Analysis of Single- and Double-Stranded DNA Damage in Osteoblastic Cells after Hyperbaric Oxygen Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Exposure to Hyperbaric Oxygen (HBO)
2.3. Single-Cell Gel Electrophoresis (Alkaline Comet Assay)
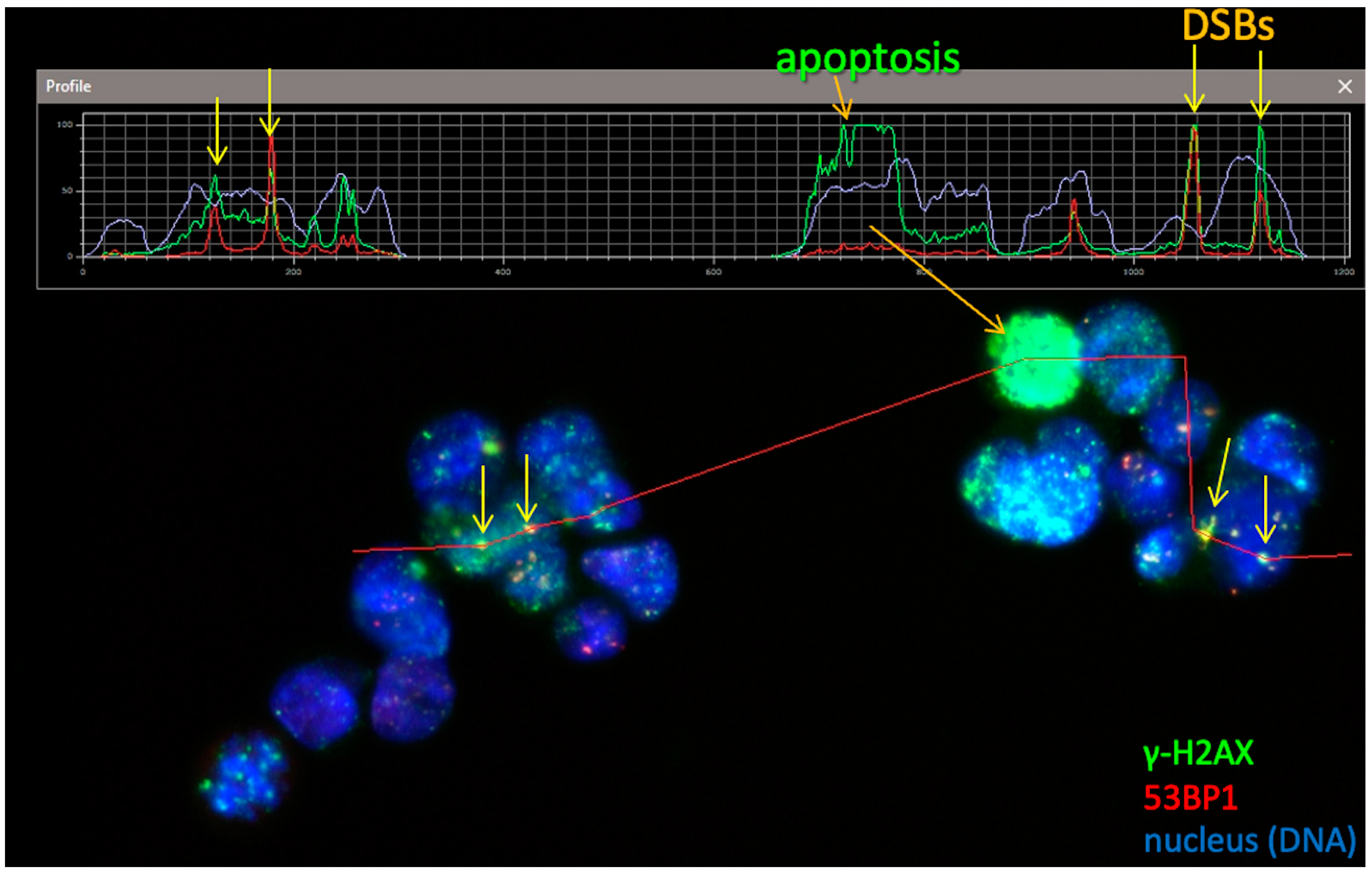
2.4. γH2AX Focus Analysis
2.5. Isolation of RNA and Synthesis of cDNA
2.6. Real-Time PCR
2.7. Statistical Analysis
3. Results
3.1. DNA Fragmentation in HOB and SAOS-2 Cells after Exposure to Hyperbaric Hyperoxia
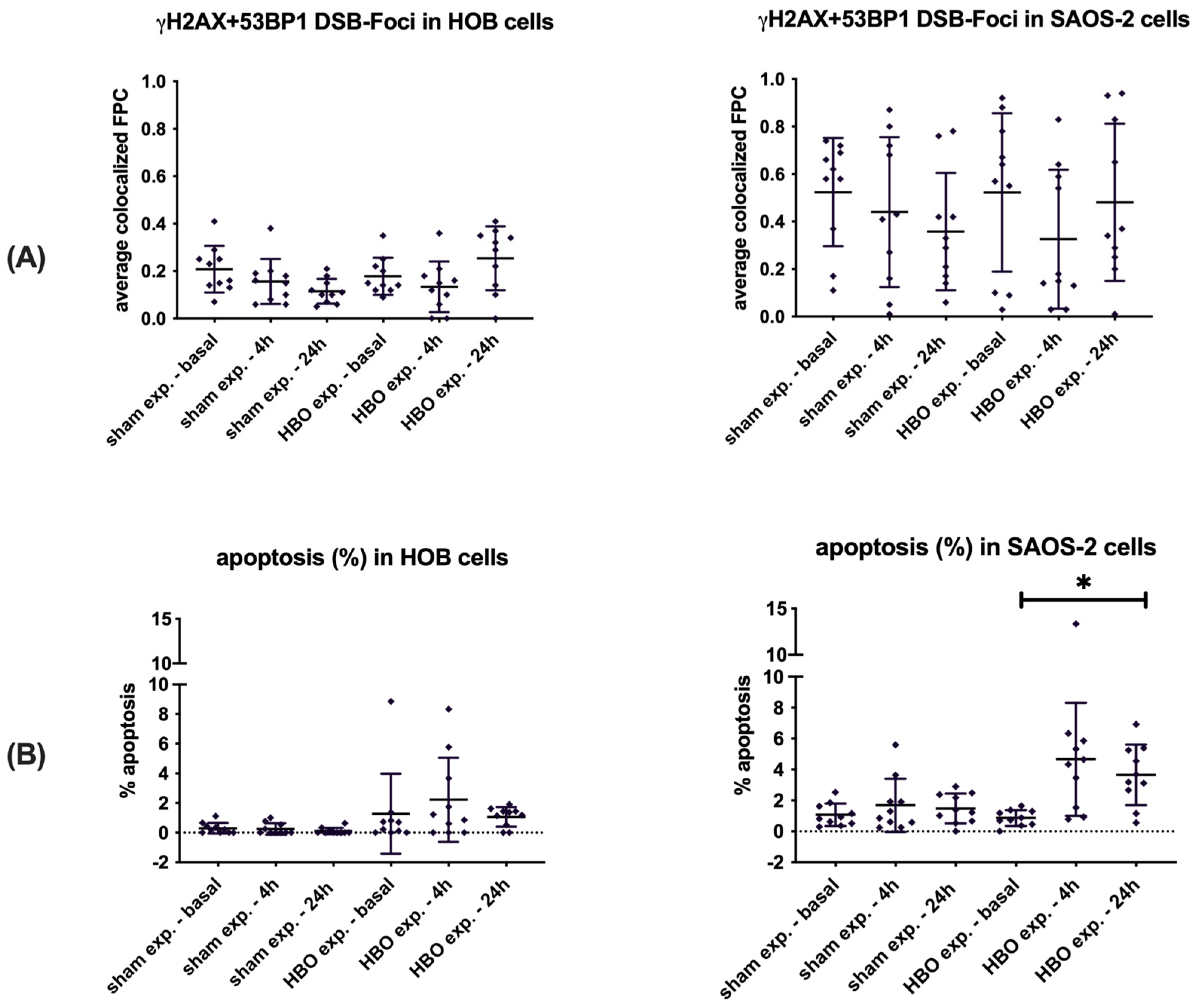
3.2. DSB Foci Detection and Apoptosis in HOB and SAOS-2 Cells
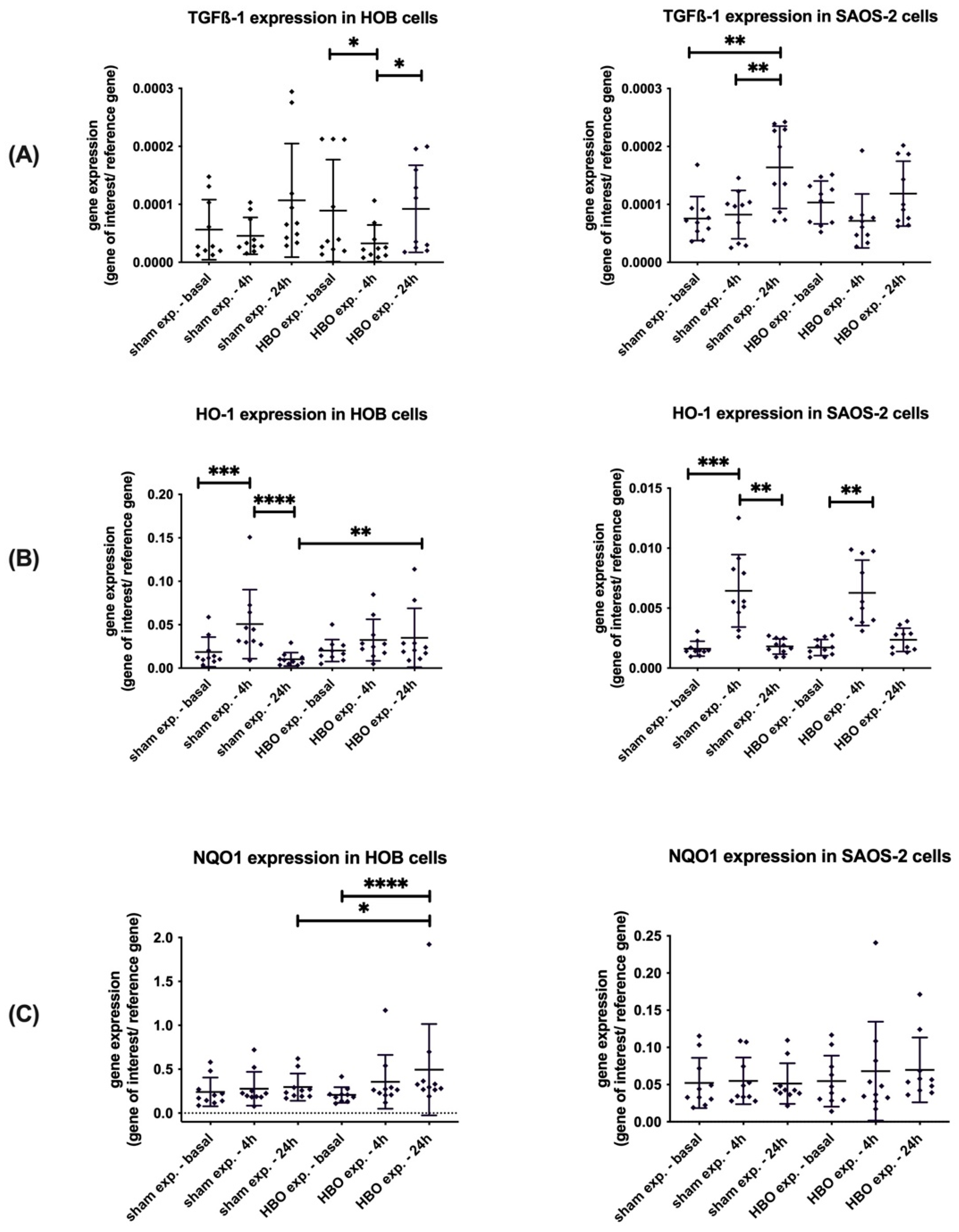
3.3. Gene Expression of Markers for Oxidative Stress in HOB and SAOS-2 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ikehata, H.; Ono, T. The Mechanisms of UV Mutagenesis. J. Radiat. Res. 2011, 52, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Hunt, C.P.; Wadhwa, M.; Sanders, L.H. DNA Damage by Oxidative Stress: Measurement Strategies for Two Genomes. Curr. Opin. Toxicol. 2018, 7, 87–94. [Google Scholar] [CrossRef]
- Ziech, D.; Franco, R.; Pappa, A.; Panayiotidis, M.I. Reactive Oxygen Species (ROS)—Induced Genetic and Epigenetic Alterations in Human Carcinogenesis. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2011, 711, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Magder, S. Reactive Oxygen Species: Toxic Molecules or Spark of Life? Crit. Care 2006, 10, 208. [Google Scholar] [CrossRef]
- Speit, G.; Dennog, C.; Radermacher, P.; Rothfuss, A. Genotoxicity of Hyperbaric Oxygen. Mutat. Res./Rev. Mutat. Res. 2002, 512, 111–119. [Google Scholar] [CrossRef]
- Dusinska, M.; Collins, A.R. The Comet Assay in Human Biomonitoring: Gene-Environment Interactions. Mutagenesis 2008, 23, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Witte, J.; Kähler, W.; Wunderlich, T.; Radermacher, P.; Wohlrab, C.; Koch, A. Dose-Time Dependency of Hyperbaric Hyperoxia-Induced DNA Strand Breaks in Human Immune Cells Visualized with the Comet Assay. Undersea Hyperb. Med. 2014, 41, 171–181. [Google Scholar]
