Abstract
In the pathogenesis of type 2 diabetes mellitus (T2DM), diet plays a key role. Individualized medical nutritional therapy, as part of lifestyle optimization, is one of the cornerstones for the management of T2DM and has been shown to improve metabolic outcomes. This paper discusses major aspects of the nutritional intervention (including macro- and micronutrients, nutraceuticals, and supplements), with key practical advice. Various eating patterns, such as the Mediterranean-style, low-carbohydrate, vegetarian or plant-based diets, as well as healthy eating plans with caloric deficits have been proven to have beneficial effects for patients with T2DM. So far, the evidence does not support a specific macronutrient distribution and meal plans should be individualized. Reducing the overall carbohydrate intake and replacing high glycemic index (GI) foods with low GI foods have been shown as valid options for patients with T2DM to improve glycemic control. Additionally, evidence supports the current recommendation to reduce the intake of free sugars to less than 10% of total energy intake, since their excessive intake promotes weight gain. The quality of fats seems to be rather important and the substitution of saturated and trans fatty acids with foods rich in monounsaturated and polyunsaturated fats lowers cardiovascular risk and improves glucose metabolism. There is no benefit of supplementation with antioxidants, such as carotene, vitamins E and C, or other micronutrients, due to the lack of consistent evidence showing efficacy and long-term safety. Some studies suggest possible beneficial metabolic effects of nutraceuticals in patients with T2DM, but more evidence about their efficacy and safety is still needed.
1. Introduction
Type 2 diabetes mellitus (T2DM) is a multifactorial disease where different genetic and/or environmental (lifestyle) factors play a role in the pathogenesis [1,2].
As for genetic factors, more than 400 polymorphisms (i.e., the presence of two or more variant forms of a specific DNA sequence) have been associated with T2DM [3]. Indeed, genome-wide association studies (GWES) detected polymorphisms related to the response to dietary intake [4] and regulation of insulin secretion and sensitivity in target tissues [5,6], which might explain the 18% risk of T2DM [3].
The main lifestyle factors associated with T2DM are as follows:
- -
- Diet. It has been long suggested that high-density energy/ultra-processed foods and Western-type diets are associated with an increased risk of T2DM, while healthy dietary patterns (such as the Mediterranean diet) are associated with a lower risk [7,8,9]. It is apparent that adherence to a diet rich in whole grains, nuts, legumes, and vegetables, as compared to one rich in refined carbohydrates (CHO), red and processed meat, and sugar-sweetened beverages, confers benefits in terms of reducing the risk of T2DM [8,10]. The quantity and quality of CHOs might be relevant for the risk of T2DM, as results from several cohort studies suggested that a higher CHO intake (>70% of total calories), higher dietary glycemic load and glycemic index, and a lower intake of dietary fiber are associated with an increased risk of diabetes [11,12,13]. Several recent meta-analyses indicated that a higher intake of vegetable fats/olive oil/linoleic acid could be beneficial for the prevention of T2DM, while long-chain omega-3 appears to have minimal or no effect on the likelihood of developing diabetes [14,15,16,17]. Regarding the micronutrients, data support the association between low serum levels of 25-hydroxyvitamin D and a higher risk of prediabetes and T2DM, as well as the role of vitamin D supplementation in reducing the risk of T2DM [18,19,20,21,22,23]. In addition, increased dietary magnesium intake seems to be associated with a reduced risk of diabetes [24,25]. Coffee and light-to-moderate alcohol consumption are inversely associated with the risk of T2DM, although the latter might increase the risk in individuals with non-alcoholic fatty liver disease (NAFLD) [26,27,28,29].
- -
- Poor Physical Activity. A sedentary lifestyle and T2DM are very strongly correlated. A recent meta-analysis, incorporating data from 1,331,468 participants, has shown an increased risk for all-cause and cardiovascular diseases (CVD) mortality and incidence of T2DM with higher levels of total sitting as well as TV viewing time [30]. In contrast, cardiovascular health, body weight, glycemic control, and the development of diabetes-related long-term complications are positively influenced by physical activity [31,32]. The focus is mainly on aerobic exercise, consisting of continuous, rhythmic movement of large muscle groups, such as walking, jogging, and cycling. The most recent guidelines of the American Diabetes Association (ADA) recommend regular physical activity (200–300 min/week) [33]. Cardiac output is improved by moderate to vigorous (65–90% of maximum heart rate) aerobic exercise training, which is associated with significantly reduced cardiovascular and overall mortality risk in patients with T2DM [34]. Moreover, a meta-analysis (846 participants: 440 in the intervention group, and 406 in the control group) reported that regular exercise improved blood glucose control and enhanced insulin sensitivity in patients with T2DM compared with physically inactive patients [35].
- -
- Smoking. There is a strong association between smoking and T2DM. Smoking leads to insulin resistance and/or inadequate compensatory insulin secretion through various underlying effects, including oxidative stress, inflammation, and endothelial dysfunction [36,37,38]. Nicotine in cigarettes may also exert a direct toxic effect on beta cell function [39]. In addition, although smoking tends to decrease weight, it leads to central adiposity, which is linked to inflammation and insulin resistance [40,41,42,43]. A meta-analysis comprising 88 prospective studies (with nearly 6 million participants and 295,446 incident T2DM cases) showed that various smoking behaviors (active and passive smoking and smoking cessation) are correlated with an increased risk of T2DM in a dose-dependent manner [44].
Other risk factors that may be influenced by diet have been shown to increase the risk of T2DM:
- -
- Sleep quality. A U-shaped relationship between sleep duration and T2DM risk has been identified by 2 meta-analyses, with lowest risk at 7–8 h per day [45,46]. Stress, depression, lower socioeconomic status, environmental chemicals, and abnormal intrauterine milieu seem to be additional contributing factors that modulate the risk [47,48].
- -
- Obesity. Weight gain, body fat distribution, and BMI are closely linked to T2DM. A primary risk factor in T2DM is the increase of adipose tissue, especially if this is accumulated in the central abdominal area. Adipocytes can secrete a variety of hormones and cytokines, namely tumor necrosis factor (TNF)-α, interleukin (IL)-6, and resistin, which are able to induce a chronic inflammatory state and insulin resistance, with consequences primarily on adipose, muscular, and hepatic tissues [49,50]. In this situation, insulin has markedly impaired anti-lipolytic effect. Consequently, there is an increased production and secretion of free fatty acids in the systemic circulation which is also responsible for insulin resistance. Furthermore, in patients with obesity and with metabolic syndrome, low adiponectin levels and leptin resistance are frequently observed [51,52,53]. Leptin, produced by adipocytes, is a hormone with an anorexigenic activity that aids in regulating energy balance by inhibiting hunger [54]. At the same time, adiponectin is a peptide synthesized by adipocytes that presents anti-inflammatory and anti-atherogenic effects, and it is also an insulin sensitizer [55]. Therefore, individuals with obesity may present a dysregulation of adipokine levels with an increased risk of overfeeding and cardiometabolic diseases.
- -
- Microbiota Dysbiosis. Normally, the gut microbiota provides numerous beneficial functions for the human host. These include involvement in lipid and CHO metabolism, hormone modulation, protection against pathogens, epithelial cell proliferation, and synthesis of vitamins and amino acids [56,57,58,59]. Additionally, gut microbiota breakdown otherwise indigestible molecules, including plant polysaccharides and some human milk oligosaccharides [56,57,58,59,60]. Several studies identified that changes in the quantity and diversity of gut microbiota have a significant impact on the progression of many metabolic disorders [61,62]. These changes in the gut microbiota composition can reshape intestinal barrier functions and induce metabolic and signaling pathways related to insulin resistance [63].
- -
- Polycystic Ovary Syndrome (PCOS). PCOS is a common hormonal disorder characterized by excessive androgen production by the ovaries, which causes irregular menstrual cycles, hirsutism, acne, and, frequently, obesity [64]. Many women with PCOS exhibit basal and glucose-stimulated hyperinsulinemia and are insulin resistant, independent of BMI [65,66]. Kakoly et al., in a recent meta-analysis including 40 studies, indicated that the incidence of impaired glucose tolerance and T2DM is higher in women with PCOS compared to women without PCOS [67].
Although all these lifestyle factors should be considered also for the management of T2DM to reduce disease progression and complications, medical nutritional therapy is the main cornerstone in the treatment of T2DM.
Therefore, the present review aimed to provide an updated and more practical evidence-based guideline for dietitians and nutritionists about the management of T2DM by medical nutritional therapy. In addition, the role of nutraceuticals/bioactive compounds was also discussed.
2. Medical Nutritional Therapy
Medical nutritional therapy plays a crucial role in the management of T2DM. The goals of nutritional therapy endorsed by the ADA 2022 [68] include the following:
- -
- To promote healthy eating patterns and appropriate portion sizes, considering individual needs and personal and cultural preferences.
- -
- To support the pleasure of eating but limiting food choices that have been associated with detrimental effects on health.
- -
- To provide the patient (and caregivers) practical tools to achieve a healthier eating pattern and improve overall diet quality.
- -
- To emphasize that the nutritional goals for patients with T2DM are similar to those recommended to the general population.
3. Eating Patterns
A variety of dietary patterns may be effective for managing T2DM [68,69]. The Mediterranean-style, low-CHO, vegetarian or plant-based, DASH (Dietary Approaches to Stop Hypertension), or macrobiotic diet are all favorable dietary patterns that have shown benefits for patients with T2DM [70,71,72,73,74,75,76,77]. For other dietary patterns (e.g., Paleo diet, very-low-fat diets, intermittent fasting, etc.) there is less consistent evidence, and data need to be substantiated. A recent meta-analysis of 7 studies (338 patients with T2DM) showed that intermittent fasting induced a greater weight loss, but no further reduction of HbA1c compared with a standard diet [78].
So far, no single eating pattern or meal composition has been identified as superior to the others. However, some common features have been proven to be beneficial (i.e., choosing non-starchy vegetables, reducing sugars and refined grains, and increasing whole foods consumption instead of highly processed foods) [79,80].
Modest persistent weight loss ameliorates glycemic control, blood pressure, and lipid profile and may minimize the need for medications to control these risk factors in patients with both T2DM and overweight or obesity [81,82]. Healthy eating plans that result in an energy deficit of 500–750 kcal/day, which in most cases means an intake of approximately 1200–1500 kcal/day for women and 1500–1800 kcal/day for men, can lead to beneficial effects in terms of glycemic control, lipid profile, and blood pressure [33]. The results of the Look AHEAD study support this approach [83]. The study included 5145 overweight/obese patients with recently diagnosed T2DM, randomized to an intensive lifestyle intervention (with the goal to obtain a weight loss of >7% by following a 1200–1800 kcal/day diet with reduced total and saturated fat and increased physical exercise) or the current standard of care for 4 years. It resulted in significantly greater weight loss (−6.15% vs. −0.88%, p < 0.0001), improved HbA1c (−0.36% vs. 0.09%, p < 0.0001) along with other cardio-metabolic parameters, and a greater proportion of patients with diabetes remission (7.3% vs. 2.0% at 4 years) in the intervention group vs. the control group [83].
Clinical benefits generally begin when achieving a ≥5% weight loss, and more intensive weight loss (i.e., 15%) may be appropriate to maximize benefits [33,84,85]. Furthermore, very-low-calorie diets, typically 800–1000 kcal/day, integrated with high-protein foods and meal replacement products, may increase the rate and/or amount of initial weight loss and glycemic improvements compared with standard behavioral interventions [81,82,83,84,85,86].
4. Macronutrients
Many studies have been carried out to define the proper macronutrient distribution. Evidence supports that, for all people with or at risk for T2DM, there is not an ideal percentage of calories from CHO, protein, and fat [33]. Therefore, macronutrient partition should be established based on the individualized assessment of current eating patterns, preferences (e.g., tradition, culture, religion, health beliefs and goals, economics), and metabolic goals to determine the best dietary plan [87,88].
4.1. Carbohydrates
CHOs are classified into three main groups by the degree of polymerization: sugars (monosaccharides: glucose, fructose, galactose; disaccharides: sucrose, lactose, maltose, trehalose; and polyols: sorbitol, mannitol, lactitol, xylitol, erythritol, isomalt, maltitol), oligosaccharides (short-chain CHOs comprise α-glucans: maltodextrins, and non-α-glucans: raffinose, stachyose, fructo- and galacto-oligosaccharides, polydextrose, inulin), and polysaccharides (long-chain carbohydrates divided into starch: amylose, amylopectin, modified starches; and non-starch polysaccharides: cellulose, hemicellulose, pectin, arabinoxylans, b-glucan, glucomannans, plant gums, mucilages, and hydrocolloids) [89]. After being digested and absorbed, CHOs are delivered to the circulation causing an elevation of the blood glucose, which stimulates insulin secretion by β-cells.
Consequently, as the primary goal for the management of T2DM is to achieve an adequate fasting and postprandial glycemic control, CHO intake needs to be primarily addressed. Most patients with T2DM report consuming a moderate amount of CHO (44–46% of total calories). However, lowering CHO intake through a low- (26–45% of total calories) or very-low-CHO diet (less than 26% of total calories) has been shown as a valid option for patients with T2DM to improve glycemia [88]. It should be noted though that very-low-CHO dietary patterns are not recommended for pregnant or lactating women, people with or at risk for eating disorders, or people who have renal disease, and they should be used with caution in patients taking sodium–glucose cotransporter (SGLT) 2 inhibitors due to the potential risk of ketoacidosis [90,91]. A recent meta-analysis of 23 studies (1357 participants, age range of 47 to 67 years) showed that patients with T2DM after 6 months of a low- and very-low-CHO diet have improved HbA1c < 6.5% and or fasting glucose levels [92]. In addition, patients had a more significant weight loss, a reduction in diabetes medication, and improved triglycerides levels and insulin resistance compared with control diets (which primarily were low-fat diets) [92].
