Biological Evaluation and Structural Analysis of Some Aminodiphenylamine Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Apparatus and Techniques Used
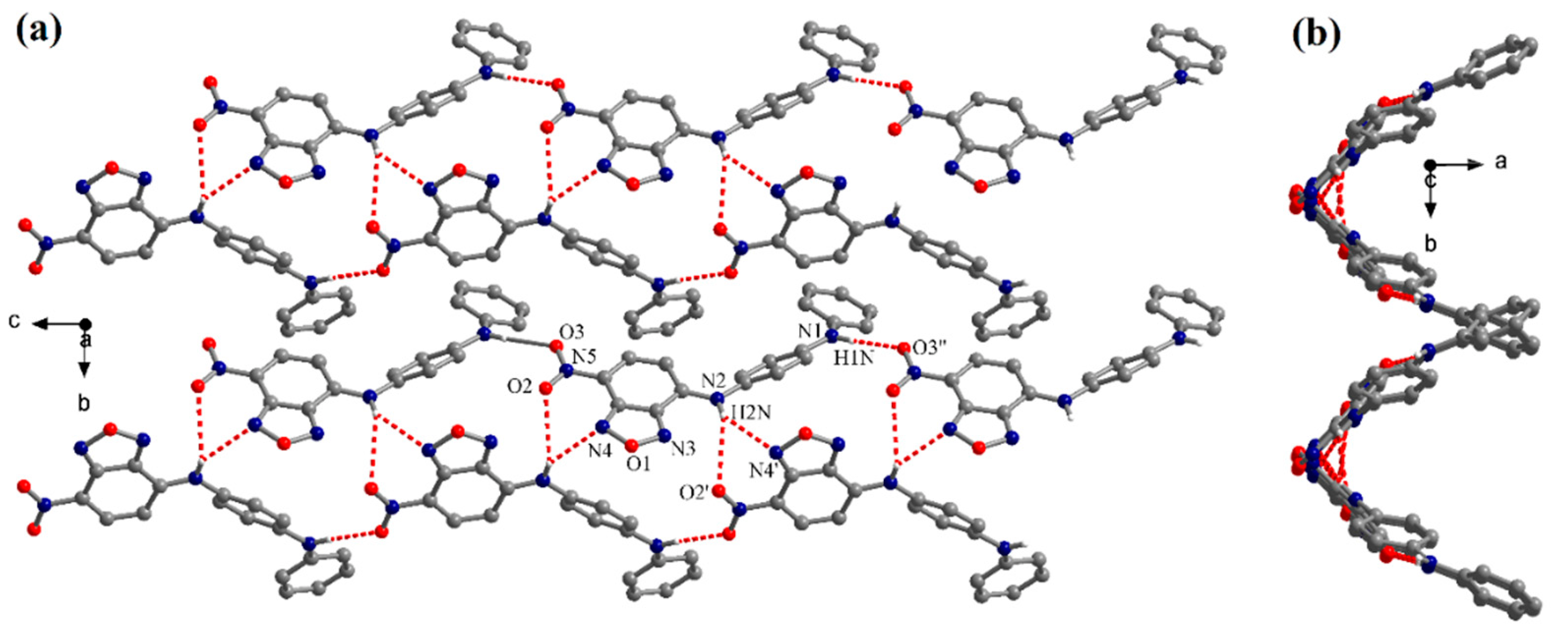
X-ray Crystallographic Analysis
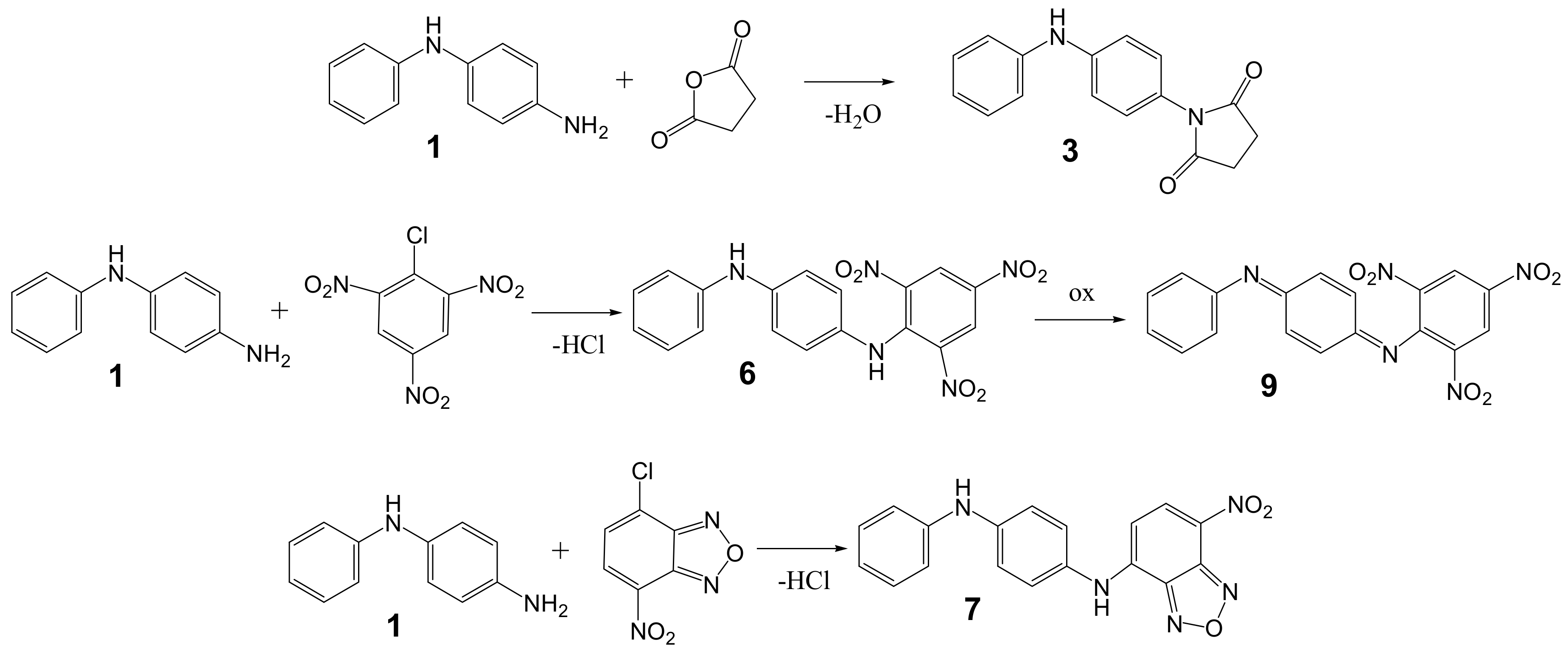
2.2. Synthesis of the Compounds 7 and 9
2.3. Experimental Lipophilicity Measurements
2.4. Total Antioxidant Capacity (TAC) Measurements
2.5. Computational Measurements
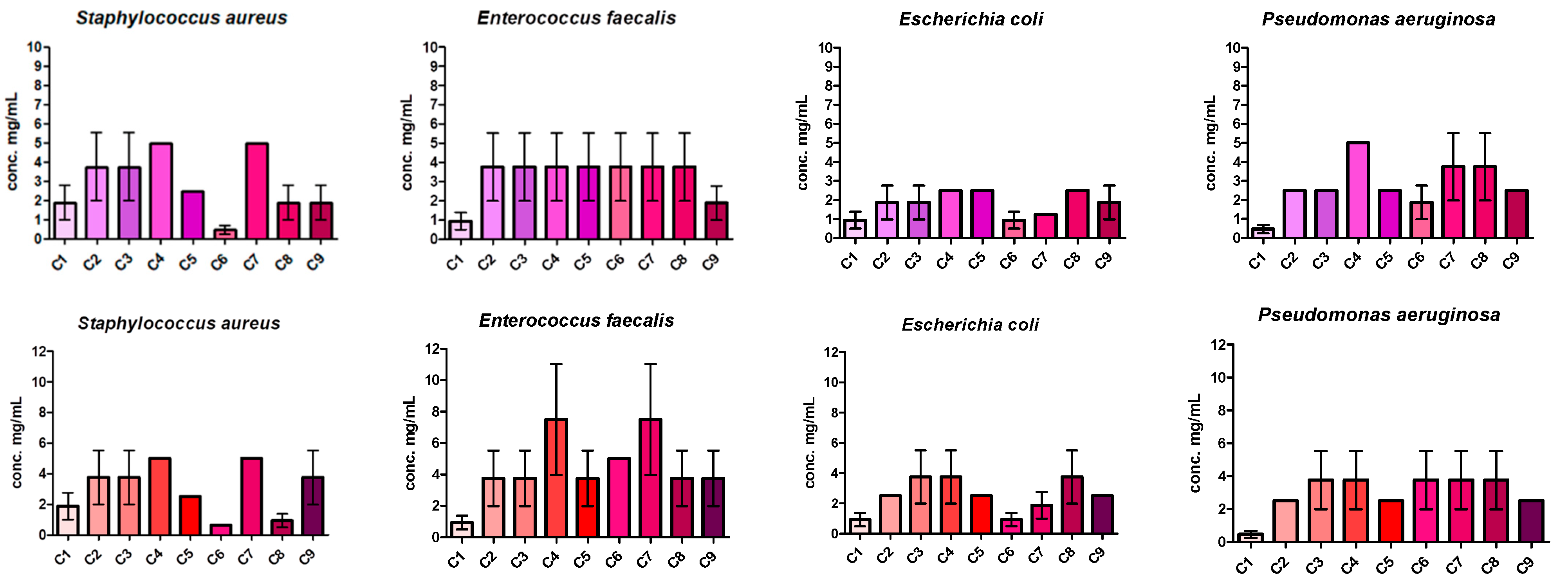
2.6. Determination of MICs and MBCs of the Compounds
2.7. Screening of the Antibiofilm Activity
3. Results and Discussion
3.1. Synthesis and Structural Analysis
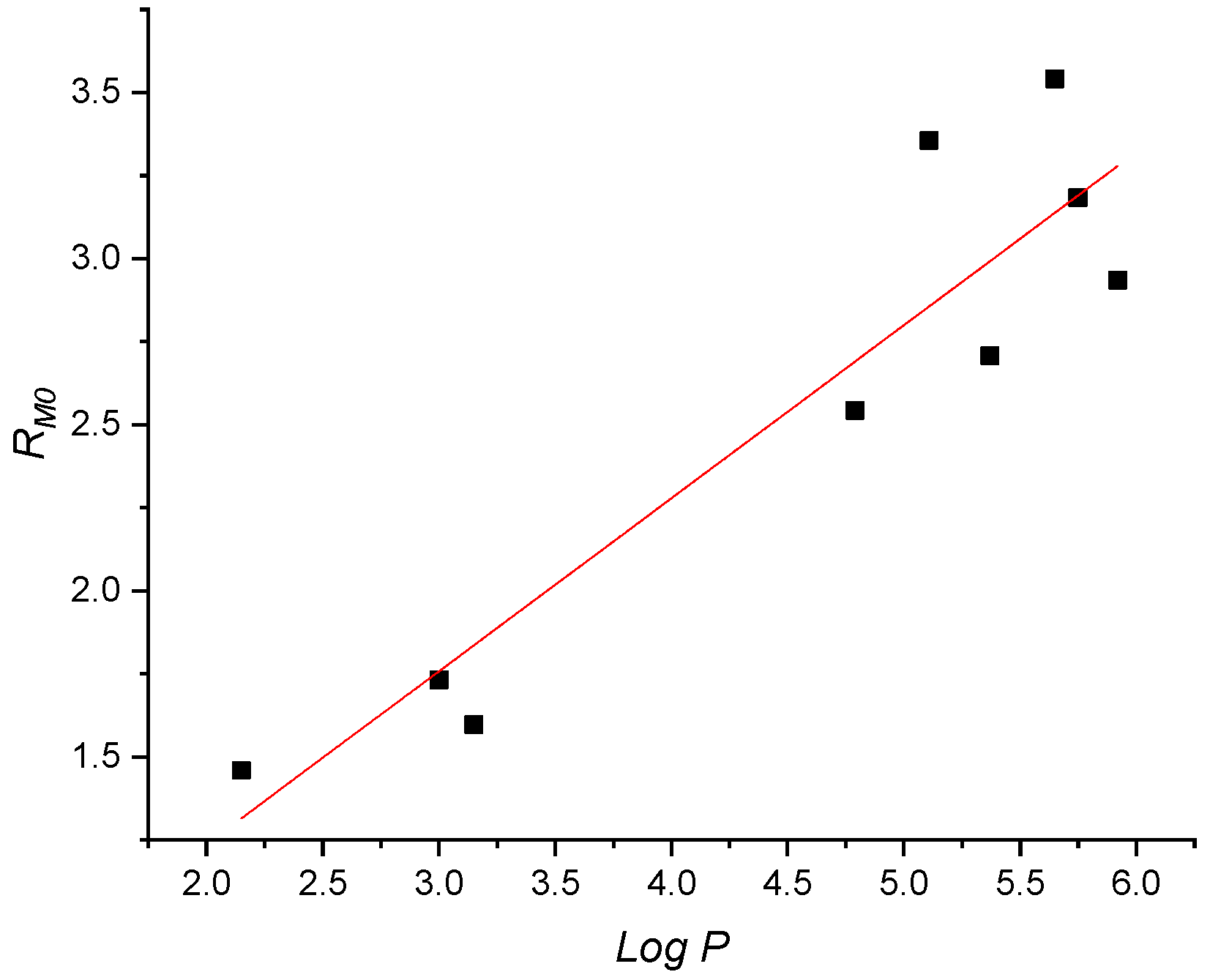
3.2. Lipophilicity and Related Measurements
3.3. Total Antioxidant Capacity
3.4. Correlations between Experimental and Computed Properties
3.5. Bioevaluation of the Compounds as Antimicrobial and Antibiofilm Agents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.-F.; Zhang, X.; Sverko, E.; Marvin, C.H.; Jobst, K.J.; Smyth, S.A.; Li, Y.F. Determination of diphenylamine antioxidants in wastewater/biosolids and sediment. Environ. Sci. Technol. Lett. 2020, 7, 102–110. [Google Scholar] [CrossRef]
