Abstract
Brain ageing is a complex physiological process that includes several mechanisms. It is characterized by neuronal/glial dysfunction, alterations in brain vasculature and barriers, and the decline in brain repair systems. These disorders are triggered by an increase in oxidative stress and a proinflammatory state, without adequate antioxidant and anti-inflammatory systems, as it occurs in young life stages. This state is known as inflammaging. Gut microbiota and the gut–brain axis (GBA) have been associated with brain function, in a bidirectional communication that can cause loss or gain of the brain’s functionality. There are also intrinsic and extrinsic factors with the ability to modulate this connection. Among the extrinsic factors, the components of diet, principally natural components such as polyphenols, are the most reported. The beneficial effects of polyphenols in brain ageing have been described, mainly due to their antioxidants and anti-inflammatory properties, including the modulation of gut microbiota and the GBA. The aim of this review was, by following the canonical methodology for a state-of-the-art review, to compose the existing evidenced picture of the impact of the gut microbiota on ageing and their modulation by polyphenols as beneficial molecules against brain ageing.
1. Introduction
Due to the globally existing demographic context characterized by the increase in life expectancy and the large proportion of elderly people worldwide, one of the current great challenges in science is the search for strategies to prevent ageing [1]. In this context, within ageing, brain ageing is one of the most studied phenomenon, due to its medical, social, and economic impacts [2]. Anti-ageing strategies can be multiple, ranging from the most interventionists with pharmacological or genetic techniques, to the less interventionists that use the modification of lifestyle [3]. In this sense, diet is being increasingly studied because its benefits have been known since ancient times, because it is an easily modifiable element with a direct effect on general physiology [4,5].
Brain ageing is a complex physiological process that includes different mechanisms, some clearly described but others under study, which makes the final integration of all of them a complex scientific challenge. Among the main known causes of brain ageing and neurodegeneration are the limited renewal capacity of neural cells, neuronal/glial dysfunction [6], alterations of the brain vasculature and the blood brain barrier (BBB) [7,8], and loss of plasticity [9,10,11,12,13], accompanied by an age-dependent decline in the brain repair systems, including adult neurogenesis [14]. The causes of this damage are related to an increase in oxidative stress levels [15], together with a proinflammatory state [16,17], without the appropriate antioxidant and anti-inflammatory responses. In gerontology, this condition is defined as inflammaging [16]. The control of this state contributes to the prevention of ageing and to the repair of the brain after damage, playing a key role in complex brain functions, such as cognition (e.g., memory [18,19,20,21,22], learning [23]), mood [24,25], or sensorial ability (e.g., olfactory [26,27]).
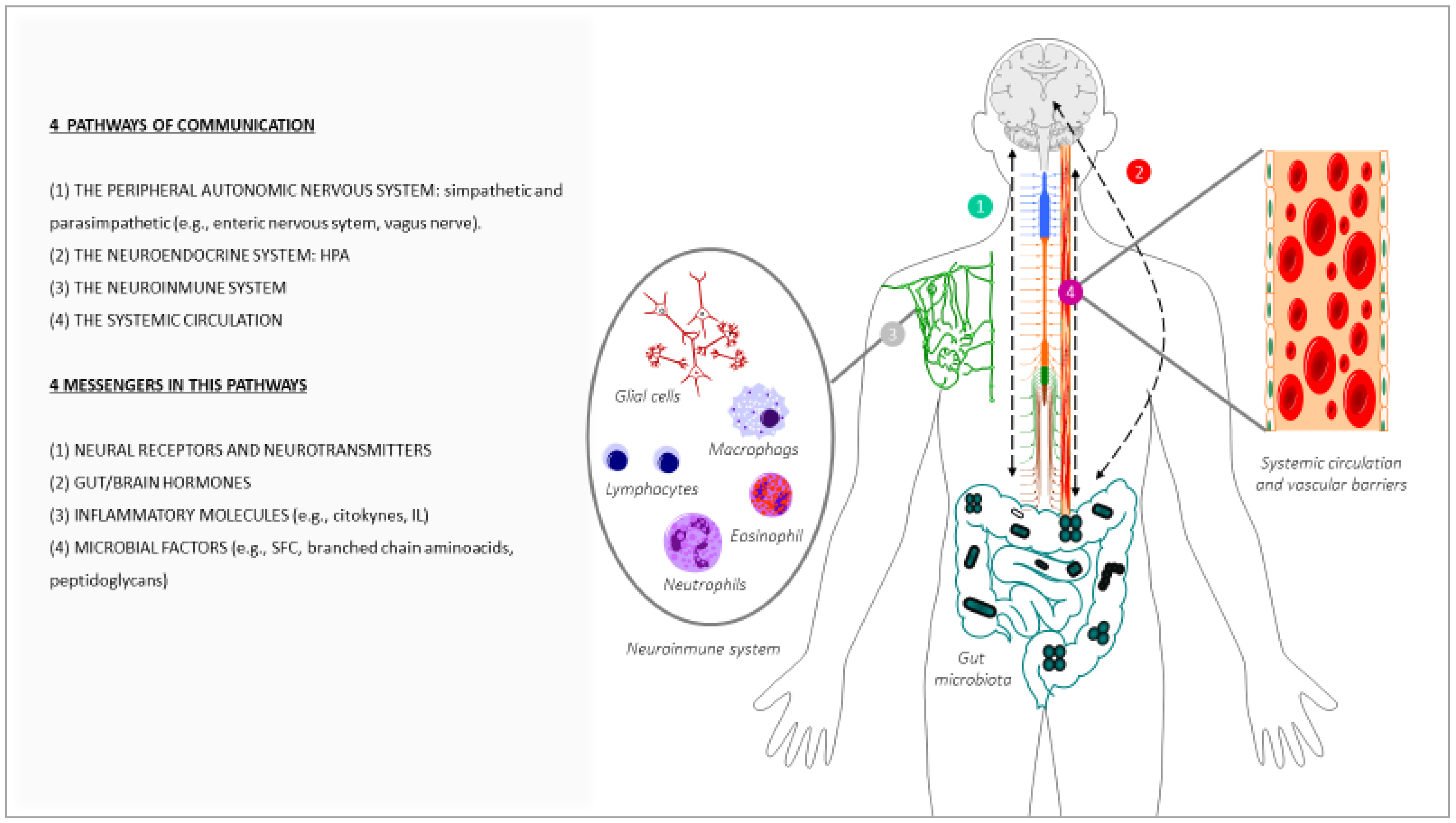
In order to address brain ageing, there are different therapeutic strategies, one of which is to consider the modifications of the gut–brain axis (GBA) as therapeutic mediators [28]. During the 1960s and 1970s, several peptides were found in both the gastrointestinal tract and the brain; subsequently, the concept of amine precursor uptake and decarboxylation hypothesis (APUD) was developed. Over time, this evolved into the concept of the GBA, based on the predominant concept of a bidirectional communication between the gut and the brain [29,30], in health and in disease conditions [31,32]. In this bidirectional communication of the GBA, four pathways of communication from the brain to the gut are involved: the peripheral autonomic nervous system (sympathetic and parasympathetic) (e.g., enteric nervous system or the vagus nerve [33]), the neuroendocrine outputs axis (e.g., hypothalamic–pituitary–adrenal axis (HPA)), the neuroimmune systems (immune cells and glia [34]), and the systemic circulation [34]. There are four main groups of messengers participating in this communication, with the ability to modify the cerebral function and behaviour, these being microbial factors (e.g., short-chain fatty acids (SCFAs), branched chain amino acids and peptidoglycans [35]), gut hormones, cytokines, and sensory neurons (Figure 1). Among the microbial factors, the gut microbiota composition is notably, and this comprises several species of microorganisms, including bacteria, yeast, and viruses [36], that live in a delicate symbiosis. The disruption of this relation, known as dysbiosis and common in ageing, can lead to aberrant neural and glial reactivity, accompanied by the loss of brain functionality, observed at a cognitive level [37]. Thus, a functional relationship links the gut microbiota and GBA with brain functions [34]. Therefore, alterations in this axis not only affect the neural regulation of the gastrointestinal tract, but also several brain functions with the onset of neurological symptomatology, for instance in mood (e.g., depression, anxiety), in neurodevelopment (e.g., autism) [38,39], and in cognition (e.g., Alzheimer’s disease) [40,41,42]. It is important to note that imbalances affecting this complex ecosystem can impact the permeability of the body barriers, including the BBB and the enteric barrier [43].
Figure 1.
Main messengers and pathways of communication involved in the crosstalk between the gut and the brain.
Many factors can influence the microbiota composition and their functionality, both intrinsic to the organism itself and to external ones. Among the intrinsic factors, genetics and the ageing process significantly affect the composition of the microbiome [44,45]. External factors include infectious processes, drug administration (including antibiotics), environmental stressors, or factors related to lifestyle, especially diet. In this context, dietary polyphenols are pointed out as ductile molecules with the capacity to counteract the impact of ageing in the brain, due to their natural antioxidant and anti-inflammatory properties [46,47], both directly in the brain due to its ability to cross the BBB, and indirectly through modulation of the microbiome and GBA [48,49,50].
The aim of this review is to assess the known data related to the connection between the gut microbiota and GBA and brain ageing, with special attention on the impact that polyphenols may have on it. To reach this aim, a canonical methodology for a state-of-the-art review was followed [51]. A list of keywords (gut microbiota; gut–brain axis; brain; ageing; neurodegeneration; polyphenols; inflammation; nutrients; antioxidants; anti-ageing strategies) was initially identified. Then, different keyword combinations, each containing the term “ageing”, were used to search the following sources: PubMed, Embase, Medline, Scopus, Web of Knowledge, and Google Scholar. Articles published in English and indexed as original articles, meta-analysis reviews, narrative reviews, clinical cases, and comment to editor, with qualitative and quantitative data, were included in the analysis. Although the time range was not limited, the most recent publications were prioritized.
