Essential Oil Composition, Antioxidant Activity and Leaf Micromorphology of Five Tunisian Eucalyptus Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Macromorphological, Micromorphological, and Anatomical Investigation
2.3. Extraction of the EOs
2.4. GC-FID and GC/MS Analyses and Identification of the Essential Oil Components
2.5. Antioxidant Activity
2.5.1. DPPH Test
2.5.2. ABTS Test
2.6. Cell Culture
2.7. Cell Viability Assay
2.8. Intracellular ROS Measurement
2.9. Quantification of Nitrite in Cell Culture Supernatants
2.10. Quantitative Real-Time PCR
- Gclc: 5′GTTGGGGTTTGTCCTCTCCC-3′; 5′-GGGGTGACGAGGTGGAGTA-3′;
- Gclm: 5′-AGGAGCTTCGGGACTGTATCC-3′; 5′-GGGACATGGTGCATTCCAAAA-3′; Hmox-1: 5′-GCCGTGTAGATATGGTACAAGGA-3′; 5′-AAGCCGAGAATGCTGAG TTCA-3′.
2.11. Statistical Analysis
3. Results
3.1. Macromorphological, Micromorphological, and Anatomical Investigation
3.2. Chemical Composition of Essential Oils
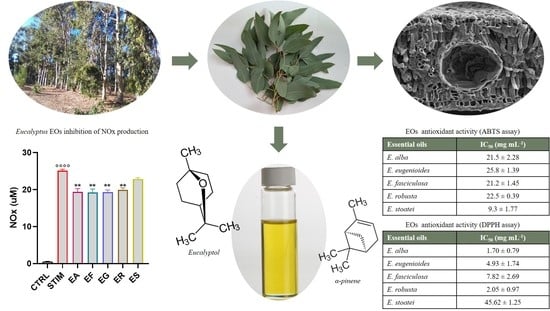