- He, J.-B.; Shi, H.; Wang, Y.; Gao, X.-L. Synthesis, Characterization, and performance evaluation of sulfur-containing diphenylamines based on intramolecular synergism. Molecules 2018, 23, 401. [Google Scholar] [CrossRef]
- Tumosienė, I.; Jonuškienė, I.; Kantminienė, K.; Mickevičius, V.; Petrikaitė, V. Novel N-substituted amino acid hydrazone-isatin derivatives: Synthesis, antioxidant activity, and anticancer activity in 2D and 3D models in vitro. Int. J. Mol. Sci. 2021, 21, 7799. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, S.; Ishihara, M.; Kadoma, Y. Antioxidant activity of diphenylamine-related compounds as scavengers of carbon radicals. Inter. Electron. J. Mol. Des. 2005, 4, 711–720. [Google Scholar]
- Krause-Heuer, A.M.; Yepuri, N.R.; Darwish, T.A.; Holden, P.J. Mild conditions for deuteration of primary and secondary arylamines for the synthesis of deuterated optoelectronic organic molecules. Molecules 2014, 19, 18604–18617. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Welsh, W.; Shi, L.; Fang, H.; Perkins, R. Structure–activity relationship approaches and applications. Environ. Toxicol. Chem. 2003, 22, 1680–1695. [Google Scholar] [CrossRef]
- Sharma, D.; Narasimhan, B.; Kumar, P.; Jalbout, A. Synthesis and QSAR evaluation of 2-(substituted phenyl)-1H-benzimidazoles and [2-(substituted phenyl)-benzimidazol-1-yl]-pyridin-3-yl-methanones. Eur. J. Med. Chem. 2009, 44, 1119–1127. [Google Scholar] [CrossRef]
- Gupta, S.; Finelli, R.; Agarwal, A.; Henkel, R. Total antioxidant capacity- relevance, methods and clinical implications. Andrologia 2021, 53, 13624. [Google Scholar] [CrossRef]
- Napolitano, G.; Fasciolo, G.; Venditti, P. The ambiguous aspects of oxygen. Oxygen 2022, 2, 382–409. [Google Scholar] [CrossRef]
- Kumar, S.; Saxena, J.; Srivastava, V.K.; Kaushik, S.; Singh, H.; Abo-EL-Sooud, K.; Abdel-Daim, M.M.; Jyoti, A.; Saluja, R. The interplay of oxidative stress and ROS scavenging: Antioxidants as a therapeutic potential in sepsis. Vaccines 2022, 10, 1575. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Mekapati, S.B.; Kurup, A. QSAR and ADME. Bioorg. Med. Chem. 2004, 12, 3391–3400. [Google Scholar] [CrossRef]