2. Evidence of the Impact of the Gut Microbiota in Brain Ageing
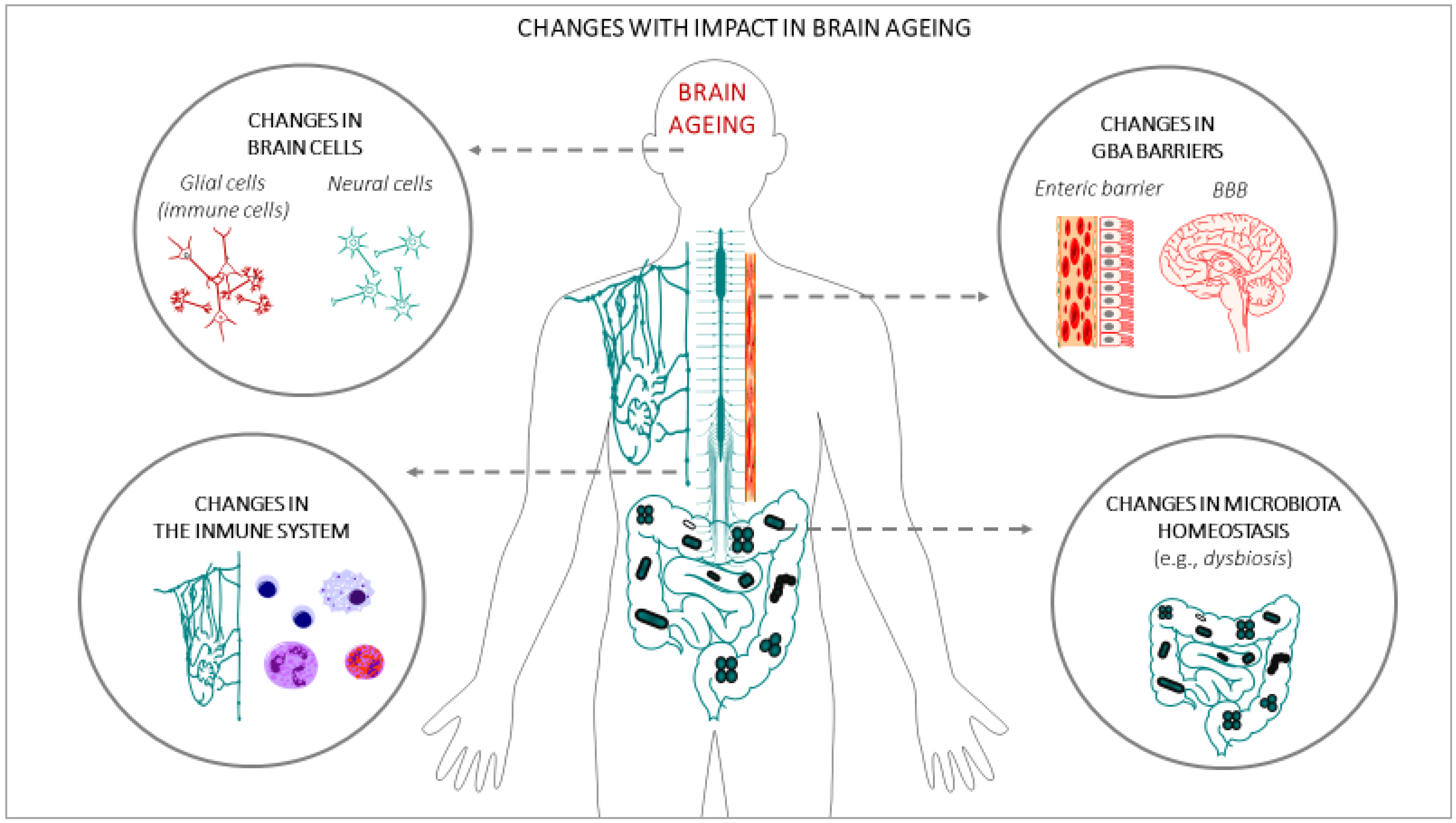
In the last few decades, gut microbiota have emerged as one of the key regulators of brain function, gaining attention for their role in brain health, brain ageing, and neurodegenerative disorders [52]. It has also been demonstrated that a crosstalk exits between gut microbiota and the ageing process [53]. A large portion of the experimental data that have demonstrated the impact of gut microbiota on brain ageing and neurodegenerative disorders are based on animal studies: germ-free (GF) animal data, infection or antibiotic drug use data, and microbiota transfer studies, but less observations from human studies can be found. Altogether, these studies support the hypothesis of a key role of the gut microbiota in the ageing process [54], and in central nervous system (CNS) disorders [55]. One of the main gut changes that has an impact on ageing is the alteration of the gut microbiota composition, accompanied by alteration of the enteric barrier. In this case, the presence of harmful microorganisms or substances could also be observed, accompanied by a loss of the beneficial ones in the microbioma environment. The main interactions between the changes in the gut microbiota and brain ageing are summarized in Figure 2.
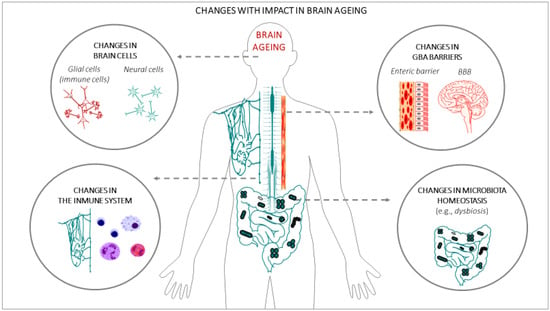
Figure 2.
Changes in body systems with an impact on brain ageing.
(a) Alterations of the gut microbiota composition with an impact on brain ageing: Throughout life, many factors can influence the composition of the gut microbiota [56], such as the type of delivery at birth [57], genetics [58], infectious processes, antibiotic drug use, lifestyle, diet, stress factors, and the development of the ageing process [44,59]. It has been demonstrated that certain age-related changes in the composition of the gut microbiota are associated with many health conditions, including increased frailty, cognitive impairment, or depression, both in humans [44,60,61,62] and in animals [62,63]. During ageing, the gut can be dominated by noxious microorganisms, producers of metabolites with adverse effects, a phenomenon known as dysbiosis. These changes, related to the microbiome, have been described in many studies and have been attributed to an acceleration of the ageing process, in turn, generating impairment in different organs of the human body [54], including the brain [64]. An example of these alterations can be seen in the fact that some specific microbial taxa, such as the Porphyromonadaceae family, have been related to the appearance of cognitive and affective disorders [65,66,67], despite large individual variations. On the contrary, others microorganisms are associated with a reduction of frailty in older populations, such as the Bacteroidetes family [68,69,70], or the species Clostridium cluster XIVa and Faecalibacterium prausnitzii [68,70,71]. Furthermore, it has been proposed that age-associated characteristic inflammatory status can be, at least in part, promoted by age-associated dysbiosis [72,73]. As age advances, a greater proinflammatory state and a lower adaptive immune response are progressively expressed [74,75,76,77,78], which contributes to accelerating the aging process and increasing susceptibility to developing age-associated chronic diseases, some of which compromise brain health and promote CNS functional decline [79]. In this sense, the relationship between the alterations of the gut microbiota pattern and some brain alterations has been demonstrated, such as anxiety and depression [62], cognitive dysfunction [66], and the development of neurodegenerative diseases such as Alzheimer [80] and Parkinson [81]. Although inflammation may not normally represent a triggering factor in neurodegenerative diseases, the severity of the neuroinflammation is certainly a factor that accelerates the progression of cognitive decline and the development of neurodegenerative diseases [81,82,83].
(b) Alterations of the enteric barrier and BBB composition with an impact on brain ageing: The enteric/gut barrier and the BBB are two barriers that belong to the GBA, whose integrity is central for axis functionality. The enteric barrier is defined as a semipermeable tissue that allows the uptake of essential nutrients and the gut immune defence, while being restrictive against pathogenic molecules and bacteria from the intestinal tract. Both structural and molecular components of the barrier act together to fulfil this essential function of the gastrointestinal tract. Histologically, the apical layer from gut epithelium is the mucus layer, which is formed by a sieve-like structure overlying the intestinal epithelium. Antimicrobial peptides and secretory immunoglobulin A (IgA) molecules are secreted into the mucus layer as immune-detectors and regulatory proteins. Above this layer, the intestinal epithelial cells, which are tightly attached to each other by junctional complexes, make a continuous monolayer. The tight junctions are located at the apical side of the cells and regulate the transport of small molecules and ions. The adherens junctions and desmosomes provide strict cell-adhesion bonds and assist in the maintenance of the integrity of the intestinal barrier. The lamina propria contains immune cells from the adaptive and innate immune system (e.g., T cells, B cells, macrophages, and dendritic cells) that participate in the immunological defence of the intestinal barrier [84]. The gut barrier is the first defence against potential harmful agents that could have been ingested with food. This barrier constantly deals with innocuous and non-innocuous food antigens, and with harmful microorganisms. The gut barrier is equipped to interact with and/or tolerate the gut microbiota, induce systemic tolerance to food antigens, and fight against possible pathogens. Deficiencies in these functions can lead to intestinal disorders such as inflammatory bowel disease and irritable bowel syndrome, food allergy or intolerance, and microbial infection, which could favour a general body inflammatory state that also impacts brain functionality and accelerates the brain aging process [85,86].
In turn, the BBB is a term used to describe the exclusive properties of the microvasculature of the CNS, characterized for being a semipermeable and extremely selective vascular wall that separates blood from the brain’s extracellular fluid. This barrier is composed mainly of capillary endothelial cells (sealed by special tight junctions), astrocytes, and pericytes, as well as some other elements, such as basement membrane and other cell types that contribute to the immunological function. These components, which are frequently referred to as the neurovascular unit, preserve a healthy BBB to guarantee the appropriate CNS activity [87]. Although the enteric barrier and the BBB provide defence in very different environments, there are many similarities in their mechanisms of action. In both cases, there is a physical barrier formed by a cellular layer that tightly regulates the movement of ions, molecules, and cells between two tissue spaces. These barriers interact with different cell types, which dynamically regulate their function, and with different immune cells that assess the physical barrier and provide innate and adaptive immunity [88]. The general postulated hypothesis is that characteristic imbalances of ageing that affect the complex ecosystem of the gut microbiota contribute to the decrease in the normal function of these barriers, impacting the permeability in such a way that it allows the flow of potentially harmful substances into the brain tissue through the GBA [34,43,89], which in turn leads to chronic inflammation. In fact, during ageing, it has been demonstrated that the integrity and function of the gastrointestinal barrier weakens [43,72], negatively affecting BBB permeability and accelerating neuroinflammation and functional decline in the CNS [89].
