Phytochemical Composition and Antioxidant, Anti-Acetylcholinesterase, and Anti-α-Glucosidase Activity of Thymus carnosus Extracts: A Three-Year Study on the Impact of Annual Variation and Geographic Location
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
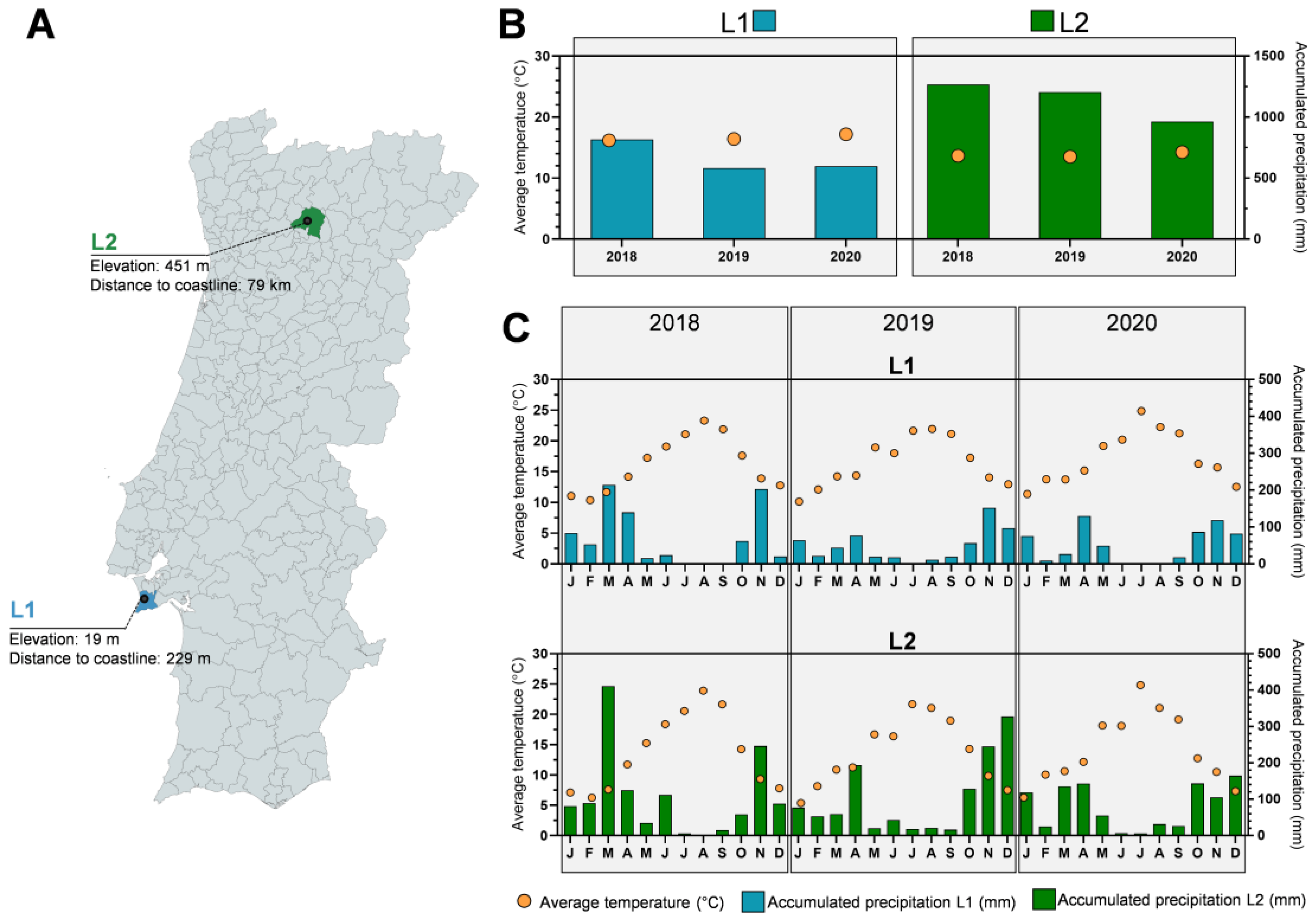
2.2. Plant Material
2.3. Preparation of Extracts
2.4. Total Phenolic Compounds, Total Flavonoids and Ortho-Diphenols Content
2.5. Phytochemical Composition Profiling and Quantification by HPLC-DAD and HPLC-ESI-MSn
2.6. In Vitro Antioxidant Activity Assessment
2.7. Enzymatic Inhibition Assays
2.8. Statistical Analysis
3. Results and Discussion
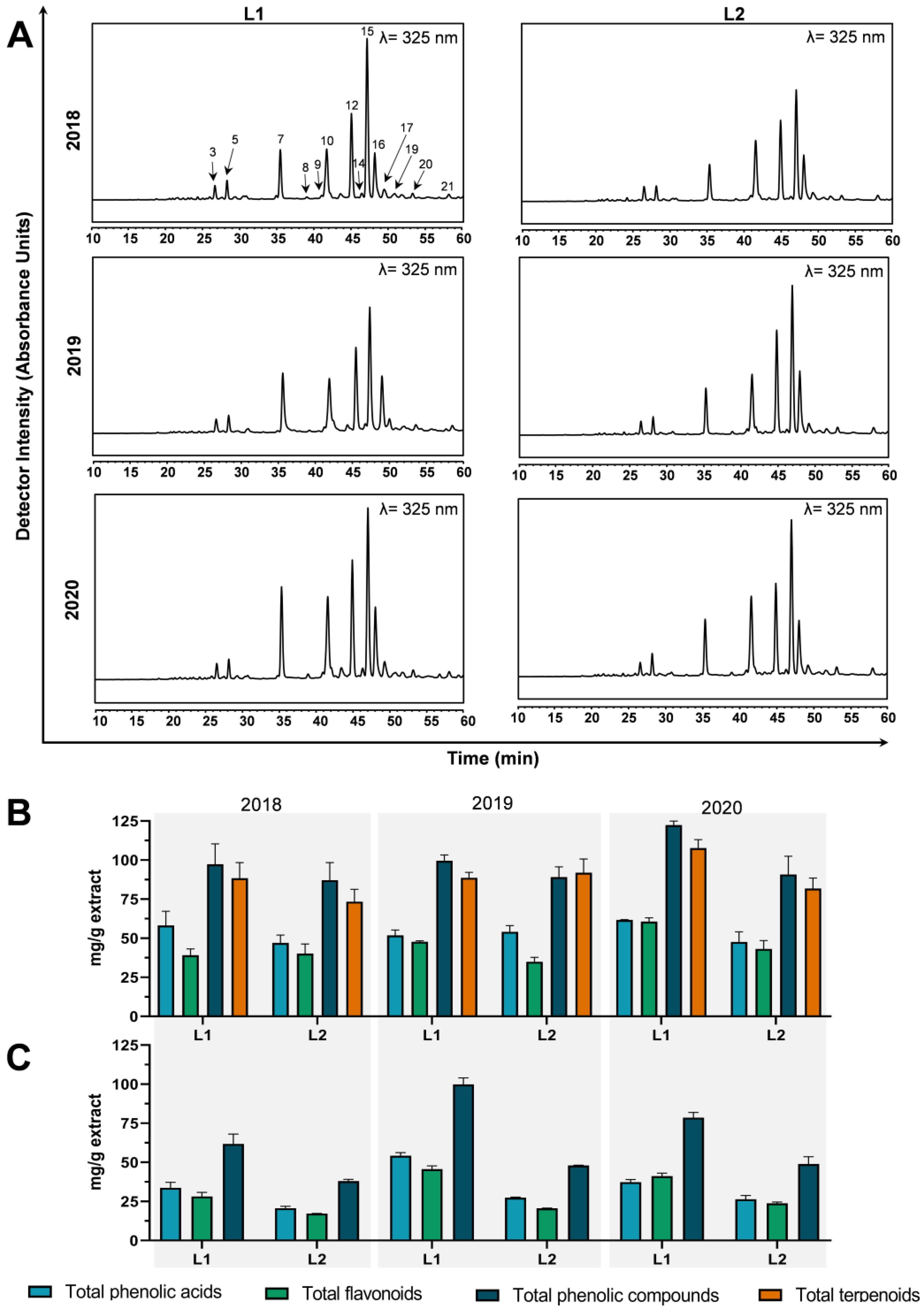
3.1. Extraction Yield, Total Phenolic, Total Flavonoid, and Ortho-Diphenols Content
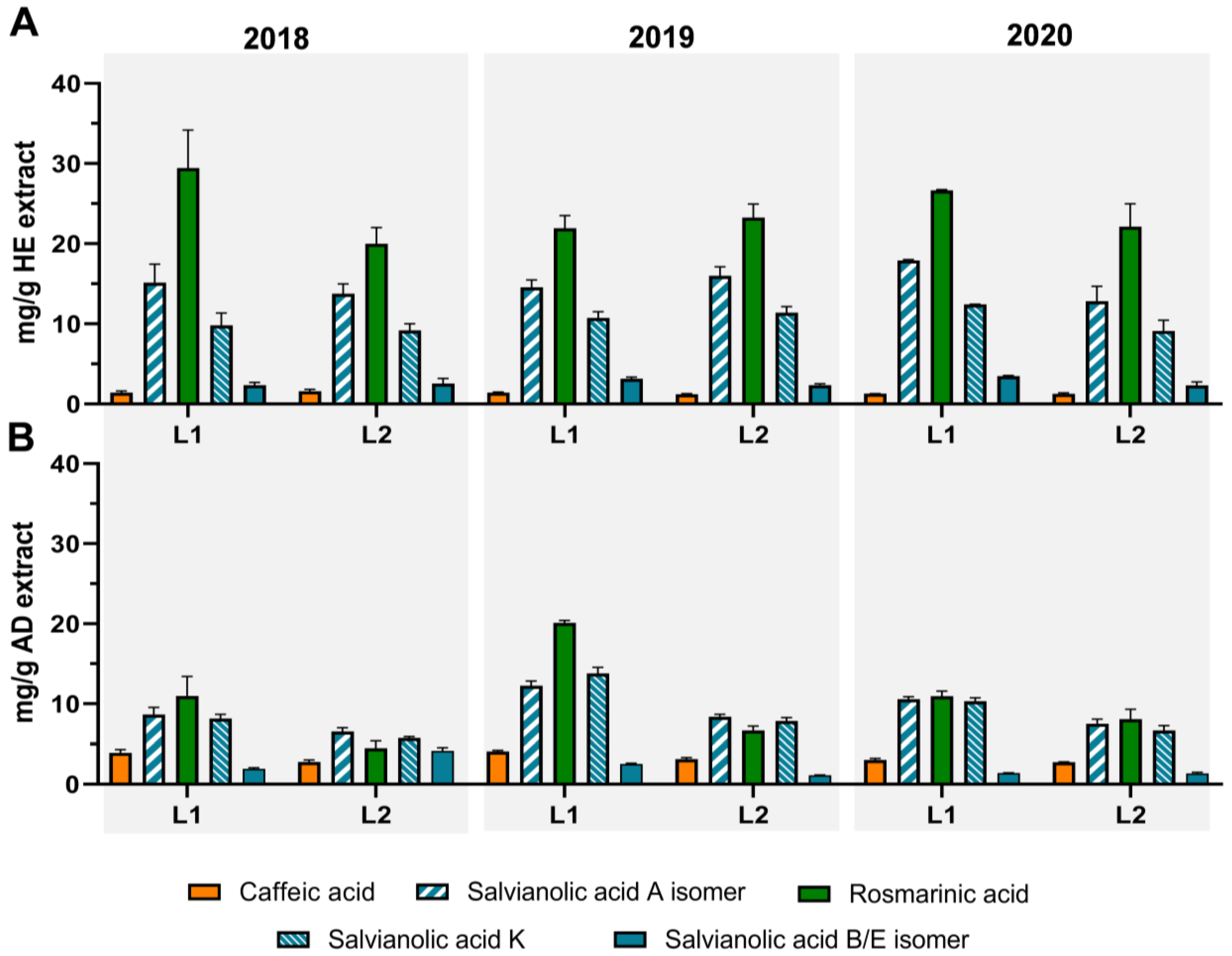
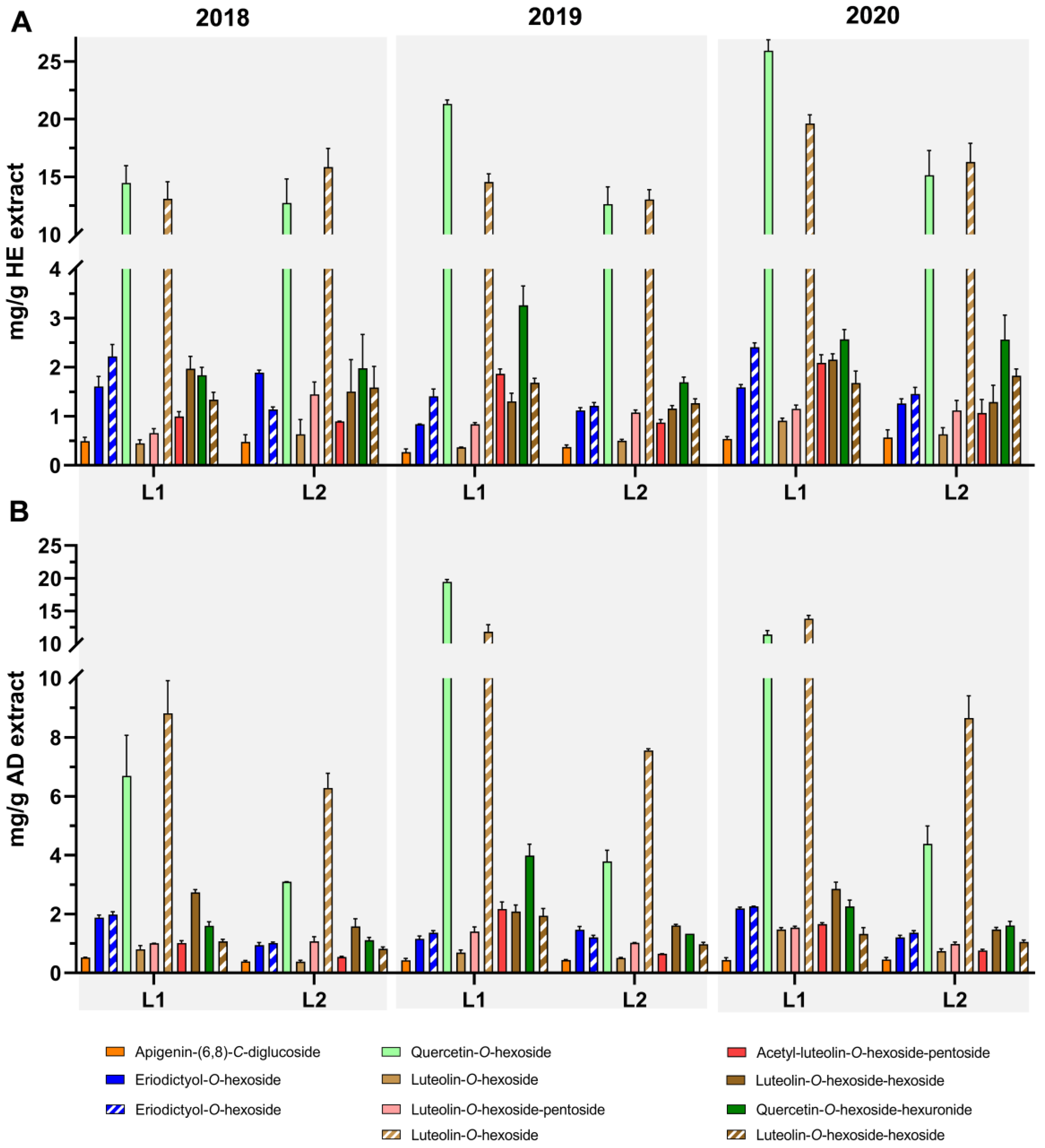
3.2. Profiling and Quantification of Individual Compounds by HPLC-DAD and HPLC-ESI-MSn
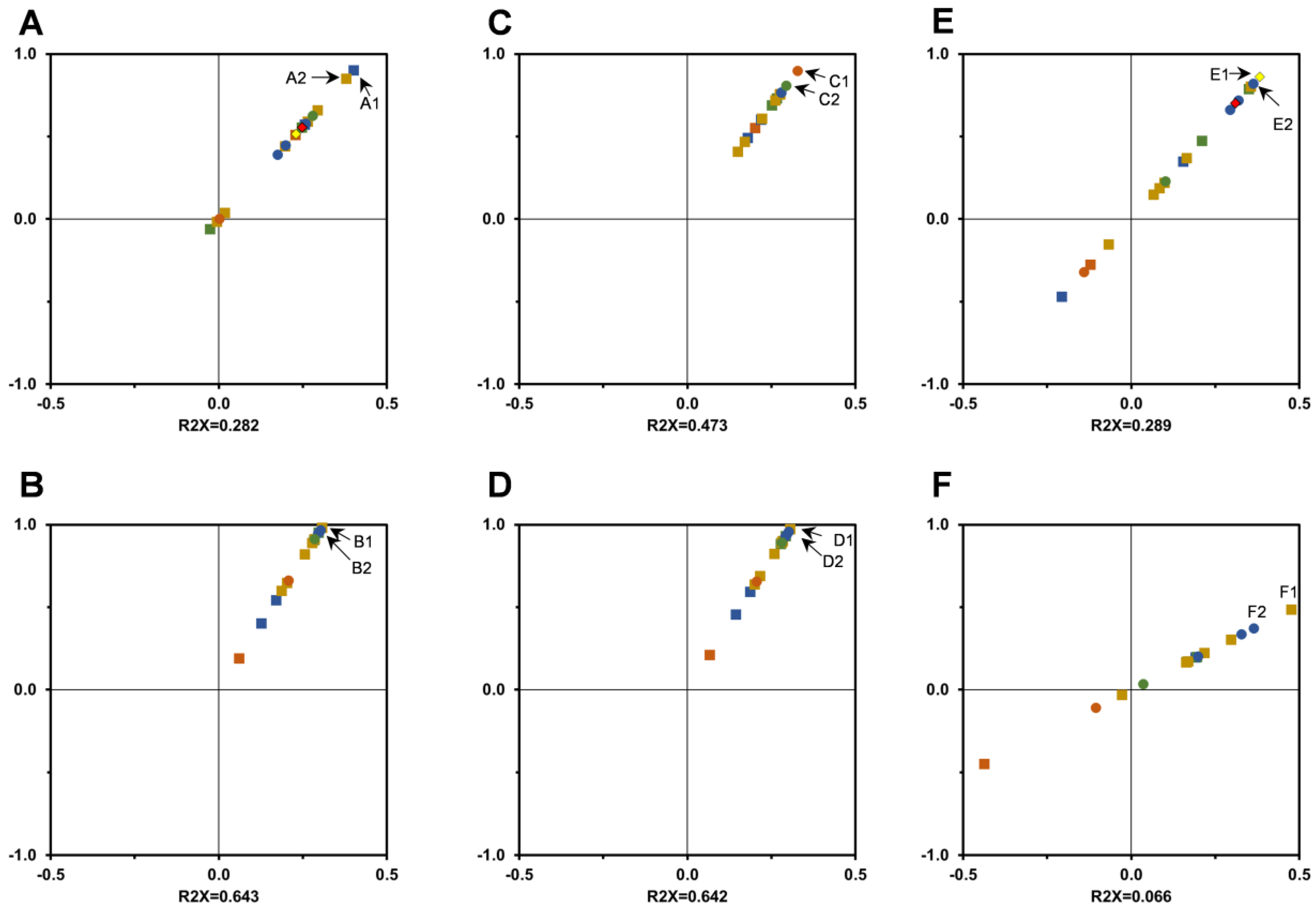
3.3. In Vitro Antioxidant Activity Assessment
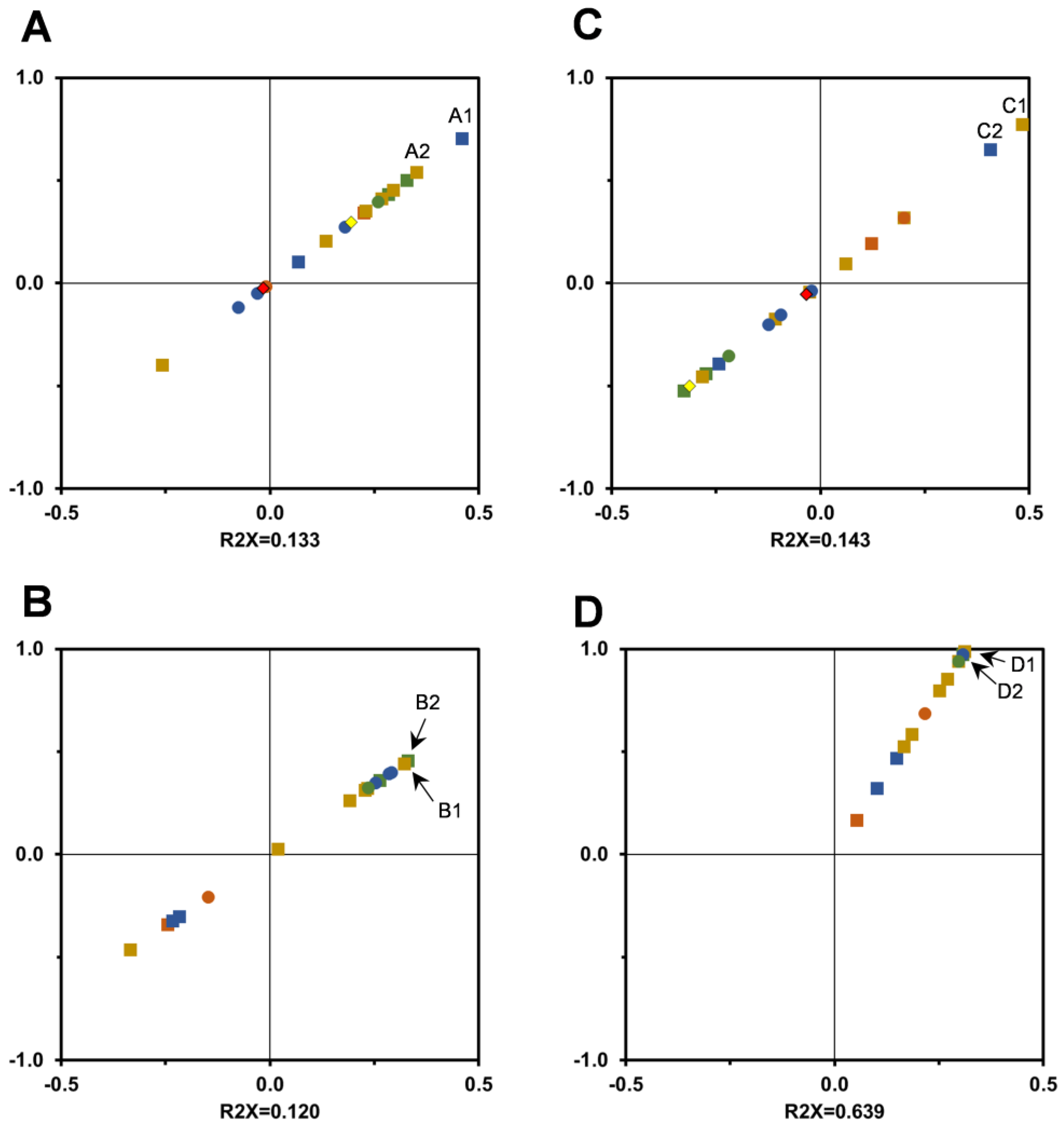