3.3. Antioxidant Activity by the DPPH Assay
3.4. Antioxidant Activity by the ABTS Assay
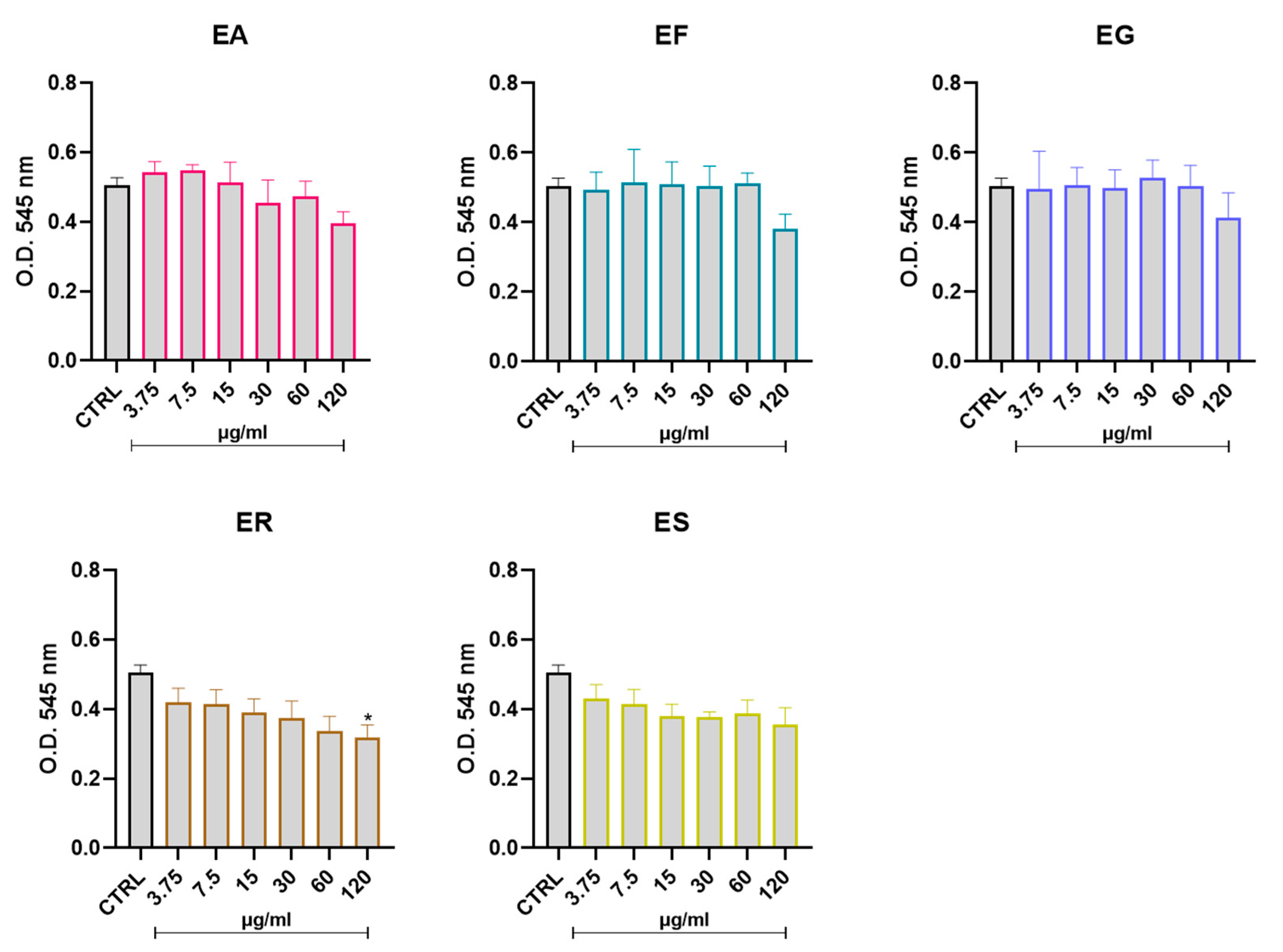
3.5. Effect of the EOs on Cell Vitality
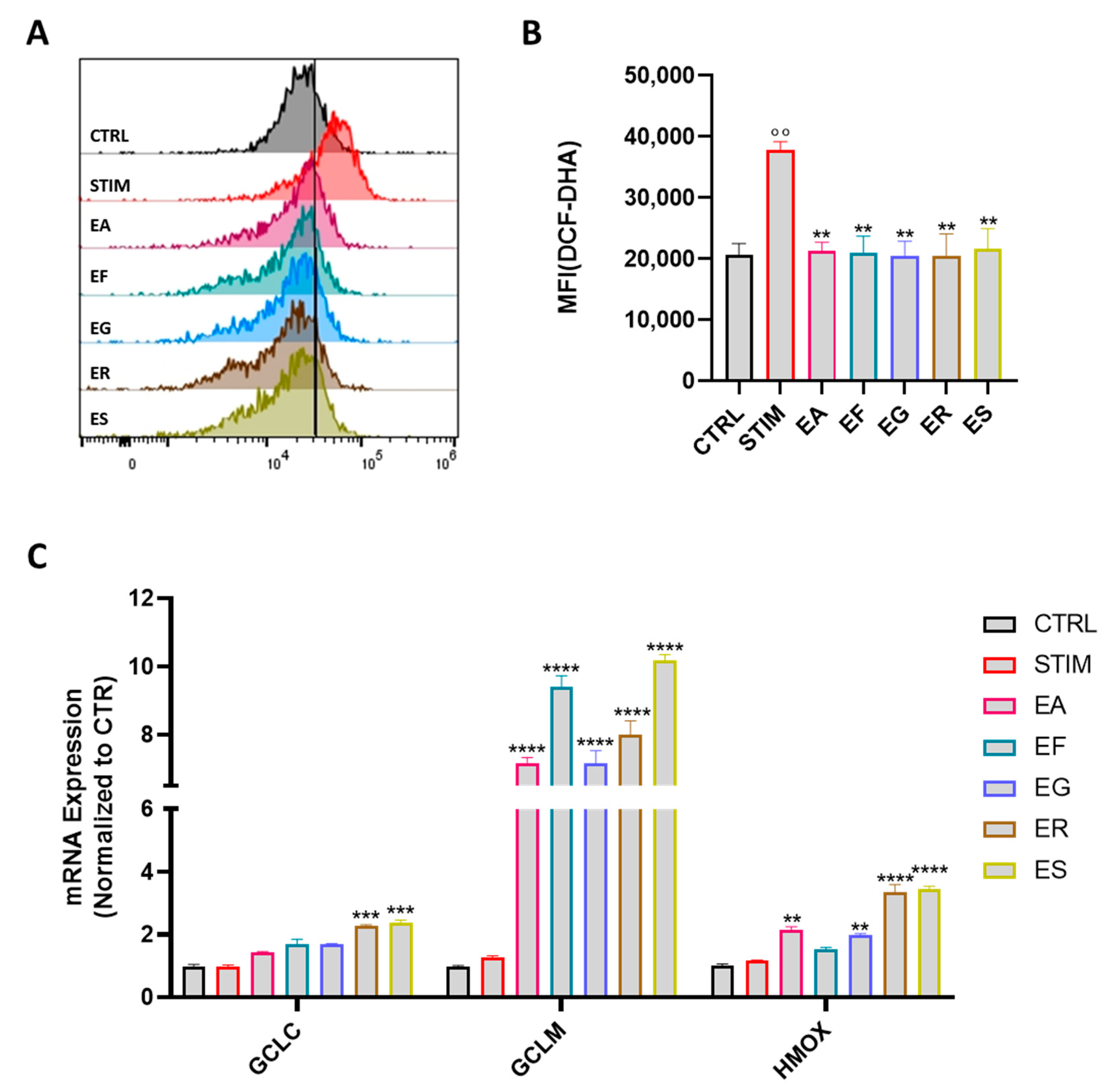
3.6. Activity on ROS Production and Modulation of the Expression of Antioxidant Enzymes
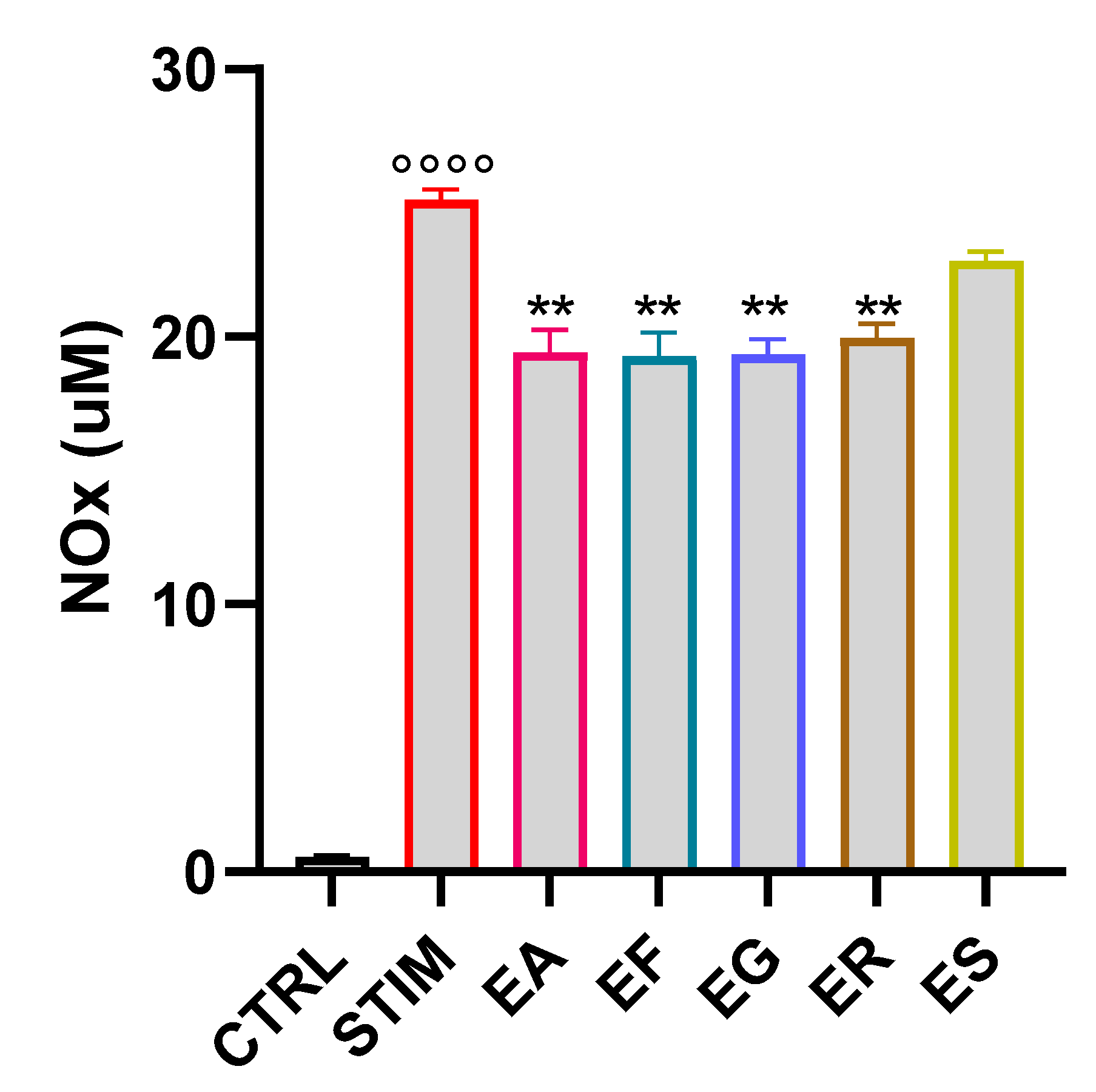
3.7. Activity of the EOs on NOx Production in M1 Murine Macrophages
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salehi, B.; Sharifi-Rad, J.; Quispe, C.; Llaique, H.; Villalobos, M.; Smeriglio, A.; Trombetta, D.; Ezzat, S.M.; Salem, M.A.; Zayed, A.; et al. Insights into Eucalyptus genus chemical constituents, biological activities and health-promoting effects. Trends Food Sci. Technol. 2019, 91, 609–624. [Google Scholar] [CrossRef]
- Nordqvist, J. Eucalyptus: What Are the Health Benefits? Medical News Today: MediLexicon,intl. 2017. Available online: https://www.medicalnewstoday.com/articles/266580.php (accessed on 15 March 2023).