(c) Metabolism-derived substances and immune cells with an impact on brain ageing: The metabolites generated in the gut as a result of digestion become circulating metabolites when they have passed the enteric barrier, and if they also cross the BBB they may enter into the CNS. These metabolites regulate the function of peripheral immune cells, which in turn may influence brain function directly or indirectly, for example via the cerebral lymphatic network [90]. This is the case in butyrate, which is produced through microbial fermentation of dietary fibres in the lower intestinal tract. Butyrate exerts its functions by acting as a histone deacetylase inhibitor or by signalling through several G protein-coupled receptors. This molecule has received particular attention for its beneficial effects on intestinal homeostasis and energy metabolism. Interestingly, butyrate enhances intestinal barrier function and mucosal immunity because of its anti-inflammatory properties [91]. Growing evidence has highlighted the impact of butyrate on the GBA, especially in the regulation of inflammation [92]. In this context, another important molecule derived from digestion is the amino acid tryptophan, which is metabolized by gut commensals, yielding compounds that affect innate immune cell functions. Tryptophan also acts on receptors that regulate the maintenance of the immune response, including lymphoid cells, promoting T helper 17 cell differentiation, and interleukin-22 production. In addition, some microbiota-derived tryptophan metabolites (e.g., indole-3-propionic acid and indole-3-aldehyde) have endothelial direct protective effects maintaining the vascular endothelium integrity and functionality, influencing the development of vascular inflammatory phenotypes [93]. Therefore, the immune system, formed by immune cells in the body and microglia cells in the brain, plays an essential role in maintaining tissue homeostasis, responding to these molecules, and also in infection and injury. In the brain, microglia are the main resident immune cell group; they constantly monitor the microenvironment and produce factors that influence surrounding neurons and astrocytes (another glial cell type with important functions for neuron activity). In brief, metabolites generated in the gut microbiota can modulate not only the peripheral immune system [56] and the CNS microglia activation [94,95], but are also important for the prevention of brain inflammation. Interestingly, both systems have been highlighted as being impaired in ageing [96]. This can be explained because, in the brain, microglial cells play multiple roles in the regulation of inflammatory responses and neuronal function, being pivotal in the pathogenesis of neurodegenerative diseases [97]. In line with this, the restoration of gut microbiota balance and inflammation can have beneficial effects on brain function and age-related diseases [98]. This idea was already pointed out by Metchnikoff more than a century ago [99]. Currently, several sequencing studies, along with those modifying the gut microbiota composition in animal models by means of the administration of antibiotics or probiotics or transferring the microbiota, not only supports the role of the microbiota in brain inflammation and diseases, but also offers new therapeutic perspectives aimed at a specific microbial modulation to attenuate ageing or brain pathologies, because the modifications of the gut microbiota have been reported to have protective effects not only on ageing [77], but also on learning, memory [100], and in the attenuation of neurodegenerative pathologies such as in Alzheimer’s disease [101].
3. Modulation of Brain Ageing by Polyphenols via the Gut Microbiota
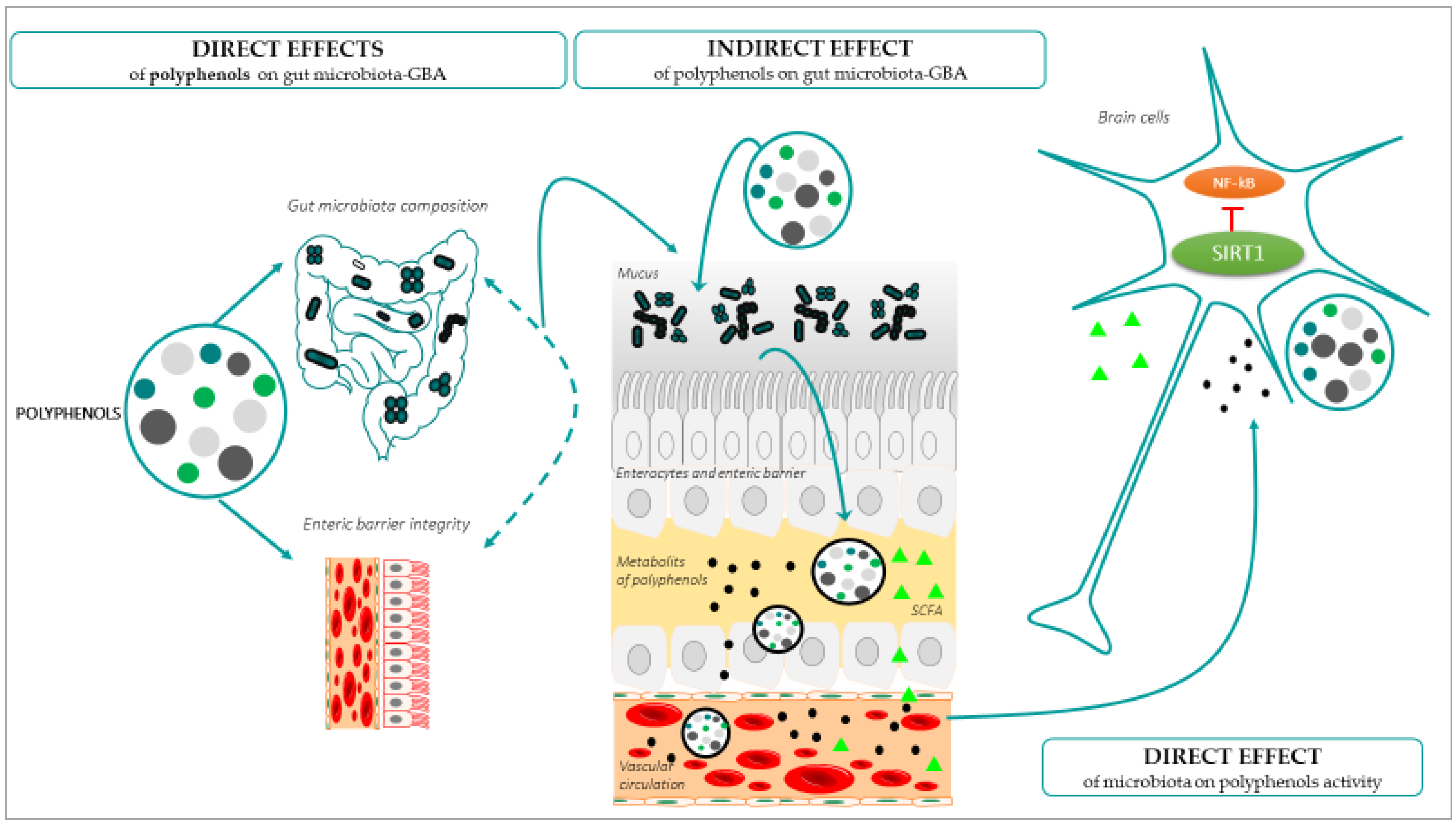
The onset and development of the ageing process can be modulated by lifestyle factors, such as it the case of diet components that interact with the gut microbiota before reaching the brain [102]. The great interest related to this interaction is because diet is an easily extrinsic modifiable factor. Polyphenols are one of the leading anti-ageing diet components, natural compounds exclusively synthesized by plants with chemical features related to phenolic substances [103]. Polyphenols can be found in plant-derived food, and are promising anti-ageing molecules, especially for the brain, because they have the ability to cross the BBB; exert antioxidant and anti-inflammatory properties [104,105]; and generate positive effects on the preservation of monoaminergic neurotransmitters [106], cognitive and motor functions [107,108], and neurogenesis [109]. All these parameters and functions can alter the brain homeostasis, and can thus promote or prevent ageing [110]. When polyphenols are obtained from food, their arrival to the brain takes place by passing through the enteric barrier, so that all the interactions of these molecules in the gut have an impact on the GBA, in the brain functionality and finally in the development of the ageing process [111]. In turn, all the age-related changes in the gut, including pathological conditions and inflammation, among others [112], have an impact on the absorption, metabolism, and the arrival and effectiveness of the polyphenols in the brain [28]. The effects of polyphenols on gut microbiota have been shown in vitro, in vivo, and in human studies (for a review, see [113]), but the mechanisms by which polyphenols modulate the gut microbiota and have an impact on brain ageing are still unknown. The main demonstrated interactions between polyphenols, the gut microbiota, and GBA, with a beneficial impact on healthy brain ageing, are described in Figure 3.
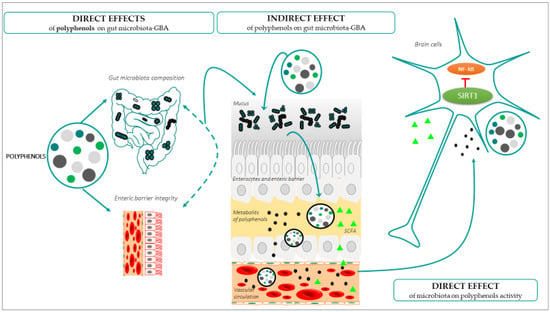
Figure 3.
Direct and indirect effects of polyphenols on gut microbiome and how these changes directly affect brain aging.
(a) Direct effects of polyphenols on the gut microbiota: Gut dysbiosis is one of the most frequently found age-related gut disturbances [114]. In this condition, although the dietary intake would be rich in polyphenols, their absorption would be deficient. The concrete effect of polyphenols on the intestinal microbiota and the GBA is not yet know, but increased evidence suggests that these molecules exert selective effects on the gut microbial biodiversity, thus preventing dysbiosis [115]. Specifically, polyphenols would have a direct effect on the gut microbioma composition by affecting the bacterial growth and metabolism, favouring an increase in beneficial bacteria and inhibiting the proliferation of pathogenic bacteria [113,116,117]. This also occurs with other types of dietry components, for instance, the increases in carbohydrate consumption favours the presence of Prevotella, being the main bacteria in the gut microbial community, or protein and saturated fat consumption increases the presence of Bacteroides [118,119]. In the case of polyphenols, at least in humans, it has been demonstrated that there are some microorganisms that especially participate in the metabolism of polyphenols and, in turn, these molecules can enhance their abundance, such as Flavonifractor plautii, Slackia equolifaciens, Slackia isoflavoniconvertens, Adlercreutzia equolifaciens, Eubacterium ramulus, Eggerthella lenta, Bifidobacterium spp., or Lactobacillus spp. (for a complete list of bacteria, see [113]). Interestingly, some of them, such as Bifidobacterium spp. and Lactobacillus spp., contribute to the gut barrier protection and to the decrease in factors related to the gut and general body inflammation and oxidative stress, for example, by blocking with their metabolites the activation of the nuclear factor-kappa B (NF-κB) or reactive oxygen species (ROS), generating an antioxidative and anti-inflammatory status of the GBA, which would be an important factor for preventing ageing [120,121,122]. This is also the case of Faecalibacterium prausnitzii, which presents anti-inflammatory action by blocking NF-κB activation. Lactobacillus spp. showed a neuroprotective role of the gut microbiota, due to its probiotic and high antioxidant activity [123]. In addition, Faecalibacterium prausnitzii presents anti-inflammatory action by blocking NF-κB activation. Lactobacillus spp. showed a neuroprotective role of the gut microbiota, due to its probiotic and high antioxidant activity [118,124], while minimally affecting—or even increasing—the population of beneficial bacteria. Altogether, they prevent inflammatory processes, which is key in ageing. Interestingly, it has been demonstrated that the effects of polyphenols on bacteria are due in part to the differences in the bacterial wall composition, Gram-positive bacteria being more sensitive to polyphenols than Gram-negative bacteria [125].