- Kähler, W.; Koch, I.; Wohlrab, C.; Kowalski, J.; Witte, J.; Koch, A. Influence of Hyperoxia and Physical Exercise on *OH-Radical Stress in Humans as Measured by Dihydroxylated Benzoates (DHB) in Urine. Undersea Hyperb. Med. 2013, 40, 231–238. [Google Scholar]
- Collins, A.R.; Oscoz, A.A.; Brunborg, G.; Gaivao, I.; Giovannelli, L.; Kruszewski, M.; Smith, C.C.; Stetina, R. The Comet Assay: Topical Issues. Mutagenesis 2008, 23, 143–151. [Google Scholar] [CrossRef]
- Wu, D.; Malda, J.; Crawford, R.; Xiao, Y. Effects of Hyperbaric Oxygen on Proliferation and Differentiation of Osteoblasts from Human Alveolar Bone. Connect. Tissue Res. 2007, 48, 206–213. [Google Scholar] [CrossRef]
- Al Hadi, H.; Smerdon, G.R.; Fox, S.W. Hyperbaric Oxygen Therapy Accelerates Osteoblast Differentiation and Promotes Bone Formation. J. Dent. 2015, 43, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R.; Ai-guo, M.; Duthie, S.J. The Kinetics of Repair of Oxidative DNA Damage (Strand Breaks and Oxidised Pyrimidines) in Human Cells. Mutat. Res./DNA Repair 1995, 336, 69–77. [Google Scholar] [CrossRef]
- Anderson, D.; Laubenthal, J. Analysis of DNA Damage via Single-Cell Electrophoresis. In DNA Electrophoresis; Makovets, S., Ed.; Humana Press: Totowa, NJ, USA, 2013; Volume 1054, pp. 209–218. ISBN 978-1-62703-564-4. [Google Scholar]
- Collins, A.R. The Comet Assay for DNA Damage and Repair: Principles, Applications, and Limitations. Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Eberlein, U.; Peper, M.; Fernández, M.; Lassmann, M.; Scherthan, H. Calibration of the γ-H2AX DNA Double Strand Break Focus Assay for Internal Radiation Exposure of Blood Lymphocytes. PLoS ONE 2015, 10, e0123174. [Google Scholar] [CrossRef] [PubMed]
- Rothkamm, K.; Barnard, S.; Moquet, J.; Ellender, M.; Rana, Z.; Burdak-Rothkamm, S. DNA Damage Foci: Meaning and Significance: DNA Damage Foci. Environ. Mol. Mutagen. 2015, 56, 491–504. [Google Scholar] [CrossRef]