3.4. Enzymatic Inhibition Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martins-Gomes, C.; Souto, E.B.; Silva, A.M. Chapter 15—Nanophytosomes: A novel approach for the delivery of herbal drugs. In Systems of Nanovesicular Drug Delivery; Nayak, A.K., Hasnain, M.S., Aminabhavi, T.M., Torchilin, V.P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 239–257. [Google Scholar]
- Anwar, F.; Abbas, A.; Mehmood, T.; Gilani, A.-H.; Rehman, N.-U. Mentha: A genus rich in vital nutra-pharmaceuticals—A review. Phytother. Res. 2019, 33, 2548–2570. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G. A Review on Applications and Uses of Thymus in the Food Industry. Plants 2020, 9, 961. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharopov, F.; Antolak, H.; Kręgiel, D.; Sen, S.; Sharifi-Rad, M.; Acharya, K.; Sharifi-Rad, R.; et al. Plants of Genus Mentha: From Farm to Food Factory. Plants 2018, 7, 70. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Ozcelik, B.; Altın, G.; Daşkaya-Dikmen, C.; Martorell, M.; Ramírez-Alarcón, K.; Alarcón-Zapata, P.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Alves Borges Leal, A.L.; et al. Salvia spp. plants-from farm to food applications and phytopharmacotherapy. Trends Food Sci. Technol. 2018, 80, 242–263. [Google Scholar] [CrossRef]
