Effects of Resveratrol on Vascular Function in Retinal Ischemia-Reperfusion Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Induction of Ischemia-Reperfusion Injury and Application of Resveratrol
2.3. Retinal Wholemounts and Cell Counting
2.4. Retinal Arteriole Reactivity Measurement
2.5. Assessment of ROS and RNS Levels
2.6. Quantitative PCR
2.7. Quantification of NOX2 Expression
2.8. Statistical Analysis
3. Results
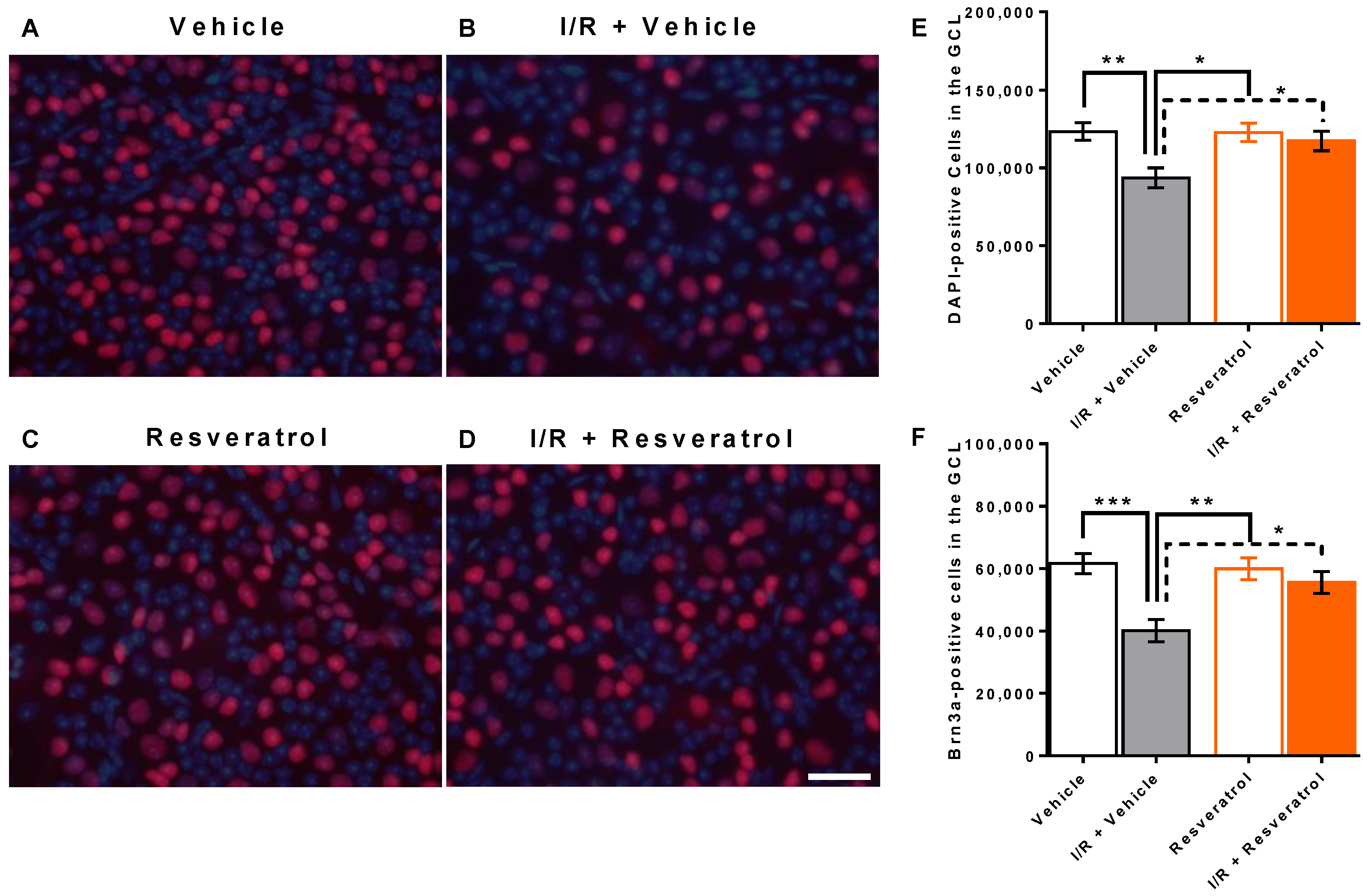
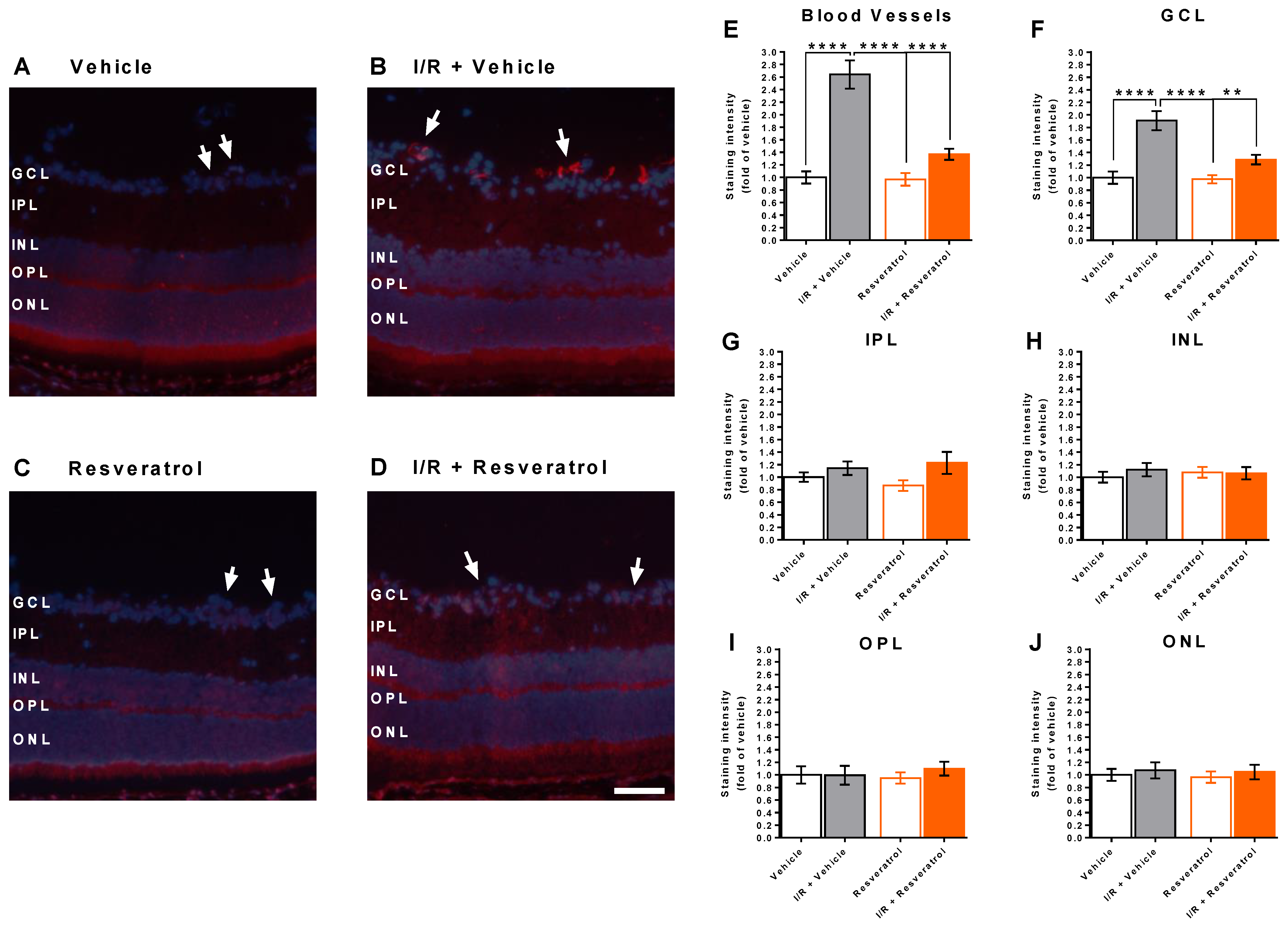
3.1. Number of Cells in the Retinal Ganglion Cell Layer
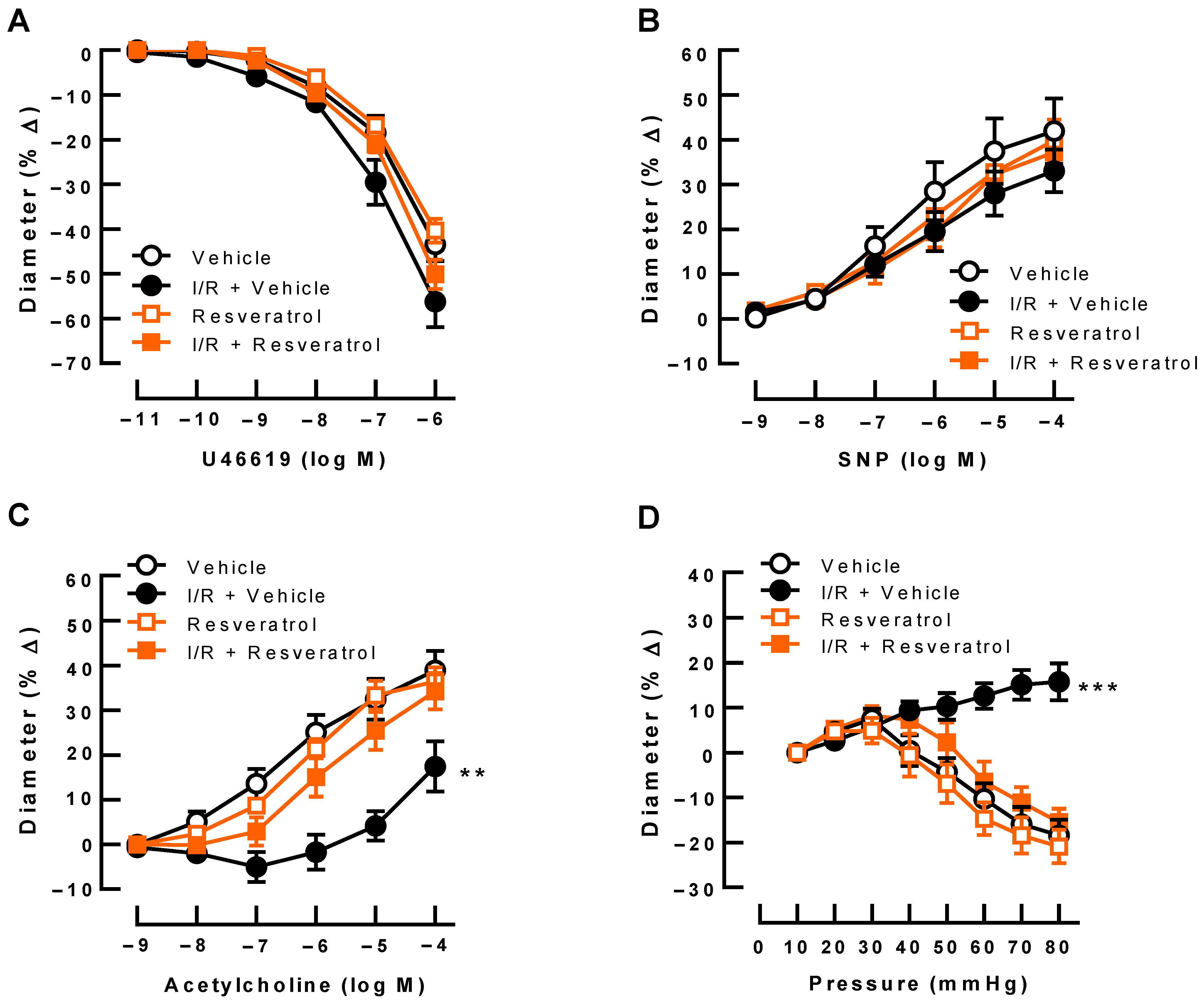
3.2. Responses of Retinal Arterioles
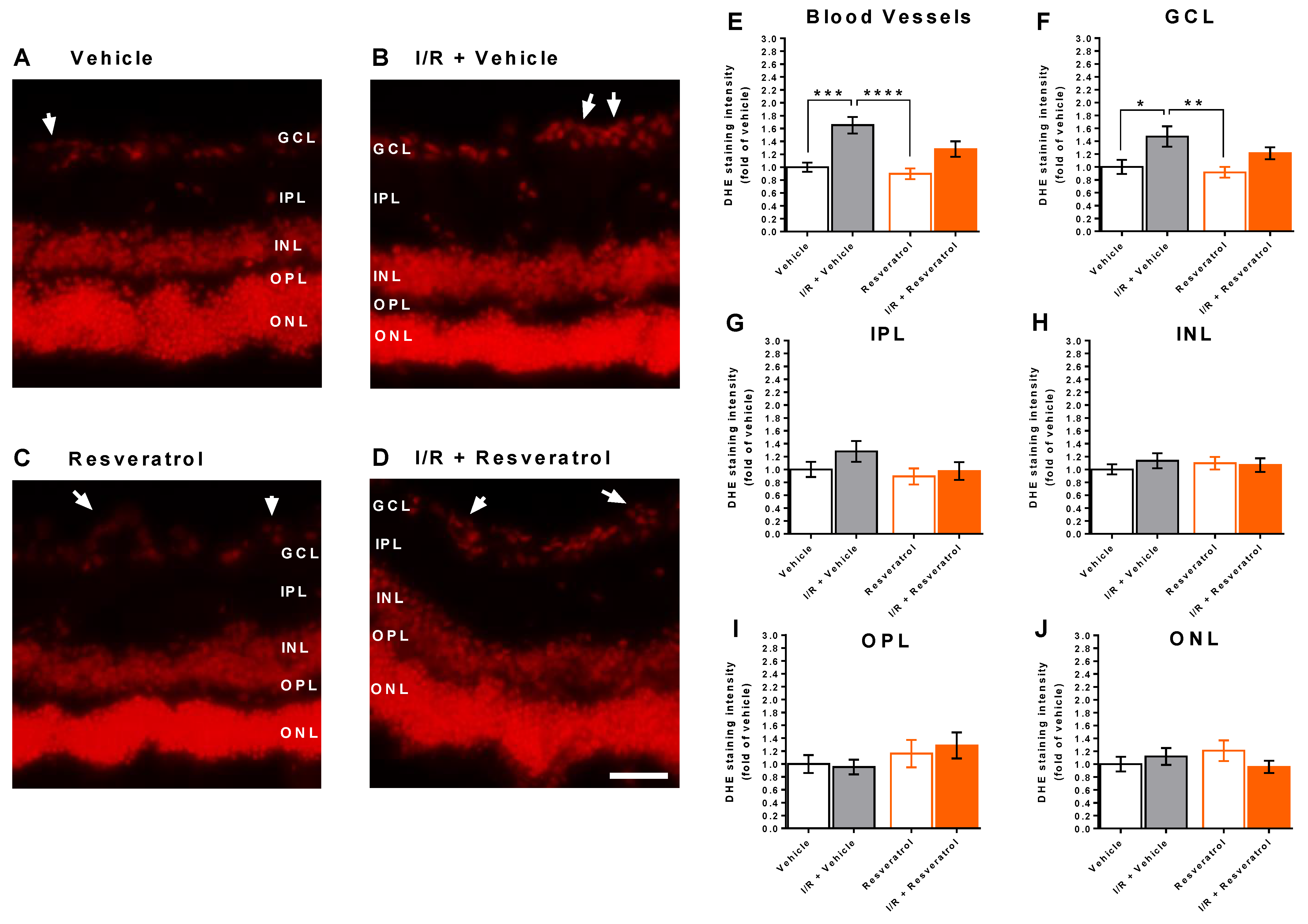
3.3. ROS Levels in the Retina
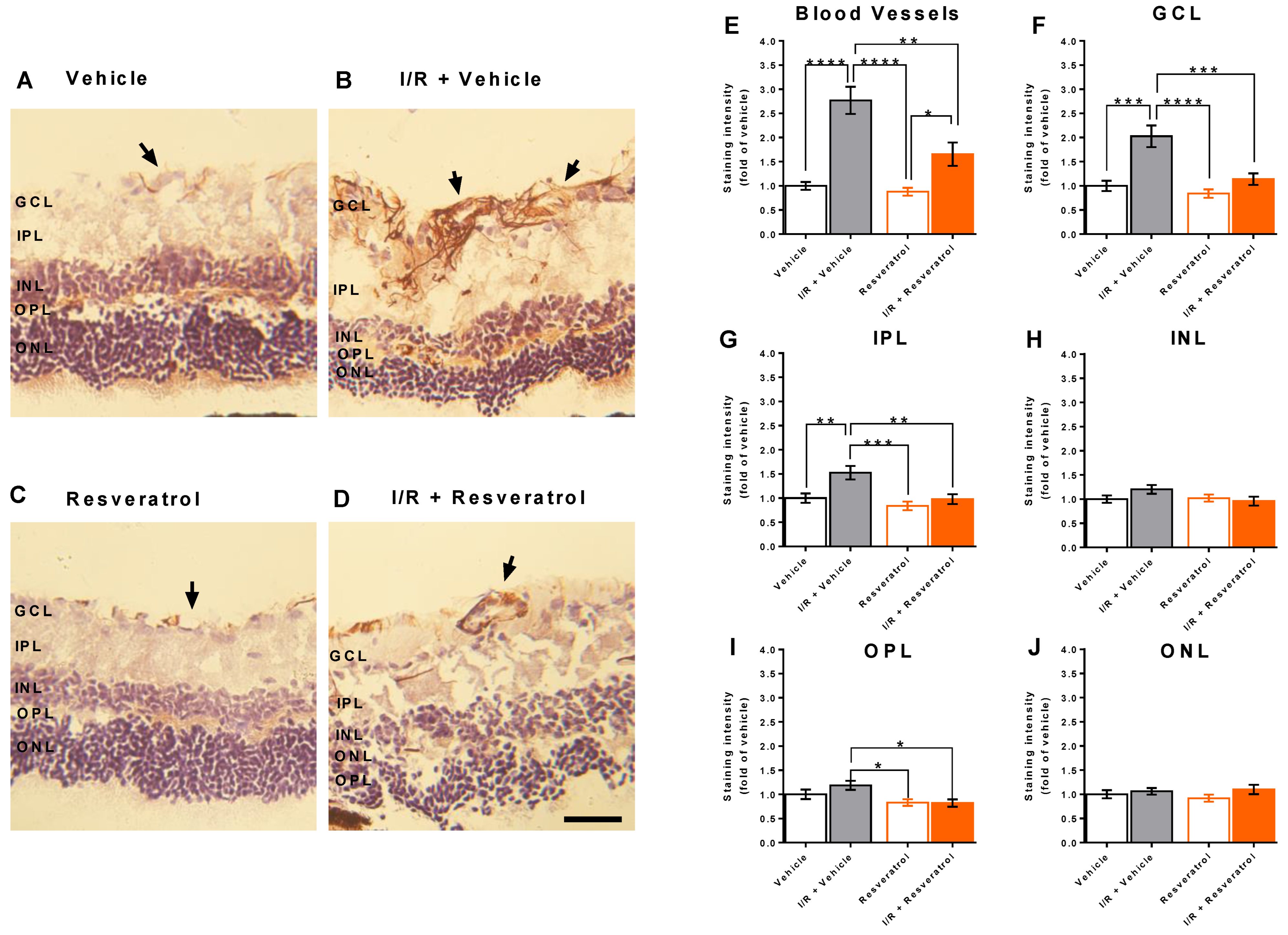
3.4. RNS Levels in the Retina
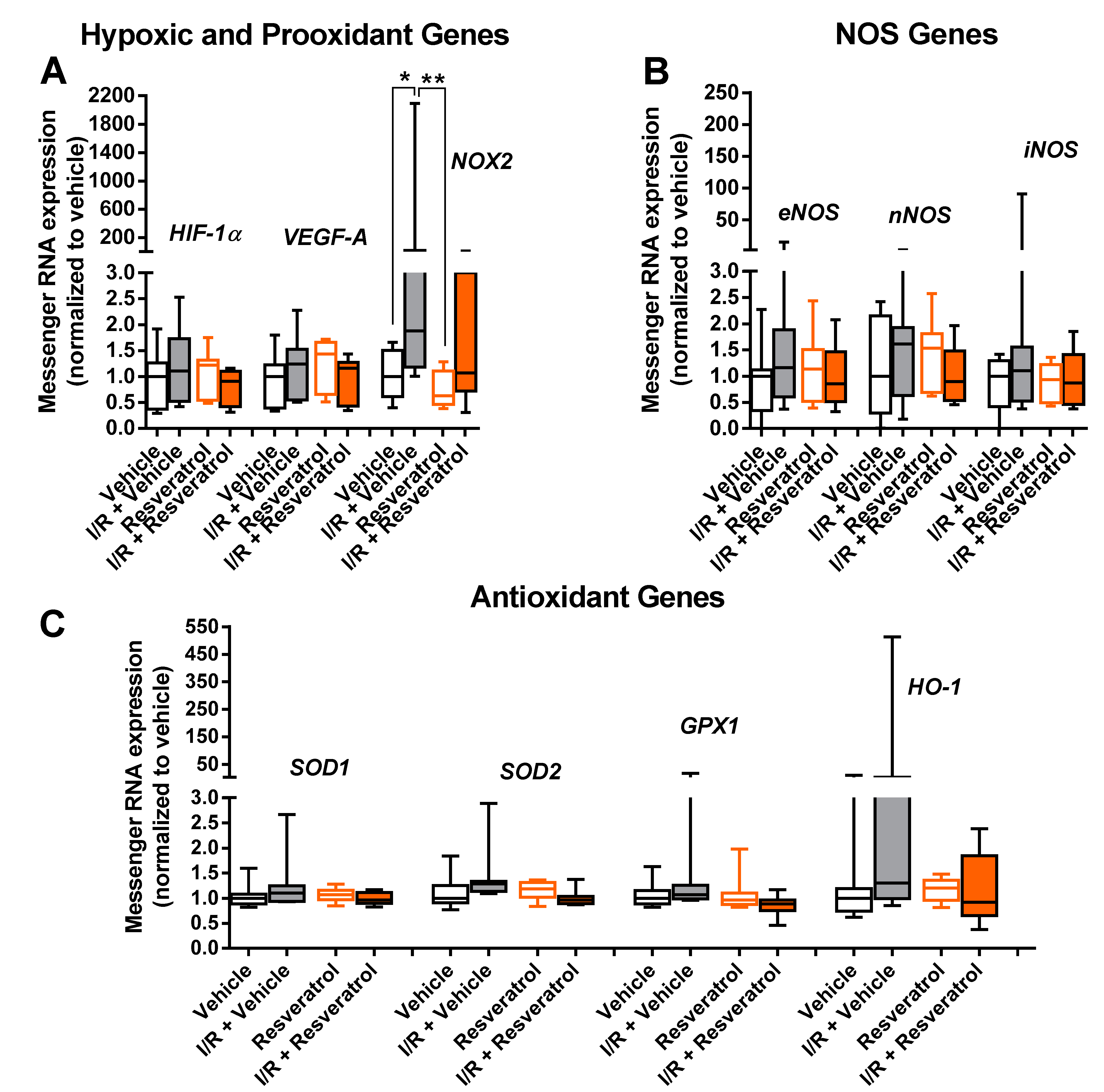
3.5. Messenger RNA Expression
3.6. NOX2 Expression in the Retina
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourne, R.R.; Stevens, G.A.; White, R.A.; Smith, J.L.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; et al. Causes of vision loss worldwide, 1990–2010: A systematic analysis. Lancet Glob. Health 2013, 1, e339–e349. [Google Scholar] [CrossRef]
- Ruan, Y.; Jiang, S.; Musayeva, A.; Gericke, A. Oxidative Stress and Vascular Dysfunction in the Retina: Therapeutic Strategies. Antioxidants 2020, 9, 761. [Google Scholar] [CrossRef]
- Tobalem, S.; Schutz, J.S.; Chronopoulos, A. Central retinal artery occlusion—Rethinking retinal survival time. BMC Ophthalmol. 2018, 18, 101. [Google Scholar] [CrossRef]
- Schumacher, M.; Schmidt, D.; Jurklies, B.; Gall, C.; Wanke, I.; Schmoor, C.; Maier-Lenz, H.; Solymosi, L.; Brueckmann, H.; Neubauer, A.S.; et al. Central retinal artery occlusion: Local intra-arterial fibrinolysis versus conservative treatment, a multicenter randomized trial. Ophthalmology 2010, 117, 1367–1375.e1. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, A.; Markeviciute, A.; Zemaitiene, R. Treatment of Macular Edema in Vascular Retinal Diseases: A 2021 Update. J. Clin. Med. 2021, 10, 5300. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Bata, A.M.; Fondi, K.; Witkowska, K.J.; Werkmeister, R.M.; Hommer, A.; Vass, C.; Resch, H.; Schmidl, D.; Popa-Cherecheanu, A.; Chua, J.; et al. Optic nerve head blood flow regulation during changes in arterial blood pressure in patients with primary open-angle glaucoma. Acta Ophthalmol. 2019, 97, e36–e41. [Google Scholar] [CrossRef]
- Flammer, J.; Orgul, S.; Costa, V.P.; Orzalesi, N.; Krieglstein, G.K.; Serra, L.M.; Renard, J.P.; Stefansson, E. The impact of ocular blood flow in glaucoma. Prog. Retin. Eye Res. 2002, 21, 359–393. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Yu, H.J.; Avery, R.L.; Ehlers, J.P.; Tadayoni, R.; Sadda, S.R. Retinal non-perfusion in diabetic retinopathy. Eye 2022, 36, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Terelak-Borys, B.; Grabska-Liberek, I.; Schoetzau, A.; Konieczka, K. Transient visual field impairment after cold provocation in glaucoma patients with Flammer syndrome. Restor. Neurol. Neurosci. 2019, 37, 31–39. [Google Scholar] [CrossRef]
