Anti-Inflammation and Anti-Melanogenic Effects of Maca Root Extracts Fermented Using Lactobacillus Strains
Abstract
1. Introduction
2. Materials and Methods
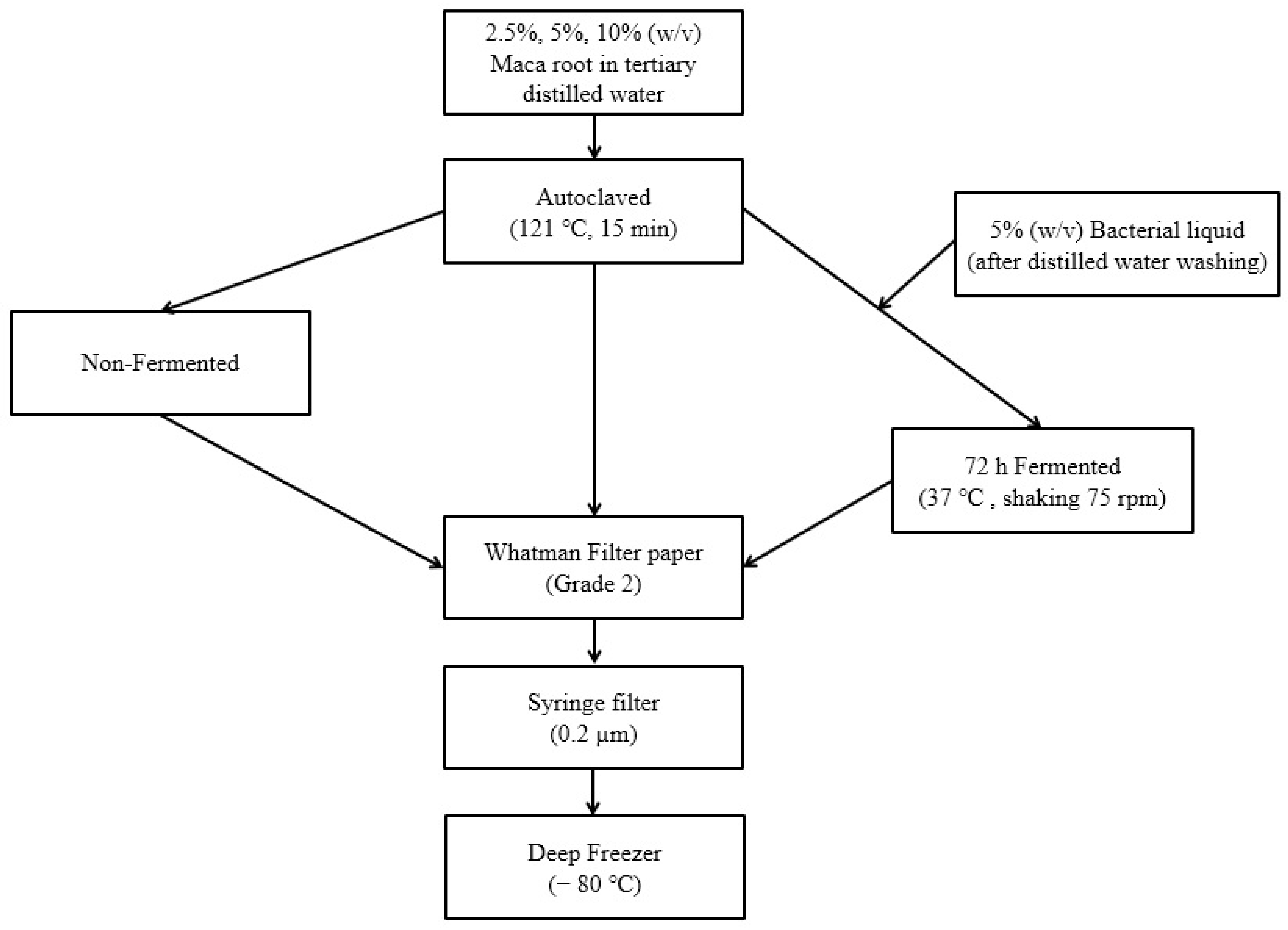
2.1. Maca Root Fermented by Lactobacillus
2.2. Cell Culture
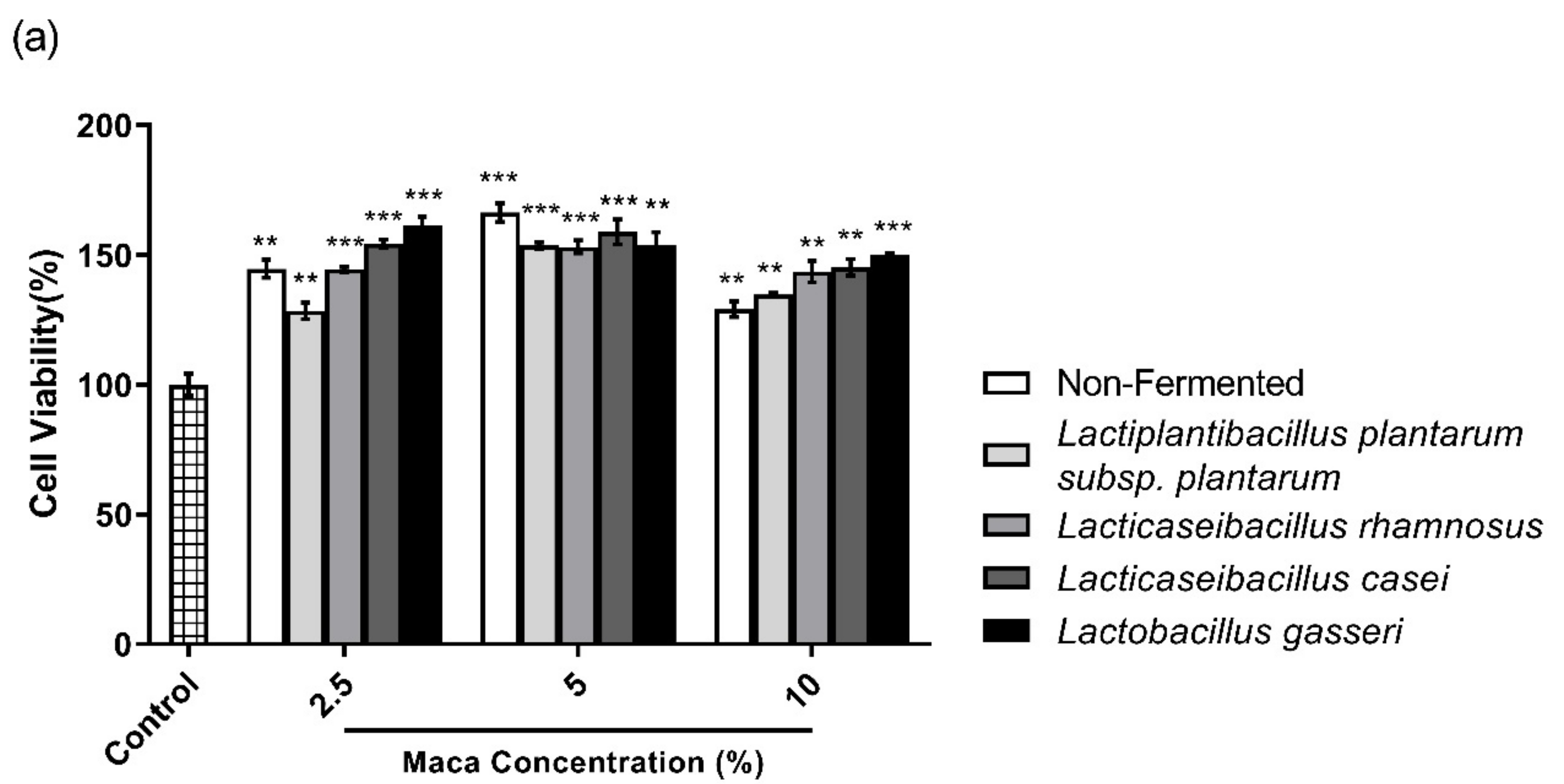
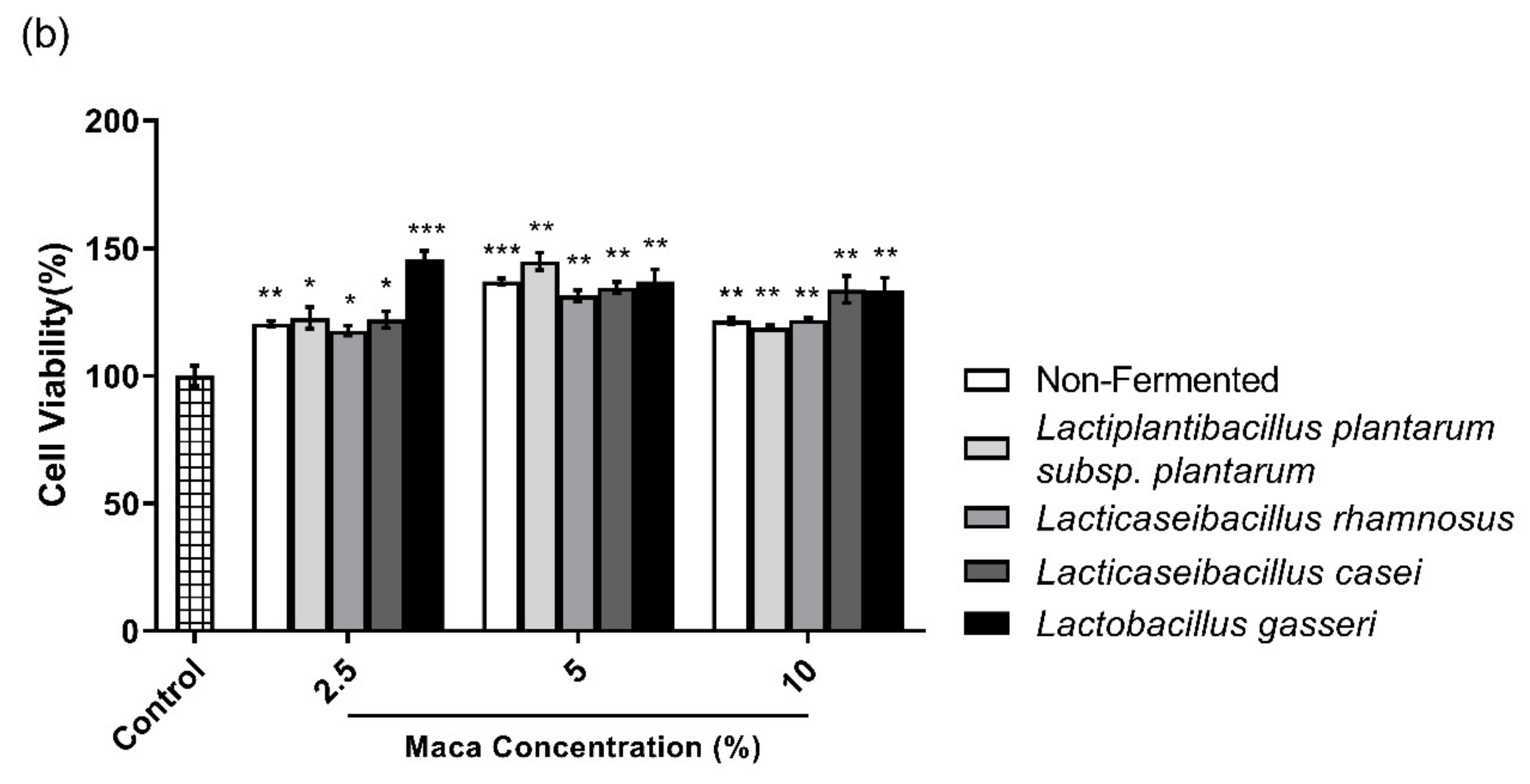
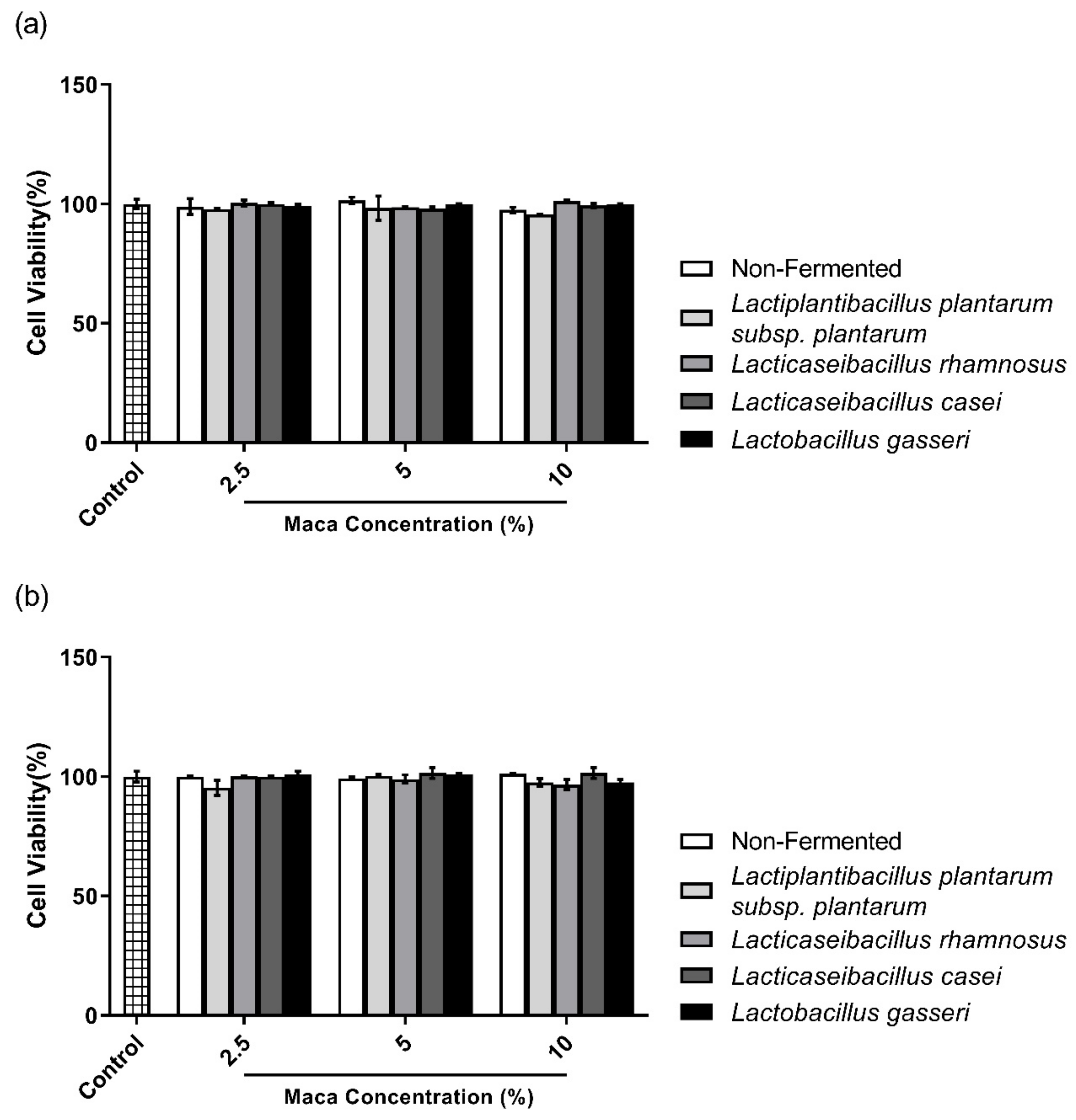
2.3. Cell Viability
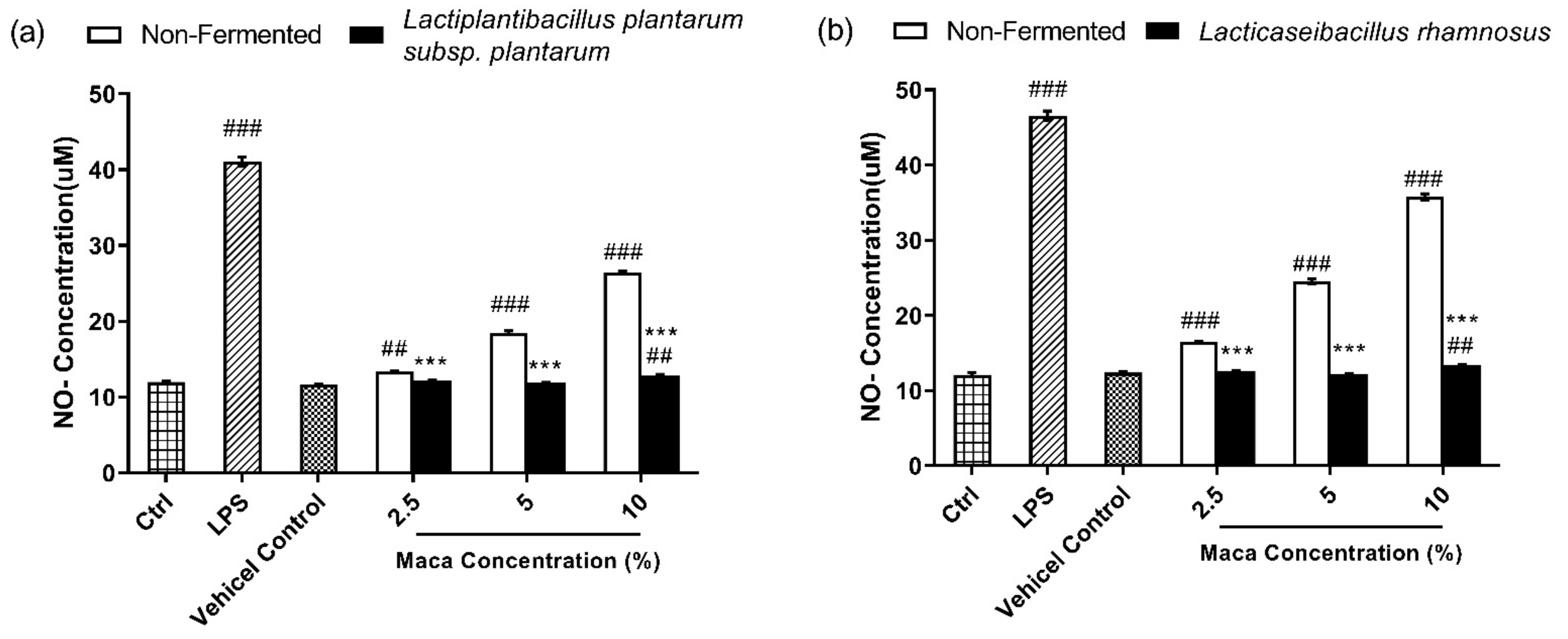
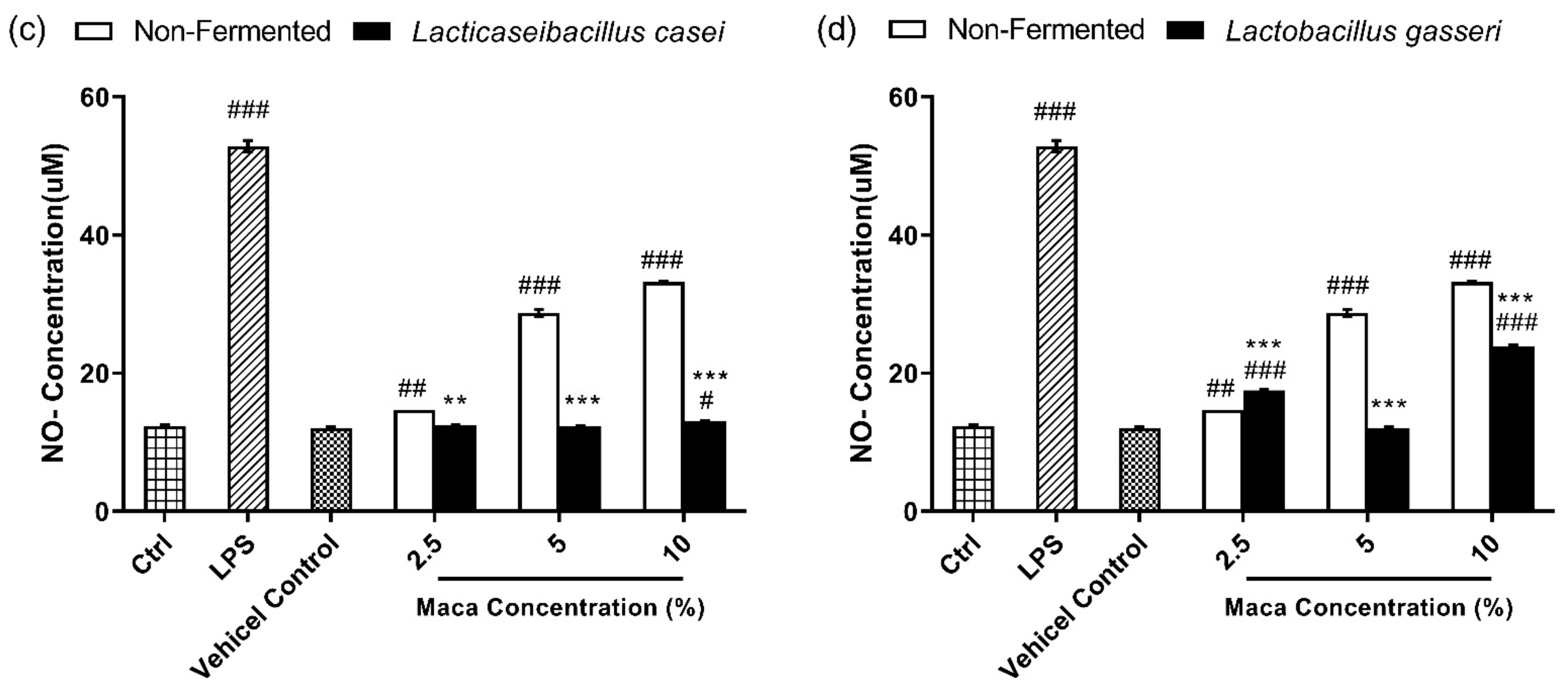
2.4. NO Assay
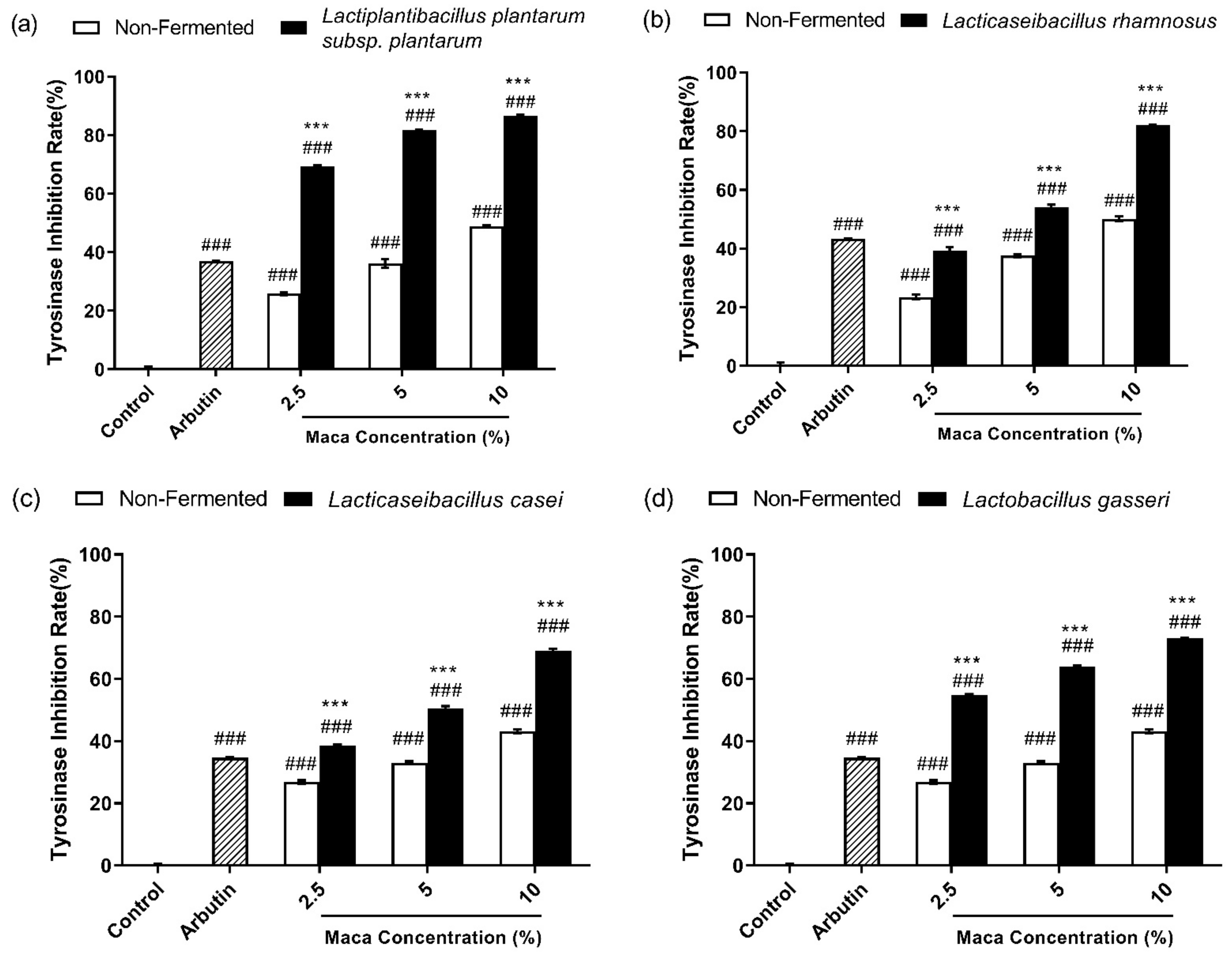
2.5. Mushroom Tyrosinase Inhibition Assay
2.6. Intracellular Melanin Content Assay
2.7. Extracellular Melanin Content Assay
2.8. Intracellular Tyrosinase Activity
2.9. Quantitative Real-Time PCR
2.10. DPPH Antioxidant Assay
2.11. Determination of Total Phenolic Content
2.12. Statistical Analysis
3. Results
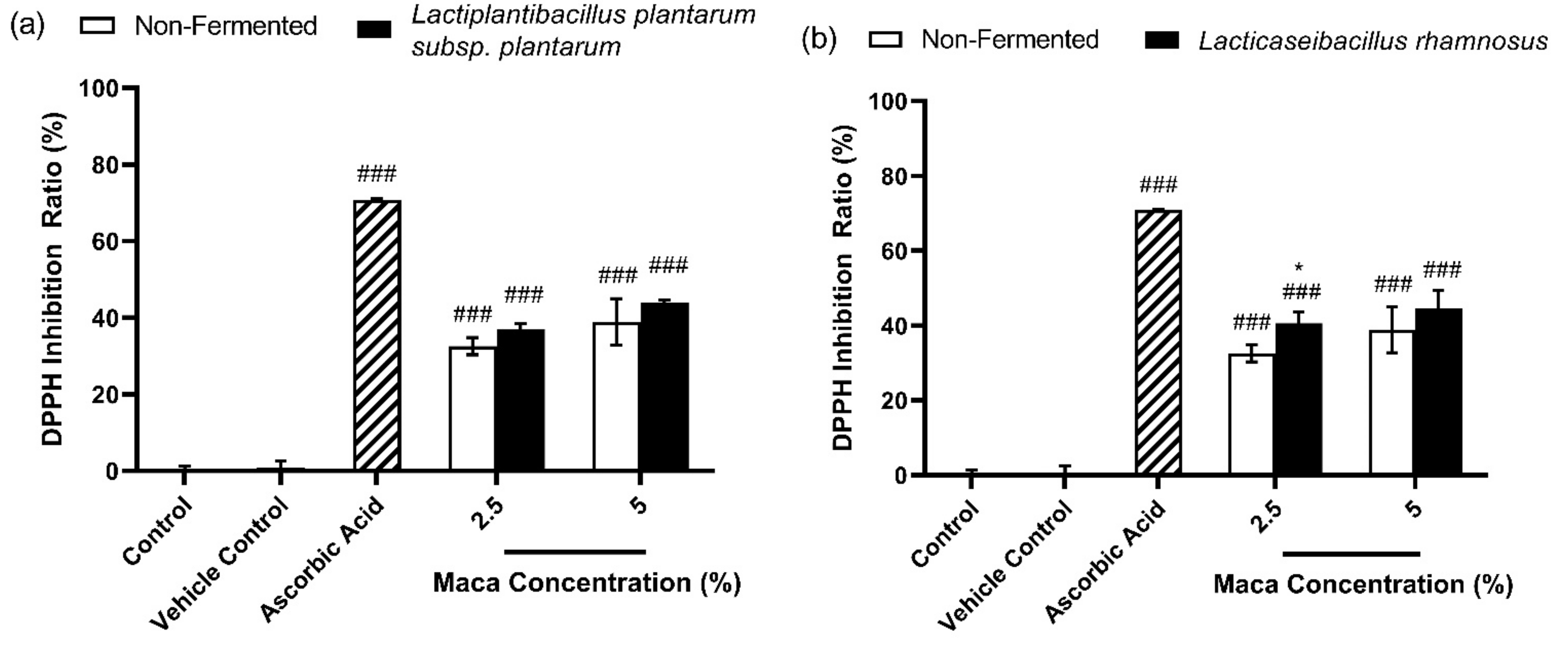
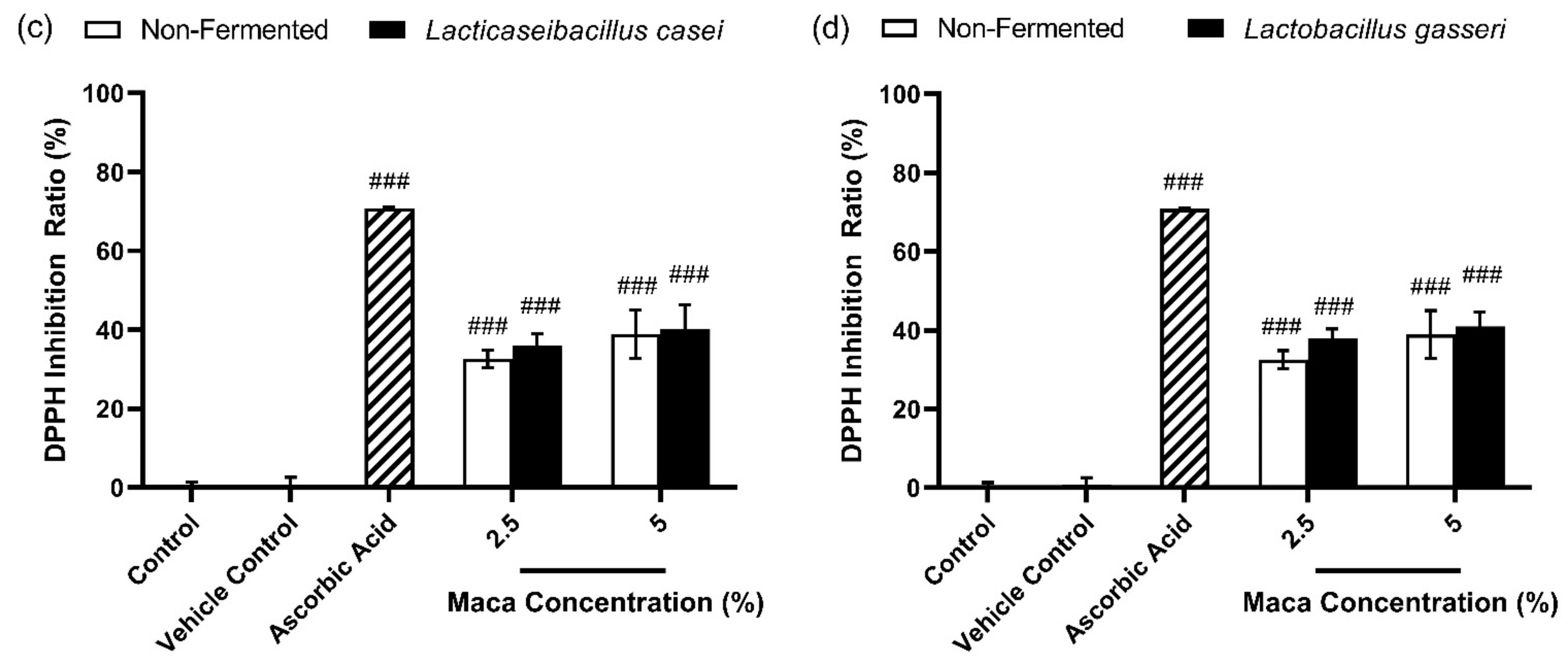
3.1. Effects of Antioxidants of Non-Fermented Maca Root Extracts Maintained through Fermentation by Lactobacilli
3.2. Total Phenolic Content of Non-Fermented Maca Root Extracts Maintained through Fermentation by Lactobacilli
3.3. Fermented Maca Root Extracts Suppresses NO Production Compared to Non-Fermented Maca Root Extracts
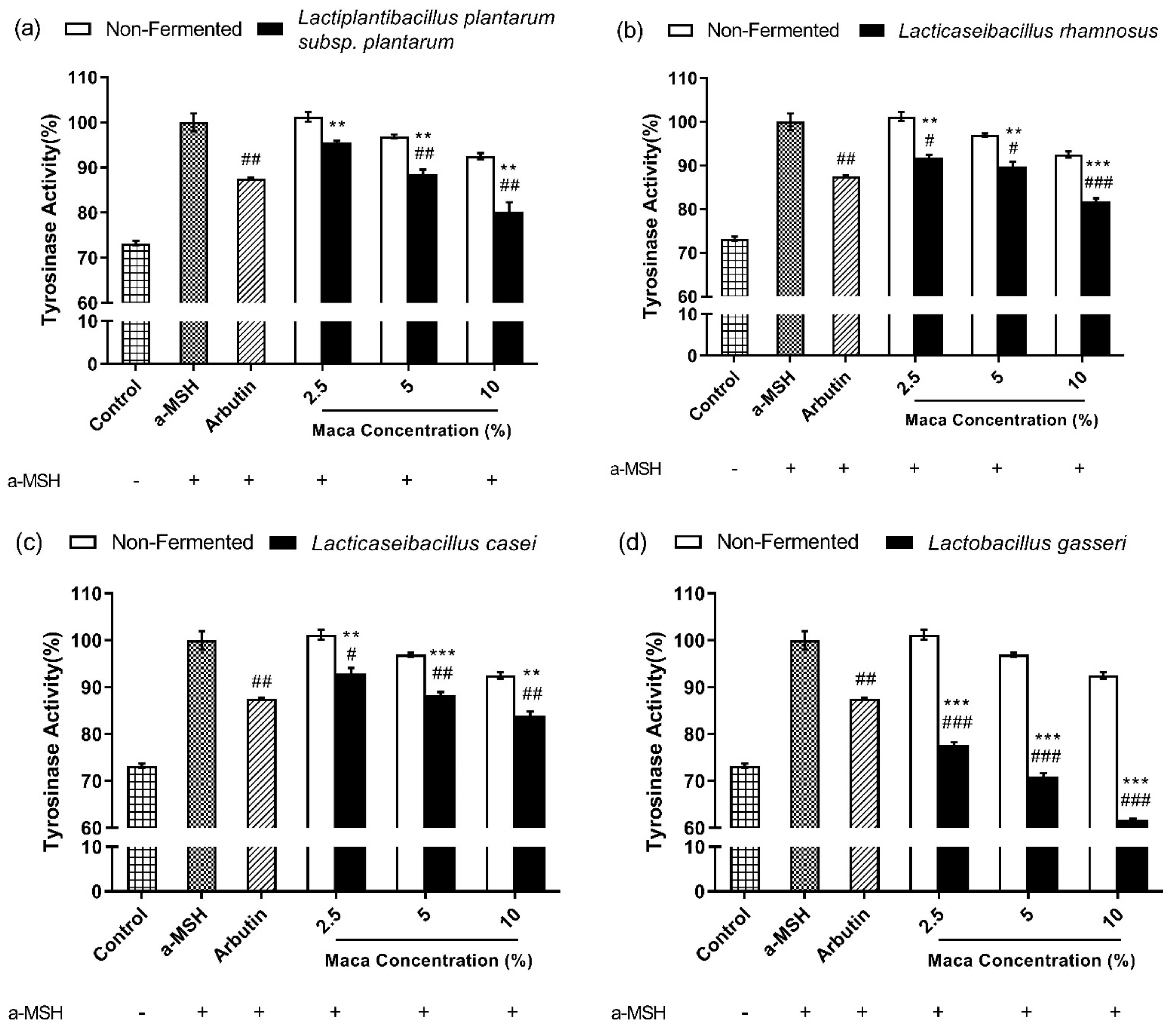
3.4. Fermented Maca Root Extracts Inhibit Mushroom Tyrosinase Activity
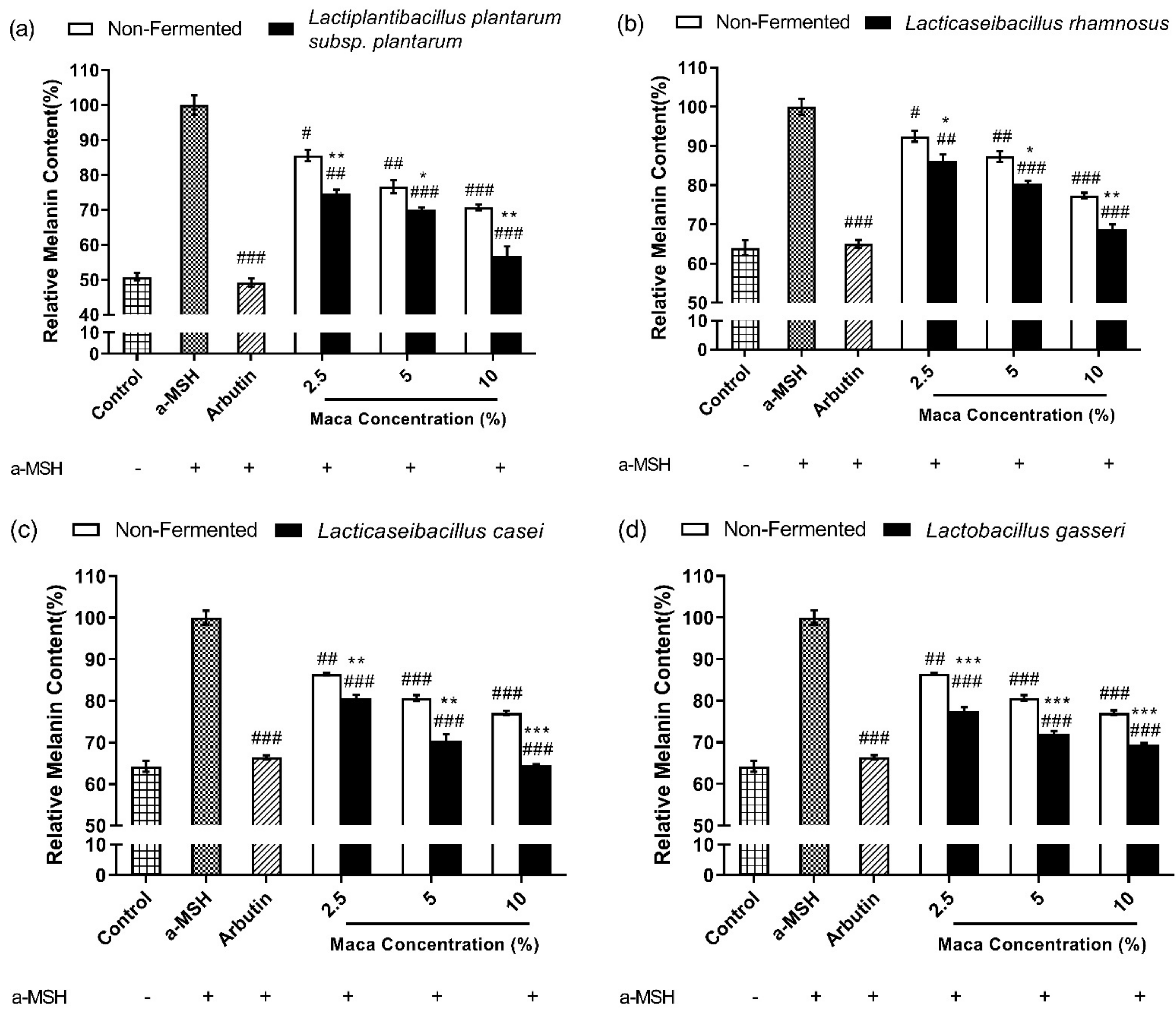
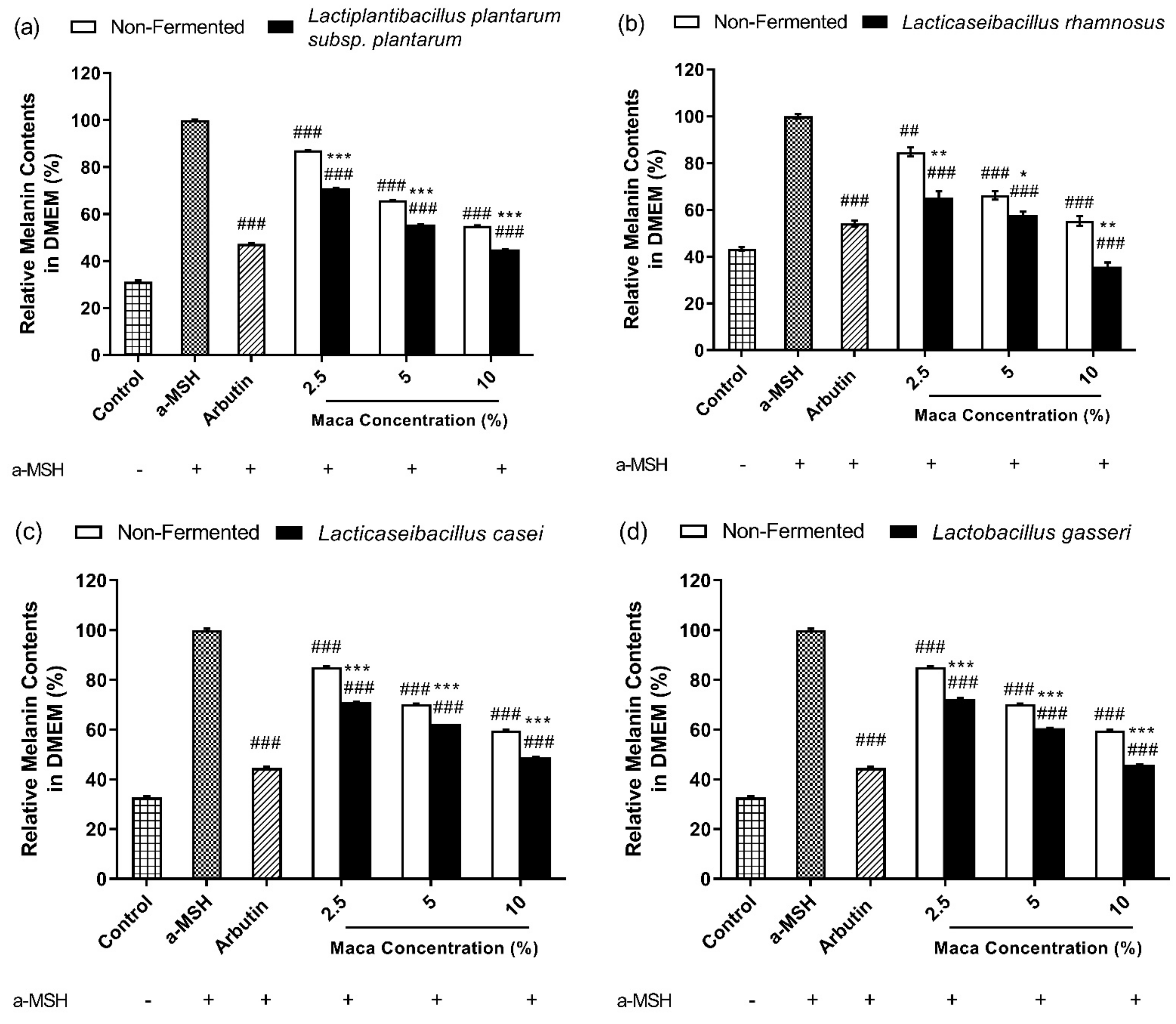
3.5. Fermented Maca Root Extracts Suppress Melanin Synthesis
3.6. Fermented Maca Root Extracts Inhibit Intracellular Tyrosinase Activity
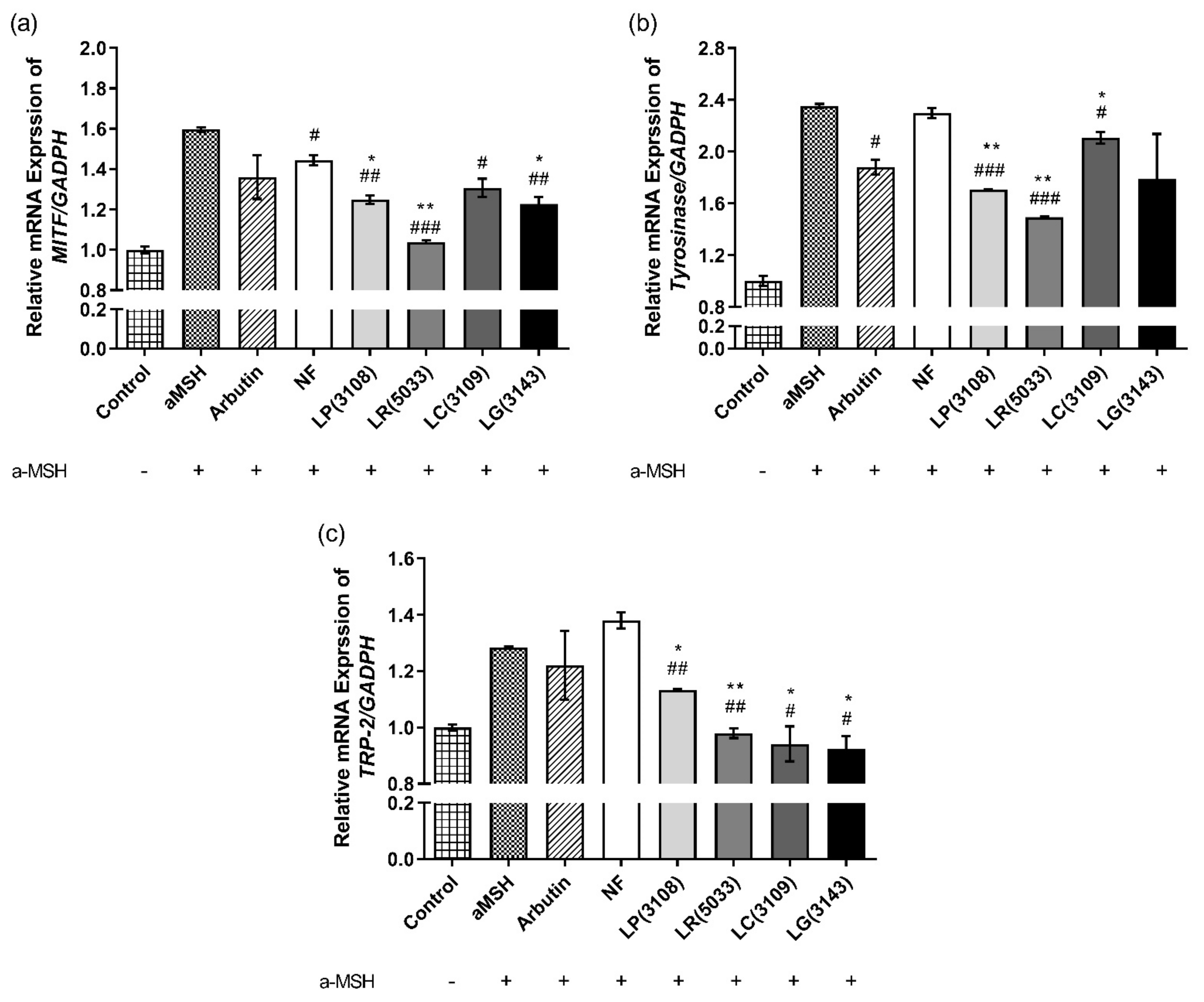
3.7. Fermented Maca Root Extracts Suppressed mRNA expression of MITF, Tyrosinase and TRP-2 in B16F10
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [PubMed]
- Zolkiewicz, J.; Marzec, A.; Ruszczynski, M.; Feleszko, W. Postbiotics-A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef] [PubMed]
- Scarpellini, E.; Rinninella, E.; Basilico, M.; Colomier, E.; Rasetti, C.; Larussa, T.; Santori, P.; Abenavoli, L. From Pre- and Probiotics to Post-Biotics: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Moore, R.J.; Stanley, D.; Chousalkar, K.K. The Gut Microbiota of Laying Hens and Its Manipulation with Prebiotics and Probiotics To Enhance Gut Health and Food Safety. Appl. Environ. Microbiol. 2020, 86, e00600-20. [Google Scholar] [CrossRef]
- Kim, S.K.; Guevarra, R.B.; Kim, Y.T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhou, D.D.; Gan, R.Y.; Huang, S.Y.; Zhao, C.N.; Shang, A.; Xu, X.Y.; Li, H.B. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef]
- Scott, E.; De Paepe, K.; Van de Wiele, T. Postbiotics and Their Health Modulatory Biomolecules. Biomolecules 2022, 12, 1640. [Google Scholar] [CrossRef]
- Hernandez-Granados, M.J.; Franco-Robles, E. Postbiotics in human health: Possible new functional ingredients? Food Res. Int. 2020, 137, 109660. [Google Scholar] [CrossRef]
- Vinderola, G.; Sanders, M.E.; Salminen, S.; Szajewska, H. Postbiotics: The concept and their use in healthy populations. Front. Nutr. 2022, 9, 1002213. [Google Scholar] [CrossRef]
- Puebla-Barragan, S.; Reid, G. Probiotics in Cosmetic and Personal Care Products: Trends and Challenges. Molecules 2021, 26, 1249. [Google Scholar] [CrossRef]
- Gueniche, A.; Liboutet, M.; Cheilian, S.; Fagot, D.; Juchaux, F.; Breton, L. Vitreoscilla filiformis Extract for Topical Skin Care: A Review. Front. Cell. Infect. Microbiol. 2021, 11, 747663. [Google Scholar] [CrossRef]