(b) Indirect effects of polyphenols on the gut microbiota: Depending on the concentration and the chemical structure of the specific polyphenol, and whether it occurs in conjugated or free form, the digestion of polyphenols produces phenolic metabolites. These metabolites have an impact on the rest of the microbiota and on the GBA [117,126]. Using in vitro models, it has been described that polyphenol permeation through the BBB is dependent on the degree of lipophilicity of each polyphenol or its metabolites, the less polar (i.e., O-methylated derivatives) being capable of greater brain uptake than the more polar ones (i.e., sulphated and glucuronidated derivatives) [127]. The arrival of these metabolites to the brain promotes neuro resilience [128]. This occurs in several ways, for example, the use of resveratrol in humans, because the resveratrol-derived metabolites, named dihydroresveratrol and lunularin, present antibacterial activity on pathological bacteria, such as Salmonella enterica, Enterococcus faecalis, and Escherichia coli [129]. These metabolites are mainly absorbed in phase II of metabolism in the small intestine, although they can also reach the colon [130]. In other cases, polyphenols favour the growth of Roseburia sp., which produces, by fermentation of fibre butyrate in the colon [131,132], a SCFA with activity as histone deacetylase inhibitor, which has anti-inflammatory and memory positive effects in rodent models [133].
To a certain extent, the demonstrated worsening of cognition and motor coordination in ageing is due to the accumulation of molecules in the brain derived from oxidative stress (mainly ROS) and inflammatory status, which produces cytokines and interleukins (IL) [134]. This status, accompanied by a lack of sufficient physiological response to counteract it, is a consequence of neural wear caused over the years [135]. Therefore, in this context, one important indirect effect of polyphenols is their action neutralizing ROS by inhibiting the major ROS-forming enzymes [136], such as monoamine oxidase or xanthine oxidase [137,138]. Furthermore, polyphenols and their in vivo metabolites do not act as conventional hydrogen-donating antioxidants, but they may exert modulatory actions in cells through actions in the protein kinase and lipid kinase signalling pathways [139] and may even involve hormetic effects to protect neurons against the oxidative and inflammatory stressors [140]. A study evaluating 45 polyphenolic compounds indicated that whilst both the flavanols (+)-catechin and (−)-epicatechin failed to inhibit NADPH oxidase, their relevant methylated metabolites exhibited strong NADPH oxidase inhibition through an apocynin-like mechanism [141]. Interestingly, other apocynin-like phenolic compounds, such as ferulic acid, homovanillin alcohol, caffeic acid, tyrosol, and vanillic acid, were also observed to inhibit NADPH oxidase activity, therefore indicating that smaller polyphenols, more structurally related to some colonic metabolites, may also serve as novel therapeutic agents in neuroinflammation.
Furthermore, polyphenols also act as a chelate of metal ions (mainly iron and copper) involved in ROS reactions [142], thus regulating the redox metal homeostasis and preventing metal deposition and neurotoxicity, with important implications for age-related neurodegenerative diseases such as dementia, Alzheimer’s, or Parkinson’s disease [143].
Polyphenols and their in vivo metabolites activate cellular stress-response pathways, resulting in the upregulation of neuroprotective genes. For instance, the polyphenol quercetin has been reported to inhibit neuroinflammation by attenuating nitric oxide production and inducible nitric oxide synthase (iNOS) gene expression in microglia [144,145], preventing inflammatory cytokine production and neuronal injury [146,147]. Polyphenols can also activate the transcription factor cAMP-response-element-binding protein (CREB), which induces the expression of brain-derived neurotrophic factor (BDNF), a mediator of neurohormesis. Finally, polyphenols can also regulate the transcription factor NF-κB, which can mediate adaptive cellular stress responses by reducing the expression of inflammatory cytokines. Activated SIRT1 may also inhibit NF-κB and so can reduce the cellular stress response, altogether modulating genes that encode antioxidant enzymes and other stress-response proteins [148].
(c) Direct effect of the gut microbiota on the activity of polyphenols: The suitable homeostasis of the gut microbiota has an impact on the activity of polyphenol, because their bioavailability depends on it [149]. If polyphenols are well digested and their metabolites arrive at the brain, they can exert brain anti-ageing effects. Gut microbiota contribute to polyphenol xenobiotic metabolisms and bioactive metabolite production, because gut microbiota-derived metabolites from polyphenols have been shown to contribute towards the metabolism of dietary polyphenols, leading to the generation of de novo and potentially bioactive compounds [150]. More than 90% of dietary polyphenols are not absorbed in the small intestine and reach the large intestine; thus, gut microbiota is critically important in turning these polyphenols into bioavailable products [151]. In general, gut microbiota metabolizes glycosylated polyphenols into lower molecular weight phenolic compounds, such as small phenolic acids [130]. Indeed, these gut microbiota-derived polyphenolic metabolites are also essential bioavailable polyphenolic acids. Polyphenols have been shown to undergo various enzymatic processes by gut microbiota, through which the polyphenol derivatives are in a form capable of being absorbed or are even more bioactive [152,153]. Interestingly, it was also demonstrated that gut bacteria can metabolize polyphenols into neurotransmitters and bioactive metabolites with pro-survival and anti-inflammatory effects for the neurons [85]. Therefore, the protective effects of polyphenols also depend on how gut microbiota metabolize these compounds. In fact, based on in vitro studies and in vivo studies focusing on the effect on immunometabolism of microbiota-derived polyphenolic metabolites, specific metabolizing-bacteria have been described, depending on the type of polyphenol that needs to be metabolized, each metabolite having different immunomodulatory effects (for more information, see [150]).
Once they have arrived at the brain, polyphenols and their metabolites can attenuate oxidative and inflammatory damage, preserving cognitive function in the ageing brain [154] by suppressing the expression of harmful molecules and senescence-related genes [155]. In this sense, the reduction of concentrations of antioxidants in both serum and brain cells is inherent to ageing [156], leading to a decline in neural survival and age-related functionality worsening, justifying the need for antioxidant and anti-inflammatory supplementation [157]. As previously mentioned, polyphenols have the abilities of antioxidants [136] and chelation of metal ions involved in ROS reactions [142], and are regulators of redox metal homeostasis, preventing neurodegenerative diseases such as dementia, Alzheimer, and Parkinson [143]. It should be noticed that the effects are accompanied by the modulation of polyphenols of signalling pathways and factors involve in cell survival preservation and neurogenesis, including SIRT1, NF-κB, Nrf2, and Wnt/β-catenin [46]. Both SIRT1 and NF-κB [158,159] are involved in the modulation of the neutralization of oxidative stress and inflammation. In response to a proinflammatory stimulus (e.g., tumour necrosis factor-α (TNFα) or IL-1), via Toll-like receptor 2 (TLR2) or cytokine receptors, NF-κB is translocated to the nucleus and activates the transcription of a cascade of proinflammatory cytokines and chemokines to induce inflammatory responses [160]. Remarkably, the activation of NF-κB-regulated gene expression is modulated by post-transcriptional modifications, such as methylation, phosphorylation, or acetylation, which can be altered upon stimulation [160,161]. The acetylation of p65/RelA, a subunit of the NF-κB protein, is of particular interest because it can either potentiate or diminish NF-κB signalling, depending on the particular acetylated lysine residue [162]. Specifically, the acetylation of lysine 310 is critical for the full activation of NF-κB transcription potential, and this can be deacetylated by SIRT1, a deacetylase and pro-survival protein [163],that can prevent inflammation by the deacetylation of the NF-κB protein [164]. In hippocampus and during ageing, the acetylated form of NF-κB has been seen to be increased, suggesting a lack of the inhibitory effect of SIRT1 against the NF-κB signalling [165]. This contributes to an inflammatory response in the brain and ageing [166], which demonstrates a key role of SIRT1 as a mediator of cognitive decline in normal ageing [166]. In this sense, polyphenols because of their antioxidant and anti-inflammatory capacity, are described as good candidates for the modulation of SIRT1 and NF-kB, which would help in preventing brain functionality decline. This is the case in polyphenols, resveratrol, silymarin, quercetin, naringenin [165], chatechin, and a diet enriched with polyphenols, which in aged rats have been described to modulate SIRT1 and activate the NF-κB signalling pathway, with a positive impact in the recovery of working memory, episodic-like memory [167], and motor coordination [168], always in ageing. Resveratrol also recovers the monoamine levels in the hippocampus and striatum [165]. It has been described that polyphenols exhibit their beneficial properties through a set of mechanisms, including the potential to modulate the triggering of neuroinflammation associated with ageing, by reducing the acetylation of NF-κB, which in turn could be due to the rise in SIRT1 levels, in key regions for cognitive processes, such as the hippocampus, as has been explained previously. The increase in SIRT1 implies not only a decrease in neuroinflammation but also a relation to the regulation of the functionality of cognitive processes, because SIRT1 regulates the expression of the neurotrophins involved in the morphology and functionality of synapses, thus regulating synaptic plasticity, adult hippocampal neurogenesis, and cognition [165]. This is accompanied by the antioxidant effects of polyphenols in the brain, because they prevent the oxidation of enzymes, such as the tryptophan hydroxylase enzyme (TPH) and the tyrosine hydroxylase enzyme (TH), and the inhibition of monoamine oxidase enzyme A (MAO-A), which together favours the increase in the synthesis and accumulation of monoamines. Moreover, it has been demonstrated that resveratrol enhanced the cholinergic system and BDNF and CREB signalling pathways in the prefrontal cortex of an Alzheimer’s disease mouse model. This can also improve physical strength [169]. Although other effects described after chronic resveratrol treatments involving the contribution of the cognitive and motor improvement observed here cannot be proven, it can be postulated that the modulation of SIRT1 and the NF-κB transcription factor would be part of this improvement. Therefore, alterations in the gut microbiota with an impact on these molecular pathways are essential for the observation of the beneficial effects previously mentioned.
4. Future Research Lines
Despite all the effects shown, more basic, clinical, and translational research regarding the effect of polyphenos is needed, especially regarding its administration by oral intake [111]. The first basic factor to study would be the changes in the gut microbiota profile, depending on the polyphenol diet composition, including the type of components and the doses. Relating to the previous idea, another question would be how polyphenols interact with the brain, depending on the human gut microbiome biodiversity, because it varies among individuals depending on genetical and environmental factors, such as diet habits [40]. In addition, the exact degree and the way that polyphenols act to prevent dysbiosis and ageing should also be addressed. All these interactions affect the metabolome, and consequently significant differences in metabolite concentrations can be observed, even if subjects consume the same diet. Furthermore, it also influences the arrival to the brain of potential beneficial metabolites from polyphenols. This could be studied in the serum/plasma of subjects recruited in clinical trials, with the aim to prove the effect of polyphenols on ageing. Great efforts should be made in order to design clinical trials to test the pharmacokinetics, safety, and efficacy of polyphenol oral intake in relation to the prevention of brain ageing. In this sense, there are many clinical trials related to young or adult populations [170], but in the aging population they are still scarce. Some clinical trials in young and adult people have been performed in patients with Type 1 diabetes, in which cocoa flavanols improved cognition and hemodynamic responses [171]. In healthy adults (aged 40–60 years old, n = 101) with overweight and obesity, the long-term (24 weeks) supplementation with anthocyanin-rich Aronia melanocarpa extract (90 mg and 150 mg) improved psychomotor speed [172]. In a double-blind controlled trial in young subjects (n = 90) with sickle cell disease, spearmint extract intake (900 mg/d) improved working memory compared to the placebo group [173]. Regarding the few trials focused on older people, it was found that patients with mild cognitive impairment saw an improvement in age-related episodic memory impairment after the consumption of flavonoids (n = 215, age: 60–70 years old, 6 months, 258 mg/d) [174]. In a randomized controlled trial with older people (n = 37, 12-week), the consumption of cherry juice improved memory and performance during learning tasks [175]. Furthermore, cognitive performance and attention were improved (n = 100, 12-week, 800 mg/d) after Lactobacillus plantarum (C29-fermented soybean) consumption [176]. The same effect was observed when Cosmos caudaus supplement (n = 23, 500 mg/d) was used [177]. Finally, another study showed that Persicaria minor extract supplement rich in polyphenols (n = 36, 6-month, 500 mg/d) improved visual memory, negative mood, and bilateral dorsolateral prefrontal cortex activation in this type of patient. However, there is a lack of trials focusing on non-pathological ageing situations. Therefore, more clinical trials are needed, such as the MaPLE trial [178], which found changes in serum metabolome in healthy elderly people after the consumption of a diet enriched with polyhpenols over 8 weeks.