- Minhas, G.; Sharma, J.; Khan, N. Cellular Stress Response and Immune Signaling in Retinal Ischemia-Reperfusion Injury. Front. Immunol. 2016, 7, 444. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Jo, Y.H.; Jeong, D.; Shon, K.; Kook, M.S. Baseline Systolic versus Diastolic Blood Pressure Dip and Subsequent Visual Field Progression in Normal-Tension Glaucoma. Ophthalmology 2019, 126, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Mingrou, L.; Guo, S.; Ho, C.T.; Bai, N. Review on chemical compositions and biological activities of peanut (Arachis hypogeae L.). J. Food Biochem. 2022, 46, e14119. [Google Scholar] [CrossRef] [PubMed]
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Abbaszadeh, H.A.; Rezaei-Tavirani, M. Resveratrol: A miraculous natural compound for diseases treatment. Food Sci. Nutr. 2018, 6, 2473–2490. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.Y.; Dun, R.L.; Yao, D.S.; Wu, F.; Qian, Y.L.; Zhou, Y.; Zhan, T.T.; Shao, M.H.; Gao, J.D.; Wang, C. Effects of resveratrol on renal ischemia-reperfusion injury: A systematic review and meta-analysis. Front. Nutr. 2022, 9, 1064507. [Google Scholar] [CrossRef]
- Zheng, M.; Bai, Y.; Sun, X.; Fu, R.; Liu, L.; Liu, M.; Li, Z.; Huang, X. Resveratrol Reestablishes Mitochondrial Quality Control in Myocardial Ischemia/Reperfusion Injury through Sirt1/Sirt3-Mfn2-Parkin-PGC-1alpha Pathway. Molecules 2022, 27, 5545. [Google Scholar] [CrossRef]
- Li, H.; Zheng, F.; Zhang, Y.; Sun, J.; Gao, F.; Shi, G. Resveratrol, novel application by preconditioning to attenuate myocardial ischemia/reperfusion injury in mice through regulate AMPK pathway and autophagy level. J. Cell. Mol. Med. 2022, 26, 4216–4229. [Google Scholar] [CrossRef]
- Xue, R.; Gao, S.; Zhang, Y.; Cui, X.; Mo, W.; Xu, J.; Yao, M. A meta-analysis of resveratrol protects against cerebral ischemia/reperfusion injury: Evidence from rats studies and insight into molecular mechanisms. Front. Pharmacol. 2022, 13, 988836. [Google Scholar] [CrossRef]
- Kiziltepe, U.; Turan, N.N.; Han, U.; Ulus, A.T.; Akar, F. Resveratrol, a red wine polyphenol, protects spinal cord from ischemia-reperfusion injury. J. Vasc. Surg. 2004, 40, 138–145. [Google Scholar] [CrossRef]
- Kaplan, S.; Bisleri, G.; Morgan, J.A.; Cheema, F.H.; Oz, M.C. Resveratrol, a natural red wine polyphenol, reduces ischemia-reperfusion-induced spinal cord injury. Ann. Thorac. Surg. 2005, 80, 2242–2249. [Google Scholar] [CrossRef]
- King, R.E.; Kent, K.D.; Bomser, J.A. Resveratrol reduces oxidation and proliferation of human retinal pigment epithelial cells via extracellular signal-regulated kinase inhibition. Chem. Biol. Interact. 2005, 151, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Guerin, K.I.; Chen, J.; Michan, S.; Stahl, A.; Krah, N.M.; Seaward, M.R.; Dennison, R.J.; Juan, A.M.; Hatton, C.J.; et al. Resveratrol inhibits pathologic retinal neovascularization in Vldlr(-/-) mice. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2809–2816. [Google Scholar] [CrossRef]
- Ghadiri Soufi, F.; Arbabi-Aval, E.; Rezaei Kanavi, M.; Ahmadieh, H. Anti-inflammatory properties of resveratrol in the retinas of type 2 diabetic rats. Clin. Exp. Pharmacol. Physiol. 2015, 42, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Wang, Y.; Huang, L.; Song, Y.; Yu, X.; Deng, B.; Zhou, X. Resveratrol inhibits neural apoptosis and regulates RAX/P-PKR expression in retina of diabetic rats. Nutr. Neurosci. 2022, 25, 2560–2569. [Google Scholar] [CrossRef] [PubMed]