- Nikolova, T.; Marini, F.; Kaina, B. Genotoxicity Testing: Comparison of the γH2AX Focus Assay with the Alkaline and Neutral Comet Assays. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2017, 822, 10–18. [Google Scholar] [CrossRef]
- Ahmed, E.A.; Agay, D.; Schrock, G.; Drouet, M.; Meineke, V.; Scherthan, H. Persistent DNA Damage after High Dose In Vivo Gamma Exposure of Minipig Skin. PLoS ONE 2012, 7, e39521. [Google Scholar] [CrossRef]
- Ma, Q.; He, X. Molecular Basis of Electrophilic and Oxidative Defense: Promises and Perils of Nrf2. Pharmacol. Rev. 2012, 64, 1055–1081. [Google Scholar] [CrossRef]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.-L. The Keap1-Nrf2 Pathway: Mechanisms of Activation and Dysregulation in Cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 System in Development, Oxidative Stress Response and Diseases: An Evolutionarily Conserved Mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Yoshimura, A.; Wakabayashi, Y.; Mori, T. Cellular and Molecular Basis for the Regulation of Inflammation by TGF-β. J. Biochem. 2010, 147, 781–792. [Google Scholar] [CrossRef]
- Yoshimura, A.; Muto, G. TGF-β Function in Immune Suppression. Curr. Top. Microbiol. Immunol. 2011, 350, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, M.; Nakatsukasa, H.; Okada, M.; Lu, Q.; Yoshimura, A. Induced Regulatory T Cells: Their Development, Stability, and Applications. Trends Immunol. 2016, 37, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Birch, M.A.; Ginty, A.F.; Walsh, C.A.; Fraser, W.D.; Gallagher, J.A.; Bilbe, G. PCR Detection of Cytokines in Normal Human and Pagetic Osteoblast-like Cells. J. Bone Miner. Res. 1993, 8, 1155–1162. [Google Scholar] [CrossRef]
- Kasagi, S.; Chen, W. TGF-Beta1 on Osteoimmunology and the Bone Component Cells. Cell Biosci. 2013, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.; Petrovska, H.; Duthie, S.; Fillion, L.; Panayiotidis, M. Comet Assay in Human Biomonitoring Studies: Reliability, Validation, and Applications. Environ. Mol. Mutagen. 1997, 30, 139–146. [Google Scholar] [CrossRef]