- Sjursnes, B.J.; Kvittingen, L.; Schmid, R. Normal and reversed-phase thin layer chromatography of green leaf extracts. J. Chem. Educ. 2015, 92, 193–196. [Google Scholar] [CrossRef]
- Paun, A.; Zarafu, I.; Caproiu, M.T.; Draghici, C.; Maganu, M.; Cotar, A.I.; Chifiriuc, M.C.; Ionita, P. Synthesis and microbiological evaluation of several benzocaine derivatives. C. R. Chim. 2013, 16, 665–671. [Google Scholar] [CrossRef]
- Hiroko, S.; Minoru, H.; Yoshiki, H.; Isao, T.; Takae, K. Infrared spectra and intramolecular hydrogen bonding of 4-(haloacetylamino)diphenylamines. Bull. Chem. Soc. Jpn. 1991, 64, 2863–2864. [Google Scholar] [CrossRef]
- Mečiarová, M.; Toma, S.; Magdolen, P. On the 3-aminophenothiazine synthesis. Synth. Commun. 2003, 33, 3049–3054. [Google Scholar] [CrossRef]
- Han, C.-C.; Balakumar, R.; Thirumalai, D.; Chung, M.-T. The different electronic natures displayed by the alkylthio groups in simple and higher conjugated aniline systems. Org. Biomol. Chem. 2006, 4, 3511–3516. [Google Scholar] [CrossRef]
- Amaya, T.; Suzuki, R.; Hirao, T. Quinonediimine-induced oxidative coupling of organomagnesium reagents. Chem. Eur. J. 2013, 20, 653–656. [Google Scholar] [CrossRef]
- Samet, S.; Ayachi, A.; Fourati, M.; Mallouli, L.; Allouche, N.; Treilhou, M.; Téné, N.; Mezghani-Jarraya, R. Antioxidant and antimicrobial activities of Erodium Arborescens aerial part extracts and characterization by lc-hesi-ms2 of its acetone extract. Molecules 2022, 27, 4399. [Google Scholar] [CrossRef] [PubMed]
- Agour, A.; Mssillou, I.; Es-Safi, I.; Conte, R.; Mechchate, H.; Slighoua, M.; Amrati, F.E.-Z.; Parvez, M.K.; Numan, O.; Bari, A.; et al. The antioxidant, analgesic, anti-inflammatory, and wound healing activities of Haplophyllum tuberculatum (Forsskal) A. Juss aqueous and ethanolic extract. Life 2022, 12, 1553. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Available online: www.cambridgesoft.com (accessed on 1 June 2022).
- Available online: www.molinspiration.com (accessed on 1 June 2022).