- Lewington, A. Plants for People; Random House: New York, NY, USA; Eden Project Books: London, UK, 2003; ISBN 1903919088, 9781903919088. [Google Scholar]
- Khouja, M.L.; Khaldi, A.; Rejeb, M.N. Results of the Eucalyptus Introduction Trials in Tunisia. In Proceedings of the International Conference of Eucalyptus in the Mediterranean Basin: Perspectives and New Utilization, Florence, Italy, 15–19 October 2000; pp. 163–168. [Google Scholar]
- Zhang, C.; Fu, S. Allelopathic effects of Eucalyptus and the establishment of mixed stands of Eucalyptus and native species. For. Ecol. Manag. 2009, 258, 1391–1396. [Google Scholar] [CrossRef]
- Gardner, R.A.W. Investigating the environmental adaptability of promising subtropical and cold-tolerant eucalypt species in the warm temperate climate zone of KwaZulu-Natal, South Africa. Sout. Hemisph. For. J. 2007, 69, 27–38. [Google Scholar] [CrossRef]
- Singh, V.; Toky, O.P. Biomass and net primary productivity in Leucaena, Acacia and Eucalyptus, short rotation, high density (‘energy’) plantations in arid India. J. Arid Environ. 1995, 31, 301–309. [Google Scholar] [CrossRef]
- Cossalter, C.; Pye-Smith, C. Fast-Wood Forestry: Myths and Realities; Center for International Forestry Research: Bogor, Indonesia, 2003. [Google Scholar]
- García-Tenesaca, M.; Navarrete, E.S.; Iturralde, G.A.; Villacrés Granda, I.M.; Tejera, E.; Beltrán-Ayala, P.; Giampieri, F.; Battino, M.; Alvarez-Suarez, J.M. Influence of Botanical Origin and Chemical Composition on the Protective Effect against Oxidative Damage and the Capacity to Reduce In Vitro Bacterial Biofilms of Monofloral Honeys from the Andean Region of Ecuador. Int. J. Mol. Sci. 2018, 19, 45. [Google Scholar] [CrossRef] [PubMed]
- Bobis, O.; Moise, A.R.; Ballesteros, I.; Reyes, E.S.; Durán, S.S.; Sánchez-Sánchez, J.; Cruz-Quintana, S.; Giampieri, F.; Battino, M.; Alvarez-Suarez, J.M. Eucalyptus honey: Quality parameters, chemical composition and health-promoting properties. Food Chem. 2020, 325, 126870. [Google Scholar] [CrossRef]
- Chalbi, H.N. Peuplement sylvo-pastoral de l’espace saharien de la Tunisie méridionale. Perspectives. In Le Projet Majeur Africain de la Grande Muraille Verte: Concepts et Mise en Œuvre; Dia, A., Duponnois, R., Eds.; IRD Editions: Montpellier, France, 2012. [Google Scholar]
- Bachir, R.G.; Benali, M. Antibacterial activity of the essential oils from the leaves of Eucalyptus globulus against Escherichia coli and Staphylococcus aureus. Asian Pac. J. Trop. Biomed. 2012, 2, 739–742. [Google Scholar] [CrossRef]
- Gakuubi, M.M.; Maina, A.W.; Wagacha, J.M. Antifungal activity of essential oil of Eucalyptus camaldulensis Dehnh. against selected Fusarium spp. Int. J. Microbiol. 2017, 2017, 8761610. [Google Scholar] [CrossRef]
- Ben Marzoug, H.N.; Romdhane, M.; Lebrihi, A.; Mathieu, F.; Couderc, F.; Abderraba, M.; Larbi Khouja, M.; Bouajila, J. Eucalyptus oleosa essential oils: Chemical composition and antimicrobial and antioxidant activities of the oils from different plant parts (stems, leaves, flowers and fruits). Molecules 2011, 16, 1695–1709. [Google Scholar] [CrossRef]
- Valeriano, C.; Oliveira, T.L.C.; Carvalho, S.M.; Cardoso, M.G.; Alves, E.; Piccoli, R.H. The sanitizing action of essential oil-based solutions against Salmonella enterica serotype Enteritidis S64 biofilm formation on AISI 304 stainless steel. Food Control 2012, 25, 673–677. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Estevinho, B.N.; Santos, L. Application of microencapsulated essential oils in cosmetic and personal healthcare products—A review. Int. J. Cosmet. Sci. 2016, 38, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Khedhri, S.; Polito, F.; Caputo, L.; Manna, F.; Khammassi, M.; Hamrouni, L.; Amri, I.; Nazzaro, F.; De Feo, V.; Fratianni, F. Chemical Composition, Phytotoxic and Antibiofilm Activity of Seven Eucalyptus Species from Tunisia. Molecules 2022, 27, 8227. [Google Scholar] [CrossRef] [PubMed]
- Danna, C.; Cornara, L.; Smeriglio, A.; Trombetta, D.; Amato, G.; Aicardi, P.; De Martino, L.; De Feo, V.; Caputo, L. Eucalyptus gunnii and Eucalyptus pulverulenta “Baby Blue” Essential Oils as Potential Natural Herbicides. Molecules 2021, 26, 6749. [Google Scholar] [CrossRef] [PubMed]
- Malaspina, P.; Papaianni, M.; Ranesi, M.; Polito, F.; Danna, C.; Aicardi, P.; Cornara, L.; Woo, S.L.; De Feo, V. Eucalyptus cinerea and E. nicholii by-Products as Source of Bioactive Compounds for Agricultural Applications. Plants 2022, 11, 2777. [Google Scholar] [CrossRef]
- Hobbs, T.J.; Bennell, M.; Huxtable, D.; Bartle, J.; Neumann, C.; George, N.; O’Sullivan, W.; McKenna, D. Potential Agroforestry Species and Regional Industries for Lower Rainfall Southern Australia: Flora Search 2. Report to the Joint Venture Agroforestry Program (JVAP) and the Future Farm Industries CRC; RIRDC: Canberra, Australia, 2008. [Google Scholar]
