Abstract
Agricultural soil degradation is occurring at unprecedented rates, not only as an indirect effect of climate change (CC) but also due to intensified agricultural practices which affect soil properties and biodiversity. Therefore, understanding the impacts of CC and soil degradation on plant physiology is crucial for the sustainable development of mitigation strategies to prevent crop productivity losses. The amino acid proline has long been recognized for playing distinct roles in plant cells undergoing osmotic stress. Due to its osmoprotectant and redox-buffering ability, a positive correlation between proline accumulation and plants’ tolerance to abiotic stress has been pointed out in numerous reviews. Indeed, proline quantification is used systematically by plant physiologists as an indicator of the degree of tolerance and a measurement of the antioxidant potential in plants under stressful conditions. Moreover, the exogenous application of proline has been shown to increase resilience to several stress factors, including those related to soil degradation such as salinity and exposure to metals and xenobiotics. However, recent data from several studies often refer to proline accumulation as a signal of stress sensitivity with no clear correlation with improved antioxidant activity or higher stress tolerance, including when proline is used exogenously as a stress reliever. Nevertheless, endogenous proline levels are strongly modified by these stresses, proving its involvement in plant responses. Hence, one main question arises—is proline augmentation always a sign of improved stress resilience? From this perspective, the present review aims to provide a more comprehensive understanding of the implications of proline accumulation in plants under abiotic stress induced by soil degradation factors, reinforcing the idea that proline quantification should not be employed as a sole indicator of stress sensitivity or resilience but rather complemented with further biochemical and physiological endpoints.
Keywords:
oxidative stress; redox homeostasis; antioxidants; soil erosion; abiotic stress; salinity; metals; xenobiotics 1. Introduction
Climate change (CC), environmental pollution, and the loss of agricultural productivity are major issues of our time, particularly because they are interconnected and aggravate one another and even more so for being the most threatening to regions of the planet that are already vulnerable [1,2,3]. The degradation of agricultural soils follows at unprecedented rates, not only due to the indirect effects of CC but also due to the use of heavy machinery, excessive and negligent irrigation, and the application of agrochemicals, which altogether lead to soil salinization and contamination and a loss of soil functions and biodiversity [4,5]. Thus, in a snowball phenomenon, large-scale agricultural production on increasingly infertile soils tends to accelerate soil erosion, indirectly contributing to the worsening of CC [5,6,7]. In this sense, it is imperative to reinvent modern agriculture, reduce its ecological footprint, and make it more resilient to these inevitable adversities [4]. Understanding the effects of CC and soil degradation on plant physiology is of the utmost importance in the research on sustainable mitigation approaches to avoiding crop productivity losses. Abiotic stress mitigators have gained attention from plant researchers, including those that aim for a tolerance to the stresses induced by soil degradation, such as salinity, metal toxicity, and exposure to xenobiotics [8]. Several compounds have been proposed as stress mitigators, including biostimulants, plant-growth-promoting microorganisms, phytohormones, trace elements, N-containing compounds, and the focus of this work: the amino acid proline [9,10,11]. In fact, the exogenous application of proline as a stress mitigator is evidenced in numerous reviews, having been used to boost plant tolerance to several abiotic stresses [10,12,13,14,15,16,17,18,19,20].
Taking advantage of the already extensive literature regarding the participation of proline in plant responses to environmental stress, the present work aims to further disclose the roles of proline in the specific cases of plant abiotic stress related to soil degradation. Providing a broader perspective, the proline metabolism responses of plants under exposure to excessive salt, metals, and xenobiotics are described below. Examples of studies in which proline accumulation was used for the mitigation of stress, either by exogenous application or by employing genetic engineering tools, are also presented. The suggested roles and implications of proline accumulation in stress signaling and tolerance are critically discussed, highlighting how this amino acid appears to have such divergent functions in cells under stress depending on the type of stress and the plant species, age, and tissue, as well as the source of proline itself.
2. Proline: Functions and Metabolism
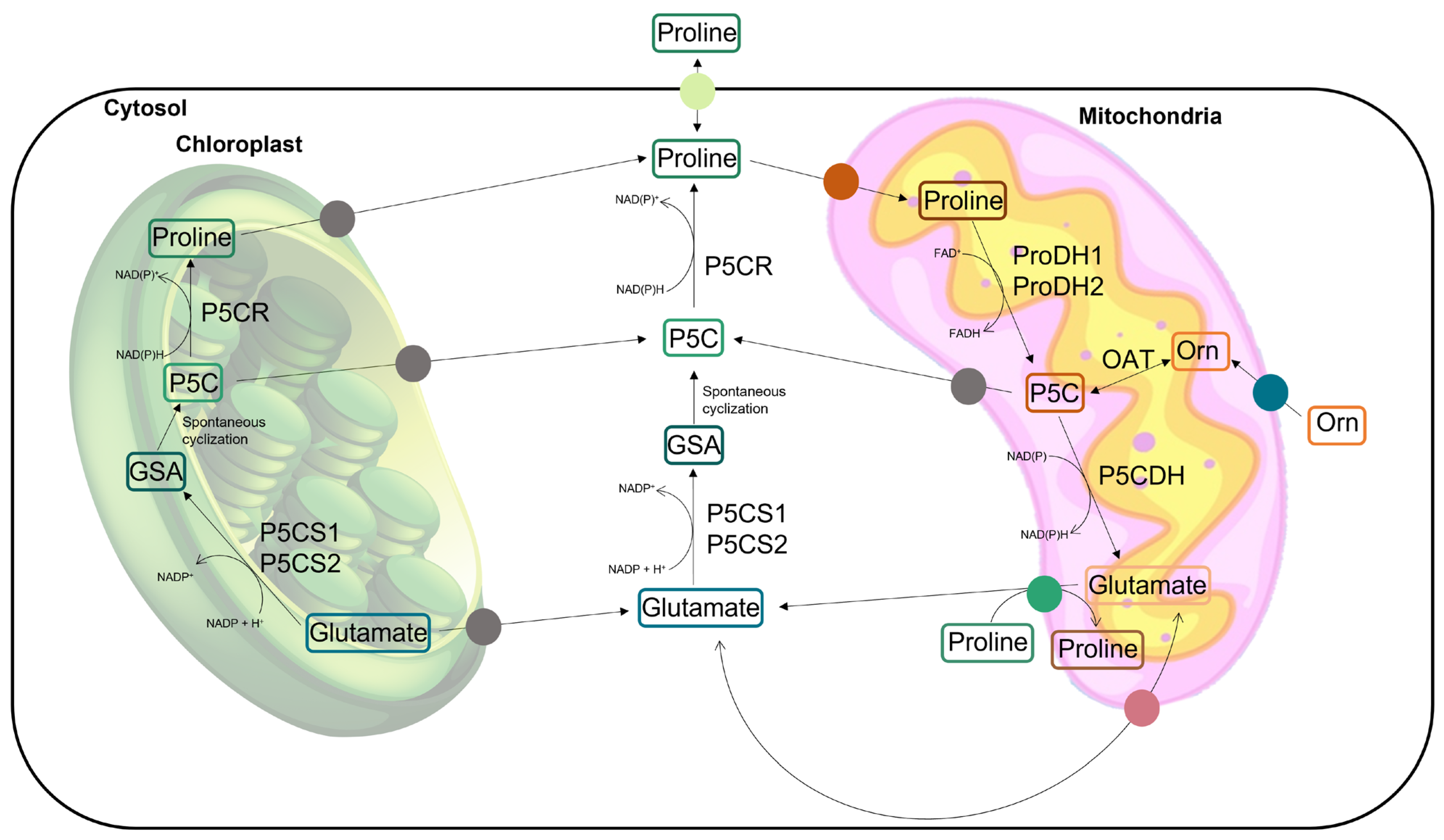
Proline is a proteinogenic amino acid with a secondary amine in the α-amino group and an unusual cyclic structure which confers conformational rigidity to the secondary structure of the proteins [18,21]. In addition to taking part in the composition of proteins, free proline has also been recognized for playing distinct roles in plant cells, especially under conditions of abiotic stress. In addition to occurring as a product of proteolysis, proline can be biosynthesized de novo in plant cells by the glutamic acid (Glu) pathway or, alternatively, by the ornithine (Orn) pathway (Figure 1). L-proline is mainly biosynthesized from Glu in the cytoplasm and/or chloroplast through the activity of two enzymes: Δ1-pyrroline-5-carboxylate-synthetase (P5CS, EC 2.7.2.11; EC 1.2.1.41) and Δ1-pyrroline-5-carboxylate-reductase (P5CR; EC 1.5.1.2). The first is responsible for the conversion of Glu into glutamic-5-semialdehyde (GSA), which then undergoes a spontaneous cyclization to form pyrroline-5-carboxylate (P5C). P5CS is encoded by two genes whose expression is differently regulated, resulting in two isoforms which are usually present in non-redundant activity, have different subcellular locations, and are reportedly sub-functionalized in their stress-responsive (P5CS1) and housekeeping (P5CS2) forms [10,18,20,22,23]. On the second enzymatic step of this biosynthetic pathway, P5CR reduces P5C into proline, preferentially using NADPH as the electron donor. In the alternative pathway, the first step encompasses the reversible transamination of Orn into GSA, which is catalyzed by the mitochondrial enzyme ornithine δ-aminotransferase (OAT; EC 2.6.1.13) (Figure 1). Similar to the Glu pathway, GSA is then spontaneously cyclized into P5C, which is converted into proline by P5CR [18,24]. The catabolism of proline takes place in the mitochondria through the activities of the enzyme proline dehydrogenase (ProDH; EC 1.5.5.2), which converts proline back to P5C. This common intermediate, P5C, is oxidized by P5C dehydrogenase (P5CDH; EC 1.2.1.88) to produce Glu. The enzyme ProDH also comes in two isoforms, which are expressed under different circumstances [25]. Although the exact mechanisms of intracellular transport are not yet well described, the compartmentalized metabolism of proline involves the active transport of proline, P5C, GSA, and Glu between the cytosol, chloroplasts, and mitochondria [26] (Figure 1). When P5C oxidation in the mitochondria is impaired, P5C can be exported to the cytosol and be reduced to proline by cytosolic P5CR, forming the recently proposed proline–P5C cycle [10,12,18,24,27,28]. A review by Ben Rejeb et al. [28] mentioned that when cells accumulate excess proline, the proline–P5C cycle is more active and more electrons are delivered to the mitochondrial electron transport chain, leading to a greater production of reactive oxygen species (ROS). For this reason, P5C has been recognized as toxic due to the induction of oxidative stress, and the process of proline degradation in plants has been associated with the generation of hypersensitive responses (HRs) and programmed cell death (PCD) [28]. The levels of free proline inside the cells are regulated by the balance between biosynthesis and degradation. Under normal conditions, the proline degradation pathway is positively regulated by its accumulation in a feedback mechanism. In fact, plants treated with exogenous proline usually show an induction of proline oxidation via the activity of ProDH and P5CDH in the mitochondria [29].
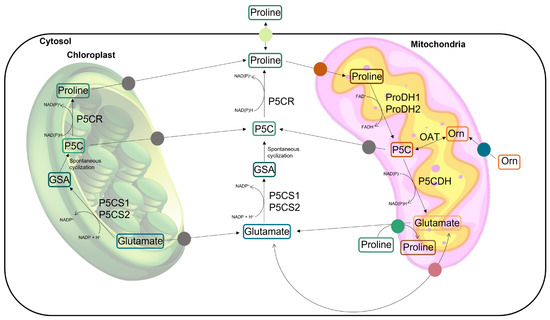
Figure 1.
Overview of the main metabolic pathways responsible for proline biosynthesis in the cytoplasm and/or chloroplast and for proline catabolism in the mitochondria. The protein transporters involved are highlighted in colored circles. Putative transporters are presented as grey circles. P5CS: Δ1-pyrroline-5-carboxylate-synthetase; P5CR: Δ1-pyrroline-5-carboxylate-reductase; GSA: glutamic-5-semialdehyde; P5C: pyrroline-5-carboxylate; ProDH: proline dehydrogenase; P5CDH: pyrroline-5-carboxylate dehydrogenase; and OAT: ornithine δ-aminotransferase.
Proline has been reported to play important roles in the developmental processes associated with plant reproduction. Under normal conditions, the highest levels of proline in a plant are found in the flowers, especially in pollen grains, and in the seeds. The lowest levels are found in the roots [30]. Proline seems to be required as an osmoprotectant during pollen development and embryogenesis, which are both desiccation-dependent processes [22]. Moreover, proline has also been found to accumulate in actively dividing meristematic tissues, such as the root tip, shoot apex, lateral buds, inflorescence, and the germinating seed, acting as a source of energy to support such metabolically demanding programs. Indeed, during proline catabolism in the mitochondria, the oxidation of one molecule of proline yields 30 ATP [22,31]. Regarding the role of proline in growth, in addition to providing the energy required for cell division, proline is also involved in cell elongation as the extracellular proteins hydroxyproline-rich O-glycoproteins (HRGPs) are important components of the plant cell wall structure and contain variations of proline–proline repeats [32]. The light-dependent reciprocal gene activation of P5CS has also been reported to cause a light-dependent accumulation of proline, showing a strong relationship between photosynthesis and proline metabolism [19]. In the absence of stress, the biosynthesis of housekeeping proline seems to take place in the cytosol because of the activity of P5CS2 [19].
Most studies on this subject report the accumulation of proline in plant cells during events of abiotic stress, especially under conditions of osmotic stress (Figure 2). Its high solubility in water and zwitterionic state allow proline to accumulate in high amounts inside cells [18], a property that proline shares with other compounds commonly known as compatible solutes, which are also built up by an array of organisms to adjust cellular osmolarity under conditions of osmotic stress [18]. Thus, proline is mostly recognized for its role as a potent osmoregulator, namely, to protect cells by increasing water uptake under conditions of low water availability and salinity stress. In addition to its role as a compatible solute, proline is also widely recognized for improving the stability of proteins and the integrity of membranes, facilitating the activation of enzymes and serving as a redox buffer, ROS scavenger, and metal chelator in the cytoplasm. Interestingly, aside from these functions, proline has been shown to act as a signaling molecule [18]. This is generally represented in Figure 2 and will be described in more detail throughout the review.
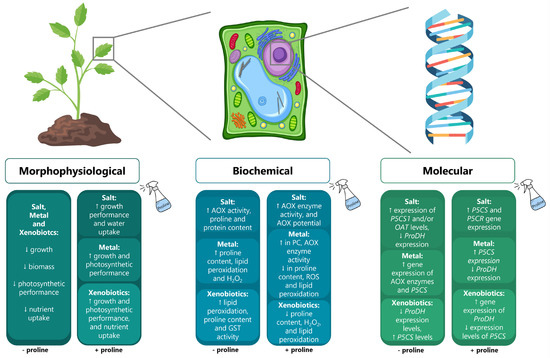
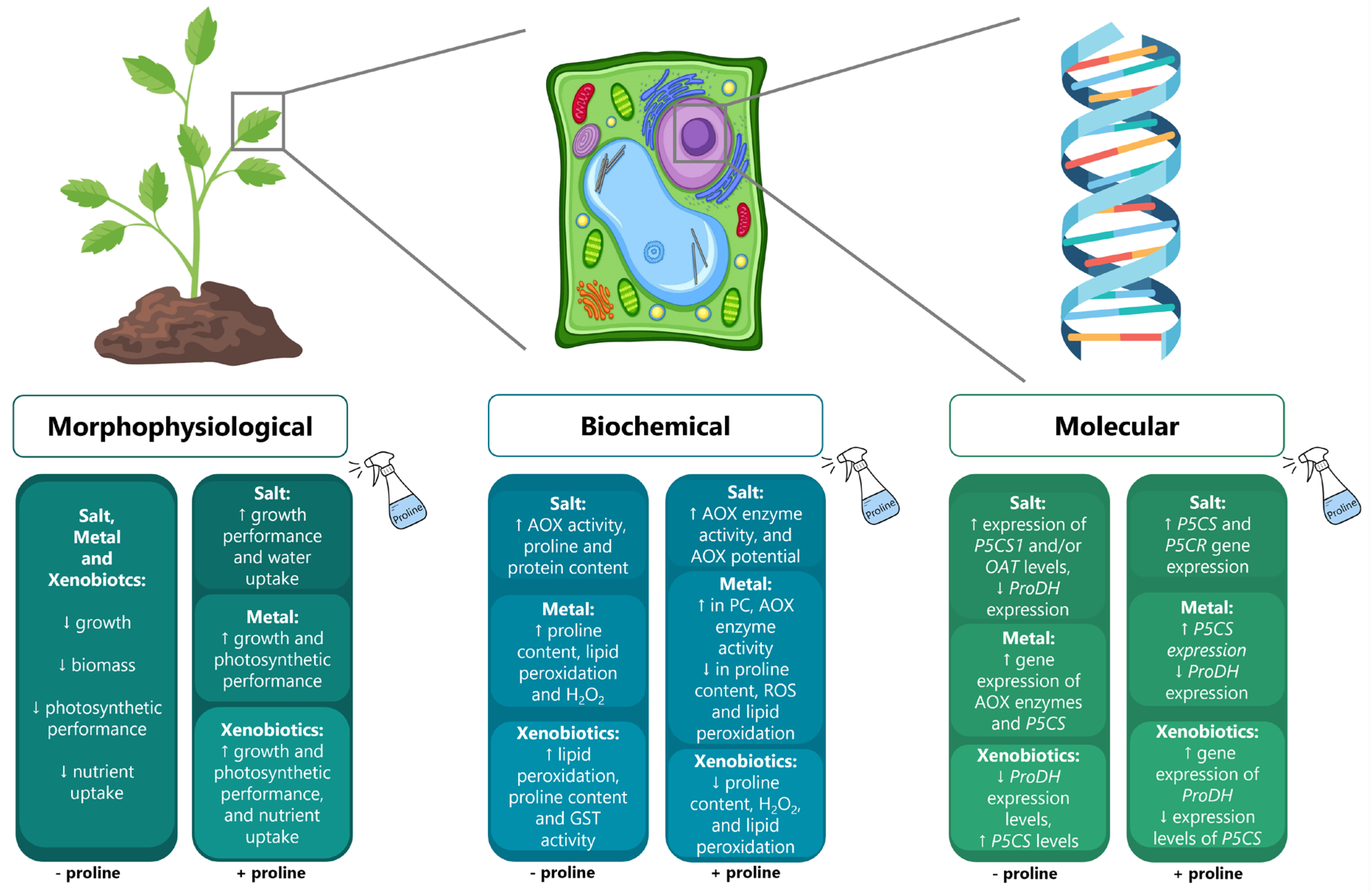
Figure 2.
