Nuciferine Effectively Protects Mice against Acetaminophen-Induced Liver Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Nuci
2.2. Animal Experiments
2.3. Liver Histopathological Examinations
2.4. Biochemical Analyses
2.5. Western Blot
2.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. Prediction of Potential Target Proteins of Nuci
2.8. mRNA-seq and Bioinformatics Analysis
2.9. Molecular Docking
2.10. Enzyme-Linked Immunosorbent Assay (ELISA)
2.11. Statistical Analysis
3. Result
3.1. GO and KEGG Enrichment of the Predicted Target Proteins of Nuci
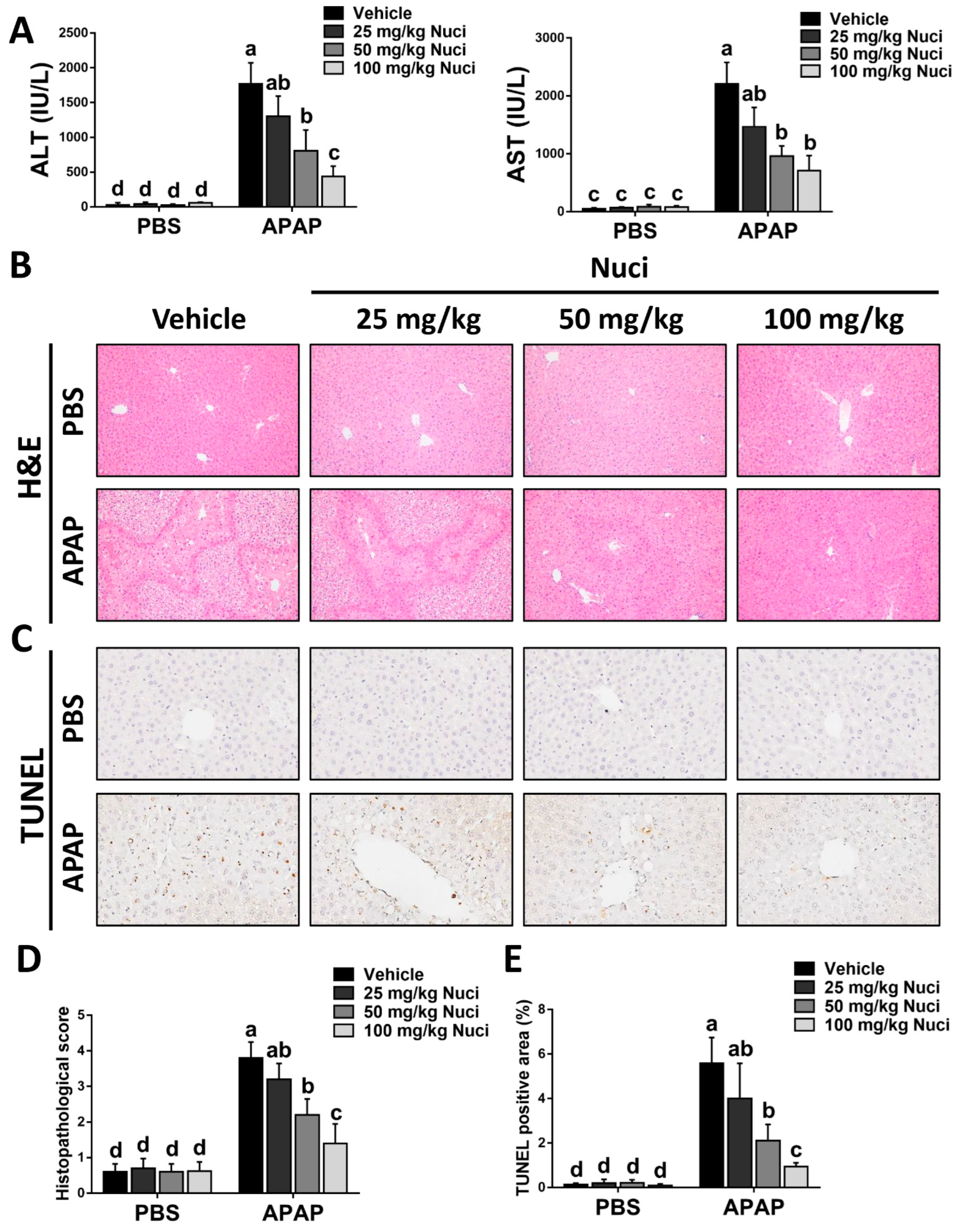
3.2. Treatment with Nuci Mitigates APAP-Evoked ALI in Mice
3.3. The Bioinformatic Analysis of mRNA-seq
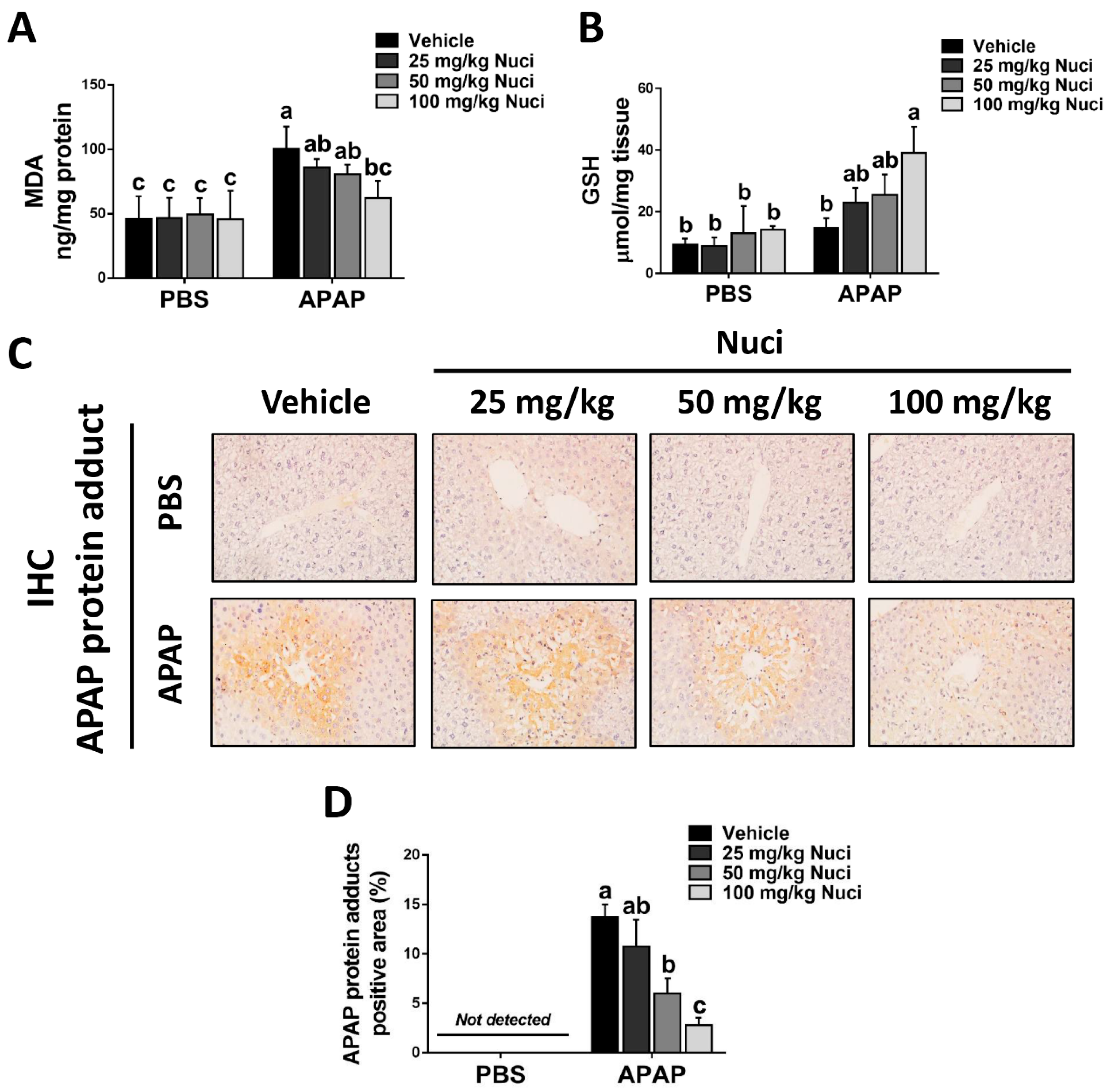
3.4. Treating Nuci Decreases the Formation of APAP Protein Adducts in Damaged Murine Livers
3.5. Nuci Promotes Hepatic Autophagy after APAP Overdose
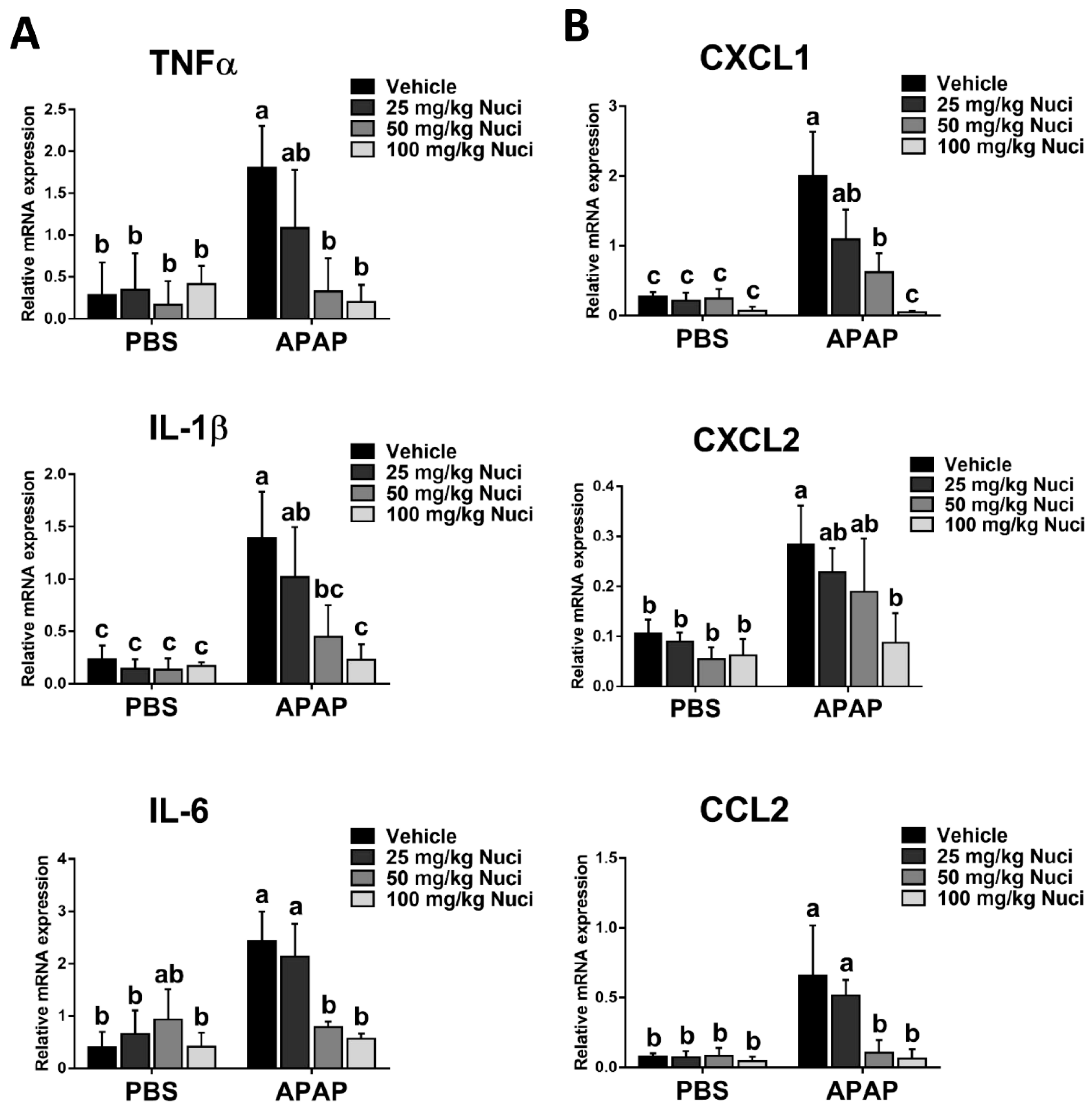
3.6. Nuci Inhibits APAP-Induced Sterile Inflammation Responses in Mice with ALI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tong, Y.; Li, Z.; Wu, Y.; Zhu, S.; Lu, K.; He, Z. Lotus leaf extract inhibits ER(-) breast cancer cell migration and metastasis. Nutr. Metab. 2021, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Luo, T.; Huang, P.; Luo, M.; Zhu, J.; Wang, X.; Lin, Q.; He, Z.; Gao, P.; Liu, S. Nuciferine administration in C57BL/6J mice with gestational diabetes mellitus induced by a high-fat diet: The improvement of glycolipid disorders and intestinal dysbacteriosis. Food Funct. 2021, 12, 11174–11189. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Fan, J.; Li, T.; Yan, X.; Jiang, Y. Nuciferine Protects Against High-Fat Diet-Induced Hepatic Steatosis via Modulation of Gut Microbiota and Bile Acid Metabolism in Rats. J. Agric. Food Chem. 2022, 70, 12014–12028. [Google Scholar] [CrossRef]
