Recent Advances in Biotechnologies for the Treatment of Environmental Pollutants Based on Reactive Sulfur Species
Abstract
1. Introduction
2. RSS Definition and Relationship with Environmental Management
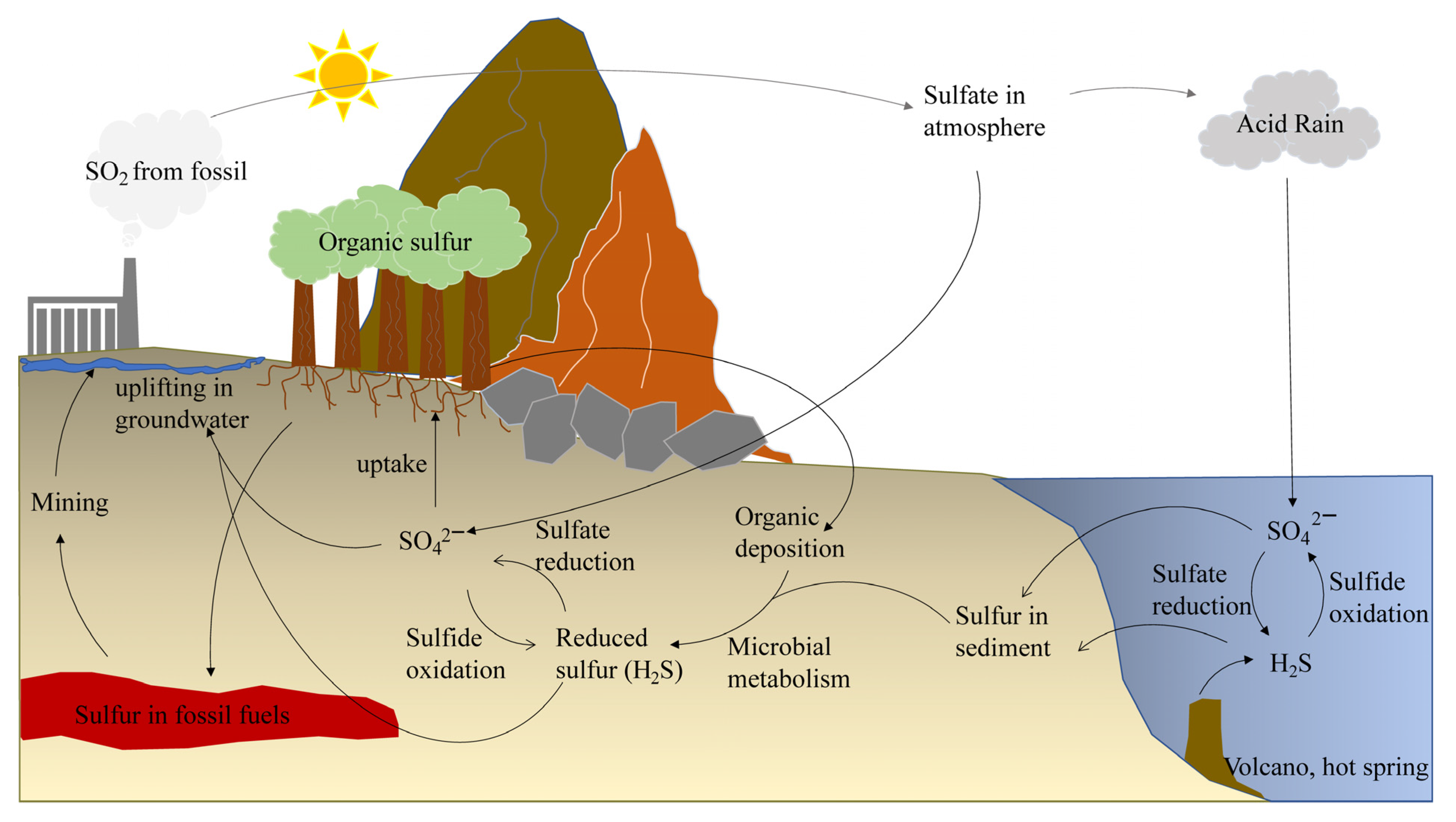
2.1. Origins and Definition of RSS
2.2. Relationship between RSS and Environmental Pollutant Management
3. RSS-Related Bioprocesses for the Treatment of Environmental Pollutants
3.1. Sulfur-Reduction-Based Biological Treatment
3.1.1. Sulfate-Reduction Bioreactors
3.1.2. Sulfate-Reducing Bacteria (SRB) and Molecular Mechanisms
3.1.3. S0-Based Reduction Bioreactors
3.1.4. Sulfur-Reducing Bacteria (S0RB) and Molecular Mechanisms
3.2. Sulfur-Oxidation-Based Biological Treatment
3.2.1. Sulfide-Oxidation Bioreactors
3.2.2. Sulfide-Oxidation Bacteria (SOB) and Molecular Mechanisms
3.2.3. S0-Based Oxidation Bioreactors
3.2.4. Sulfur-Oxidation Bacteria (S0OB) and Molecular Mechanisms
4. Prospects and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raiswell, R.; Canfield, D.E. The iron biogeochemical cycle past and present. Geochem. Perspect. 2012, 1, 1–220. [Google Scholar] [CrossRef]
- Olson, K.R.; Straub, K.D. The role of hydrogen sulfide in evolution and the evolution of hydrogen sulfide in metabolism and signaling. Physiology 2016, 31, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Schopf, J.W. Geological evidence of oxygenic photosynthesis and the biotic response to the 2400-2200 Ma “Great Oxidation Event”. Biochem.-Mosc. 2014, 79, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P.; Sies, H. The Redox Code. Antioxid. Redox Signal. 2015, 23, 734–746. [Google Scholar] [CrossRef]
- Grman, M.; Nasim, M.J.; Leontiev, R.; Misak, A.; Jakusova, V.; Ondrias, K.; Jacob, C. Inorganic reactive sulfur-nitrogen species: Intricate release mechanisms or cacophony in yellow, blue and red? Antioxidants 2017, 6, 14. [Google Scholar] [CrossRef]
- Giles, G.I.; Jacob, C. Reactive sulfur species: An emerging concept in oxidative stress. Biol. Chem. 2002, 383, 375–388. [Google Scholar] [CrossRef]
- Olson, K.R. Reactive oxygen species or reactive sulfur species: Why we should consider the latter. J. Exp. Biol. 2020, 223, 196352. [Google Scholar] [CrossRef]
- Czerewko, M.A.; Cripps, J.C.; Reid, J.M.; Duffell, C.G. Sulfur species in geological materials—sources and quantification. Cem. Concr. Compos. 2003, 25, 657–671. [Google Scholar] [CrossRef]
- Fike, D.A.; Bradley, A.S.; Rose, C.V. Rethinking the Ancient Sulfur Cycle. In Annual Review of Earth and Planetary Sciences; Jeanloz, R., Freeman, K.H., Eds.; Annual Reviews: Palo Alto, CA, USA, 2015; Volume 43, pp. 593–622. [Google Scholar]
- Giles, G.I.; Tasker, K.M.; Jacob, C. Hypothesis: The role of reactive sulfur species in oxidative stress. Free Radic. Biol. Med. 2001, 31, 1279–1283. [Google Scholar] [CrossRef]
- Brannan, R.G. Reactive sulfur species act as prooxidants in liposomal and skeletal muscle model systems. J. Agric. Food Chem. 2010, 58, 3767–3771. [Google Scholar] [CrossRef]
- Gruhlke, M.C.H.; Slusarenko, A.J. The biology of reactive sulfur species (RSS). Plant Physiol. Biochem. 2012, 59, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Ingenbleek, Y. The nutritional relationship linking sulfur to nitrogen in living organisms. J. Nutr. 2006, 136S, 1641S–1651S. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Bailey, S.M. Redox biology of hydrogen sulfide: Implications for physiology, pathophysiology, and pharmacology. Redox Biol. 2013, 1, 32–39. [Google Scholar] [CrossRef]
- Czyzewski, B.K.; Wang, D. Identification and characterization of a bacterial hydrosulphide ion channel. Nature 2012, 483, 155–494. [Google Scholar] [CrossRef] [PubMed]
- Maki, J.S. Bacterial intracellular sulfur globules: Structure and function. J. Plant Biochem. Biotechnol. 2013, 23, 270–280. [Google Scholar] [CrossRef]
- Chen, Z.; Xia, Y.; Liu, H.; Liu, H.; Xun, L. The mechanisms of thiosulfate toxicity against Saccharomyces cerevisiae. Antioxidants 2021, 10, 646. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xuan, G.; Liu, H.; Xia, Y.; Xun, L. Sulfane sulfur is a strong inducer of the multiple antibiotic resistance regulator MarR in Escherichia coli. Antioxidants 2021, 10, 1778. [Google Scholar] [CrossRef]
- Toohey, J.I.; Cooper, A.J.L. Thiosulfoxide (sulfane) sulfur: New chemistry and new regulatory roles in biology. Molecules 2014, 19, 12789–12813. [Google Scholar] [CrossRef]
- Lau, N.; Pluth, M.D. Reactive sulfur species (RSS): Persulfides, polysulfides, potential, and problems. Curr. Opin. Chem. Biol. 2019, 49, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.; Xin, Y.; Chen, Z.; Xia, Y.; Xun, L.; Liu, H. Sulfide-quinone oxidoreductase is required for cysteine synthesis and indispensable to mitochondrial health. Redox Biol. 2021, 47, 102169. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Z.; Zhang, X.; Xin, Y.; Xia, Y.; Xun, L.; Liu, H. Rhodanese Rdl2 produces reactive sulfur species to protect mitochondria from reactive oxygen species. Free Radic. Biol. Med. 2021, 177, 287–298. [Google Scholar] [CrossRef]