Common effects observed in plants exposed to conditions of salinity, metal contamination, and pollution by xenobiotics at morphophysiological, biochemical, and molecular levels (left boxes) in comparison to the response of stressed plants to the exogenous application of proline (right boxes). ↑ and ↓ symbolize the reported increase or decrease of a certain parameter, respectively.
3. How Is Proline Homeostasis Affected by Stress?
The induction of proline accumulation under stress usually occurs due to the increased de novo synthesis and does not seem to rely as much on proteolysis events [29]. The reduction in turgor is probably the main trigger for proline accumulation in plants under osmotic-related stresses such as drought, heat stress, and salinity [29]. These conditions usually induce stomatal closure, limiting carbon uptake and subsequently decreasing NADPH consumption by the Calvin cycle, depleting the pool of the electron acceptor NADP+. This leads to singlet oxygen (1O2) production and the accumulation of ROS [28]. Under abiotic stress, there is an activation of the biosynthetic enzyme P5CS1 but not of P5CS2 [30]. There is also a stimulated import of P5CS1 and P5CR enzymes into the chloroplasts, directing the buildup of proline to these organelles during stress [15,19,28,30,33]. Abiotic stress has also been shown to inhibit proline degradation pathways through the inhibition of the ProDH and P5CDH enzymes at expression and protein activity levels [20,30]. This overrides the feedback inhibition mechanism in order to maintain high levels of the newly synthesized proline. Moreover, plants under stress also show changes to the permeability of the mitochondrial membrane that prevent proline from reaching its site of oxidation. They also demonstrate an impaired incorporation of proline into proteins to maintain high levels of free proline in the cytoplasm and chloroplasts [29].
The spatial distribution of proline seems to play an important role in the functional diversification of this amino acid [34] (Figure 1). For instance, the compartmentalized biosynthesis of proline in chloroplasts is light-dependent and is stimulated during osmotic stress. It is associated with the protection of the photosynthetic apparatus [19] as it is a reductive pathway that requires NADPH to reduce Glu and P5C, restoring the pool of NADP+ and sustaining the electron flow between photosynthetic excitation centers. In this way, proline synthesis may lower the production of ROS in the chloroplasts to help maintain redox balance and avoid oxidative damage and photoinhibition.
Verbruggen and Hermans [30], Kavi Kishor and Sreenivasulu [27], and others have highlighted that the stress-induced inhibition of proline oxidation ceases once the stress is relieved. The pools of stress-induced proline supply a reduced potential for the mitochondria through the oxidation of proline by ProDH and P5CDH, providing electrons for the respiratory chain [19]. Once stress is relieved, the degradation of the highly accumulated proline provides a significant supply of energy that could be used by cells to resume growth [19,27]. In addition, during the imposition of drought and/or salinity stress, some proline has been shown to be translocated from the shoots to the roots, where its subsequent degradation is thought to be used as an energy reservoir that could support root growth, allowing the roots to reach deeper in the soil profile in the search for areas of higher moisture [27]. This raises an interesting question on the involvement of proline in the perception of stress, suggesting that the catabolic enzymes ProDH and P5CDH are not as inhibited by stress in the root tissues as they are in the leaf tissues, where proline is so promptly synthesized.
4. The Involvement of Proline in Plants Facing Issues of Soil Degradation
4.1. Salinity Stress
Soil salinity problems have accompanied humanity throughout known history, with historical reports showing the downfall of many civilizations due to the inability of agriculturally based societies to deal with a loss of soil viability through salinization [35]. Due to natural and anthropogenic factors, either at an individual (e.g., the rise of seawater levels, excessive fertilization, or poor management practices) or synergic (e.g., reduced precipitation and excessive irrigation) level, over 1.5 Mha of arable land is becoming unsuitable for crop production per year, with up to half of all cultivable lands throughout the world being expected to become inviable for agriculture by 2050 [6,36].
Indeed, it is well known that exposure to a high salt content severely affects crop yield by decreasing the plants’ water uptake, disrupting the ionic balance, inducing oxidative stress, and limiting photosynthetic efficiency, ultimately leading to decreased productivity or even the death of the plants [37]. For these reasons, extensive research has been conducted on the physiological effects of salinity stress and uncovering possible tolerance traits.
4.1.1. Role of Proline in Salt-Stressed Plants
Although salt stress can affect plants in different ways, one of the most common responses is an exacerbated accumulation of proline [38,39,40,41,42,43,44]. Plant researchers have been dedicated to understanding whether this ubiquitous increase in proline content could be explored as a sign of tolerance or as a sign of stress susceptibility. Despite this, and as further reviewed below, a consensus is yet to be reached regarding the role of proline accumulation under saline conditions.
Although some authors show a preference for the role of the OAT pathway in mediating proline synthesis under salt stress [45], most works to date report a high upregulation of the P5CS pathways [46,47,48,49,50] that is sometimes coupled with a slight increase in OAT activity [51,52]. Curiously, Wang et al. [53] showed that when Saussurea amara (L.) DC. plants were exposed to NaCl concentrations ranging from 100 to 400 mM, P5CS takes the leading role in proline biosynthesis only up to 200 mM, with the increased proline accumulation under higher salt concentrations being ascribed mainly to OAT. Contrasting results were found by Bagdi et al. [54] in which an OAT pathway in a salt-tolerant variety of rice was induced in a generalized manner but P5CS was only induced for the highest NaCl concentrations. Although P5CS appears to be the preferential pathway for proline biosynthesis under saline conditions, there appears to be no observable pattern between different species and experimental conditions. Even when comparing salt-sensitive and tolerant cultivars, the proline biosynthetic pathways are not a useful indicator of a plant’s ability to cope with salt stress. de la Torre-González et al. [45] showed that a tolerant tomato cultivar presented a salt-induced OAT enhancement, while the proline accumulation in the sensitive cultivar was only related to reduced proline degradation. Indeed, Xue et al. [55] stated that the activation of the OAT pathway depends on the severity of the stress. Yusuf et al. [52] and Arabbeigi et al. [47] showed that sensitive and tolerant genotypes equally presented an increased activity of the proline biosynthetic pathways under salt stress (especially P5CS). However, Goharrizi et al. [46] reported that salt-tolerant wheat cultivars had higher P5CS activity induction in response to salinity than the sensitive ones.
In this sense, proline de novo generation is probably not induced merely by salt exposure and cannot be taken as a sign of either tolerance or susceptibility. Moreover, proline biosynthesis can also be inhibited through negative feedback related to endogenous proline levels. Indeed, ProDH activity can decrease when plants are exposed to saline conditions, limiting the proline degradation rate and contributing to its accumulation with proline possibly binding to the γ-GK domain of P5CS and inhibiting its activity [19,56]. This might explain the unaltered P5CS activity levels described by de la Torre-González et al. [45] and Naliwajski and Skłodowska [56]. Nonetheless, the most common response when plants are subjected to salt stress is the additive effect of increased P5CS or OAT and decreased ProDH activity [48,49,50,53], indicating a tight regulation between both enzymes to ensure an adequate stress response. This was reinforced by the work of Wang et al. [51], which reported that short-term proline accumulation occurs due to the inhibition of ProDH and the activation of OAT, while in long-term salt stress or during the recovery process, biosynthetic pathways are activated to ensure a proline supply but the activity of ProDH increases or reverts to basal levels, possibly to provide energy and electrons for the respiratory chain.
4.1.2. Proline Functions in Salt-Stressed Plants—Increased Tolerance or Higher Susceptibility?
Throughout the years, it has been widely believed that this overaccumulation of proline in response to stress must be related to an increased tolerance. As an osmolyte, proline’s most well-regarded function in plants under salt stress is its ability to ensure proper osmotic pressure [12,57] by limiting the accumulation of Na+ and Cl- in the cytosol while also being able to protect plant cells from oxidative damage. However, many authors have debated if proline accumulation is a trait for an increased salt tolerance or just a symptom of stress. For instance, various authors have reported very little contribution from proline in the osmotic adjustment of salt-stressed plants (see Mansour and Ali [57] and references therein). As a more direct approach to settle this debate, there are several research papers focused on understanding the specific differences in proline metabolism between tolerant and sensitive genotypes. For instance, Forlani et al. [58] studied the response of 17 rice genotypes and found no correlation between basal or stress-induced proline levels and the tolerance index. However, a significant interaction was found between the relative increase in proline content and the ability of rice plants to tolerate salt stress. Here, the authors postulated that the role of proline in salt tolerance is not mainly related to its ability to act as an osmolyte or ROS scavenger but to activating several signaling pathways related to redox balance, NADP+/NADPH ratio, and the maintenance of the oxidative pentose phosphate pathway through the recently proposed proline–P5C cycle, as reviewed by Kavi Kishor and Sreenivasulu [27]. Moreover, Gharsallah et al. [59] showed that proline accumulation was higher in salt-tolerant tomato cultivars and was positively correlated with a lower Na+ content and improved antioxidant efficiency. Despite this, through an analysis of the existing literature it can be seen this assessment does not apply to all case studies. Even within the same species, inconsistent behavior has been observed between genotypes. While Ashrafi et al. [60] and Badran et al. [61] found that tolerant Medicago sativa genotypes accumulated more proline than the sensitive cultivars, X. S. Wang and Han [62] reported the opposite, pointing to proline accumulation as a symptom of injury and not a sign of tolerance. Other inconsistencies in the role of proline were found by Carpici et al. [63], Ghoulam et al. [64], Wu et al. [65], Poustini et al. [66], Kanawapee et al. [67], Lutts et al. [68], Ashraf et al. [69] and Ali et al. [70]. It becomes evident that the plant tolerance index is not always correlated to higher proline levels, but there is a clear involvement of the proline metabolism in several key pathways of the stress response. Thus, considering the important functions of this amino acid, the artificial modulation of proline levels can modify, in either a positive or negative way, the ability of plants to withstand salt stress, helping to elucidate the protective role of this compound. In addition, proline levels usually increase when salt stress mitigation strategies are employed (Table S1), although it is still unclear whether this proline accumulation is part of the tolerance-inducing mechanism put into action by the mitigator or simply a consequence of an altered redox balance and hormonal metabolism. It is worth mentioning, however, that this is not observed in mitigation strategies for other types of abiotic stress, as can be seen later in this work (Section 5).
4.1.3. Exogenous Application of Proline to Mitigate Salt Stress
As previously mentioned, proline has also been explored as a stress mitigator itself. The supplementation of proline, either by foliar spray or through irrigation, is the easiest method of inducing proline accumulation in plants, and several authors have highlighted its osmoprotectant role and benefits in improving plant tolerance to salt stress. For instance, a decrease in the Na+ (and Cl−)/K+ ratio was observed in several species when proline was applied exogenously [71,72,73,74,75,76,77,78,79]. These authors suggested that proline helps in avoiding Na+ xylem loading as well as efficiently induces the compartmentalization of Na+ excess, allowing for a greater influx of K+ and restoring the K+/Na+ ratio. Messedi et al. [80] showed that the exogenous foliar application of proline induced no differences in the Na+ content but enhanced the K+ content in Cakile maritima L. plants under saline stress, suggesting that proline may stimulate the root uptake of K+ to the detriment of Na+ by protecting the activity of K+ transporters [80]. On the other hand, Huang et al. [81] reported a decrease in Na+ content and in the Na+/K+ ratio in addition to an improvement in the plant’s water content, while no differences were observed in the K+ content. In this case, the positive role of proline seems to rely mainly on the improvement of water uptake, limiting the salt’s phytotoxic effects. This mechanism was also described by [73,79,80,82,83,84,85,86,87]. This facet of exogenously applied proline, which improves plant water relations under salinity stress, may be linked to the accumulation of some organic and inorganic compounds, such as endogenous proline, sugars, glycine betaine, and K+ (see Table S2 and the references therein), given that these substances can promote the adjustment of osmotic potential. Additionally, exogenous proline application further improves plant tolerance to saline stress by increasing the antioxidant potential, favoring redox homeostasis (Figure 2; Table S2). For instance, Reza et al. [88] showed that a 15 mM proline supply to salt-stressed barley (Hordeum vulgare L.) plants prevented lipid peroxidation (LP) and increased catalase (CAT; EC 1.11.1.6), glutathione reductase (GR; EC 1.8.1.7), and peroxidase (POD; EC 1.11.1.7) activity. Similarly, M. A. Hossain and Fujita [89] also reported a decrease in malondialdehyde (MDA) and hydrogen peroxide (H2O2) content and an increase in glutathione (GSH) content and GR activity. Most studies also reported an increase in the activities of the antioxidant enzymes superoxide dismutase (SOD; EC 1.15.1.1) and ascorbate peroxidase (APX; EC 1.11.1.11) in addition to CAT, GR, and POD in salt-stressed plants treated with proline [71,84,90,91]; this increase is usually followed by an enhanced capacity to scavenge O2•− and H2O2 and enhanced contents of non-enzymatic antioxidants (see Table S2 and the references therein).
As can be seen in Figure 2, proline functions can be found at different levels of plant responses. In addition to its biochemical effects at the cellular level, proline is also recognized for affecting broader physiological mechanisms in plants such as photosynthesis, mineral nutrition, and growth. Indeed, apart from its antioxidant action, proline’s role also extends to the positive regulation of photosynthetic parameters as proline supplementation to salt-stressed plants results in higher levels of chlorophylls and carotenoids. Proline supplementation also leads to improved photosynthetic rates, such as stomatal conductance, transpiration rate, PSII yield, non-photochemical quenching, electron transport rate, and intercellular CO2 levels [80,83,85] (Table S2). The positive influence of exogenous proline on the photosynthetic performance of plants seems to rely significantly on the role of proline in maintaining chloroplastic redox homeostasis and protecting the PSII from salt-induced osmotic and oxidative stress, allowing for proper electron transport activity [80]. Additionally, the positive effects of proline supply are also associated with a better nutrient uptake. In fact, proline application to salt-stressed plants increased the content of N, P, K, NO3−, NO2−, and Ca2+ in Phaseolus vulgaris [71], Cucumis melo [76], Zea mays [92], and Sorghum bicolor [73]. In addition to nutrient uptake, the assimilation of N can be improved by exogenous proline supply, as shown by el Sabagh et al. [93].
Together, these findings suggest that the application of exogenous proline positively affects the performance of plants under salt stress by restoring the K+/Na+ ratio, regulating water relations, maintaining the redox homeostasis, enhancing photosynthetic efficiency, and promoting a proper nutrient balance while endogenous proline levels increase. As such, it is clear that the exogenous proline supplementation helps to counteract the effects of salt stress in several plants, improving their growth and performance under these adverse conditions (Figure 2).