- Jung, Y.O.; Jeong, H.; Cho, Y.; Lee, E.O.; Jang, H.W.; Kim, J.; Nam, K.; Lim, K.M. Lysates of a Probiotic, Lactobacillus rhamnosus, Can Improve Skin Barrier Function in a Reconstructed Human Epidermis Model. Int. J. Mol. Sci. 2019, 20, 4289. [Google Scholar] [CrossRef]
- Gueniche, A.; Bastien, P.; Ovigne, J.M.; Kermici, M.; Courchay, G.; Chevalier, V.; Breton, L.; Castiel-Higounenc, I. Bifidobacterium longum lysate, a new ingredient for reactive skin. Exp. Dermatol. 2010, 19, e1–e8. [Google Scholar] [CrossRef]
- Gueniche, A.; Knaudt, B.; Schuck, E.; Volz, T.; Bastien, P.; Martin, R.; Rocken, M.; Breton, L.; Biedermann, T. Effects of nonpathogenic gram-negative bacterium Vitreoscilla filiformis lysate on atopic dermatitis: A prospective, randomized, double-blind, placebo-controlled clinical study. Br. J. Dermatol. 2008, 159, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Catic, T.; Pehlivanovic, B.; Pljakic, N.; Balicevac, A. The Moisturizing Efficacy of a Proprietary Dermo-Cosmetic Product (CLS02021) Versus Placebo in a 4-week Application Period. Med. Arch. 2022, 76, 108–114. [Google Scholar] [CrossRef]
- Nam, Y.; Kim, J.; Baek, J.; Kim, W. Improvement of Cutaneous Wound Healing via Topical Application of Heat-Killed Lactococcus chungangensis CAU 1447 on Diabetic Mice. Nutrients 2021, 13, 2666. [Google Scholar] [CrossRef]
- Duarte, M.; Oliveira, A.L.; Oliveira, C.; Pintado, M.; Amaro, A.; Madureira, A.R. Current postbiotics in the cosmetic market-an update and development opportunities. Appl. Microbiol. Biotechnol. 2022, 106, 5879–5891. [Google Scholar] [CrossRef]
- Gueniche, A.; Perin, O.; Bouslimani, A.; Landemaine, L.; Misra, N.; Cupferman, S.; Aguilar, L.; Clavaud, C.; Chopra, T.; Khodr, A. Advances in Microbiome-Derived Solutions and Methodologies Are Founding a New Era in Skin Health and Care. Pathogens 2022, 11, 121. [Google Scholar] [CrossRef]
- Tejada-Simon, M.V.; Pestka, J.J. Proinflammatory cytokine and nitric oxide induction in murine macrophages by cell wall and cytoplasmic extracts of lactic acid bacteria. J. Food Prot. 1999, 62, 1435–1444. [Google Scholar] [CrossRef]
- Matsuguchi, T.; Takagi, A.; Matsuzaki, T.; Nagaoka, M.; Ishikawa, K.; Yokokura, T.; Yoshikai, Y. Lipoteichoic acids from Lactobacillus strains elicit strong tumor necrosis factor alpha-inducing activities in macrophages through Toll-like receptor 2. Clin. Diagn. Lab. Immunol. 2003, 10, 259–266. [Google Scholar] [CrossRef]
- Kim, H.G.; Lee, S.Y.; Kim, N.R.; Lee, H.Y.; Ko, M.Y.; Jung, B.J.; Kim, C.M.; Lee, J.M.; Park, J.H.; Han, S.H.; et al. Lactobacillus plantarum lipoteichoic acid down-regulated Shigella flexneri peptidoglycan-induced inflammation. Mol. Immunol. 2011, 48, 382–391. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, A.; Gupta, M.; Padwad, Y.; Sharma, R. Cell-Free Culture Supernatant of Probiotic Lactobacillus fermentum Protects Against H(2)O(2)-Induced Premature Senescence by Suppressing ROS-Akt-mTOR Axis in Murine Preadipocytes. Probiotics Antimicrob. Proteins 2020, 12, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Niu, M.; Song, D.; Song, X.; Zhao, J.; Wu, Y.; Lu, B.; Niu, G. Preparation, partial characterization and biological activity of exopolysaccharides produced from Lactobacillus fermentum S1. J. Biosci. Bioeng. 