On the other hand, animal models could also be interesting to study the amount of metabolites of polyphenols in the brain after oral consumption of polyphenols, or how this affects monoamine concentration as they are brain activity modulators. They could also be used to investigate the molecular pathways involved in ageing, including the expression of the main proteins involved in inflammaging. Moreover, the study of the effects of polyphenols on metagenomics, anatomy gut diversity, and the relation with metabolomics should also be addressed. For example, several studies have indicated a high interindividual variability, at least in humans, regarding polyphenol metabolism, two profiles of people being recognized regarding their response to metabolizing polyphenols in the gut: “producers” and the “non-producers”. The first group would be people who produce metabolites (e.g., equol and O-desmethylangolensin from isoflavones), and the “non-producers” would be people who do not produce them [179,180]. Finally, it could be addressed if the described effects of polyphenols at the molecular level in brain cells are due to an indirect mechanism of action on SIRT1, or to an enzyme direct action, as occured in the in vitro experiments [181,182]. Thus, the knowledge of the mechanism of action of polyphenols would help in the design of new drugs and more specific and effective anti-ageing therapies.
5. Conclusions
The gut microbiota and the brain are bidirectionally communicated via the GBA. This implies that changes in this communication are the causes of the loss or the gain of brain homeostasis and, consequently, they have an impact on brain ageing. There are extrinsic factors, such as diet, which have the ability to affect this gut–brain connection. Natural components of diet, such as polyphenols, due to their antioxidants and anti-inflammatory properties, have been highlighted as modulators of brain ageing, one of the ways being the regulation of gut microbiota and the GBA. Numerous studies in animals and some in humans have shown these favourable effects of polyphenols on brain ageing via the gut microbiome and GBA, indicating the need for further research in order to develop therapeutical strategies against ageing based on the oral intake of polyphenols. This topic is an actual and prevailing research line, focusing on the prevention of brain ageing.
Author Contributions
Conceptualization, F.S. and S.E.; methodology, F.S. and S.E.; validation F.S. and S.E.; formal analysis F.S. and S.E.; investigation F.S. and S.E.; resources, F.S. and S.E.; original draft preparation, F.S. and S.E.; writing—review and editing, D.M., S.T. and M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Government Delegation for the National Plan on Drugs (2021I022, Ministry of Health, Spain) and the Program of Promotion of Biomedical Research and Health Sciences, Instituto de Salud Carlos III (CIBEROBN CB12/03/30038).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Lamas for her contribution to the revision of the English language.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| APUD | amine precursor uptake and decarboxylation hypothesis |
| BBB | blood–brain barrier |
| BDNF | brain-derived neurotrophic factor |
| CNS | central nervous system |
| CREB | cAMP response element-binding protein |
| GBA | gut–brain axis |
| GF | germ-free |
| HPA | hypothalamic–pituitary–adrenal axis |
| IgA | immunoglobulin A |
| iNOS | inducible nitric oxide synthase S |
| IL | interleukin |
| MAO-A | monoamine oxidase enzyme A |
| ROS | reactive oxygen species |
| SCFAs | short-chain fatty acids |
| TH | tyrosine hydroxylase enzyme |
| TNFα | tumour necrosis factor-α |
| TLR2 | toll-like receptor 2 |
| TPH | tryptophan hydroxylase enzyme |
References
- World Health Organization. Website: WHO; Media Centre: New York, NY, USA, 2018. [Google Scholar]
- Wimo, A.; Jönsson, L.; Bond, J.; Prince, M.; Winblad, B. The worldwide economic impact of dementia 2010. Alzheimers Dement. 2013, 9, 1–11.e3. [Google Scholar] [CrossRef]
- Anisimov, V.N. Aging and age-related diseases: Prevent, postpone, or threat? J. Bioenerg. Biomembr. 2018, 50, 515–517. [Google Scholar]
- Melzer, T.M.; Manosso, L.M.; Yau, S.Y.; Gil-Mohapel, J.; Brocardo, P.S. In pursuit of healthy aging: Effects of nutrition on brain function. Int. J. Mol. Sci. 2021, 22, 5026. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Hughes, C.F.; Ward, M.; Hoey, L.; McNulty, H. Diet, nutrition and the ageing brain: Current evidence and new directions. Proc. Nutr. Soc. 2018, 77, 2. [Google Scholar] [CrossRef]
- Hanslik, K.; Marino, K.; Ulland, T. Modulation of Glial Function in Health, Aging, and Neurodegenerative Disease. Front. Cell. Neurosci. 2021, 15, 718324. [Google Scholar] [CrossRef]
- Bondolfi, L.; Ermini, F.; Long, J.M.; Ingram, D.K.; Jucker, M. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol. Aging 2004, 25, 333–340. [Google Scholar] [CrossRef]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef]
- Apple, D.M.; Solano-Fonseca, R.; Kokovay, E. Neurogenesis in the aging brain. Biochem. Pharmacol. 2017, 141, 77–85. [Google Scholar] [CrossRef]
- Klempin, F.; Kempermann, G. Adult hippocampal neurogenesis and aging. Eur. Arch. Psychiatry Clin. Neurosci. 2007, 257, 271–280. [Google Scholar] [CrossRef]
- Riddle, D.; Lichtenwalner, R. Brain Aging: Models, Methods, and Mechanisms; Riddle, D.R., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Isaev, N.K.; Stelmashook, E.V.; Genrikhs, E.E. Neurogenesis and brain aging. Rev. Neurosci. 2019, 30, 573–580. [Google Scholar] [CrossRef]
- Mathews, K.J.; Allen, K.M.; Boerrigter, D.; Ball, H.; Shannon Weickert, C.; Double, K.L. Evidence for reduced neurogenesis in the aging human hippocampus despite stable stem cell markers. Aging Cell 2017, 16, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Cotman, C.W. The Aging Mind: Opportunities in Cognitive Research; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Yuan, T.-F.; Gu, S.; Shan, C.; Marchado, S.; Arias-Carrion, O. Oxidative Stress and Adult Neurogenesis. Stem Cell Rev. Rep. 2015, 11, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Ekdahl, C.T.; Claasen, J.H.; Bonde, S.; Kokaia, Z.; Lindvall, O. Inflammation is detrimental for neurogenesis in adult brain. Proc. Natl. Acad. Sci. USA 2003, 100, 13632–13637. [Google Scholar] [CrossRef]
- Chevalier, G.; Siopi, E.; Guenin-Macé, L.; Pascal, M.; Laval, T.; Rifflet, A.; Boneca, I.G.; Demangel, C.; Colsch, B.; Pruvost, A.; et al. Effect of gut microbiota on depressive-like behaviors in mice is mediated by the endocannabinoid system. Nat. Commun. 2020, 11, 6363. [Google Scholar] [CrossRef]
- Colangelo, A.; Cirillo, G.; Alberghina, L.; Papa, M.; Westerhoff, H. Neural plasticity and adult neurogenesis: The deep biology perspective. Neural Regen. Res. 2019, 14, 201–205. [Google Scholar] [CrossRef]
- Ogbonnaya, E.S.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F.; O’Leary, O.F. Adult Hippocampal Neurogenesis is Regulated by the Microbiome. Biol. Psychiatry 2015, 78, e7–e9. [Google Scholar] [CrossRef]
- Gage, F.H. Mammalian neural stem cells. Science 2000, 287, 1433–1438. [Google Scholar] [CrossRef]
- Deng, W.; Aimone, J.B.; Gage, F.H. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 2010, 11, 339–350. [Google Scholar] [CrossRef]
- Leuner, B.; Gould, E.; Shors, T.J. Is there a link between adult neurogenesis and learning? Hippocampus 2006, 16, 216–224. [Google Scholar] [CrossRef]
- Anacker, C.; Hen, R. Adult hippocampal neurogenesis and cognitive flexibility–linking memory and mood. Nat. Rev. Neurosci. 2017, 18, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Dokter, M.; von Bohlen und Halbach, O. Neurogenesis within the adult hippocampus under physiological conditions and in depression. Neural Regen. Res. 2012, 7, 552. [Google Scholar]
- Lazarini, F.; Lledo, P.M. Is adult neurogenesis essential for olfaction? Trends Neurosci. 2011, 34, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.M.G.; Jessberger, S. Adult neurogenesis: Mechanisms and functional significance. Development 2014, 141, 1983–1986. [Google Scholar] [CrossRef]
- Mohajeri, M.H. Brain aging and gut-brain axis. Nutrients 2019, 11, 424. [Google Scholar] [CrossRef]
- Pearse, A.G. The cytochemistry and ultrastructure of polypeptide hormone-producing cells of the APUD series and the embryologic, physiologic and pathologic implications of the concept. J. Histochem. Cytochem. Off. J. Histochem. Soc. 1969, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P.; Farzi, A. Neuropeptides and the microbiota- Gut-brain axis. Adv. Exp. Med. Biol. 2014, 817, 195–219. [Google Scholar]
- Mayer, E.A.; Tillisch, K. The brain-gut Axis in abdominal pain syndromes. Annu. Rev. Med. 2011, 62, 381–396. [Google Scholar] [CrossRef]
- Holzer, P.; Reichmann, F.; Farzi, A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides 2012, 46, 6. [Google Scholar] [CrossRef]
- Rao, M.; Gershon, M.D. The bowel and beyond: The enteric nervous system in neurological disorders. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-chain fatty acids: Microbial metabolites that alleviate stress-induced brain–gut axis alterations. J. Physiol. 2018, 596, 4923–4944. [Google Scholar] [CrossRef] [PubMed]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Liu, C.; Yang, S.Y.; Wang, L.; Zhou, F. The gut microbiome: Implications for neurogenesis and neurological diseases. Neural Regen. Res. 2022, 17, 53–58. [Google Scholar] [PubMed]