4.1.4. Modulation of Endogenous Proline Levels in Response to Salt Stress—Genetic Engineering Approaches
Aside from understanding how plants respond to the external supplementation of proline, it is also important to unravel how genetic engineering of the proline-related pathways can be used as an efficient method of inducing plant tolerance to salt stress. For example, Guerzoni et al. [94] and Guan et al. [95] reported that transgenic sugarcane and switchgrass plants that overexpressed a form of P5CS accumulated more proline under salt stress and presented an increased K+/Na+ ratio in the leaf and root tissues, which was correlated with improved stress tolerance. The overexpression of P5CS under salt stress has also been reported to improve the maximum quantum efficiency of photosystem II, photosynthetic rate, stomatal conductance, transpiration rate, water use efficiency, and chlorophyll fluorescence in sugarcane [94] and sorghum [96]. Such protective effects of proline might be related to its ability to scavenge ROS and thus protect the thylakoid membranes against photodamage while also avoiding ROS-mediated chlorophyll degradation. Indeed, the increased growth performance in plants that over-accumulate proline is also associated with an enhanced antioxidant activity and decreased levels of ROS and MDA, highlighting the role of proline as a redox buffer and membrane stabilizer [97]. Accordingly, the knockdown of P5CS1 and P5CS2 leads to a reduced proline accumulation, increased ROS and MDA content, the inhibition of antioxidant enzymes, and an increased Na+/K+ ratio, affecting plant growth performance [95].
Reinforcing the idea that the two different isoforms of P5CS have sub-functionalization, a study by Funck et al. [23] showed that salt-stressed p5cs1-4 mutants presented heavily impaired proline accumulation in response to NaCl, indicating that P5CS1 contributed more to the stress-induced proline production. On the other hand, the germination and root growth of the p5cs2-1 mutants were impaired under control condition; this was reverted by exogenous proline application, corroborating the role of P5CS2-induced proline production in normal plant development. The p5cs2-1 mutants, which could still produce considerable levels of proline under stress, displayed a high salt tolerance, no signs of Na+ accumulation, and a much more efficient photosynthetic machinery.
Moreover, proline-biosynthesis-related genes have binding sites for several transcription factors for which engineering has been explored to enhance salt stress tolerance, as reviewed by Zarattini and Forlani [98]. For instance, Zhang et al. [99] achieved a higher salt tolerance in soybeans when overexpressing GmMYB84, presenting an increased proline content, improved antioxidant efficiency, and reduced Na+ accumulation. Similar results were found by overexpressing VvWRKY30 and WRKY8 in salt-stressed Arabidopsis [100] and tomato plants [101]. J. Liang et al. [102] and Alshareef et al. [103] tested the overexpression of JUB1 NAC transcription factors in strawberry and tomato plants, respectively. Here, J. Liang et al. [102] reported that the overexpression of the strawberry FaNAC2 gene in Nicotiana benthamiana plants altered their proline metabolism, with an increased P5CS1 and decreased ProDH2 and P5CDH expression and an increased growth and antioxidant capacity under salt stress conditions. Alshareef et al. [103], found that transgenic tomato plants that overexpressed JUB1 NAC transcription factors were more tolerant than the wild type under high salt levels. This was demonstrated by an improved biomass and increased chlorophyll content, although the increase in proline content had no effect on the leaves’ osmotic potential or on the accumulation of Na+ and K+. In contrast, the inhibition of P5CS1 via the overexpression of MYC2 (a bHLH transcriptional activator of abscisic acid (ABA)) in Arabidopsis [104] and the inhibition of P5CS1 and P5CS2 through the overexpression of BpERF11 (an ethylene-responsive factor) in Betula platyphylla [105], respectively, might lead to a higher sensitivity to salt stress. Curiously, Verma et al. [104] showed that the overexpression or silencing of MYC2 caused higher or lower salt sensitivity, respectively, and were accompanied by decreased or increased proline biosynthesis after 24 h of salt stress. However, when the stress duration was extended for one month, the myc2 mutants were more sensitive to salinity than the wild-type plants, indicating the presence of complex cascade mechanisms that regulate long-term stress response, especially when the effects on the downregulation of ABA biosynthesis and ABA- and stress-responsive genes are considered. Accordingly, Li et al. [106] reported that although salt-stressed alfalfa overexpressing MsHB7 (an ABA-inducible homeodomain-leucine zipper I gene) presented a higher increase in proline content (presumably due to the inhibition of ABA-mediated signaling), their tolerance to salt stress was severely reduced. Nonetheless, Zhang et al. [105] showed that salt-tolerant erf11 mutant lines of Betula platyphylla still presented an improved performance after 4 weeks of exposure to salt stress. They also presented a better antioxidant efficiency and water status through the decreased stomatal aperture and increased proline levels associated with a higher expression of P5CS1/2 and a lower expression of ProDH and P5CDH, contrasting with the opposite results that were found for the sensitive lines overexpressing ERF11. It is worth mentioning that the expression of P5CS can be positively regulated by ABA at expression and post-translation levels, although both ABA-dependent and independent signaling mechanisms have been reported to regulate the accumulation of proline under conditions of osmotic stress [26,98].
4.2. Metal-Induced Phytotoxicity
Considering the unprecedented rate of population growth in recent decades and the consequent intensification of all industrial, agricultural, urban, and technological anthropogenic activities, numerous global problems have arisen. Many of these problems are related to environmental pollution. One case that deserves special attention is the contamination of agroecosystems with concerning levels of metals [107]. Metals include high-density metallic elements and metalloids which, despite their important roles in soil constitution and nutrient cycles, tend to accumulate in these environmental matrices, causing toxicity to several organisms.
At very low concentrations, some metals are essential micronutrients for plant development and growth as they are involved in vital redox reactions. This is the case for molybdenum (Mo), copper (Cu), nickel (Ni), manganese (Mn), iron (Fe), and zinc (Zn). On the other hand, once accumulated in the soil at levels above a certain metal-specific threshold, these micronutrients can become unsafe for plant growth, putting agriculture at as much risk as the presence of other non-essential pollutants such as lead (Pb), arsenic (As), cadmium (Cd), mercury (Hg), strontium (Sr), titanium (Ti), vanadium (V), lanthanum (La), thallium (Tl), and chromium (Cr). In this sense, the response of plants to metal-induced toxicity is not only dependent on species- and environment-related traits but also on the metal concentration, properties, and bioavailability [108,109,110]. As can be seen in Table S3, several authors reported an overall reduction in plant growth due to metal-induced toxicity, including As, Cd, Cr, Pb and Ni, which is recognized as the consequence of a combination of the following effects: (i) the induction of nutritional imbalances due to interference with nutrient uptake and assimilation; (ii) the disruption of the photosynthetic machinery; (iii) the alteration of the plants’ water relations; and (iv) the induction of oxidative stress [107,111]. Regarding photosynthetic traits, metals can damage or inhibit the biosynthesis of pigments; disrupt the electron transport chain, CO2 fixation, and enzyme activity in the Calvin cycle; cause the disorganization of the granum and thylakoid membranes; and induce stomatal closure. Altogether, these alterations culminate in major growth inhibitions and yield losses, threatening food production at global level.
4.2.1. Proline Homeostasis in Response to Metal Stress
When plants are exposed to toxic levels of metals, several biochemical adjustments take place within the plant cells. These adjustments include the accumulation of proline, especially in the shoot tissues [112]. As previously mentioned, such proline increases are often seen as a positive response of a plant’s defense system, suggesting that the proline is being used to counteract the effects of metal toxicity due to its general properties as an osmoprotectant, antioxidant, and metal chelator (Figure 2; Table S3). This was the case in the reports by [113,114], who observed increases in proline levels in plants under Ni- and Mo-stress, and by Chaturvedi et al. [115], who observed increases in proline levels in response to Pb- and Zn-stress. The authors noticed that the plants’ physiological improvements seemed to be related to the higher levels of proline production. Soares et al. [116] also related the high accumulation of proline in the roots of Solanum nigrum with the improved resilience of the plants to Ni toxicity, and the authors Siddiqui et al. [117] and Sirhindi et al. [118] noticed that Ni-stressed wheat and soybean plants treated with gibberellin, calcium, and jasmonic acid accumulated higher levels of endogenous proline, probably as an indirect result of the positive influence of these stress mitigators in enhancing the internal defenses of the plants. Further examples of proline accumulation in response to stress can be found in several reviews [19,20,29,119,120]. Indeed, most reports link this biochemical adjustment with stress resilience, indicating that plant species that accumulate more proline under stress show better physiological performance and survival (as reviewed by Heuer [29]). Nonetheless, the stress-induced accumulation of proline in plant tissues is not always perceived as a fully positive outcome. In many studies regarding plants under metal stress, proline accumulated to levels several folds higher than in control plants while the plants still suffered considerable growth inhibitions, phytotoxicity symptoms, and severe oxidative damages that did not seem to be efficiently counteracted by endogenous proline [108,121,122,123] (Table S3). Moreover, some authors found that proline accumulation in response to metal stress should even be seen as a signal of stress sensitivity rather than a defense mechanism [20,27,33,124]. During normal plant development, the highest proline levels are usually found in the pollen grains and seeds, where this amino acid seems to be needed to protect cells from osmotic stress during desiccation-dependent events [22]. Apart from these tissues, proline levels usually increase more in the shoots than in the roots under metal stress [112]. This is the case with tomato [122] and wheat [113], for instance. Under stress conditions, high levels of proline can also be found in the phloem, indicating that proline can also be translocated from the shoots to the roots [27].
Very curious outcomes have been noticed with respect to protein accumulation in metal-tolerant plants. It has been shown that metal-tolerant plants present overall higher constitutive levels of proline than their closest sensitive species or ecotypes, demonstrating considerable differences especially in the root tissues. In such tolerant genotypes, including S. nigrum (a well-known metal hyperaccumulator species—Rehman et al. [125]), the stress-induced increase in proline tend to be more tenuous and usually occurs in the roots instead of shoots [126,127]. For instance, Sun et al. [127] found that under control conditions, S. nigrum had higher constitutive levels of proline than the sensitive species from the same genus, Solanum melogena. The same authors found that proline was also more accumulated in the roots than in the shoots of S. nigrum during exposure to Cd [127]. Similarly, Soares et al. [116] also found a greater increase in proline in the roots than in the shoot tissues of S. nigrum under Ni stress. In a study using the metal-accumulator species Atriplex halimus, an exposure to toxic Cd levels boosted proline accumulation in both organs, but proline accumulation was boosted to a much higher extent in the roots than in the shoots [126]. In agreement with this pattern, Matsunami et al. [128] found that proline accumulation was upregulated in the roots of the stress-tolerant rice cultivar IR58. Schat et al. [129] noticed that in a metal-tolerant ecotype of Silene vulgaris grown under normal conditions, the constitutive proline concentration was five to six times higher than in the nontolerant ecotype. Additionally, the authors could not find increases in proline in the tolerant ecotype under exposure to toxic levels of Cu or Zn, while the sensitive ecotype accumulated high levels of proline under the same conditions of metal stress. The Cd stress only induced proline levels in the tolerant genotype at concentrations at the mM level, hundreds of times higher than in the sensitive plant in which proline was built up in response to 5 µM Cd. Cd stress disturbs water relations in plants by affecting aquaporin function, altering the structure and permeability of membranes, and affecting the transpiration rate, thus reducing the water potential. The authors suggested that proline accumulation in the shoots of the sensitive species occurred not in response to the metal per se but rather as a response to the metal-induced water deficit in the leaves [129]. Overall, metal-tolerant plants show higher constitutive levels of proline than sensitive genotypes and do not seem to undergo such drastic peaks in proline production during stress events, especially in the leaves, demonstrating more notorious increases in the roots. It could be inferred that the mechanism leading to proline accumulation in these tissues could be somehow part of a defensive strategy that makes these plants more resilient to stress. In fact, the roots are the first organ to contact soil contaminants and to perceive stressful conditions of salinity and drought; therefore, it would not be a surprise if stress-tolerant species had better root-related attributes to deal with abiotic stress. Bearing in mind how free proline can be used as an energy reservoir to provide ATP during its degradation in the mitochondria, one could infer that the accumulation of proline in the root tissues could be used as a strategic method of sustaining or stimulating root growth and elongation under stress [27], which could be used as a tolerance adaptation response to help plant roots search for unpolluted soil areas.
4.2.2. Exogenous Application of Proline to Mitigate Metal-Induced Stress
The application of proline as an external stress mitigator to metal-exposed plants has shown very positive results in general, either by preventing the oxidative damage induced by these contaminants and/or through the stimulation of plant defense mechanisms to overcome metal toxicity [10,120] (Table S2). Although there are several methods for the exogenous application of this amino acid, foliar spraying and seed priming are the most effective [10,120]. The foliar application of proline to maize plants grown under Cd and Pb improved growth performance, delayed leaf and flower senescence, and improved photosynthesis, antioxidant enzyme activity, and levels of phenolic compounds, leading to an increased seed yield [119,130]. The exogenous application of proline to an olive tree and wheat plants promoted the uptake of nutrients under stress induced by Cd and Cu, respectively [131,132]. On one hand, proline contributes to the protection of cells from the toxic effects of metals; on the other hand, proline sometimes also seems to play a role in metal detoxification. Examples include studies by several authors [131,132,133,134] in which the positive influence of proline was partially due to restricting the uptake or translocation of the respective metal (Table S2). Chandrakar et al. [135] reported that the proline-mediated reduction of metal root-to-shoot translocation seemed to be related to the induced synthesis of phytochelatins, which are well-recognized molecules for their metal-chelating properties and their role in metal detoxification. Curiously, as can be seen in Table S2, some works reported that a supply of proline sometimes stimulated the uptake and increased the accumulation of metals in plants [136,137,138]. For instance, the work by Xu et al. [137] on S. nigrum under Cd toxicity showed that the application of proline enhanced the uptake of Cd by a phytochelatin-mediated mechanism. The authors suggested that proline, by helping to maintain the redox balance in the cytoplasm, alleviated the GSH depletion, favoring the synthesis of phytochelatin (see review by X. Liang et al. [31]). In an aside, exogenous proline has been shown to exert an effect on the levels of GSH, increasing the GSH/GSSG ratio in the leaves and roots of B. juncea [139], validating the indirect effect of proline in stimulating another multifunctional compound, GSH, and strengthening downstream mechanisms of stress tolerance. In fact, Xu et al. [137] suggested that in this way, the conjugation of Cd with phytochelatin would be more easily sequestered in the vacuoles, enhancing the Cd accumulation ability and the phytoremediation potential of these plants [137]. Yu et al. [138] also suggested that proline might have prevented Cr (VI) from reaching the shoot tissues by “storing” it in the root apoplast. Indeed, the compartmentalization of metals in root cell walls is an effective strategy for avoiding metal toxicity and is commonly implemented by metal-accumulator species, conferring the protection of the photosynthetically active tissues and decreasing metal-induced oxidative damages [140]. Regarding the proline-mediated stimulation of metal uptake, Ullah et al. [141] found that the accumulation of Pb and Cr was enhanced in plants that accumulated higher levels of endogenous proline, especially in the roots.
In terms of photosynthesis, the application of proline has also been shown to attenuate the negative effects induced by metals [83,119,142,143]. Exogenously applied proline was reported to stabilize not only the electron transport chain between cell membranes and mitochondria but also the 3D structure of proteins, preserving the activity of important, photosynthesis-related enzymes such as ribulose-1,5-bisphosphate carboxylase-oxygenase (RuBisCO; EC 4.1.1.39) [119]. Furthermore, proline promoted stomatal opening in plants subjected to Ni and Pb by blocking the connection of ABA and specific proteins on cell guard membranes [143] and by increasing the levels of K+ on guard cells [130], respectively, thus favoring the stomatal opening. In eggplants (S. melongena) exposed to As, the exogenously applied proline seemed to act as an electron donor for PS II [143]. The CO2 assimilation rate in metal-stressed plants was also restored upon the addition of exogenous proline by an increase in photosynthetic gas exchange attributes and by enhanced biosynthesis and/or the decreased degradation of chlorophylls and carotenoids [139,142].
Regarding the redox homeostasis, the application of proline, either by foliar spraying or through irrigation, usually leads to an enhanced antioxidant potential [120,130,135,139,142,144,145,146]. This happens not only due to the direct role of proline as an antioxidant per se but also because of its ability to trigger other defensive pathways, including antioxidants. For instance, the contents of GSH, phenolic acids, ascorbic acid, flavonoids, and polyamines have been reported to increase in response to proline treatment in Coriandrum sativum, Zea mays, Olea europaea, and Pisum sativum under Pb, Hg, or Ni stress [83,120,146,147,148,149]. The gene expression and activity of antioxidant enzymes such as SOD, CAT, APX, and POX [83,130,135,136,145,148] were also reported to increase, helping to avoid the excessive build-up of ROS [97]. In fact, exogenous proline could decrease the levels of H2O2 and/or O2.- in pea, brown mustard, common bean, eggplant, olive tree, brown mustard, pigeon pea, soybean, wheat, and black nightshade plants exposed different metals such as Ni, Cd, Pb, Se, and As [83,135,137,139,142,143,144,145,146,149,150]. In plants exposed to Cd, Ni, Pb, and Se, proline treatment led to lower degrees of oxidative damage, observed by the decline in contents of MDA, thiobarbituric-acid-reactive substances (TBARS), and electrolyte leakage (EL) [83,120,144,145,146]. Furthermore, proline has been reported to stabilize membranes, making them less porous and leaky and less susceptible to the oxidative damage caused by these contaminants [135,144,150].
