Subcritical Water Extraction of Rosmarinic Acid from Lemon Balm (Melissa officinalis L.) and Its Effect on Plant Cell Wall Constituents
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Proximate Composition Analysis of Plant Material
2.3. Extraction of RA and Phenolic Components
2.3.1. Reference Extraction Procedure of RA and Phenolic Components
2.3.2. Static Maceration (Preparation of Tincture)
2.3.3. Temperature-Assisted Dynamic Maceration
2.3.4. Subcritical Water Extraction
2.4. Total Polyphenol Content
2.5. Antioxidant Activity Assays
2.6. High Performance Liquid Chromatography (HPLC) Determination of RA
- A1 = area of the peak due to RA in the chromatogram obtained with the test solution;
- A2 = area of the peak due to RA in the chromatogram obtained with reference solution;
- M1 = mass of the plant material/extract to be examined used to prepare the test solution, in grams;
- M2 = mass of RA used to prepare reference solution, in grams;
- P = percentage content of RA in standard rosmarinic acid.
2.7. HPLC Determination of Other Phenolic Compounds
2.8. HPLC Determination of Free Sugars
2.9. Uronic Acid, Cellulose, and Lignin Content
2.10. Isolation of the Polysaccharide Fractions
2.11. Preparation of Freeze-Dried Extracts
2.12. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition Analysis of Lemon Balm Leaves
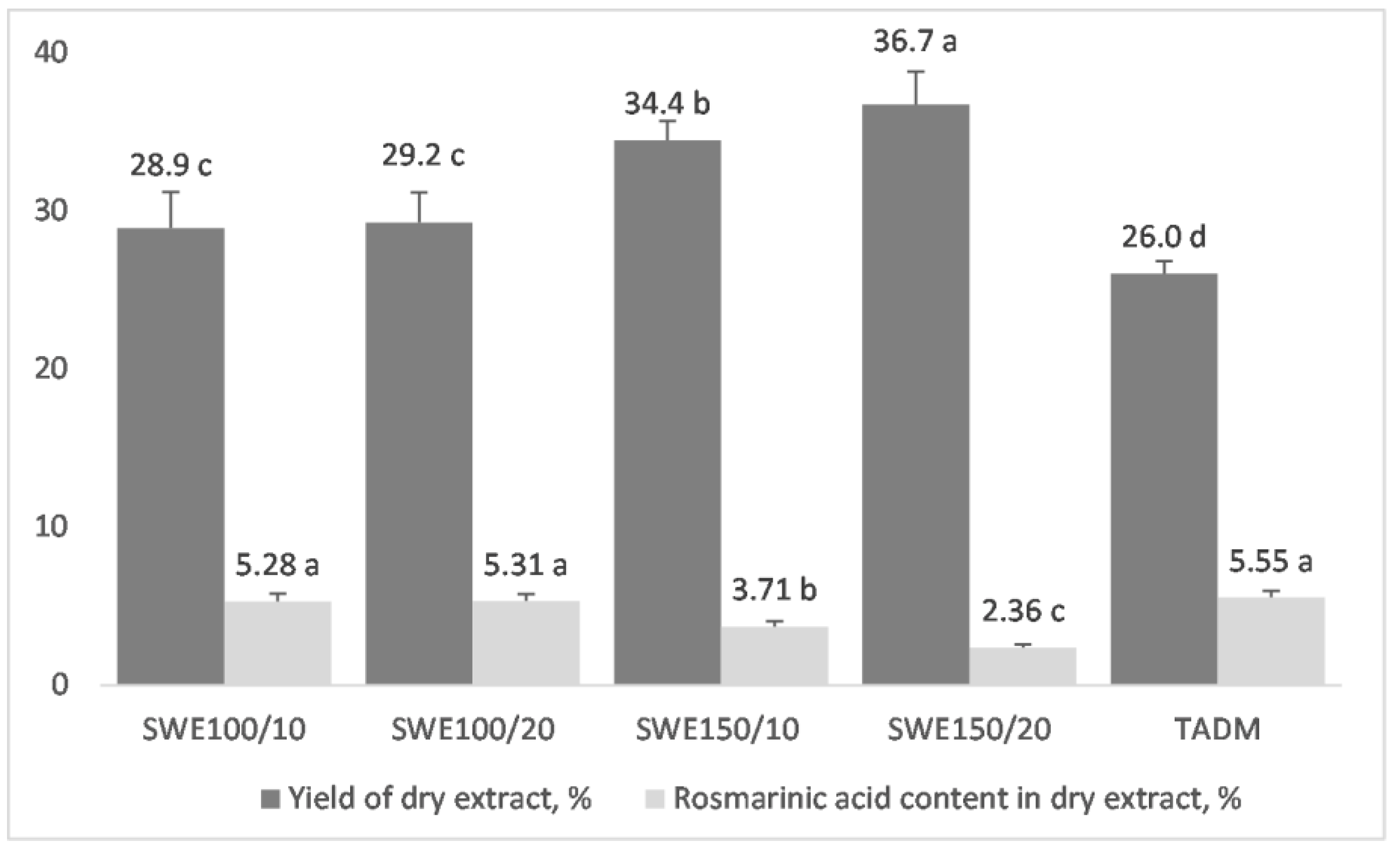
3.2. Recovery of Rosmarinic Acid from Lemon Balm Leaves by Subcritical Water and Conventional Hydro-Alcoholic Extraction
3.3. Characterization of Lemon Balm Dried Extracts, Obtained by Subcritical Water or Hydro-Alcoholic Extraction
3.4. Influence of Subcritical Water Extraction on Primary and Secondary Cell Wall Constituents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shekarchi, M.; Hajimehdipoor, H.; Saeidnia, S.; Gohari, A.R.; Hamedani, M.P. Comparative study of rosmarinic acid content in some plants of Labiatae family. Pharmacogn. Mag. 2012, 8, 37–41. [Google Scholar]
- Kintzios, S.; Nikolaou, A.; Skoula, M. Somatic embryogenesis and in vitro rosmarinic acid accumulation in Salvia officinalis and S. fruticosa leaf callus cultures. Plant Cell Rep. 1999, 18, 462–466. [Google Scholar] [CrossRef]
- Petersen, M. Rosmarinic acid: New aspects. Phytochem. Rev. 2013, 12, 207–227. [Google Scholar] [CrossRef]
- Lagouri, V.; Nisteropoulou, E. Antioxidant properties of O. onites, T. vulgaris and O. basilicum species grown in Greece and their total phenol and rosmarinic acid content. J. Food Lipids 2009, 16, 484–498. [Google Scholar] [CrossRef]
- Cao, H.; Cheng, W.X.; Li, C.; Pan, X.L.; Xie, X.G.; Li, T.H. DFT study on the antioxidant activity of rosmarinic acid. J. Mol. Struct. Theochem. 2005, 71, 177–183. [Google Scholar] [CrossRef]