- Wang, F.X.; Zhu, N.; Zhou, F.; Lin, D.X. Natural Aporphine Alkaloids with Potential to Impact Metabolic Syndrome. Molecules 2021, 26, 6117. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Florez, I.D.; Tamayo, M.E.; Mbuagbaw, L.; Vanniyasingam, T.; Veroniki, A.A.; Zea, A.M.; Zhang, Y.; Sadeghirad, B.; Thabane, L. Association of Placebo, Indomethacin, Ibuprofen, and Acetaminophen With Closure of Hemodynamically Significant Patent Ductus Arteriosus in Preterm Infants: A Systematic Review and Meta-analysis. JAMA 2018, 319, 1221–1238. [Google Scholar] [CrossRef]
- Wilson, C.G.; Clarke, C.P.; Starkey, Y.Y.; Clarke, G.D. Comparison of a novel fast-dissolving acetaminophen tablet formulation (FD-APAP) and standard acetaminophen tablets using gamma scintigraphy and pharmacokinetic studies. Drug Dev. Ind. Pharm. 2011, 37, 747–753. [Google Scholar] [CrossRef]
- Mohar, I.; Stamper, B.D.; Rademacher, P.M.; White, C.C.; Nelson, S.D.; Kavanagh, T.J. Acetaminophen-induced liver damage in mice is associated with gender-specific adduction of peroxiredoxin-6. Redox Biol. 2014, 2, 377–387. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, X.; Jiao, T.; Li, W.; Chen, P.; Jiang, Y.; Sun, J.; Chen, Y.; Chen, P.; Guan, L.; et al. SIRT6 as a key event linking P53 and NRF2 counteracts APAP-induced hepatotoxicity through inhibiting oxidative stress and promoting hepatocyte proliferation. Acta Pharm. Sin. B 2021, 11, 89–99. [Google Scholar] [CrossRef]
- Zafeiri, A.; Mitchell, R.T.; Hay, D.C.; Fowler, P.A. Over-the-counter analgesics during pregnancy: A comprehensive review of global prevalence and offspring safety. Hum. Reprod. Update 2021, 27, 67–95. [Google Scholar] [CrossRef]
- Rushworth, G.F.; Megson, I.L. Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014, 141, 150–159. [Google Scholar] [CrossRef]