- Vin, A.P.; Hu, H.; Zhai, Y.; Von Zee, C.L.; Logeman, A.; Stubbs, E.B., Jr.; Perlman, J.I.; Bu, P. Neuroprotective effect of resveratrol prophylaxis on experimental retinal ischemic injury. Exp. Eye Res. 2013, 108, 72–75. [Google Scholar] [CrossRef]
- Liu, X.Q.; Wu, B.J.; Pan, W.H.; Zhang, X.M.; Liu, J.H.; Chen, M.M.; Chao, F.P.; Chao, H.M. Resveratrol mitigates rat retinal ischemic injury: The roles of matrix metalloproteinase-9, inducible nitric oxide, and heme oxygenase-1. J. Ocul. Pharmacol. Ther. 2013, 29, 33–40. [Google Scholar] [CrossRef]
- Ji, K.; Li, Z.; Lei, Y.; Xu, W.; Ouyang, L.; He, T.; Xing, Y. Resveratrol attenuates retinal ganglion cell loss in a mouse model of retinal ischemia reperfusion injury via multiple pathways. Exp. Eye Res. 2021, 209, 108683. [Google Scholar] [CrossRef]
- Zadeh, J.K.; Garcia-Bardon, A.; Hartmann, E.K.; Pfeiffer, N.; Omran, W.; Ludwig, M.; Patzak, A.; Xia, N.; Li, H.; Gericke, A. Short-Time Ocular Ischemia Induces Vascular Endothelial Dysfunction and Ganglion Cell Loss in the Pig Retina. Int. J. Mol. Sci. 2019, 20, 4685. [Google Scholar] [CrossRef]
- Musayeva, A.; Unkrig, J.C.; Zhutdieva, M.B.; Manicam, C.; Ruan, Y.; Laspas, P.; Chronopoulos, P.; Gobel, M.L.; Pfeiffer, N.; Brochhausen, C.; et al. Betulinic Acid Protects from Ischemia-Reperfusion Injury in the Mouse Retina. Cells 2021, 10, 2440. [Google Scholar] [CrossRef]
- Sehirli, O.; Tozan, A.; Omurtag, G.Z.; Cetinel, S.; Contuk, G.; Gedik, N.; Sener, G. Protective effect of resveratrol against naphthalene-induced oxidative stress in mice. Ecotoxicol. Environ. Saf. 2008, 71, 301–308. [Google Scholar] [CrossRef]
- Xia, N.; Daiber, A.; Habermeier, A.; Closs, E.I.; Thum, T.; Spanier, G.; Lu, Q.; Oelze, M.; Torzewski, M.; Lackner, K.J.; et al. Resveratrol reverses endothelial nitric-oxide synthase uncoupling in apolipoprotein E knockout mice. J. Pharmacol. Exp. Ther. 2010, 335, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Anzai, T.; Morisawa, M.; Kohno, T.; Nagai, T.; Anzai, A.; Takahashi, T.; Shimoda, M.; Sasaki, A.; Maekawa, Y.; et al. Resveratrol prevents the development of abdominal aortic aneurysm through attenuation of inflammation, oxidative stress, and neovascularization. Atherosclerosis 2011, 217, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; McNeill, J.H. To scale or not to scale: The principles of dose extrapolation. Br. J. Pharmacol. 2009, 157, 907–921. [Google Scholar] [CrossRef]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef]
- Laspas, P.; Sniatecki, J.J.; Brochhausen, C.; Steege, A.; Goloborodko, E.; Kordasz, M.L.; Grus, F.H.; Pfeiffer, N.; Gericke, A. Effect of the M1 Muscarinic Acetylcholine Receptor on Retinal Neuron Number Studied with Gene-Targeted Mice. J. Mol. Neurosci. 2015, 56, 472–479. [Google Scholar] [CrossRef]
- Gericke, A.; Goloborodko, E.; Pfeiffer, N.; Manicam, C. Preparation Steps for Measurement of Reactivity in Mouse Retinal Arterioles Ex Vivo. J. Vis. Exp. 2018, 135, 56199. [Google Scholar] [CrossRef]