However, efforts to modify usual dietary patterns are often unsuccessful in the long-term, as people generally tend go back to their usual macronutrient distribution. Thus, the recommended approach is to individualize dietary plans with a macronutrient distribution that is more consistent with personal preference and usual intake to increase the likelihood of long-term maintenance. Patients with T2DM are encouraged to reduce the consumption of refined CHOs and added sugars, and instead choose CHOs from vegetables, legumes, fruits, dairy (milk and yogurt), and whole grains [68].
4.1.1. Glycemic Index and Glycemic Load
The utilization of the glycemic index (GI) was introduced by Jenkins D. et al. in 1981 as an instrument for patients with T2DM to select foods [93]. Foods containing CHOs have a different blood glucose-raising potential, defined as GI, resulting in a distinct glycemic response (GR). GR refers to the appearance of glucose in the bloodstream following eating, which is a regular physiological event that depends on the rate of glucose entry into the circulation, the amount of glucose absorbed, the rate of disappearance from the circulation due to tissue uptake, and hepatic regulation of glucose release [94]. Consequently, GI provides information on the GR that might be expected when a person eats food containing CHOs. Glycemic load (GL) considers GI and the total CHO content of a food (GL = GI × available CHO in a given amount of food) [95].
In applying these concepts, foods have been classified by GI into low (GI ≤ 55), medium (GI 56–69), and high (GI ≥ 70) categories, and classified by GL as being low (GL ≤ 10), medium (GL 11–19), and high (GL ≥ 20) [96]. Foods with a low GI value (55 or less) are more slowly digested, absorbed, and metabolized, and cause a lower and slower rise in blood glucose and, therefore, insulin levels. On average, refined grain products and potatoes have a higher GI, legumes and whole grains have a moderate GI, and non-starchy fruits and vegetables have a low GI. Methods of cooking, the physical state of food, dietary protein, fat, and fiber content can affect the GI, which, in turn, can alter rates of glucose absorption [97]. Replacing high GI foods with low GI foods has been shown to improve glycemic control, insulin sensitivity, and HbA1C in patients with T2DM [98,99].
4.1.2. Dietary Fibers
Dietary fibers are complex CHOs, soluble or insoluble, found in plants, that are not hydrolyzed by human enzymes and, therefore, are not digested or absorbed [100]. Insoluble fibers (IF) include cellulose, some hemicellulose, and lignin, while soluble fibers (SF) include mucilage extracted from psyllium husk, gums, pectin, β-glucan, some hemicellulose, and fructans [101]. SF absorb water and form viscous gels capable of increasing food transit time, delaying gastric emptying, decreasing nutrient absorption, slowing digestion, and promoting satiety. SF are found in vegetables (broccoli, onion, carrots, artichokes), fruits (bananas, apples, pears, and berries), legumes, oats, and barley. IF’s food sources are nuts and seeds, wheat, whole grain, bran, some fruits, and vegetables [102]. In addition, some type of SF (β-glucan, arabinoxylans, and pectins) can be easily fermented by the gut microbiota, thus providing a source of short-chain fatty acids, which have been demonstrated to improve glucose control by several mechanisms (reducing the gastric emptying, modifying the release of digestion-related hormones, inhibiting the amylase activity) [103].
Patients with T2DM should consume the amount of dietary fibers generally recommended by the Dietary Guidelines for Americans 2020–2025: a minimum of 14 g of fiber/1000 kcal [68]. Reynolds et al., in their meta-analysis, including 2 prospective cohort studies (8300 adults with T1DM or T2DM residing in 22 countries followed for a mean duration of 8.8 years) and 42 controlled trials (1789 participants with prediabetes, T1DM, or T2DM), demonstrated that an increase in dietary fibers by 1 to 45 g per day improved glycemic control, blood lipids, body weight, and inflammation, and was associated with a reduction in premature mortality in patients with T2DM [104]. Moreover, regular intake of adequate dietary fibers was associated with lower all-cause mortality in patients with T2DM, and prospective cohort studies have found dietary fibers consumption is inversely related to the risk of T2DM [104,105,106,107].
4.1.3. Nutritive Sweeteners
Nutritive sweeteners (NS) generally found in foods and beverages are glucose, fructose, and sucrose [108].
Sucrose, the common commercial sugar, is naturally contained in fruits and vegetables, and is often added to many processed foods [109]. It is hydrolyzed in the small intestine to release an equimolar mixture of glucose and fructose and provides 4 kcal/g [110].
Fructose, a monosaccharide contained in sucrose, is found in fruits, some vegetables, honey, and high fructose corn syrup (HFCS) (up to 55% fructose) [111]. Fructose, mainly as HFCS, is commonly used in beverages and processed foods due to its intrinsically greater sweetness and capacity to improve the appearance and texture of baked foods, and it represents a less expensive alternative to sucrose [111]. Fructose, when consumed in large doses, is a lipogenic sugar, as it increases hepatic de novo lipogenesis through several metabolic pathways. This results in selective deposition of ectopic and visceral fat, and thus adverse effects on lipid metabolism, blood pressure, and insulin sensitivity [112]. As a result, patients with T2DM should limit or avoid consuming sugar-sweetened beverages and processed foods (from any caloric sweetener, including HFCS and sucrose) to reduce the risk for weight gain and worsening of cardiometabolic risk profile [88]. A meta-analysis of 16 controlled feeding trials (236 subjects) showed that fructose at doses > 60 g/day or >10% of energy in isocaloric exchange for other CHOs increased serum triglyceride levels in patients with T2DM [113]. The meta-analysis by Livesey and Taylor also demonstrated a consistent triglyceride-raising effect of fructose at high doses (>100 g/day) across different subject types [114]. In contrast, data from a meta-analysis of 18 short-term controlled feeding trials (209 subjects) indicated that isocaloric fructose replacement by other CHOs resulted in clinically significant improvements in glycemic control, equivalent to a ~0.53% reduction in HbA1c, without significantly affecting insulin in patients with T2DM. This benefit was seen across a full dose range (20–160 g/day) of fructose [115]. Thus, recommendations about the ideal quantity of dietary fructose remain disputed due to potential metabolic consequences leading to further insulin resistance and obesity [115].
The current dietary debate is about the amounts and thresholds of NS consumption. Recognizing that many foods containing NS, such as vegetables, fruits, and milk, are beneficial for health and help prevent T2DM, the debate focuses on “added sugar” or “free sugars” (glucose, fructose, and sucrose, commonly as refined sugar, corn syrup, or honey) added to foods and meals at home or in food manufacturing. Commercially available fruit juices or jams may contain only fruit sugars or may contain added sugars. Therefore, it is recommended to prefer products that contain only sugars naturally occurring in fruit. In 2015, the World Health Organization limited its NS intake recommendations [116]. Evidence supports the current recommendation to reduce intake of free sugars to less than 10% of total energy intake since excessive NS promotes adiposity and weight gain [117,118].
Table 1 summarises the recommendations for CHO distribution for individuals with T2DM.

Table 1.
Recommended CHO distribution for patients with T2DM.
4.1.4. Non-Nutritive Sweeteners
Non-nutritive sweeteners (NNS) present high sweetening properties but lower calorie content than NS (sucrose or HFSC) [119]. They are added in several foods (i.e., diet yogurts, desserts, diet beverages, chewing gum) but can also be found also as table-top sweeteners [119]. The U.S. Food and Drug Administration (FDA) has reviewed several types of NNS for safety, and approved them for consumption by the public, including patients with T2DM [120]:
- Saccharine is 200 to 700 times sweeter than commercial sugar. The acceptable daily intake (ADI) for saccharine is maximum 5 mg/kg/day [121].
- Acesulfame K is 200 times sweeter than sugar and is often combined with other sweeteners to decrease its bitter aftertaste. Its ADI is 15 mg/kg/day.
- Sucralose is 600 times sweeter than commercial sugar. Its ADI is set at 5 mg/kg/day.
- Aspartame is 200 times sweeter than table sugar. It is the only NNS providing energy (4 kcal/g), but a small amount of it is needed to achieve desired sweetness levels due to its high sweetening power. The ADI recommended by the FDA is 50 mg/kg/d. It is a methyl ester of aspartic acid and phenylalanine dipeptide, thus providing aspartic acid, phenylalanine, and methanol when broken down in the intestine. For this reason, the FDA requires any foods containing aspartame to have an informational label statement: “Phenylketonurics: contains phenylalanine”. Patients with phenylketonuria should avoid products containing aspartame.
- Neotame is closely related to aspartame in the chemical constitution. It is approximately 7.000 to 13.000 times sweeter than table sugar. The recommended ADI is 0.3 mg/kg/d.
- Stevia is a non-caloric natural sweetener derived from the leaves of Stevia rebaudiana, a plant native to South America [122,123]. Its sweetness potency is 200 to 400 times sweeter than sucrose [122,123].
In conclusion, NNS can be used as substitutes for NS, to reduce the daily intake of CHOs and calories.
4.1.5. Polyols
Polyols are sugar alcohols obtained via the catalytic hydrogenation of monosaccharides. They are naturally contained in some vegetables and fruits, but they are commonly added into candies, chewing gum, and beverages and provide about 2.4 kcal/g. Polyols constitute an interesting alternative to sucrose, since they do not promote tooth decay, and are slowly and incompletely absorbed in the small intestine [124]. Therefore, they can be used to reduce blood glucose rise and for the management of body weight [124,125,126,127,128,129].
To date, the FDA has approved the use of eight polyols for consumption: erythritol, hydrogenated starch hydrolysates, sorbitol, lactitol, isomalt, maltitol, mannitol and xylitol [130]. Nevertheless, polyols are less sweet than sucrose, and a high amount is required to match the degree of sweetness of sucrose [111]. It is worth mentioning that excessive polyol intake (≥20 g) can induce gastrointestinal symptoms and exert laxative effects [130].
4.2. Fat
Dietary fats are represented by fatty acids and cholesterol. Fatty acids (FAs) are organic acids with at least one carboxylic group attached to a carbonaceous chain whose links may form single or double bonds [131]. Generally, naturally occurring FAs have an even number (4–28) of carbon atoms. Specifically, FAs are mainly derived from triglycerides and phospholipids, which are the principal components of food fats. FAs are categorized into saturated fatty acids (SFAs), with no double bonds, monounsaturated fatty acids (MUFAs) with one double bond, polyunsaturated fatty acids (PUFAs) with more than one double bond, and trans fatty acids (TFAs) with single or one or more isolated double bonds in a trans configuration. PUFA can be further classified in omega-6 PUFAs and omega-3 PUFAs. This variant depends on the position of the first double bond from the methyl-end of the molecule; moreover, there are also omega-7 and omega-9 PUFAs, which are not essential [131].
Data are uncertain about an ideal total fat intake for patients with T2DM [132]. Indeed, there is no established standard percentage of calories from fat for patients with T2DM, while more attention has been addressed to fat quality [133].
Due to the high risk of cardiovascular disease in patients with T2DM, it is recommended to promote the substitution of SFAs and TFAs with healthier oils and foods higher in unsaturated FAs, such as MUFAs, and PUFAs [133].
4.2.1. Saturated Fatty Acids
SFAs are FAs that have no double bonds. Fats containing more SFAs are typically solid or nearly solid at ambient temperature. SFAs are found in all animal fats such as butter, and some plant oils, such as coconut, palm kernel, and palm oil [134]. In addition, processed and fast foods have an elevated quantity of SFAs. Tropical oils, such as coconut and palm, are usually advertised as beneficial SFAs because they originated from plants, but this is not correct [134]. The American Heart Association advises choosing non-tropical vegetable oils when cooking, and suggests replacing saturated with unsaturated fats, especially PUFAs, which may prevent CVD [134,135].
Nutritional guidelines recommend that patients with T2DM should be advised to follow the guidelines for the general population for the intake of SFAs [33,68]. The consumption of SFAs should not exceed 10% of total calories per day and be substituted with unsaturated fats, particularly PUFAs [134]. Conversely, it has been shown that a diet high in SFAs may increase CVD risk. Substituting SFAs with PUFAs, significantly lowers total cholesterol and LDL-cholesterol (LDL-C), and substituting SFAs with MUFAs from plant sources, for example, olive oil and nuts, decreases CVD risk [134,135]. Likewise, substituting SFAs with CHOs lowers total cholesterol and LDL-C, even if it significantly raises triglycerides and reduces HDL-cholesterol (HDL-C) [88]. In addition, switching SFAs with CHOs does not reduce the risk of CVD [134,135].
A recent systematic review and meta-analysis including 5 RCTs (for a total of 22,591 participants, with or without TD2M) showed that the substitution of SFAs with PUFAs or CHOs is correlated with a decrease in cardiovascular events [136]. Another recent meta-analysis comprising 15 RCTs (56,675 participants) suggested that decreasing SFA consumption for at least 2 years triggers a possible significant reduction in cardiovascular events, asserting that the transition from SFAs to PUFAs or CHOs appears to be a helpful approach, while the consequences of substitution with MUFAs are uncertain [137]. In summary, it is advisable to reduce the consumption of SFAs to no more than 10% of total daily caloric intake and substitute them with unsaturated fats.
4.2.2. Trans Fatty Acids
TFAs are fats generated when oils are “partially hydrogenated” [132]. According to the FDA, TFAs are defined as the amount of all unsaturated FAs that include one or more isolated (non-conjugated) double bond in a trans configuration. The European Food Safety Authority (EFSA) provides a distinct classification of TFAs, to distinguish them as trans-MUFAs (that are the most common TFAs in the human diet) or trans-PUFAs (composed by as a minimum one trans double bond, and thus could additionally have double bonds in the cis configuration) [138]. The EFSA reported that TFAs might derive from various sources. However, a first classification distinguishes between natural (also called “ruminant”) and artificial (or “industrially produced”) sources [138]. The first include the bacterial conversion of unsaturated fatty acids into TFAs in the rumen of ruminants (such as cattle and sheep) that produce dairy products, while the second include industrial hydrogenation, a procedure that shifts the chemical composition of unsaturated fats by inserting hydrogen atoms. This technique is used by manufacturers to produce semi-liquid and solid fats, which enhances product steadiness and shelf-life, and hence it is less likely to become spoiled or rancid.