- Orandi, B.J.; McLeod, M.C.; MacLennan, P.A.; Lee, W.M.; Fontana, R.J.; Karvellas, C.J.; McGuire, B.M.; Lewis, C.E.; Terrault, N.M.; Locke, J.E. Association of FDA Mandate Limiting Acetaminophen (Paracetamol) in Prescription Combination Opioid Products and Subsequent Hospitalizations and Acute Liver Failure. JAMA 2023, 329, 735–744. [Google Scholar] [CrossRef]
- Tittarelli, R.; Pellegrini, M.; Scarpellini, M.G.; Marinelli, E.; Bruti, V.; di Luca, N.M.; Busardò, F.P.; Zaami, S. Hepatotoxicity of paracetamol and related fatalities. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 95–101. [Google Scholar]
- Jaeschke, H.; Akakpo, J.Y.; Umbaugh, D.S.; Ramachandran, A. Novel Therapeutic Approaches Against Acetaminophen-induced Liver Injury and Acute Liver Failure. Toxicol. Sci. Off. J. Soc. Toxicol. 2020, 174, 159–167. [Google Scholar] [CrossRef]
- Chao, X.; Wang, H.; Jaeschke, H.; Ding, W.X. Role and mechanisms of autophagy in acetaminophen-induced liver injury. Liver Int. Off. J. Int. Assoc. Study Liver 2018, 38, 1363–1374. [Google Scholar] [CrossRef]
- Ning, Q.; Wang, Y.; Zhang, Y.; Shen, G.; Xie, Z.; Pang, J. Nuciferine Prevents Hepatic Steatosis by Regulating Lipid Metabolismin Diabetic Rat Model. Open Life Sci. 2019, 14, 699–706. [Google Scholar] [CrossRef]
- Zhou, Z.; Qi, J.; Zhao, J.; Seo, J.H.; Shin, D.G.; Cha, J.D.; Lim, C.W.; Kim, J.W.; Kim, B. Orostachys japonicus ameliorates acetaminophen-induced acute liver injury in mice. J. Ethnopharmacol. 2021, 265, 113392. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017, 45, W356–W360. [Google Scholar] [CrossRef]
- Wu, Y.; Bao, W.; Ren, J.; Li, C.; Chen, M.; Zhang, D.; Zhu, A. Integrated Profiles of Transcriptome and mRNA m6A Modification Reveal the Intestinal Cytotoxicity of Aflatoxin B1 on HCT116 Cells. Genes 2022, 14, 79. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed]