- Amit, S.K.; Uddin, M.M.; Rahman, R.; Islam, S.M.R.; Khan, M.S. A review on mechanisms and commercial aspects of food preservation and processing. Agric. Food Secur. 2017, 6, 51. [Google Scholar] [CrossRef]
- Vasileva, I.; Denkova, R.; Chochkov, R.; Teneva, D.; Denkova, Z.; Dessev, T.; Denev, P.; Slavov, A. Effect of lavender (Lavandula angustifolia) and melissa (Melissa officinalis) waste on quality and shelf life of bread. Food Chem. 2018, 253, 13–21. [Google Scholar] [CrossRef]
- Farahmandfar, R.; Naeli, M.H.; Naderi, M.; Asnaashari, M. Stabilizing corn oil using the lemon balm (Melissa officinalis) antioxidants extracted by subcritical water. J. Food Sci. Technol. 2019, 56, 695–704. [Google Scholar] [CrossRef]
- Vara, S.; Karnena, M.K.; Dwarapureddi, B.K. Natural Preservatives for Nonalcoholic Beverages. In Preservatives and Preservation Approaches in Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2019; Volume 15, pp. 179–201. [Google Scholar]
- Caleja, C.; Barros, L.; Barreira, J.C.M.; Ciric, A.; Sokovic, M.; Calhelha, R.C.; Beatrizb, M.; Oliveira, P.P.; Ferreira, I.C.F.R. Suitability of lemon balm (Melissa officinalis L.) extract rich in rosmarinic acid as a potential enhancer of functional properties in cupcakes. Food Chem. 2018, 250, 67–74. [Google Scholar] [CrossRef]
- Klisurova, D.; Petrova, I.; Ognyanov, M.; Georgiev, Y.; Kratchanova, M.; Denev, P. Co-pigmentation of black chokeberry (Aronia melanocarpa) anthocyanins with phenolic co-pigments and herbal extracts. Food Chem. 2019, 279, 162–170. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Natural food additives: Quo vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Costa, P.; Sarmento, B.; Gonçalves, S.; Romano, A. Protective effects of Lavandula viridis L’her extracts and rosmarinic acid against H2O2-induced oxidative damage in A172 human astrocyte cell line. Ind. Crops Prod. 2013, 50, 361–365. [Google Scholar] [CrossRef]
- Wang, S.J.; Chen, Q.; Liu, M.Y.; Yu, H.Y.; Xu, J.Q.; Wu, J.Q.; Zhang, Y.; Wang, T. Regulation effects of rosemary (Rosmarinus officinalis Linn.) on hepatic lipid metabolism in OA induced NAFLD rats. Food Funct. 2019, 10, 7356–7365. [Google Scholar] [CrossRef]
- Georgiev, M.; Pastore, S.; Lulli, D.; Alipieva, K.; Kostyuk, V. Verbascum xanthophoeniceum-derived phenylethanoid glycosides are potent inhibitors of inflammatory chemokines in dormant and interferon-gamma-stimulated human keratinocytes. J. Ethnopharmacol. 2012, 144, 754–760. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Liu, G.; Hao, S.; Wang, C.; Wang, Y. Black rice anthocyanin-rich extract and rosmarinic acid, alone and in combination, protect against DSS-induced colitis in mice. Food Funct. 2018, 9, 2796–2808. [Google Scholar] [CrossRef]