- Zettel, V.; Hitzmann, B. Applications of chia (Salvia hispanica L.) in food products. Trends Food Sci. Technol. 2018, 80, 43–50. [Google Scholar] [CrossRef]
- Maleš, I.; Dragović-Uzelac, V.; Jerković, I.; Zorić, Z.; Pedisić, S.; Repajić, M.; Garofulić, I.E.; Dobrinčić, A. Non-Volatile and Volatile Bioactives of Salvia officinalis L., Thymus serpyllum L. and Laurus nobilis L. Extracts with Potential Use in the Development of Functional Beverages. Antioxidants 2022, 11, 1140. [Google Scholar] [CrossRef]
- Chohan, M.; Forster-Wilkins, G.; Opara, E.I. Determination of the antioxidant capacity of culinary herbs subjected to various cooking and storage processes using the ABTS(*+) radical cation assay. Plant Foods Hum. Nutr. 2008, 63, 47–52. [Google Scholar] [CrossRef]
- Martins-Gomes, C.; Taghouti, M.; Schäfer, J.; Bunzel, M.; Silva, A.M.; Nunes, F.M. Chemical characterization and bioactive properties of decoctions and hydroethanolic extracts of Thymus carnosus Boiss. J. Funct. Foods 2018, 43, 154–164. [Google Scholar] [CrossRef]