- Khomtchouk, B.B.; Van Booven, D.J.; Wahlestedt, C. HeatmapGenerator: High performance RNAseq and microarray visualization software suite to examine differential gene expression levels using an R and C++ hybrid computational pipeline. Source Code Biol. Med. 2014, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, X.; Bao, W.; Hong, X.; Li, C.; Lu, J.; Zhang, D.; Zhu, A. Effect of Humantenine on mRNA m6A Modification and Expression in Human Colon Cancer Cell Line HCT116. Genes 2022, 13, 781. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, A.; Sun, Y.; Zhang, W.; Zhang, T.; Gao, Y.; Shan, D.; Wang, S.; Li, G.; Zeng, K.; et al. Beneficial effects of sappanone A on lifespan and thermotolerance in Caenorhabditis elegans. Eur. J. Pharmacol. 2020, 888, 173558. [Google Scholar] [CrossRef]
- Zou, L.; Bao, W.; Gao, Y.; Chen, M.; Wu, Y.; Wang, S.; Li, C.; Zhang, J.; Zhang, D.; Wang, Q.; et al. Integrated Analysis of Transcriptome and microRNA Profile Reveals the Toxicity of Euphorbia Factors toward Human Colon Adenocarcinoma Cell Line Caco-2. Molecules 2022, 27, 6931. [Google Scholar] [CrossRef]
- Zhu, A.; Ji, Z.; Zhao, J.; Zhang, W.; Sun, Y.; Zhang, T.; Gao, S.; Li, G.; Wang, Q. Effect of Euphorbia factor L1 on intestinal barrier impairment and defecation dysfunction in Caenorhabditis elegans. Phytomedicine 2019, 65, 153102. [Google Scholar] [CrossRef]
- Li, D.; Liu, B.; Fan, Y.; Liu, M.; Han, B.; Meng, Y.; Xu, X.; Song, Z.; Liu, X.; Hao, Q.; et al. Nuciferine protects against folic acid-induced acute kidney injury by inhibiting ferroptosis. Br. J. Pharmacol. 2021, 178, 1182–1199. [Google Scholar] [CrossRef]
- Wu, H.; Yang, Y.; Guo, S.; Yang, J.; Jiang, K.; Zhao, G.; Qiu, C.; Deng, G. Nuciferine Ameliorates Inflammatory Responses by Inhibiting the TLR4-Mediated Pathway in Lipopolysaccharide-Induced Acute Lung Injury. Front. Pharmacol. 2017, 8, 939. [Google Scholar] [CrossRef]