- Topal, M.; Gulcin, I. Evaluation of the in vitro antioxidant, antidiabetic and anticholinergic properties of rosmarinic acid from rosemary (Rosmarinus officinalis L.). Biocatal. Agric. Biotechnol. 2022, 43, 102417. [Google Scholar] [CrossRef]
- Vasileva, L.V.; Savova, M.S.; Tews, D.; Wabitsch, M.; Georgiev, M.I. Rosmarinic acid attenuates obesity and obesity-related inflammation in human adipocytes. Food Chem. Toxicol. 2021, 149, 112002. [Google Scholar] [CrossRef]
- Elufioyea, T.O.; Habtemariamb, S. Hepatoprotective effects of rosmarinic acid: Insight into its mechanisms of action. Biomed. Pharmacother. 2019, 112, 108600. [Google Scholar] [CrossRef]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A Review of the anti-inflammatory effects of rosmarinic acid on inflammatory diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Stansbury, J. Rosmarinic acid as a novel agent in the treatment of allergies and asthma. J. Restor. Med. 2014, 3, 121–126. [Google Scholar] [CrossRef]
- Noguchi-Shinohara, M.; Ono, K.; Hamaguchi, T.; Iwasa, K.; Nagai, T.; Kobayashi, S.; Nakamura, H.; Yamada, M. Pharmacokinetics, safety and tolerability of Melissa officinalis extract which contained rosmarinic acid in healthy individuals: A randomized controlled trial. PLoS ONE 2015, 10, e0126422. [Google Scholar] [CrossRef] [PubMed]
- Verlag, G.T. The Scientific Foundation for Herbal Medicinal Products, 2nd ed.; ESCOP Monographs; ESCOP: Stuttgart, Germany; Thieme: New York, NY, USA, 2003; Volume 1. [Google Scholar]
- Hänsel, R.; Keller, K.; Rimpler, H.; Schneider, G. (Eds.) Hagers Handbuch; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Zhao, J.; Xu, L.; Jin, D.; Xin, Y.; Tian, L.; Wang, T.; Zhao, D.; Wang, Z.; Wang, J. Rosmarinic acid and related dietary supplements: Potential applications in the prevention and treatment of cancer. Biomolecules 2022, 12, 1410. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.O.; Hong, J.T.; Oh, K.W. Rosmarinic acid potentiates pentobarbital-induced sleep behaviors and non-rapid eye movement (NREM) sleep through the activation of GABAA-ergic systems. Biomol. Ther. 2017, 25, 105–111. [Google Scholar] [CrossRef] [PubMed]
- European Medicine Agency, Committee on Herbal Medicinal Products (HMPC). Community Herbal Monograph on Melissa officinalis L., Folium, 196745/2012. 2013. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/final-community-herbal-monograph-melissa-officinalis-l-folium_en.pdf (accessed on 5 February 2023).