- Bohm, E.W.; Pfeiffer, N.; Wagner, F.M.; Gericke, A. Methods to measure blood flow and vascular reactivity in the retina. Front. Med. 2022, 9, 1069449. [Google Scholar] [CrossRef]
- Gericke, A.; Sniatecki, J.J.; Goloborodko, E.; Steege, A.; Zavaritskaya, O.; Vetter, J.M.; Grus, F.H.; Patzak, A.; Wess, J.; Pfeiffer, N. Identification of the muscarinic acetylcholine receptor subtype mediating cholinergic vasodilation in murine retinal arterioles. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7479–7484. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, J.K.; Zhutdieva, M.B.; Laspas, P.; Yuksel, C.; Musayeva, A.; Pfeiffer, N.; Brochhausen, C.; Oelze, M.; Daiber, A.; Xia, N.; et al. Apolipoprotein E Deficiency Causes Endothelial Dysfunction in the Mouse Retina. Oxid. Med. Cell. Longev. 2019, 2019, 5181429. [Google Scholar] [CrossRef] [PubMed]
- Musayeva, A.; Jiang, S.; Ruan, Y.; Zadeh, J.K.; Chronopoulos, P.; Pfeiffer, N.; Muller, W.E.G.; Ackermann, M.; Xia, N.; Li, H.; et al. Aged Mice Devoid of the M(3) Muscarinic Acetylcholine Receptor Develop Mild Dry Eye Disease. Int. J. Mol. Sci. 2021, 22, 6133. [Google Scholar] [CrossRef] [PubMed]
- Drager, U.C.; Olsen, J.F. Ganglion cell distribution in the retina of the mouse. Investig. Ophthalmol. Vis. Sci. 1981, 20, 285–293. [Google Scholar]
- Schlamp, C.L.; Montgomery, A.D.; Mac Nair, C.E.; Schuart, C.; Willmer, D.J.; Nickells, R.W. Evaluation of the percentage of ganglion cells in the ganglion cell layer of the rodent retina. Mol. Vis. 2013, 19, 1387–1396. [Google Scholar]
- Crow, J.P.; Beckman, J.S. Reactions between nitric oxide, superoxide, and peroxynitrite: Footprints of peroxynitrite in vivo. Adv. Pharmacol. 1995, 34, 17–43. [Google Scholar] [CrossRef] [PubMed]
- Seong, H.; Jeong, J.Y.; Ryu, J.; Park, J.; Han, Y.S.; Cho, H.K.; Kim, S.J.; Park, J.M.; Kang, S.S.; Seo, S.W. Resveratrol prevents hypoxia-induced retinal ganglion cell death related with ErbB2. Int. J. Ophthalmol. 2022, 15, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Seong, H.; Ryu, J.; Yoo, W.S.; Kim, S.J.; Han, Y.S.; Park, J.M.; Kang, S.S.; Seo, S.W. Resveratrol Ameliorates Retinal Ischemia/Reperfusion Injury in C57BL/6J Mice via Downregulation of Caspase-3. Curr. Eye Res. 2017, 42, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Kant, S.; Sellke, F.; Feng, J. Metabolic regulation and dysregulation of endothelial small conductance calcium activated potassium channels. Eur. J. Cell Biol. 2022, 101, 151208. [Google Scholar] [CrossRef] [PubMed]
- Dal-Ros, S.; Oswald-Mammosser, M.; Pestrikova, T.; Schott, C.; Boehm, N.; Bronner, C.; Chataigneau, T.; Geny, B.; Schini-Kerth, V.B. Losartan prevents portal hypertension-induced, redox-mediated endothelial dysfunction in the mesenteric artery in rats. Gastroenterology 2010, 138, 1574–1584. [Google Scholar] [CrossRef]
- Laspas, P.; Goloborodko, E.; Sniatecki, J.J.; Kordasz, M.L.; Manicam, C.; Wojnowski, L.; Li, H.; Patzak, A.; Pfeiffer, N.; Gericke, A. Role of nitric oxide synthase isoforms for ophthalmic artery reactivity in mice. Exp. Eye Res. 2014, 127, 1–8. [Google Scholar] [CrossRef]