- Zhang, C.; Deng, J.; Liu, D.; Tuo, X.; Xiao, L.; Lai, B.; Yao, Q.; Liu, J.; Yang, H.; Wang, N. Nuciferine ameliorates hepatic steatosis in high-fat diet/streptozocin-induced diabetic mice through a PPARα/PPARγ coactivator-1α pathway. Br. J. Pharmacol. 2018, 175, 4218–4228. [Google Scholar] [CrossRef]
- Zhou, Z.; Qi, J.; Yang, D.; Yang, M.S.; Jeong, H.; Lim, C.W.; Kim, J.W.; Kim, B. Exogenous activation of toll-like receptor 5 signaling mitigates acetaminophen-induced hepatotoxicity in mice. Toxicol. Lett. 2021, 342, 58–72. [Google Scholar] [CrossRef]
- Ni, H.M.; McGill, M.R.; Chao, X.; Du, K.; Williams, J.A.; Xie, Y.; Jaeschke, H.; Ding, W.X. Removal of acetaminophen protein adducts by autophagy protects against acetaminophen-induced liver injury in mice. J. Hepatol. 2016, 65, 354–362. [Google Scholar] [CrossRef]
- Zhu, A.; Chang, X.H.; Sun, Y.B.; Zou, L.Y.; Su, L.; Sun, Y.F.; Li, S.; Liu, S.K.; Sun, Y.Y.; Zhou, H.H.; et al. Role of Oxidative Stress and Inflammatory Response in Subchronic Pulmonary Toxicity Induced by Nano Nickel Oxide in Rats. J. Nanosci. Nanotechnol. 2017, 17, 1753–1761. [Google Scholar] [CrossRef]
- Graham, G.G.; Scott, K.F. Mechanism of action of paracetamol. Am. J. Ther. 2005, 12, 46–55. [Google Scholar] [CrossRef]
- Larson, A.M. Acetaminophen hepatotoxicity. Clin. Liver Dis. 2007, 11, 525–548. [Google Scholar] [CrossRef]
- Woolbright, B.L.; Jaeschke, H. Role of the inflammasome in acetaminophen-induced liver injury and acute liver failure. J. Hepatol. 2017, 66, 836–848. [Google Scholar] [CrossRef]
- Bower, W.A.; Johns, M.; Margolis, H.S.; Williams, I.T.; Bell, B.P. Population-based surveillance for acute liver failure. Am. J. Gastroenterol. 2007, 102, 2459–2463. [Google Scholar] [CrossRef]