- Melissa Leaf. In Ph Eur Monograph 1447, European Pharmacopoeia, 7th ed.; Council of Europe, European Directorate for the Quality of Medicines and Healthcare: Strasbourg, France, 2013.
- Melissa Leaf Dry Extract. In Ph Eur Monograph 2524, European Pharmacopoeia, 7th ed.; Council of Europe, European Directorate for the Quality of Medicines and Healthcare: Strasbourg, France, 2013.
- Marchev, A.S.; Vasileva, L.V.; Amirova, K.M.; Savova, M.S.; Koycheva, I.K.; Balcheva-Sivenova, Z.P.; Vasileva, S.M.; Georgiev, M.I. Rosmarinic acid—From bench to valuable applications in food industry. Trends Food Sci. Technol. 2021, 117, 182–193. [Google Scholar] [CrossRef]
- Iwaia, M.; Ohta, M.; Tsuchiyac, H.; Suzukia, T. Enhanced accumulation of caffeic acid, rosmarinic acid and luteolin-glucoside in red perilla cultivated under red diode laser and blue LED illumination followed by UV-A irradiation. J. Funct. Foods 2010, 2, 66–70. [Google Scholar] [CrossRef]
- Hadi, N.; Sefidkon, F.; Shojaeiyan, A.; Šiler, B.; Jafari, A.A. Phenolics’ composition in four endemic Nepeta species from Iran cultivated under experimental field conditions: The possibility of the exploitation of Nepeta germplasm. Ind. Crops Prod. 2017, 95, 475–484. [Google Scholar] [CrossRef]
- Nourozi, E.; Hosseini, B.; Maleki, R.; Mandoulakani, B. Pharmaceutical important phenolic compounds overproduction and gene expression analysis in Dracocephalum kotschyi hairy roots elicited by SiO2 nanoparticle. Ind. Crops Prod. 2019, 133, 435–446. [Google Scholar] [CrossRef]
- Chen, S.Y.; Wang, G.Y.; Lin, J.H.; Yen, G.C. Antioxidant and anti-inflammatory activities and bioactive compounds of the leaves of Trichodesma khasianum Clarke. Ind. Crops Prod. 2020, 151, e112447. [Google Scholar] [CrossRef]
- Mabrouki, H.; Duarte, C.M.M.; Akretche, D.E. Estimation of total phenolic contents and in vitro antioxidant and antimicrobial activities of various solvent extracts of Melissa officinalis L. Arab. J. Sci. Eng. 2018, 43, 3349–3357. [Google Scholar] [CrossRef]
- Khalaja, A.; Khanib, S. Spasmolytic effects of hydro-alcoholic extract of Melissa officinalis on isolated rat ileum. J. Rep. Pharm. Sci. 2018, 7, 260–269. [Google Scholar]
- Hong, E.; Kim, G. Comparison of extraction conditions for phenolic, flavonoid content and determination of rosmarinic acid from Perilla frutescens var. acuta. Int. J. Food Sci. 2010, 45, 1353–1359. [Google Scholar] [CrossRef]
- Ngoa, Y.L.; Laua, C.H.; Chua, L.S. Review on rosmarinic acid extraction, fractionation and its anti-diabetic potential. Food Chem. Toxicol. 2018, 121, 687–700. [Google Scholar] [CrossRef]
- Mirona, T.L.; Herrerob, M.E.; Nezb, I. Enrichment of antioxidant compounds from lemon balm (Melissa officinalis) by pressurized liquid extraction and enzyme-assisted extraction. J. Chromatogr. A 2013, 1288, 1–9. [Google Scholar] [CrossRef]
- Quintana, S.; Villanueva-Bermejo, D.; Reglero, G.; García-Risco, M.; Fornari, T. Supercritical antisolvent particle precipitation and fractionation of rosemary (Rosmarinus officinalis L.) extracts. J. CO2 Util. 2019, 34, 479–489. [Google Scholar] [CrossRef]
- Hirondart, M.; Rombaut, N.; Fabiano-Tixier, A.S.; Bily, A.; Chemat, F. Comparison between pressurized liquid extraction and conventional soxhlet extraction for rosemary antioxidants, yield, composition, and environmental footprint. Foods 2020, 9, 584. [Google Scholar] [CrossRef]
- Liu, T.; Sui, X.; Zhang, R.; Yang, L.; Zu, Y.; Zhang, L.; Zhang, Y.; Zhang, Z. Application of ionic liquids based microwave-assisted simultaneous extraction of carnosic acid, rosmarinic acid and essential oil from Rosmarinus officinalis. J. Chromatogr. A 2011, 1218, 8480–8489. [Google Scholar] [CrossRef]
- Saad, E.; El Gohary, N.; Abdel-Halim, M.; Handoussa, H.; El Nashar, R.; Mizaikoff, B. Molecularly imprinted polymers for selective extraction of rosmarinic acid from Rosmarinus officinalis L. Food Chem. 2021, 335, 127644. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical water extraction of natural products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef]
- Plaza, M.; Turner, C. Pressurized hot water extraction of bioactives. TrAC Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef]
- GB 5009.5-2016; Determination of Protein in Foods. National Food Safety Standard (NFSS) of the People’s Republic of China. China National Center for Food Safety Risk Assessment: Beijing, China, 2016.