- Manicam, C.; Staubitz, J.; Brochhausen, C.; Grus, F.H.; Pfeiffer, N.; Gericke, A. The Gatekeepers in the Mouse Ophthalmic Artery: Endothelium-Dependent Mechanisms of Cholinergic Vasodilation. Sci. Rep. 2016, 6, 20322. [Google Scholar] [CrossRef]
- Manicam, C.; Ginter, N.; Li, H.; Xia, N.; Goloborodko, E.; Zadeh, J.K.; Musayeva, A.; Pfeiffer, N.; Gericke, A. Compensatory Vasodilator Mechanisms in the Ophthalmic Artery of Endothelial Nitric Oxide Synthase Gene Knockout Mice. Sci. Rep. 2017, 7, 7111. [Google Scholar] [CrossRef] [PubMed]
- Birk, M.; Baum, E.; Zadeh, J.K.; Manicam, C.; Pfeiffer, N.; Patzak, A.; Helmstadter, J.; Steven, S.; Kuntic, M.; Daiber, A.; et al. Angiotensin II Induces Oxidative Stress and Endothelial Dysfunction in Mouse Ophthalmic Arteries via Involvement of AT1 Receptors and NOX2. Antioxidants 2021, 10, 1238. [Google Scholar] [CrossRef]
- Gericke, A.; Goloborodko, E.; Sniatecki, J.J.; Steege, A.; Wojnowski, L.; Pfeiffer, N. Contribution of nitric oxide synthase isoforms to cholinergic vasodilation in murine retinal arterioles. Exp. Eye Res. 2013, 109, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Gericke, A.; Wolff, I.; Musayeva, A.; Zadeh, J.K.; Manicam, C.; Pfeiffer, N.; Li, H.; Xia, N. Retinal arteriole reactivity in mice lacking the endothelial nitric oxide synthase (eNOS) gene. Exp. Eye Res. 2019, 181, 150–156. [Google Scholar] [CrossRef]
- Knott, A.B.; Bossy-Wetzel, E. Nitric oxide in health and disease of the nervous system. Antioxid. Redox Signal. 2009, 11, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I.; Lushchak, O. Interplay between reactive oxygen and nitrogen species in living organisms. Chem. Biol. Interact. 2021, 349, 109680. [Google Scholar] [CrossRef] [PubMed]
- Canto, A.; Olivar, T.; Romero, F.J.; Miranda, M. Nitrosative Stress in Retinal Pathologies: Review. Antioxidants 2019, 8, 543. [Google Scholar] [CrossRef]
- Ischiropoulos, H.; Zhu, L.; Chen, J.; Tsai, M.; Martin, J.C.; Smith, C.D.; Beckman, J.S. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch. Biochem. Biophys. 1992, 298, 431–437. [Google Scholar] [CrossRef]
- Beckman, J.S. Oxidative damage and tyrosine nitration from peroxynitrite. Chem. Res. Toxicol. 1996, 9, 836–844. [Google Scholar] [CrossRef]
- Lei, Y.; Gao, Y.; Song, M.; Cao, W.; Sun, X. Peroxynitrite is a novel risk factor and treatment target of glaucoma. Nitric Oxide 2020, 99, 17–24. [Google Scholar] [CrossRef]
- Gacar, G.; Gocmez, S.S.; Halbutogullari, Z.S.; Kilic, K.C.; Kaya, A.; Yazir, Y.; Utkan, T. Resveratrol improves vascular endothelial dysfunction in the unpredictable chronic mild stress model of depression in rats by reducing inflammation. Behav. Brain Res. 2023, 438, 114186. [Google Scholar] [CrossRef]
- Wiedenhoeft, T.; Tarantini, S.; Nyul-Toth, A.; Yabluchanskiy, A.; Csipo, T.; Balasubramanian, P.; Lipecz, A.; Kiss, T.; Csiszar, A.; Csiszar, A.; et al. Fusogenic liposomes effectively deliver resveratrol to the cerebral microcirculation and improve endothelium-dependent neurovascular coupling responses in aged mice. Geroscience 2019, 41, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Gocmez, S.S.; Scarpace, P.J.; Whidden, M.A.; Erdos, B.; Kirichenko, N.; Sakarya, Y.; Utkan, T.; Tumer, N. Age Impaired endothelium-dependent vasodilation is improved by resveratrol in rat mesenteric arteries. J. Exerc. Nutr. Biochem. 2016, 20, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Wallerath, T.; Deckert, G.; Ternes, T.; Anderson, H.; Li, H.; Witte, K.; Forstermann, U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 2002, 106, 1652–1658. [Google Scholar] [CrossRef] [PubMed]