- Su, C.; Ran, X.; Hu, J.; Shao, C. Photocatalytic process of simultaneous desulfurization and denitrification of flue gas by tio2-polyacrylonitrile nanofibers. Environ. Sci. Technol. 2013, 47, 11562–11568. [Google Scholar] [CrossRef] [PubMed]
- Guimera, X.; Mora, M.; Dorado, A.D.; Bonsfills, A.; Gabriel, D.; Gamisans, X. Optimization of SO2 and NOx sequential wet absorption in a two-stage bioscrubber for elemental sulphur valorisation. Environ. Sci. Pollut. Res. 2021, 28, 24605–24617. [Google Scholar] [CrossRef] [PubMed]
- Crowe, S.A.; Paris, G.; Katsev, S.; Jones, C.; Kim, S.; Zerkle, A.L.; Nomosatryo, S.; Fowle, D.A.; Adkins, J.F.; Sessions, A.L.; et al. Sulfate was a trace constituent of Archean seawater. Science 2014, 346, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Hao, T.; Xiang, P.; Mackey, H.R.; Chi, K.; Lu, H.; Chui, H.; van Loosdrecht, M.C.M.; Chen, G. A review of biological sulfate conversions in wastewater treatment. Water Res. 2014, 65, 1–21. [Google Scholar] [CrossRef]
- Zhang, L.; Qiu, Y.; Zhou, Y.; Chen, G.; Loosdrecht, M.C.M.V.; Jiang, F. Elemental sulfur as electron donor and/or acceptor: Mechanisms, applications and perspectives for biological water and wastewater treatment. Water Res. 2021, 202, 117373. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Wang, J. Various electron donors for biological nitrate removal: A review. Sci. Total Environ. 2021, 794, 148699. [Google Scholar] [CrossRef]
- Wang, J.; Lu, H.; Chen, G.; Lau, G.N.; Tsang, W.L.; van Loosdrecht, M.C.M. A novel sulfate reduction, autotrophic denitrification, nitrification integrated (SANI) process for saline wastewater treatment. Water Res. 2009, 43, 2363–2372. [Google Scholar] [CrossRef]
- Jin, Q.; Bethke, C.M. Cellular energy conservation and the rate of microbial sulfate reduction. Geology 2009, 37, 1027–1030. [Google Scholar] [CrossRef]
- Sorensen, J.; Christensen, D.; Jorgensen, B.B. Volatile fatty acids and hydrogen as substrates for sulfate-reducing bacteria in anaerobic marine sediment. Appl. Environ. Microbiol. 1981, 42, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Nandi, M.; Pakshirajan, K. Recent advances in heavy metal recovery from wastewater by biogenic sulfide precipitation. J. Environ. Manage. 2021, 278, 111555. [Google Scholar] [CrossRef]
- Mendez-Garcia, C.; Pelaez, A.I.; Mesa, V.; Sanchez, J.; Golyshina, O.V.; Ferrer, M. Microbial diversity and metabolic networks in acid mine drainage habitats. Front. Microbiol. 2015, 6, 475. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Wu, J.; Mo, P. Efficiency and mechanisms of antimony removal from wastewater using mixed cultures of iron-oxidizing bacteria and sulfate-reducing bacteria based on scrap iron. Sep. Purif. Technol. 2020, 246, 116756. [Google Scholar] [CrossRef]
- Xi, Y.; Lan, S.; Li, X.; Wu, Y.; Yuan, X.; Zhang, C.; Liu, Y.; Huang, Y.; Quan, B.; Wu, S. Bioremediation of antimony from wastewater by sulfate-reducing bacteria: Effect of the coexisting ferrous ion. Int. Biodeterior. Biodegrad. 2020, 148, 104912. [Google Scholar] [CrossRef]
- Xie, P.; Chen, C.; Zhang, C.; Su, G.; Ren, N.; Ho, S. Revealing the role of adsorption in ciprofloxacin and sulfadiazine elimination routes in microalgae. Water Res. 2020, 172, 115475. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Khanal, S.K.; Shu, H.; Zhang, H.; Chen, G.; Lu, H. Ciprofloxacin degradation in anaerobic sulfate-reducing bacteria (SRB) sludge system: Mechanism and pathways. Water Res. 2018, 136, 64–74. [Google Scholar] [CrossRef]
- Zhang, H.; Song, S.; Jia, Y.; Wu, D.; Lu, H. Stress-responses of activated sludge and anaerobic sulfate-reducing bacteria sludge under long-term ciprofloxacin exposure. Water Res. 2019, 164, 114964. [Google Scholar] [CrossRef]
- Boopathy, R. Anaerobic phenol degradation by microorganisms of swine manure. Curr. Microbiol. 1997, 35, 64–67. [Google Scholar] [CrossRef]
- Abu Laban, N.; Selesi, D.; Jobelius, C.; Meckenstock, R.U. Anaerobic benzene degradation by Gram-positive sulfate-reducing bacteria. Fems Microbiol. Ecol. 2009, 68, 300–311. [Google Scholar] [CrossRef]
- Guo, X.J.; Lu, Z.Y.; Wang, P.; Li, H.; Huang, Z.Z.; Lin, K.F.; Liu, Y.D. Diversity and degradation mechanism of an anaerobic bacterial community treating phenolic wastewater with sulfate as an electron acceptor. Environ. Sci. Pollut. Res. 2015, 22, 16121–16132. [Google Scholar] [CrossRef]
- Xie, X.; Mueller, N. Enzymes involved in the anaerobic degradation of phenol by the sulfate-reducing bacterium Desulfatiglans anilini. BMC Microbiol. 2018, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Finke, N.; Vandieken, V.; Jorgensen, B.B. Acetate, lactate, propionate, and isobutyrate as electron donors for iron and sulfate reduction in Arctic marine sediments, Svalbard. Fems Microbiol. Ecol. 2007, 59, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Muyzer, G.; Stams, A.J.M. The ecology and biotechnology of sulphate-reducing bacteria. Nat. Rev. Microbiol. 2008, 6, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Gittel, A.; Mussmann, M.; Sass, H.; Cypionka, H.; Koenneke, M. Identity and abundance of active sulfate-reducing bacteria in deep tidal flat sediments determined by directed cultivation and CARD-FISH analysis. Environ. Microbiol. 2008, 10, 2645–2658. [Google Scholar] [CrossRef]