- El-Hadary, A.E.; Taha, M. Pomegranate peel methanolic-extract improves the shelf-life of edible-oils under accelerated oxidation conditions. Food Sci. Nutr. 2020, 8, 1798–1811. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant Activity of Essential Oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Silva, J.; Abebe, W.; Sousa, S.M.; Duarte, V.G.; Machado, M.I.L.; Matos, F.J.A. Analgesic and anti-inflammatory effects of essential oils of Eucalyptus. J. Ethnopharmacol. 2003, 89, 277–283. [Google Scholar] [CrossRef]
- Zhao, Q.; Bowles, E.J.; Zhang, H.Y. Antioxidant Activities of Eleven Australian Essential Oils. Nat. Prod. Commun. 2008, 3, 837–842. [Google Scholar] [CrossRef]
- Choi, H.S.; Song, H.S.; Ukeda, H.; Sawamura, M. Radical scavenging activities of citrus essential oils and their components: Detection using 1,1-diphenyl-2-picrylhydrazyl. J. Agric. Food Chem. 2000, 48, 4156–4161. [Google Scholar] [CrossRef]
- Kim, H.J.; Chen, F.; Wu, C.Q.; Wang, X.; Chung, H.Y.; Jin, Z.Y. Evaluation of antioxidant activity of Australian tea tree (Melaleuca alternifolia) oil and its components. J. Agric. Food Chem. 2004, 52, 2849–2854. [Google Scholar] [CrossRef]
- Siramon, P.; Ohtani, Y. Antioxidative and antiradical activities of Eucalyptus camaldulensis leaf oils from Thailand. J. Wood Sci. 2007, 53, 498–504. [Google Scholar] [CrossRef]
- Rejeb, M.N.; Khaldi, A.; Khouja, M.L.; Garchi, S.; Ben Mansoura, A.; Nouri, M. Guide pour le choix des espèces de reboisement: Espèces forestières et pastorales. In INGREF; 1996; 137p. [Google Scholar]
- FAO. 1954. Les Eucalyptus Dans les Reboisements; FAO: Rome, Italy, 1982; ISBN 92-5-200570-6. [Google Scholar]
- Mueller, F. Descriptions of fifty new Australian plants, chiefly from the colony of Victoria. In Transactions and Proceedings of the Victorian Institute for the Advancement of Science; Victorian Institute for the Advancement of Science: Melbourne, Australia, 1855; pp. 28–48. [Google Scholar]
- Chieco, C.; Rotondi, A.; Morrone, L.; Rapparini, F.; Baraldi, R. An ethanol-based fixation method for anatomical and micro-morphological characterization of leaves of various tree species. Biotech. Histochem. 2013, 88, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.H. Fuchsin staining with NaOH clearing for lignified elements ofwhole plants or plants organs. Stain Technol. 1963, 38, 141–144. [Google Scholar] [CrossRef]
- Migacz, I.P.; Raeski, P.A.; Paes de Almeida, V.; Raman, V.; Nisgoski, S.; Bolzón de Muniz, G.I.; Farago, P.V.; Khan, I.A.; Budel, J.M. Comparative leaf morpho-anatomy of six species of Eucalyptus cultivated in Brazil. Rev. Bras. Farmacogn. 2018, 28, 273–281. [Google Scholar] [CrossRef]
- Pathan, A.K.; Bond, J.; Gaskin, R.E. Sample preparation for scanning electron microscopy of plant surfaces. Horses for courses. Micron 2008, 39, 1049–1061. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 5th ed.; Council of Europe: Strasbourg, France, 2014; Volume I, pp. 217–218. [Google Scholar]
- Jennings, W.; Shibamoto, T. Qualitative Analyisis of Flavour and Fragrance Volatiles by Glass Capillary Gas Chromatography; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicone and Carbowas 20M phases. J. Chromatogr. 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 1998. [Google Scholar]
- Goodner, K.I. Practical retention index models of OV-101, DB-1, DB-5, and DB-Wax for flavour and fragrance compounds. LWT Food Sci. Technol. 2008, 41, 951–958. [Google Scholar] [CrossRef]
- McLafferty, F.W. The Wiley Registry of Mass Spectral Data, with Nist Spectral Data CD Rom, 7th ed.; John Wiley & Sons: New York, NY, USA, 1998. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Tartaglione, L.; Gambuti, A.; De Cicco, P.; Ercolano, G.; Formisano, C.; Ianaro, O.; Taglialatela-Scafati, O.; Moio, L.; Forino, M. NMR-based phytochemical analysis of Vitis vinifera cv Falanghina leaves. Characterization of a previously undescribed biflavonoid with antiproliferative activity. Fitoterapia 2018, 125, 13–17. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, P.; Busà, E.; Ercolano, G.; Formisano, C.; Allegra, M.; Taglialatela-Scafati, O.; Ianaro, A. Inhibitory effects of cynaropicrin on human melanoma progression by targeting MAPK, NF-κB, and Nrf-2 signaling pathways in vitro. Phytother. Res. 2021, 35, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Hunam Macrophage polarization in inflammatiom during infectious diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Ghosheh, Y.; Bala Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS–) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef] [PubMed]
- Pauzer, M.S.; Borsato, T.D.O.; de Almeida, V.P.; Raman, V.; Justus, B.; Pereira, C.B.; Flores, T.B.; Maia, B.H.L.N.S.; Meneghetti, E.K.; Kanunfre, C.C.; et al. Eucalyptus cinerea: Microscopic Profile, Chemical Composition of Essential Oil and its Antioxidant, Microbiological and Cytotoxic Activities. Braz. Arch. Biol. Technol. 2021, 64, e21200772. [Google Scholar] [CrossRef]
- Santos, L.D.T.; Thadeo, M.; Iarema, L.; Meira, R.M.S.A.; Ferreira, F.A. Foliar anatomy and histochemistry in seven species of Eucalyptus. Rev. Arvore 2008, 32, 769–779. [Google Scholar] [CrossRef]