Several studies showed that increased consumption of industrial TFAs is associated with a higher risk of CVD. Indeed, industrial TFAs increase the circulating concentrations of total and LDL-C and reduce HDL-C concentrations, resulting in a stronger atherogenicity compared with SFAs or cis-unsaturated FAs. Moreover, they may promote atherogenesis by activating inflammation and reactive oxygen species production and injuring endothelial cells [139]. A meta-analysis of 7 RCTs studies (208 subjects), showed that TFA intake did not result in significant changes in glucose and insulin concentrations, but the increase in TFA intake led to a significant increase in total and LDL-C (3.1%) [140].
Therefore, TFA intake should be avoided; the recommended intake is less than 1% of total energy.
4.2.3. Monounsaturated Fatty Acids
MUFAs are FAs with one double bond and are usually liquid at room temperature. MUFAs are also called omega-9, as their only unsaturation is at position 9 concerning the methyl terminal; for reference, there are also omega-7 MUFAs [141]. The most common dietary MUFA is oleic acid, found primarily in olive oil, followed by high oleic-sunflower and safflower oil, and canola oil. MUFAs are also present in avocado, some fatty fish, nuts, and nut-based butters [141].
MUFAs have been shown to improve insulin sensitivity by inhibiting endoplasmic reticulum (ER) stress and anti-inflammatory activities. Moreover, they enhance β cell survival and inhibit the reduction of the insulin signaling pathway [142].
A meta-analysis of 24 RCTs, evaluating diets rich in MUFAs vs. CHOs or PUFAs, showed that high-MUFA-containing diets might improve metabolic factors in patients with T2DM, i.e., fasting insulin and glucose level, homeostasis model of assessment for insulin resistance (HOMA-IR) and β-cell function, HDL-C levels, and triglyceride levels [143].
There are currently no daily limits on MUFAs intake for the general population or patients with T2DM, except recommendations to maintain total fat intake between 25% to 35% of total daily calories and to prefer foods that contain MUFAs or PUFAs instead of SFAs or TFAs [133].
4.2.4. Polyunsaturated Fatty Acids
PUFAs are fatty acids with two or more double bonds and are usually liquid at room temperature. PUFAs provide essential fats, such as omega-3 and omega-6 FAs, found in significant amounts in sunflower, corn, soybean, and cottonseed oils, walnuts, pine nuts, sesame, pumpkin, and flax seeds. Only a small amount of PUFA is found in animal fats
Recently, a meta-analysis of 102 RCTs (4660 participants) showed different results regarding macronutrients’ effects on glucose–insulin homeostasis and concluded that the most compelling positive effects were observed for PUFAs compared to CHOs, MUFAs, or SFAs, as PUFA replacement resulted in improved blood glucose levels, insulin resistance, and insulin secretion capacity [144]. Another meta-analysis comprising 15 RCTs (667 participants) reported that the consumption of walnuts (a good source of PUFAs) prevented the onset of T2DM and improved blood glucose levels [145]. Furthermore, the intake of pistachios for three months also significantly reduced triglycerides (mean difference [MD] −0.28 mmol/L), and other nuts (almonds and hazelnuts) reduced fasting blood sugar and glycated hemoglobin (MD −0.26 mmol/L and −0.11% respectively) [145]. However, a systematic review and meta-analysis including 83 RCTs assessed the effects of increased PUFAs on glucose metabolism in patients with T2DM over 24 weeks, which demonstrated that long-chain omega-3 PUFAs have minimal or no effect on glucose metabolism [14]. Moreover, harmful outcomes might occur when the amount of supplemental long-chain omega-3 PUFAs exceeds 4.4 g/day [14].
Regarding omega-6 PUFAs, current data show that linoleic acid (LA) consumption is frequently lower than the recommended amounts. Therefore, there are currently no daily limits on omega-6 PUFA intake for the general population or patients with T2DM.
4.2.5. Omega-3 PUFAs
Omega-3 polyunsaturated fatty acids (PUFAs) include α-linolenic acid (ALA; 18:3 ω-3), stearidonic acid (SDA; 18:4 ω-3), eicosapentaenoic acid (EPA; 20:5 ω-3), docosapentaenoic acid (DPA; 22:5 ω-3), and docosahexaenoic acid (DHA; 22:6 ω-3). Since humans do not have the enzymes necessary to produce omega-3 PUFAs, they are deemed essential FAs, so they must be taken from the diet [146,147]. The form of omega-3 PUFAs in plants is alpha-linolenic acid (ALA, 18:3), and the primary sources include soybean oil, canola oil, walnuts, flaxseed, and chia seeds. The animal sources of omega-3 are eicosapentaenoic acid (EPA, 20:5) and docosahexaenoic acid (DHA, 22:6), which are very-long-chain n-3 FAs contained in seafood, such as fish and shellfish. Fish with a high level of omega-3 include salmon, albacore tuna, mackerel, sardines, herring, and lake trout [134]. At the metabolic level, ALA can be transformed into both DHA and EPA, but this reaction’s rate is very low, indicating the need for direct food intake of DHA [147,148]. Omega-3 PUFAs reduce triglycerides, which are additional risk factors for CVD, and are usually dysregulated in patients with T2DM. The mechanism of action of omega-3 PUFAs on lowering triglycerides is not completely identified yet but is supposed to inhibit the expression of lipogenic genes, improve beta-oxidation of FAs, and enhance the expression of lipoprotein-lipase (LPL) [149,150,151]. Additional mechanisms of action may explain the potentially beneficial properties of omega-3 PUFAs on T2DM. Specifically, omega-3 PUFAs can regulate numerous inflammatory pathways, including the suppression of pro-inflammatory cytokines (such as TNF-alpha, IL-1, IL-6), the suppression of cyclo-oxygenase function (and its consequent eicosanoid production from arachidonic acid, such as leukotrienes and prostaglandins), the suppression of pro-inflammatory transcription nuclear factor kappa B (NF-ĸB), and the activation of peroxisome proliferator-activated receptors (PPARs) [152,153,154,155].
However, data on the effect of omega-3 PUFAs from food in patients with T2DM are insufficient and inconclusive. A recent meta-analysis suggested an association between reduced incidence of T2DM and a higher intake of vegetable fats, particularly of ALA of vegetable origin, up to a maximum of 560 mg per day, and lower amounts for total PUFAs [16]. In contrast, long-chain omega-3 PUFAs of animal origin were related to an increased incidence of T2DM, although geographic differences were found [16].
Previous data did not consistently support recommending omega-3 PUFA (EPA and DHA) supplements in patients with T2DM to prevent or manage CVD [88]. In fact, in the ASCEND trial (carried out on 15,480 patients with T2DM and no CVD), the use of omega-3 PUFAs (1 g/day) did not bring cardiovascular advantages [156]. Similar findings were reported in the VITAL trial (in which 13% of the 25,871 participants were patients with T2DM), as the supplementation with 1 g of omega-3 PUFAs did not decrease the frequency of major cardiovascular outcomes [157]. Nevertheless, in the REDUCE-IT trial (in which 57% of the 8179 participants were patients with T2DM), 2 g of prescribed icosapentethyl twice a day significantly decreased cardiovascular outcomes by 25% [158].
Nonetheless, at present the FDA has authorized the use of two omega-3 PUFA-containing products: omega-3-acid ethyl esters (icosapent ethyl) and omega-3-carboxylic acids [146]. The supplements mentioned above are approved for adults (≥18 years of age) with hypertriglyceridemia (≥500 mg/dl) in combination with diet to decrease triglyceride levels and reduce the risk of cardiovascular events [159,160,161]. Furthermore, the FDA advises that the total daily consumption of omega-3 PUFAs should not exceed 3g of combined EPA and DHA per day, with no more than 2 g/day taken from supplementation [146].
4.2.6. Dietary Cholesterol
Cholesterol is a natural sterol present in all animal tissues, and it is a waxy fat-like substance. Cholesterol derives from an external source through food and an internal source through its endogenous synthesis by the liver and extrahepatic tissues. Furthermore, it is the precursor of steroid hormones and an essential component of the cell membranes. Cholesterol circulates into the bloodstream through molecular aggregates called lipoproteins. HDL-C carries cholesterol from the tissues to the liver, while LDL-C goes in the opposite direction, transporting cholesterol from the liver to the tissues. Food sources of cholesterol are egg yolks, chicken, pork, beef burgers, and cheese [162]. It is absent in plant products such as fruit, vegetables, and cereals [162].
Nutritional guidelines recommend consuming no more than 300 mg/day of cholesterol from food sources, a limit that drops to 200 mg/day if the patients have a high risk of developing CVD [33]. Usually, the intake of dietary cholesterol is associated with a greater intake of SFAs, which, in turn, can increase LDL-C levels with a consequent risk of CVD [136]. However, a meta-analysis including six studies of TD2M patients, suggested no correlation between dietary cholesterol (contained in eggs) and cardiovascular disease in T2DM patients [163]. Therefore, there is no solid evidence for the limitation of egg consumption that was imposed in the past. Nevertheless, low-fat dairy products should be consumed in order to limit the total cholesterol intake [163].
Table 2 summarises the recommendations for dietary fats distribution for individuals with T2DM.

Table 2.
Recommendation regarding dietary fat intake for patients with T2DM.
4.3. Protein
Proteins are molecules made up of amino acids, linked to one another by peptide bonds in long chains. There are 20 different amino acids that can be combined to form every type of protein in the body. These amino acids fall into two major categories: essential amino acids, obtained from food, and non-essential amino acids, synthesized by cells [164]. Proteins are found in plant and animal foods, such as legumes, dairy products (milk, cheese, and yogurt), eggs, meats, poultry, nuts, seafood (fish and shellfish), soy products, whole grains, and vegetables [164].
Many studies focus on the amount of dietary protein and their relationship with glycemic control in patients with T2DM. A randomized crossover study on eight patients with T2DM showed that a high-protein-low-CHO diet improves glycemic control with statistically significant results [165]. A recent cross-sectional study of 212 participants (most participants were between the ages of 45 and 75, overweight or obese, with prediabetes or T2DM) suggested that the mean value of HOMA-IR is lower in those with a protein intake higher than recommendations as compared to those whose protein intake was lower [166]. A meta-analysis of 9 short-term studies (418 subjects with T2DM) showed that high-protein diets resulted in a better glycemic control (decreased HbA1c by 0.52%) and weight loss but did not affect fasting blood glucose or lipid levels [167].
However, there is limited evidence that adjusting the daily level of protein intake (typically 1–1.5 g/kg body weight/day or 15–20% of total calories) may improve glycemic goals in patients with T2DM. A meta-analysis by Dong and colleagues found successful management of T2DM with a moderately higher level of proteins, at 20–30% of total calories, which may increase satiety in these patients [167].
Additionally, replacing animal with plant proteins could confer some glycemic control benefits in patients with T2DM. A meta-analysis of 13 RCTs (280 middle-aged adults) showed that replacing animal with plant proteins led to modest improvements in glycemic control in patients with T2DM, as the HbA1c, fasting glucose, and fasting insulin were decreased in diets that replaced the animal with plant proteins at a median level of ~35% of total protein per day [168]. A potential explanation could be the reduction of heme iron intake, found only in animal protein sources, which is significantly associated with an increased risk of T2DM, as opposed to non-heme iron intake, found both in plant and animal foods, which is inversely associated with or not associated with T2DM incidence [168].
Moreover, the daily intake of proteins for patients with T2DM should be individualized. Those with diabetic kidney disease need to restrict protein intake < 0.8 g/kg of body weight [169]. The National Kidney Foundation recommends maintaining the daily protein intake at no more than 0.8 g/kg of body weight for patients with T2DM who have kidney complications, while a meta-analysis of the Cochrane Database advises a protein intake of 20–30% of total calories for people without kidney complications [170].
Table 3 summarises the recommendations for dietary proteins distribution for individuals with T2DM.

Table 3.
Recommendations regarding dietary proteins for patients with T2DM.
5. Micronutrients
Despite T2DM being associated with increased oxidative stress, current studies report no benefit of routine supplementation with antioxidants, such as carotene, or vitamins E and C, due to lack of evidence showing efficacy and long-term safety [68]. In addition, scientific evidence does not support the regular use of herbal supplements and micronutrients, such as curcumin, cinnamon, aloe vera, vitamin D, zinc, magnesium, or chromium, to improve glycemia or treat diabetes complications in patients with T2DM without a documented deficiency [68]. A multivitamin supplement may be necessary for special populations, including pregnant or lactating women, older adults, patients with celiac disease, vegetarians, and people following very-low-calorie or low-CHO diets [88].
Blood testing of vitamin B12 levels should be considered in metformin-treated patients, particularly those with anemia or peripheral neuropathy [171]. The exact cause of B12 deficiency in patients taking metformin is unclear. The standard of care is B12 injections, but some studies suggest that high-dose oral supplementation may be as effective [172,173].
6. Fluid Intake
Recommendations about total daily fluid intake are less common and accurate than food recommendations. Increasing water intake during a meal could contribute to a sense of fullness, thus decreasing energy intake [174,175,176]. Moreover, replacing diet beverages (NNS beverages) or sugar-sweetened beverages with water is an effective goal for overweight/obese patients with T2DM in order to improve weight loss and glycemic control, since water contains zero calories [176,177]. It is imperative to conduct more extensive and more rigorous trials to answer whether drinking water can improve glycemic parameters in patients with T2DM, as there is lack of proper evidence in this regard. It is, however, reasonable to recommend a water intake similar to the general population.
Regarding alcohol consumption, nutritional guidelines recommend no more than one drink/day for women and two drinks/day for men, as for the general population (one drink refers to 12 oz = 341 mL beer, 5 oz = 142 mL glass of wine, or 1.5 oz= 43 mL of distilled spirits) [178]. Even though moderate alcohol consumption may have cardiovascular benefits, alcohol intake may increase the risk for delayed hypoglycemia in patients with T2DM, due to its effect of preventing the liver from releasing glucose. This is particularly relevant for patients using insulin or insulin secretagogues, as they can experience delayed nocturnal or fasting hypoglycemia after evening alcohol consumption. The risk of hypoglycemia can be minimized by consuming alcohol with food.