2020, 129, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Tsilingiri, K.; Barbosa, T.; Penna, G.; Caprioli, F.; Sonzogni, A.; Viale, G.; Rescigno, M. Probiotic and postbiotic activity in health and disease: Comparison on a novel polarised ex-vivo organ culture model. Gut 2012, 61, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Merghni, A.; Dallel, I.; Noumi, E.; Kadmi, Y.; Hentati, H.; Tobji, S.; Ben Amor, A.; Mastouri, M. Antioxidant and antiproliferative potential of biosurfactants isolated from Lactobacillus casei and their anti-biofilm effect in oral Staphylococcus aureus strains. Microb. Pathog. 2017, 104, 84–89. [Google Scholar] [CrossRef]
- Teame, T.; Wang, A.; Xie, M.; Zhang, Z.; Yang, Y.; Ding, Q.; Gao, C.; Olsen, R.E.; Ran, C.; Zhou, Z. Paraprobiotics and Postbiotics of Probiotic Lactobacilli, Their Positive Effects on the Host and Action Mechanisms: A Review. Front. Nutr. 2020, 7, 570344. [Google Scholar] [CrossRef]
- Ong, J.S.; Taylor, T.D.; Yong, C.C.; Khoo, B.Y.; Sasidharan, S.; Choi, S.B.; Ohno, H.; Liong, M.T. Lactobacillus plantarum USM8613 Aids in Wound Healing and Suppresses Staphylococcus aureus Infection at Wound Sites. Probiotics Antimicrob. Proteins 2020, 12, 125–137. [Google Scholar] [CrossRef]
- Alves, E.; Gregorio, J.; Rijo, P.; Rosado, C.; Rodrigues, L.M. The Impact of Kefir on Epidermal Water Homeostasis in Healthy Human Skin. Life 2022, 12, 1075. [Google Scholar] [CrossRef]
- Kimoto-Nira, H. New lactic acid bacteria for skin health via oral intake of heat-killed or live cells. Anim. Sci. J. 2018, 89, 835–842. [Google Scholar] [CrossRef]
- Lukic, J.; Chen, V.; Strahinic, I.; Begovic, J.; Lev-Tov, H.; Davis, S.C.; Tomic-Canic, M.; Pastar, I. Probiotics or pro-healers: The role of beneficial bacteria in tissue repair. Wound Repair Regen. 2017, 25, 912–922. [Google Scholar] [CrossRef]
- Zettam, A.; Taleb, A.; Sauvage, S.; Boithias, L.; Belaidi, N.; Sanchez-Perez, J.M. Applications of a SWAT model to evaluate the contribution of the Tafna catchment (north-west Africa) to the nitrate load entering the Mediterranean Sea. Environ. Monit. Assess. 2020, 192, 510. [Google Scholar] [CrossRef]
- Baquerizo Nole, K.L.; Yim, E.; Keri, J.E. Probiotics and prebiotics in dermatology. J. Am. Acad. Dermatol. 2014, 71, 814–821. [Google Scholar] [CrossRef]
- Al-Ghazzewi, F.H.; Tester, R.F. Impact of prebiotics and probiotics on skin health. Benef. Microbes 2014, 5, 99–107. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, F. Chemical composition and health effects of maca (Lepidium meyenii). Food Chem. 2019, 288, 422–443. [Google Scholar] [CrossRef]
- Huarancca Reyes, T.; Esparza, E.; Crestani, G.; Limonchi, F.; Cruz, R.; Salinas, N.; Scartazza, A.; Guglielminetti, L.; Cosio, E. Physiological responses of maca (Lepidium meyenii Walp.) plants to UV radiation in its high-altitude mountain ecosystem. Sci. Rep. 2020, 10, 2654. [Google Scholar] [CrossRef] [PubMed]
- Zha, S.; Zhao, Q.; Chen, J.; Wang, L.; Zhang, G.; Zhang, H.; Zhao, B. Extraction, purification and antioxidant activities of the polysaccharides from maca (Lepidium meyenii). Carbohydr. Polym. 2014, 111, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Caicai, K.; Limin, H.; Liming, Z.; Zhiqiang, Z.; Yongwu, Y. Isolation, purification and antioxidant activity of polysaccharides from the leaves of maca (Lepidium Meyenii). Int. J. Biol. Macromol. 2018, 107, 2611–2619. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wei, J.; Gao, Y.; Chen, R. Antioxidant and antitumoral activities of isolated macamide and macaene fractions from Lepidium meyenii (Maca). Talanta 2021, 221, 121635. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, F.; Ge, F. Effects of combined extracts of Lepidium meyenii and Allium tuberosum Rottl. on erectile dysfunction. BMC Complement. Altern. Med. 2019, 19, 135. [Google Scholar] [CrossRef]