- Stravitz, R.T.; Lee, W.M. Acute liver failure. Lancet 2019, 394, 869–881. [Google Scholar] [CrossRef]
- Poddighe, D.; Brambilla, I.; Licari, A.; Marseglia, G.L. Ibuprofen for Pain Control in Children: New Value for an Old Molecule. Pediatr. Emerg. Care 2019, 35, 448–453. [Google Scholar] [CrossRef]
- Ye, H.; Chen, C.; Wu, H.; Zheng, K.; Martín-Adrados, B.; Caparros, E.; Francés, R.; Nelson, L.J.; Gómez Del Moral, M.; Asensio, I.; et al. Genetic and pharmacological inhibition of XBP1 protects against APAP hepatotoxicity through the activation of autophagy. Cell Death Dis. 2022, 13, 143. [Google Scholar] [CrossRef]
- Russell, R.C.; Tian, Y.; Yuan, H.; Park, H.W.; Chang, Y.Y.; Kim, J.; Kim, H.; Neufeld, T.P.; Dillin, A.; Guan, K.L. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 2013, 15, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Meng, L.; Li, X.; Li, D.; Liu, Q.; Chen, Y.; Li, X.; Bu, W.; Sun, H. Regulation of FN1 degradation by the p62/SQSTM1-dependent autophagy-lysosome pathway in HNSCC. Int. J. Oral Sci. 2020, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Shen, Z.; Song, F. Autophagy and acetaminophen-induced hepatotoxicity. Arch. Toxicol. 2018, 92, 2153–2161. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Sun, Y.; Zhong, Q.; Yang, J.; Zhang, T.; Zhao, J.; Wang, Q. Effect of euphorbia factor L1 on oxidative stress, apoptosis, and autophagy in human gastric epithelial cells. Phytomedicine 2019, 64, 152929. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zheng, X.; Zhang, M.; Yin, H.; Jiang, K.; Wu, H.; Dai, A.; Yang, S. Nuciferine alleviates LPS-induced mastitis in mice via suppressing the TLR4-NF-κB signaling pathway. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2018, 67, 903–911. [Google Scholar] [CrossRef]