- Lamkowski, A.; Forcheron, F.; Agay, D.; Ahmed, E.A.; Drouet, M.; Meineke, V.; Scherthan, H. DNA Damage Focus Analysis in Blood Samples of Minipigs Reveals Acute Partial Body Irradiation. PLoS ONE 2014, 9, e87458. [Google Scholar] [CrossRef]
- Speit, G.; Rothfuss, A. The Comet Assay: A Sensitive Genotoxicity Test for the Detection of DNA Damage and Repair. In DNA Repair Protocols; Bjergbæk, L., Ed.; Humana Press: Totowa, NJ, USA, 2012; Volume 920, pp. 79–90. ISBN 978-1-61779-997-6. [Google Scholar]
- Kuo, L.J.; Yang, L.-X. γ-H2AX—A Novel Biomarker for DNA Double-Strand Breaks. In Vivo 2008, 22, 305–309. [Google Scholar] [PubMed]
- Fairbairn, D.W.; Olive, P.L.; O’Neill, K.L. The Comet Assay: A Comprehensive Review. Mutat. Res./Rev. Genet. Toxicol. 1995, 339, 37–59. [Google Scholar] [CrossRef]
- Halliwell, B. Cell Culture, Oxidative Stress, and Antioxidants: Avoiding Pitfalls. Biomed. J. 2014, 37, 99–105. [Google Scholar] [CrossRef]
- Pani, G.; Colavitti, R.; Bedogni, B.; Anzevino, R.; Borrello, S.; Galeotti, T. A Redox Signaling Mechanism for Density-Dependent Inhibition of Cell Growth. J. Biol. Chem. 2000, 275, 38891–38899. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; 5. Auflage; Oxford University Press: Oxford, UK, 2015; ISBN 978-0-19-871748-5. [Google Scholar]
- Scherthan, H.; Wagner, S.-Q.; Grundhöfer, J.; Matejka, N.; Müller, J.; Müller, S.; Rudigkeit, S.; Sammer, M.; Schoof, S.; Port, M.; et al. Planar Proton Minibeam Irradiation Elicits Spatially Confined DNA Damage in a Human Epidermis Model. Cancers 2022, 14, 1545. [Google Scholar] [CrossRef] [PubMed]
- Dianov, G.L.; Hübscher, U. Mammalian Base Excision Repair: The Forgotten Archangel. Nucleic Acids Res. 2013, 41, 3483–3490. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.J.; Ashworth, A. The DNA Damage Response and Cancer Therapy. Nature 2012, 481, 287–294. [Google Scholar] [CrossRef]
- Tubbs, A.; Nussenzweig, A. Endogenous DNA Damage as a Source of Genomic Instability in Cancer. Cell 2017, 168, 644–656. [Google Scholar] [CrossRef]