- Wallerath, T.; Poleo, D.; Li, H.; Forstermann, U. Red wine increases the expression of human endothelial nitric oxide synthase: A mechanism that may contribute to its beneficial cardiovascular effects. J. Am. Coll. Cardiol. 2003, 41, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Wallerath, T.; Li, H.; Godtel-Ambrust, U.; Schwarz, P.M.; Forstermann, U. A blend of polyphenolic compounds explains the stimulatory effect of red wine on human endothelial NO synthase. Nitric Oxide 2005, 12, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M.; Wickramasinghe, N.S.; Ivanova, M.M.; Dougherty, S.M. Resveratrol stimulates nitric oxide production by increasing estrogen receptor alpha-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J. 2008, 22, 2185–2197. [Google Scholar] [CrossRef]
- Mattagajasingh, I.; Kim, C.S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.B.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [CrossRef]
- Arunachalam, G.; Yao, H.; Sundar, I.K.; Caito, S.; Rahman, I. SIRT1 regulates oxidant- and cigarette smoke-induced eNOS acetylation in endothelial cells: Role of resveratrol. Biochem. Biophys. Res. Commun. 2010, 393, 66–72. [Google Scholar] [CrossRef]
- Penumathsa, S.V.; Koneru, S.; Samuel, S.M.; Maulik, G.; Bagchi, D.; Yet, S.F.; Menon, V.P.; Maulik, N. Strategic targets to induce neovascularization by resveratrol in hypercholesterolemic rat myocardium: Role of caveolin-1, endothelial nitric oxide synthase, hemeoxygenase-1, and vascular endothelial growth factor. Free Radic. Biol. Med. 2008, 45, 1027–1034. [Google Scholar] [CrossRef]
- Penumathsa, S.V.; Thirunavukkarasu, M.; Zhan, L.; Maulik, G.; Menon, V.P.; Bagchi, D.; Maulik, N. Resveratrol enhances GLUT-4 translocation to the caveolar lipid raft fractions through AMPK/Akt/eNOS signalling pathway in diabetic myocardium. J. Cell. Mol. Med. 2008, 12, 2350–2361. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, R.; Ye, X.; Zhang, C.; Jin, X.; Yamori, Y.; Hao, L.; Sun, X.; Ying, C. Resveratrol ameliorates high-glucose-induced hyperpermeability mediated by caveolae via VEGF/KDR pathway. Genes Nutr. 2013, 8, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Forstermann, U. Uncoupling of endothelial NO synthase in atherosclerosis and vascular disease. Curr. Opin. Pharmacol. 2013, 13, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xia, N.; Hasselwander, S.; Daiber, A. Resveratrol and Vascular Function. Int. J. Mol. Sci. 2019, 20, 2155. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Bagi, Z.; Feher, A.; Recchia, F.A.; Sonntag, W.E.; Pearson, K.; de Cabo, R.; Csiszar, A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H18–H24. [Google Scholar] [CrossRef]
- Ungvari, Z.; Orosz, Z.; Rivera, A.; Labinskyy, N.; Xiangmin, Z.; Olson, S.; Podlutsky, A.; Csiszar, A. Resveratrol increases vascular oxidative stress resistance. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2417–H2424. [Google Scholar] [CrossRef]
