [Google Scholar] [CrossRef]
- Howatson, G.; Snaith, G.C.; Kimble, R.; Cowper, G.; Keane, K.M. Improved endurance running performance following haskap berry (Lonicera caerulea L.) ingestion. Nutrients 2022, 14, 780. [Google Scholar] [CrossRef]
- Jakobek, L.; Matic, P.; Istuk, J.; Barron, A.R. Study of interactions between individual phenolics of Aronia with barley beta-glucan. Pol. J. Food Nutr. Sci. 2021, 71, 187–196. [Google Scholar] [CrossRef]
- Cujic, N.; Trifkovic, K.; Bugarski, B.; Ibric, S.; Pljevljakusic, D.; Savikin, K. Chokeberry (Aronia melanocarpa L.) extract loaded in alginate and alginate/inulin system. Ind. Crop. Prod. 2016, 86, 120–131. [Google Scholar] [CrossRef]
- Zhang, J.; Celli, G.B.; Brooks, M.S. Natural sources of anthocyanins. Food Chem. Funct. Anal. 2019, 12, 3–33. [Google Scholar]
- Rupasinghe, H.P.V.; Arumuggam, N. Health benefits of anthocyanins. Food Chem. Funct. Anal. 2019, 12, 123–158. [Google Scholar]
- Lee, Y.S.; Park, E.J.; Kim, S.M.; Kim, J.Y.; Lee, H.J. Anti-sarcopenic obesity effects of lonicera caerulea extract in high-fat diet-fed mice. Antioxidants 2021, 10, 1633. [Google Scholar] [CrossRef]
- Klimaszewska, E.; Zieba, M.; Gregorczyk, K.; Markuszewski, L. Application of blue honeysuckle powder obtained by an innovative method of low-temperature drying in skincare face masks. Molecules 2021, 26, 7184. [Google Scholar] [CrossRef]
- Jurcaga, L.; Bobko, M.; Kolesarova, A.; Bobkova, A.; Demianova, A.; Hascik, P.; Belej, L.; Mendelova, A.; Bucko, O.; Krocko, M.; et al. Blackcurrant (Ribes nigrum L.) and kamchatka honeysuckle (Lonicera caerulea var. Kamtschatica) extract effects on technological properties, sensory quality, and lipid oxidation of raw-cooked meat product (frankfurters). Foods 2021, 10, 2957. [Google Scholar] [CrossRef]
- Celli, G.B.; Ghanem, A.; Brooks, M.S.L. Optimized encapsulation of anthocyanin-rich extract from haskap berries (Lonicera caerulea L.) in calcium-alginate microparticles. J. Berry Res. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Meng, X.L.; Cao, H.; Li, H.; Li, K.K.; Yang, G.K.; Zhang, Y.M.; Chang, X.L.; Zhang, X.D.; Zhang, J.X. Effect of dietary honeysuckle (lonicera caerulea L.) supplementation on lipid metabolism, immunity and intestinal microbiota in grass carp (ctenopharyngodon idellus). Aquacult. Rep. 2022, 23, 101063. [Google Scholar] [CrossRef]
- Chong, K.Y.; Yuryev, Y.; Jain, A.; Mason, B.; Brooks, M.S.L. Development of pea protein films with haskap (Lonicera caerulea) leaf extracts from aqueous two-phase systems. Food Bioprocess. Tech. 2021, 14, 1733–1750. [Google Scholar] [CrossRef]
- Li, B.; Bao, Y.W.; Li, J.X.; Bi, J.F.; Chen, Q.Q.; Cui, H.J.; Wang, Y.X.; Tian, J.L.; Shu, C.; Wang, Y.H.; et al. A sub-freshness monitoring chitosan/starch-based colorimetric film for improving color recognition accuracy via controlling the pH value of the film-forming solution. Food Chem. 2022, 388, 132975. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, D.; Chun, Y.S.; Kim, J.K.; Lee, J.O.; Ku, S.K.; Shim, S.M. Effects of blue honeysuckle containing anthocyanin on anti-diabetic hypoglycemia and hyperlipidemia in ob/ob mice. J. Funct. Foods 2022, 89, 104959. [Google Scholar] [CrossRef]
- Amararathna, M.; Hoskin, D.W.; Rupasinghe, H.P.V. Cyanidin-3-o-glucoside-rich haskap berry administration suppresses carcinogen-induced lung tumorigenesis in a/jcr mice. Molecules 2020, 25, 3823. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Hu, Y.F.; Li, X.; Mei, Z.X.; Wu, S.; He, Y.; Jiang, X.W.; Sun, J.X.; Xiao, J.B.; Deng, L.H.; et al. Nanoencapsulation of cyanidin-3-o-glucoside enhances protection against uvb-induced epidermal damage through regulation of p53-mediated apoptosis in mice. J. Agric. Food Chem. 2018, 66, 5359–5367. [Google Scholar] [CrossRef] [PubMed]