- Balao, F.; Caldas, F.B.; Casimiro-Soriguer Solanas, F.; Sánchez-Gullón, E.; Talavera Lozano, S. Thymus Carnosus. The IUCN Red List of Threatened Species 2011: E.T162164A5551116. Available online: https://doi.org/10.2305/IUCN.UK.2011-1.RLTS.T162164A5551116.en (accessed on 22 November 2022).
- Roxo, M.; Zuzarte, M.; Gonçalves, M.J.; Alves-Silva, J.M.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. Antifungal and anti-inflammatory potential of the endangered aromatic plant Thymus albicans. Sci. Rep. 2020, 10, 18859. [Google Scholar] [CrossRef] [PubMed]
- Buira, A.; Carapeto, A.; García Murillo, P.G.; Monteiro-Henriques, T. Thymus Albicans. The IUCN Red List of Threatened Species 2017: E.T103496011A103496020. Available online: https://doi.org/10.2305/IUCN.UK.2017-3.RLTS.T103496011A103496020.en (accessed on 22 November 2022).
- International Union for Conservation of Nature’s Red List of Threatened Species. Table 1b—Numbers of Threatened Species by Major Groups of Organisms (1996–2022). Available online: https://nc.iucnredlist.org/redlist/content/attachment_files/2022-2_RL_Stats_Table_1b.pdf (accessed on 4 January 2023).