- da Silva Leitao Peres, N.; Cabrera Parra Bortoluzzi, L.; Medeiros Marques, L.L.; Formigoni, M.; Fuchs, R.H.B.; Droval, A.A.; Reitz Cardoso, F.A. Medicinal effects of Peruvian maca (Lepidium meyenii): A review. Food Funct. 2020, 11, 83–92. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, L.; Li, H.; Xie, W.; Liu, J.; Zhang, Y.; Li, Y.; Wang, C. In vivo and in vitro neuroprotective effects of maca polysaccharide. Front. Biosci. Landmark Ed. 2022, 27, 8. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, X.; Wang, L.; Xin, X.; Zhang, M. Effects of two polysaccharides from Lepidium meyenii (maca) on intestinal immunity and inflammation in vitro. Food Funct. 2022, 13, 3441–3452. [Google Scholar] [CrossRef]
- He, P.; Pan, L.; Wu, H.; Zhang, L.; Zhang, Y.; Zhang, Y.; Yang, J.; Lin, Z.; Zhang, M. Isolation, Identification, and Immunomodulatory Mechanism of Peptides from Lepidium meyenii (Maca) Protein Hydrolysate. J. Agric. Food Chem. 2022, 70, 4328–4341. [Google Scholar] [CrossRef]
- Castaneda-Alarcon, M.; Bell-Cortez, C.; Hidalgo-Ascensios, J.; Moreno-Exebio, L. Photoprotective activity of a cream containing lyophilized aqueous extract of Lepidium meyenii (MACA) against ultraviolet irradiation on mouse skin. Rev. Peru Med. Exp. Salud Publica 2021, 38, 434–441. [Google Scholar] [CrossRef]
- Nunez, D.; Olavegoya, P.; Gonzales, G.F.; Gonzales-Castaneda, C. Red Maca (Lepidium meyenii), a Plant from the Peruvian Highlands, Promotes Skin Wound Healing at Sea Level and at High Altitude in Adult Male Mice. High Alt. Med. Biol. 2017, 18, 372–383. [Google Scholar] [CrossRef]
- Tsai, W.H.; Chou, C.H.; Chiang, Y.J.; Lin, C.G.; Lee, C.H. Regulatory effects of Lactobacillus plantarum-GMNL6 on human skin health by improving skin microbiome. Int. J. Med. Sci. 2021, 18, 1114–1120. [Google Scholar] [CrossRef]
- Saito, Y.; Mihara, T.; Maruyama, K.; Saito, J.; Ikeda, M.; Tomonaga, A.; Kumagai, T. Effects of intake of Lactobacillus casei subsp. casei 327 on skin conditions: A randomized, double-blind, placebo-controlled, parallel-group study in women. Biosci. Microbiota Food Health 2017, 36, 111–120. [Google Scholar] [CrossRef]
- Lee, K.; Kim, H.J.; Kim, S.A.; Park, S.D.; Shim, J.J.; Lee, J.L. Exopolysaccharide from Lactobacillus plantarum HY7714 Protects against Skin Aging through Skin-Gut Axis Communication. Molecules 2021, 26, 1651. [Google Scholar] [CrossRef]
- Moreira, C.F.; Cassini-Vieira, P.; Canesso, M.C.C.; Felipetto, M.; Ranfley, H.; Teixeira, M.M.; Nicoli, J.R.; Martins, F.S.; Barcelos, L.S. Lactobacillus rhamnosus CGMCC 1.3724 (LPR) Improves Skin Wound Healing and Reduces Scar Formation in Mice. Probiotics Antimicrob. Proteins 2021, 13, 709–719. [Google Scholar] [CrossRef]
- Morais, I.M.C.; Cordeiro, A.L.; Teixeira, G.S.; Domingues, V.S.; Nardi, R.M.D.; Monteiro, A.S.; Alves, R.J.; Siqueira, E.P.; Santos, V.L. Biological and physicochemical properties of biosurfactants produced by Lactobacillus jensenii P(6A) and Lactobacillus gasseri P(65). Microb. Cell Fact. 2017, 16, 155. [Google Scholar] [CrossRef]