4.3. Exposure to Xenobiotics
4.3.1. Xenobiotic Exposure and Proline Metabolism—Is There a Connection?
Environmental degradation caused by the accumulation of organic compounds called xenobiotics is paradoxically an old yet emergent issue currently affecting ecosystem dynamics [151,152]. Indeed, due to long-lasting and recent anthropic activities such as those related to the industry, nanotechnology, healthcare, cosmetics, and agri–food systems, soils and surface waters are becoming sinks of innumerous synthetic compounds, including drugs and pesticides, jeopardizing the normal functioning of agro-ecosystems [153].
With the exception of herbicides, most of these products are not designed to directly affect plant cells; therefore; plants are often overlooked in risk assessments of xenobiotics when compared to animal systems. Still, especially over the last 10–15 years, considerable effort has been placed on understanding the effects of different kinds of xenobiotics on plant metabolism [154,155,156,157,158,159,160,161]. Given their generalized use and their recognition as emergent pollutants, attention has been mainly centered on pesticides and pharmaceuticals [154,155,156,160,162,163,164]. In opposition to several inorganic contaminants such as essential metals (e.g., Cu and Zn), plants do not usually present specific mechanisms to uptake organic contaminants; nonetheless, they are able to uptake them using non-selective mechanisms and can accumulate these compounds to a certain extent, leading to a series of physiological and biochemical disturbances that usually affect growth and productivity. Once accumulated inside plant tissues, xenobiotics can interact with several cellular players such as proteins and nucleic acids, inducing a cascade of downstream effects which usually involve the disruption of redox homeostasis [155,165]. For instance, Gomes et al. [166] and Soares et al. [167,168], when reporting the biochemical and cellular effects of glyphosate (GLY) exposure on willow (Salix miyabeana Seemen) and tomato (Solanum lycopersicum L.) plants, revealed that the plants underwent a state of oxidative stress, measured by the increase in LP and the overaccumulation of ROS, while also experiencing changes to the antioxidant system, including an increase in proline levels. The upregulation of proline production has also been reported in response to pharmaceuticals [158], although proline metabolism seems to fall into a species-specific regulation in response to these organic pollutants. As reported for other types of stress previously detailed in this review, proline is often considered a good biomarker of xenobiotic exposure [169,170]. Works performed with several plant models ranging from monocots to dicots revealed that proline accumulation is usually modulated by the presence of these organic compounds, although a constant response cannot always be found (Table S4).
4.3.2. Impacts of Pharmaceuticals-Induced Stress on Proline Endogenous Levels
Of the wide range of organic contaminants found in soils and surface waters, pharmaceuticals and cosmetic-related compounds are among the most common due to their widespread utilization for both human and animal health care purposes [164]. Usually, plants contact these pollutants directly via the roots, where these substances exert their first toxic symptoms. When studying the phytotoxic hazards of several drugs on alfalfa plants, individually or combined (diclofenac, sulfamethoxazole, trimethoprim, and 17a-ethinylestradiol, all tested), Christou et al. [171] revealed distinctive responses depending on the analyzed organ (shoot or root) and the chemical itself. Interestingly, although no substantial effects were observed in plant growth performance, substantial changes were recorded subcellularly, probably because of xenobiotic bioaccumulation and an interference with the normal cellular metabolism. In general, alfalfa seedlings exposed to these contaminants (especially diclofenac), individually or combined, demonstrated a lowered accumulation of proline in both the shoots and roots and an aggravation of lipid peroxidation in the roots [171]. In fact, the modulation of proline content in response to these types of contaminants has also been observed in other works [157,158,172]. Martins et al. [157] reported that the accumulation of proline in barley plants followed a dose-response manner, being noticeable only for higher tested concentrations of diclofenac. Moreover, it should be noted that, upon exposure to diclofenac, the maintenance of proline levels reported by Pawłowska et al. [172] was not followed by a rise in lipid peroxidation or H2O2. In contrast, when studying the toxic effects of diclofenac on tomato seedlings, Sousa et al. [158] observed a boost in proline levels paired with an overaccumulation of H2O2, which culminated in increased membrane damage in the roots. Given the high variability found in the literature, it appears that, at least with respect to diclofenac-induced stress, proline metabolism does not seem to be a key signature point and most probably occurs as a downstream consequence caused by, for instance, xenobiotic-induced effects on enzyme activity. Based on the assumption of Christou et al. [171], the modulation of proline levels in response to diclofenac may arise as a result of xenobiotic interference with its metabolic pathway, either by favoring proline catabolic enzymes and/or inhibiting those responsible for its biosynthesis [171]. This hypothesis was further supported by Martins et al. [157], whose work revealed that under diclofenac stress, the production of proline by tomato plants is ensured by an alternative pathway related to the GS/GOGAT cycle as diclofenac suppressed glutamine synthetase (GS; EC 6.3.1.2) but enhanced glutamate dehydrogenase (GDH; EC EC 1.4.1.2) as an additional source of glutamate, the main precursor of proline in conditions of stress. Proline accumulation as a response to paracetamol, one of the most commonly utilized drugs worldwide, has also been reported [173]. Barley plants growing in paracetamol-contaminated soils showed no clear signs of oxidative damage, despite the strong growth inhibitions observed [173], which the authors suggested were in relation to the observed accumulation of proline, especially in the leaves, which seemed to have played a key role in protecting the cell membranes. Curiously, in that paper, upon treatment with silicon dioxide nanomaterials (nano-SiO2), proline levels were not changed in the barley leaves but were decreased in the roots when compared to plants that were only exposed to paracetamol, suggesting that proline accumulation under single exposure was indeed a protective mechanism and was no longer required after the co-treatment [173]. An upsurge of proline does not always seem to be accompanied by an increased tolerance. A former study with two macrophytes (Lemna minor L. and L. gibba) established a correlation between the accumulation of proline and the degree of tolerance—while L. minor, which is particularly sensitive to acetaminophen, did not alter the proline content, L. gibba curiously decreased the levels of this amino acid and showed no signs of oxidative damage [174].
Although it has been explored less, the phytotoxicity of other pharmaceuticals, such as tetracycline and naproxen, has also been assessed. An enhanced accumulation of proline took place in barley leaves, especially when exposed to high concentrations of naproxen (1000 mg kg−1). This was paired with a clear oxidative disruption and increases in ROS and MDA content [172]. Regarding tetracycline, in a work performed with two aquatic plants (Hydrocharis dubia (Bl.) Backer and Trapa bispinosa roxb.), the authors observed a time- and species-dependent response of proline homeostasis [175]. According to the authors, the ability of T. bispinosa plants to maintain higher levels of proline (along with protein) during long-term exposure to tetracycline should be interpreted as an indicator of the higher stress tolerance of T. bispinosa in relation to H. dubia, which is associated with the major role of proline as an efficient osmoprotectant [175].
4.3.3. Impacts of Pesticide-Induced Stress on Proline Endogenous Levels
Another class of xenobiotics that has been gaining attention from both ecotoxicological and phytotoxic researchers are the agrochemicals. Indeed, a growing number of studies have been unravelling the effects of several pesticides used in agriculture on the growth and redox homeostasis of non-target plant species through the analysis of ROS production and antioxidant metabolism [155,176,177,178,179]. Such studies have been conducted for the insecticides deltamethrin, imidacloprid, phosalone, emamectin benzoate, α-cypermethrin, chlorpyrifos, omethoate, and dimethoate; the fungicides metalaxyl, acetophenone, chlorothalonil, tricyclazole, and thiabendazole; and herbicides such as bentazon, prometryn, butachlor, paraquat, fluroxypyr, clopyralid, atrazine, and glyphosate. A thorough comparison of the outcomes of these articles highlights, in most cases, the accumulation of proline as a common effect of pesticide-induced stress, regardless of the xenobiotic and plant species considered (Figure 2). At the same time, these increased proline levels are often accompanied by enhanced activities of GST and SOD enzymes, despite the harsh oxidative damages and growth inhibitions observed in plants under pesticide-induced stress. Altogether, these results suggest that the accumulation of proline in plants exposed to pesticides seems to be tightly associated not with its antioxidant properties of membrane stabilization and ROS scavenging but with its action as a signaling molecule, as noted by Shopova et al. [180]. From this perspective, the increased levels of proline seem to act as a stress signal that triggers the expression of genes related to the antioxidant defense system and the xenobiotic detoxification pathway, counteracting two of the most common consequences of pesticide exposure: the overproduction of ROS and the bioaccumulation of pesticides in plant tissues, respectively. Although the existence of a direct interaction between proline accumulation and pesticide toxicity is hard to establish, it has been shown that the overexpression of a bacterial D-amino acid oxidase (DAAO), a glyphosate-degrading enzyme, in Arabidopsis thaliana seedlings increased plant resistance to the herbicide, lowering the overaccumulation of ROS and significantly reducing the levels of proline in comparison to wild-type plants [181], thereby allowing for plant survival upon exposure to glyphosate.
4.3.4. Xenobiotic Exposure vs. Mitigation Strategies—Is There Room for Proline Action?
Given the important role that different pharmaceuticals and pesticide classes play in human health and agri–food production, respectively, more than characterizing their negative effects on plant physiology, it is crucial to develop strategies to alleviate the stress induced by these xenobiotics in order to minimize their impact on crop yield. In comparison to other types of abiotic stresses, there are few strategies targeting the alleviation of xenobiotic-induced stress in plants, especially targeting specific cellular and biochemical mechanisms involved. Even so, several authors have tested multiple alternatives that could be implemented in real agronomic scenarios by either seed or foliar application to help plants deal with the environmental presence of different organic pollutants. Although the outcomes are not always straightforward, the beneficial effects observed are usually associated with an overall improvement of the antioxidant system and detoxification responses as oxidative stress is often the common language between plant cells and stress exposure.
In general, the exogenous treatment of plants exposed to different herbicides with salicylic acid, nano-SiO2, Na2SiO3·5H2O, sodium nitroprusside (SNP), or proline led to the reduction of endogenous proline levels in comparison with the stress factor alone, which demonstrated a significant increase in proline content in relation to the control (Figure 2; Table S4). However, the mechanisms behind this common effect seem to rely mainly on the type of treatment applied rather than the plant species or the class of xenobiotics. For instance, while it has been reported that the root uptake of herbicides such as glyphosate can be reduced upon treatment with salicylic acid, the application of Si-containing compounds, NO donors (SNP), and salicylic acid once more efficiently alleviate herbicide-induced stress, likely due to the stimulation of the antioxidant system through both increased levels of carotenoids and GSH and the activation of the enzymes SOD, CAT, APX, and GST. Moreover, upon exogenous administration of different phytoprotective compounds, such as Si-containing formulations, salicylic acid, and NO-donors, the glyphosate-induced accumulation of proline is frankly repressed, which is accompanied by a better growth performance and an increased activity of antioxidant and detoxification mechanisms [167,168,182]. Altogether, these observations strengthen the premise that proline is used as an early warning signal of herbicide exposure, mediating a series of stress responses rather than tolerance mechanisms.
When exploring the exogenous application of proline (Table S2), Ould Said et al. [179] suggested that the external supply of proline inhibited the accumulation of endogenous proline in plants exposed to the herbicide, promoting the activation of a proline catabolic pathway to degrade its excess. This was evidenced by an increase in the activity of ProDH. Despite the concomitant and generalized reduction of the activity of other components of the antioxidant system, plants exposed to bentazon showed a better growth performance, highlighting the powerful antioxidant activity of proline. In contrast, the foliar application of brassinosteroids to mitigate pesticide-induced stress did not cause a consistent change in proline levels compared to the stress factor alone [180,183]. In fact, both studies concluded that brassinosteroid treatment activated the pesticide detoxification pathway, with GST playing a leading role in this outcome.
5. Concluding Remarks and Future Perspectives
Extensive reviews on the roles of proline in plants under abiotic stress emphasize its positive effects as a key player in inducing plant stress tolerance [10,12,13,14,15,16,31], recognizing its clear involvement in osmoprotection, the scavenging of ROS, redox buffering, enzyme protection, membrane stabilization, the activation of antioxidant mechanisms, and the stimulation of metal chelation. In general, the exogenous application of proline as a stress mitigator has been revealed to be a promising strategy for enhancing the resilience of plants to stress factors derived from soil degradation effects, including salinity and contamination by metals and xenobiotics (Figure 2; Table S2). However, there were also a considerable number of studies that could not find such optimal results using proline as a stress mitigator [19,20], and other studies even reported toxic effects of exogenous proline [184]. Moreover, despite a significant amount of evidence regarding the role of proline accumulation in mediating stress tolerance, there is still much discussion on whether exacerbated levels of proline can be directly correlated with improved performance. Still, in most cases, proline levels are significantly modulated by stress, meaning that this amino acid must be playing a role in the stress response. Some authors suggest that the occurrence of peaks in proline accumulation during stress, especially in the shoot tissues, occur as a symptom of high sensitivity to stress, serving as an alarm that signals the imposition of stress without necessarily being involved in cell protection [124].
Future studies should be careful to discern the patterns of proline accumulation in stress-sensitive vs. tolerant genotypes. They should also discern the roles and mechanisms affected by proline when this amino acid is accumulated in sudden peaks upon the imposition of stress from when this stress-induced accumulation occurs more gradually over time. The quantification of proline should not be used as a mere indicator of stress sensitivity or stress defense, and proline quantification should always be accompanied by assessments of other biochemical and physiological endpoints. Moreover, the relative accumulation of proline in shoot and root tissues and the occurrence of proline translocation events between plant organs should be taken into consideration. Additionally, assumptions about the roles of endogenous proline should not be made based on the action of proline that is artificially accumulated through genetic engineering or exogenous application. Indeed, the importance of proline in plants under abiotic stress is still very much a matter of debate. In this sense, proline metabolism and homeostasis in response to stress should be more carefully studied, including further conditions of abiotic stress and times of exposure, and taking specificities of stress, species, tissue, and organs into account. An investigation of the effects of abiotic stress on the kinetics of proline metabolism with respect to the enzymes related to proline synthesis and catabolism is highly encouraged.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox12030666/s1. Table S1: Effects of applying stress mitigators to plants facing salinity-induced stress, namely on the endogenous levels of proline; Table S2: Physiological effects observed in plants under abiotic stress caused by exposure to contaminant levels of salt, heavy metals, and xenobiotics and in response to the exogenous application of proline; Table S3: Effects on overall plant physiology and on the proline accumulation in plants facing heavy-metal-induced stress; and Table S4: Effects of applying stress mitigators to plants facing xenobiotics-induced stress, namely on the endogenous levels of proline. References [185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218] are cited in the supplementary materials.
Author Contributions
Conceptualization, S.S., P.N., C.S. and F.F.; writing—original draft preparation, S.S., P.N., F.S., M.P., M.M., B.S. and C.S.; writing—review & editing, S. S., C.S. and F.F.; supervision, F.F.; project administration, F.F.; funding acquisition, C.S. and F.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação para a Ciência e Tecnologia (FCT) through the research project PEST(bio)CIDE (PCIF/GVB/0150/2018) and by Fundação La Caixa/BPI in cooperation with FCT through the research project CC&NUTS within the scope of the fourth edition of Programa Promove (PD21-00007). This research was also supported through FCT/MCTES within the scope of UIDB/05748/2020 and UIDP/05748/2020 (GreenUPorto). S.S., F.S., M.P., M.M., and B.S. also acknowledge FCT for providing Ph.D. scholarships (S.S.: 2021.07825.BD; F.S.: 2021.04941.BD; M.P.: 2021.07342.BD; M.M.: SFRH/BD/143268/2019; and B.S.: 2020/07826/BD).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and supplementary materials.