In addition, weekly alcohol consumption has been associated with an increased risk for death, disability, and social problems, mainly triggered by cancers, liver disease, unintentional injuries, and violence. Finally, it is worth mentioning that alcohol consumption (2 drinks/week) can increase the risk for some cancers, especially breast and colon [178].
Therefore, it is important that patients with T2DM are advised about a low-to-moderate and sensible alcohol consumption for people choosing to consume alcohol, considering also individual tolerance [88]. People should not be encouraged to start drinking alcohol.
7. Nutraceuticals and Supplements
7.1. Phytosterols
Phytosterols are a group of chemical compounds of plant origin, including stanols, sterols, and esters. Structurally, phytosterols are like cholesterol, but they are poorly absorbed in the gut [179]. They are not synthesized in humans and therefore are introduced exclusively through the diet. Phytosterols are present in dried fruit such as almonds but also in vegetable oils, mainly corn oil, sunflower oil, soybean oil, and olive oil, and in cereals (wheat germ and wheat bran), and fruits and vegetables (cauliflower, passion fruit, and orange) [180].
Phytosterols can help manage hypercholesterolemia, particularly LDL-cholesterol concentrations, by reducing intestinal cholesterol absorption in the intestine through several mechanisms [181,182,183]: (1) competition with cholesterol through solubilization in mixed micelles in the intestinal lumen; (2) reduction of cholesterol esterification in the enterocyte; (3) reduction of cholesterol through trans-intestinal excretion; (4) modification of gene expression encoding sterol-carrying proteins, such as the Niemann-Pick C1-like 1 (NPC1-L1) protein, reducing the transport of cholesterol to the enterocyte.
The proportion of phytosterols in certain foods cannot exert a therapeutic effect. Thus, many food items (i.e., yogurt, cereals, and margarine) are fortified with phytosterols. Indeed, the cholesterol-lowering effect is dose-dependent for phytosterol intake below 3 g/day. Notably, above this dose no further reduction in LDL cholesterol is observed [179]. However, few studies have analyzed the long-term effects in patients with T2DM treated with phytosterols. A meta-analysis of 5 RCT (n: 148 individuals with T2DM) reported a significant LDL-cholesterol reduction (−12 mg/dL), with no effect on HDL fraction [184].
Regarding the cholesterol-lowering effect, an RCT carried out on 200 patients with impaired glucose regulation showed that phytosterols (3 g/day) can significantly reduce triglyceride concentrations and inflammation (measured by c-reactive protein) [185].
Nevertheless, an excessive intake of phytosterols may reduce the absorption of fat-soluble vitamins. In addition, individuals with heterozygous sitosterolemia (a rare autosomal recessive disease characterized by phytosterol accumulation due to ABCG5 or ABCG8 gene mutations) should not take phytosterols. Meanwhile, others can tolerate the intake of phytosterols with no occurrence of health problems such as hypercholesterolemia, early atherosclerosis development, or increased cardiovascular morbidity and mortality. However, the threshold of safe intake has not yet been defined [179]. According to the available evidence, no side effects for regular consumption of 2 g/day of phytosterols have been recorded.
Therefore, phytosterols supplementation (above 3 g/day) could be advised under medical supervision to patients with T2DM to reduce LDL-cholesterol concentrations.
7.2. Polyphenols
Polyphenols are one of the largest family of phytochemical compounds found in nature [186]. They can be found in a variety of plant foods, such as berries, fruits and citrus fruits, green leafy vegetables, spices and herbs, green tea and coffee, olive oil, and nuts. In addition, they can be easily supplemented by adding their extracts into food items or pills [186].
Polyphenols have an ample diversity of chemical structures, with at least one or more phenolic groups; they typically present antioxidant, anti-inflammatory, neuroprotective, and anti-obesity/weight-reducing effects [187,188]. As for their role in the management of T2DM, polyphenols have been shown to hamper CHO digestion in the intestine, decreasing glucose absorption and influencing glucose metabolism [189]. They can also enhance insulin sensitivity and β cell function [186]. Furthermore, it has been reported that they control the islet amyloid polypeptide (IAPP) or amylin secretion [190].
Nevertheless, these effects have been demonstrated only for some classes of polyphenols.
Resveratrol is one of the most studied phenolic compounds. It belongs to the stilbenes subclass [191]. Several meta-analyses of RCTs in patients with T2DM or pre-diabetes have reported a dose–response effect of resveratrol (≥500mg/day) in the improvement of outcomes related to glucose metabolism (fasting glucose and insulin, HbA1c) [192,193,194], cholesterol and triglyceride concentrations [195,196], and blood pressure [197].
Quercetin, one of the most common flavonols, has been shown to reduce hyperglycemia and its associated micro- and macrovascular complications [191,198]. As recently summarized by Ansari and colleagues, in vitro and animal models demonstrated that quercetin could reduce oxidative damage and inflammation in the pancreatic beta cells and in other tissues [198]. In addition, it can influence GLUT2 expression and sodium-dependent glucose uptake in the gut, thus reducing glucose absorption. However, few clinical studies have been carried out with inconclusive results [198].
Oleuropein complex and phenolic alcohols (tyrosol and hydroxytyrosol) are found in olive derivatives (fruits, leaves, branches, and oil). Olive extract supplementation has been shown to influence glucose metabolism in healthy subjects as well as in individuals with type 2 diabetes [199]. These effects are mainly related to the improvement of insulin secretion, likely due to the increase of glucagon-like peptide-1 (GLP-1). Notably, olive oil polyphenols (5 mg/day) bear a health claim by the EFSA for the protection of LDL particles from oxidative damage [200].
Among other polyphenols, anthocyanins and flavan-3-ols have been shown to significantly improve insulin sensitivity and lipid metabolism [201,202] whereas catechins can influence weight loss and maintenance, likely through increased oxidation in adipocytes, inhibition of lipogenesis, and increased energy expenditure [203]. In addition, catechins, in particular epigallocatechin gallate, have been appointed a sharp antioxidant activity that reduces oxidative stress, endothelial dysfunction, and consequent inflammation [204].
It is worth mentioning that cocoa polyphenols (200 mg of flavan-3-ols, 2,5 g cocoa powder, or 10 g of dark chocolate) bear a health claim by the EFSA for the maintenance of normal endothelium-dependent vasodilation [200].
Despite these promising results, the current evidence does not allow establishing the dose of polyphenols or specific phenolic compounds to be recommended for the management of T2DM.
Indeed, the studies available so far present several methodological limitations. First, duration and dose of polyphenol supplementation in very heterogeneous among the studies. Furthermore, no information is provided on the bioavailability of some phenolic compounds when they are ingested as food items or extracts.
Nevertheless, the daily consumption of naturally polyphenol-rich food items might be advised as part of a healthy diet according to the current nutritional dietary recommendation.
7.3. Inositol
Inositol is a carbocyclic sugar that can be synthesized by both prokaryotic and eukaryotic cells. In mammals, it is obtained from dietary sources (as free inositol, phosphatidyl-inositol or inositol-6-phosphate) or the endogenous synthesis from glucose [205].
The main dietary sources are plant foods, such as soybean and legumes, cereals (in particular buckwheat), and pumpkin [206]. Inositol has nine stereoisomers, but the compounds with biological activity are myo-inositol and D-chiro-inositol [207].
Dietary inositol is mainly in the form of myo-inositol, and after intake it is converted to D-chiro-inositol by an NAD–NADH-dependent epimerase, under the stimulus of insulin. Under physiological conditions, the myo-inositol/D-chiro-inositol ratio is 40:1 in plasma [207].
All cell membranes have inositol phospholipids, which are part of intracellular messengers, and they can modulate the members of insulin signaling pathways [208]. In particular, inositols are second messengers that favor the translocation of GLUT4 (glucotransporter 4) and glucose uptake in the cells [208]. In addition, D-chiro-inositol can increase glycogen synthesis and storage in the liver or skeletal muscle. Finally, D-chiro-inositol can influence the expression of key protein participating in insulin and other hormone signal transduction (i.e., IRS2, PI3K, and AKT) [206]. Therefore, inositols have been used as insulin sensitizers in many clinical settings characterized by the occurrence of insulin resistance, i.e., metabolic syndrome, PCOS, GDM, and other individuals with any abnormal glucose homeostasis [205,207].
A recent meta-analysis reported that the supplementation with myo-inositol (1–4 g/day) in 1319 pregnant women significantly reduced the incidence of GDM [209]. However, the studies presented a huge variability in doses, duration, and timing of administration of myo-inositol [209].
As for the evidence in patients with T2DM, a meta-analysis considering 20 RCTs (n= 1239 individuals with T2DM or prediabetes) showed that the supplementation with inositol (1.2–4 g/day) improved fasting blood glucose and insulin sensitivity (HOMA-IR), independently of body weight loss [210].
Notably, no serious side effects have been reported for the use of inositol in these studies. Mild gastrointestinal side effects (i.e., nausea, flatus, and diarrhea) have been reported in studies with higher doses of myo-inositol (12 g/day) [211].
In conclusion, the studies available so far suggest that inositol can improve glucose metabolism, likely increasing insulin sensitivity. However, further studies are needed to establish the most effective combination of inositol isomers (myo-inositol and D-chiro-inositol) to formulate a clear recommendation for its use in the management of T2DM.
7.4. Microalgae
Microalgae are unicellular microorganisms located in saline and freshwater. They are rich in proteins (phycobiliproteins), CHOs (polysaccharides), lipids (ω-3 PUFAs, sterols), nucleic acids, and many bioactive components such as dietary fiber, antioxidants (polyphenols, tocopherols), and pigments (carotenoids, phycocyanine, chlorophylls, xanthophylls) [212,213,214]. Even though there is a vast variety of microalgae, the ones that have been approved by the EFSA to be used for human nutrition are Arthrospira (also known as Spirulina), Chlorella, and Tetraselmis [214]. Edible microalgae have a particular structure that might affect insulin resistance onset and pancreatic cell function [214]. ω-3 PUFAs and carotenoids, by their antioxidant and anti-inflammatory properties, help to compensate for systemic inflammation and pancreatic impairment. Microalgae’s prebiotic polysaccharides contribute to conserving the intestine’s integrity, and its phenolics may have a part by directly impacting gut microbiota, or concomitantly by the intervention of the endogenous antioxidant defense system [214]. In this respect, a meta-analysis of 7 clinical and 27 preclinical studies, about the effects of Arthrospira (Spirulina) supplementation on glucose metabolism and glycemic control showed that in clinical studies it decreased fasting blood glucose, triglycerides, and total cholesterol levels, and increased HDL-C for patients with T2DM, but it did not have an impact on HbA1c, while in animal studies it decreased fasting blood glucose and HbA1c [215]. However, further studies are needed to fully elucidate their potential benefit on glucose control in T2DM.
7.5. Berberine
Berberine, an alkaloid component obtained from roots and rhizomes, has a variety of therapeutic applications for inflammation, infections, and diabetes [216,217]. Regarding the latter, it has been demonstrated that berberine increases insulin sensitivity and insulin expression, decreases blood insulin levels, stimulates beta cell regeneration, has antioxidant enzyme activity, and reduces lipid peroxidation [216,217,218]. A recent systematic review (18 RCTs) evidenced that berberine decreased HOMA-IR and fasting plasma glucose, and improved lipid profile [217]. Another meta-analysis of 28 RCTs (2313 patients with T2DM) demonstrated that the use of berberine was related to better blood glucose control, including HbA1c, and that its combination with oral hypoglycemic agents provided a more significant reduction of glycemia than using each of them alone [219]. Nevertheless, almost all studies with berberine supplementation have been performed in Asian populations, making difficult the transferability of the results to other populations. In addition, no information on the bioavailability of berberine among different formulations is available.
Therefore, there is still the need for more evidence about the efficacy and safety of berberine.
8. Conclusions
Individualized medical nutritional therapy, as part of lifestyle optimization, is the cornerstone for the management of T2DM and has been shown to improve metabolic outcomes.
To date, scientific evidence does not support a specific macronutrient distribution and meal plans should be individualized. Nevertheless, reducing the overall CHO intake and replacing high GI foods with low GI foods have been shown as valid options for patients with T2DM to improve glycemic control. In addition, the intake of free sugars must not exceed the 10% of total energy intake to avoid hyperglycemia and weight gain. The substitution of saturated and trans fatty acids for monounsaturated and polyunsaturated fats reduces cardiovascular risk and improves glucose metabolism.
As for antioxidant supplementation (i.e., carotene, vitamins E and C) and nutraceuticals (i.e., phytosterols, polyphenols, microalgae, inositols, and berberine), further studies are needed to evaluate the efficacy and the long-term safety in patients with T2DM. The key practical advice is summarized in Figure 1.
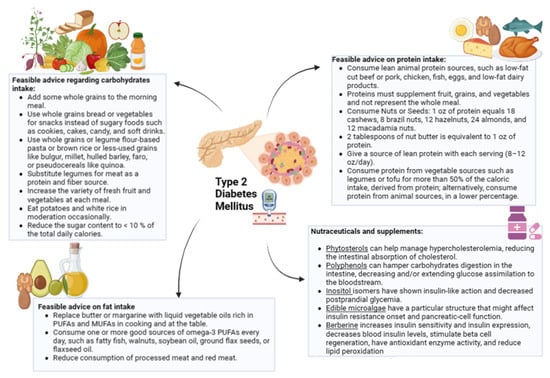
Figure 1.
Graphic representation of dietary and integrative treatment for patients with type 2 diabetes mellitus. PUFAs, polyunsaturated fatty acids; MUFAs, monounsaturated fatty acids.