- Jochum, L.M.; Chen, X.; Lever, M.A.; Loy, A.; Jorgensen, B.B.; Schramm, A.; Kjeldsen, K.U. Depth distribution and assembly of sulfate-reducing microbial communities in marine sediments of Aarhus bay. Appl. Environ. Microbiol. 2017, 83, e01547-17. [Google Scholar] [CrossRef]
- Chen, C.; Ren, N.; Wang, A.; Yu, Z.; Lee, D. Microbial community of granules in expanded granular sludge bed reactor for simultaneous biological removal of sulfate, nitrate and lactate. Appl. Microbiol. Biotechnol. 2008, 79, 1071–1077. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, C.; Liang, B.; Huang, C.; Zhao, Y.; Xu, X.; Tan, W.; Zhou, X.; Gao, S.; Sun, D.; et al. Fine-tuning key parameters of an integrated reactor system for the simultaneous removal of COD, sulfate and ammonium and elemental sulfur reclamation. J. Hazard. Mater. 2014, 269, 56–67. [Google Scholar] [CrossRef]
- Madani, R.M.; Liang, J.; Cui, L.; Zhang, D.; Otitoju, T.A.; Elsalahi, R.H.; Song, X. Novel simultaneous anaerobic ammonium and sulfate removal process: A review. Environ. Technol. Innov. 2021, 23, 101661. [Google Scholar] [CrossRef]
- Peck, H.D.J. Enzymatic basis for assimilatory and dissimilatory sulfate reduction. J. Bacteriol. 1961, 82, 933–939. [Google Scholar] [CrossRef]
- Prior, A.; Uhrig, J.F.; Heins, L.; Wiesmann, A.; Lillig, C.H.; Stoltze, C.; Soll, J.; Schwenn, J.D. Structural and kinetic properties of adenylyl sulfate reductase from Catharanthus roseus cell cultures. Biochim. Biophys. Acta Protein Struct. Molecul. Enzymol. 1999, 1430, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Fritz, G.; Buchert, T.; Huber, H.; Stetter, K.O.; Kroneck, P. Adenylylsulfate reductases from archaea and bacteria are 1:1 alpha beta-heterodimeric iron-sulfur flavoenzymes—high similarity of molecular properties emphasizes their central role in sulfur metabolism. Febs Lett. 2000, 473, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.A.; Venceslau, S.S.; Grein, F.; Leavitt, W.D.; Dahl, C.; Johnston, D.T.; Pereira, I.A.C. A protein trisulfide couples dissimilatory sulfate reduction to energy conservation. Science 2015, 350, 1541–1545. [Google Scholar] [CrossRef]
- Mueller, A.L.; Kjeldsen, K.U.; Rattei, T.; Pester, M.; Loy, A. Phylogenetic and environmental diversity of DsrAB-type dissimilatory (bi) sulfite reductases. Isme J. 2015, 9, 1152–1165. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.M.; Bisher, M.E.; Pratt, L.M.; Fong, J.; Southam, G.; Pfiffner, S.M.; Reches, Z.; Onstott, T.C. Sulfur isotope enrichment during maintenance metabolism in the thermophilic sulfate-reducing bacterium Desulfotomaculum putei. Appl. Environ. Microbiol. 2009, 75, 5621–5630. [Google Scholar] [CrossRef]
- Wing, B.A.; Halevy, I. Intracellular metabolite levels shape sulfur isotope fractionation during microbial sulfate respiration. Proc. Natl. Acad. Sci. USA. 2014, 111, 18116–18125. [Google Scholar] [CrossRef]
- Xu, X.; Chen, C.; Wang, A.; Fang, N.; Yuan, Y.; Ren, N.; Lee, D. Enhanced elementary sulfur recovery in integrated sulfate-reducing, sulfur-producing rector under micro-aerobic condition. Bioresour. Technol. 2012, 116, 517–521. [Google Scholar] [CrossRef]
- Beulig, F.; Roy, H.; Glombitza, C.; Jorgensen, B.B. Control on rate and pathway of anaerobic organic carbon degradation in the seabed. Proc. Natl. Acad. Sci. USA. 2018, 115, 367–372. [Google Scholar] [CrossRef]
- Das, B.K.; Gauri, S.S.; Bhattacharya, J. Sweetmeat waste fractions as suitable organic carbon source for biological sulfate reduction. Int. Biodeterior. Biodegrad. 2013, 82, 215–223. [Google Scholar] [CrossRef]
- Hussain, A.; Iqbal, M.A.; Javid, A.; Razaq, A.; Aslam, S.; Hasan, A.; Akmal, M.; Qazi, J.I. Application of fruit wastes as cost-effective carbon sources for biological sulphate reduction. Iran. J. Sci. Technol. Trans. A-Sci. 2019, 43, 33–41. [Google Scholar] [CrossRef]
- Jorgensen, B.B.; Findlay, A.J.; Pellerin, A. The biogeochemical sulfur cycle of marine sediments. Front. Microbiol. 2019, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Mardanov, A.V.; Kadnikov, V.V.; Beletsky, A.V.; Ravin, N.V. Sulfur and methane-oxidizing microbial community in a terrestrial mud volcano revealed by metagenomics. Microorganisms 2020, 8, 1333. [Google Scholar] [CrossRef] [PubMed]
- Shahsavari, S.; Seth, R.; Chaganti, S.R.; Biswas, N. Inhibition of anaerobic biological sulfate reduction process by copper precipitates. Chemosphere 2019, 236, 124246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, X.; Zhang, Z.; Chen, G.; Jiang, F. Elemental sulfur as an electron acceptor for organic matter removal in a new high-rate anaerobic biological wastewater treatment process. Chem. Eng. J. 2018, 331, 16–22. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Hu, C.; Liu, H.; Qu, J. Denitrification of groundwater using a sulfur-oxidizing autotrophic denitrifying anaerobic fluidized-bed MBR: Performance and bacterial community structure. Appl. Microbiol. Biotechnol. 2015, 99, 2815–2827. [Google Scholar] [CrossRef]