Acknowledgments
The authors would like to acknowledge GreenUPorto-Sustainable Agrifood Production Research Centre & INOV4AGRO.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Korres, N.E.; Norsworthy, J.K.; Tehranchian, P.; Gitsopoulos, T.K.; Loka, D.A.; Oosterhuis, D.M.; Gealy, D.R.; Moss, S.R.; Burgos, N.R.; Miller, M.R.; et al. Cultivars to Face Climate Change Effects on Crops and Weeds: A Review. Agron. Sustain. Dev. 2016, 36, 12. [Google Scholar] [CrossRef]
- Becklin, K.M.; Anderson, J.T.; Gerhart, L.M.; Wadgymar, S.M.; Wessinger, C.A.; Ward, J.K. Examining Plant Physiological Responses to Climate Change through an Evolutionary Lens. Plant Physiol. 2016, 172, 635–649. [Google Scholar] [CrossRef] [PubMed]
- FAO. FAO Strategy on Climate Change; FAO: Rome, Italy, 2017. [Google Scholar]
- FAO. FAO’s Work on Climate Change. In Proceedings of the United Nations Climate Change Conference 2018, Food and Agriculture Organization of the United Nations, Katowice, Poland, 2–14 December 2018. [Google Scholar]
- Prăvălie, R.; Patriche, C.; Borrelli, P.; Panagos, P.; Roșca, B.; Dumitraşcu, M.; Nita, I.A.; Săvulescu, I.; Birsan, M.V.; Bandoc, G. Arable Lands under the Pressure of Multiple Land Degradation Processes. A Global Perspective. Environ. Res. 2021, 194, 110697. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil Salinity under Climate Change: Challenges for Sustainable Agriculture and Food Security. J. Environ. Manag. 2021, 280, 111736. [Google Scholar] [CrossRef] [PubMed]
- Talukder, B.; Ganguli, N.; Matthew, R.; van Loon, G.W.; Hipel, K.W.; Orbinski, J. Climate Change-Triggered Land Degradation and Planetary Health: A Review. Land Degrad. Dev. 2021, 32, 4509–4522. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Lin, K.H.; Ho, S.L.; Chiang, C.M.; Yang, C.M. Enhancing the Abiotic Stress Tolerance of Plants: From Chemical Treatment to Biotechnological Approaches. Physiol. Plant 2018, 164, 452–466. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Alam, M.A.; Syed, M.A.; Hossain, J.; Sarkar, S.; Saha, S.; Bhadra, P.; et al. Consequences and Mitigation Strategies of Abiotic Stresses in Wheat (Triticum aestivum L.) under the Changing Climate. Agronomy 2021, 11, 241. [Google Scholar] [CrossRef]
- Hosseinifard, M.; Stefaniak, S.; Javid, M.G.; Soltani, E.; Wojtyla, Ł.; Garnczarska, M. Contribution of Exogenous Proline to Abiotic Stresses Tolerance in Plants: A Review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef]
- Fleming, T.R.; Fleming, C.C.; Levy, C.C.B.; Repiso, C.; Hennequart, F.; Nolasco, J.B.; Liu, F. Biostimulants Enhance Growth and Drought Tolerance in Arabidopsis thaliana and Exhibit Chemical Priming Action. Ann. Appl. Biol. 2019, 174, 153–165. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of Proline under Changing Environments: A Review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Dar, M.I.; Naikoo, M.I.; Rehman, F.; Naushin, F.; Khan, F.A. Proline Accumulation in Plants: Roles in Stress Tolerance and Plant Development. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Springer: New Delhi, India, 2015; pp. 155–166. ISBN 9788132226161. [Google Scholar]
- Aslam, M. Specific Role of Proline Against Heavy Metals Toxicity in Plants. Int. J. Pure Appl. Biosci. 2017, 5, 27–34. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a Multifaceted Signalling Molecule in Plant Responses to Abiotic Stress: Understanding the Physiological Mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K. The Significance of Amino Acids and Amino Acid-Derived Molecules in Plant Responses and Adaptation to Heavy Metal Stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef] [PubMed]
- EL-Bauome, H.A.; Abdeldaym, E.A.; Abd El-Hady, M.A.M.; Darwish, D.B.E.; Alsubeie, M.S.; El-Mogy, M.M.; Basahi, M.A.; Al-Qahtani, S.M.; Al-Harbi, N.A.; Alzuaibr, F.M.; et al. Exogenous Proline, Methionine, and Melatonin Stimulate Growth, Quality, and Drought Tolerance in Cauliflower Plants. Agriculture 2022, 12, 1301. [Google Scholar] [CrossRef]
- Verslues, P.E.; Sharma, S. Proline Metabolism and Its Implications for Plant-Environment Interaction. Arab. Book 2010, 8, e0140. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Alvarez, M.E.; Savouré, A.; Szabados, L. Proline Metabolism as Regulatory Hub. Trends Plant Sci. 2022, 27, 39–55. [Google Scholar] [CrossRef]
- Williamson, M.P. The Structure and Function of Proline-Rich Regions in Proteins. Biochem. J. 1994, 297, 249. [Google Scholar] [CrossRef]
- Mattioli, R.; Costantino, P.; Trovato, M. Proline Accumulation in Plants: Not Only Stress. Plant Signal. Behav. 2009, 4, 1016–1018. [Google Scholar] [CrossRef]
- Funck, D.; Baumgarten, L.; Stift, M.; von Wirén, N.; Schönemann, L. Differential Contribution of P5CS Isoforms to Stress Tolerance in Arabidopsis. Front. Plant Sci. 2020, 11, 1483. [Google Scholar] [CrossRef]
- Meena, M.; Divyanshu, K.; Kumar, S.; Swapnil, P.; Zehra, A.; Shukla, V.; Yadav, M.; Upadhyay, R.S. Regulation of L-Proline Biosynthesis, Signal Transduction, Transport, Accumulation and Its Vital Role in Plants during Variable Environmental Conditions. Heliyon 2019, 5, e02952. [Google Scholar] [CrossRef] [PubMed]
- Ribarits, A.; Abdullaev, A.; Tashpulatov, A.; Richter, A.; Heberle-Bors, E.; Touraev, A. Two Tobacco Proline Dehydrogenases Are Differentially Regulated and Play a Role in Early Plant Development. Planta 2007, 225, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Szepesi, Á.; Szőllősi, R. Mechanism of Proline Biosynthesis and Role of Proline Metabolism Enzymes Under Environmental Stress in Plants. In Plant Metabolites and Regulation under Environmental Stress; Academic Press: Cambridge, MA, USA, 2018; pp. 337–353. ISBN 9780128126899. [Google Scholar]
- Kavi Kishor, P.B.; Sreenivasulu, N. Is Proline Accumulation per Se Correlated with Stress Tolerance or Is Proline Homeostasis a More Critical Issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Rejeb, K.B.; Abdelly, C.; Savouré, A. How Reactive Oxygen Species and Proline Face Stress Together. Plant Physiol. Biochem. 2014, 80, 278–284. [Google Scholar] [CrossRef]
- Heuer, B. Role of Proline in Plant Response to Drought and Salinity. In Handbook of Plant and Crop Stress, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 213–238. ISBN 9781439813997. [Google Scholar]
- Verbruggen, N.; Hermans, C. Proline Accumulation in Plants: A Review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline Mechanisms of Stress Survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef]
- Kavi Kishor, P.B.; Kumari, P.H.; Sunita, M.S.L.; Sreenivasulu, N. Role of Proline in Cell Wall Synthesis and Plant Development and Its Implications in Plant Ontogeny. Front. Plant Sci. 2015, 6, 544. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A. Metabolic Implications of Stress-Induced Proline Accumulation in Plants; Kluwer Academic Publishers: Alphen am Rhein, The Netherlands, 1997; Volume 21. [Google Scholar]
- Kaur, G.; Asthir, B. Proline: A Key Player in Plant Abiotic Stress Tolerance. Biol. Plant 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil Salinity: Historical Perspectives and a World Overview of the Problem. Guidel. Salin. Assess. Mitig. Adapt. Using Nucl. Relat. Tech. 2018, 2018, 43–53. [Google Scholar] [CrossRef]
- Hossain, M.S. Present Scenario of Global Salt Affected Soils, Its Management and Importance of Salinity Research. Int. Res. J. Biol. Sci. 2019, 1, 1–3. Available online: https://www.researchgate.net/profile/Md-Hossain-72/publication/334773002_Present_Scenario_of_Global_Salt_Affected_Soils_its_Management_and_Importance_of_Salinity_Research/links/5d7150a292851cacdb23c8d9/Present-Scenario-of-Global-Salt-Affected-Soils-its-Management-and-Importance-of-Salinity-Research.pdf (accessed on 2 February 2023).
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of Salinity Stress on Plants and Its Tolerance Strategies: A Review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Ami, K.; Planchais, S.; Cabassa, C.; Guivarc’h, A.; Very, A.A.; Khelifi, M.; Djebbar, R.; Abrous-Belbachir, O.; Carol, P. Different Proline Responses of Two Algerian Durum Wheat Cultivars to in Vitro Salt Stress. Acta Physiol. Plant 2020, 42, 21. [Google Scholar] [CrossRef]
- ben Ahmed, C.; ben Rouina, B.; Sensoy, S.; Boukhriss, M.; ben Abdullah, F. Saline Water Irrigation Effects on Antioxidant Defense System and Proline Accumulation in Leaves and Roots of Field-Grown Olive. J. Agric. Food Chem. 2009, 57, 11484–11490. [Google Scholar] [CrossRef]
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity Stress Affects Photosynthesis, Malondialdehyde Formation, and Proline Content in Portulaca oleracea L. Plants 2021, 10, 845. [Google Scholar] [CrossRef]
- Izadi, M.H.; Rabbani, J.; Emam, Y.; Pessarakli, M.; Tahmasebi, A. Effects of Salinity Stress on Physiological Performance of Various Wheat and Barley Cultivars. J. Plant Nutr. 2014, 37, 520–531. [Google Scholar] [CrossRef]
- Moghaddam, M.; Farhadi, N.; Panjtandoust, M.; Ghanati, F. Seed Germination, Antioxidant Enzymes Activity and Proline Content in Medicinal Plant Tagetes minuta under Salinity Stress. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2019, 154, 835–842. [Google Scholar] [CrossRef]
- Shin, Y.K.; Bhandari, S.R.; Cho, M.C.; Lee, J.G. Evaluation of Chlorophyll Fluorescence Parameters and Proline Content in Tomato Seedlings Grown under Different Salt Stress Conditions. Hortic. Environ. Biotechnol. 2020, 61, 433–443. [Google Scholar] [CrossRef]
- Sousa, B.; Rodrigues, F.; Soares, C.; Martins, M.; Azenha, M.; Lino-Neto, T.; Santos, C.; Cunha, A.; Fidalgo, F. Impact of Combined Heat and Salt Stresses on Tomato Plants—Insights into Nutrient Uptake and Redox Homeostasis. Antioxidants 2022, 11, 478. [Google Scholar] [CrossRef] [PubMed]
- de la Torre-González, A.; Montesinos-Pereira, D.; Blasco, B.; Ruiz, J.M. Influence of the Proline Metabolism and Glycine Betaine on Tolerance to Salt Stress in Tomato (Solanum lycopersicum L.) Commercial Genotypes. J. Plant Physiol. 2018, 231, 329–336. [Google Scholar] [CrossRef]
- Goharrizi, K.J.; Baghizadeh, A.; Afroushteh, M.; Amirmahani, F.; Kermani, S.G. Effects of Salinity Stress on Proline Content and Expression of Δ1-Pyrroline-5-Carboxylate Synthase and Vacuolar-Type H+ Subunit E Genes in Wheat. Plant Genet. Resour. 2020, 18, 334–342. [Google Scholar] [CrossRef]
- Arabbeigi, M.; Arzani, A.; Majidi, M.M. Expression Profiles of P5CS and DREB2 Genes under Salt Stress in Aegilops cylindrica. Russ. J. Plant Physiol. 2019, 66, 583–590. [Google Scholar] [CrossRef]
- Singh, M.; Singh, V.P.; Prasad, S.M. Responses of Photosynthesis, Nitrogen and Proline Metabolism to Salinity Stress in Solanum lycopersicum under Different Levels of Nitrogen Supplementation. Plant Physiol. Biochem. 2016, 109, 72–83. [Google Scholar] [CrossRef]
- Singh, M.; Singh, V.P.; Prasad, S.M. Nitrogen Modifies NaCl Toxicity in Eggplant Seedlings: Assessment of Chlorophyll a Fluorescence, Antioxidative Response and Proline Metabolism. Biocatal. Agric. Biotechnol. 2016, 7, 76–86. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, X.; Zhang, J.; He, Y.; Zhu, X.; Zhou, X.; Gong, H.; Yin, J.; Liu, Y. Silicon Confers Cucumber Resistance to Salinity Stress through Regulation of Proline and Cytokinins. Plant Physiol. Biochem. 2020, 156, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tang, X.; Wang, H.; Shao, H.B. Proline Accumulation and Metabolism-Related Genes Expression Profiles in Kosteletzkya virginica Seedlings under Salt Stress. Front. Plant Sci. 2015, 6, 792. [Google Scholar] [CrossRef]
- Yusuf, M.; Fariduddin, Q.; Khan, T.A.; Hayat, S. Epibrassinolide Reverses the Stress Generated by Combination of Excess Aluminum and Salt in Two Wheat Cultivars through Altered Proline Metabolism and Antioxidants. S. Afr. J. Bot. 2017, 112, 391–398. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Y.; Dong, K.; Dong, J.; Kang, J.; Yang, Q.; Zhou, H.; Sun, Y. The Effect of NaCl on Proline Metabolism in Saussurea amara Seedlings. Afr. J. Biotechnol. 2011, 10, 2886–2893. [Google Scholar] [CrossRef]
- Bagdi, D.L.; Shaw, B.B.; Purohit, G.K. Real Time PCR Expression Analysis of Gene Encoding P5CS Enzyme and Proline Metabolism under NaCl Salinity in Rice. J. Environ. Biol. 2015, 36, 955–961. [Google Scholar]
- Xue, X.; Liu, A.; Hua, X. Proline Accumulation and Transcriptional Regulation of Proline Biothesynthesis and Degradation in Brassica napus. BMB Rep. 2009, 42, 28–34. [Google Scholar] [CrossRef]
- Naliwajski, M.; Skłodowska, M. The Relationship between the Antioxidant System and Proline Metabolism in the Leaves of Cucumber Plants Acclimated to Salt Stress. Cells 2021, 10, 609. [Google Scholar] [CrossRef]
- Mansour, M.M.F.; Ali, E.F. Evaluation of Proline Functions in Saline Conditions. Phytochemistry 2017, 140, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Forlani, G.; Bertazzini, M.; Cagnano, G. Stress-Driven Increase in Proline Levels, and Not Proline Levels Themselves, Correlates with the Ability to Withstand Excess Salt in a Group of 17 Italian Rice Genotypes. Plant Biol. 2019, 21, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Gharsallah, C.; Fakhfakh, H.; Grubb, D.; Gorsane, F. Effect of Salt Stress on Ion Concentration, Proline Content, Antioxidant Enzyme Activities and Gene Expression in Tomato Cultivars. AoB Plants 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, E.; Razmjoo, J.; Zahedi, M.; Pessarakli, M. Selecting Alfalfa Cultivars for Salt Tolerance Based on Some Physiochemical Traits. Agron. J. 2014, 106, 1758–1764. [Google Scholar] [CrossRef]