- Bernardini, S.; Tiezzi, A.; Laghezza Masci, V.; Ovidi, E. Natural products for human health: An historical overview of the drug discovery approaches. Nat. Prod. Res. 2018, 32, 1926–1950. [Google Scholar] [CrossRef]
- Martins-Gomes, C.; Souto, E.B.; Cosme, F.; Nunes, F.M.; Silva, A.M. Thymus carnosus extracts induce anti-proliferative activity in Caco-2 cells through mechanisms that involve cell cycle arrest and apoptosis. J. Funct. Foods 2019, 54, 128–135. [Google Scholar] [CrossRef]
- Lemos, M.F.; Lemos, M.F.; Pacheco, H.P.; Guimarães, A.C.; Fronza, M.; Endringer, D.C.; Scherer, R. Seasonal variation affects the composition and antibacterial and antioxidant activities of Thymus vulgaris. Ind. Crops Prod. 2017, 95, 543–548. [Google Scholar] [CrossRef]
- Vaičiulytė, V.; Butkienė, R.; Ložienė, K. Effects of meteorological conditions and plant growth stage on the accumulation of carvacrol and its precursors in Thymus pulegioides. Phytochemistry 2016, 128, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Hazzit, M.; Baaliouamer, A. Variation of Essential Oil Yield and Composition of Thymus pallescens de Noé from Algeria. J. Essent. Oil Res. 2009, 21, 162–165. [Google Scholar] [CrossRef]
- Martínez, R.M.; Jordán, M.J.; Quílez, M.; Sotomayor, J.A. Effects of Edaphoclimatic Conditions on Thymus hyemalis L. Essential Oil Yield and Composition. J. Essent. Oil Res. 2005, 17, 614–618. [Google Scholar] [CrossRef]
- Galasso, S.; Pacifico, S.; Kretschmer, N.; Pan, S.-P.; Marciano, S.; Piccolella, S.; Monaco, P.; Bauer, R. Influence of seasonal variation on Thymus longicaulis C. Presl chemical composition and its antioxidant and anti-inflammatory properties. Phytochemistry 2014, 107, 80–90. [Google Scholar] [CrossRef]
- Raudone, L.; Zymone, K.; Raudonis, R.; Vainoriene, R.; Motiekaityte, V.; Janulis, V. Phenological changes in triterpenic and phenolic composition of Thymus L. species. Ind. Crops Prod. 2017, 109, 445–451. [Google Scholar] [CrossRef]
- Fonseca, A.R.; Santos, J.A. High-Resolution Temperature Datasets in Portugal from a Geostatistical Approach: Variability and Extremes. J. Appl. Meteorol. Climatol. 2018, 57, 627–644. [Google Scholar] [CrossRef]
- Taghouti, M.; Martins-Gomes, C.; Schäfer, J.; Santos, J.A.; Bunzel, M.; Nunes, F.M.; Silva, A.M. Chemical Characterization and Bioactivity of Extracts from Thymus mastichina: A Thymus with a Distinct Salvianolic Acid Composition. Antioxidants 2020, 9, 34. [Google Scholar] [CrossRef]
- Taghouti, M.; Martins-Gomes, C.; Schäfer, J.; Félix, L.M.; Santos, J.A.; Bunzel, M.; Nunes, F.M.; Silva, A.M. Thymus pulegioides L. as a rich source of antioxidant, anti-proliferative and neuroprotective phenolic compounds. Food Funct. 2018, 9, 3617–3629. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Neto, R.T.; Silva, A.M.S.; Cardoso, S.M. Health-Promoting Effects of Thymus herba-barona, Thymus pseudolanuginosus, and Thymus caespititius Decoctions. Int. J. Mol. Sci. 2017, 18, 1879. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Martins-Gomes, C.; Coutinho, T.E.; Fangueiro, J.F.; Sanchez-Lopez, E.; Pashirova, T.N.; Andreani, T.; Souto, E.B. Soft Cationic Nanoparticles for Drug Delivery: Production and Cytotoxicity of Solid Lipid Nanoparticles (SLNs). Appl. Sci. 2019, 9, 4438. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Silva, P.; Silva, A.M.; Nunes, F.M. Effect of harvesting year and elderberry cultivar on the chemical composition and potential bioactivity: A three-year study. Food Chem. 2020, 302, 125366. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Silva, E.; Brito, C.; Martins-Gomes, C.; Gonçalves, A.; Arrobas, M.; Rodrigues, M.Â.; Correia, C.M.; Nunes, F.M. Zeolites and Biochar Modulate Olive Fruit and Oil Polyphenolic Profile. Antioxidants 2022, 11, 1332. [Google Scholar] [CrossRef]
- Silva, A.M.; Martins-Gomes, C.; Souto, E.B.; Schäfer, J.; Santos, J.A.; Bunzel, M.; Nunes, F.M. Thymus zygis subsp. zygis an Endemic Portuguese Plant: Phytochemical Profiling, Antioxidant, Anti-Proliferative and Anti-Inflammatory Activities. Antioxidants 2020, 9, 482. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Félix, L.M.; Teixeira, I.; Martins-Gomes, C.; Schäfer, J.; Souto, E.B.; Santos, D.J.; Bunzel, M.; Nunes, F.M. Orange thyme: Phytochemical profiling, in vitro bioactivities of extracts and potential health benefits. Food Chem. X 2021, 12, 100171. [Google Scholar] [CrossRef]
- Taghouti, M.; Martins-Gomes, C.; Félix, L.M.; Schäfer, J.; Santos, J.A.; Bunzel, M.; Nunes, F.M.; Silva, A.M. Polyphenol composition and biological activity of Thymus citriodorus and Thymus vulgaris: Comparison with endemic Iberian Thymus species. Food Chem. 2020, 331, 127362. [Google Scholar] [CrossRef] [PubMed]