- No, J.K.; Soung, D.Y.; Kim, Y.J.; Shim, K.H.; Jun, Y.S.; Rhee, S.H.; Yokozawa, T.; Chung, H.Y. Inhibition of tyrosinase by green tea components. Life Sci 1999, 65, PL241–PL246. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.H.; Jang, G.Y.; Ji, Y.J.; Lee, J.H.; Choi, S.J.; Hyun, T.K.; Kim, H.D. Antioxidant and Anti-Melanogenic Activities of Heat-Treated Licorice (Wongam, Glycyrrhiza glabra × G. uralensis) Extract. Curr. Issues Mol. Biol. 2021, 43, 1171–1187. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.I.; Yun, J.M.; Park, E.J.; Kim, Y.S.; Lee, Y.M.; Lim, J.H. Plumbagin Suppresses alpha-MSH-Induced Melanogenesis in B16F10 Mouse Melanoma Cells by Inhibiting Tyrosinase Activity. Int. J. Mol. Sci. 2017, 18, 320. [Google Scholar] [CrossRef] [PubMed]
- Eghbali-Feriz, S.; Taleghani, A.; Al-Najjar, H.; Emami, S.A.; Rahimi, H.; Asili, J.; Hasanzadeh, S.; Tayarani-Najaran, Z. Anti-melanogenesis and anti-tyrosinase properties of Pistacia atlantica subsp. mutica extracts on B16F10 murine melanoma cells. Res. Pharm. Sci. 2018, 13, 533–545. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, H.; Chu, J.H.; Chou, G.X.; Yu, Z.L. Activation of p38 MAPK pathway contributes to the melanogenic property of apigenin in B16 cells. Exp. Dermatol. 2011, 20, 755–757. [Google Scholar] [CrossRef]
- Tachibana, M. Cochlear melanocytes and MITF signaling. J. Investig. Dermatol. Symp. Proc. 2001, 6, 95–98. [Google Scholar] [CrossRef]
- Jung, H.J.; Park, S.H.; Cho, K.M.; Jung, K.I.; Cho, D.; Kim, T.S. Threonyl-tRNA Synthetase Promotes T Helper Type 1 Cell Responses by Inducing Dendritic Cell Maturation and IL-12 Production via an NF-kappaB Pathway. Front. Immunol. 2020, 11, 571959. [Google Scholar] [CrossRef]
- Kang, W.; Choi, D.; Park, S.; Park, T. Carvone Decreases Melanin Content by Inhibiting Melanoma Cell Proliferation via the Cyclic Adenosine Monophosphate (cAMP) Pathway. Molecules 2020, 25, 5191. [Google Scholar] [CrossRef]
- Lee, J.; Renita, M.; Fioritto, R.J.; St Martin, S.K.; Schwartz, S.J.; Vodovotz, Y. Isoflavone characterization and antioxidant activity of ohio soybeans. J. Agric. Food Chem. 2004, 52, 2647–2651. [Google Scholar] [CrossRef]
- Hatami, T.; Emami, S.A.; Miraghaee, S.S.; Mojarrab, M. Total Phenolic Contents and Antioxidant Activities of Different Extracts and Fractions from the Aerial Parts of Artemisia biennis Willd. Iran. J. Pharm. Res. 2014, 13, 551–559. [Google Scholar]
- Yang, T.; Li, Y.; Lyu, Z.; Huang, K.; Corrigan, C.J.; Ying, S.; Wang, W.; Wang, C. Characteristics of Proinflammatory Cytokines and Chemokines in Airways of Asthmatics: Relationships with Disease Severity and Infiltration of Inflammatory Cells. Chin. Med. J. 2017, 130, 2033–2040. [Google Scholar] [CrossRef]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.C.; Plummer, A.L.; Taylor, D.R.; American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels for Clinical Applications. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Schiaffino, M.V. Signaling pathways in melanosome biogenesis and pathology. Int. J. Biochem. Cell Biol. 2010, 42, 1094–1104. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Xin, Y.; Zheng, M.; Xu, F.; Xi, X.; Cao, H.; Cui, X.; Guo, H.; Han, C. Maca Cosmetics: A Review on Constituents, Therapeutics and Advantages. J. Oleo Sci. 2018, 67, 789–800. [Google Scholar] [CrossRef]