Author Contributions
Conceptualization, C.V., L.B. and G.M.; methodology, G.M. and L.B.; validation, G.M.; investigation and data curation, C.V., L.V., D.T., E.F.-T., F.C., S.C., A.D. and C.G.; writing—original draft preparation, C.V., L.V., E.F.-T., F.C., S.C., A.D., D.T. and C.G; writing—review and editing, G.M., L.V., C.V. and L.B.; visualization, A.C. and S.S.; supervision, A.C. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors thank Angelo Ferrara, Pasquale Labanchi, and Antimo Puca for technical assessment. All individuals included in this section have consented to the acknowledgement.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaul, N.; Ali, S. Genes, Genetics, and Environment in Type 2 Diabetes: Implication in Personalized Medicine. DNA Cell Biol. 2016, 35, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yu, W.; Hu, C. Genetics of Type 2 Diabetes: Insights into the Pathogenesis and Its Clinical Application. Biomed. Res. Int. 2014, 2014, 926713. [Google Scholar] [CrossRef] [PubMed]
- Laakso, M. Biomarkers for type 2 diabetes. Mol. Metab. 2019, 27S, S139–S146. [Google Scholar] [CrossRef] [PubMed]
- Westerman, K.E.; Walker, M.E.; Gaynor, S.M.; Wesse, J.; DiCorpo, D.; Ma, J.; Alonso, A.; Aslibekyan, S.; Baldridge, A.S.; Bertoni, A.G.; et al. Investigating gene-diet interactions impacting the association between macronutrient intake and glycemic traits. Diabetes 2023, db220851. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Taliun, D.; Thurner, M.; Robertson, N.R.; Torres, J.M.; Rayner, N.W.; Payne, A.J.; Steinthorsdottir, V.; Scott, R.A.; Grarup, N.; et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat. Genet. 2018, 50, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Visscher, P.M.; Wray, N.R.; Zhang, Q.; Sklar, P.; McCarthy, M.I.; Brown, M.A.; Yang, J. 10 Years of GWAS Discovery: Biology, Function, and Translation. Am. J. Hum. Genet. 2017, 101, 5–22. [Google Scholar] [CrossRef]
- McEvoy, C.T.; Cardwell, C.R.; Woodside, J.V.; Young, I.S.; Hunter, S.J.; McKinley, M.C. A Posteriori Dietary Patterns Are Related to Risk of Type 2 Diabetes: Findings from a Systematic Review and Meta-Analysis. J. Acad. Nutr. Diet. 2014, 114, 1759–1775.e4. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.; Fanidi, A.; Bishop, T.R.P.; Sharp, S.J.; Imamura, F.; Dietrich, S.; Akbaraly, T.; Bes-Rastrollo, M.; Beulens, J.W.J.; Byberg, L.; et al. Associations of Total Legume, Pulse, and Soy Consumption with Incident Type 2 Diabetes: Federated Meta-Analysis of 27 Studies from Diverse World Regions. J. Nutr. 2021, 151, 1231–1240. [Google Scholar] [CrossRef]
- Srour, B.; Fezeu, L.K.; Kesse-Guyot, E.; Allès, B.; Debras, C.; Druesne-Pecollo, N.; Chazelas, E.; Deschasaux, M.; Hercberg, S.; Galan, P.; et al. Ultraprocessed Food Consumption and Risk of Type 2 Diabetes among Participants of the NutriNet-Santé Prospective Cohort. JAMA Intern. Med. 2020, 180, 283–291. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.-M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef]
- Halton, T.L.; Liu, S.; Manson, J.E.; Hu, F.B. Low-carbohydrate-diet score and risk of type 2 diabetes in women. Am. J. Clin. Nutr. 2008, 87, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, F.; Jayedi, A.; Khan, T.A.; Shab-Bidar, S. Dietary carbohydrate and the risk of type 2 diabetes: An updated systematic review and dose–response meta-analysis of prospective cohort studies. Sci. Rep. 2022, 12, 2491. [Google Scholar] [CrossRef] [PubMed]
- Sluijs, I.; Van der Schouw, Y.T.; Van der A, D.L.; Spijkerman, A.M.; Hu, F.B.; Grobbee, D.E.; Beulens, J.W. Carbohydrate quantity and quality and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition–Netherlands (EPIC-NL) study. Am. J. Clin. Nutr. 2010, 92, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.J.; Brainard, J.; Song, F.; Wang, X.; Abdelhamid, A.; Hooper, L. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: Systematic review and meta-analysis of randomised controlled trials. BMJ 2019, 366, l4697. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Jalilpiran, Y.; Karimi, E.; Aune, D.; Larijani, B.; Mozaffarian, D.; Willett, W.C.; Esmaillzadeh, A. Dietary Intake of Linoleic Acid, Its Concentrations, and the Risk of Type 2 Diabetes: A Systematic Review and Dose-Response Meta-analysis of Prospective Cohort Studies. Diabetes Care 2021, 44, 2173–2181. [Google Scholar] [CrossRef] [PubMed]
- Neuenschwander, M.; Barbaresko, J.; Pischke, C.R.; Iser, N.; Beckhaus, J.; Schwingshackl, L.; Schlesinger, S. Intake of dietary fats and fatty acids and the incidence of type 2 diabetes: A systematic review and dose-response meta-analysis of prospective observational studies. PLoS Med. 2020, 17, e1003347. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Lampousi, A.-M.; Portillo, M.P.; Romaguera, D.; Hoffmann, G.; Boeing, H. Olive oil in the prevention and management of type 2 diabetes mellitus: A systematic review and meta-analysis of cohort studies and intervention trials. Nutr. Diabetes 2017, 7, e262. [Google Scholar] [CrossRef]
- Barbarawi, M.; Zayed, Y.; Barbarawi, O.; Bala, A.; Alabdouh, A.; Gakhal, I.; Rizk, F.; Alkasasbeh, M.; Bachuwa, G.; Manson, J.E. Effect of Vitamin D Supplementation on the Incidence of Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2020, 105, 2857–2868. [Google Scholar] [CrossRef]
- Rafiq, S.; Jeppesen, P.B. Is Hypovitaminosis D Related to Incidence of Type 2 Diabetes and High Fasting Glucose Level in Healthy Subjects: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2018, 10, 59. [Google Scholar] [CrossRef]
- Seida, J.C.; Mitri, J.; Colmers, I.N.; Majumdar, S.R.; Davidson, M.B.; Edwards, A.L.; Hanley, D.A.; Pittas, A.G.; Tjosvold, L.; Johnson, J.A. Effect of Vitamin D3 Supplementation on Improving Glucose Homeostasis and Preventing Diabetes: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2014, 99, 3551–3560. [Google Scholar] [CrossRef]
- Song, Y.; Wang, L.; Pittas, A.G.; Del Gobbo, L.C.; Zhang, C.; Manson, J.E.; Hu, F.B. Blood 25-Hydroxy Vitamin D Levels and Incident Type 2 Diabetes: A meta-analysis of prospective studies. Diabetes Care 2013, 36, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhai, Y.; Shen, S. Association between vitamin D and prediabetes: A PRISMA-compliant meta-analysis. Medicine 2020, 99, e19034. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, H.; Tang, J.; Li, J.; Chong, W.; Hai, Y.; Feng, Y.; Lunsford, L.D.; Xu, P.; Jia, D.; et al. Effects of Vitamin D Supplementation on Prevention of Type 2 Diabetes in Patients with Prediabetes: A Systematic Review and Meta-analysis. Diabetes Care 2020, 43, 1650–1658. [Google Scholar] [CrossRef]
- Fang, X.; Wang, K.; Han, D.; He, X.; Wei, J.; Zhao, L.; Imam, M.U.; Ping, Z.; Li, Y.; Xu, Y.; et al. Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: A dose–response meta-analysis of prospective cohort studies. BMC Med. 2016, 14, 210. [Google Scholar] [CrossRef]
- Zhao, B.; Zeng, L.; Zhao, J.; Wu, Q.; Dong, Y.; Zou, F.; Gan, L.; Wei, Y.; Zhang, W. Association of magnesium intake with type 2 diabetes and total stroke: An updated systematic review and meta-analysis. BMJ Open 2020, 10, e032240. [Google Scholar] [CrossRef]
- Carlström, M.; Larsson, S. Coffee consumption and reduced risk of developing type 2 diabetes: A systematic review with meta-analysis. Nutr. Rev. 2018, 76, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Knott, C.; Bell, S.; Britton, A. Alcohol Consumption and the Risk of Type 2 Diabetes: A Systematic Review and Dose-Response Meta-analysis of More than 1.9 Million Individuals From 38 Observational Studies. Diabetes Care 2015, 38, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.; Rovira, P.; Llamosas-Falcón, L.; Shield, K.D. Dose–Response Relationships between Levels of Alcohol Use and Risks of Mortality or Disease, for All People, by Age, Sex, and Specific Risk Factors. Nutrients 2021, 13, 2652. [Google Scholar] [CrossRef]
- Xu, L.; Xie, J.; Chen, S.; Chen, Y.; Yang, H.; Miao, M.; Zhu, Z.; Li, Y.; Yu, C.; Xu, C. Light-to-Moderate Alcohol Consumption Is Associated with Increased Risk of Type 2 Diabetes in Individuals with Nonalcoholic Fatty Liver Disease: A Nine-Year Cohort Study. Am. J. Gastroenterol. 2020, 115, 876–884. [Google Scholar] [CrossRef]
- Patterson, R.; McNamara, E.; Tainio, M.; de Sá, T.H.; Smith, A.D.; Sharp, S.J.; Edwards, P.; Woodcock, J.; Brage, S.; Wijndaele, K. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: A systematic review and dose response meta-analysis. Eur. J. Epidemiol. 2018, 33, 811–829. [Google Scholar] [CrossRef]
- Kirwan, J.P.; Solomon, T.P.J.; Wojta, D.M.; Staten, M.A.; Holloszy, J.O. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E151–E156. [Google Scholar] [CrossRef] [PubMed]
- Winnick, J.J.; Sherman, W.M.; Habash, D.L.; Stout, M.B.; Failla, M.L.; Belury, M.A.; Schuster, D.P. Short-Term Aerobic Exercise Training in Obese Humans with Type 2 Diabetes Mellitus Improves Whole-Body Insulin Sensitivity through Gains in Peripheral, not Hepatic Insulin Sensitivity. J. Clin. Endocrinol. Metab. 2008, 93, 771–778. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 8. Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45 (Suppl. S1), S113–S124. [Google Scholar] [CrossRef] [PubMed]
- Sluik, D.; Buijsse, B.; Muckelbauer, R.; Kaaks, R.; Teucher, B.; Johnsen, N.F.; Tjønneland, A.; Overvad, K.; Østergaard, J.N.; Amiano, P.; et al. Physical Activity and Mortality in Individuals with Diabetes Mellitus: A Prospective Study and Meta-analysis. Arch. Intern. Med. 2012, 172, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.S.; Maiya, A.G.; Shastry, B.; Vaishali, K.; Ravishankar, N.; Hazari, A.; Gundmi, S.; Jadhav, R. Exercise and insulin resistance in type 2 diabetes mellitus: A systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2019, 62, 98–103. [Google Scholar] [CrossRef]
- Attvall, S.; Fowelin, J.; Lager, I.; Schenck, H.; Smith, U. Smoking induces insulin resistance-a potential link with the insulin resistance syndrome. J. Intern. Med. 1993, 233, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Van Der Vaart, H.; Postma, D.S.; Timens, W.; Hacken, N.H.T.T. Acute effects of cigarette smoke on inflammation and oxidative stress: A review. Thorax 2004, 59, 713–721. [Google Scholar] [CrossRef]
- Yanbaeva, D.G.; Dentener, M.A.; Creutzberg, E.C.; Wesseling, G.; Wouters, E.F.M. Systemic Effects of Smoking. Chest 2007, 131, 1557–1566. [Google Scholar] [CrossRef]
- Chowdhury, P.; MacLeod, S.; Udupa, K.B.; Rayford, P.L. Pathophysiological Effects of Nicotine on the Pancreas: An Update. Exp. Biol. Med. 2002, 227, 445–454. [Google Scholar] [CrossRef]
- Barrett-Connor, E.; Khaw, K.-T. Cigarette Smoking and Increased Central Adiposity. Ann. Intern. Med. 1989, 111, 783–787. [Google Scholar] [CrossRef]
- Bastard, J.-P.; Maachi, M.; Lagathu, C.; Kim, M.J.; Caron, M.; Vidal, H.; Capeau, J.; Feve, B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006, 17, 4–12. [Google Scholar] [PubMed]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Troisi, R.; Heinold, J.; Vokonas, P.; Weiss, S. Cigarette smoking, dietary intake, and physical activity: Effects on body fat distribution—The Normative Aging Study. Am. J. Clin. Nutr. 1991, 53, 1104–1111. [Google Scholar] [CrossRef]
- Pan, A.; Wang, Y.; Talaei, M.; Hu, F.B.; Wu, T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015, 3, 958–967. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Quantity and Quality of Sleep and Incidence of Type 2 Diabetes: A systematic review and meta-analysis. Diabetes Care 2010, 33, 414–420. [Google Scholar] [CrossRef]
- Shan, Z.; Majewski, C.; Xie, M.; Yan, P.; Guo, Y.; Bao, W.; Rong, Y.; Jackson, C.L.; Hu, F.B.; Liu, L. Sleep Duration and Risk of Type 2 Diabetes: A Meta-analysis of Prospective Studies. Diabetes Care 2015, 38, 529–537. [Google Scholar] [CrossRef]
- Kolb, H.; Martin, S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017, 15, 131. [Google Scholar] [CrossRef]
- Kuo, C.-C.; Moon, K.; Thayer, K.A.; Navas-Acien, A. Environmental Chemicals and Type 2 Diabetes: An Updated Systematic Review of the Epidemiologic Evidence. Curr. Diabetes Rep. 2013, 13, 831–849. [Google Scholar] [CrossRef]
- Guerre-Millo, M. Adipose tissue hormones. J. Endocrinol. Investig. 2002, 25, 855–861. [Google Scholar] [CrossRef]
- Yadav, A.; Jyoti, P.; Jain, S.K.; Bhattacharjee, J. Correlation of Adiponectin and Leptin with Insulin Resistance: A Pilot Study in Healthy North Indian Population. Indian J. Clin. Biochem. 2011, 26, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Jalovaara, K.; Santaniemi, M.; Timonen, M.; Jokelainen, J.; Kesäniemi, Y.A.; Ukkola, O.; Keinänen-Kiukaanniemi, S.; Rajala, U. Low serum adiponectin level as a predictor of impaired glucose regulation and type 2 diabetes mellitus in a middle-aged Finnish population. Metabolism 2008, 57, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Sears, B.; Perry, M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015, 14, 121. [Google Scholar] [CrossRef]
- Zhang, Y.; Scarpace, P.J. The role of leptin in leptin resistance and obesity. Physiol. Behav. 2006, 88, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, T.; Kelesidis, I.; Chou, S.; Mantzoros, C.S. Narrative Review: The Role of Leptin in Human Physiology: Emerging Clinical Applications. Ann. Intern. Med. 2010, 152, 93–100. [Google Scholar] [CrossRef]