- Formisano, C.; Delfine, S.; Oliviero, F.; Tenore, G.C.; Rigano, D.; Senatore, F. Correlation among environmental factors, chemical composition and antioxidative properties of essential oil and extracts of chamomile (Matricaria chamomilla L.) collected in Molise (South-central Italy). Ind. Crops Prod. 2015, 63, 256–263. [Google Scholar] [CrossRef]
- Kumar, S.; Yadav, A.; Yadav, M.; Yadav, J.P. Effect of climate change on phytochemical diversity, total phenolic content and in vitro antioxidant activity of Aloe vera (L.) Burm.f. BMC Res. Notes 2017, 10, 60. [Google Scholar] [CrossRef]
- Liebelt, D.J.; Jordan, J.T.; Doherty, C.J. Only a matter of time: The impact of daily and seasonal rhythms on phytochemicals. Phytochem. Rev. 2019, 18, 1409–1433. [Google Scholar] [CrossRef]
- Jordán, M.J.; Martínez, R.M.; Goodner, K.L.; Baldwin, E.A.; Sotomayor, J.A. Seasonal variation of Thymus hyemalis Lange and Spanish Thymus vulgaris L. essential oils composition. Ind. Crops Prod. 2006, 24, 253–263. [Google Scholar] [CrossRef]
- Ghasemi Pirbalouti, A.; Hashemi, M.; Ghahfarokhi, F.T. Essential oil and chemical compositions of wild and cultivated Thymus daenensis Celak and Thymus vulgaris L. Ind. Crops Prod. 2013, 48, 43–48. [Google Scholar] [CrossRef]
- Ferreira, J.; Hartmann, A.; Martins-Gomes, C.; Nunes, F.M.; Souto, E.B.; Santos, D.L.; Abreu, H.; Pereira, R.; Pacheco, M.; Gaivão, I.; et al. Red seaweeds strengthening the nexus between nutrition and health: Phytochemical characterization and bioactive properties of Grateloupia turuturu and Porphyra umbilicalis extracts. J. Appl. Phycol. 2021, 33, 3365–3381. [Google Scholar] [CrossRef]
- Yadav, B.; Jogawat, A.; Rahman, M.S.; Narayan, O.P. Secondary metabolites in the drought stress tolerance of crop plants: A review. Gene Rep. 2021, 23, 101040. [Google Scholar] [CrossRef]
- Costa, R.; Fraga, H.; Fernandes, P.M.; Santos, J.A. Implications of future bioclimatic shifts on Portuguese forests. Reg. Environ. Change 2017, 17, 117–127. [Google Scholar] [CrossRef]
- Cosme, F.; Vilela, A.; Moreira, L.; Moura, C.; Enríquez, J.A.P.; Filipe-Ribeiro, L.; Nunes, F.M. Terroir Effect on the Phenolic Composition and Chromatic Characteristics of Mencía/Jaen Monovarietal Wines: Bierzo D.O. (Spain) and Dão D.O. (Portugal). Molecules 2020, 25, 6008. [Google Scholar] [CrossRef]
- Soare, J.R.; Dinis, T.C.P.; Cunha, A.P.; Almeida, L. Antioxidant Activities of Some Extracts of Thymus zygis. Free Radic. Res. 1997, 26, 469–478. [Google Scholar] [CrossRef]
- Kim, I.-S.; Yang, M.-R.; Lee, O.-H.; Kang, S.-N. Antioxidant Activities of Hot Water Extracts from Various Spices. Int. J. Mol. Sci. 2011, 12, 4120–4131. [Google Scholar] [CrossRef]
- Barros, L.; Heleno, S.A.; Carvalho, A.M.; Ferreira, I.C.F.R. Lamiaceae often used in Portuguese folk medicine as a source of powerful antioxidants: Vitamins and phenolics. LWT—Food Sci. Technol. 2010, 43, 544–550. [Google Scholar] [CrossRef]
- Fernandes, Â.S.F.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Lipophilic and hydrophilic antioxidants, lipid peroxidation inhibition and radical scavenging activity of two Lamiaceae food plants. Eur. J. Lipid Sci. Technol. 2010, 112, 1115–1121. [Google Scholar] [CrossRef]
- Sati, P.; Pandey, A.; Rawat, S.; Rani, A. Phytochemicals and antioxidants in leaf extracts of Ginkgo biloba with reference to location, seasonal variation and solvent system. J. Pharm. Res. 2013, 7, 804–809. [Google Scholar] [CrossRef]
- Guesmi, F.; Saidi, I.; Bouzenna, H.; Hfaiedh, N.; Landoulsi, A. Phytocompound variability, antioxidant and antibacterial activities, anatomical features of glandular and aglandular hairs of Thymus hirtus Willd. Ssp. algeriensis Boiss. and Reut. over developmental stages. S. Afr. J. Bot. 2019, 127, 234–243. [Google Scholar] [CrossRef]
- Albano, S.M.; Lima, A.S.; Miguel, M.G.; Pedro, L.G.; Barroso, J.G.; Figueiredo, A.C. Antioxidant, anti-5-lipoxygenase and antiacetylcholinesterase activities of essential oils and decoction waters of some aromatic plants. Rec. Nat. Prod. 2012, 6, 35. [Google Scholar]
- Vladimir-Knežević, S.; Blažeković, B.; Kindl, M.; Vladić, J.; Lower-Nedza, A.D.; Brantner, A.H. Acetylcholinesterase Inhibitory, Antioxidant and Phytochemical Properties of Selected Medicinal Plants of the Lamiaceae Family. Molecules 2014, 19, 767. [Google Scholar] [CrossRef]