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Nenov, N.; Govedarov, M.; Baldjieva, T. Pressurized hot water system for green extraction of valuable compounds from biomass and its performance. J. Balkan Ecol. 2022, 25, 81–91. [Google Scholar]
- Singleton, V.; Rossi, J. Colorimetry of total phenolic with phosphomolibdiphosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescence probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Denev, P.; Ciz, M.; Ambrozova, G.; Lojek, A.; Yanakieva, I.; Kratchanova, M. Solid-phase extraction of berries’ anthocyanins and evaluation of their antioxidative properties. Food Chem. 2010, 123, 1055–1061. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Deemer, E.K.; Prior, R.L.; Huang, D. Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the probe. J. Agric. Food Chem. 2002, 50, 2772–2777. [Google Scholar] [CrossRef]
- Updegraff, D.M. Semimicro determination of cellulose in biological materials. Anal. Biochem. 1969, 32, 420–424. [Google Scholar] [CrossRef]
- Dence, C.W. The determination of lignin. In Methods in Lignin Chemistry; Lin, S.Y., Dence, C.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 33–61. [Google Scholar]
- Fukushima, R.S.; Kerley, M.S.; Ramos, M.H.; Porter, J.H.; Kallenbach, R.L. Comparison of acetyl bromide lignin with acid detergent lignin and Klason lignin and correlation with in vitro forage degradability. Anim. Feed Sci. Technol. 2015, 201, 25–37. [Google Scholar] [CrossRef]
- Putnik, P.; Kovačević, D.B.; Penić, M.; Fegeš, M.; Dragović-Uzelac, V. Microwave-assisted extraction (MAE) of dalmatian sage leaves for the optimal yield of polyphenols: HPLC-DAD identification and quantification. Food Anal. Methods 2016, 9, 2385–2394. [Google Scholar] [CrossRef]
- Caleja, C.; Barros, L.; Prieto, M.A.; Barreiro, M.F.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Extraction of rosmarinic acid from Melissa officinalis L. by heat-, microwave- and ultrasound-assisted extraction techniques: A comparative study through response surface analysis. Sep. Purif. Technol. 2017, 186, 297–308. [Google Scholar] [CrossRef]
- Zhang, Y.; Smuts, J.P.; Dodbiba, E.; Rangarajan, R.; Lang, J.C.; Armstrong, D.W. Degradation study of carnosic acid, carnosol, rosmarinic acid, and rosemary extract (Rosmarinus officinalis L.) assessed using HPLC. J. Agric. Food Chem. 2012, 60, 9305–9314. [Google Scholar] [CrossRef]
- Jeong, G.H.; Cho, J.H.; Jo, C.; Lee, S.; Lee, S.S.; Bai, H.W.; Chung, B.Y.; Kim, T.H. Gamma irradiation-assisted degradation of rosmarinic acid and evaluation of structures and anti-adipogenic properties. Food Chem. 2018, 258, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, V.; Madureira, A.; Sarmento, B.; Gomes, A.; Pintado, M. Study of the interactions between rosmarinic acid and bovine milk whey protein α-lactalbumin, β-lactoglobulin and lactoferrin. Food Res. Int. 2015, 77, 450–459. [Google Scholar] [CrossRef]