- Sun, R.; Li, Y.; Lin, N.; Ou, C.; Wang, X.; Zhang, L.; Jiang, F. Removal of heavy metals using a novel sulfidogenic AMD treatment system with sulfur reduction: Configuration, performance, critical parameters and economic analysis. Environ. Int. 2020, 136, 105457. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhang, L.; Mu, X.; Li, G.; Guan, X.; Hong, J.; Jiang, F. Overlooked pathways of denitrification in a sulfur-based denitrification system with organic supplementation. Water Res. 2020, 169, 115084. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, X.; Zhang, R.; Shao, B.; Fan, K.; Zhao, L.; Ji, X.; Ren, N.; Lee, D.; Chen, C. The mixed/mixotrophic nitrogen removal for the effective and sustainable treatment of wastewater: From treatment process to microbial mechanism. Water Res. 2022, 226, 119269. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Sun, R.; Liang, S.; Chen, G.; Jiang, F. Self-accelerating sulfur reduction via polysulfide to realize a high-rate sulfidogenic reactor for wastewater treatment. Water Res. 2018, 130, 161–167. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Li, L.; Chen, G.; Jiang, F. A novel elemental sulfur reduction and sulfide oxidation integrated process for wastewater treatment and sulfur recycling. Chem. Eng. J. 2018, 342, 438–445. [Google Scholar] [CrossRef]
- Li, G.; Liang, Z.; Sun, J.; Qiu, Y.; Qiu, C.; Liang, X.; Zhu, Y.; Wang, P.; Li, Y.; Jiang, F. A pilot-scale sulfur-based sulfidogenic system for the treatment of Cu-laden electroplating wastewater using real domestic sewage as electron donor. Water Res. 2021, 195, 116999. [Google Scholar] [CrossRef] [PubMed]
- Gorny, J.; Billon, G.; Lesven, L.; Dumoulin, D.; Made, B.; Noiriel, C. Arsenic behavior in river sediments under redox gradient: A review. Sci. Total Environ. 2015, 505, 423–434. [Google Scholar] [CrossRef] [PubMed]
- de Matos, L.P.; Costa, P.F.; Moreira, M.; Silva Gomes, P.C.; Silva, S.D.Q.; Alves Gurgel, L.V.; Teixeira, M.C. Simultaneous removal of sulfate and arsenic using immobilized non-traditional SRB mixed culture and alternative low-cost carbon sources. Chem. Eng. J. 2018, 334, 1630–1641. [Google Scholar] [CrossRef]
- Sun, J.; Hong, Y.; Guo, J.; Yang, J.; Huang, D.; Lin, Z.; Jiang, F. Arsenite removal without thioarsenite formation in a sulfidogenic system driven by sulfur reducing bacteria under acidic conditions. Water Res. 2019, 151, 362–370. [Google Scholar] [CrossRef]
- King, J.K.; Harmon, S.M.; Fu, T.T.; Gladden, J.B. Mercury removal, methylmercury formation, and sulfate-reducing bacteria profiles in wetland mesocosms. Chemosphere 2002, 46, 859–870. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Kang, Y.; Chen, G.; Jiang, F. Long-term feeding of elemental sulfur alters microbial community structure and eliminates mercury methylation potential in sulfate-reducing bacteria abundant activated sludge. Environ. Sci. Technol. 2018, 52, 4746–4753. [Google Scholar] [CrossRef]
- Sun, J.; Li, L.; Zhou, G.; Wang, X.; Zhang, L.; Liu, Y.; Yang, J.; Lu, X.; Jiang, F. Biological sulfur reduction to generate H2S as a reducing agent to achieve simultaneous catalytic removal of so2 and no and sulfur recovery from flue gas. Environ. Sci. Technol. 2018, 52, 4754–4762. [Google Scholar] [CrossRef]
- Qiu, Y.; Gong, X.; Zhang, L.; Zhou, S.; Li, G.; Jiang, F. Achieving a novel polysulfide-involved sulfur-based autotrophic denitrification process for high-rate nitrogen removal in elemental sulfur-packed bed reactors. ACS EsT Eng. 2022, 2, 1504–1513. [Google Scholar] [CrossRef]
- Hedderich, R.; Klimmek, O.; Kroger, A.; Dirmeier, R.; Keller, M.; Stetter, K.O. Anaerobic respiration with elemental sulfur and with disulfides. Fems Microbiol. Rev. 1998, 22, 353–381. [Google Scholar] [CrossRef]
- Schauder, R.; Kroger, A. Bacterial sulfur respiration. Arch. Microbiol. 1993, 159, 491–497. [Google Scholar] [CrossRef]
- Segerer, A.; Neuner, A.; Kristjansson, J.K.; Stetter, K.O. Acidianus infernus gen-nov, sp-nov, and Acidianus brierleyi comb-nov—facultatively aerobic, extremely acidophilic thermophilic sulfur-metabolizing archaebacteria. Int. J. Syst. Bacteriol. 1986, 36, 559–564. [Google Scholar] [CrossRef]
- Fischer, F.; Zillig, W.; Stetter, K.O.; Schreiber, G. Chemolithoautotrophic metabolism of anaerobic extremely thermophilic archaebacteria. Nature 1983, 301, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.M.; Masuchi, Y.; Robb, F.T.; Ammerman, J.W.; Maeder, D.L.; Yanagibayashi, M.; Tamaoka, J.; Kato, C. Pyrococcus horikoshii sp. nov., a hyperthermophilic archaeon isolated from a hydrothermal vent at the Okinawa Trough. Extremophiles 1998, 2, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Deppenmeier, U.; Lienard, T.; Gottschalk, G. Novel reactions involved in energy conservation by methanogenic archaea. Febs Lett. 1999, 457, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Wilharm, T.; Huber, D.; Trincone, A.; Burggraf, S.; Konig, H.; Rachel, R.; Rockinger, I.; Fricke, H.; Stetter, K.O. Aquifex pyrophilus gen-nov sp-nov represents a novel group of marine hyperthermophilic hydrogen-oxidizing bacteria. Syst. Appl. Microbiol. 1992, 15, 340–351. [Google Scholar] [CrossRef]