- Saulle, C.C.; Raman, V.; Oliveira, A.V.G.; Lameiro de Noronha Sales Maia, B.H.; Meneghetti, E.K.; Flores, T.B.; Farago, P.V.; Khan, I.A.; Budel, J.M. Anatomy and volatile oil chemistry of Eucalyptus saligna cultivated in South Brazil. Rev. Bras. Farmacogn. 2018, 28, 125–134. [Google Scholar] [CrossRef]
- Retamales, H.A.; Scharaschkin, T. Comparative leaf anatomy and micromorphology of the Chilean Myrtaceae: Taxonomic and ecological implications. Flora 2015, 217, 138–154. [Google Scholar] [CrossRef]
- Elaissi, A.; Moumni, S.; Roeleveld, K.; Larbi Khouja, M. Chemical characterization of five Tunisian Eucalyptus essential oils species. Chem. Biodivers. 2020, 17, e1900378. [Google Scholar] [CrossRef]
- Mondello, L.; Verzera, A.; Bonaccorsi, I.; Chowdhury, J.U.; Yusef, M.; Begum, J. Studies in the Essential Oil Bearing Plants of Bangladesh. Part V. Composition of the Leaf Oils of Eucalyptus citriodora Hook and E. alba Reinw. ex Blume. J. Essent. Oil Res. 1198, 10, 185–188. [Google Scholar] [CrossRef]
- Cimanga, K.; Kambu, K.; Tona, L.; Apers, S.; De Bruyne, T.; Hermans, N.; Totté, J.; Pieters, L.; Vlietinck, A.J. Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the Democratic Republic of Congo. J. Ethnopharmacol. 2002, 79, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Tine, Y.; Diallo, A.; Diop, A.; Costa, J.; Boye, C.S.B.; Wélé, A.; Paolini, J. The Essential oil of Eucalyptus alba L. growing on the Salt Zone of Fatick (Senegal) as a Source of 1, 8− Cineole and Their Antibacterial Activity. J. Drug Deliv. Ther. 2020, 10, 140–143. [Google Scholar] [CrossRef]
- Barka Ndiaye, E.H.; Talla Gueye, M.; Ndiaye, I.; Diop, S.M.; Diop, M.B.; Fauconnier, M.L.; Lognay, G. Chemical composition of essential oils and hydrosols of three Eucalyptus species from Senegal: Eucalyptus alba Renv, Eucalyptus camaldulensis Dehnh and Eucalyptus tereticornis Hook. Am. J. Essent. Oils Nat. Prod. 2017, 5, 1–7. [Google Scholar]
- Samaté, A.D.; Nacro, M.; Menut, C.; Lamaty, G.; Bessiere, J.M. Aromatic plants of tropical West Africa. VII. Chemical composition of the essential oils of two eucalyptus species (Myrtaceae) from Burkina Faso: Eucalyptus alba Muell. and Eucalyptus camaldulensis Dehnardt. J. Essent. Oil Res. 1998, 10, 321–324. [Google Scholar] [CrossRef]
- Bignell, C.M.; Dunlop, P.J.; Brophy, J.J. Volatile leaf oils of some Queensland and northern Australian species of the genus Eucalyptus (series II). Part II. Subgenera (a) Blakella, (b) Corymbia,(c) Unnamed,(d) Idiogenes,(e) Monocalyptus and (f) Symphyomyrtus. Flavour Fragr. J. 1997, 12, 277–284. [Google Scholar] [CrossRef]
- Elaissi, A.; Medini, H.; Simmonds, M.; Lynen, F.; Farhat, F.; Chemli, R.; Harzallah-Skhiri, F.; Khouja, M.L. Variation in volatile leaf oils of seven Eucalyptus species harvested from Zerniza arboreta (Tunisia). Chem. Biodivers. 2011, 8, 362–372. [Google Scholar] [CrossRef]
- Elaissi, A.; Salah, K.H.; Mabrouk, S.; Larbi, K.M.; Chemli, R.; Harzallah-Skhiri, F. Antibacterial activity and chemical composition of 20 Eucalyptus species’ essential oils. Food Chem. 2011, 129, 1427–1434. [Google Scholar] [CrossRef]
- Bignell, C.M.; Dunlop, P.J.; Brophy, J.J.; Jackson, J.F. Volatile leaf oils of some South-western and Southern Australian species of the genus Eucalyptus part VI—Subgenus Symphyomyrtus, section Adnataria. Flavour Fragr. J. 1995, 10, 359–364. [Google Scholar] [CrossRef]
- Sartorelli, P.; Marquioreto, A.D.; Amaral-Baroli, A.; Lima, M.E.L.; Moreno, P.R.H. Chemical composition and antimicrobial activity of the essential oils from two species of Eucalyptus. Phytother. Res. 2007, 21, 231–233. [Google Scholar] [CrossRef]
- Bachheti, R.K.; Joshi, A.; Singh, A. Oil Content variation and antimicrobial activity of Eucalyptus leaves oils of three different Species of Dehradun, Uttarakhand, India. Int. J. ChemTech Res. 2011, 3, 625–628. [Google Scholar]
- Liu, Z.L.; Yu, M.; Li, X.M.; Wan, T.; Chu, S.S. Repellent activity of eight essential oils of Chinese medicinal herbs to Blattella germanica L. Rec. Nat. Prod. 2011, 5, 176–183. [Google Scholar]
- Liu, X.C.; Liu, Q.Z.; Shi, W.P.; Liu, Z.L. Evaluation of insecticidal activity of the essential oil of Eucalyptus robusta Smith leaves and its constituent compound against overwintering Cacopsylla chinensis (Yang et Li) (Hemiptera: Psyllidae). J. Entomol. Zool. Stud. 2014, 2, 27–31. [Google Scholar]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Lucia, A.; Juan, L.W.; Zerba, E.N.; Harrand, L.; Marcó, M.; Masuh, H.M. Validation of models to estimate the fumigant and larvicidal activity of Eucalyptus essential oils against Aedes aegypti (Diptera: Culicidae). Parasitol. Res. 2012, 110, 1675–1686. [Google Scholar] [CrossRef]
- Ameur, E.; Sarra, M.; Yosra, D.; Mariem, K.; Nabil, A.; Lynen, F.; Larbi, K.M. Chemical composition of essential oils of eight Tunisian Eucalyptus species and their antibacterial activity against strains responsible for otitis. BMC Complement. Med. Ther. 2021, 21, 209. [Google Scholar]