- Badran, A.E.; Elsherebeny, E.A.M.; Salama, Y.A. Performance of Some Alfalfa Cultivars under Salinity Stress Conditions. J. Agric. Sci. 2015, 7, 281. [Google Scholar] [CrossRef]
- Wang, X.S.; Han, J.G. Changes of Proline Content, Activity, and Active Isoforms of Antioxidative Enzymes in Two Alfalfa Cultivars Under Salt Stress. Agric. Sci. China 2009, 8, 431–440. [Google Scholar] [CrossRef]
- Carpici, E.B.; Celik, N.; Bayram, G. The Effects of Salt Stress on the Growth, Biochemical Parameter and Mineral Element Content of Some Maize (Zea mays L.) Cultivars. Afr. J. Biotechnol. 2010, 9, 6937–6942. [Google Scholar] [CrossRef]
- Ghoulam, C.; Foursy, A.; Fares, K. Effects of Salt Stress on Growth, Inorganic Ions and Proline Accumulation in Relation to Osmotic Adjustment in Five Sugar Beet Cultivars. Environ. Exp. Bot. 2002, 47, 39–50. [Google Scholar] [CrossRef]
- Wu, G.Q.; Liang, N.; Feng, R.J.; Zhang, J.J. Evaluation of Salinity Tolerance in Seedlings of Sugar Beet (Beta vulgaris L.) Cultivars Using Proline, Soluble Sugars and Cation Accumulation Criteria. Acta Physiol. Plant 2013, 35, 2665–2674. [Google Scholar] [CrossRef]
- Poustini, K.; Siosemardeh, A.; Ranjbar, M. Proline Accumulation as a Response to Salt Stress in 30 Wheat (Triticum aestivum L.) Cultivars Differing in Salt Tolerance. Genet. Resour. Crop Evol. 2007, 54, 925–934. [Google Scholar] [CrossRef]
- Kanawapee, N.; Sanitchon, J.; Lontom, W.; Threerakulpisut, P. Evaluation of Salt Tolerance at the Seedling Stage in Rice Genotypes by Growth Performance, Ion Accumulation, Proline and Chlorophyll Content. Plant Soil 2012, 358, 235–249. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. Effects of Various Salts and of Mannitol on Ion and Proline Accumulation in Relation to Osmotic Adjustment in Rice (Oryza sativa L.) Callus Cultures. J. Plant Physiol. 1996, 149, 186–195. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Ashraf, M.; Shahbaz, M. Growth Stage-Based Modulation in Antioxidant Defense System and Proline Accumulation in Two Hexaploid Wheat (Triticum aestivum L.) Cultivars Differing in Salinity Tolerance. Flora Morphol. Distrib. Funct. Ecol. Plants 2012, 207, 388–397. [Google Scholar] [CrossRef]
- Ali, S.G.; Rab, A.; Khan, N.U.; Nawab, K. Enhanced Proline Synthesis May Determine Resistance to Salt Stress in Tomato Cultivars. Pak. J. Bot. 2011, 43, 2707–2710. [Google Scholar]
- Abdelhamid, M.T.; Rady, M.M.; Osman, A.S.; Abdalla, M.A. Exogenous Application of Proline Alleviates Salt-Induced Oxidative Stress in Phaseolus vulgaris L. Plants. J. Hortic. Sci. Biotechnol. 2015, 88, 439–446. [Google Scholar] [CrossRef]
- Bhusan, D.; Das, D.K.; Hossain, M.; Murata, Y.; Hoque, M.A. Improvement of Salt Tolerance in Rice (“Oryza sativa” L.) by Increasing Antioxidant Defense Systems Using Exogenous Application of Proline. Aust. J. Crop Sci. 2016, 10, 50–56. [Google Scholar]
- de Freitas, P.A.F.; de Carvalho, H.H.; Costa, J.H.; Miranda, R.D.S.; Saraiva, K.D.D.C.; de Oliveira, F.D.B.; Coelho, D.G.; Prisco, J.T.; Gomes-Filho, E. Salt Acclimation in Sorghum Plants by Exogenous Proline: Physiological and Biochemical Changes and Regulation of Proline Metabolism. Plant Cell Rep. 2019, 38, 403–416. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, P.A.F.; de Souza Miranda, R.; Marques, E.C.; Prisco, J.T.; Gomes-Filho, E. Salt Tolerance Induced by Exogenous Proline in Maize Is Related to Low Oxidative Damage and Favorable Ionic Homeostasis. J. Plant Growth Regul. 2018, 37, 911–924. [Google Scholar] [CrossRef]
- Heuer, B. Influence of Exogenous Application of Proline and Glycinebetaine on Growth of Salt-Stressed Tomato Plants. Plant Sci. 2003, 165, 693–699. [Google Scholar] [CrossRef]
- Kaya, C.; Tuna, A.L.; Ashraf, M.; Altunlu, H. Improved Salt Tolerance of Melon (Cucumis melo L.) by the Addition of Proline and Potassium Nitrate. Environ. Exp. Bot. 2007, 60, 397–403. [Google Scholar] [CrossRef]
- Mahboob, W.; Khan, M.; Bot, M.S.; Pak, J. Induction of salt tolerance in wheat (Triticum aestivum L.) seedlings through exogenous application of proline. Pak. J. Bot. 2016, 48, 861–867. [Google Scholar]
- Medeiros, M.J.L.; de Silva, M.M.A.; Granja, M.M.C.; de Souza, E.S.G.; Camara, T.; Willadino, L. Effect of Exogenous Proline in Two Sugarcane Genotypes Grown in Vitro under Salt Stress. Acta Biológica Colomb. 2015, 20, 57–63. [Google Scholar] [CrossRef]
- Wu, G.-Q.; Du, Y.-Y.; Wu, G.-Q.; Feng, R.-J.; Li, S.-J.; Du, Y.-Y. Exogenous Application of Proline Alleviates Salt-Induced Toxicity in Sainfoin Seedlings. J. Anim. Plant Sci. 2017, 27, 246–251. [Google Scholar]
- Messedi, D.; Farhani, F.; Hamed, K.; Trabelsi, N.; Bot, R.K.-P.J. Highlighting the Mechanisms by Which Proline Can Confer Tolerance to Salt Stress in Cakile maritima. Pak. J. Bot. 2016, 48, 417–427. [Google Scholar]
- Huang, Y.; Bie, Z.; Liu, Z.; Zhen, A.; Wang, W. Protective Role of Proline against Salt Stress Is Partially Related to the Improvement of Water Status and Peroxidase Enzyme Activity in Cucumber. Soil Sci. Plant Nutr. 2010, 55, 698–704. [Google Scholar] [CrossRef]
- ben Ahmed, C.; Magdich, S.; ben Rouina, B.; Sensoy, S.; Boukhris, M.; ben Abdullah, F. Exogenous Proline Effects on Water Relations and Ions Contents in Leaves and Roots of Young Olive. Amino Acids 2011, 40, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.A.; Balal, R.M.; Pervez, M.A.; Abbas, T.; Aqeel, M.A.; Javaid, M.M.; Garcia-Sanchez, F. Exogenous Proline and Proline-Enriched Lolium perenne Leaf Extract Protects against Phytotoxic Effects of Nickel and Salinity in Pisum Sativum by Altering Polyamine Metabolism in Leaves. Turk. J. Bot. 2014, 38, 914–926. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Hasanuzzaman, M.; Nahar, K.; Fujita, M. Exogenous Proline and Glycine Betaine Mediated Upregulation of Antioxidant Defense and Glyoxalase Systems Provides Better Protection against Salt-Induced Oxidative Stress in Two Rice (Oryza sativa L.) Varieties. Biomed. Res. Int. 2014, 2014, 757219. [Google Scholar] [CrossRef]
- Wani, A.S.; Ahmad, A.; Hayat, S.; Tahir, I. Is Foliar Spray of Proline Sufficient for Mitigation of Salt Stress in Brassica juncea Cultivars? Environ. Sci. Pollut. Res. 2016, 23, 13413–13423. [Google Scholar] [CrossRef]
- Wani, A.S.; Ahmad, A.; Hayat, S.; Tahir, I. Epibrassinolide and Proline Alleviate the Photosynthetic and Yield Inhibition under Salt Stress by Acting on Antioxidant System in Mustard. Plant Physiol. Biochem. 2019, 135, 385–394. [Google Scholar] [CrossRef]
- Zheng, J.L.; Zhao, L.Y.; Wu, C.W.; Shen, B.; Zhu, A.Y. Exogenous Proline Reduces NaCl-Induced Damage by Mediating Ionic and Osmotic Adjustment and Enhancing Antioxidant Defense in Eurya emarginata. Acta Physiol. Plant 2015, 37, 181. [Google Scholar] [CrossRef]
- Reza, S.; Heidari, R.; Zare, S.; Norastehnia, A. Antioxidant Response of Two Salt-Stressed Barley Varieties in the Presence or Absence of Exogenous Proline. Gen. Appl. Plant Physiol. 2006, 32, 233–251. [Google Scholar]
- Hossain, M.A.; Fujita, M. Evidence for a Role of Exogenous Glycinebetaine and Proline in Antioxidant Defense and Methylglyoxal Detoxification Systems in Mung Bean Seedlings under Salt Stress. Physiol. Mol. Biol. Plants 2010, 16, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Agami, R.A. Applications of Ascorbic Acid or Proline Increase Resistance to Salt Stress in Barley Seedlings. Biol. Plant 2014, 58, 341–347. [Google Scholar] [CrossRef]
- ben Ahmed, C.; ben Rouina, B.; Sensoy, S.; Boukhriss, M.; ben Abdullah, F. Exogenous Proline Effects on Photosynthetic Performance and Antioxidant Defense System of Young Olive Tree. J. Agric. Food Chem. 2010, 58, 4216–4222. [Google Scholar] [CrossRef]
- Alam, R.; Das, D.K.; Islam, M.R.; Murata, Y.; Hoque, M.A. Exogenous Proline Enhances Nutrient Uptake and Confers Tolerance to Salt Stress in Maize (Zea mays L.). Progress. Agric. 2016, 27, 409–417. [Google Scholar] [CrossRef]
- el Sabagh, A.; Sorour, S.; Ragab, A.; Saneoka, H.; Islam, M.S.; Sabagh, A. The Effect of Exogenous Application of Proline and Glycine Betaine on the Nodule Activity of Soybean Under Saline Condition. J. Agric. Biotechnol. 2017, 2, 300–310. [Google Scholar] [CrossRef]
- Guerzoni, J.T.S.; Belintani, N.G.; Moreira, R.M.P.; Hoshino, A.A.; Domingues, D.S.; Filho, J.C.B.; Vieira, L.G.E. Stress-Induced Δ1-Pyrroline-5-Carboxylate Synthetase (P5CS) Gene Confers Tolerance to Salt Stress in Transgenic Sugarcane. Acta Physiol. Plant 2014, 36, 2309–2319. [Google Scholar] [CrossRef]
- Guan, C.; Cui, X.; Liu, H.Y.; Li, X.; Li, M.Q.; Zhang, Y.W. Proline Biosynthesis Enzyme Genes Confer Salt Tolerance to Switchgrass (Panicum virgatum L.) in Cooperation With Polyamines Metabolism. Front. Plant Sci. 2020, 11, 46. [Google Scholar] [CrossRef]
- Reddy, P.S.; Jogeswar, G.; Rasineni, G.K.; Maheswari, M.; Reddy, A.R.; Varshney, R.K.; Kavi Kishor, P.B. Proline Over-Accumulation Alleviates Salt Stress and Protects Photosynthetic and Antioxidant Enzyme Activities in Transgenic Sorghum [Sorghum bicolor (L.) Moench]. Plant Physiol. Biochem. 2015, 94, 104–113. [Google Scholar] [CrossRef]
- Soares, C.; Carvalho, M.E.A.; Azevedo, R.A.; Fidalgo, F. Plants Facing Oxidative Challenges—A Little Help from the Antioxidant Networks. Environ. Exp. Bot. 2019, 161, 4–25. [Google Scholar] [CrossRef]
- Zarattini, M.; Forlani, G. Toward Unveiling the Mechanisms for Transcriptional Regulation of Proline Biosynthesis in the Plant Cell Response to Biotic and Abiotic Stress Conditions. Front. Plant Sci. 2017, 8, 927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, N.; Yang, J.; Guo, H.; Liu, Z.; Zheng, X.; Li, S.; Xiang, F. The Salt-Induced Transcription Factor GmMYB84 Confers Salinity Tolerance in Soybean. Plant Sci. 2020, 291, 110326. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Hou, L.; Xiao, P.; Guo, Y.; Deyholos, M.K.; Liu, X. VvWRKY30, a Grape WRKY Transcription Factor, Plays a Positive Regulatory Role under Salinity Stress. Plant Sci. 2019, 280, 132–142. [Google Scholar] [CrossRef]
- Gao, Y.F.; Liu, J.K.; Yang, F.M.; Zhang, G.Y.; Wang, D.; Zhang, L.; Ou, Y.B.; Yao, Y.A. The WRKY Transcription Factor WRKY8 Promotes Resistance to Pathogen Infection and Mediates Drought and Salt Stress Tolerance in Solanum lycopersicum. Physiol. Plant 2020, 168, 98–117. [Google Scholar] [CrossRef]
- Liang, J.; Zheng, J.; Wu, Z.; Wang, H. Strawberry FaNAC2 Enhances Tolerance to Abiotic Stress by Regulating Proline Metabolism. Plants 2020, 9, 1417. [Google Scholar] [CrossRef]
- Alshareef, N.O.; Wang, J.Y.; Ali, S.; Al-Babili, S.; Tester, M.; Schmöckel, S.M. Overexpression of the NAC Transcription Factor JUNGBRUNNEN1 (JUB1) Increases Salinity Tolerance in Tomato. Plant Physiol. Biochem. 2019, 140, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Jalmi, S.K.; Bhagat, P.K.; Verma, N.; Sinha, A.K. A BHLH Transcription Factor, MYC2, Imparts Salt Intolerance by Regulating Proline Biosynthesis in Arabidopsis. FEBS J. 2020, 287, 2560–2576. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, G.; Mu, D.; Li, H.; Zang, D.; Xu, H.; Zou, X.; Wang, Y. An Ethylene-Responsive Factor BpERF11 Negatively Modulates Salt and Osmotic Tolerance in Betula platyphylla. Sci. Rep. 2016, 6, 23085. [Google Scholar] [CrossRef]
- Li, X.; Hou, Y.; Li, M.; Zhang, F.; Yi, F.; Kang, J.; Yang, Q.; Long, R. Overexpression of an ABA-Inducible Homeodomain-Leucine Zipper I Gene MsHB7 Confers Salt Stress Sensitivity to Alfalfa. Ind. Crops Prod. 2022, 177, 114463. [Google Scholar] [CrossRef]
- Noor, I.; Sohail, H.; Sun, J.; Nawaz, M.A.; Li, G.; Hasanuzzaman, M.; Liu, J. Heavy Metal and Metalloid Toxicity in Horticultural Plants: Tolerance Mechanism and Remediation Strategies. Chemosphere 2022, 303, 135196. [Google Scholar] [CrossRef]
- Handique, G.K.; Handique, A.K. Proline Accumulation in Lemongrass (Cymbopogon flexuosus Stapf.) Due to Heavy Metal Stress. J. Environ. Biol. 2009, 30, 299–302. [Google Scholar]
- Zengin, F.K.; Munzuroglu, O. Effects of Some Heavy Metals on Content of Chlorophyll, Proline and Some Antioxidant Chemicals in Bean (Phaseolus vulgaris L.) Seedlings. Acta Biol. Crac. Ser. Bot. 2005, 47, 157–164. [Google Scholar]
- Bhagyawant, S.S.; Narvekar, D.T.; Gupta, N.; Bhadkaria, A.; Koul, K.K.; Srivastava, N. Variations in the Antioxidant and Free Radical Scavenging under Induced Heavy Metal Stress Expressed as Proline Content in Chickpea. Physiol. Mol. Biol. Plants 2019, 25, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Ameen, N.; Amjad, M.; Murtaza, B.; Abbas, G.; Shahid, M.; Imran, M.; Naeem, M.A.; Niazi, N.K. Biogeochemical Behavior of Nickel under Different Abiotic Stresses: Toxicity and Detoxification Mechanisms in Plants. Environ. Sci. Pollut. Res. 2019, 26, 10496–10514. [Google Scholar] [CrossRef] [PubMed]
- Ghori, N.H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy Metal Stress and Responses in Plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Gajewska, E.; Skłodowska, M. Differential Biochemical Responses of Wheat Shoots and Roots to Nickel Stress: Antioxidative Reactions and Proline Accumulation. Plant Growth Regul. 2008, 54, 179–188. [Google Scholar] [CrossRef]