- Haladyn, K.; Tkacz, K.; Wojdylo, A.; Nowicka, P. The types of polysaccharide coatings and their mixtures as a factor affecting the stability of bioactive compounds and health-promoting properties expressed as the ability to inhibit the alpha-amylase and alpha-glucosidase of chokeberry extracts in the microencapsulation process. Foods 2021, 10, 1994. [Google Scholar]
- Wang, M.Y.; Li, L.; Wan, M.Z.; Lin, Y.; Tong, Y.Q.; Cui, Y.M.; Deng, H.T.; Tan, C.; Kong, Y.W.; Meng, X.J. Preparing, optimising, and evaluating chitosan nanocapsules to improve the stability of anthocyanins from Aronia melanocarpa. Rsc. Adv. 2021, 11, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Kaloudi, T.; Tsimogiannis, D.; Oreopoulou, V. Aronia melanocarpa: Identification and exploitation of its phenolic components. Molecules 2022, 27, 4375. [Google Scholar] [CrossRef]
- Tong, Y.Q.; Ma, Y.; Kong, Y.W.; Deng, H.T.; Wan, M.Z.; Tan, C.; Wang, M.Y.; Li, L.; Meng, X.J. Pharmacokinetic and excretion study of Aronia melanocarpa anthocyanins bound to amylopectin nanoparticles and their main metabolites using high-performance liquid chromatography-tandem mass spectrometry. Food Funct. 2021, 12, 10917–10925. [Google Scholar] [CrossRef] [PubMed]
- Nemetz, N.J.; Schieber, A.; Weber, F. Application of crude pomace powder of chokeberry, bilberry, and elderberry as a coloring foodstuff. Molecules 2021, 26, 2689. [Google Scholar] [CrossRef]
- Buda, V.; Brezoiu, A.M.; Berger, D.; Pavel, I.Z.; Muntean, D.; Minda, D.; Dehelean, C.A.; Soica, C.; Diaconeasa, Z.; Folescu, R.; et al. Biological evaluation of black chokeberry extract free and embedded in two mesoporous silica-type matrices. Pharmaceutics 2020, 12, 838. [Google Scholar] [CrossRef] [PubMed]
- Cujic-Nikolic, N.; Stanisavljevic, N.; Savikin, K.; Kaluevic, A.; Nedovic, V.; Samardzic, J.; Jankovic, T. Chokeberry polyphenols preservation using spray drying: Effect of encapsulation using maltodextrin and skimmed milk on their recovery following in vitro digestion. J. Microencapsul. 2019, 36, 693–703. [Google Scholar] [CrossRef]
- Wilkowska, A.; Ambroziak, W.; Adamiec, J.; Czyzowska, A. Preservation of antioxidant activity and polyphenols in chokeberry juice and wine with the use of microencapsulation. J. Food Process. Pres. 2017, 41, e12924. [Google Scholar] [CrossRef]
- Gawalek, J.; Domian, E.; Ryniecki, A.; Bakier, S. Effects of the spray drying conditions of chokeberry (Aronia melanocarpa L.) juice concentrate on the physicochemical properties of powders. Int. J. Food Sci. Tech. 2017, 52, 1933–1941. [Google Scholar] [CrossRef]
- Young, K.N.; Yong, L.H. Skin anti-inflammatory activity of nano-encapsulated Aronia melanocarpa extracts. Res. J. Biotechnol. 2015, 10, 62–74. [Google Scholar]
- Schmid, V.; Mayer-Miebach, E.; Behsnilian, D.; Briviba, K.; Karbstein, H.P.; Emin, M.A. Enrichment of starch-based extruded cereals with chokeberry (Aronia melanocarpa) pomace: Influence of processing conditions on techno-functional and sensory related properties, dietary fibre and polyphenol content as well as in vitro digestibility. Lwt-Food Sci. Technol. 2022, 154, 112610. [Google Scholar] [CrossRef]
- Oun, A.A.; Shin, G.H.; Kim, J.T. Antimicrobial, antioxidant, and ph-sensitive polyvinyl alcohol/chitosan-based composite films with Aronia extract, cellulose nanocrystals, and grapefruit seed extract. Int. J. Biol. Macromol. 2022, 213, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.W.; Lee, J.S. Characterization of electrospun Aronia melanocarpa fruit extracts loaded polyurethane nanoweb. Fash. Text. 2021, 8, 12. [Google Scholar] [CrossRef]
- Lee, K.H.; Chun, Y.; Jang, Y.W.; Lee, S.K.; Kim, H.R.; Lee, J.H.; Kim, S.W.; Park, C.; Yoo, H.Y. Fabrication of functional bioelastomer for food packaging from Aronia (Aronia melanocarpa) juice processing by-products. Foods 2020, 9, 1565. [Google Scholar] [CrossRef]
- Bao, T.; Karim, N.; Xie, L.H.; Xie, J.H.; Chen, W. Simulated gastrointestinal digestion and colonic fermentation of blue honeysuckle: Phenolic profile and protectivity on ethyl carbamate-induced oxidative damage. Process. Biochem. 2022, 120, 74–84. [Google Scholar] [CrossRef]