- L’Haridon, S.; Cilia, V.; Messner, P.; Raguénès, G.; Gambacorta, A.; Sleytr, U.B.; Prieur, D.; Jeanthon, C. Desulfurobacterium thermolithotrophum gen. nov., sp. nov., a novel autotrophic, sulphur-reducing bacterium isolated from a deep-sea hydrothermal vent. Int. J. Syst. Evol. Microbiol. 1998, 48, 701–711. [Google Scholar] [CrossRef]
- Pfennig, N.; Biebl, H. Desulfuromonas acetoxidans gen. nov. and sp. nov., a new anaerobic, sulfur-reducing, acetate-oxidizing bacterium. Arch. Microbiol. 1976, 110, 3–12. [Google Scholar] [CrossRef]
- Liesack, W.; Finster, K. Phylogenetic analysis of five strains of gram-negative, obligately anaerobic, sulfur-reducing bacteria and description of Desulfuromusa gen. nov., including Desulfuromusa kysingii sp. nov., Desulfuromusa bakii sp. nov., and Desulfuromusa succinoxidans sp. Int. J. Syst. Evol. Microbiol. 1994, 44, 753–758. [Google Scholar] [CrossRef]
- Huber, R.; Woese, C.R.; Langworthy, T.A.; Kristjansson, J.K.; Stetter, K.O. Fervidobacterium-islandicum sp-nov, a new extremely thermophilic eubacterium belonging to the thermotogales. Arch. Microbiol. 1990, 154, 105–111. [Google Scholar] [CrossRef]
- Caccavo, F.; Lonergan, D.J.; Lovley, D.R.; Davis, M.; Stolz, J.F.; Mcinerney, M.J. Geobacter sulfurreducens sp-nov, a hydrogen-oxidizing and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl. Environ. Microbiol. 1994, 60, 3752–3759. [Google Scholar] [CrossRef]
- Wolfe, R.S.; Penning, N. Reduction of sulfur by spirillum 5175 and syntrophism with Chlorobium. Appl. Environ. Microbiol. 1977, 33, 427–433. [Google Scholar] [CrossRef]
- Windberger, E.; Huber, R.; Trincone, A.; Fricke, H.; Stetter, K.O. Thermotoga thermarum sp-nov and Thermotoga neapolitana occurring in African continental solfataric springs. Arch. Microbiol. 1989, 151, 506–512. [Google Scholar] [CrossRef]
- Huber, R.; Woese, C.R.; Langworthy, T.A.; Fricke, H.; Stetter, K.O. Thermosipho africanus gen-nov, represents a new genus of thermophilic eubacteria within the thermotogales. Syst. Appl. Microbiol. 1989, 12, 32–37. [Google Scholar] [CrossRef]
- Macy, J.M.; Schroder, I.; Thauer, R.K.; Kroger, A. Growth the Wolinella succinogenes on H2S plus fumarate and on formate plus sulfur as energy sources. Arch. Microbiol. 1986, 144, 147–150. [Google Scholar] [CrossRef]
- Jelen, B.; Giovannelli, D.; Falkowski, P.G.; Vetriani, C. Elemental sulfur reduction in the deep-sea vent thermophile, Thermovibrio ammonificans. Environ. Microbiol. 2018, 20, 2301–2316. [Google Scholar] [CrossRef] [PubMed]
- Koschorreck, M. Microbial sulphate reduction at a low pH microbial sulphate reduction at a low pH. Fems Microbiol. Ecol. 2008, 64, 329–342. [Google Scholar] [CrossRef]
- Grubba, D.; Yin, Z.; Majtacz, J.; Al-Hazmi, H.E.; Makinia, J. Incorporation of the sulfur cycle in sustainable nitrogen removal systems-A review. J. Clean Prod. 2022, 372, 133495. [Google Scholar] [CrossRef]
- Huang, C.; Liu, Q.; Li, Z.; Ma, X.; Hou, Y.; Ren, N.; Wang, A. Relationship between functional bacteria in a denitrification desulfurization system under autotrophic, heterotrophic, and mixotrophic conditions. Water Res. 2021, 188, 116526. [Google Scholar] [CrossRef]
- Bi, Z.; Zhang, Q.; Xu, X.; Yuan, Y.; Ren, N.; Lee, D.; Chen, C. Perspective on inorganic electron donor-mediated biological denitrification process for low C/N wastewaters. Bioresour. Technol. 2022, 363, 127890. [Google Scholar] [CrossRef]
- Fernandez, M.; Ramirez, M.; Maria Perez, R.; Manuel Gomez, J.; Canter, D. Hydrogen sulphide removal from biogas by an anoxic biotrickling filter packed with Pall rings. Chem. Eng. J. 2013, 225, 456–463. [Google Scholar] [CrossRef]
- Ramos, I.; Perez, R.; Fdz-Polanco, M. Microaerobic desulphurisation unit: A new biological system for the removal of H2S from biogas. Bioresour. Technol. 2013, 142, 633–640. [Google Scholar] [CrossRef]
- Jung, H.; Kim, D.; Choi, H.; Lee, C. A review of technologies for in-situ sulfide control in anaerobic digestion. Renew. Sust. Energ. Rev. 2022, 157, 112068. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, R.; Huang, Z.; Chen, C.; Xu, X.; Zhou, X.; Yin, T.; Wang, A.; Lee, D.; Ren, N. Performance of a novel IAHD-DSR process with methane and sulfide as co-electron donors. J. Hazard. Mater. 2020, 386, 121657. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Avila, J.S.; Razo-Flores, E.; Gomez, J. Simultaneous biological removal of nitrogen, carbon and sulfur by denitrification. Water Res. 2004, 38, 3313–3321. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ren, N.; Wang, A.; Yu, Z.; Lee, D. Simultaneous biological removal of sulfur, nitrogen and carbon using EGSB reactor. Appl. Microbiol. Biotechnol. 2008, 78, 1057–1063. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, X.; Chen, C.; Xing, D.; Shao, B.; Liu, W.; Wang, A.; Lee, D.; Ren, N. Interactions of functional bacteria and their contributions to the performance in integrated autotrophic and heterotrophic denitrification. Water Res. 2018, 143, 355–366. [Google Scholar] [CrossRef]