- Hadi, F.; Ali, N.; Fuller, M.P. Molybdenum (Mo) Increases Endogenous Phenolics, Proline and Photosynthetic Pigments and the Phytoremediation Potential of the Industrially Important Plant Ricinus communis L. for Removal of Cadmium from Contaminated Soil. Environ. Sci. Pollut. Res. 2016, 23, 20408–20430. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, R.; Favas, P.; Pratas, J.; Varun, M.; Paul, M.S. EDTA-Assisted Metal Uptake in Raphanus sativus L. and Brassica oleracea L.: Assessment of Toxicity and Food Safety. Bull. Environ. Contam. Toxicol. 2019, 103, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.; de Sousa, A.; Pinto, A.; Azenha, M.; Teixeira, J.; Azevedo, R.A.; Fidalgo, F. Effect of 24-Epibrassinolide on ROS Content, Antioxidant System, Lipid Peroxidation and Ni Uptake in Solanum nigrum L. under Ni Stress. Environ. Exp. Bot. 2016, 122, 115–125. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Basalah, M.O. Interactive Effect of Calcium and Gibberellin on Nickel Tolerance in Relation to Antioxidant Systems in Triticum aestivum L. Protoplasma 2011, 248, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Sirhindi, G.; Mir, M.A.; Abd-Allah, E.F.; Ahmad, P.; Gucel, S. Jasmonic Acid Modulates the Physio-Biochemical Attributes, Antioxidant Enzyme Activity, and Gene Expression in Glycine max under Nickel Toxicity. Front. Plant Sci. 2016, 7, 591. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Ahmad, A. Proline Enhances Antioxidative Enzyme Activity, Photosynthesis and Yield of Cicer arietinum L. Exposed to Cadmium Stress. Acta Bot. Croat. 2013, 72, 323–335. [Google Scholar] [CrossRef]
- Zouari, M.; ben Hassena, A.; Trabelsi, L.; ben Rouina, B.; Decou, R.; Labrousse, P. Exogenous Proline-Mediated Abiotic Stress Tolerance in Plants: Possible Mechanisms. Osmoprotectant Mediat. Abiotic Stress Toler. Plants 2019, 2019, 99–121. [Google Scholar] [CrossRef]
- Rizwan, M.; Imtiaz, M.; Dai, Z.; Mehmood, S.; Adeel, M.; Liu, J.; Tu, S. Nickel Stressed Responses of Rice in Ni Subcellular Distribution, Antioxidant Production, and Osmolyte Accumulation. Environ. Sci. Pollut. Res. 2017, 24, 20587–20598. [Google Scholar] [CrossRef] [PubMed]
- Spormann, S.; Soares, C.; Martins, V.; Azenha, M.; Gerós, H.; Fidalgo, F. Early Activation of Antioxidant Responses in Ni-Stressed Tomato Cultivars Determines Their Resilience Under Co-Exposure to Drought. J. Plant Growth Regul. 2022, 42, 877–891. [Google Scholar] [CrossRef]
- Tiwari, K.K.; Singh, N.K.; Rai, U.N. Chromium Phytotoxicity in Radish (Raphanus sativus): Effects on Metabolism and Nutrient Uptake. Bull. Environ. Contam. Toxicol. 2013, 91, 339–344. [Google Scholar] [CrossRef]
- Cia, M.C.; Guimarães, A.C.R.; Medici, L.O.; Chabregas, S.M.; Azevedo, R.A. Antioxidant Responses to Water Deficit by Drought-Tolerant and -Sensitive Sugarcane Varieties. Ann. Appl. Biol. 2012, 161, 313–324. [Google Scholar] [CrossRef]
- ur Rehman, M.Z.; Rizwan, M.; Ali, S.; Ok, Y.S.; Ishaque, W.; Saifullah; Nawaz, M.F.; Akmal, F.; Waqar, M. Remediation of Heavy Metal Contaminated Soils by Using Solanum nigrum: A Review. Ecotoxicol. Environ. Saf. 2017, 143, 236–248. [Google Scholar] [CrossRef]
- Nedjimi, B.; Daoud, Y. Cadmium Accumulation in Atriplex halimus Subsp. Schweinfurthii and Its Influence on Growth, Proline, Root Hydraulic Conductivity and Nutrient Uptake. Flora Morphol. Distrib. Funct. Ecol. Plants 2009, 204, 316–324. [Google Scholar] [CrossRef]
- Sun, R.L.; Zhou, Q.X.; Sun, F.H.; Jin, C.X. Antioxidative Defense and Proline/Phytochelatin Accumulation in a Newly Discovered Cd-Hyperaccumulator, Solanum nigrum L. Environ. Exp. Bot. 2007, 60, 468–476. [Google Scholar] [CrossRef]
- Matsunami, M.; Toyofuku, K.; Kimura, N.; Plants, A.O. Osmotic Stress Leads to Significant Changes in Rice Root Metabolic Profiles between Tolerant and Sensitive Genotypes. Plants 2020, 9, 1503. [Google Scholar] [CrossRef]
- Schat, H.; Sharma, S.S.; Schat, R.V.; Sharma, H.; Vooijs, S.S. Heavy Metal-Induced Accumulation of Free Proline in a Metal-Tolerant and a Nontolerant Ecotype of Silene vuigaris. Physiol. Plant. 1997, 101, 477–482. [Google Scholar] [CrossRef]
- Sofy, M.R.; Seleiman, M.F.; Alhammad, B.A.; Alharbi, B.M.; Mohamed, H.I. Minimizing Adverse Effects of Pb on Maize Plants by Combined Treatment with Jasmonic, Salicylic Acids and Proline. Agronomy 2020, 10, 699. [Google Scholar] [CrossRef]
- Noreen, S.; Akhter, M.S.; Yaamin, T.; Arfan, M. The Ameliorative Effects of Exogenously Applied Proline on Physiological and Biochemical Parameters of Wheat (Triticum aestivum L.) Crop under Copper Stress Condition. J. Plant Interact. 2018, 13, 221–230. [Google Scholar] [CrossRef]
- Zouari, M.; Elloumi, N.; ben Ahmed, C.; Delmail, D.; ben Rouina, B.; ben Abdallah, F.; Labrousse, P. Exogenous Proline Enhances Growth, Mineral Uptake, Antioxidant Defense, and Reduces Cadmium-Induced Oxidative Damage in Young Date Palm (Phoenix dactylifera L.). Ecol. Eng. 2016, 86, 202–209. [Google Scholar] [CrossRef]
- Wang, F.; Zeng, B.; Sun, Z.; Zhu, C. Relationship between Proline and Hg2+-Induced Oxidative Stress in a Tolerant Rice Mutant. Arch. Environ. Contam. Toxicol. 2009, 56, 723–731. [Google Scholar] [CrossRef]
- Chen, C.T.; Chen, T.H.; Lo, K.F.; Chiu, C.Y. Effects of Proline on Copper Transport in Rice Seedlings under Excess Copper Stress. Plant Sci. 2004, 166, 103–111. [Google Scholar] [CrossRef]
- Chandrakar, V.; Parkhey, S.; Dubey, A.; Keshavkant, S. Modulation in Arsenic-Induced Lipid Catabolism in Glycine max Using Proline, 24-Epibrassinolide and Diphenylene Iodonium. Biologia 2017, 72, 292–299. [Google Scholar] [CrossRef]
- Khatun, M.; Matsushima, D.; Rhaman, M.S.; Okuma, E.; Nakamura, T.; Nakamura, Y.; Munemasa, S.; Murata, Y. Exogenous Proline Enhances Antioxidant Enzyme Activities but Does Not Mitigate Growth Inhibition by Selenate Stress in Tobacco BY-2 Cells. Biosci. Biotechnol. Biochem. 2020, 84, 2281–2292. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yin, H.; Li, X. Protective Effects of Proline against Cadmium Toxicity in Micropropagated Hyperaccumulator, Solanum nigrum L. Plant Cell Rep. 2009, 28, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Z.; Lin, Y.J.; Fan, W.J.; Lu, M.R. The Role of Exogenous Proline in Amelioration of Lipid Peroxidation in Rice Seedlings Exposed to Cr(VI). Int. Biodeterior. Biodegrad. 2017, 123, 106–112. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, P.; Chang, L.; Yue, Z.; Zeng, C.; Li, M.; Liu, Z.; Dong, X.; Yan, M. Exogenous Proline Mitigates Toxic Effects of Cadmium via the Decrease of Cadmium Accumulation and Reestablishment of Redox Homeostasis in Brassica juncea. BMC Plant Biol. 2022, 22, 182. [Google Scholar] [CrossRef] [PubMed]
- Nedjimi, B. Phytoremediation: A Sustainable Environmental Technology for Heavy Metals Decontamination. SN Appl. Sci. 2021, 3, 286. [Google Scholar] [CrossRef]
- Ullah, R.; Hadi, F.; Ahmad, S.; Jan, A.U.; Rongliang, Q. Phytoremediation of Lead and Chromium Contaminated Soil Improves with the Endogenous Phenolics and Proline Production in Parthenium, Cannabis, Euphorbia, and Rumex Species. Water Air Soil Pollut. 2019, 230, 40. [Google Scholar] [CrossRef]
- Balal, R.M.; Shahid, M.A.; Javaid, M.M.; Anjum, M.A.; Ali, H.H.; Mattson, N.S.; Garcia-Sanchez, F. Foliar Treatment with Lolium perenne (Poaceae) Leaf Extract Alleviates Salinity and Nickel-Induced Growth Inhibition in Pea. Rev. Bras. De Bot. 2016, 39, 453–463. [Google Scholar] [CrossRef]
- Singh, M.; Pratap Singh, V.; Dubey, G.; Mohan Prasad, S. Exogenous Proline Application Ameliorates Toxic Effects of Arsenate in Solanum melongena L. Seedlings. Ecotoxicol. Environ. Saf. 2015, 117, 164–173. [Google Scholar] [CrossRef]
- Aggarwal, M.; Sharma, S.; Kaur, N.; Pathania, D.; Bhandhari, K.; Kaushal, N.; Kaur, R.; Singh, K.; Srivastava, A.; Nayyar, H. Exogenous Proline Application Reduces Phytotoxic Effects of Selenium by Minimising Oxidative Stress and Improves Growth in Bean (Phaseolus vulgaris L.) Seedlings. Biol. Trace Elem. Res. 2011, 140, 354–367. [Google Scholar] [CrossRef]
- Hayat, K.; Khan, J.; Khan, A.; Ullah, S.; Ali, S.; Salahudin; Fu, Y. Ameliorative Effects of Exogenous Proline on Photosynthetic Attributes, Nutrients Uptake, and Oxidative Stresses under Cadmium in Pigeon Pea (Cajanus cajan L.). Plants 2021, 10, 796. [Google Scholar] [CrossRef]
- Zouari, M.; Elloumi, N.; Labrousse, P.; ben Rouina, B.; ben Abdallah, F.; ben Ahmed, C. Olive Trees Response to Lead Stress: Exogenous Proline Provided Better Tolerance than Glycine Betaine. S. Afr. J. Bot. 2018, 118, 158–165. [Google Scholar] [CrossRef]
- Adejumo, S.A.; Awoyemi, V.; Togun, A.O. Exogenous Proline and Hormone in Combination with Compost Improves Growth and Tolerance of Maize under Heavy Metal Stress. Plants Environ. 2020, 2, 40–53. [Google Scholar] [CrossRef]
- Kapoor, D.; Kavani, K.; Rattan, A.; Landi, M.; Sharma, A. Ameliorative Role of Pre-Sowing Proline Treatment in Coriandrum sativum L. Seedlings under Mercury Toxicity. Phyton Int. J. Exp. Bot. 2021, 90, 489–501. [Google Scholar] [CrossRef]
- Shahbaz, A.K.; Adnan Ramzani, P.M.; Saeed, R.; Turan, V.; Iqbal, M.; Lewińska, K.; Abbas, F.; Saqib, M.; Tauqeer, H.M.; Iqbal, M.; et al. Effects of Biochar and Zeolite Soil Amendments with Foliar Proline Spray on Nickel Immobilization, Nutritional Quality and Nickel Concentrations in Wheat. Ecotoxicol. Environ. Saf. 2019, 173, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.M.; Elrys, A.S.; Abo El-Maati, M.F.; Desoky, E.S.M. Interplaying Roles of Silicon and Proline Effectively Improve Salt and Cadmium Stress Tolerance in Phaseolus vulgaris Plant. Plant Physiol. Biochem. 2019, 139, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gupta, V.K.; Kumar, A.; Sharma, B. Synergistic Effects of Heavy Metals and Pesticides in Living Systems. Front. Chem. 2017, 5, 70. [Google Scholar] [CrossRef]
- Godheja, J.; Shekhar, S.K.; Siddiqui, S.A. Xenobiotic Compounds Present in Soil and Water: A Review on Remediation Strategies. J. Environ. Anal. Toxicol. 2016, 6, 1000392. [Google Scholar] [CrossRef]
- Duarte, R.M.B.O.; Matos, J.T.V.; Senesi, N. Organic Pollutants in Soils. Soil Pollut. Monit. Remediat. 2018, 103–126. [Google Scholar] [CrossRef]
- Soares, C.; Branco-Neves, S.; de Sousa, A.; Pereira, R.; Fidalgo, F. Ecotoxicological Relevance of Nano-NiO and Acetaminophen to Hordeum vulgare L.: Combining Standardized Procedures and Physiological Endpoints. Chemosphere 2016, 165, 442–452. [Google Scholar] [CrossRef]
- Soares, C.; Pereira, R.; Spormann, S.; Fidalgo, F. Is Soil Contamination by a Glyphosate Commercial Formulation Truly Harmless to Non-Target Plants?—Evaluation of Oxidative Damage and Antioxidant Responses in Tomato. Environ. Pollut. 2019, 247, 256–265. [Google Scholar] [CrossRef]
- Soares, C.; Pereira, R.; Martins, M.; Tamagnini, P.; Serôdio, J.; Moutinho-Pereira, J.; Cunha, A.; Fidalgo, F. Glyphosate-Dependent Effects on Photosynthesis of Solanum lycopersicum L.—An Ecophysiological, Ultrastructural and Molecular Approach. J. Hazard. Mater. 2020, 398, 122871. [Google Scholar] [CrossRef]
- Martins, M.; Sousa, B.; Lopes, J.; Soares, C.; Machado, J.; Carvalho, S.; Fidalgo, F.; Teixeira, J. Diclofenac Shifts the Role of Root Glutamine Synthetase and Glutamate Dehydrogenase for Maintaining Nitrogen Assimilation and Proline Production at the Expense of Shoot Carbon Reserves in Solanum lycopersicum L. Environ. Sci. Pollut. Res. 2020, 27, 29130–29142. [Google Scholar] [CrossRef] [PubMed]
- Sousa, B.; Lopes, J.; Leal, A.; Martins, M.; Soares, C.; Valente, I.M.; Rodrigues, J.A.; Fidalgo, F.; Teixeira, J. Response of Solanum lycopersicum L. to Diclofenac—Impacts on the Plant’s Antioxidant Mechanisms. Environ. Pollut. 2020, 258, 113762. [Google Scholar] [CrossRef]
- Branco-Neves, S.; Soares, C.; de Sousa, A.; Martins, V.; Azenha, M.; Gerós, H.; Fidalgo, F. An Efficient Antioxidant System and Heavy Metal Exclusion from Leaves Make Solanum cheesmaniae More Tolerant to Cu than Its Cultivated Counterpart. Food Energy Secur. 2017, 6, 123–133. [Google Scholar] [CrossRef]
- Gomes, M.P.; le Manac’h, S.G.; Moingt, M.; Smedbol, E.; Paquet, S.; Labrecque, M.; Lucotte, M.; Juneau, P. Impact of Phosphate on Glyphosate Uptake and Toxicity in Willow. J. Hazard. Mater. 2016, 304, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Bartha, B.; Huber, C.; Harpaintner, R.; Schröder, P. Effects of Acetaminophen in Brassica juncea L. Czern.: Investigation of Uptake, Translocation, Detoxification, and the Induced Defense Pathways. Environ. Sci. Pollut. Res. 2010, 17, 1553–1562. [Google Scholar] [CrossRef]
- Soares, C.; Fernandes, B.; Paiva, C.; Nogueira, V.; Cachada, A.; Fidalgo, F.; Pereira, R. Ecotoxicological Relevance of Glyphosate and Flazasulfuron to Soil Habitat and Retention Functions—Single vs. Combined Exposures. J. Hazard. Mater. 2023, 442, 130128. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Datta, S.; Wani, A.B.; Dhanjal, D.S.; Romero, R.; Singh, J. Glyphosate Uptake, Translocation, Resistance Emergence in Crops, Analytical Monitoring, Toxicity and Degradation: A Review. Environ. Chem. Lett. 2020, 18, 663–702. [Google Scholar] [CrossRef]
- Sun, C.; Dudley, S.; Trumble, J.; Gan, J. Pharmaceutical and Personal Care Products-Induced Stress Symptoms and Detoxification Mechanisms in Cucumber Plants. Environ. Pollut. 2018, 234, 39–47. [Google Scholar] [CrossRef]
- Gomes, M.P.; Smedbol, E.; Chalifour, A.; Hénault-Ethier, L.; Labrecque, M.; Lepage, L.; Lucotte, M.; Juneau, P. Alteration of Plant Physiology by Glyphosate and Its By-Product Aminomethylphosphonic Acid: An Overview. J. Exp. Bot. 2014, 65, 4691–4703. [Google Scholar] [CrossRef]
- Gomes, M.P.; le Manac’h, S.G.; Hénault-Ethier, L.; Labrecque, M.; Lucotte, M.; Juneau, P. Glyphosate-Dependent Inhibition of Photosynthesis in Willow. Front. Plant Sci. 2017, 8, 207. [Google Scholar] [CrossRef]