- Wang, M.X.; Liu, Y.L.; Yang, Y.; Zhang, D.M.; Kong, L.D. Nuciferine restores potassium oxonate-induced hyperuricemia and kidney inflammation in mice. Eur. J. Pharmacol. 2015, 747, 59–70. [Google Scholar] [CrossRef]
- Wu, L.; Chen, C.; Li, Y.; Guo, C.; Fan, Y.; Yu, D.; Zhang, T.; Wen, B.; Yan, Z.; Liu, A. UPLC-Q-TOF/MS-Based Serum Metabolomics Reveals the Anti-Ischemic Stroke Mechanism of Nuciferine in MCAO Rats. ACS Omega 2020, 5, 33433–33444. [Google Scholar] [CrossRef]
- Zhang, C.; Deng, J.; Liu, D.; Tuo, X.; Yu, Y.; Yang, H.; Wang, N. Nuciferine Inhibits Proinflammatory Cytokines via the PPARs in LPS-Induced RAW264.7 Cells. Molecules 2018, 23, 2723. [Google Scholar] [CrossRef]
- Wang, F.; Cao, J.; Hou, X.; Li, Z.; Qu, X. Pharmacokinetics, tissue distribution, bioavailability, and excretion of nuciferine, an alkaloid from lotus, in rats by LC/MS/MS. Drug Dev. Ind. Pharm. 2018, 44, 1557–1562. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, L.; Zhu, A.; Liu, X.; Wang, T.; Wan, M.; Yang, X.; Zhang, Y.; Zhang, Y. Metabolic Profiling of Nuciferine In Vivo and In Vitro. J. Agric. Food Chem. 2020, 68, 14135–14147. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, Y.; Hua, W.; Yan, X.; Li, L.; Zhu, A.; Qi, J. Sappanone A ameliorates acetaminophen-induced acute liver injury in mice. Toxicology 2022, 480, 153336. [Google Scholar] [CrossRef]




| Gene | Gene Accession Number | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|---|
| CYP2e1 | NM_021282 | AAGCGCTTCGGGCCAG | TAGCCATGCAGGACCACGA |
| CYP1a2 | NM_009993.3 | GGTCAGAAAGCCGTGGTTG | GACATGGCCTAACGTGCAG |
| CYP3a11 | NM_007818.3 | CGCCTCTCCTTGCTGTCACA | CTTTGCCTTCTGCCTCAAGT |
| TNFα | NM_013693.3 | GTCTACTCCCAGGTTTCTCTTCAAGG | GCAAATCGGCTGACGGTGTG |
| IL-1β | NM_008361.4 | CTCGCAGCAGCACATCAACA | CCACGGGAAAGACACAGGTA |
| IL-6 | NM_001314054.1 | CAACGATGATGCACTTGCAGA | CTCCAGGTAGCTATGGTACTCCAGA |
| CXCL1 | NM_008176.3 | TGCACCCAAACCGAAGTC | GTCAGAAGCCAGCGTTCACC |