- Finegold, S.M.; Downes, J.; Summanen, P.H. Microbiology of regressive autism. Anaerobe 2012, 18, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Patusco, R.; Ziegler, J. Role of probiotics in managing gastrointestinal dysfunction in children with autism spectrum disorder: An update for practitioners. Adv. Nutr. 2018, 9, 637–650. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Köhler, C.; Maes, M.; Slyepchenko, A.; Berk, M.; Solmi, M.; Lanctôt, K.; Carvalho, A. The Gut-Brain Axis, Including the Microbiome, Leaky Gut and Bacterial Translocation: Mechanisms and Pathophysiological Role in Alzheimer’s Disease. Curr. Pharm. Des. 2016, 22, 6152–6166. [Google Scholar] [CrossRef]
- Sun, J.; Xu, J.; Ling, Y.; Wang, F.; Gong, T.; Yang, C.; Ye, S.; Ye, K.; Wei, D.; Song, Z.; et al. Fecal microbiota transplantation alleviated Alzheimer’s disease-like pathogenesis in APP/PS1 transgenic mice. Transl. Psychiatry 2019, 9, 189. [Google Scholar] [CrossRef]
- Saffrey, M.J. Cellular changes in the enteric nervous system during ageing. Dev. Biol. 2013, 382, 344–355. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Zhao, J.; Wu, L.; Carru, C.; Biagi, E.; Franceschi, C. Microbiomes other than the gut: Inflammaging and age-related diseases. Semin. Immunopathol. 2020, 42, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, F.; Moranta, D.; Pani, G. Dietary polyphenols and neurogenesis: Molecular interactions and implication for brain ageing and cognition. Neurosci. Biobehav. Rev. 2018, 90, 456–470. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Yau, Y.F.; Leung, K.S.; El-Nezami, H.; Lee, J.C.Y. Interaction of polyphenols as antioxidant and anti-inflammatory compounds in brain–liver–gut axis. Antioxidants 2020, 9, 669. [Google Scholar] [CrossRef]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci. Rep. 2017, 7, 11456. [Google Scholar] [CrossRef]
- Shimazu, R.; Anada, M.; Miyaguchi, A.; Nomi, Y.; Matsumoto, H. Evaluation of Blood-Brain Barrier Permeability of Polyphenols, Anthocyanins, and Their Metabolites. J. Agric. Food Chem. 2021, 69, 11676–11686. [Google Scholar] [CrossRef]
- Velásquez-Jiménez, D.; Corella-Salazar, D.A.; Zuñiga-Martínez, B.S.; Domínguez-Avila, J.A.; Montiel-Herrera, M.; Salazar-López, N.J.; Rodrigo-Garcia, J.; Villegas-Ochoa, M.A.; González-Aguilar, G.A. Phenolic compounds that cross the blood-brain barrier exert positive health effects as central nervous system antioxidants. Food Funct. 2021, 12, 21. [Google Scholar] [CrossRef]
- Grant, M.; Booth, A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Inf. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Choi, J.; Hur, T.-Y.; Hong, Y. Influence of Altered Gut Microbiota Composition on Aging and Aging-Related Diseases. J. Lifestyle Med. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Seidel, J.; Valenzano, D.R. The role of the gut microbiome during host ageing. F1000Research 2018, 7, 1086. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Li, G.; Huang, P.; Liu, Z.; Zhao, B. The Gut Microbiota and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 58, 1–15. [Google Scholar] [CrossRef]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 2. [Google Scholar]
- Polidano, C.; Zhu, A.; Bornstein, J.C. The relation between cesarean birth and child cognitive development. Sci. Rep. 2017, 7, 11483. [Google Scholar] [CrossRef]
- Santoro, A.; Ostan, R.; Candela, M.; Biagi, E.; Brigidi, P.; Capri, M.; Franceschi, C. Gut microbiota changes in the extreme decades of human life: A focus on centenarians. Cell. Mol. Life Sci. 2018, 58, 1. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef]
- Claesson, M.J.; Cusack, S.; O’Sullivan, O.; Greene-Diniz, R.; De Weerd, H.; Flannery, E.; Marchesi, J.R.; Falush, D.; Dinan, T.; Fitzgerald, G.; et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4586–4591. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.; Jeffery, I.B.; Beaumont, M.; Bell, J.T.; Clark, A.G.; Ley, R.E.; O’Toole, P.W.; Spector, T.D.; Steves, C.J. Signatures of early frailty in the gut microbiota. Genome Med. 2016, 8, 8. [Google Scholar] [CrossRef]
- Simpson, C.A.; Diaz-Arteche, C.; Eliby, D.; Schwartz, O.S.; Simmons, J.G.; Cowan, C.S.M. The gut microbiota in anxiety and depression—A systematic review. Clin. Psychol. Rev. 2021, 83. [Google Scholar] [CrossRef]
- Fröhlich, E.E.; Farzi, A.; Mayerhofer, R.; Reichmann, F.; Jačan, A.; Wagner, B.; Zinser, E.; Bordag, N.; Magnes, C.; Fröhlich, E.; et al. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav. Immun. 2016, 56, 140–155. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Bäckhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 10. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 11. [Google Scholar]
- Scott, K.A.; Ida, M.; Peterson, V.L.; Prenderville, J.A.; Moloney, G.M.; Izumo, T.; Murphy, K.; Murphy, A.; Ross, R.P.; Stanton, C.; et al. Revisiting Metchnikoff: Age-related alterations in microbiota-gut-brain axis in the mouse. Brain Behav. Immun. 2017, 65, 20–32. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Ridlon, J.M.; Hylemon, P.B.; Thacker, L.R.; Heuman, D.M.; Smith, S.; Sikaroodi, M.; Gillevet, P.M. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G168–G175. [Google Scholar] [CrossRef]
- Bartosch, S.; Fite, A.; Macfarlane, G.T.; McMurdo, M.E.T. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl. Environ. Microbiol. 2004, 70, 3575–3581. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.J.; Sharp, R.; Macfarlane, G.T. Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut 2001, 48, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Van Tongeren, S.P.; Slaets, J.P.J.; Harmsen, H.J.M.; Welling, G.W. Fecal microbiota composition and frailty. Appl. Environ. Microbiol. 2005, 71, 6241–6246. [Google Scholar] [CrossRef]
- Zwielehner, J.; Liszt, K.; Handschur, M.; Lassl, C.; Lapin, A.; Haslberger, A.G. Combined PCR-DGGE fingerprinting and quantitative-PCR indicates shifts in fecal population sizes and diversity of Bacteroides, bifidobacteria and Clostridium cluster IV in institutionalized elderly. Exp. Gerontol. 2009, 44, 440–446. [Google Scholar] [CrossRef]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.C.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P.; et al. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe 2017, 21, 455–466.e4. [Google Scholar] [CrossRef]
- Parker, A.; Romano, S.; Ansorge, R.; Aboelnour, A.; Le Gall, G.; Savva, G.M.; Pontifex, M.G.; Telatin, A.; Baker, D.; Jones, E.; et al. Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain. Microbiome 2022, 10, 68. [Google Scholar] [CrossRef]
- Clark, R.I.; Salazar, A.; Yamada, R.; Fitz-Gibbon, S.; Morselli, M.; Alcaraz, J.; Rana, A.; Rera, M.; Pellegrini, M.; Ja, W.W.; et al. Distinct Shifts in Microbiota Composition during Drosophila Aging Impair Intestinal Function and Drive Mortality. Cell Rep. 2015, 12, 1656–1667. [Google Scholar] [CrossRef] [PubMed]
- Fransen, F.; van Beek, A.A.; Borghuis, T.; El Aidy, S.; Hugenholtz, F.; van der Gaast de Jongh, C.; Savelkoul, H.F.J.; de Jonge, M.I.; Boekschoten, M.V.; Smidt, H.; et al. Aged gut microbiota contributes to systemical inflammaging after transfer to germ-free mice. Front. Immunol. 2017, 8, 1385. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.I.; Meehan, C.J.; Koenig, J.E.; Dhanani, A.S.; Rose, R.A.; Howlett, S.E.; Beiko, R.G. Microbial shifts in the aging mouse gut. Microbiome 2014, 2, 50. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Willemsen, D.; Popkes, M.; Metge, F.; Gandiwa, E.; Reichard, M.; Valenzano, D.R. Regulation of life span by the gut microbiota in the short-lived african turquoise killifish. Elife 2017, 6, e27014. [Google Scholar] [CrossRef] [PubMed]
- Stebegg, M.; Silva-Cayetano, A.; Innocentin, S.; Jenkins, T.P.; Cantacessi, C.; Gilbert, C.; Linterman, M.A. Heterochronic faecal transplantation boosts gut germinal centres in aged mice. Nat. Commun. 2019, 10, 2443. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, X.; Zheng, S.; Khanabdali, R.; Kalionis, B.; Wu, J.; Wan, W.; Tai, X. An Update on Inflamm-Aging: Mechanisms, Prevention, and Treatment. J. Immunol. Res. 2016, 2016, 8426874. [Google Scholar] [CrossRef]
- Minter, M.R.; Zhang, C.; Leone, V.; Ringus, D.L.; Zhang, X.; Oyler-Castrillo, P.; Musch, M.W.; Liao, F.; Ward, J.F.; Holtzman, D.M.; et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci. Rep. 2016, 6, 30028. [Google Scholar] [CrossRef]
- Petrov, V.A.; Saltykova, I.V.; Zhukova, I.A.; Alifirova, V.M.; Zhukova, N.G.; Dorofeeva, Y.B.; Tyakht, A.V.; Kovarsky, B.A.; Alekseev, D.G.; Kostryukova, E.S.; et al. Analysis of gut microbiota in patients with parkinson’s disease. Bull. Exp. Biol. Med. 2017, 162, 734–737. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms Underlying Inflammation in Neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Bevan-Jones, W.R.; Cope, T.E.; Jones, P.S.; Kaalund, S.S.; Passamonti, L.; Allinson, K.; Green, O.; Hong, Y.T.; Fryer, T.D.; Arnold, R.; et al. Neuroinflammation and protein aggregation co-localize across the frontotemporal dementia spectrum. Brain 2020, 143, 1010–1026. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 9. [Google Scholar]
- Houser, M.C.; Tansey, M.G. The gut-brain axis: Is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Park. Dis. 2017, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.; Almeida, L.M.; Dinis, T.C.P. The Impact of Chronic Intestinal Inflammation on Brain Disorders: The Microbiota-Gut-Brain Axi. Mol. Neurobiol. 2019, 56, 10. [Google Scholar] [CrossRef] [PubMed]
- Alahmari, A. Blood-Brain Barrier Overview: Structural and Functional Correlation. Neural Plast. 2021, 2021, 6564585. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Rescigno, M. The Gut Immune Barrier and the Blood-Brain Barrier: Are They So Different? Immunity 2009, 31, 5. [Google Scholar] [CrossRef]