- De Bels, D.; Tillmans, F.; Corazza, F.; Bizzarri, M.; Germonpre, P.; Radermacher, P.; Orman, K.G.; Balestra, C. Hyperoxia Alters Ultrastructure and Induces Apoptosis in Leukemia Cell Lines. Biomolecules 2020, 10, 282. [Google Scholar] [CrossRef]
- Weber, S.U.; Koch, A.; Kankeleit, J.; Schewe, J.-C.; Siekmann, U.; Stüber, F.; Hoeft, A.; Schröder, S. Hyperbaric Oxygen Induces Apoptosis via a Mitochondrial Mechanism. Apoptosis 2009, 14, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, B.J.; Tonomura, N.; Benson, R.M.; Osborne, B.A.; Granowitz, E.V. Hyperbaric Oxygen Enhances Apoptosis in Hematopoietic Cells. Apoptosis 2002, 7, 499–510. [Google Scholar] [CrossRef]
- Ross, D.; Siegel, D. NQO1 in Protection against Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 67–72. [Google Scholar] [CrossRef]
- Ryter, S.W.; Choi, A.M.K. Heme Oxygenase-1: Redox Regulation of a Stress Protein in Lung and Cell Culture Models. Antioxid. Redox Signal. 2005, 7, 80–91. [Google Scholar] [CrossRef]
- Loboda, A.; Jazwa, A.; Grochot-Przeczek, A.; Rutkowski, A.J.; Cisowski, J.; Agarwal, A.; Jozkowicz, A.; Dulak, J. Heme Oxygenase-1 and the Vascular Bed: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid. Redox Signal. 2008, 10, 1767–1812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yi, H.; Xia, X.-P.; Zhao, Y. Transforming Growth Factor-Beta: An Important Role in CD4+CD25+ Regulatory T Cells and Immune Tolerance. Autoimmunity 2006, 39, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Qadan, M.; Battista, C.; Gardner, S.A.; Anderson, G.; Akca, O.; Polk, H.C. Oxygen and Surgical Site Infection. Anesthesiology 2010, 113, 369–377. [Google Scholar] [CrossRef]
- Al-Waili, N.S.; Butler, G.J.; Petrillo, R.L.; Carrey, Z.; Hamilton, R.W.B. Hyperbaric Oxygen and Lymphoid System Function: A Review Supporting Possible Intervention in Tissue Transplantation. Technol. Health Care 2006, 14, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Kiers, D.; Gerretsen, J.; Janssen, E.; John, A.; Groeneveld, R.; van der Hoeven, J.G.; Scheffer, G.-J.; Pickkers, P.; Kox, M. Short-Term Hyperoxia Does Not Exert Immunologic Effects during Experimental Murine and Human Endotoxemia. Sci. Rep. 2015, 5, 17441. [Google Scholar] [CrossRef]