- Li, Y.; Qi, H.; Fan, M.; Zhu, Z.; Zhan, S.; Li, L. Quantifying the efficiency of o-benzoquinones reaction with amino acids and related nucleophiles by cyclic voltammetry. Food Chem. 2020, 317, 126454. [Google Scholar] [CrossRef]
- Patora, J.; Klimek, B. Flavonoids from lemon balm (Melissa officinalis L., Lamiaceae). Acta Pol. Pharm. 2002, 59, 139–144. [Google Scholar]
- Barros, L.; Dueñas, M.; Dias, M.I.; Sousa, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic profiles of cultivated, in vitro cultured and commercial samples of Melissa officinalis L. infusions. Food Chem. 2013, 136, 1–8. [Google Scholar] [CrossRef]
- Georgiev, Y.N.; Paulsen, B.S.; Kiyohara, H.; Ciz, M.; Ognyanov, M.H.; Vasicek, O.; Rise, F.; Yamada, H.; Denev, P.N.; Lojek, A.; et al. The common lavender (Lavandula angustifolia Mill.) pectic polysaccharides modulate phagocytic leukocytes and intestinal Peyer’s patch cells. Carbohydr. Polym. 2017, 174, 948–959. [Google Scholar] [CrossRef]

| A. Moisture, % | 10.0 ± 0.1 |
|---|---|
| B. Crude protein (N × 6.25), % | 18.4 ± 0.5 |
| C. Total lipids, % | 2.2 ± 0.1 |
| D. Total carbohydrates, % | 22.0 ± 0.5 |
| Glucose (Glc) | 1.8 ± 0.1 |
| Fructose (Fru) | 1.9 ± 0.2 |
| Sucrose (Suc) | 0.02 ± 0.0 |
| Maltose (Mal) | 0.3 ± 0.0 |
| Total uronic acids | 5.4 ± 0.1 |
| Cellulose | 5.3 ± 0.6 |
| E. Ash, % | 18.1 ± 0.4 |
| F. Lignin (Klason), % | 18.7 ± 0.3 |
| G. Phenolics *, mg/100 g | |
| Total polyphenols | 11,491.1 ± 186.8 |
| Rosmarinic acid | 1599.9 ± 45 |
| Neochlorogenic acid | 96.6 ± 3.3 |
| Caffeic acid | 15.5 ± 1.1 |
| Luteolin | 23.8 ± 0.1 |
| H. Antioxidant activity * | |
| ORAC, µmol TE/g DW | 1814.6 ± 51.9 |
| HORAC, µmol GAE/g DW | 950.2 ± 15.9 |
| Extract | Total Polyphenols, mg/100 g DW | Caffeic Acid, mg/100 g DW | Neochlorogenic Acid, mg/100 g DW | Luteolin, mg/100 g DW | ORAC, µmol TE/g DW | HORAC, µmol GAE/g DW |
|---|---|---|---|---|---|---|
| SWE100/10 | 30,933 a ± 267 | 60.5 b ± 0.9 | 408.3 c ± 1.1 | 13.4 c ± 2.1 | 3796 c ± 77 | 1902 b ± 95 |
| SWE100/20 | 30,212 a ± 622 | 63.1 b ± 2.5 | 412.3 c ± 25.2 | 14.1 c ± 1.3 | 3784 c ± 45 | 1879 b ± 30 |
| SWE150/10 | 26,102 c ± 88 | 292.6 a ± 18.2 | 784.9 b ± 49.2 | 92.7 b ± 2.0 | 3952 b ± 50 | 1877 b ± 30 |
| SWE150/20 | 27,972 b ± 221 | 173.5 b± 11.1 | 953.1 a ± 81.3 | 112.1 a ± 9.1 | 4480 a ± 91 | 2207 a ± 86 |
| TADM | 27,426 b ± 163 | 47.0 c ± 3.9 | 177.7 d ± 1.2 | 10.4 c ± 1.2 | 4347 a ± 59 | 2080 ab ± 78 |
| Constituents | SWE100/10 | SWE100/20 | SWE150/10 | SWE150/20 |
|---|---|---|---|---|