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L.; et al. Blood-Brain barrier breakdown in the aging human hippocampus. Neuron 2015, 85, 296–302. [Google Scholar] [CrossRef]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A double-edged sword for health? Adv. Nutr. 2018, 9, 1. [Google Scholar] [CrossRef]
- Gonçalves, P.; Araújo, J.R.; Di Santo, J.P. A cross-talk between microbiota-derived short-chain fatty acids and the host mucosal immune system regulates intestinal homeostasis and inflammatory bowel disease. Inflamm. Bowel Dis. 2018, 24, 558–572. [Google Scholar] [CrossRef]
- Paeslack, N.; Mimmler, M.; Becker, S.; Gao, Z.; Khuu, M.P.; Mann, A.; Malinarich, F.; Regen, T.; Reinhardt, C. Microbiota-derived tryptophan metabolites in vascular inflammation and cardiovascular disease. Amino Acids 2022, 54, 1339–1356. [Google Scholar] [CrossRef]
- Erny, D.; De Angelis, A.L.H.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Kabouridis, P.S.; Lasrado, R.; McCallum, S.; Chng, S.H.; Snippert, H.J.; Clevers, H.; Pettersson, S.; Pachnis, V. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron 2015, 85, 289–295. [Google Scholar] [CrossRef]
- Weyand, C.M.; Goronzy, J.J. Aging of the immune system: Mechanisms and therapeutic targets. Ann. Am. Thorac. Soc. 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T. Microglia take centre stage in neurodegenerative disease. Nat. Rev. Immunol. 2019, 19, 79–80. [Google Scholar] [CrossRef]
- Boehme, M.; van de Wouw, M.; Bastiaanssen, T.F.S.; Olavarría-Ramírez, L.; Lyons, K.; Fouhy, F.; Golubeva, A.V.; Moloney, G.M.; Minuto, C.; Sandhu, K.V.; et al. Mid-life microbiota crises: Middle age is associated with pervasive neuroimmune alterations that are reversed by targeting the gut microbiome. Mol. Psychiatry 2020, 25, 2567–2583. [Google Scholar] [CrossRef] [PubMed]
- Mackowiak, P.A. Recycling Metchnikoff: Probiotics, the intestinal microbiome and the quest for long life. Front. Public Health 2013, 1, 52. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hu, X.; Liang, S.; Li, W.; Wu, X.; Wang, L.; Jin, F. Lactobacillus fermentum NS9 restores the antibiotic induced physiological and psychological abnormalities in rats. Benef. Microbes 2015, 6, 707–717. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, Y.; Choi, H.; Kim, W.; Park, S.; Lee, D.; Kim, D.K.; Kim, H.J.; Choi, H.; Hyun, D.W.; et al. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut 2020, 69, 283–294. [Google Scholar] [CrossRef]
- Badal, V.D.; Vaccariello, E.D.; Murray, E.R.; Yu, K.E.; Knight, R.; Jeste, D.V.; Nguyen, T.T. The gut microbiome, aging, and longevity: A systematic review. Nutrients 2020, 12, 3759. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Corredor, C. Antioxidantes, cuarta edición. Metab. Nutr. Shock 2006, 293. [Google Scholar]
- Joseph, J.; Cole, G.; Head, E.; Ingram, D. Nutrition, brain aging, and neurodegeneration. J. Neurosci. 2009, 29, 12795–12801. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, F.; Moranta, D.; Ramis, M.; Kienzer, C.; Esteban, S.; Miralles, A. SIRT1 and Monoamine Levels in Hippocampus of Aged-Rats after Chronic Polyphenol Treatments. In Conference Proceeding of FEPS; Wiley-Blackwell: Kaunas, Lituania, 2015. [Google Scholar]
- Sarubbo, F.; Esteban, S.; Miralles, A.; Moranta, D. Effects of Resveratrol and other Polyphenols on Sirt1: Relevance to Brain Function during Aging. Curr. Neuropharmacol. 2018, 16, 126–136. [Google Scholar] [CrossRef]
- Ramis, M.; Sarubbo, F.; Terrasa, J.; Moranta, D.; Aparicio, S.; Miralles, A.; Esteban, S. Chronic α-tocopherol increases central monoamines synthesis and improves cognitive and motor abilities in old rats. Rejuvenation Res. 2015, 19, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, F.; Cavallucci, V.; Pani, G. The Influence of Gut Microbiota on Neurogenesis: Evidence and Hopes. Cells 2022, 11, 382. [Google Scholar] [CrossRef]
- Sarubbo, F.; Moranta, D.; Asensio, V.J.; Miralles, A.; Esteban, S. Effects of Resveratrol and Other Polyphenols on the Most Common Brain Age-Related Diseases. Curr. Med. Chem. 2017, 24, 4245–4266. [Google Scholar] [CrossRef]
- Filosa, S.; Di Meo, F.; Crispi, S. Polyphenols-gut microbiota interplay and brain neuromodulation. Neural Regen. Res. 2018, 13, 2055–2059. [Google Scholar]
- Ratto, D.; Roda, E.; Romeo, M.; Venuti, M.T.; Desiderio, A.; Lupo, G.; Capelli, E.; Sandionigi, A.; Rossi PRatto, D.; Roda, E.; et al. The Many Ages of Microbiome-Gut-Brain Axis. Nutrients 2022, 14, 2937. [Google Scholar] [CrossRef]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef]
- Haran, J.P.; McCormick, B.A. Aging, Frailty, and the Microbiome—How Dysbiosis Influences Human Aging and Disease. Gastroenterology 2021, 160, 507–523. [Google Scholar] [CrossRef]
- Davinelli, S.; Scapagnini, G. Interactions between dietary polyphenols and aging gut microbiota: A review. BioFactors 2022, 48, 2. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; Muñoz-González, I.; Cueva, C.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolomé, B. A survey of modulation of gut microbiota by dietary polyphenols. BioMed Res. Int. 2015, 2015, 850902. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 15. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, U.; Fernández-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J. Agric. Food Chem. 2013, 61, 40. [Google Scholar] [CrossRef]
- Moco, S.; Martin, F.P.J.; Rezzi, S.J. Metabolomics view on gut microbiome modulation by polyphenol-rich foods. Proteome Res. 2012, 11, 10. [Google Scholar] [CrossRef]
- Candela, M.; Perna, F.; Carnevali, P.; Vitali, B.; Ciati, R.; Gionchetti, P.; Rizzello, F.; Campieri, M.; Brigidi, P. Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: Adhesion properties, competition against enteropathogens and modulation of IL-8 production. Int. J. Food Microbiol. 2008, 125, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.W.; Myers, L.E.S.; Ray, L.; Song, S.C.; Nasr, T.R.; Berardinelli, A.J.; Kundu, K.; Murthy, N.; Hansen, J.M.; Neish, A.S. Lactobacillus rhamnosus blocks inflammatory signaling in vivo via reactive oxygen species generation. Free Radic. Biol. Med. 2009, 47, 1205–1211. [Google Scholar] [CrossRef]
- Tien, M.-T.; Girardin, S.E.; Regnault, B.; Le Bourhis, L.; Dillies, M.-A.; Coppée, J.-Y.; Bourdet-Sicard, R.; Sansonetti, P.J.; Pédron, T. Anti-inflammatory effect of Lactobacillus casei on Shigella-infected human intestinal epithelial cells. J. Immunol. 2006, 176, 1228–1237. [Google Scholar] [CrossRef]
- Cheon, M.J.; Lim, S.M.; Lee, N.K.; Paik, H.D. Probiotic properties and neuroprotective effects of lactobacillus buchneri ku200793 isolated from korean fermented foods. Int. J. Mol. Sci. 2020, 21, 1227. [Google Scholar] [CrossRef]
- Hochma, E.; Yarmolinsky, L.; Khalfin, B.; Nisnevitch, M.; Ben-Shabat, S.; Nakonechny, F. Antimicrobial Effect of Phytochemicals from Edible Plants. Processes 2021, 9, 2089. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I.J. Benefits of polyphenols on gut microbiota and implications in human health. Nutr. Biochem. 2013, 24, 8. [Google Scholar] [CrossRef] [PubMed]
- Mosele, J.I.; Macià, A.; Motilva, M.J. Metabolic and microbial modulation of the large intestine ecosystem by non-absorbed diet phenolic compounds: A review. Molecules 2015, 20, 17429–17468. [Google Scholar] [CrossRef]
- Youdim, K.A.; Dobbie, M.S.; Kuhnle, G.; Proteggente, A.R.; Abbott, N.J.; Rice-Evans, C. Interaction between flavonoids and the blood-brain barrier: In vitro studies. J. Neurochem. 2003, 85, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Raval, U.; Harary, J.M.; Zeng, E.; Pasinetti, G.M. The dichotomous role of the gut microbiome in exacerbating and ameliorating neurodegenerative disorders. Expert Rev. Neurother. 2020, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Bird, J.K.; Raederstorff, D.; Weber, P.; Steinert, R.E. Cardiovascular and antiobesity effects of resveratrol mediated through the gut microbiota. Adv. Nutr. 2017, 8, 6. [Google Scholar] [CrossRef]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.A.P.; Kulling, S.E. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Sánchez-Alcoholado, L.; Pérez-Martínez, P.; Andrés-Lacueva, C.; Cardona, F.; Tinahones, F.; Queipo-Ortuño, M.I. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct. 2016, 7, 1775–1787. [Google Scholar] [CrossRef]
- Matt, S.M.; Allen, J.M.; Lawson, M.A.; Mailing, L.J.; Woods, J.A.; Johnson, R.W. Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice. Front. Immunol. 2018, 9, 1832. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef]
- Barrientos, R.M.; Kitt, M.M.; Watkins, L.R.; Maier, S.F. Neuroinflammation in the normal aging hippocampus. Neuroscience 2015, 309, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H.; Cassidy, A.; Comte, B.; Heinonen, M.; Richelle, M.; Richling, E.; Serafini, M.; Scalbert, A.; Sies, H.; Vidry, S. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J. Nutr. 2011, 141, 989S–1009S. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Acuña, C.; Ferreira, J.; Speisky, H. Polyphenols and mitochondria: An update on their increasingly emerging ROS-scavenging independent actions. Arch. Biochem. Biophys. 2014, 559, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Shandilya, S.; Kumar, S.; Kumar Jha, N.; Kumar Kesari, K.; Ruokolainen, J. Interplay of gut microbiota and oxidative stress: Perspective on neurodegeneration and neuroprotection. J. Adv. Res. 2022, 559, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Iavicoli, I.; Di Paola, R.; Koverech, A.; Cuzzocrea, S.; Rizzarelli, E.; Calabrese, E.J. Cellular stress responses, hormetic phytochemicals and vitagenes in aging and longevity. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 5. [Google Scholar] [CrossRef] [PubMed]