- Spanier, G.; Xu, H.; Xia, N.; Tobias, S.; Deng, S.; Wojnowski, L.; Forstermann, U.; Li, H. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4). J. Physiol. Pharmacol. 2009, 60 (Suppl. 4), 111–116. [Google Scholar]
- Liu, J.C.; Chen, J.J.; Chan, P.; Cheng, C.F.; Cheng, T.H. Inhibition of cyclic strain-induced endothelin-1 gene expression by resveratrol. Hypertension 2003, 42, 1198–1205. [Google Scholar] [CrossRef]
- Ungvari, Z.; Labinskyy, N.; Mukhopadhyay, P.; Pinto, J.T.; Bagi, Z.; Ballabh, P.; Zhang, C.; Pacher, P.; Csiszar, A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1876–H1881. [Google Scholar] [CrossRef]
- Zadeh, J.K.; Ruemmler, R.; Hartmann, E.K.; Ziebart, A.; Ludwig, M.; Patzak, A.; Xia, N.; Li, H.; Pfeiffer, N.; Gericke, A. Responses of retinal arterioles and ciliary arteries in pigs with acute respiratory distress syndrome (ARDS). Exp. Eye Res. 2019, 184, 152–161. [Google Scholar] [CrossRef]
- Yokota, H.; Narayanan, S.P.; Zhang, W.; Liu, H.; Rojas, M.; Xu, Z.; Lemtalsi, T.; Nagaoka, T.; Yoshida, A.; Brooks, S.E.; et al. Neuroprotection from retinal ischemia/reperfusion injury by NOX2 NADPH oxidase deletion. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8123–8131. [Google Scholar] [CrossRef]
- Gericke, A.; Mann, C.; Zadeh, J.K.; Musayeva, A.; Wolff, I.; Wang, M.; Pfeiffer, N.; Daiber, A.; Li, H.; Xia, N.; et al. Elevated Intraocular Pressure Causes Abnormal Reactivity of Mouse Retinal Arterioles. Oxid. Med. Cell. Longev. 2019, 2019, 9736047. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, H.; Xia, N.; Li, H.; van Beers, T.; Gericke, A.; Prokosch, V. Intraocular Pressure-Induced Endothelial Dysfunction of Retinal Blood Vessels Is Persistent, but Does Not Trigger Retinal Ganglion Cell Loss. Antioxidants 2022, 11, 1864. [Google Scholar] [CrossRef]
- Rojas, M.; Lemtalsi, T.; Toque, H.A.; Xu, Z.; Fulton, D.; Caldwell, R.W.; Caldwell, R.B. NOX2-Induced Activation of Arginase and Diabetes-Induced Retinal Endothelial Cell Senescence. Antioxidants 2017, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Kindelin, A.; Mahady, L.; Bhatia, K.; Hoda, M.N.; Ducruet, A.F.; Ahmad, S. Remote Ischemic Post-Conditioning Therapy is Protective in Mouse Model of Traumatic Optic Neuropathy. Neuromolecular. Med. 2021, 23, 371–382. [Google Scholar] [CrossRef]
- Laspas, P.; Zhutdieva, M.B.; Brochhausen, C.; Musayeva, A.; Zadeh, J.K.; Pfeiffer, N.; Xia, N.; Li, H.; Wess, J.; Gericke, A. The M1 muscarinic acetylcholine receptor subtype is important for retinal neuron survival in aging mice. Sci. Rep. 2019, 9, 5222. [Google Scholar] [CrossRef]
- Toth, P.; Tarantini, S.; Tucsek, Z.; Ashpole, N.M.; Sosnowska, D.; Gautam, T.; Ballabh, P.; Koller, A.; Sonntag, W.E.; Csiszar, A.; et al. Resveratrol treatment rescues neurovascular coupling in aged mice: Role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H299–H308. [Google Scholar] [CrossRef]