| CXCL2 | NM_009140.2 | GCCAAGGGTTGACTTCAAGAACA | AGGCTCCTCCTTTCCAGGTCA |
| CCL2 | NM_011333.3 | AGCAGCAGGTGTCCCAAAGA | GTGCTGAAGACCTTAGGGCAGA |
| GAPDH | NM_001411840.1 | ACGGCAAATTCAACGGCACAG | GAAGACTCCACGACATACTCAGCAC |
| Protein | PDB ID | Total Score | Crash | Polar | H-Bond Number | Residues Involved in H-Bond Formation | Hydrophobic Contacts Number | Residues Involved in Hydrophobic Contacts |
|---|---|---|---|---|---|---|---|---|
| Beclin 1 | 7BL1 | 5.895 | −0.558 | 1.366 | 1 | Lys266 | 2 | Thr267, Lys263 |
| ATG5 | 7W36 | 4.832 | −2.124 | 0.004 | 0 | - | 10 | Leu157, Asn159, Lys51, Leu37, Val48, Pro40, Leu47, Leu39, Asp160, Gln158 |
| LC3-Ⅱ | 5MS2 | 5.588 | −1.523 | 0 | 0 | - | 11 | Asn84, Lys39, Arg37, Tyr38, Glu36, Val112, Ile34, Tyr110, Met111, Leu82, Gly85 |
| p62 | 6JM4 | 6.713 | −1.534 | 0.892 | 1 | Gly61 | 9 | Gly62, Phe63, Gln64, Ser78, Glu45, Ser49, Leu48, Ala52, Pro60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Qi, J.; Wu, Y.; Li, C.; Bao, W.; Lin, X.; Zhu, A. Nuciferine Effectively Protects Mice against Acetaminophen-Induced Liver Injury. Antioxidants 2023, 12, 949. https://doi.org/10.3390/antiox12040949
Zhou Z, Qi J, Wu Y, Li C, Bao W, Lin X, Zhu A. Nuciferine Effectively Protects Mice against Acetaminophen-Induced Liver Injury. Antioxidants. 2023; 12(4):949. https://doi.org/10.3390/antiox12040949
Chicago/Turabian StyleZhou, Zixiong, Jing Qi, Yajiao Wu, Chutao Li, Wenqiang Bao, Xiaohuang Lin, and An Zhu. 2023. "Nuciferine Effectively Protects Mice against Acetaminophen-Induced Liver Injury" Antioxidants 12, no. 4: 949. https://doi.org/10.3390/antiox12040949
APA StyleZhou, Z., Qi, J., Wu, Y., Li, C., Bao, W., Lin, X., & Zhu, A. (2023). Nuciferine Effectively Protects Mice against Acetaminophen-Induced Liver Injury. Antioxidants, 12(4), 949. https://doi.org/10.3390/antiox12040949