- Wang, H.; Gong, J.; Tang, J.; Zhou, H.; Mao, X. Effects of compound of cinnamon essential oil and Eucalyptus robusta leaves essential oil on quality maintenance of “Xia hei” grape. J. Food Saf. Qual. 2016, 7, 3703–3709. [Google Scholar]
- Benayache, S.; Benayache, F.; Benyahia, S.; Chalchat, J.C.; Garry, R.P. Leaf oils of some Eucalyptus species growing in Algeria. J. Essent. Oil Res. 2001, 13, 210–213. [Google Scholar] [CrossRef]
- Traoré, N.; Sidibé, L.; Figuérédo, G.; Chalchat, J.C. Chemical composition of five essential oils of Eucalyptus species from Mali: E. houseana FV Fitzg. ex-Maiden, E. citriodora Hook., E. raveretiana F. Muell., E. robusta Smith and E. urophylla ST Blake. J. Essent. Oil Res. 2010, 22, 510–513. [Google Scholar] [CrossRef]
- Lassak, E.V.; Brophy, J.J. Steam volatile leaf oils of some Western Australian species of the family Myrtaceae. Flavour Fragr. J. 2004, 19, 12–16. [Google Scholar] [CrossRef]
- Bignell, C.M.; Dunlop, P.J.; Brophy, J.J.; Jackson, J.F. Volatile leaf oils of some south-western and southern Australian species of the genus Eucalyptus. Part I: Subgenus Symphyomyrtus, section Dumaria, series Incrassatae. Flavour Fragr. J. 1994, 9, 113–117. [Google Scholar] [CrossRef]
- Brophy, J.J.; Boland, D.J. Leaf essential oil of two chemotypes of Eucalyptus cloetiana F. Muell. J. Essent. Oil Res. 1990, 2, 87–90. [Google Scholar] [CrossRef]
- Mishra, A.K.; Sahu, N.; Mishra, A.; Ghosh, A.K.; Jha, S.; Chattopadhyay, P. Phytochemical screening and antioxidant activity of essential oil of Eucalyptus leaf. Pharmacogn. J. 2010, 2, 25–28. [Google Scholar] [CrossRef]
- Adnan, M. Bioactive potential of essential oil extracted from the leaves of Eucalyptus globulus (Myrtaceae). J. Pharmacogn. Phytochem. 2019, 8, 213–216. [Google Scholar]
- Cahaya, C.; Taufik, M.; Alfian, Z.; Lenny, S.; Hidayati, R. Identification and Analysis of Potential Antioxidants from Leaves of Eucalyptus robusta. In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), Medan, Indonesia, 18–19 July 2019; Volume 1, pp. 245–248. [Google Scholar]
- Singh, H.P.; Mittal, S.; Kaur, S.; Batish, D.R.; Kohli, R.K. Characterization and antioxidant activity of essential oils from fresh and decaying leaves of Eucalyptus tereticornis. J. Agric. Food Chem. 2009, 57, 6962–6966. [Google Scholar] [CrossRef]
- Wang, W.; Wu, N.; Zu, Y.G.; Fu, Y.J. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008, 108, 1019–1022. [Google Scholar] [CrossRef]
- Wei, A.; Shibamoto, T. Antioxidant activities and volatile constituents of various essential oils. J. Agric. Food Chem. 2007, 55, 1737–1742. [Google Scholar] [CrossRef]
- Wojtunik, K.A.; Ciesla, L.M.; Waksmundzka-Hajnos, M. Model studies on the antioxidant activity of common terpenoid constituents of essential oils by means of the 2, 2-diphenyl-1-picrylhydrazyl method. J. Agric. Food Chem. 2014, 62, 9088–9094. [Google Scholar] [CrossRef] [PubMed]
- Noacco, N.; Rodenak-Kladniew, B.; de Bravo, M.G.; Castro, G.R.; Islan, G.A. Simple colorimetric method to determine the in vitro antioxidant activity of different monoterpenes. Anal. Biochem. 2018, 555, 59–66. [Google Scholar] [CrossRef]
- Juergens, L.J.; Tuleta, I.; Stoeber, M.; Racké, K.; Juergens, U.R. Regulation of monocyte redox balance by 1,8-cineole (eucalyptol) controls oxidative stress and pro-inflammatory responses in vitro: A new option to increase the antioxidant effects of combined respiratory therapy with budesonide and formoterol? Synergy 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Fazelan, Z.; Hoseini, S.M.; Yousefi, M.; Khalili, M.; Hoseinifar, S.H.; Van Doan, H. Effects of dietary eucalyptol administration on antioxidant and inflammatory genes in common carp (Cyprinus carpio) exposed to ambient copper. Aquaculture 2020, 520, 734988. [Google Scholar] [CrossRef]
- Tiwari, M.; Kakkar, P. Plant derived antioxidants–geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicol. In Vitro 2009, 23, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Su, J.; Li, L.; Li, B.; Li, W. A new source of natural D-borneol and its characteristic. J. Med. Plants Res. 2011, 5, 3440–3447. [Google Scholar]
- Quassinti, L.; Lupidi, G.; Maggi, F.; Sagratini, G.; Papa, F.; Vittori, S.; Bianco, A.; Bramucci, M. Antioxidant and antiproliferative activity of Hypericum hircinum L. subsp. majus (Aiton) N. Robson essential oil. Nat. Prod. Res. 2013, 27, 862–868. [Google Scholar]
- Zardi-Bergaoui, A.; Jelassi, A.; Daami-Remadi, M.; Harzallah-Skhiri, F.; Flamini, G.; Ascrizzi, R.; Ben Jannet, H. Chemical composition and bioactivities of essential oils from Pulicaria vulgaris subsp. dentate (Sm.) Batt. growing in Tunisia. J. Essent. Oil Res. 2020, 32, 111–120. [Google Scholar] [CrossRef]