- Steffen, Y.; Gruber, C.; Schewe, T.; Sies, H. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Arch. Biochem. Biophys. 2008, 469, 209–219. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Lakey-Beitia, J.; Berrocal, R.; Rao, K.S.; Durant, A.A. Polyphenols as Therapeutic Molecules in Alzheimer’s Disease through Modulating Amyloid Pathways. Mol. Neurobiol. 2015, 51, 466–479. [Google Scholar] [CrossRef]
- Abramov, A.Y.; Jacobson, J.; Wientjes, F.; Hothersall, J.; Canevari, L.; Duchen, M.R. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J. Neurosci. 2005, 25, 9176–9184. [Google Scholar] [CrossRef]
- Kao, T.K.; Ou, Y.C.; Raung, S.L.; Lai, C.Y.; Liao, S.L.; Chen, C.J. Inhibition of nitric oxide production by quercetin in endotoxin/cytokine-stimulated microglia. Life Sci. 2010, 86, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Mishra, M.; Ghosh, S.; Tewari, R.; Basu, A.; Seth, P.; Sen, E. Modulation of interleukin-1β mediated inflammatory response in human astrocytes by flavonoids: Implications in neuroprotection. Brain Res. Bull. 2007, 73, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Bureau, G.; Longpré, F.; Martinoli, M.G. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J. Neurosci. Res. 2008, 86, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D. Dietary polyphenols as modulators of brain functions: Biological actions and molecular mechanisms underpinning their beneficial effects. Oxid. Med. Cell. Longev. 2012, 2012, 914273. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef]
- Man, A.W.C.; Zhou, Y.; Xia, N.; Li, H. Involvement of gut microbiota, microbial metabolites and interaction with polyphenol in host immunometabolism. Nutrients 2020, 12, 3054. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Dietary polyphenols and the prevention of diseases. Am. J. Clin. Nutr. 2005, 4, 287–306. [Google Scholar]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef]
- Liu, J.; He, Z.; Ma, N.; Chen, Z.Y. Beneficial Effects of Dietary Polyphenols on High-Fat Diet-Induced Obesity Linking with Modulation of Gut Microbiota. J. Agric. Food Chem. 2020, 68, 1. [Google Scholar] [CrossRef]
- Lau, F.; Shukitt-Hale, B.; Joseph, J. The beneficial effects of fruit polyphenols on brain aging. Neurobiol. Aging 2005, 26, 128–132. [Google Scholar] [CrossRef]
- Liu, J.; Killilea, D.; Ames, B. Age-associated mitochondrial oxidative decay: Improvement of carnitine acetyltransferase substrate-binding affinity and activity in brain by feeding old rats acetyl-L- carnitine and/or R.-alpha-lipoic acid. Proc. Natl. Acad. Sci. USA 2002, 99, 1876–1881. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, P.; Polidori, M.; Metastasio, A.; Mariani, E.; Mattioli, P.; Cherubini, A.; Catani, M.; Cecchetti, R.; Senin, U.; Mecocci, P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol. Aging 2003, 24, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Beckervordersandforth, R. Mitochondrial Metabolism-Mediated Regulation of Adult Neurogenesis. Brain Plast. 2017, 3, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, Y.; Mueller-Steiner, S.; Chen, L.F.; Kwon, H.; Yi, S.; Mucke, L.; Gan, L. SIRT1 protects against microglia-dependent amyloid-β toxicity through inhibiting NF-κB signaling. J. Biol. Chem. 2005, 280, 40364–40374. [Google Scholar] [CrossRef]
- Stacchiotti, A.; Favero, G.; Rezzani, R. Resveratrol and SIRT1 Activators for the Treatment of Aging and Age-Related Diseases. Resveratrol-Adding Life to Years, not Adding Years to Life; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Goh, F.G.; Midwood, K.S. Intrinsic danger: Activation of Toll-like receptors in rheumatoid arthritis. Rheumatology 2012, 51, 1. [Google Scholar] [CrossRef]
- Yang, X.-D.; Huang, B.; Li, M.; Lamb, A.; Kelleher, N.; Chen, L.-F. Negative regulation of NF-kappaB action by Set9-mediated lysine methylation of the RelA subunit. EMBO J. 2009, 28, 1055–1066. [Google Scholar] [CrossRef]
- Chen, L.; Mu, Y.; Greene, W.C. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-κB. EMBO J. 2002, 21, 6539–6548. [Google Scholar] [CrossRef]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, W.; Pan, H.; Feldser, H.G.; Lainez, E.; Miller, C.; Leung, S.; Zhong, Z.; Zhao, H.; Sweitzer, S.; et al. SIRT1 Activators Suppress Inflammatory Responses through Promotion of p65 Deacetylation and Inhibition of NF-κB Activity. PLoS ONE 2012, 7, e46364. [Google Scholar] [CrossRef]
- Sarubbo, F.; Ramis, M.R.; Kienzer, C.; Aparicio, S.; Esteban, S.; Miralles, A.; Moranta, D. Chronic Silymarin, Quercetin and Naringenin Treatments Increase Monoamines Synthesis and Hippocampal Sirt1 Levels Improving Cognition in Aged Rats. J. Neuroimmune Pharmacol. 2017, 13, 24–38. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Crosstalk between oxidative stress and SIRT1: Impact on the aging process. Int. J. Mol. Sci. 2013, 14, 3834–3859. [Google Scholar] [CrossRef] [PubMed]
- Veronica Witte, A.; Kerti, L.; Margulies, D.S.; Flöel, A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J. Neurosci. 2014, 34, 7862–7870. [Google Scholar] [CrossRef] [PubMed]
- Socci, D.J.; Crandall, B.M.; Arendash, G.W. Chronic antioxidant treatment improves the cognitive performance of aged rats. Brain Res. 1995, 693, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Zocchi, L.; Sassone-Corsi, P. SIRT1-mediated deacetylation of MeCP2 contributes to BDNF expression. Epigenetics 2012, 7, 695–700. [Google Scholar] [CrossRef]
- Shin, J.H.; Kim, C.S.; Cha, L.; Kim, S.; Lee, S.; Chae, S.; Chun, W.Y.; Shin, D.M. Consumption of 85% cocoa dark chocolate improves mood in association with gut microbial changes in healthy adults: A randomized controlled trial. J. Nutr. Biochem. 2022, 99, 108854. [Google Scholar] [CrossRef]
- Decroix, L.; van Schuerbeek, P.; Tonoli, C.; van Cutsem, J.; Soares, D.D.; Heyman, E.; Vanderhasselt, T.; Verrelst, R.; Raeymaekers, H.; de Mey, J.; et al. The effect of acute cocoa flavanol intake on the BOLD response and cognitive function in type 1 diabetes: A randomized, placebo-controlled, double-blinded cross-over pilot study. Psychopharmacology 2019, 236, 3421–3428. [Google Scholar] [CrossRef]
- Ahles, S.; Stevens, Y.R.; Joris, P.J.; Vauzour, D.; Adam, J.; de Groot, E.; Plat, J. The effect of long-term aronia melanocarpa extract supplementation on cognitive performance, mood, and vascular function: A randomized controlled trial in healthy, middle-aged individuals. Nutrients 2020, 12, 2475. [Google Scholar] [CrossRef]
- Herrlinger, K.A.; Nieman, K.M.; Sanoshy, K.D.; Fonseca, B.A.; Lasrado, J.A.; Schild, A.L.; Maki, K.C.; Wesnes, K.A.; Ceddia, M.A. Spearmint Extract Improves Working Memory in Men and Women with Age-Associated Memory Impairment. J. Altern. Complement. Med. 2018, 24, 37–47. [Google Scholar] [CrossRef]
- Bensalem, J.; Dudonné, S.; Etchamendy, N.; Pellay, H.; Amadieu, C.; Gaudout, D.; Dubreuil, S.; Paradis, M.E.; Pomerleau, S.; Capuron, L.; et al. Polyphenols from Grape and Blueberry Improve Episodic Memory in Healthy Elderly with Lower Level of Memory Performance: A Bicentric Double-Blind, Randomized, Placebo-Controlled Clinical Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019, 74, 996–1007. [Google Scholar] [CrossRef]
- Kent, K.; Charlton, K.; Roodenrys, S.; Batterham, M.; Potter, J.; Traynor, V.; Gilbert, H.; Morgan, O.; Richards, R. Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia. Eur. J. Nutr. 2017, 56, 333–341. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Park, S.; Paik, J.W.; Chae, S.W.; Kim, D.H.; Jeong, D.G.; Ha, E.; Kim, M.; Hong, G.; Park, S.H.; et al. Efficacy and safety of lactobacillus plantarum C29-fermented soybean (DW2009) in individuals with mild cognitive impairment: A 12-week, multi-center, randomized, double-blind, placebo-controlled clinical trial. Nutrients 2019, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.; Shahar, S.; Mohamad, M.; Rajab, N.F.; Yahya, H.M.; Din, N.C.; Hamid, H.A. The effects of six months Persicaria minor extract supplement among older adults with mild cognitive impairment: A double-blinded, randomized, and placebo-controlled trial. BMC Complement. Med. Ther. 2020, 20, 315. [Google Scholar] [CrossRef] [PubMed]
- Peron, G.; Gargari, G.; Meroño, T.; Miñarro, A.; Lozano, E.V.; Escuder, P.C.; González-Domínguez, R.; Hidalgo-Liberona, N.; Del Bo, C.; Bernardi, S.; et al. Crosstalk among intestinal barrier, gut microbiota and serum metabolome after a polyphenol-rich diet in older subjects with “leaky gut”: The MaPLE trial. Clin. Nutr. 2021, 40, 5288–5297. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Martín, A.; García-Villalba, R.; González-Sarrías, A.; Romo-Vaquero, M.; Loria-Kohen, V.; Ramírez-De-Molina, A.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. The gut microbiota urolithin metabotypes revisited: The human metabolism of ellagic acid is mainly determined by aging. Food Funct. 2018, 9, 4100–4106. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.L.; Costa, G.R.; Dörr, F.A.; Ong, T.P.; Pinto, E.; Lajolo, F.M.; Hassimotto, N.M.A. Potential antiproliferative activity of polyphenol metabolites against human breast cancer cells and their urine excretion pattern in healthy subjects following acute intake of a polyphenol-rich juice of grumixama (Eugenia brasiliensis Lam.). Food Funct. 2017, 8, 2266–2274. [Google Scholar] [CrossRef]
- Gertz, M.; Nguyen, G.T.T.; Fischer, F.; Suenkel, B.; Schlicker, C.; Fränzel, B.; Tomaschewski, J.; Aladini, F.; Becker, C.; Wolters, D.; et al. A Molecular Mechanism for Direct Sirtuin Activation by Resveratrol. PLoS ONE 2012, 7, e49761. [Google Scholar] [CrossRef]
- Hubbard, B.P.; Gomes, A.P.; Dai, H.; Li, J.; Case, A.W.; Considine, T.; Riera, T.V.; Lee, J.E.; E, S.Y.; Lamming, D.W.; et al. Evidence for a Common Mechanism of SIRT1 Regulation by Allosteric Activators. Science 2013, 339, 1216–1219. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).