- Esmaili, S.; Hemmati, M.; Karamian, M. Physiological role of adiponectin in different tissues: A review. Arch. Physiol. Biochem. 2020, 126, 67–73. [Google Scholar] [CrossRef]
- Brooks, L.; Viardot, A.; Tsakmaki, A.; Stolarczyk, E.; Howard, J.K.; Cani, P.D.; Everard, A.; Sleeth, M.L.; Psichas, A.; Anastasovskaj, J.; et al. Fermentable carbohydrate stimulates FFAR2-dependent colonic PYY cell expansion to increase satiety. Mol. Metab. 2017, 6, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef]
- Alam, C.; Bittoun, E.; Bhagwat, D.; Valkonen, S.; Saari, A.; Jaakkola, U.; Eerola, E.; Huovinen, P.; Hänninen, A. Effects of a germ-free environment on gut immune regulation and diabetes progression in non-obese diabetic (NOD) mice. Diabetologia 2011, 54, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-T.; Nie, Y.-Z.; Yang, Z.; Lu, N.-H. The crosstalk between gut microbiota and obesity and related metabolic disorders. Future Microbiol. 2016, 11, 825–836. [Google Scholar] [CrossRef]
- Sharma, S.; Tripathi, P. Gut microbiome and type 2 diabetes: Where we are and where to go? J. Nutr. Biochem. 2019, 63, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Frias-Toral, E.; Garcia-Velasquez, E.; Carignano, M.D.L.A.; Rodriguez-Veintimilla, D.; Alvarado-Aguilera, I.; Bautista-Litardo, N. Polycystic ovary syndrome and obesity: Clinical aspects and nutritional management. Minerva Endocrinol. 2022, 47, 215–241. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R. Polycystic Ovary Syndrome. Obstet. Gynecol. 2018, 132, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Frias-Toral, E.; Verde, L.; Ceriani, F.; Cucalón, G.; Garcia-Velasquez, E.; Moretti, D.; Savastano, S.; Colao, A.; Muscogiuri, G. PCOS and nutritional approaches: Differences between lean and obese phenotype. Metab. Open 2021, 12, 100123. [Google Scholar] [CrossRef]
- Kakoly, N.S.; Khomami, M.B.; Joham, A.E.; Cooray, S.; Misso, M.L.; Norman, R.; Harrison, C.L.; Ranasinha, S.; Teede, H.; Moran, L.J. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: A systematic review and meta-regression. Hum. Reprod. Update 2018, 24, 455–467. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 5. Facilitating Behavior Change and Well-being to Improve Health Outcomes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45 (Suppl. S1), S60–S82. [Google Scholar] [CrossRef]
- MacLeod, J.; Franz, M.J.; Handu, D.; Gradwell, E.; Brown, C.; Evert, A.; Reppert, A.; Robinson, M. Academy of Nutrition and Dietetics Nutrition Practice Guideline for Type 1 and Type 2 Diabetes in Adults: Nutrition Intervention Evidence Reviews and Recommendations. J. Acad. Nutr. Diet. 2017, 117, 1637–1658. [Google Scholar] [CrossRef]
- De Carvalho, G.B.; Dias-Vasconcelos, N.L.; Santos, R.K.F.; Brandão-Lima, P.N.; Da Silva, D.G.; Pires, L.V. Effect of different dietary patterns on glycemic control in individuals with type 2 diabetes mellitus: A systematic review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1999–2010. [Google Scholar] [CrossRef]
- Esposito, K.; Maiorino, M.I.; Ciotola, M.; Di Palo, C.; Scognamiglio, P.; Gicchino, M.; Petrizzo, M.; Saccomanno, F.; Beneduce, F.; Ceriello, A.; et al. Effects of a Mediterranean-style diet on the need for antihyperglycemic drug therapy in patients with newly diagnosed type 2 diabetes: A randomized trial. Ann. Intern. Med. 2009, 151, 306–314. [Google Scholar] [CrossRef]
- Fallucca, F.; Fontana, L.; Fallucca, S.; Pianesi, M. Gut microbiota and Ma-Pi 2 macrobiotic diet in the treatment of type 2 diabetes. World J. Diabetes 2015, 6, 403–411. [Google Scholar] [CrossRef]
- Kahleova, H.; Salas-Salvadó, J.; Rahelić, D.; Kendall, C.W.; Rembert, E.; Sievenpiper, J.L. Dietary Patterns and Cardiometabolic Outcomes in Diabetes: A Summary of Systematic Reviews and Meta-Analyses. Nutrients 2019, 11, 2209. [Google Scholar] [CrossRef] [PubMed]
- Papamichou, D.; Panagiotakos, D.; Itsiopoulos, C. Dietary patterns and management of type 2 diabetes: A systematic review of randomised clinical trials. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 531–543. [Google Scholar] [CrossRef]
- Rinaldi, S.; Campbell, E.E.; Fournier, J.; O’Connor, C.; Madill, J. A Comprehensive Review of the Literature Supporting Recommendations From the Canadian Diabetes Association for the Use of a Plant-Based Diet for Management of Type 2 Diabetes. Can. J. Diabetes 2016, 40, 471–477. [Google Scholar] [CrossRef]
- Sainsbury, E.; Kizirian, N.V.; Partridge, S.R.; Gill, T.; Colagiuri, S.; Gibson, A.A. Effect of dietary carbohydrate restriction on glycemic control in adults with diabetes: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2018, 139, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Chaimani, A.; Hoffmann, G.; Schwedhelm, C.; Boeing, H. A network meta-analysis on the comparative efficacy of different dietary approaches on glycaemic control in patients with type 2 diabetes mellitus. Eur. J. Epidemiol. 2018, 33, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Borgundvaag, E.; Mak, J.; Kramer, C.K. Metabolic Impact of Intermittent Fasting in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis of Interventional Studies. J. Clin. Endocrinol. Metab. 2021, 106, 902–911. [Google Scholar] [CrossRef]
- Emadian, A.; Andrews, R.C.; England, C.Y.; Wallace, V.; Thompson, J. The effect of macronutrients on glycaemic control: A systematic review of dietary randomised controlled trials in overweight and obese adults with type 2 diabetes in which there was no difference in weight loss between treatment groups. Br. J. Nutr. 2015, 114, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Korsmo-Haugen, H.-K.; Brurberg, K.G.; Mann, J.; Aas, A.-M. Carbohydrate quantity in the dietary management of type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes. Metab. 2019, 21, 15–27. [Google Scholar] [CrossRef]
- Lean, M.E.J.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019, 7, 344–355. [Google Scholar] [CrossRef]
- Magkos, F.; Hjorth, M.F.; Astrup, A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 545–555. [Google Scholar] [CrossRef]
- Look, A.R.G.; Wing, R.R. Long-term Effects of a Lifestyle Intervention on Weight and Cardiovascular Risk Factors in Individuals with Type 2 Diabetes Mellitus: Four-year results of the Look AHEAD trial. Arch. Intern. Med. 2010, 170, 1566–1575. [Google Scholar] [CrossRef]
- Lingvay, I.; Sumithran, P.; Cohen, R.V.; le Roux, C.W. Obesity management as a primary treatment goal for type 2 diabetes: Time to reframe the conversation. Lancet. 2022, 399, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.J.; Boucher, J.L.; Rutten-Ramos, S.; VanWormer, J.J. Lifestyle Weight-Loss Intervention Outcomes in Overweight and Obese Adults with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Acad. Nutr. Diet. 2015, 115, 1447–1463. [Google Scholar] [CrossRef]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet 2018, 391, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Vetrani, C.; Caprio, M.; El Ghoch, M.; Frias-Toral, E.; Mehta, R.J.; Mendez, V.; Moriconi, E.; Paschou, S.A.; Pazderska, A.; et al. Nutritional management of type 2 diabetes in subjects with obesity: An international guideline for clinical practice. Crit. Rev. Food Sci. Nutr. 2021, 1–13. [Google Scholar] [CrossRef]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults with Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef]
- Carbohydrates in Human Nutrition. Report of a Joint FAO/WHO Expert Consultation. FAO Food Nutr. Pap. 1998, 66, 1–140. [Google Scholar]
- FDA. FDA Drug Safety Communication: FDA Revises Labels of SGLT2 Inhibitors for Diabetes to Include Warnings about Too Much Acid in the Blood and Serious Urinary Tract Infections. Available online: https://cacmap.fda.gov/drugs/drug-safety-and-availability/fda-revises-labels-sglt2-inhibitors-diabetes-include-warnings-about-too-much-acid-blood-and-serious (accessed on 14 September 2022).
- Blau, J.E.; Tella, S.H.; Taylor, S.I.; Rother, K.I. Ketoacidosis associated with SGLT2 inhibitor treatment: Analysis of FAERS data. Diabetes/Metabolism Res. Rev. 2017, 33, e2924. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, J.Z.; Day, A.; Brinkworth, G.D.; Sato, J.; Yamada, S.; Jönsson, T.; Beardsley, J.; Johnson, J.A.; Thabane, L.; Johnston, B.C. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: Systematic review and meta-analysis of published and unpublished randomized trial data. BMJ 2021, 372, m4743. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef]
- Triplitt, C.L. Examining the mechanisms of glucose regulation. Am. J. Manag. Care 2012, 18 (Suppl. S1), S4–S10. [Google Scholar]
- Salmerón, J.; Manson, J.E.; Stampfer, M.J.; Colditz, G.; Wing, A.L.; Willett, W.C. Dietary Fiber, Glycemic Load, and Risk of Non—Insulin-dependent Diabetes Mellitus in Women. JAMA 1997, 277, 472–477. [Google Scholar] [CrossRef]
- Vega-López, S.; Venn, B.J.; Slavin, J.L. Relevance of the Glycemic Index and Glycemic Load for Body Weight, Diabetes, and Cardiovascular Disease. Nutrients 2018, 10, 1361. [Google Scholar] [CrossRef]
- Sydney, U.O. Glycemic Index Research. Available online: www.glycemicindex.com (accessed on 15 September 2022).
- Marsh, K.; Barclay, A.; Colagiuri, S.; Brand-Miller, J. Glycemic Index and Glycemic Load of Carbohydrates in the Diabetes Diet. Curr. Diabetes Rep. 2011, 11, 120–127. [Google Scholar] [CrossRef]
- Zafar, M.I.; Mills, K.E.; Zheng, J.; Regmi, A.; Hu, S.Q.; Gou, L.; Chen, L.-L. Low-glycemic index diets as an intervention for diabetes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2019, 110, 891–902. [Google Scholar] [CrossRef]
- Eastwood, M.A.; Kay, R.M. An hypothesis for the action of dietary fiber along the gastrointestinal tract. Am. J. Clin. Nutr. 1979, 32, 364–367. [Google Scholar] [CrossRef]
- McRorie, J.W., Jr.; McKeown, N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef]
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef]
- Salamone, D.; Rivellese, A.A.; Vetrani, C. The relationship between gut microbiota, short-chain fatty acids and type 2 diabetes mellitus: The possible role of dietary fibre. Acta Diabetol. 2021, 58, 1131–1138. [Google Scholar] [CrossRef]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef]
- Burger, K.N.J.; Beulens, J.W.J.; Van Der Schouw, Y.T.; Sluijs, I.; Spijkerman, A.M.W.; Sluik, D.; Boeing, H.; Kaaks, R.; Teucher, B.; Dethlefsen, C.; et al. Dietary Fiber, Carbohydrate Quality and Quantity, and Mortality Risk of Individuals with Diabetes Mellitus. PLoS ONE 2012, 7, e43127. [Google Scholar] [CrossRef]
- He, M.; van Dam, R.; Rimm, E.B.; Hu, F.B.; Qi, L. Whole-Grain, Cereal Fiber, Bran, and Germ Intake and the Risks of All-Cause and Cardiovascular Disease-Specific Mortality among Women with Type 2 Diabetes Mellitus. Circulation 2010, 121, 2162–2168. [Google Scholar] [CrossRef]
- Partula, V.; Deschasaux, M.; Druesne-Pecollo, N.; Latino-Martel, P.; Desmetz, E.; Chazelas, E.; Kesse-Guyot, E.; Julia, C.; Fezeu, L.K.; Galan, P.; et al. Associations between consumption of dietary fibers and the risk of cardiovascular diseases, cancers, type 2 diabetes, and mortality in the prospective NutriNet-Santé cohort. Am. J. Clin. Nutr. 2020, 112, 195–207. [Google Scholar] [CrossRef]
- Fitch, C.; Keim, K.S. Position of the Academy of Nutrition and Dietetics: Use of Nutritive and Nonnutritive Sweeteners. J. Acad. Nutr. Diet. 2012, 112, 739–758. [Google Scholar] [CrossRef]
- Magnuson, B.A.; Roberts, A.; Nestmann, E.R. Critical review of the current literature on the safety of sucralose. Food Chem. Toxicol. 2017, 106, 324–355. [Google Scholar] [CrossRef]
- Robayo–Torres, C.C.; Quezada–Calvillo, R.; Nichols, B.L. Disaccharide Digestion: Clinical and Molecular Aspects. Clin. Gastroenterol. Hepatol. 2006, 4, 276–287. [Google Scholar] [CrossRef]
- Hanover, L.M.; White, J.S. Manufacturing, composition, and applications of fructose. Am. J. Clin. Nutr. 1993, 58 (Suppl. S5), 724S–732S. [Google Scholar] [CrossRef]
- Taskinen, M.-R.; Söderlund, S.; Bogl, L.H.; Hakkarainen, A.; Matikainen, N.; Pietiläinen, K.H.; Räsänen, S.; Lundbom, N.; Björnson, E.; Eliasson, B.; et al. Adverse effects of fructose on cardiometabolic risk factors and hepatic lipid metabolism in subjects with abdominal obesity. J. Intern. Med. 2017, 282, 187–201. [Google Scholar] [CrossRef]
- Sievenpiper, J.L.; Carleton, A.J.; Chatha, S.; Jiang, H.Y.; de Souza, R.J.; Beyene, J.; Kendall, C.W.; Jenkins, D.J. Heterogeneous Effects of Fructose on Blood Lipids in Individuals with Type 2 Diabetes: Systematic review and meta-analysis of experimental trials in humans. Diabetes Care 2009, 32, 1930–1937. [Google Scholar] [CrossRef]
- Livesey, G.; Taylor, R. Fructose consumption and consequences for glycation, plasma triacylglycerol, and body weight: Meta-analyses and meta-regression models of intervention studies. Am. J. Clin. Nutr. 2008, 88, 1419–1437. [Google Scholar] [PubMed]
- Cozma, A.I.; Sievenpiper, J.L.; de Souza, R.J.; Chiavaroli, L.; Ha, V.; Wang, D.D.; Mirrahimi, A.; Yu, M.E.; Carleton, A.J.; Di Buono, M.; et al. Effect of Fructose on Glycemic Control in Diabetes: A systematic review and meta-analysis of controlled feeding trials. Diabetes Care 2012, 35, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guideline: Sugars Intake for Adults and Children; WHO Guidelines Approved by the Guidelines Review Committee; WHO: Geneva, Switzerland, 2015.