- Huang, C.; Li, Z.; Chen, F.; Liu, Q.; Zhao, Y.; Gao, L.; Chen, C.; Zhou, J.; Wang, A. Efficient regulation of elemental sulfur recovery through optimizing working height of upflow anaerobic sludge blanket reactor during denitrifying sulfide removal process. Bioresour. Technol. 2016, 200, 1019–1023. [Google Scholar] [CrossRef]
- Huang, C.; Liu, W.; Li, Z.; Zhang, S.; Chen, F.; Yu, H.; Shao, S.; Nan, J.; Wang, A. High recycling efficiency and elemental sulfur purity achieved in a biofilm formed membrane filtration reactor. Water Res. 2018, 130, 1–12. [Google Scholar] [CrossRef]
- Marques, E.L.S.; Dias, J.C.T.; Gross, E.; de Cerqueira E Silva, A.B.; de Moura, S.R.; Rezende, R.P. Purple sulfur bacteria dominate microbial community in Brazilian limestone cave. Microorganisms 2019, 7, 29. [Google Scholar] [CrossRef]
- Gregersen, L.H.; Bryant, D.A.; Frigaard, N. Mechanisms and evolution of oxidative sulfur metabolism in green sulfur bacteria. Front. Microbiol. 2011, 2, 116. [Google Scholar] [CrossRef]
- Karr, E.A.; Sattley, W.M.; Jung, D.O.; Madigan, M.T.; Achenbach, L.A. Remarkable diversity of phototrophic purple bacteria in a permanently frozen Antarctic lake. Appl. Environ. Microbiol. 2003, 69, 4910–4914. [Google Scholar] [CrossRef]
- Madigan, M.T. Anoxygenic phototrophic bacteria from extreme environments. Photosynth. Res. 2003, 76, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Eckert, C.A.; Freed, E.; Wawrousek, K.; Smolinski, S.; Yu, J.; Maness, P. Inactivation of the uptake hydrogenase in the purple non-sulfur photosynthetic bacterium Rubrivivax gelatinosus CBS enables a biological water-gas shift platform for H-2 production. J. Ind. Microbiol. Biotechnol. 2019, 46, 993–1002. [Google Scholar] [CrossRef]
- Chan, L.; Morgan-Kiss, R.; Hanson, T.E. Sulfur oxidation in Chlorobium tepidum (syn. Chlorobaculum tepidum) Genetic and proteomic analyses. In Microbial Sulfur Metabolism; Springer: Munster, Germany, 2008; p. 117. [Google Scholar]
- Stout, J.; De Smet, L.; Vergauwen, B.; Savvides, S.; Van Beeumen, J. Structural insights into component SoxY of the thiosulfate-oxidizing multienzyme system of Chlorobaculum thiosulfatiphilum. In Microbial Sulfur Metabolism; Springer: Munster, Germany, 2008; p. 127. [Google Scholar]
- Serrano, W.; Schruebbers, J.; Amann, R.; Fischer, U. Allochromatium humboldtianum sp. nov., isolated from soft coastal sediments. Int. J. Syst. Evol. Microbiol. 2015, 65, 2980. [Google Scholar] [CrossRef] [PubMed]
- Caumette, P.; Baulaigue, R.; Matheron, R. Characterization of Chromatium salexigens sp. nov., a Halophilic Chromatiaceae Isolated from Mediterranean Salinas—ScienceDirect. Syst. Appl. Microbiol. 1988, 10, 284–292. [Google Scholar] [CrossRef]
- Bertini, I.; Gaudemer, A.; Luchinat, C.; Piccioli, M. Electron self-exchange in high-potential iron-sulfur proteins. Characterization of protein I from Ectothiorhodospira vacuolata. Biochemistry 1993, 32, 12887–12893. [Google Scholar] [CrossRef] [PubMed]
- Hensen, D.; Sperling, D.; Trüper, H.G.; Brune, D.C.; Dahl, C. Thiosulphate oxidation in the phototrophic sulphur bacterium Allochromatium vinosum. Mol. Microbiol. 2010, 62, 794–810. [Google Scholar] [CrossRef]
- Harwood, C.S.; Gibson, J. Anaerobic and aerobic metabolism of diverse aromatic compounds by the photosynthetic bacterium Rhodopseudomonas palustris. Appl. Environ. Microbiol. 1988, 54, 712–717. [Google Scholar] [CrossRef]
- Pfennig, N. Rhodocyclus purpureus gen. nov. and sp. nov., a Ring-Shaped, Vitamin B12-Requiring Member of the Family Rhodospirillaceae. Int. J. Syst. Bacteriol. 1978, 30, 283–288. [Google Scholar]
- Dziewit, L.; Baj, J.; Szuplewska, M.; Maj, A.; Tabin, M.; Czyzkowska, A.; Skrzypczyk, G.; Adamczuk, M.; Sitarek, T.; Stawinski, P. Insights into the transposable mobilome of Paracoccus spp. (Alphaproteobacteria). PLoS ONE 2012, 7, e32277. [Google Scholar] [CrossRef]
- Valdés, J.; Pedroso, I.; Quatrini, R.; Dodson, R.J.; Tettelin, H.; Blake, R.; Eisen, J.A.; Holmes, D.S. Acidithiobacillus ferrooxidans metabolism: From genome sequence to industrial applications. BMC Genomics 2008, 9, 597. [Google Scholar] [CrossRef]
- Takai, K. Thiomicrospira thermophila sp. nov., a novel microaerobic, thermotolerant, sulfur-oxidizing chemolithomixotroph isolated from a deep-sea hydrothermal fumarole in the TOTO caldera, Mariana Arc, Western Pacific. Int. J. Syst. Evol. Microbiol. 2004, 54, 2325. [Google Scholar]