- Kassem, M.; Kveiborg, M.; Eriksen, E.F. Production and Action of Transforming Growth Factor-β in Human Osteoblast Cultures: Dependence on Cell Differentiation and Modulation by Calcitriol: Effects of TGF-β on Human Osteoblasts in Vitro. Eur. J. Clin. Investig. 2000, 30, 429–437. [Google Scholar] [CrossRef]
- Speit, G. Genotoxic and Protective Effects of Hyperbaric Oxygen in A549 Lung Cells. Mutagenesis 2003, 18, 545–548. [Google Scholar] [CrossRef]
- Scott, T.L.; Rangaswamy, S.; Wicker, C.A.; Izumi, T. Repair of Oxidative DNA Damage and Cancer: Recent Progress in DNA Base Excision Repair. Antioxid. Redox Signal. 2014, 20, 708–726. [Google Scholar] [CrossRef]
- Liu, T.; Qian, W.-J.; Gritsenko, M.A.; Camp, D.G.; Monroe, M.E.; Moore, R.J.; Smith, R.D. Human Plasma N-Glycoproteome Analysis by Immunoaffinity Subtraction, Hydrazide Chemistry, and Mass Spectrometry. J. Proteome Res. 2005, 4, 2070–2080. [Google Scholar] [CrossRef]
- Joseph, P.; Xie, T.; Xu, Y.; Jaiswal, A.K. NAD(P)H:Quinone Oxidoreductase1 (DT-Diaphorase): Expression, Regulation, and Role in Cancer. Oncol. Res. 1994, 6, 525–532. [Google Scholar]
- Tillmans, F.; Sharghi, R.; Noy, T.; Kähler, W.; Klapa, S.; Sartisohn, S.; Sebens, S.; Koch, A. Effect of Hyperoxia on the Immune Status of Oxygen Divers and Endurance Athletes. Free Radic. Res. 2019, 53, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Rothfuss, A.; Radermacher, P.; Speit, G. Involvement of Heme Oxygenase-1 (HO-1) in the Adaptive Protection of Human Lymphocytes after Hyperbaric Oxygen (HBO) Treatment. Carcinogenesis 2001, 22, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Tohidnezhad, M.; Wruck, C.-J.; Slowik, A.; Kweider, N.; Beckmann, R.; Bayer, A.; Houben, A.; Brandenburg, L.-O.; Varoga, D.; Sönmez, T.-T.; et al. Role of Platelet-Released Growth Factors in Detoxification of Reactive Oxygen Species in Osteoblasts. Bone 2014, 65, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.; Siegel, D. Functions of NQO1 in Cellular Protection and CoQ10 Metabolism and Its Potential Role as a Redox Sensitive Molecular Switch. Front. Physiol. 2017, 8, 595. [Google Scholar] [CrossRef]