- Soares, C.; Nadais, P.; Sousa, B.; Pinto, E.; Ferreira, I.M.P.L.V.O.; Pereira, R.; Fidalgo, F. Silicon Improves the Redox Homeostasis to Alleviate Glyphosate Toxicity in Tomato Plants—Are Nanomaterials Relevant? Antioxidants 2021, 10, 1320. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.; Rodrigues, F.; Sousa, B.; Pinto, E.; Ferreira, I.M.P.L.V.O.; Pereira, R.; Fidalgo, F. Foliar Application of Sodium Nitroprusside Boosts Solanum lycopersicum L. Tolerance to Glyphosate by Preventing Redox Disorders and Stimulating Herbicide Detoxification Pathways. Plants 2021, 10, 1862. [Google Scholar] [CrossRef]
- Stuchlíková, L.R.; Skálová, L.; Szotáková, B.; Syslová, E.; Vokřál, I.; Vaněk, T.; Podlipná, R. Biotransformation of Flubendazole and Fenbendazole and Their Effects in the Ribwort Plantain (Plantago lanceolata). Ecotoxicol. Environ. Saf. 2018, 147, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Navrátilová, M.; Raisová Stuchlíková, L.; Skálová, L.; Szotáková, B.; Langhansová, L.; Podlipná, R. Pharmaceuticals in Environment: The Effect of Ivermectin on Ribwort Plantain (Plantago lanceolata L.). Environ. Sci. Pollut. Res. 2020, 27, 31202–31210. [Google Scholar] [CrossRef]
- Christou, A.; Antoniou, C.; Christodoulou, C.; Hapeshi, E.; Stavrou, I.; Michael, C.; Fatta-Kassinos, D.; Fotopoulos, V. Stress-Related Phenomena and Detoxification Mechanisms Induced by Common Pharmaceuticals in Alfalfa (Medicago sativa L.) Plants. Sci. Total Environ. 2016, 557–558, 652–664. [Google Scholar] [CrossRef]
- Pawłowska, B.; Telesiński, A.; Biczak, R. Effect of Diclofenac and Naproxen and Their Mixture on Spring Barley Seedlings and Heterocypris incongruens. Environ. Toxicol. Pharmacol. 2021, 88, 103746. [Google Scholar] [CrossRef]
- Soares, C.; Branco-Neves, S.; de Sousa, A.; Teixeira, J.; Pereira, R.; Fidalgo, F. Can Nano-SiO2 Reduce the Phytotoxicity of Acetaminophen?—A Physiological, Biochemical and Molecular Approach. Environ. Pollut. 2018, 241, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Nunes, B.; Pinto, G.; Martins, L.; Gonçalves, F.; Antunes, S.C. Biochemical and Standard Toxic Effects of Acetaminophen on the Macrophyte Species Lemna minor and Lemna gibba. Environ. Sci. Pollut. Res. 2014, 21, 10815–10822. [Google Scholar] [CrossRef]
- Liu, Y.; Pang, Y.; Yang, L.; Ning, S.; Wang, D.; Wu, Z. Responses of Hydrocharis dubia (Bl.) Backer and Trapa Bispinosa Roxb. to Tetracycline Exposure. Ecotoxicol. Environ. Saf. 2020, 202, 110890. [Google Scholar] [CrossRef]
- Wu, G.L.; Cui, J.; Tao, L.; Yang, H. Fluroxypyr Triggers Oxidative Damage by Producing Superoxide and Hydrogen Peroxide in Rice (Oryza sativa). Ecotoxicology 2010, 19, 124–132. [Google Scholar] [CrossRef]
- Moreira, J.T.; Moreira, T.M.; Cunha, J.B.; Azenha, M.; Fidalgo, F.; Teixeira, J. Differential Effects of Acetophenone on Shoots’ and Roots’ Metabolism of Solanum nigrum L. Plants and Implications in Its Phytoremediation. Plant Physiol. Biochem. 2018, 130, 391–398. [Google Scholar] [CrossRef]
- Shakir, S.K.; Irfan, S.; Akhtar, B.; ur Rehman, S.; Daud, M.K.; Taimur, N.; Azizullah, A. Pesticide-Induced Oxidative Stress and Antioxidant Responses in Tomato (Solanum lycopersicum) Seedlings. Ecotoxicology 2018, 27, 919–935. [Google Scholar] [CrossRef] [PubMed]
- Ould Said, C.; Boulahia, K.; Eid, M.A.M.; Rady, M.M.; Djebbar, R.; Abrous-Belbachir, O. Exogenously Used Proline Offers Potent Antioxidative and Osmoprotective Strategies to Re-Balance Growth and Physio-Biochemical Attributes in Herbicide-Stressed Trigonella foenum-graecum. J. Soil Sci. Plant Nutr. 2021, 21, 3254–3268. [Google Scholar] [CrossRef]
- Shopova, E.; Katerova, Z.; Brankova, L.; Dimitrova, L.; Sergiev, I.; Todorova, D.; Talaat, N.B. Modulation of Physiological Stress Response of Triticum aestivum L. to Glyphosate by Brassinosteroid Application. Life 2021, 11, 1156. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Zhu, B.; Fu, X.; You, S.; Wang, B.; Li, Z.; Zhao, W.; Peng, R.; Yao, Q. Overexpression of D-Amino Acid Oxidase from Bradyrhizobium japonicum, Enhances Resistance to Glyphosate in Arabidopsis thaliana. Plant Cell. Rep. 2015, 34, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Spormann, S.; Soares, C.; Fidalgo, F. Salicylic Acid Alleviates Glyphosate-Induced Oxidative Stress in Hordeum vulgare L. J. Environ. Manag. 2019, 241, 226–234. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Y.; Peng, X.; Xu, S.; Zhang, H.; Gao, J.; Xi, Z. Exogenous 24-Epibrassinolide Regulates Antioxidant and Pesticide Detoxification Systems in Grapevine after Chlorothalonil Treatment. Plant Growth Regul. 2017, 81, 455–466. [Google Scholar] [CrossRef]
- Hellmann, H.; Funck, D.; Rentsch, D.; Frommer, W.B. Hypersensitivity of an Arabidopsis Sugar Signaling Mutant toward Exogenous Proline Application. Plant Physiol. 2000, 122, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Alqarawi, A.A.; Radhakrishnan, R.; Al-Arjani, A.-B.F.; Aldehaish, H.A.; Egamberdieva, D.; Abd_Allah, E.F. Arbuscular Mycorrhizal Fungi Regulate the Oxidative System, Hormones and Ionic Equilibrium to Trigger Salt Stress Tolerance in Cucumis sativus L. Saudi J. Biol. Sci. 2018, 25, 1102–1114. [Google Scholar] [CrossRef]
- Latef, A.A.A.; Akter, A.; Tahjib-Ul-Arif, M. Foliar Application of Auxin or Cytokinin Can Confer Salinity Stress Tolerance in Vicia faba L. Agronomy 2021, 11, 228–233. [Google Scholar] [CrossRef]
- Siddiqui, H.; Yusuf, M.; Faraz, A.; Faizan, M.; Sami, F.; Hayat, S. 24-Epibrassinolide Supplemented with Silicon Enhances the Photosynthetic Efficiency of Brassica juncea under Salt Stress. S. Afr. J. Bot. 2018, 118, 120–128. [Google Scholar] [CrossRef]
- Kaur, H.; Hussain, S.J.; Kaur, G.; Poor, P.; Alamri, S.; Siddiqui, M.H.; Khan, M.I.R. Salicylic Acid Improves Nitrogen Fixation, Growth, Yield and Antioxidant Defence Mechanisms in Chickpea Genotypes Under Salt Stress. J. Plant Growth Regul. 2022, 41, 2034–2047. [Google Scholar] [CrossRef]
- Ahmad, S.; Cui, W.; Kamran, M.; Ahmad, I.; Meng, X.; Wu, X.; Su, W.; Javed, T.; El-Serehy, H.A.; Jia, Z.; et al. Exogenous Application of Melatonin Induces Tolerance to Salt Stress by Improving the Photosynthetic Efficiency and Antioxidant Defense System of Maize Seedling. J. Plant Growth Regul. 2021, 40, 1270–1283. [Google Scholar] [CrossRef]
- Shah, W.H.; Rasool, A.; Tahir, I.; Rehman, R.U. Exogenously Applied Selenium (Se) Mitigates the Impact of Salt Stress in Setaria italica L. and Panicum miliaceum L. Nucleus 2020, 63, 327–339. [Google Scholar] [CrossRef]
- Hurtado, A.C.; Chiconato, D.A.; Prado, R.d.M.; Sousa Junior, G.d.S.; Gratão, P.L.; Felisberto, G.; Olivera Viciedo, D.; Mathias dos Santos, D.M. Different Methods of Silicon Application Attenuate Salt Stress in Sorghum and Sunflower by Modifying the Antioxidative Defense Mechanism. Ecotoxicol. Environ. Saf. 2020, 203, 110964. [Google Scholar] [CrossRef] [PubMed]
- Sobahan, M.A.; Arias, C.R.; Okuma, E.; Shimoishi, Y.; Nakamura, Y.; Hirai, Y.; Mori, I.C.; Murata, Y. Exogenous Proline and Glycinebetaine Suppress Apoplastic Flow to Reduce Na+ Uptake in Rice Seedlings. Biosci. Biotechnol. Biochem. 2009, 73, 2037–2042. [Google Scholar] [CrossRef]
- Nawaz, K.; Talat, A.; Hussain, K.; Majeed, A. Induction of Salt Tolerance in Two Cultivars of Sorghum (Sorghum bicolor L.) by Exogenous Application of Proline at Seedling Stage. World Appl. Sci. J. 2010, 10, 93–99. [Google Scholar]
- Deivanai, S.; Xavier, R.; Vinod, V.; Timalata, K.; Lim, O.F. Role of Exogenous Proline in Ameliorating Salt Stress at Early Stage in Two Rice Cultivars. J. Stress Physiol. Biochem. 2011, 7, 157–174. [Google Scholar]
- Sakr, M.T.; El-Sarkassy, N.M.; Fuller, M.P. Osmoregulators Proline and Glycine Betaine Counteract Salinity Stress in Canola. Agron. Sustain. Dev. 2012, 32, 747–754. [Google Scholar] [CrossRef]
- Sobahan, M.A.; Akter, N.; Ohno, M.; Okuma, E.; Hirai, Y.; Mori, I.C.; Nakamura, Y.; Murata, Y. Effects of Exogenous Proline and Glycinebetaine on the Salt Tolerance of Rice Cultivars. Biosci. Biotechnol. Biochem 2012, 76, 1568–1570. [Google Scholar] [CrossRef]
- Nounjan, N.; Nghia, P.T.; Theerakulpisut, P. Exogenous Proline and Trehalose Promote Recovery of Rice Seedlings from Salt-Stress and Differentially Modulate Antioxidant Enzymes and Expression of Related Genes. J. Plant Physiol. 2012, 169, 596–604. [Google Scholar] [CrossRef]
- Nounjan, N.; Theerakulpisut, P. Effects of Exogenous Proline and Trehalose on Physiological Responses in Rice Seedlings during Salt-Stress and after Recovery. Plant Soil Environ. 2012, 58, 309–315. [Google Scholar] [CrossRef]
- Hua-long, L.; Han-jing, S.; Jing-guo, W.; Yang, L.; De-tang, Z.; Hong-wei, Z. Effect of Seed Soaking with Exogenous Proline on Seed Germination of Rice Under Salt Stress. J. Northeast. Agric. Univ. (Engl. Ed.) 2014, 21, 1–6. [Google Scholar] [CrossRef]
- El Sabagh, A.; Sorour, S.; Omar, A.E.; Ragab, A.; Islam, M.S.; Barutçular, C.; Ueda, A.; Saneoka, H. Alleviation of Adverse Effects of Salt Stress on Soybean (Glycine max L.) by Using Osmoprotectants and Organic Nutrients. Int. J. Agric. Biosyst. Eng. 2015, 9, 1014–1018. [Google Scholar]
- Teh, C.Y.; Mahmood, M.; Shaharuddin, N.A.; Ho, C.L. In Vitro Rice Shoot Apices as Simple Model to Study the Effect of NaCl and the Potential of Exogenous Proline and Glutathione in Mitigating Salinity Stress. Plant Growth Regul. 2015, 75, 771–781. [Google Scholar] [CrossRef]
- Rady, M.M.; Hemida, K.A. Sequenced Application of Ascorbate-Proline-Glutathione Improves Salt Tolerance in Maize Seedlings. Ecotoxicol. Environ. Saf. 2016, 133, 252–259. [Google Scholar] [CrossRef]
- Sobahan, M.A.; Akter, N.; Murata, Y.; Munemasa, S. Exogenous Proline and Glycinebetaine Mitigate the Detrimental Effect of Salt Stress on Rice Plants. Sci. Eng. Health Stud. 2016, 10, 38–43. [Google Scholar] [CrossRef]
- Singh, M.; Nehal, N.; Sharma, N.; Monika Singh, C.; Singh, A. Effect of Proline on Germination and Seedling Growth of Rice (Oryza sativa L.) under Salt Stress. J. Pharmacogn. Phytochem. 2018, 7, 2449–2452. [Google Scholar]
- Shafiq, F.; Raza, S.H.; Bibi, A.; Khan, I.; Iqbal, M. Influence of Proline Priming on Antioxidative Potential and Ionic Distribution and Its Relationship with Salt Tolerance of Wheat. Cereal Res. Commun. 2018, 46, 287–300. [Google Scholar] [CrossRef]
- Islam, M.M.; Hoque, M.A.; Okuma, E.; Banu, M.N.A.; Shimoishi, Y.; Nakamura, Y.; Murata, Y. Exogenous Proline and Glycinebetaine Increase Antioxidant Enzyme Activities and Confer Tolerance to Cadmium Stress in Cultured Tobacco Cells. J. Plant Physiol. 2009, 166, 1587–1597. [Google Scholar] [CrossRef]
- Min, S.O.N.G.; Wen-jing, X.U.; Xiang-yong, P.E.N.G.; Fan-hua, K.O.N.G. Effects of Exogenous Proline on the Growth of Wheat Seedlings under Cadmium Stress. Yingyong Shengtai Xuebao 2013, 24, 129–134. [Google Scholar]
- Rasheed, R.; Ashraf, M.A.; Hussain, I.; Haider, M.Z.; Kanwal, U.; Iqbal, M. Exogenous Proline and Glycinebetaine Mitigate Cadmium Stress in Two Genetically Different Spring Wheat (Triticum aestivum L.) Cultivars. Rev. Bras. Bot. 2014, 37, 399–406. [Google Scholar] [CrossRef]
- Zouari, M.; ben Ahmed, C.; Elloumi, N.; Bellassoued, K.; Delmail, D.; Labrousse, P.; ben Abdallah, F.; ben Rouina, B. Impact of Proline Application on Cadmium Accumulation, Mineral Nutrition and Enzymatic Antioxidant Defense System of Olea europaea L. Cv Chemlali Exposed to Cadmium Stress. Ecotoxicol. Environ. Saf. 2016, 128, 195–205. [Google Scholar] [CrossRef]
- Alyemeni, M.N.; Hayat, Q.; Hayat, S.; Faizan, M.; Faraz, A. Exogenous Proline Application Enhances the Efficiency of Nitrogen Fixation and Assimilation in Chickpea Plants Exposed to Cadmium. Legume Res. 2016, 39, 221–227. [Google Scholar] [CrossRef]
- Konotop, Y.; Kovalenko, M.; Matušíková, I.; Batsmanova, L.; Taran, N. Proline Application Triggers Temporal Redox Imbalance, but Alleviates Cadmium Stress in Wheat Seedlings. Pak. J. Bot. 2017, 49, 2145–2151. [Google Scholar]
- Singh, V.; Bhatt, I.; Aggarwal, A.; Tripathi, B.N.; Munjal, A.K.; Sharma, V. Proline Improves Copper Tolerance in Chickpea (Cicer arietinum). Protoplasma 2010, 245, 173–181. [Google Scholar] [CrossRef]
- Stetsenko, L.A.; Shevyakova, N.I.; Rakitin, V.Y.; Kuznetsov, V.v. Proline Protects Atropa belladonna Plants against Nickel Salt Toxicity. Russ. J. Plant Physiol. 2011, 58, 275–282. [Google Scholar] [CrossRef]
- Sadeghipour, O. The Effect of Common Bean (Phaseolus vulgaris L.) Seed Pretreatment with Proline and Glycine Betaine on Tolerance to Lead Stress. Environ. Stress. Crop Sci. 2019, 12, 319–329. [Google Scholar] [CrossRef]
- Mishra, S.; Dubey, R.S. Inhibition of Ribonuclease and Protease Activities in Arsenic Exposed Rice Seedlings: Role of Proline as Enzyme Protectant. J. Plant Physiol. 2006, 163, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Ghahremani, B.; Hassannejad, S.; Alizadeh, K.; Eslam, B.P. Salicylic Acid Alleviates Oxidative Stress and Lipid Peroxidation Caused by Clopyralid Herbicide in Indian Mustard Plants. Acta Physiol. Plant 2022, 44, 1–10. [Google Scholar] [CrossRef]
- Soares, C.; Spormann, S.; Fidalgo, F. Salicylic Acid Improves the Performance of the Enzymatic Antioxidant System of Barley Exposed to Glyphosate. Free Radic. Biol. Med. 2018, 120, S157. [Google Scholar] [CrossRef]
- Tripthi, D.K.; Varma, R.K.; Singh, S.; Sachan, M.; Guerriero, G.; Kushwaha, B.K.; Bhardwaj, S.; Ramawat, N.; Sharma, S.; Singh, V.P.; et al. Silicon Tackles Butachlor Toxicity in Rice Seedlings by Regulating Anatomical Characteristics, Ascorbate-Glutathione Cycle, Proline Metabolism and Levels of Nutrients. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).