- Nelson, D.C.; Jrgensen, B.B.; Revsbech, N.P. Growth pattern and yield of a chemoautotrophic Beggiatoa sp. in oxygen-sulfide microgradients. Appl. Environ. Microbiol. 1986, 52, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Zhang, T.; Fang, H.H. Sulfur-driven autotrophic denitrification: Diversity, biochemistry, and engineering applications. Appl. Microbiol. Biotechnol. 2010, 88, 1027–1042. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Lu, C.; Hou, N.; Xin, Y.; Liu, J.; Liu, H.; Xun, L. Sulfide production and oxidation by heterotrophic bacteria under aerobic conditions. ISME J. 2017, 11, 2754–2766. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Liu, H.; Cui, F.; Liu, H.; Xun, L. Recombinant Escherichia coli with sulfide: Quinone oxidoreductase and persulfide dioxygenase rapidly oxidises sulfide to sulfite and thiosulfate via a new pathway. Environ. Microbiol. 2016, 18, 5123–5136. [Google Scholar] [CrossRef]
- Lu, C.; Xia, Y.; Liu, D.; Zhao, R.; Gao, R.; Liu, H.; Xun, L. Cupriavidus necator H16 uses flavocytochrome c sulfide dehydrogenase to oxidize self-produced and added sulfide. Appl. Environ. Microbiol. 2017, 83, e01610-17. [Google Scholar] [CrossRef]
- Xin, Y.; Gao, R.; Cui, F.; Lu, C.; Liu, H.; Liu, H.; Xia, Y.; Xun, L. The heterotrophic bacterium Cupriavidus pinatubonensis JMP134 oxidizes sulfide to sulfate with thiosulfate as a key intermediate. Appl. Environ. Microbiol. 2020, 86, e01835-20. [Google Scholar] [CrossRef]
- Fan, K.; Xu, X.; Xu, F.; Shi, J.; Sun, K.; Fedorova, I.; Ren, N.; Lee, D.; Chen, C. A novel intra- and extracellular distribution pattern of elemental sulfur in Pseudomonas sp. C27-driven denitrifying sulfide removal process. Environ. Res. 2022, 213, 113674. [Google Scholar] [CrossRef]
- Jorgensen, B.B. Ecology of the bacteria of the sulphur cycle with special reference to anoxic-oxic interface environments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1982, 298, 543–561. [Google Scholar] [CrossRef]
- Luther, G.W.I.; Findlay, A.J.; Macdonald, D.J.; Owings, S.M.; Hanson, T.E.; Beinart, R.A.; Girguis, P.R. Thermodynamics and kinetics of sulfide oxidation by oxygen: A look at inorganically controlled reactions and biologically mediated processes in the environment. Front. Microbiol. 2011, 2, 62. [Google Scholar] [CrossRef]
- Marazioti, C.; Kornaros, M.; Lyberatos, G. Kinetic modeling of a mixed culture of Pseudomonas denitrificans and Bacillus subtilis under aerobic and anoxic operating conditions. Water Res. 2003, 37, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, R.; Xu, X.; Fang, N.; Wang, A.; Ren, N.; Lee, D. Enhanced performance of denitrifying sulfide removal process at high carbon to nitrogen ratios under micro-aerobic condition. Bioresour. Technol. 2017, 232, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Lohwacharin, J.; Annachhatre, A.P. Biological sulfide oxidation in an airlift bioreactor. Bioresour. Technol. 2010, 101, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chen, C.; Shao, B.; Wang, W.; Xu, X.; Zhou, X.; Xiang, Y.; Zhao, L.; Lee, D.; Ren, N. Heterotrophic sulfide-oxidizing nitrate-reducing bacteria enables the high performance of integrated autotrophic-heterotrophic denitrification (IAHD) process under high sulfide loading. Water Res. 2020, 178, 115848. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Zhang, L.; Jiang, F. Indirect sulfur reduction via polysulfide contributes to serious odor problem in a sewer receiving nitrate dosage. Water Res. 2016, 100, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Biswal, B.K.; Guo, G.; Deng, Y.; Huang, H.; Chen, G.; Wu, D. Biological nitrogen removal from wastewater using sulphur-driven autotrophic denitrification. Appl. Microbiol. Biotechnol. 2019, 103, 6023–6039. [Google Scholar] [CrossRef]
- Sahinkaya, E.; Yurtsever, A.; Aktas, O.; Ucar, D.; Wang, Z. Sulfur-based autotrophic denitrification of drinking water using a membrane bioreactor. Chem. Eng. J. 2015, 268, 180–186. [Google Scholar] [CrossRef]
- Sahinkaya, E.; Kilic, A.; Duygulu, B. Pilot and full scale applications of sulfur-based autotrophic denitrification process for nitrate removal from activated sludge process effluent. Water Res. 2014, 60, 210–217. [Google Scholar] [CrossRef]
- Jamieson-Hanes, J.H.; Gibson, B.D.; Lindsay, M.B.J.; Kim, Y.; Ptacek, C.J.; Blowes, D.W. Chromium isotope fractionation during reduction of CR(VI) under saturated flow conditions. Environ. Sci. Technol. 2012, 46, 6783–6789. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, B.; Qiu, R.; Lai, C.; Jiang, Y.; He, C.; Guo, J. Microbial chromate reduction coupled to anaerobic oxidation of elemental sulfur or zerovalent iron. Environ. Sci. Technol. 2019, 53, 3198–3207. [Google Scholar] [CrossRef]
- Loy, A.; Duller, S.; Wagner, M. Evolution and ecology of microbes dissimilating sulfur compounds Insights from siroheme sulfite reductases. In Microbial Sulfur Metabolism; Springer: Berlin/Heidelberg, Germany, 2008; pp. 46–59. [Google Scholar] [CrossRef]
- Dahl, C. Cytoplasmic sulfur trafficking in sulfur-oxidizing prokaryotes. IUBMB Life 2015, 67, 268–274. [Google Scholar] [CrossRef] [PubMed]