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; M100-S25; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022. [Google Scholar]
- Limban, C.; Marutescu, L.; Chifiriuc, M.C. Synthesis, spectroscopic properties and antipathogenic activity of new thiourea derivatives. Molecules 2011, 16, 7593–7607. [Google Scholar] [CrossRef] [PubMed]
- Matei, I.; Culita, D.C.; Tecuceanu, V.; Hanganu, A.; Ionita, P. N,N-Bis(7-nitrobenz[c][1,2,5]oxadiazol-4-yl)cystamine. Molbank 2022, 2022, M1423. [Google Scholar] [CrossRef]
- Cserhati, T.; Forgacs, E.; Hajos, G. Determination of the lipophilicity of fused-ring nitrogen-heterocycles by reversed-phase thin layer chromatography-the effect of pH. J. Planar Chromatogr. Modern TLC 1998, 11, 64–69. [Google Scholar]
- Cserháti, T. Study of the absorption characteristics of a zeolite support in normal and reversed-phase thin-layer chromatography. JPC-J. Planar Chromatogr. 2007, 20, 381–384. [Google Scholar] [CrossRef]
- Chmiel, T.; Mieszkowska, A.; Kempińska-Kupczyka, D.; Kot-Wasik, A.; Namieśnik, J.; Mazerska, Z. The impact of lipophilicity on environmental processes, drug delivery and bioavailability of food components. Microchem. J. 2019, 146, 393–406. [Google Scholar] [CrossRef]
- Hubicka, U.; Zuromska-Witek, B.; Komsta, L.; Krzek, J. Lipophilicity study of fifteen fluoroquinolones by reversed-phase thin-layer chromatography. Anal. Methods 2015, 7, 3841–3848. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2010, 53, 4290–4302. [Google Scholar] [CrossRef]
- Sarbu, C.; Sekulic, T.D.; Perisic-Janjic, N. Evaluation of lipophilicity of some benzimidazole and benztriazole derivatives by RP HPTLC and PCA. J. Pharm. Biomed. Anal. 2002, 30, 739–745. [Google Scholar] [CrossRef]
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef] [PubMed]
- Lynch, B.M.; Macdonald, B.C.; Webb, J.G.K. NMR spectra of aromatic amines and amides-I Correlations of amino proton shifts with Hammett substituents constants with Hückel electron densities. Tetrahedron 1968, 24, 3595–3605. [Google Scholar] [CrossRef]
- Woller, T.; Geerlings, P.; De Proft, F.; Champagne, B.; Alonso, M. Aromaticity as a guiding concept for spectroscopic features and nonlinear optical properties of porphyrinoids. Molecules 2018, 23, 1333. [Google Scholar] [CrossRef]
- Shaik, S.; Danovich, D.; Hiberty, P.C. Valence bond theory—Its birth, struggles with molecular orbital theory, its present state and future prospects. Molecules 2021, 26, 1624. [Google Scholar] [CrossRef]
- Perušković, D.S. Quantitative structure-activity relationship study of some antipsychotics by multiple linear regressions. Am. J. Anal. Chem. 2007, 5, 335–342. [Google Scholar] [CrossRef]
- Starek, M.; Plenis, A.; Zagrobelna, M.; Dąbrowska, M. Assessment of lipophilicity descriptors of selected nsaids obtained at different tlc stationary phases. Pharmaceutics 2021, 13, 440. [Google Scholar] [CrossRef] [PubMed]
- Czeszak, A.; Resztak, M.; Czyrski, A.; Nowak, I. Determination of the partition coefficient of isoquinoline alkaloids from Chelidonium majus by reversed phase thin layer chromatography. New J. Chem. 2020, 44, 7484–7489. [Google Scholar] [CrossRef]