- Ceponis, P.; Keilman, C.; Guerry, C.; Freiberger, J. Hyperbaric Oxygen Therapy and Osteonecrosis. Oral Dis. 2017, 23, 141–151. [Google Scholar] [CrossRef]
- Savvidou, O.D.; Kaspiris, A.; Bolia, I.K.; Chloros, G.D.; Goumenos, S.D.; Papagelopoulos, P.J.; Tsiodras, S. Effectiveness of Hyperbaric Oxygen Therapy for the Management of Chronic Osteomyelitis: A Systematic Review of the Literature. Orthopedics 2018, 41, 193–199. [Google Scholar] [CrossRef]
- Mathieu, D.; Marroni, A.; Kot, J. Tenth European Consensus Conference on Hyperbaric Medicine: Recommendations for Accepted and Non-Accepted Clinical Indications and Practice of Hyperbaric Oxygen Treatment. Diving Hyperb. Med. 2017, 47, 24–32. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schönrock, N.; Tillmans, F.; Sebens, S.; Kähler, W.; Klapa, S.; Rieger, B.; Scherthan, H.; Koch, A. Analysis of Single- and Double-Stranded DNA Damage in Osteoblastic Cells after Hyperbaric Oxygen Exposure. Antioxidants 2023, 12, 851. https://doi.org/10.3390/antiox12040851
Schönrock N, Tillmans F, Sebens S, Kähler W, Klapa S, Rieger B, Scherthan H, Koch A. Analysis of Single- and Double-Stranded DNA Damage in Osteoblastic Cells after Hyperbaric Oxygen Exposure. Antioxidants. 2023; 12(4):851. https://doi.org/10.3390/antiox12040851
Chicago/Turabian StyleSchönrock, Nele, Frauke Tillmans, Susanne Sebens, Wataru Kähler, Sebastian Klapa, Bente Rieger, Harry Scherthan, and Andreas Koch. 2023. "Analysis of Single- and Double-Stranded DNA Damage in Osteoblastic Cells after Hyperbaric Oxygen Exposure" Antioxidants 12, no. 4: 851. https://doi.org/10.3390/antiox12040851
APA StyleSchönrock, N., Tillmans, F., Sebens, S., Kähler, W., Klapa, S., Rieger, B., Scherthan, H., & Koch, A. (2023). Analysis of Single- and Double-Stranded DNA Damage in Osteoblastic Cells after Hyperbaric Oxygen Exposure. Antioxidants, 12(4), 851. https://doi.org/10.3390/antiox12040851