- Zhao, X.; Hui, Q.C.; Xu, R.; Gao, N.; Cao, P. Resveratrol: A new approach to ameliorate hyperhomocysteinaemia-induced renal dysfunction. Exp. Ther. Med. 2022, 24, 510. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.; Silverman, S.M.; Liu, Y.; Wang, X.; Kim, B.J.; Tang, L.; Clark, A.F.; Liu, X.; Pang, I.H. Rapid repeatable in vivo detection of retinal reactive oxygen species. Exp. Eye Res. 2017, 161, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Milic, M.; Gericke, A.; Mercieca, K.; Liu, H.; Ruan, Y.; Jiang, S.; van Beers, T.; von Pein, H.D.; Muller, M.B.; et al. Chronic social defeat stress causes retinal vascular dysfunction. Exp. Eye Res. 2021, 213, 108853. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Z.; Yang, S.; Yin, T.; Zhang, Y.; Qin, Y.; Weinreb, R.N.; Sun, X. Tissue Distribution of trans-Resveratrol and Its Metabolites after Oral Administration in Human Eyes. J. Ophthalmol. 2017, 2017, 4052094. [Google Scholar] [CrossRef]
- Popescu, M.; Bogdan, C.; Pintea, A.; Rugina, D.; Ionescu, C. Antiangiogenic cytokines as potential new therapeutic targets for resveratrol in diabetic retinopathy. Drug Des. Devel. Ther. 2018, 12, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
| Gene | Accession Number | Forward | Reverse |
|---|---|---|---|
| HIF-1α | NM_010431 | TCATCAGTTGCCACTTCCCCAC | CCGTCATCTGTTAGCACCATCAC |
| VEGF-A | NM_001025250.3 | ACTTGTGTTGGGAGGAGGATGTC | AATGGGTTTGTCGTGTTTCTGG |
| NOX2 | NM_007807.2 | CCAACTGGGATAACGAGTTCA | GAGAGTTTCAGCCAAGGCTTC |
| eNOS | NM_008713 | CCTTCCGCTACCAGCCAGA | CAGAGATCTTCACTGCATTGGCTA |
| iNOS | NM_010927 | CAGCTGGGCTGTACAAACCTT | CATTGGAAGTGAAGCGTTTCG |
| nNOS | NM_008712 | TCCACCTGCCTCGAAACC | TTGTCGCTGTTGCCAAAAAC |
| GPX1 | NM_008160 | CCCGTGCGCAGGTACAG | GGGACAGCAGGGTTTCTATGTC |
| HO-1 | NM_010442 | GGTGATGCTGACAGAGGAACAC | TAGCAGGCCTCTGACGAAGTG |
| SOD1 | NM_011434.1 | CCAGTGCAGGACCTCATTTTAAT | TCTCCAACATGCCTCTCTTCATC |
| SOD2 | NM_013671 | CCTGCTCTAATCAGGACCCATT | CGTGCTCCCACACGTCAAT |
| TBP | NM_013684 | CTTCGTGCAAGAAATGCTGAAT | CAGTTGTCCGTGGCTCTCTTATT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chronopoulos, P.; Manicam, C.; Zadeh, J.K.; Laspas, P.; Unkrig, J.C.; Göbel, M.L.; Musayeva, A.; Pfeiffer, N.; Oelze, M.; Daiber, A.; et al. Effects of Resveratrol on Vascular Function in Retinal Ischemia-Reperfusion Injury. Antioxidants 2023, 12, 853. https://doi.org/10.3390/antiox12040853
Chronopoulos P, Manicam C, Zadeh JK, Laspas P, Unkrig JC, Göbel ML, Musayeva A, Pfeiffer N, Oelze M, Daiber A, et al. Effects of Resveratrol on Vascular Function in Retinal Ischemia-Reperfusion Injury. Antioxidants. 2023; 12(4):853. https://doi.org/10.3390/antiox12040853
Chicago/Turabian StyleChronopoulos, Panagiotis, Caroline Manicam, Jenia Kouchek Zadeh, Panagiotis Laspas, Johanna Charlotte Unkrig, Marie Luise Göbel, Aytan Musayeva, Norbert Pfeiffer, Matthias Oelze, Andreas Daiber, and et al. 2023. "Effects of Resveratrol on Vascular Function in Retinal Ischemia-Reperfusion Injury" Antioxidants 12, no. 4: 853. https://doi.org/10.3390/antiox12040853
APA StyleChronopoulos, P., Manicam, C., Zadeh, J. K., Laspas, P., Unkrig, J. C., Göbel, M. L., Musayeva, A., Pfeiffer, N., Oelze, M., Daiber, A., Li, H., Xia, N., & Gericke, A. (2023). Effects of Resveratrol on Vascular Function in Retinal Ischemia-Reperfusion Injury. Antioxidants, 12(4), 853. https://doi.org/10.3390/antiox12040853