| Component | Chemical Formula | Valence of Sulfur |
|---|---|---|
| Inorganic sulfur species: | ||
| Sulfide | H2S/HS−/S2− | −2 |
| Pyretic sulfur | FeS/FeS2 | −2 and −1 |
| Inorganic polysulfides | H-Sn-H/Sn2− (n ≥ 2) | −1 and 0 |
| Elemental sulfur | S/S8/S0 | 0 |
| Thiosulfate | S2O32− | +2 |
| Sulfur dioxide | SO2 | +4 |
| Sulfite | SO32− | +4 |
| Sulfate | SO42− | +6 |
| Organic sulfur species: | ||
| Reduced organic sulfur compounds | Cysteine, methionine | −2 |
| Organic polysulfides | R-Sn-R/R-SnH (n ≥ 2) | 0 |
| Taxonomic Category | Electron Donor | Reference |
|---|---|---|
| Archaea | ||
| Crenarchaeota: | ||
| Acidianus | H2 | [82] |
| Thermoproteus | H2, peptides, maltose, formate, fumarate, ethanol, malate, methanol, glycogen, starch, amylopectin, formamide | [83] |
| Euryarchaeota: | ||
| Pyrococcus | Complex substrates, amino acids, starch, maltose, pyruvate | [84] |
| Methanococcus | H2, formate | [85] |
| Bacteria | ||
| Aquifex | H2, sulfur, thiosulfate | [86] |
| Desulfurobacterium | H2 | [87] |
| Desulfuromonas | Acetate, pyruvate, ethanol | [88] |
| Desulfuromusa | Acetate, propionate | [89] |
| Fervidobacterium | Sugars, pyruvate, yeast extract | [90] |
| Geobacter | Acetate | [91] |
| Sulfospirillum | H2, formate | [92] |
| Thermotoga | Sugars, peptone, yeast extract, bacterial and archaeal cell homogenates | [93] |
| Thermosipho | Yeast extract, brain heart infusion, peptone, tryptone | [94] |
| Wolinella | H2, formate | [95] |
| Taxonomic Category | Representative Species | Metabolic Features | Sulfur Oxidation Genes | Distributed Environment | Reference |
|---|---|---|---|---|---|
| GSB Chlorobi | Chlorobaculum tepidum, Chlorobaculum thiosulfatiphilum | Obligate phototrophy; S2–, S0, or S2O32− as e− donors for reduction of CO2; extracellular S0 globules; potential mixotrophy | SoxXAYZB, APS reductase, Qmo complex, and Fcc | Anaerobic waters, oceans, soils, the Yellowstone hot springs and sediments | [115,116] |
| PSB Chromatiaceae | Allochromatium warmingi Isochromatium buderi | Photoautotrophy except for Rheinheimera spp.; S2− and S0 as e− donors of photosynthesis; intracellular S0 globules | - | Oceans, stagnant aquifers, eutrophic lakes with water bodies, and extreme environments rich in sulfides | [117,118] |
| Ectothiorhodospiraceae | Allochromatium vinosum Ectothiorhodospira vacuolata | Oxidation of S2− for all the members; extracellular S0 globules; polysulfides under alkaline conditions; some can oxidize S2O32− to SO42− | SoxXAYZB, Sqr, DsrABEFHCMKLJOPNRS, APS reductase, and Fcc | [119,120] | |
| PNSB Alphaproteobacteria | Rhodopseudomonas palustris | The preferred photoheterotrophy under anaerobic conditions; photolithoautotrophy with S2−/S2O32− | SoxXAYZBCD, SoxEF, and Sqr | Waste ponds, coastal lagoons and other aquatic-habitat stagnant areas, sediments, wet soils, and rice paddies | [121] |
| Betaproteobacteria | Rhodocyclus purpureus | Chemoorganotrophy/chemolithoautotrophy under aerobic or microaerobic conditions | - | [122] | |
| CSB Alphaproteobacteria | Paracoccus spp. | Facultative chemolithoautotrophy; oxidation of S2−, S0, S2O32−, or SO32− to SO42− | SoxXAYZBCD and SoxEF | Activated sludge, wastewater treatment systems, farmland, and natural ecological environment such as orchards | [123] |
| Acidithiobacillia | Acidithiobacillus ferrooxidans | Obligate chemolithoautotrophy; oxidation of S0, S2O32−, or S4O62− by the incomplete Sox system; S0 globules as intermediates | SoxXAYZB and Sqr | [124] | |
| Gammaproteobacteria | Thiomicrospira crunogena | Obligate chemolithoautotrophy; extracellular S0 globules under low oxygen/pH; transient accumulation of SO32− or polythionate during S0 globules or S2O32− oxidation | SoxXAYZBCD and Sqr | [125] | |
| Gammaproteobacteria | Beggiatoa spp. | Chemolithoheterotrophy/mixotrophy; intracellular S0 globules | Dsr, Sqr, and APS reductase | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, K.; Wang, W.; Xu, X.; Yuan, Y.; Ren, N.; Lee, D.-J.; Chen, C. Recent Advances in Biotechnologies for the Treatment of Environmental Pollutants Based on Reactive Sulfur Species. Antioxidants 2023, 12, 767. https://doi.org/10.3390/antiox12030767
Fan K, Wang W, Xu X, Yuan Y, Ren N, Lee D-J, Chen C. Recent Advances in Biotechnologies for the Treatment of Environmental Pollutants Based on Reactive Sulfur Species. Antioxidants. 2023; 12(3):767. https://doi.org/10.3390/antiox12030767
Chicago/Turabian StyleFan, Kaili, Wei Wang, Xijun Xu, Yuan Yuan, Nanqi Ren, Duu-Jong Lee, and Chuan Chen. 2023. "Recent Advances in Biotechnologies for the Treatment of Environmental Pollutants Based on Reactive Sulfur Species" Antioxidants 12, no. 3: 767. https://doi.org/10.3390/antiox12030767
APA StyleFan, K., Wang, W., Xu, X., Yuan, Y., Ren, N., Lee, D.-J., & Chen, C. (2023). Recent Advances in Biotechnologies for the Treatment of Environmental Pollutants Based on Reactive Sulfur Species. Antioxidants, 12(3), 767. https://doi.org/10.3390/antiox12030767