- de Christo Scherer, M.M.; Marques, F.M.; Figueira, M.M.; Peisino, M.C.O.; Schmitt, E.F.P.; Kondratyuk, T.P.; Endringer, D.C.; Scherer, R.; Fronza, M. Wound healing activity of terpinolene and α-phellandrene by attenuating inflammation and oxidative stress in vitro. J. Tissue Viability 2019, 28, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Abd-ElGawad, A.M.; El-Amier, Y.A.; Bonanomi, G.; Gendy, A.E.N.G.E.; Elgorban, A.M.; Alamery, S.F.; Elshamy, A.I. Chemical composition of Kickxia aegyptiaca essential oil and its potential antioxidant and antimicrobial activities. Plants 2022, 11, 594. [Google Scholar] [CrossRef]
- Kumar, P.; Sati, S.C.; Khulbe, K.; Pant, P.; Tripathi, A.N.; Sarvendra, K. Phytochemical constituents, antimicrobial and antioxidant activities of Kumaun Himalayan Hoop Pine bark extract. Nat. Prod. Res. 2022, 36, 1095–1099. [Google Scholar] [CrossRef]
- do Nascimento, K.F.; Moreira, F.M.F.; Santos, J.A.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; do Carmo Vieira, M.; Tasca Gòis Ruiz, A.L.; Foglio, M.A.; de Carvalho, J.E.; et al. Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef]
- Li, Y.; Tan, B.; Cen, Z.; Fu, Y.; Zhu, X.; He, H.; Kong, D.; Wu, H. The variation in essential oils composition, phenolic acids and flavonoids is correlated with changes in antioxidant activity during Cinnamomum loureirii bark growth. Arab. J. Chem. 2021, 14, 103249. [Google Scholar] [CrossRef]
- Ahluwalia, V.; Sisodia, R.; Walia, S.; Sati, O.P.; Kumar, J.; Kundu, A. Chemical analysis of essential oils of Eupatorium adenophorum and their antimicrobial, antioxidant and phytotoxic properties. J. Pest Sci. 2014, 87, 341–349. [Google Scholar] [CrossRef]
- Noumi, V.D.; Nguimbou, R.M.; Tsague, M.V.; Deli, M.; Rup-Jacques, S.; Amadou, D.; Baudelaire, E.N.; Sokeng, S.; Njintang, N.Y. Phytochemical Profile and In Vitro Antioxidant Properties of Essential Oils from Powder Fractions of Eucalyptus camaldulensis Leaves. Am. J. Plant Sci. 2021, 12, 108053. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef] [PubMed]
- Ben-Marzoug, H.N.; Bouajila, J.; Ennajar, M.; Lebrihi, A.; Romdhane, M. Eucalyptus (gracilis, oleosa, salubris, and salmonophloia) essential oils: Their chemical composition and antioxidant and antimicrobial activities. J. Med. Food 2010, 13, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Ciesla, L.M.; Wojtunik-Kulesza, K.A.; Oniszczuk, A.; Waksmundzka-Hajnos, M. Antioxidant synergism and antagonism between selected monoterpenes using the 2,2-diphenyl-1-picrylhydrazyl method. Flavour Fragr. J. 2016, 31, 412–419. [Google Scholar] [CrossRef]
- Aazza, S.; Lyoussi, B.; Miguel, M.G. Antioxidant and antiacetylcholinesterase activities of some commercial essential oils and their major compounds. Molecules 2011, 16, 7672–7690. [Google Scholar] [CrossRef]
- Čmiková, N.; Galovičová, L.; Schwarzová, M.; Vukic, M.; Vukovic, N.L.; Kowalczewski, P.Ł.; Bakay, L.; Kluz, M.I.; Puchalski, C.; Kačániová, M. Chemical composition and biological activities of Eucalyptus globulus essential oil. Plants 2023, 12, 1076. [Google Scholar] [CrossRef] [PubMed]
- Diloksumpun, S.; Wongkattiya, N.; Buaban, K.; Saleepochn, T.; Suttiarporn, P.; Luangkamin, S. Variation in the antibacterial and antioxidant activities of essential oils of five new Eucalyptus urophylla S.T.Blake clones in Thailand. Molecules 2022, 27, 680. [Google Scholar] [CrossRef]
- Vigo, E.; Cepeda, A.; Perez-Fernandez, R.; Gualillo, O. In-vitro anti-inflammatory effect of Eucalyptus globulus and Thymus vulgaris: Nitric oxide inhibition in J774A.1 murine macrophages. J. Pharm. Pharmacol. 2004, 56, 257–263. [Google Scholar] [CrossRef]
- Tsai, M.L.; Lin, C.C.; Lin, W.C.; Yang, C.H. Antimicrobial, antioxidant, and anti-inflammatory activities of essential oils from five selected herbs. Biosci. Biotechnol. Biochem. 2011, 75, 1977–1983. [Google Scholar] [CrossRef]
- Gbenou, J.D.; Ahounou, J.F.; Akakpo, H.B.; Laleye, A.; Yayi, E.; Gbaguidi, F.; Baba-Moussa, L.; Darboux, R.; Dansou, P.; Moudachirou, M.; et al. Phytochemical composition of Cymbopogon citratus and Eucalyptus citriodora essential oils and their anti-inflammatory and analgesic properties on Wistar rats. Molec. Biol. Rep. 2013, 40, 1127–1134. [Google Scholar] [CrossRef]
- Ho, C.L.; Li, L.H.; Weng, Y.C.; Hua, K.F.; Ju, T.C. Eucalyptus essential oils inhibit the lipopolysaccharide-induced inflammatory response in RAW264. 7 macrophages through reducing MAPK and NF-κB pathways. BMC Complement. Med. Ther. 2020, 20, 200. [Google Scholar] [CrossRef] [PubMed]
- Sahouo, G.B.; Tonzibo, Z.F.; Boti, B.; Chopard, C.; Mahy, J.P.; N’guessan, Y.T. Anti-inflammatory and analgesic activities: Chemical constituents of essential oils of Ocimum gratissimum, Eucalyptus citriodora and Cymbopogon giganteus inhibited lipoxygenase L-1 and cyclooxygenase of PGHS. Bull. Chem. Soc. Ethiopia 2003, 17, 191–197. [Google Scholar]
- Belkhodja, H.; Meddah, B.; Sidelarbi, K.; Bouhadi, D.; Medjadel, B.; Brakna, A. In vitro and in vivo anti-inflammatory potential of Eucalyptus globulus essential oil. J. Appl. Biol. Sci. 2022, 16, 80–88. [Google Scholar]
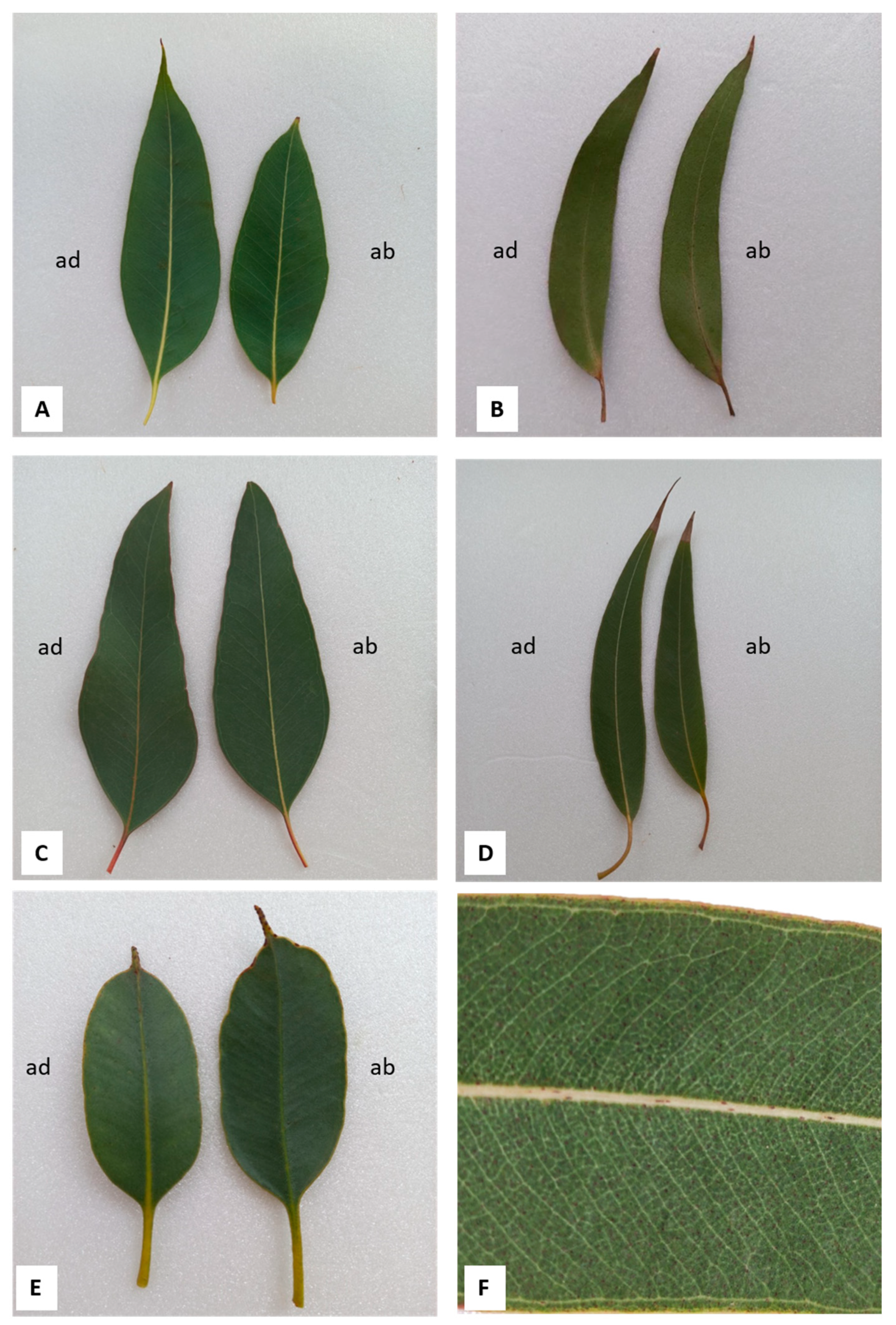

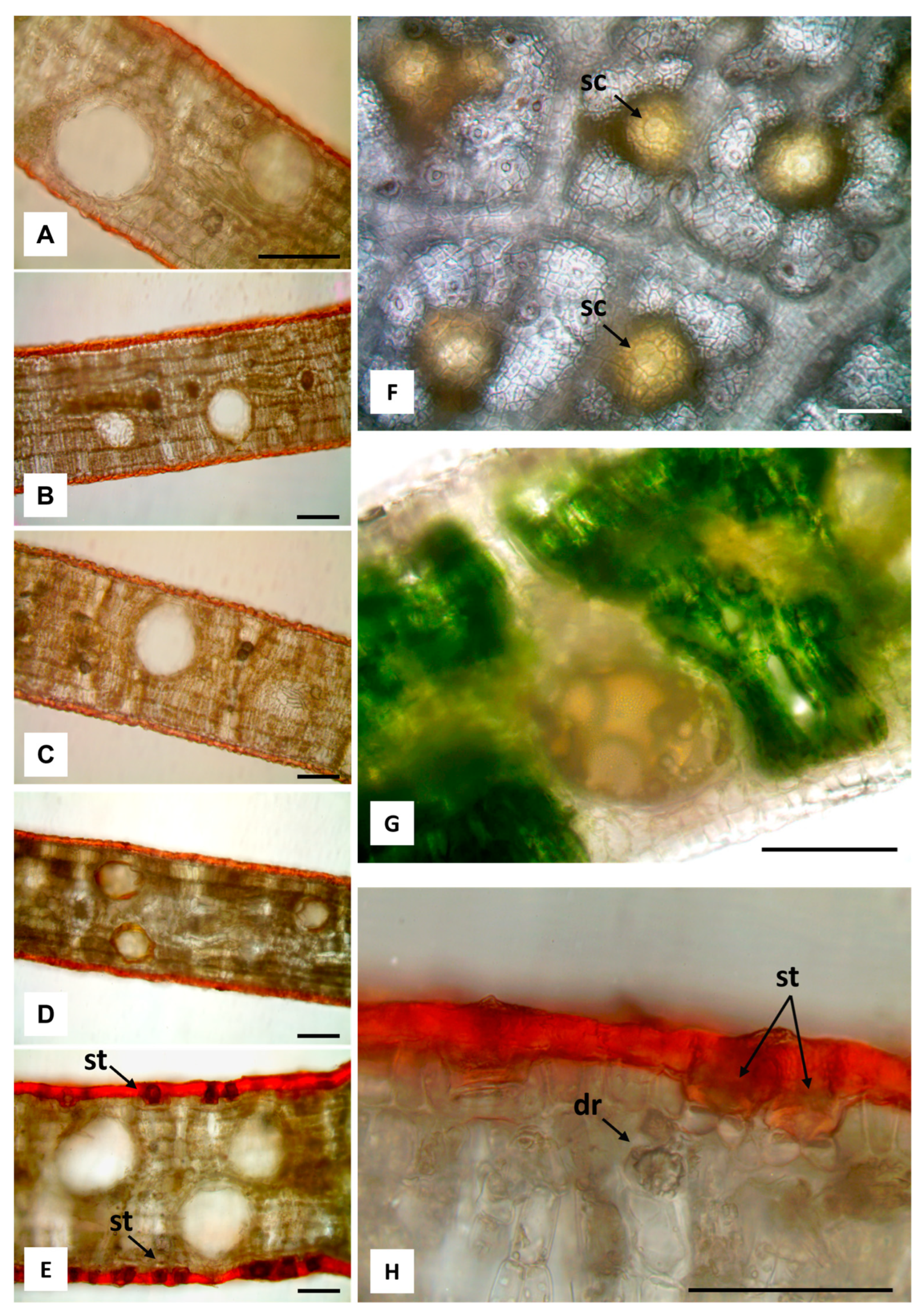
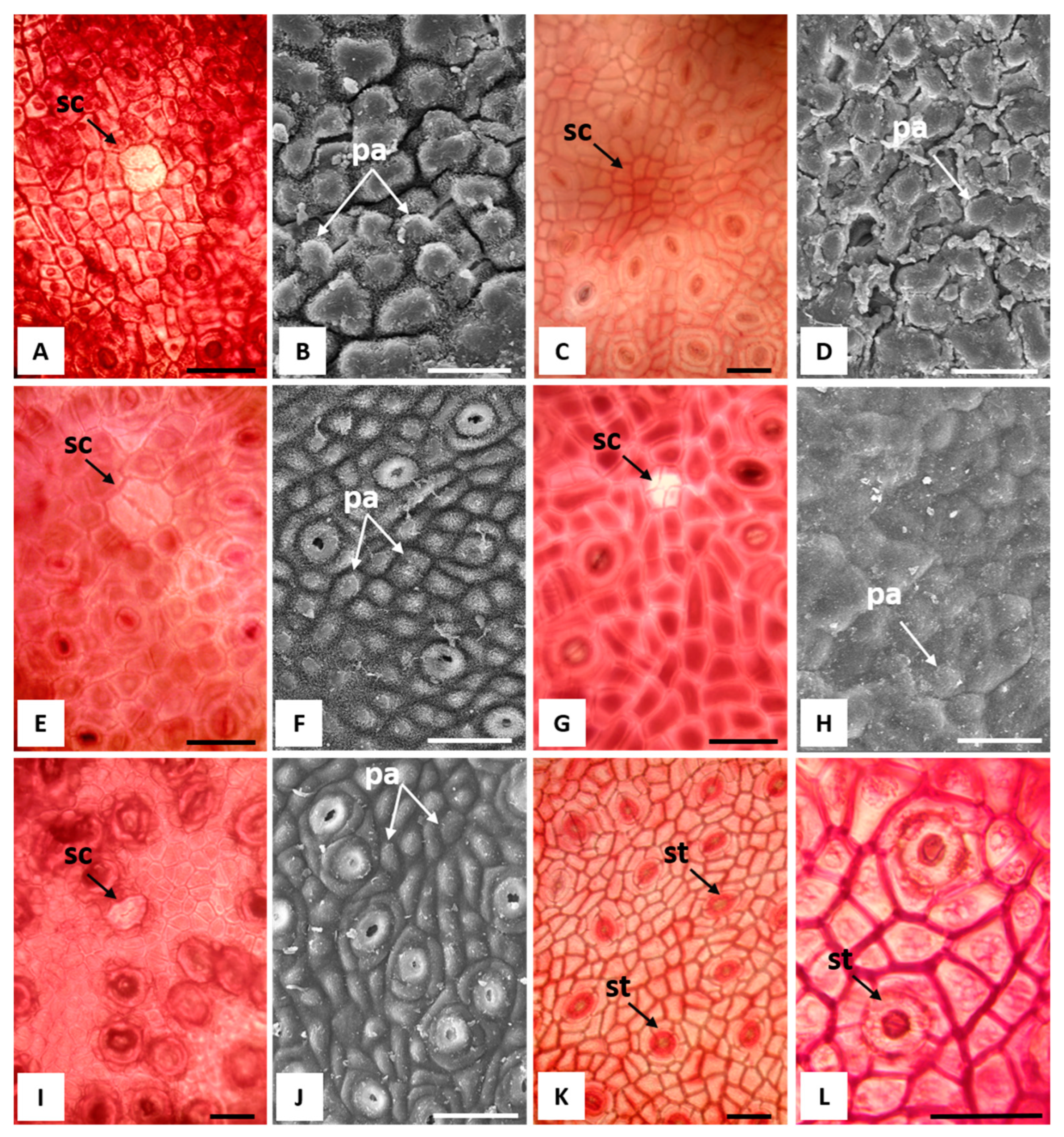
| Leaf Features | E. alba | E. eugenioides | E. fascicolosa | E. robusta | E. stoatei |