- Wu, C.F.; Wu, C.Y.; Lin, C.F.; Liu, Y.W.; Lin, T.C.; Liao, H.J.; Chang, G.R. The anticancer effects of cyanidin 3-o-glucoside combined with 5-fluoro- uracil on lung large-cell carcinoma in nude mice. Biomed. Pharm. 2022, 151, 113128. [Google Scholar] [CrossRef]
- Golubev, D.; Zemskaya, N.; Shevchenko, O.; Shaposhnikov, M.; Kukuman, D.; Patov, S.; Punegov, V.; Moskalev, A. Honeysuckle extract (Lonicera pallasii L.) exerts antioxidant properties and extends the lifespan and healthspan of drosophila melanogaster. Biogerontology 2022, 23, 215–235. [Google Scholar] [CrossRef]
- Lopalco, A.; Denora, N. Nanoformulations for drug delivery: Safety, toxicity, and efficacy. Comput. Toxicol. Methods Protoc. 2018, 1800, 347–365. [Google Scholar]
- Boraschi, D.; Li, D.J.; Li, Y.; Italiani, P. In vitro and in vivo models to assess the immune-related effects of nanomaterials. Int. J. Int. J. Environ. Res. Public Health 2021, 18, 11769. [Google Scholar] [CrossRef] [PubMed]
| Encapsulation System | Tested Components | Source/Extract | Reference |
|---|---|---|---|
| Alginate particles | Anthocyanin rich extract | L. caerulea | [28,174] |
| Protein/lipid particles | Water extract | L. caerulea | [175] |
| Pea protein film | Leaf extracts | L. caerulea | [176] |
| Chitosan/starch | Anthocyanins | L. caerulea | [177] |
| SiO2 | Anthocyanins | L. caerulea | [178] |
| Chitosan | Cyanidin-3-O-glucoside | L. caerulea | [179,180] |
| Alginate microparticles | Anthocyanins | A. melanocarpa | [168,181] |
| Chitosan nanocapsules | Anthocyanins | A. melanocarpa | [182,183] |
| Amylopectin nanoparticles | Anthocyanins | A. melanocarpa | [184,185] |
| MCM-41/ZnO | Polyphenols and flavonoids | A. melanocarpa | [186] |
| Maltodextrin microparticles | Polyphenols and anthocyanins | A. melanocarpa | [187,188,189] |
| Skimmed milk microparticles | Polyphenols and anthocyanins | A. melanocarpa | [187] |
| β-Cyclodextrin | Polyphenols | A. melanocarpa | [188] |
| Lecithin | Ethanol extract | A. melanocarpa | [190] |
| Starch extrudate | Polyphenols | A. melanocarpa | [191] |
| Polyvinyl alcohol/chitosan film | Polyphenols | A. melanocarpa | [192] |
| Polyurethane nanoweb | Polyphenols and flavonoids | A. melanocarpa | [193] |
| Polydimethylsiloxane | Anthocyanins | A. melanocarpa | [194] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negreanu-Pirjol, B.-S.; Oprea, O.C.; Negreanu-Pirjol, T.; Roncea, F.N.; Prelipcean, A.-M.; Craciunescu, O.; Iosageanu, A.; Artem, V.; Ranca, A.; Motelica, L.; et al. Health Benefits of Antioxidant Bioactive Compounds in the Fruits and Leaves of Lonicera caerulea L. and Aronia melanocarpa (Michx.) Elliot. Antioxidants 2023, 12, 951. https://doi.org/10.3390/antiox12040951
Negreanu-Pirjol B-S, Oprea OC, Negreanu-Pirjol T, Roncea FN, Prelipcean A-M, Craciunescu O, Iosageanu A, Artem V, Ranca A, Motelica L, et al. Health Benefits of Antioxidant Bioactive Compounds in the Fruits and Leaves of Lonicera caerulea L. and Aronia melanocarpa (Michx.) Elliot. Antioxidants. 2023; 12(4):951. https://doi.org/10.3390/antiox12040951
Chicago/Turabian StyleNegreanu-Pirjol, Bogdan-Stefan, Ovidiu Cristian Oprea, Ticuta Negreanu-Pirjol, Florentina Nicoleta Roncea, Ana-Maria Prelipcean, Oana Craciunescu, Andreea Iosageanu, Victoria Artem, Aurora Ranca, Ludmila Motelica, and et al. 2023. "Health Benefits of Antioxidant Bioactive Compounds in the Fruits and Leaves of Lonicera caerulea L. and Aronia melanocarpa (Michx.) Elliot" Antioxidants 12, no. 4: 951. https://doi.org/10.3390/antiox12040951
APA StyleNegreanu-Pirjol, B.-S., Oprea, O. C., Negreanu-Pirjol, T., Roncea, F. N., Prelipcean, A.-M., Craciunescu, O., Iosageanu, A., Artem, V., Ranca, A., Motelica, L., Lepadatu, A.-C., Cosma, M., & Popoviciu, D. R. (2023). Health Benefits of Antioxidant Bioactive Compounds in the Fruits and Leaves of Lonicera caerulea L. and Aronia melanocarpa (Michx.) Elliot. Antioxidants, 12(4), 951. https://doi.org/10.3390/antiox12040951