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019, 30, 67–77.e63. [Google Scholar] [CrossRef] [PubMed]
- Te Morenga, L.; Mallard, S.; Mann, J. Dietary sugars and body weight: Systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 2012, 345, e7492. [Google Scholar] [CrossRef]
- Lohner, S.; de Gaudry, D.K.; Toews, I.; Ferenci, T.; Meerpohl, J.J. Non-nutritive sweeteners for diabetes mellitus. Cochrane Database Syst. Rev. 2020, 2020, CD012885. [Google Scholar] [CrossRef]
- FDA Additional Information about High-Intensity Sweeteners Permitted for Use in Food in the United States. Available online: https://www.fda.gov/food/food-additives-petitions/additional-information-about-high-intensity-sweeteners-permitted-use-food-united-states (accessed on 15 September 2022).
- Weihrauch, M.R.; Diehl, V. Artificial sweeteners—Do they bear a carcinogenic risk? Ann. Oncol. 2004, 15, 1460–1465. [Google Scholar] [CrossRef]
- Arumugam, B.; Subramaniam, A.; Alagaraj, P. Stevia as a Natural Sweetener: A Review. Cardiovasc Hematol Agents Med Chem. 2020, 18, 94–103. [Google Scholar] [CrossRef]
- Samuel, P.; Ayoob, K.T.; Magnuson, B.A.; Wölwer-Rieck, U.; Jeppesen, P.B.; Rogers, P.J.; Rowland, I.; Mathews, R. Stevia Leaf to Stevia Sweetener: Exploring Its Science, Benefits, and Future Potential. J. Nutr. 2018, 148, 1186S–1205S. [Google Scholar] [CrossRef]
- Grembecka, M. Natural sweeteners in a human diet. Rocz. Panstw. Zakl. Hig. 2015, 66, 195–202. [Google Scholar]
- Grabitske, H.A.; Slavin, J.L. Low-Digestible Carbohydrates in Practice. J. Am. Diet. Assoc. 2008, 108, 1677–1681. [Google Scholar] [CrossRef]
- Journal EFSA. Scientific Opinion on the Substantiation of Health Claims Related to the Sugar Replacers Xylitol, Sorbitol, Mannitol, Maltitol, Lactitol, Isomalt, Erythritol, D-Tagatose, Isomaltulose, Sucralose and Polydextrose and Maintenance of Tooth Mineralisation by Decreasing Tooth Demineralisation. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/2076 (accessed on 15 September 2022).
- Nakamura, S. Bioavailability of cellobiose and other non-digestible and/or non-absorbable sugar substitutes and related topics. Nutrition 2005, 21, 1158–1159. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.L.; Pi-Sunyer, F.X. Carbohydrate Issues: Type and Amount. J. Am. Diet. Assoc. 2008, 108 (Suppl. 1), S34–S39. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, N.; Padwal, R.; Field, C.; Marks, S.; Jacobs, R.; Tonelli, M. A systematic review on the effect of sweeteners on glycemic response and clinically relevant outcomes. BMC Med. 2011, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Lenhart, A.; Chey, W.D. A Systematic Review of the Effects of Polyols on Gastrointestinal Health and Irritable Bowel Syndrome. Adv. Nutr. 2017, 8, 587–596. [Google Scholar] [CrossRef] [PubMed]
- White, B. Dietary fatty acids. Am Fam Physician. 2009, 80, 345–350. [Google Scholar]
- Gray, A.; Threlkeld, R.J. Nutritional Recommendations for Individuals with Diabetes. Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; Endotext: South Dartmouth, MA, USA, 2000. [Google Scholar]
- American Heart Association. Healthy Eating. Available online: https://www.heart.org/en/healthy-living/healthy-eating (accessed on 15 September 2022).
- American Heart Association. Saturated Fat. Available online: https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/fats/saturated-fats (accessed on 15 September 2022).
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e563–e595. [Google Scholar] [CrossRef]
- Schwab, U.; Reynolds, A.N.; Sallinen, T.; Rivellese, A.A.; Risérus, U. Dietary fat intakes and cardiovascular disease risk in adults with type 2 diabetes: A systematic review and meta-analysis. Eur. J. Nutr. 2021, 60, 3355–3363. [Google Scholar] [CrossRef]
- Hooper, L.; Martin, N.; Jimoh, O.F.; Kirk, C.; Foster, E.; Abdelhamid, A.S. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst. Rev. 2020, 5, CD011737. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on Dietary Reference Values for Fats, Including Saturated Fatty Acids, Polyunsaturated Fatty Acids, Monounsaturated Fatty Acids, Trans Fatty Acids, and Cholesterol. Available online: https://www.efsa.europa.eu/it/efsajournal/pub/1461 (accessed on 15 September 2022).
- Oteng, A.-B.; Kersten, S. Mechanisms of Action of trans Fatty Acids. Adv. Nutr. 2020, 11, 697–708. [Google Scholar] [CrossRef]
- Aronis, K.N.; Khan, S.M.; Mantzoros, C.S. Effects of trans fatty acids on glucose homeostasis: A meta-analysis of randomized, placebo-controlled clinical trials. Am. J. Clin. Nutr. 2012, 96, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- American Heart Association. Monounsaturated Fat. Available online: https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/fats/monounsaturated-fats (accessed on 15 September 2022).
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vázquez-Carrera, M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Korat, A.A.; Malik, V.; Hu, F.B. Metabolic Effects of Monounsaturated Fatty Acid–Enriched Diets Compared with Carbohydrate or Polyunsaturated Fatty Acid–Enriched Diets in Patients with Type 2 Diabetes: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Diabetes Care 2016, 39, 1448–1457. [Google Scholar] [CrossRef]
- Imamura, F.; Micha, R.; Wu, J.H.Y.; de Oliveira Otto, M.C.; Otite, F.O.; Abioye, A.I.; Mozaffarian, D. Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials. PLoS Med. 2016, 13, e1002087. [Google Scholar] [CrossRef] [PubMed]
- Muley, A.; Fernandez, R.; Ellwood, L.; Muley, P.; Shah, M. Effect of tree nuts on glycemic outcomes in adults with type 2 diabetes mellitus: A systematic review. JBI Évid. Synth. 2021, 19, 966–1002. [Google Scholar] [CrossRef]
- Krupa, K.; Fritz, K.; Parmar, M. Omega-3 Fatty Acids; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Anderson, B.M.; Ma, D.W. Are all n-3 polyunsaturated fatty acids created equal? Lipids Health Dis. 2009, 8, 33. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Backes, J.; Anzalone, D.; Hilleman, D.; Catini, J. The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis. 2016, 15, 118. [Google Scholar] [CrossRef]
- Bays, H.E.; Tighe, A.P.; Sadovsky, R.; Davidson, M.H. Prescription omega-3 fatty acids and their lipid effects: Physiologic mechanisms of action and clinical implications. Expert Rev. Cardiovasc. Ther. 2008, 6, 391–409. [Google Scholar] [CrossRef]
- Noreen, E.E.; Sass, M.J.; Crowe, M.L.; Pabon, V.A.; Brandauer, J.; Averill, L.K. Effects of supplemental fish oil on resting metabolic rate, body composition, and salivary cortisol in healthy adults. J. Int. Soc. Sports Nutr. 2010, 7, 31. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, T.; Yoshida, M.; Arita, M. Omega-3 fatty acid-derived mediators that control inflammation and tissue homeostasis. Int. Immunol. 2019, 31, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Bilotto, S.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Devi, K.P.; Loizzo, M.R.; Tundis, R. Omega-3 polyunsaturated fatty acids and cancer: Lessons learned from clinical trials. Cancer Metast. Rev. 2015, 34, 359–380. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Saotome, M.; Iguchi, K.; Maekawa, Y. Marine-Derived Omega-3 Polyunsaturated Fatty Acids and Heart Failure: Current Understanding for Basic to Clinical Relevance. Int. J. Mol. Sci. 2019, 20, 4025. [Google Scholar] [CrossRef]
- Ascend Study Collaborative Group; Bowman, L.; Mafham, M.; Wallendszus, K.; Stevens, W.; Buck, G.; Barton, J.; Murphy, K.; Aung, T.; Haynes, R.; et al. Effects of n-3 fatty acid supplements in diabetes mellitus. N. Engl. J. Med. 2018, 379, 1540–1550. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n−3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 38, 11–22. [Google Scholar] [CrossRef]
- Ito, M.K. A Comparative Overview of Prescription Omega-3 Fatty Acid Products. P T Peer-Rev. J. Formul. Manag. 2015, 40, 826–857. [Google Scholar]
- Li, Y.R.; Jia, Z.; Zhu, H. Dietary Supplementation with Anti-Inflammatory Omega-3 Fatty Acids for Cardiovascular Protection: Help or Hoax? React. Oxyg. Species 2019, 7, 78–85. [Google Scholar] [CrossRef]
- Skulas-Ray, A.C.; Wilson, P.W.; Harris, W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K.; Jacobson, T.A.; Engler, M.B.; Miller, M.; Robinson, J.G.; et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory From the American Heart Association. Circulation 2019, 140, e673–e691. [Google Scholar] [CrossRef]
- National Cancer Institute. Identification of Top Food Sources of Various Dietary Components. Available online: https://epi.grants.cancer.gov/diet/foodsources (accessed on 15 September 2022).
- Tran, N.L.; Barraj, L.; Heilman, J.; Scrafford, C. Egg consumption and cardiovascular disease among diabetic individuals: A systematic review of the literature. Diabetes Metab. Syndr. Obes. 2014, 7, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Protein Structure and Function. Biochemistry 2002, 262, 159–173. [Google Scholar]
- Gannon, M.C.; Nuttall, F.Q. Effect of a High-Protein, Low-Carbohydrate Diet on Blood Glucose Control in People with Type 2 Diabetes. Diabetes 2004, 53, 2375–2382. [Google Scholar] [CrossRef]
- Akhavan, N.S.; Pourafshar, S.; Johnson, S.A.; Foley, E.M.; George, K.S.; Munoz, J.; Siebert, S.; Clark, E.A.; Basiri, R.; Hickner, R.C.; et al. The Relationship between Protein Intake and Source on Factors Associated with Glycemic Control in Individuals with Prediabetes and Type 2 Diabetes. Nutrients 2020, 12, 2031. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.-Y.; Zhang, Z.-L.; Wang, P.-Y.; Qin, L.-Q. Effects of high-protein diets on body weight, glycaemic control, blood lipids and blood pressure in type 2 diabetes: Meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Viguiliouk, E.; Stewart, S.E.; Jayalath, V.H.; Ng, A.P.; Mirrahimi, A.; De Souza, R.J.; Hanley, A.J.; Bazinet, R.P.; Mejia, S.B.; Leiter, L.A.; et al. Effect of Replacing Animal Protein with Plant Protein on Glycemic Control in Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2015, 7, 9804–9824. [Google Scholar] [CrossRef] [PubMed]
- NK Foundation. Protein in Our Diet—Variety and Moderation is the Key. Available online: https://www.kidney.org/news/monthly/protein-in-our-diet (accessed on 15 September 2022).