| A. Yield of cell wall material, % | 77.5 b ± 1.0 | 80.5 a ± 0.5 | 79.1 a ± 1.1 | 77.2 b ± 0.7 |
| B. Yield of residue, % | 71 a ± 1 | 66 b ± 1 | 53 c ± 0 | 48 d ± 0 |
| C. Crude protein (N×6.25) | 21.3 ab ± 0.5 | 21.2 b ± 0.2 | 21.8 a ± 0.1 | 21.2 b ± 0.1 |
| Recovery, % | 84 | 77 | 64 | 56 |
| D. Total uronic acids | 8.9 a ± 0.2 | 7.3 b ± 0.1 | 3.3 c ± 0.0 | 3.2 c ± 0.1 |
| Recovery, % | 116 | 89 | 32 | 28 |
| E. Cellulose | 7.9 c ± 0.6 | 7.3 c ± 0.2 | 9.3 a ± 0.1 | 8.7 b ± 0.1 |
| Recovery, % | 105 | 91 | 93 | 79 |
| F. Lignin (Klason) | 22.8 d ± 0.3 | 28.3 c ± 0.2 | 35.3 b ± 0.3 | 40.5 a ± 0.1 |
| Recovery, % | 88 | 99 | 100 | 104 |
| Constituents | SWE100/10 | SWE100/20 | SWE150/10 | SWE150/20 |
|---|---|---|---|---|
| Polysaccharide, % * (g/100 mL extract) | 2.0 d ± 0.0 (0.10) | 2.6 c ± 0.1 (0.13) | 3.2 b ± 0.1 (0.16) | 3.6 a ± 0.1 (0.18) |
| Total carbohydrates of PS | 31 d ± 0.5 | 37 c ± 0.3 | 66 a ± 0.8 | 49 b ± 0.1 |
| Total uronic acids of PS | 26 b ± 0.2 | 29 a ± 0.2 | 21 c ± 0.0 | 13 d ± 0.0 |
| Recovery, % (100-%) | 9.8 (90.2) | 14.1 (85.9) | 12.6 (87.4) | 8.6 (91.4) |
| Glucose (Glc) | 1.5 a ± 0.1 | 1.5 a ± 0.1 | 1.7 a ± 0.2 | 0.9 b ± 0.1 |
| Fructose (Fru) | 1.9 a ± 0.0 | 1.9 a ± 0.2 | 1.8 a ± 0.1 | 1.0 b ± 0.1 |
| Sucrose (Suc) | 1.0 a ± 0.1 | 1.1 a ± 0.0 | 0.8 ± b 0.0 | 0.2 c ± 0.0 |
| Maltose (Mal) | 0.2 b ± 0.0 | 0.1 c ± 0.0 | 0.2 b ± 0.0 | 0.4 a ± 0.0 |
| Total | 4.7 | 4.6 | 4.5 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atanasova, A.; Petrova, A.; Teneva, D.; Ognyanov, M.; Georgiev, Y.; Nenov, N.; Denev, P. Subcritical Water Extraction of Rosmarinic Acid from Lemon Balm (Melissa officinalis L.) and Its Effect on Plant Cell Wall Constituents. Antioxidants 2023, 12, 888. https://doi.org/10.3390/antiox12040888
Atanasova A, Petrova A, Teneva D, Ognyanov M, Georgiev Y, Nenov N, Denev P. Subcritical Water Extraction of Rosmarinic Acid from Lemon Balm (Melissa officinalis L.) and Its Effect on Plant Cell Wall Constituents. Antioxidants. 2023; 12(4):888. https://doi.org/10.3390/antiox12040888
Chicago/Turabian StyleAtanasova, Ana, Ani Petrova, Desislava Teneva, Manol Ognyanov, Yordan Georgiev, Nenko Nenov, and Petko Denev. 2023. "Subcritical Water Extraction of Rosmarinic Acid from Lemon Balm (Melissa officinalis L.) and Its Effect on Plant Cell Wall Constituents" Antioxidants 12, no. 4: 888. https://doi.org/10.3390/antiox12040888
APA StyleAtanasova, A., Petrova, A., Teneva, D., Ognyanov, M., Georgiev, Y., Nenov, N., & Denev, P. (2023). Subcritical Water Extraction of Rosmarinic Acid from Lemon Balm (Melissa officinalis L.) and Its Effect on Plant Cell Wall Constituents. Antioxidants, 12(4), 888. https://doi.org/10.3390/antiox12040888