- Mata, A.T.; Proença, C.; Ferreira, A.R.; Serralheiro, M.L.M.; Nogueira, J.M.F.; Araújo, M.E.M. Antioxidant and antiacetylcholinesterase activities of five plants used as Portuguese food spices. Food Chem. 2007, 103, 778–786. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Rababah, T.; Johargy, A.; Gammoh, S.; Ereifej, K.; Alhamad, M.N.; Brewer, M.S.; Saati, A.A.; Kubow, S.; Rawshdeh, M. Extraction, optimisation and characterisation of phenolics from Thymus vulgaris L.: Phenolic content and profiles in relation to antioxidant, antidiabetic and antihypertensive properties. Int. J. Food Sci. Technol. 2016, 51, 720–730. [Google Scholar] [CrossRef]
- Tang, H.; Ma, F.; Zhao, D.; Xue, Z. Exploring the effect of salvianolic acid C on α-glucosidase: Inhibition kinetics, interaction mechanism and molecular modelling methods. Process Biochem. 2019, 78, 178–188. [Google Scholar] [CrossRef]
- Yin, G.; Li, Y.M.; Wei, W.; Jiang, S.H.; Zhu, D.Y.; Du, W.H. Interactions of acetylcholinesterase with salvianolic acid B and rosmarinic acid from Salvia miltiorhiza water extract investigated by NMR relaxation rate. Chin. Chem. Lett. 2008, 19, 747–751. [Google Scholar] [CrossRef]
- Loesche, A.; Köwitsch, A.; Lucas, S.D.; Al-Halabi, Z.; Sippl, W.; Al-Harrasi, A.; Csuk, R. Ursolic and oleanolic acid derivatives with cholinesterase inhibiting potential. Bioorganic Chem. 2019, 85, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Taslimi, P.; Gulçin, İ. Antidiabetic potential: In vitro inhibition effects of some natural phenolic compounds on α-glycosidase and α-amylase enzymes. J. Biochem. Mol. Toxicol. 2017, 31, e21956. [Google Scholar] [CrossRef]
- Zhang, B.-w.; Xing, Y.; Wen, C.; Yu, X.-x.; Sun, W.-l.; Xiu, Z.-l.; Dong, Y.-s. Pentacyclic triterpenes as α-glucosidase and α-amylase inhibitors: Structure-activity relationships and the synergism with acarbose. Bioorganic Med. Chem. Lett. 2017, 27, 5065–5070. [Google Scholar] [CrossRef] [PubMed]

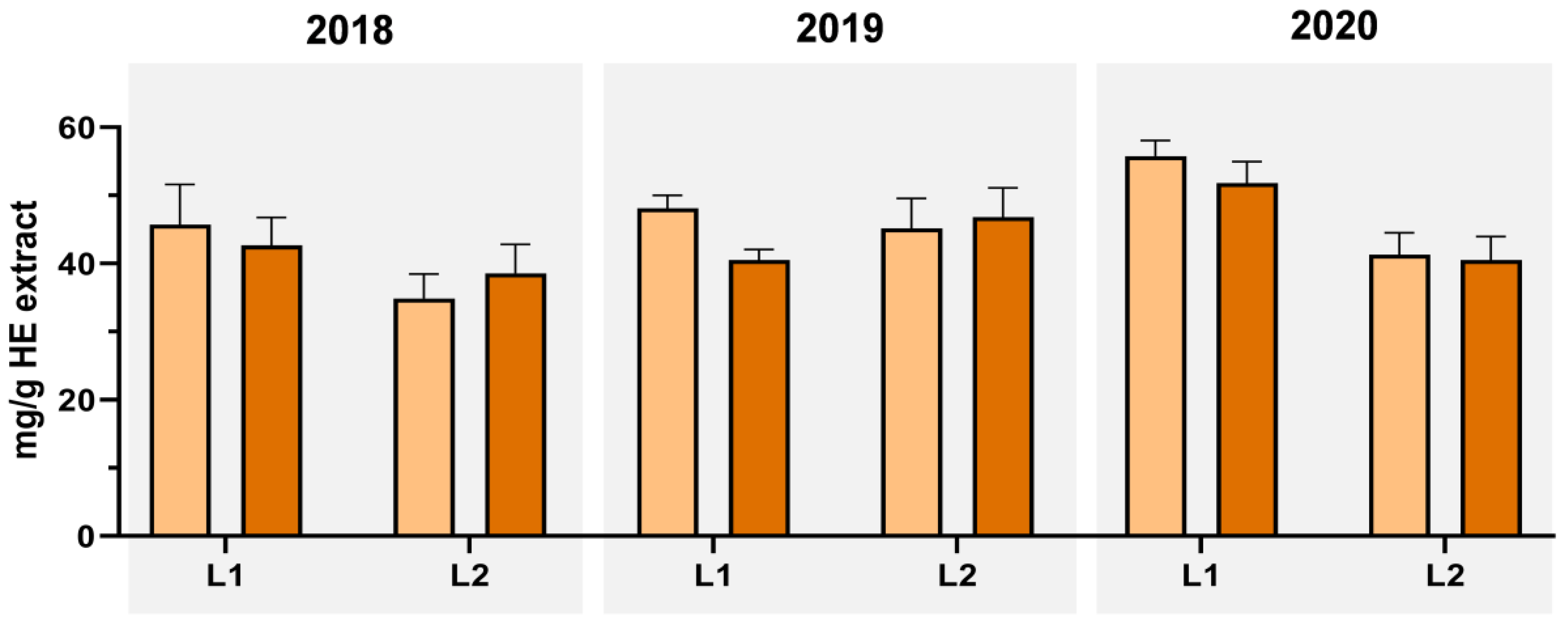
| Year | Loc. | E. M. | Extraction Yield | Total Phenols (mg Caffeic Acid eq./g) | Ortho-Diphenols (mg Caffeic Acid eq./g) | Total Flavonoids (mg Catechin eq./g) | |||
|---|---|---|---|---|---|---|---|---|---|
| % w/w | mg/g Extract | mg/g DP | mg/g Extract | mg/g DP | mg/g Extract | mg/g Dry DP | |||
| 2018 | L1 | AD | 17.90 ± 0.70 Aa | 210.93 ± 7.45 | 37.76 ± 1.33 Aa* | 95.43 ± 5.44 | 17.08 ± 0.97 Aa* | 146.85 ± 6.64 | 26.29 ± 1.19 Aa* |
| HE | 18.98 ± 0.77 ABa | 167.60 ± 14.03 | 31.81 ± 2.66 Aa* | 118.29 ± 6.97 | 22.45 ± 1.32 Aa* | 149.79 ± 0.38 | 28.43 ± 0.07 Aa* | ||
| L2 | AD | 22.78 ± 1.77 Ab | 164.74 ± 1.84 | 37.53 ± 0.42 ABa | 67.50 ± 1.75 | 15.38 ± 0.40 Ab* | 104.62 ± 8.43 | 23.83 ± 1.92 Aa* | |
| HE | 25.43 ± 2.83 Ab | 152.90 ± 13.89 | 38.88 ± 3.53 Aa | 94.50 ± 9.65 | 24.03 ± 2.45 Aa* | 146.35 ± 3.58 | 37.22 ± 0.9 Ab* | ||
| 2019 | L1 | AD | 18.98 ± 1.21 Aa | 258.65 ± 9.06 | 49.09 ± 1.72 Ba* | 126.11 ± 1.97 | 23.93 ± 0.37 Ba | 186.35 ± 5.16 | 35.37 ± 0.98 Aa* |
| HE | 21.35 ± 1.91 Aa | 173.52 ± 14.04 | 37.05 ± 2.98 Aa* | 110.57 ± 15.75 | 23.61 ± 3.36 Aa | 144.93 ± 1.38 | 30.94 ± 0.29 Ba* | ||
| L2 | AD | 21.03 ± 0.52 Aa | 194.90 ± 16.08 | 40.99 ± 3.38 Ab* | 82.50 ± 5.25 | 17.35 ± 1.10 Ab* | 131.92 ± 6.26 | 27.74 ± 1.32 Bb | |
| HE | 22.24 ± 1.53 ABa | 150.42 ± 17.31 | 33.45 ± 3.85 ABb* | 93.00 ± 7.27 | 20.68 ± 1.62 ABa* | 123.70 ± 8.44 | 27.51 ± 1.88 Bb | ||
| 2020 | L1 | AD | 19.08 ± 0.54 Aa | 242.81 ± 5.95 | 46.33 ± 1.13 Ba* | 118.43 ± 3.54 | 22.60 ± 0.67 Ba | 162.38 ± 4.38 | 30.98 ± 0.84 Ca |
| HE | 17.82 ± 0.68 Ba | 202.34 ± 7.16 | 36.06 ± 1.28 Aa* | 127.07 ± 6.54 | 22.64 ± 1.17 Aa | 176.92 ± 1.84 | 31.53 ± 0.34 Ba | ||
| L2 | AD | 19.27 ± 0.13 Ba | 184.59 ± 2.65 | 35.57 ± 0.51 Bb* | 80.36 ± 2.61 | 15.49 ± 0.50 Ab* | 118.08 ± 5.51 | 22.75 ± 1.06 Ab* | |
| HE | 19.61 ± 2.03 Ba | 154.62 ± 6.45 | 30.32 ± 1.27 Bb* | 97.43 ± 1.61 | 19.11 ± 0.31 Bb* | 135.77 ± 5.89 | 26.62 ± 1.15 Bb* | ||
| Year | Loc. | E.M. | ABTS•+ | Hydroxyl (•OH) | Nitric Oxide (NO•) | Superoxide (O2•−) | β-Carotene Bleaching | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mmol Trolox eq/DP | % Inhibition wo/EDTA | IC50 (mg/mL) | % Inhibition w/EDTA | IC50 | % Inhibition | IC50 (mg/mL) | % Inhibition | IC50 (mg/mL) | % Inhibition | IC50 (mg/mL) | |||
| 2018 | L1 | AD | 0.19 ± 0.01 Aa* | 54.92 ± 2.86 ABa | 0.87 ± 0.04 AB | 33.77 ± 0.78 Aa | - | 65.89 ± 1.01 Aa | 0.74 ± 0.01 Aa | 33.83 ± 4.14 Aa | 1.67 ± 0.16 Aa | 70.33 ± 3.46 Aa | 0.15 ± 0.02 ABa |
| HE | 0.16 ± 0.01 Aa* | - | - | - | - | - | - | 31.41 ± 5.60 Aa | 1.67 ± 0.19 Aa | 77.95 ± 8.82 Aa | 0.16 ± 0.02 Aa | ||
| L2 | AD | 0.20 ± 0.01 Aa | 38.51 ± 1.42 Ab | - | 31.91 ± 1.20 Aa | - | 53.29 ± 3.92 Ab | 0.97 ± 0.03 Ab | 41.85 ± 0.22 Ab | 1.51 ± 0.11 Aa | 78.09 ± 1.73 Ab | 0.12 ± 0.01 Aa | |
| HE | 0.21 ± 0.01 Ab | - | - | - | - | - | - | 42.31 ± 1.31 Ab | 1.35 ± 0.08 Ab | 77.54 ± 1.18 Aa | 0.12 ± 0.02 Aa | ||