- Kim, J.H.; Bae, J.T.; Song, M.H.; Lee, G.S.; Choe, S.Y.; Pyo, H.B. Biological activities of Fructus arctii fermented with the basidiomycete Grifola frondosa. Arch. Pharm. Res. 2010, 33, 1943–1951. [Google Scholar] [CrossRef]
- Park, J.Y.; Song, M.W.; Kim, K.T.; Paik, H.D. Improved Antioxidative, Anti-Inflammatory, and Antimelanogenic Effects of Fermented Hydroponic Ginseng with Bacillus Strains. Antioxidants 2022, 11, 1848. [Google Scholar] [CrossRef]
- Bae, J.T.; Ko, H.J.; Kim, G.B.; Pyo, H.B.; Lee, G.S. Protective effects of fermented Citrus unshiu peel extract against ultraviolet-A-induced photoageing in human dermal fibrobolasts. Phytother. Res. 2012, 26, 1851–1856. [Google Scholar] [CrossRef]
- Hsu, M.F.; Chiang, B.H. Stimulating effects of Bacillus subtilis natto-fermented Radix astragali on hyaluronic acid production in human skin cells. J. Ethnopharmacol. 2009, 125, 474–481. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, M.R.; Park, Y.; Park, H.J.; Chang, U.J.; Kim, S.Y.; Suh, H.J. Fermenting red ginseng enhances its safety and efficacy as a novel skin care anti-aging ingredient: In vitro and animal study. J. Med. Food 2012, 15, 1015–1023. [Google Scholar] [CrossRef]
- Sangkaew, O.; Yompakdee, C. Fermented Unpolished Black Rice (Oryza sativa L.) Inhibits Melanogenesis via ERK, p38, and AKT Phosphorylation in B16F10 Melanoma Cells. J. Microbiol. Biotechnol. 2020, 30, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Kwak, Y.J.; Kim, K.S.; Kim, K.M.; Yu, H.Y.; Chung, E.; Kim, S.J.; Cha, J.Y.; Lee, Y.C.; Lee, J.H. Fermented Viola mandshurica inhibits melanogenesis in B16 melanoma cells. Biosci. Biotechnol. Biochem. 2011, 75, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Chae, G.Y.; Ha, B.J. The Comparative Evaluation of Fermented and Non-fermented Soybean Extract on Antioxidation and Whitening. Toxicol. Res. 2011, 27, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.L.; Jang, H.J.; Kim, K.B. Anti-wrinkle effect of fermented black ginseng on human fibroblasts. Int. J. Mol. Med. 2017, 39, 681–686. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef]
- Sales, P.M.; Souza, P.M.; Simeoni, L.A.; Silveira, D. alpha-Amylase inhibitors: A review of raw material and isolated compounds from plant source. J. Pharm. Pharm. Sci. 2012, 15, 141–183. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Filannino, P.; Cavoski, I.; Thlien, N.; Vincentini, O.; De Angelis, M.; Silano, M.; Gobbetti, M.; Di Cagno, R. Lactic Acid Fermentation of Cactus Cladodes (Opuntia ficus-indica L.) Generates Flavonoid Derivatives with Antioxidant and Anti-Inflammatory Properties. PLoS ONE 2016, 11, e0152575. [Google Scholar] [CrossRef]
- Hwang, J.E.; Kim, K.T.; Paik, H.D. Improved Antioxidant, Anti-inflammatory, and Anti-adipogenic Properties of Hydroponic Ginseng Fermented by Leuconostoc mesenteroides KCCM 12010P. Molecules 2019, 24, 3359. [Google Scholar] [CrossRef]
- Xu, J.; Hussain, M.; Su, W.; Yao, Q.; Yang, G.; Zhong, Y.; Zhou, L.; Huang, X.; Wang, Z.; Gu, Q.; et al. Effects of novel cellulase (Cel 906) and probiotic yeast fermentation on antioxidant and anti-inflammatory activities of vine tea (Ampelopsis grossedentata). Front. Bioeng. Biotechnol. 2022, 10, 1006316. [Google Scholar] [CrossRef]