- Hahn, D.; Hodson, E.M.; Fouque, D. Low protein diets for non-diabetic adults with chronic kidney disease. Cochrane Database Syst. Rev. 2018, 10, CD001892. [Google Scholar] [CrossRef]
- Aroda, V.R.; Edelstein, S.L.; Goldberg, R.B.; Knowler, W.C.; Marcovina, S.M.; Orchard, T.; Bray, G.A.; Schade, D.S.; Temprosa, M.G.; White, N.H.; et al. Long-term Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study. J. Clin. Endocrinol. Metab. 2016, 101, 1754–1761. [Google Scholar] [CrossRef]
- Butler, C.C.; Vidal-Alaball, J.; Cannings-John, R.; McCaddon, A.; Hood, K.; Papaioannou, A.; Mcdowell, I.; Goringe, A. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency: A systematic review of randomized controlled trials. Fam. Pract. 2006, 23, 279–285. [Google Scholar] [CrossRef]
- Vidal-Alaball, J.; Butler, C.; Cannings-John, R.; Goringe, A.; Hood, K.; McCaddon, A.; McDowell, I.; Papaioannou, A. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency. Cochrane Database Syst. Rev. 2005, CD004655. [Google Scholar] [CrossRef]
- Davy, B.M.; Dennis, E.A.; Dengo, A.L.; Wilson, K.L.; Davy, K.P. Water Consumption Reduces Energy Intake at a Breakfast Meal in Obese Older Adults. J. Am. Diet. Assoc. 2008, 108, 1236–1239. [Google Scholar] [CrossRef] [PubMed]
- Na Jeong, J. Effect of Pre-meal Water Consumption on Energy Intake and Satiety in Non-obese Young Adults. Clin. Nutr. Res. 2018, 7, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Tate, D.F.; Turner-McGrievy, G.; Lyons, E.; Stevens, J.; Erickson, K.; Polzien, K.; Diamond, M.; Wang, X.; Popkin, B. Replacing caloric beverages with water or diet beverages for weight loss in adults: Main results of the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am. J. Clin. Nutr. 2012, 95, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Madjd, A.; Taylor, M.A.; Delavari, A.; Malekzadeh, R.; Macdonald, I.A.; Farshchi, H.R. Beneficial effects of replacing diet beverages with water on type 2 diabetic obese women following a hypo-energetic diet: A randomized, 24-week clinical trial. Diabetes Obes. Metab. 2017, 19, 125–132. [Google Scholar] [CrossRef]
- Paradis, C.; Butt, P.; Shield, K.; Poole, N.; Wells, S.; Naimi, T.; Sherk, A. The Low-Risk Alcohol Drinking Guidelines Scientific Expert Panels. In Canada’s Guidance on Alcohol and Health: Final Report; Canadian Centre on Substance Use and Addiction: Ottawa, ON, Canada, 2011. [Google Scholar]
- Pirro, M.; Vetrani, C.; Bianchi, C.; Mannarino, M.; Bernini, F.; Rivellese, A. Joint position statement on “Nutraceuticals for the treatment of hypercholesterolemia” of the Italian Society of Diabetology (SID) and of the Italian Society for the Study of Arteriosclerosis (SISA). Nutr. Metab. Cardiovasc. Dis. 2017, 27, 2–17. [Google Scholar] [CrossRef]
- Gupta, A.K.; Savopoulos, C.G.; Ahuja, J.; Hatzitolios, A.I. Role of phytosterols in lipid-lowering: Current perspectives. QJM Int. J. Med. 2011, 104, 301–308. [Google Scholar] [CrossRef] [PubMed]
- AbuMweis, S.S.; Marinangeli, C.P.; Frohlich, J.; Jones, P.J. Implementing Phytosterols Into Medical Practice as a Cholesterol-Lowering Strategy: Overview of Efficacy, Effectiveness, and Safety. Can. J. Cardiol. 2014, 30, 1225–1232. [Google Scholar] [CrossRef]
- Cohn, J.S.; Kamili, A.; Wat, E.; Chung, R.W.; Tandy, S. Reduction in intestinal cholesterol absorption by various food components: Mechanisms and implications. Atheroscler. Suppl. 2010, 11, 45–48. [Google Scholar] [CrossRef]
- Rocha, M.; Bañuls, C.; Bellod, L.; Jover, A.; Victor, V.; Mijares, A.H. A Review on the Role of Phytosterols: New Insights Into Cardiovascular Risk. Curr. Pharm. Des. 2011, 17, 4061–4075. [Google Scholar] [CrossRef]
- Baker, W.L.; Baker, E.L.; Coleman, C.I. The effect of plant sterols or stanols on lipid parameters in patients with type 2 diabetes: A meta-analysis. Diabetes Res. Clin. Pract. 2009, 84, e33–e37. [Google Scholar] [CrossRef]
- Wang, J.-F.; Zhang, H.-M.; Li, Y.-Y.; Xia, S.; Wei, Y.; Yang, L.; Wang, D.; Ye, J.-J.; Li, H.-X.; Yuan, J.; et al. A combination of omega-3 and plant sterols regulate glucose and lipid metabolism in individuals with impaired glucose regulation: A randomized and controlled clinical trial. Lipids Health Dis. 2019, 18, 106. [Google Scholar] [CrossRef] [PubMed]
- Egbuna, C.; Awuchi, C.G.; Kushwaha, G.; Rudrapal, M.; Patrick-Iwuanyanwu, K.C.; Singh, O.; Odoh, U.E.; Khan, J.; Jeevanandam, J.; Kumarasamy, S.; et al. Bioactive Compounds Effective Against Type 2 Diabetes Mellitus: A Systematic Review. Curr. Top. Med. Chem. 2021, 21, 1067–1095. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Singh Yadav, S. A review on health benefits of phenolics derived from dietary spices. Curr. Res. Food Sci. 2022, 5, 1508–1523. [Google Scholar] [CrossRef] [PubMed]
- Palma-Duran, S.A.; Vlassopoulos, A.; Lean, M.; Govan, L.; Combet, E. Nutritional intervention and impact of polyphenol on glycohemoglobin (HbA1c) in non-diabetic and type 2 diabetic subjects: Systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2017, 57, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Giacco, R.; Costabile, G.; Fatati, G.; Frittitta, L.; Maiorino, M.I.; Marelli, G.; Parillo, M.; Pistis, D.; Tubili, C.; Vetrani, C.; et al. Effects of polyphenols on cardio-metabolic risk factors and risk of type 2 diabetes. A joint position statement of the Diabetes and Nutrition Study Group of the Italian Society of Diabetology (SID), the Italian Association of Dietetics and Clinical Nutrition (ADI) and the Italian Association of Medical Diabetologists (AMD). Nutr. Metab. Cardiovasc. Dis. 2020, 30, 355–367. [Google Scholar] [CrossRef]
- Nedumpully-Govindan, P.; Kakinen, A.; Pilkington, E.H.; Davis, T.P.; Ke, P.C.; Ding, F. Stabilizing off-pathway Oligomers by Polyphenol Nanoassemblies for IAPP Aggregation Inhibition. Sci. Rep. 2016, 6, srep19463. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Abdelhaleem, I.A.; Brakat, A.M.; Adayel, H.M.; Asla, M.M.; Rizk, M.A.; Aboalfetoh, A.Y. The effects of resveratrol on glycemic control and cardiometabolic parameters in patients with T2DM: A systematic review and meta-analysis. Med. Clin. 2022, 158, 576–585. [Google Scholar] [CrossRef]
- García-Martínez, B.I.; Ruiz-Ramos, M.; Pedraza-Chaverri, J.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Influence of Age and Dose on the Effect of Resveratrol for Glycemic Control in Type 2 Diabetes Mellitus: Systematic Review and Meta-Analysis. Molecules 2022, 27, 5232. [Google Scholar] [CrossRef]
- Zeraattalab-Motlagh, S.; Jayedi, A.; Shab-Bidar, S. The effects of resveratrol supplementation in patients with type 2 diabetes, metabolic syndrome, and nonalcoholic fatty liver disease: An umbrella review of meta-analyses of randomized controlled trials. Am. J. Clin. Nutr. 2021, 114, 1675–1685. [Google Scholar] [CrossRef]
- Cao, X.; Liao, W.; Xia, H.; Wang, S.; Sun, G. The Effect of Resveratrol on Blood Lipid Profile: A Dose-Response Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 3755. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Song, A.; Zhang, Y.; Shu, L.; Song, G.; Ma, H. Effect of Resveratrol on Blood Lipid Levels in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Obesity 2019, 27, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Nyambuya, T.; Nkambule, B.; Mazibuko-Mbeje, S.; Mxinwa, V.; Mokgalaboni, K.; Orlando, P.; Silvestri, S.; Louw, J.; Tiano, L.; Dludla, P. A Meta-Analysis of the Impact of Resveratrol Supplementation on Markers of Renal Function and Blood Pressure in Type 2 Diabetic Patients on Hypoglycemic Therapy. Molecules 2020, 25, 5645. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Choudhury, S.T.; Seidel, V.; Bin Rahman, A.; Aziz, A.; Richi, A.E.; Rahman, A.; Jafrin, U.H.; Hannan, J.M.A.; Abdel-Wahab, Y.H.A. Therapeutic Potential of Quercetin in the Management of Type-2 Diabetes Mellitus. Life 2022, 12, 1146. [Google Scholar] [CrossRef]
- Zheng, S.; Huang, K.; Tong, T. Efficacy and Mechanisms of Oleuropein in Mitigating Diabetes and Diabetes Complications. J. Agric. Food Chem. 2021, 69, 6145–6155. [Google Scholar] [CrossRef]
- The European Food Safety Authority (EFSA). Scientific Opinion on the Modification of the Authorisation of a Health Claim Related to Cocoa Flavanols and Maintenance of Normal Endothelium-Dependent Vasodilation Pursuant to Article 13(5) of Regulation (EC) No 1924/2006 Following a Request in Accordance with Article 19 of Regulation (EC) No 1924/2006. 2014. Available online: https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2014.3654 (accessed on 15 September 2022).
- Vetrani, C.; Vitale, M.; Bozzetto, L.; Della Pepa, G.; Cocozza, S.; Costabile, G.; Mangione, A.; Cipriano, P.; Annuzzi, G.; Rivellese, A.A. Association between different dietary polyphenol subclasses and the improvement in cardiometabolic risk factors: Evidence from a randomized controlled clinical trial. Acta Diabetol. 2018, 55, 149–153. [Google Scholar] [CrossRef]
- Della Pepa, G.; Vetrani, C.; Vitale, M.; Bozzetto, L.; Costabile, G.; Cipriano, P.; Mangione, A.; Patti, L.; Riccardi, G.; Rivellese, A.A.; et al. Effects of a diet naturally rich in polyphenols on lipid composition of postprandial lipoproteins in high cardiometabolic risk individuals: An ancillary analysis of a randomized controlled trial. Eur. J. Clin. Nutr. 2020, 74, 183–192. [Google Scholar] [CrossRef]
- Vetrani, C.; Costabile, G.; Vitale, M.; Giacco, R. (Poly)phenols and cardiovascular diseases: Looking in to move forward. J. Funct. Foods 2020, 71, 104013. [Google Scholar] [CrossRef]
- Wen, L.; Wu, D.; Tan, X.; Zhong, M.; Xing, J.; Li, W.; Li, D.; Cao, F. The Role of Catechins in Regulating Diabetes: An Update Review. Nutrients 2022, 14, 4681. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Palomba, S.; Laganà, A.S.; Orio, F. Erratum to “Inositols in the Treatment of Insulin-Mediated Diseases”. Int. J. Endocrinol. 2016, 2016, 6189820. [Google Scholar] [CrossRef]
- Dinicola, S.; Unfer, V.; Facchinetti, F.; Soulage, C.O.; Greene, N.D.; Bizzarri, M.; Laganà, A.S.; Chan, S.-Y.; Bevilacqua, A.; Pkhaladze, L.; et al. Inositols: From Established Knowledge to Novel Approaches. Int. J. Mol. Sci. 2021, 22, 10575. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Unfer, V.; Dewailly, D.; Kamenov, Z.A.; Diamanti-Kandarakis, E.; Laganà, A.S.; Nestler, J.E.; Soulage, C.O.; Group of ‘Inositol in PCOS and Reproduction’. Inositols in Polycystic Ovary Syndrome: An Overview on the Advances. Trends Endocrinol. Metab. 2020, 31, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Croze, M.L.; Soulage, C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef] [PubMed]
- Motuhifonua, S.K.; Lin, L.; Alsweiler, J.; Crawford, T.J.; Crowther, C.A. Antenatal dietary supplementation with myo-inositol for preventing gestational diabetes. Cochrane Database Syst. Rev. 2023, 2, CD011507. [Google Scholar] [CrossRef] [PubMed]
- Miñambres, I.; Cuixart, G.; Gonçalves, A.; Corcoy, R. Effects of inositol on glucose homeostasis: Systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2019, 38, 1146–1152. [Google Scholar] [CrossRef]
- Carlomagno, G.; Unfer, V. Inositol safety: Clinical evidences. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 931–936. [Google Scholar]
- Conde, T.A.; Zabetakis, I.; Tsoupras, A.; Medina, I.; Costa, M.; Silva, J.; Neves, B.; Domingues, P.; Domingues, M.R. Microalgal Lipid Extracts Have Potential to Modulate the Inflammatory Response: A Critical Review. Int. J. Mol. Sci. 2021, 22, 9825. [Google Scholar] [CrossRef]
- Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar. Drugs 2021, 19, 484. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Torrella, J.; Pagès, T.; Viscor, G.; Torres, J. Edible Microalgae and Their Bioactive Compounds in the Prevention and Treatment of Metabolic Alterations. Nutrients 2021, 13, 563. [Google Scholar] [CrossRef]
- Ghanbari, F.; Amerizadeh, A.; Behshood, P.; Moradi, S.; Asgary, S. Effect of Microalgae Arthrospira on Biomarkers of Glycemic Control and Glucose Metabolism: A Systematic Review and Meta-analysis. Curr. Probl. Cardiol. 2022, 47, 100942. [Google Scholar] [CrossRef]
- Barzkar, F.; Baradaran, H.R.; Khamseh, M.E.; Azad, R.V.; Koohpayehzadeh, J.; Moradi, Y. Medicinal plants in the adjunctive treatment of patients with type-1 diabetes: A systematic review of randomized clinical trials. J. Diabetes Metab. Disord. 2020, 19, 1917–1929. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Liu, X.; Wu, N.; Han, Y.; Wang, J.; Yu, Y.; Chen, Q. Efficacy and Safety of Berberine Alone for Several Metabolic Disorders: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Front. Pharmacol. 2021, 12, 653887. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, A.; Li, S.; Liu, B.; Li, H.; Yan, Y. Efficacy and safety of berberine in the treatment of type 2 diabetes with insulin resistance: Protocol for a systematic review. Medicine 2019, 98, e16947. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xu, X.; Yin, M.; Zhang, Y.; Huang, L.; Chen, R.; Ni, J. Effects of berberine on blood glucose in patients with type 2 diabetes mellitus: A systematic literature review and a meta-analysis. Endocr. J. 2019, 66, 51–63. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).