| 2019 | L1 | AD | 0.25 ± 0.01 Ba* | 54.92 ± 0.50 Aa | 0.80 ± 0.03 A | 30.83 ± 2.37 Aa | - | 73.31 ± 1.06 Ba | 0.57 ± 0.01 Ba | 49.72 ± 1.10 Ba* | 0.96 ± 0.03 Ba | 82.53 ± 2.88 Ba* | 0.16 ± 0.01 Aa |
| HE | 0.16 ± 0.01 Aa* | - | - | - | - | - | - | 47.46 ± 0.47 Ba* | 1.04 ± 0.05 Ba | 90.57 ± 0.96 Ba* | 0.14 ± 0.01 Aa | ||
| L2 | AD | 0.21 ± 0.02 Aa* | 45.34 ± 5.01 Ab | - | 29.21 ± 1.31 Aa | - | 64.35 ± 2.66 Bb | 0.76 ± 0.01 Bb | 46.48 ± 1.96 Ba | 1.11 ± 0.04 Bb | 90.85 ± 3.32 Bb | 0.16 ± 0.02 Ba | |
| HE | 0.17 ± 0.01 Ba* | - | - | - | - | - | - | 43.34 ± 2.05 Ab | 1.21 ± 0.06 Ab | 91.16 ± 1.27 Ba | 0.15 ± 0.02 Aa | ||
| 2020 | L1 | AD | 0.24 ± 0.02 Ba* | 50.08 ± 1.88 Ba | 0.89 ± 0.04 B | 29.96 ± 1.14 Aa | - | 72.77 ± 1.19 Ba | 0.65 ± 0.01 Ca | 45.91 ± 1.25 Ba | 1.16 ± 0.07 Ca | 87.52 ± 4.16 Ba* | 0.13 ± 0.01 Ba |
| HE | 0.17 ± 0.01 Aa* | - | - | - | - | - | - | 43.55 ± 1.11 Ba | 1.23 ± 0.08 Ca | 96.26 ± 2.94 Ca* | 0.11 ± 0.01 Ba | ||
| L2 | AD | 0.18 ± 0.01 Ab* | 39.56 ± 0.60 Ab | - | 29.55 ± 2.38 Aa | - | 54.34 ± 1.84 Ab | 0.91 ± 0.02 Cb | 33.98 ± 6.11 Ab | 1.79 ± 0.18 Ab | 72.55 ± 1.44 Cb | 0.12 ± 0.02 ABa | |
| HE | 0.15 ± 0.01 Ba* | - | - | - | - | - | - | 33.52 ± 7.20 Aa | 1.77 ± 0.26 Bb | 80.03 ± 4.71 Ab | 0.11 ± 0.01 Ba | ||
| Positive control | Trolox (mg/mL) IC50 = 0.24 ± 0.01 | Rosmarinic acid (45 µg/mL; wo/EDTA) 43.27 ± 3.50% inhibition | Rosmarinic acid (15 µg/mL) 44.43 ± 2.62% inhibition | Rosmarinic acid (120 µg/mL) 95.71 ± 8.55% inhibition | Rosmarinic acid (µg/mL) IC50 = 22.05 ± 1.02 | ||||||||
| Year | Loc. | E.M. | AChE | Tyrosinase | Elastase | α-Amylase | α-Glucosidase |
|---|---|---|---|---|---|---|---|
| % Inhibition | % Inhibition | % Inhibition | % Inhibition | % Inhibition | |||
| 2018 | L1 | AD | 60.38 ± 6.17 ABa* | 18.07 ± 4.63 Aa* | 6.3 ± 0.29 Aa | 0.23 ± 0.12 Aa* | 18.89 ± 3.46 Aa |
| HE | 44.26 ± 2.56 Aa* | 38.33 ± 0.90 Aa* | - | 3.74 ± 0.23 Aa* | 15.79 ± 0.93 Aa | ||
| L2 | AD | 46.31 ± 2.56 Ab* | 19.11 ± 4.40 Aa* | 5.15 ± 3.38 Aa | 0.41 ± 0.06 Aa* | 12.28 ± 2.36 Aa* | |
| HE | 38.68 ± 1.63 Abb* | 33.53 ± 1.40 Ab* | - | 3.65 ± 0.16 Aa* | 26.64 ± 0.76 Ab* | ||
| 2019 | L1 | AD | 61.47 ± 1.88 Aa* | 26.63 ± 1.63 Ba* | 7.32 ± 3.22 Aa | 1.49 ± 0.08 Ba* | 27.37 ± 2.37 Ba* |
| HE | 41.98 ± 6.00 Aa* | 33.25 ± 2.58 Ba* | - | 3.76 ± 0.59 Aa* | 12.2 ± 3.34 Aa* | ||
| L2 | AD | 57.36 ± 2.56 Ba* | 20.56 ± 2.36 Ab* | 5.56 ± 0.47 Aa | 0 ± 0 Bb* | 15.44 ± 3.79 ABb* | |
| HE | 31.97 ± 4.65 Ab* | 38.33 ± 0.45 Bb* | - | 5.46 ± 0.38 Bb* | 21.26 ± 1.91 Bb* | ||
| 2020 | L1 | AD | 54.10 ± 2.56 Ba* | 21.55 ± 0.06 Aa* | 6.50 ± 1.08 Aa | 0.59 ± 0.01 Ca* | 19.75 ± 3.76 Aa |
| HE | 43.03 ± 1.63 Aa* | 30.50 ± 0.83 Ba* | - | 3.26 ± 0.28 Aa* | 18.24 ± 0.01 Ba | ||
| L2 | AD | 61.06 ± 1.42 Bb* | 11.27 ± 1.91 Bb* | 1.22 ± 0.57 Bb | 0.43 ± 0.08 Ab* | 18.41 ± 1.6 Ba | |
| HE | 43.44 ± 2.12 Ba* | 42.54 ± 1.46 Cb* | - | 3.90 ± 0.11 Ab* | 15.77 ± 1.85 Ca | ||
| Positive control (% inhibition) | Quercetin (120 µg/mL) 48.61 ± 3.50% | Kojic acid (1 mg/mL) 97.04 ± 1.09% | Quercetin (120 µg/mL) 51.20 ± 7.20% | Acarbose (1 mg/mL) 79.48 ± 3.62% | Acarbose (1 mg/mL) 76.67 ± 1.33% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins-Gomes, C.; Steck, J.; Keller, J.; Bunzel, M.; Santos, J.A.; Nunes, F.M.; Silva, A.M. Phytochemical Composition and Antioxidant, Anti-Acetylcholinesterase, and Anti-α-Glucosidase Activity of Thymus carnosus Extracts: A Three-Year Study on the Impact of Annual Variation and Geographic Location. Antioxidants 2023, 12, 668. https://doi.org/10.3390/antiox12030668
Martins-Gomes C, Steck J, Keller J, Bunzel M, Santos JA, Nunes FM, Silva AM. Phytochemical Composition and Antioxidant, Anti-Acetylcholinesterase, and Anti-α-Glucosidase Activity of Thymus carnosus Extracts: A Three-Year Study on the Impact of Annual Variation and Geographic Location. Antioxidants. 2023; 12(3):668. https://doi.org/10.3390/antiox12030668
Chicago/Turabian StyleMartins-Gomes, Carlos, Jan Steck, Judith Keller, Mirko Bunzel, João A. Santos, Fernando M. Nunes, and Amélia M. Silva. 2023. "Phytochemical Composition and Antioxidant, Anti-Acetylcholinesterase, and Anti-α-Glucosidase Activity of Thymus carnosus Extracts: A Three-Year Study on the Impact of Annual Variation and Geographic Location" Antioxidants 12, no. 3: 668. https://doi.org/10.3390/antiox12030668
APA StyleMartins-Gomes, C., Steck, J., Keller, J., Bunzel, M., Santos, J. A., Nunes, F. M., & Silva, A. M. (2023). Phytochemical Composition and Antioxidant, Anti-Acetylcholinesterase, and Anti-α-Glucosidase Activity of Thymus carnosus Extracts: A Three-Year Study on the Impact of Annual Variation and Geographic Location. Antioxidants, 12(3), 668. https://doi.org/10.3390/antiox12030668