- Albertini, B.; Schoubben, A.; Guarnaccia, D.; Pinelli, F.; Della Vecchia, M.; Ricci, M.; Di Renzo, G.C.; Blasi, P. Effect of fermentation and drying on cocoa polyphenols. J. Agric. Food. Chem. 2015, 63, 9948–9953. [Google Scholar] [CrossRef]
- Gonzales, G.F. Ethnobiology and Ethnopharmacology of Lepidium meyenii (Maca), a Plant from the Peruvian Highlands. Evid. Based Complement. Alternat. Med. 2012, 2012, 193496. [Google Scholar] [CrossRef]
- Li, Y.; Xu, F.; Zheng, M.; Xi, X.; Cui, X.; Han, C. Maca polysaccharides: A review of compositions, isolation, therapeutics and prospects. Int. J. Biol. Macromol. 2018, 111, 894–902. [Google Scholar] [CrossRef]
- Boo, Y.C. Up- or Downregulation of Melanin Synthesis Using Amino Acids, Peptides, and Their Analogs. Biomedicines 2020, 8, 322. [Google Scholar] [CrossRef]
- Sonthalia, S.; Daulatabad, D.; Sarkar, R. Glutathione as a skin whitening agent: Facts, myths, evidence and controversies. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 262–272. [Google Scholar] [CrossRef]
- Lorin, J.; Zeller, M.; Guilland, J.C.; Cottin, Y.; Vergely, C.; Rochette, L. Arginine and nitric oxide synthase: Regulatory mechanisms and cardiovascular aspects. Mol. Nutr. Food Res. 2014, 58, 101–116. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, G.; Lai, F.; Wu, H. Structural Characterization and Immunomodulatory Activity of a Novel Polysaccharide from Lepidium meyenii. J. Agric. Food Chem. 2016, 64, 1921–1931. [Google Scholar] [CrossRef]
- Sugahara, H.; Nagayama, K.; Ikeda, S.; Hirota, T.; Nakamura, Y. D- and l-amino acid concentrations in culture broth of Lactobacillus are highly dependent on the phylogenetic group of Lactobacillus. Biochem. Biophys. Rep. 2021, 27, 101073. [Google Scholar] [CrossRef]
- Biz, C.; Fantoni, I.; Crepaldi, N.; Zonta, F.; Buffon, L.; Corradin, M.; Lissandron, A.; Ruggieri, P. Clinical practice and nursing management of pre-operative skin or skeletal traction for hip fractures in elderly patients: A cross-sectional three-institution study. Int. J. Orthop. Trauma Nurs. 2019, 32, 32–40. [Google Scholar] [CrossRef]
- Lee, I.C.; Caggianiello, G.; van Swam, I.I.; Taverne, N.; Meijerink, M.; Bron, P.A.; Spano, G.; Kleerebezem, M. Strain-Specific Features of Extracellular Polysaccharides and Their Impact on Lactobacillus plantarum-Host Interactions. Appl. Environ. Microbiol. 2016, 82, 3959–3970. [Google Scholar] [CrossRef]
- Ganzle, M.G.; Follador, R. Metabolism of oligosaccharides and starch in lactobacilli: A review. Front. Microbiol. 2012, 3, 340. [Google Scholar] [CrossRef] [PubMed]
- Kimoto-Nira, H.; Aoki, R.; Sasaki, K.; Suzuki, C.; Mizumachi, K. Oral intake of heat-killed cells of Lactococcus lactis strain H61 promotes skin health in women. J. Nutr. Sci. 2012, 1, e18. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.H.; Chen, C.W.; Yi, T.H.; Huang, Y.F.; Kuo, Y.W.; Lin, J.H.; Chen, J.F.; Tsai, S.Y.; Chan, L.P.; Liang, C.H. Novel application of a Co-Fermented postbiotics of TYCA06/AP-32/CP-9/collagen in the improvement of acne vulgaris-A randomized clinical study of efficacy evaluation. J. Cosmet. Dermatol. 2022, 21, 6249–6260. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Guo, C.; Wang, Q.; Feng, C.; Duan, Z. A pilot study on the efficacy of topical lotion containing anti-acne postbiotic in subjects with mild-to-moderate acne. Front. Med. 2022, 9, 1064460. [Google Scholar] [CrossRef]