- Morak, B.; Nowak, M.; Pluta, K. Determination of the lipophilicity parameters rm0 and logp of new azaphenothiazines by reversed-phase thin-layer chromatography. J. Liq. Chromatogr. Rel. Technol. 2005, 30, 1845–1854. [Google Scholar] [CrossRef]



| Compound | 7 |
| Chemical formula | C18H13N5O3 |
| M (g mol−1) | 347.33 |
| Temperature, (K) | 293 (2) |
| Wavelength, (Å) | 0.71073 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| a (Å) | 13.0162(8) |
| b (Å) | 9.6307 (5) |
| c (Å) | 12.7982 (7) |
| α (°) | 90 |
| β (°) | 93.333 (5) |
| γ (°) | 90 |
| V (Å3) | 1601.61 (16) |
| Z | 4 |
| Dc (g cm−3) | 1.440 |
| μ (mm−1) | 0.103 |
| F(000) | 720 |
| Goodness-of-fit on F2 | 1.058 |
| Final R1, wR2 [I > 2σ(I)] | 0.0363, 0.0958 |
| R1, wR2 (all data) | 0.0439, 0.1001 |
| Cmp. | λmax (nm) | NMR a (δ ppm) | Huckel AC c | log P d | PSA d (A2) | RM0 | b | r2 | TAC f (%) | TAC g (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 285 | 5.39/3.51 b | 0.327/0.062 | 3.00 | 38.05 | 1.732 | −2.5949 | 0.96 | 87 | 75 |
| 2 | 297 | 5.36/7.25 | 0.347/0.312 | 3.15 | 41.12 | 1.597 | −2.8039 | 0.94 | 43 | 90 |
| 3 | 291 | 5.96/- | 0.351/0.534 | 2.15 | 49.41 | 1.459 | −2.4863 | 0.97 | 84 | 77 |
| 4 | 293 | 8.04/- | 0.351/0.495 | 4.79 | 51.1 | 2.543 | −3.4316 | 0.99 | - e | 42 |
| 5 | 301 | 7.88/7.88 b | 0.338/0.338 | 5.92 | 24.05 | 2.935 | −3.7514 | 0.99 | 81 | 61 |
| 6 | 422 | 5.82/10.29 | 0.383/0.485 | 5.65 | 161.53 | 3.541 | −4.4791 | 0.97 | 60 | 66 |
| 7 | 497 | 7.78/10.64 | 0.377/0.518 | 5.75 | 108.8 | 3.184 | −4.1715 | 0.97 | 47 | - e |
| 8 | 440 | -/- | 0.158/0.158 | 5.37 | 24.73 | 2.707 | −3.4197 | 0.98 | - e | 15 |
| 9 | 400 | -/- | 0.596/−0.308 | 5.11 | 162.2 | 3.356 | −4.1594 | 0.97 | - e | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bujor, A.; Hanganu, A.; Tecuceanu, V.; Madalan, A.M.; Tudose, M.; Marutescu, L.; Popa, M.; Chifiriuc, C.M.; Zarafu, I.; Ionita, P. Biological Evaluation and Structural Analysis of Some Aminodiphenylamine Derivatives. Antioxidants 2023, 12, 713. https://doi.org/10.3390/antiox12030713
Bujor A, Hanganu A, Tecuceanu V, Madalan AM, Tudose M, Marutescu L, Popa M, Chifiriuc CM, Zarafu I, Ionita P. Biological Evaluation and Structural Analysis of Some Aminodiphenylamine Derivatives. Antioxidants. 2023; 12(3):713. https://doi.org/10.3390/antiox12030713
Chicago/Turabian StyleBujor, Alexandru, Anamaria Hanganu, Victorita Tecuceanu, Augustin M. Madalan, Madalina Tudose, Luminita Marutescu, Marcela Popa, Carmen Mariana Chifiriuc, Irina Zarafu, and Petre Ionita. 2023. "Biological Evaluation and Structural Analysis of Some Aminodiphenylamine Derivatives" Antioxidants 12, no. 3: 713. https://doi.org/10.3390/antiox12030713
APA StyleBujor, A., Hanganu, A., Tecuceanu, V., Madalan, A. M., Tudose, M., Marutescu, L., Popa, M., Chifiriuc, C. M., Zarafu, I., & Ionita, P. (2023). Biological Evaluation and Structural Analysis of Some Aminodiphenylamine Derivatives. Antioxidants, 12(3), 713. https://doi.org/10.3390/antiox12030713