|---|---|---|---|---|---|
| Leaf size (Length × width in cm) n = 10 | 9.7–15.4 × 2.3–3.7 | 7.5–10.4 × 1.6–2.3 | 9.9–11.7 × 3.0–4.8 | 12.3–16.3 × 1.9–2.9 | 5.7–7.3 × 2.5–3.1 |
| Leaf shape | Lanceolate | Lanceolate/ falcate | Broadly lanceolate/ovate | Broadly lanceolate/slightly falcate | Elliptical/oblong |
| Texture | Papery | Papery/Coriaceous | Coriaceous | Coriaceous | Coriaceous/Leathery |
| Stomata | Actinocytic | Actinocytic/ anomocytic | Anomocytic | Anomocytic | Anomocytic |
| Wax and cutin depositions in the cuticle | ++ | +++ | ++ | +++ | +++ |
| Papillae | Present | Scarcely visible | Present | Scarcely visible | Present |
| E. alba | E. eugenioides | E. fasciculosa | E. robusta | E. stoatei | Ki a | Ki b | Identification c | |
|---|---|---|---|---|---|---|---|---|
| α-Pinene | 54.1 | - | 0.1 | - | 13.6 | 857 | 1036 | 1,2,3 |
| Camphene | 3.9 | - | - | - | - | 869 | 1075 | 1,2,3 |
| Thuja-2,4(10)-diene | - | - | - | - | 0.2 | 875 | 1115 | 1,2 |
| β-Pinene | 0.4 | - | - | - | 0.1 | 894 | 1136 | 1,2,3 |
| α-Phellandrene | - | - | 1.5 | - | - | 922 | 1177 | 1,2,3 |
| α-Terpinene | - | - | 0.6 | - | - | 933 | 1170 | 1,2,3 |
| Eucalyptol (1,8-cineole) | 25.6 | 95.9 | 72.8 | 64.4 | 71.1 | 943 | 1210 | 1,2,3 |
| cis-Sabinene hydrate | - | - | - | 0.7 | - | 955 | 1070 | 1,2 |
| 1,3,8-p-Menthatriene | - | 1.6 | - | - | - | 958 | - | 1,2 |
| exo-Fenchol | 0.3 | - | - | - | - | 1019 | 1591 | 1,2 |
| Borneol | 3.4 | - | - | - | - | 1067 | 1615 | 1,2,3 |
| β-Panasinsene | - | - | 0.1 | - | 0.1 | 1275 | - | 1,2 |
| α-Gurjunene | - | - | 0.3 | - | - | 1288 | 1535 | 1,2 |
| (Z)-Caryophyllene | - | - | 1.0 | - | - | 1296 | 1617 | 1,2 |
| β-Cedrene | - | - | 0.1 | 0.1 | - | 1298 | 1625 | 1,2 |
| β-Copaene | - | - | 0.1 | 2.7 | - | 1304 | 1628 | 1,2 |
| Aromadendrene | 0.8 | - | 3.7 | - | 4.7 | 1308 | 1631 | 1,2 |
| β-Longipinene | - | - | - | 0.7 | - | 1323 | - | 1,2 |
| allo-Aromadendrene | 0.8 | - | 2.8 | - | 0.9 | 1330 | 1660 | 1,2 |
| Dehydro-Aromadendrene | - | - | 2.7 | - | - | 1331 | 1642 | 1,2 |
| γ-Gurjunene | 1.7 | - | - | - | - | 1343 | 1687 | 1,2 |
| δ-Selinene | - | - | - | - | 0.2 | 1350 | 1707 | 1,2 |
| γ-Amorphene | - | - | 0.6 | - | - | 1358 | 1695 | 1,2 |
| cis-β-Guaiene | 4.1 | - | - | - | - | 1361 | 1748 | 1,2 |
| trans-β-Guaiene | - | - | - | - | 0.6 | 1366 | 1723 | 1,2 |
| Viridiflorene | - | - | 6.1 | - | 2.9 | 1367 | 1713 | 1,2 |
| α-Muurolene | - | - | - | 6.4 | - | 1373 | 1744 | 1,2 |
| γ-Cadinene | - | - | - | 3.9 | - | 1385 | 1752 | 1,2 |
| δ-Cadinene | - | - | - | 15.7 | - | 1396 | 1755 | 1,2 |
| Germacrene B | - | - | 0.2 | - | - | 1433 | 1795 | 1,2 |
| Spathulenol | 1.2 | - | 1.1 | - | - | 1452 | 2127 | 1,2 |
| Total | 96.3 | 97.5 | 93.8 | 94.6 | 94.4 | |||
| Monoterpene hydrocarbons | 58.4 | 1.6 | 2.2 | - | 13.9 | |||
| Oxygenated monoterpenes | 29.3 | 95.9 | 72.8 | 65.1 | 71.1 | |||
| Total monoterpenes | 87.7 | 97.5 | 75.0 | 65.1 | 85.0 | |||
| Sesquiterpene hydrocarbons | 7.4 | - | 17.7 | 29.5 | 9.4 | |||
| Oxygenated sesquiterpenes | 1.2 | - | 1.1 | - | - | |||
| Total sesquiterpenes | 8.6 | - | 18.8 | 29.5 | 9.4 | |||
| Ratio of monoterpenes/sesquiterpenes | 10.2 | - | 4.0 | 2.2 | 9.0 |
| Essential Oil | IC50 (mg mL−1) |
|---|---|
| E. alba | 1.70 ± 0.79 |
| E. eugenioides | 4.93 ± 1.74 |
| E. fasciculosa | 7.82 ± 2.69 |
| E. robusta | 2.05 ± 0.97 |
| E. stoatei | 45.62 ± 1.25 |
| Essential Oil | TEAC (µM gr−1) |
|---|---|
| E. alba | 21.5 ± 2.28 |
| E. eugenioides | 25.8 ± 1.39 |
| E. fasciculosa | 21.2 ± 1.45 |
| E. robusta | 22.5 ± 0.39 |
| E. stoatei | 9.3 ± 1.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polito, F.; Fratianni, F.; Nazzaro, F.; Amri, I.; Kouki, H.; Khammassi, M.; Hamrouni, L.; Malaspina, P.; Cornara, L.; Khedhri, S.; et al. Essential Oil Composition, Antioxidant Activity and Leaf Micromorphology of Five Tunisian Eucalyptus Species. Antioxidants 2023, 12, 867. https://doi.org/10.3390/antiox12040867
Polito F, Fratianni F, Nazzaro F, Amri I, Kouki H, Khammassi M, Hamrouni L, Malaspina P, Cornara L, Khedhri S, et al. Essential Oil Composition, Antioxidant Activity and Leaf Micromorphology of Five Tunisian Eucalyptus Species. Antioxidants. 2023; 12(4):867. https://doi.org/10.3390/antiox12040867
Chicago/Turabian StylePolito, Flavio, Florinda Fratianni, Filomena Nazzaro, Ismail Amri, Habiba Kouki, Marwa Khammassi, Lamia Hamrouni, Paola Malaspina, Laura Cornara, Sana Khedhri, and et al. 2023. "Essential Oil Composition, Antioxidant Activity and Leaf Micromorphology of Five Tunisian Eucalyptus Species" Antioxidants 12, no. 4: 867. https://doi.org/10.3390/antiox12040867
APA StylePolito, F., Fratianni, F., Nazzaro, F., Amri, I., Kouki, H., Khammassi, M., Hamrouni, L., Malaspina, P., Cornara, L., Khedhri, S., Romano, B., Maresca, D. C., Ianaro, A., Ercolano, G., & De Feo, V. (2023). Essential Oil Composition, Antioxidant Activity and Leaf Micromorphology of Five Tunisian Eucalyptus Species. Antioxidants, 12(4), 867. https://doi.org/10.3390/antiox12040867