- Millington, G.W. Proopiomelanocortin (POMC): The cutaneous roles of its melanocortin products and receptors. Clin. Exp. Dermatol. 2006, 31, 407–412. [Google Scholar] [CrossRef]
- Huang, H.C.; Chou, Y.C.; Wu, C.Y.; Chang, T.M. [8]-Gingerol inhibits melanogenesis in murine melanoma cells through down-regulation of the MAPK and PKA signal pathways. Biochem. Biophys. Res. Commun. 2013, 438, 375–381. [Google Scholar] [CrossRef]
- Hacker, E.; Boyce, Z.; Kimlin, M.G.; Wockner, L.; Pollak, T.; Vaartjes, S.A.; Hayward, N.K.; Whiteman, D.C. The effect of MC1R variants and sunscreen on the response of human melanocytes in vivo to ultraviolet radiation and implications for melanoma. Pigment. Cell Melanoma Res. 2013, 26, 835–844. [Google Scholar] [CrossRef]
- Rouzaud, F.; Kadekaro, A.L.; Abdel-Malek, Z.A.; Hearing, V.J. MC1R and the response of melanocytes to ultraviolet radiation. Mutat. Res. 2005, 571, 133–152. [Google Scholar] [CrossRef]
- Feng, Z.C.; Riopel, M.; Popell, A.; Wang, R. A survival Kit for pancreatic beta cells: Stem cell factor and c-Kit receptor tyrosine kinase. Diabetologia 2015, 58, 654–665. [Google Scholar] [CrossRef]
- Li, P.H.; Liu, L.H.; Chang, C.C.; Gao, R.; Leung, C.H.; Ma, D.L.; David Wang, H.M. Silencing Stem Cell Factor Gene in Fibroblasts to Regulate Paracrine Factor Productions and Enhance c-Kit Expression in Melanocytes on Melanogenesis. Int. J. Mol. Sci. 2018, 19, 1475. [Google Scholar] [CrossRef]
- Liu, L.; Fu, M.; Pei, S.; Zhou, L.; Shang, J. R-Fluoxetine Increases Melanin Synthesis Through a 5-HT1A/2A Receptor and p38 MAPK Signaling Pathways. Int. J. Mol. Sci. 2018, 20, 80. [Google Scholar] [CrossRef]
| Gene Name | Sequence | Base Pairs |
|---|---|---|
| mGAPDH [57] | F: 5′—ACATCAAGAAGGTGGTGAAG—3′ R: 5′—ATTCAAGAGAGTAGGGAGGG—3′ | 392 bp |
| mMITF [58] | F: 5′—AGCGTGTATTTTCCCCACAG—3′ R: 5′—TAGCTCCTTAATGCGGTCGT—3′ | 124 bp |
| mTyrosinase | F: 5′—CAGGCTCCCATCTTCAGCAGAT—3′ R: 5′—ATCCCTGTGAGTGGACTGGCAA—3′ | 132 bp |
| mTRP-2 | F: 5′—GCAAGATTGCCTGTCTCTCCAG—3′ R: 5′—CTTGAGAGTCCAGTGTTCCGTC—3′ | 119 bp |
| Treatment | Total Phenolic Content (mg GAE/100 µL Extracts) | ||
|---|---|---|---|
| 2.5% of Maca Root | 5% of Maca Root | 10% of Maca Root | |
| Non-fermented | 3.06 ± 0.02 | 4.91 ± 0.04 | 9.19 ± 0.01 |
| L. plantarum KCTC 3108 | 3.01 ± 0.06 | 5.06 ± 0.06 | 8.93 ± 0.16 |
| L. rhamnosus KCTC 5033 | 3.14 ± 0.10 | 5.05 ± 0.09 | 8.95 ± 0.02 |
| L. casei KCTC 3109 | 2.94 ± 0.05 | 4.95 ± 0.08 | 8.97 ± 0.05 |
| L. gasseri KCTC 3143 | 3.1 ± 00.09 | 5.18 ± 0.06 | 8.91 ± 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Cho, H.; Gil, M.; Kim, K.E. Anti-Inflammation and Anti-Melanogenic Effects of Maca Root Extracts Fermented Using Lactobacillus Strains. Antioxidants 2023, 12, 798. https://doi.org/10.3390/antiox12040798
Yang J, Cho H, Gil M, Kim KE. Anti-Inflammation and Anti-Melanogenic Effects of Maca Root Extracts Fermented Using Lactobacillus Strains. Antioxidants. 2023; 12(4):798. https://doi.org/10.3390/antiox12040798
Chicago/Turabian StyleYang, Jisun, Hyeijin Cho, Minchan Gil, and Kyung Eun Kim. 2023. "Anti-Inflammation and Anti-Melanogenic Effects of Maca Root Extracts Fermented Using Lactobacillus Strains" Antioxidants 12, no. 4: 798. https://doi.org/10.3390/antiox12040798
APA StyleYang, J., Cho, H., Gil, M., & Kim, K. E. (2023). Anti-Inflammation and Anti-Melanogenic Effects of Maca Root Extracts Fermented Using Lactobacillus Strains. Antioxidants, 12(4), 798. https://doi.org/10.3390/antiox12040798







