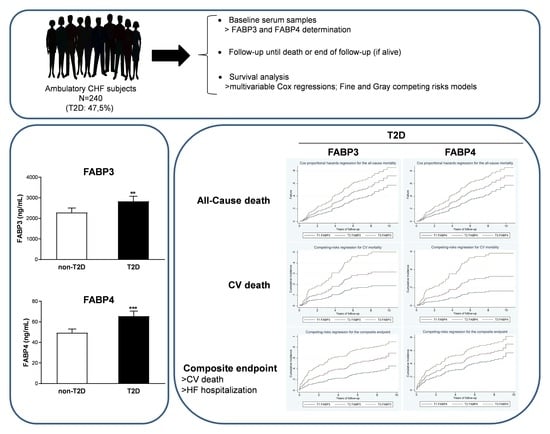
Fatty Acid Binding Proteins 3 and 4 Predict Both All-Cause and Cardiovascular Mortality in Subjects with Chronic Heart Failure and Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical and Biochemical Data
2.3. Serum FABPs Determination
2.4. T2D Diagnosis
2.5. Statistical Analysis
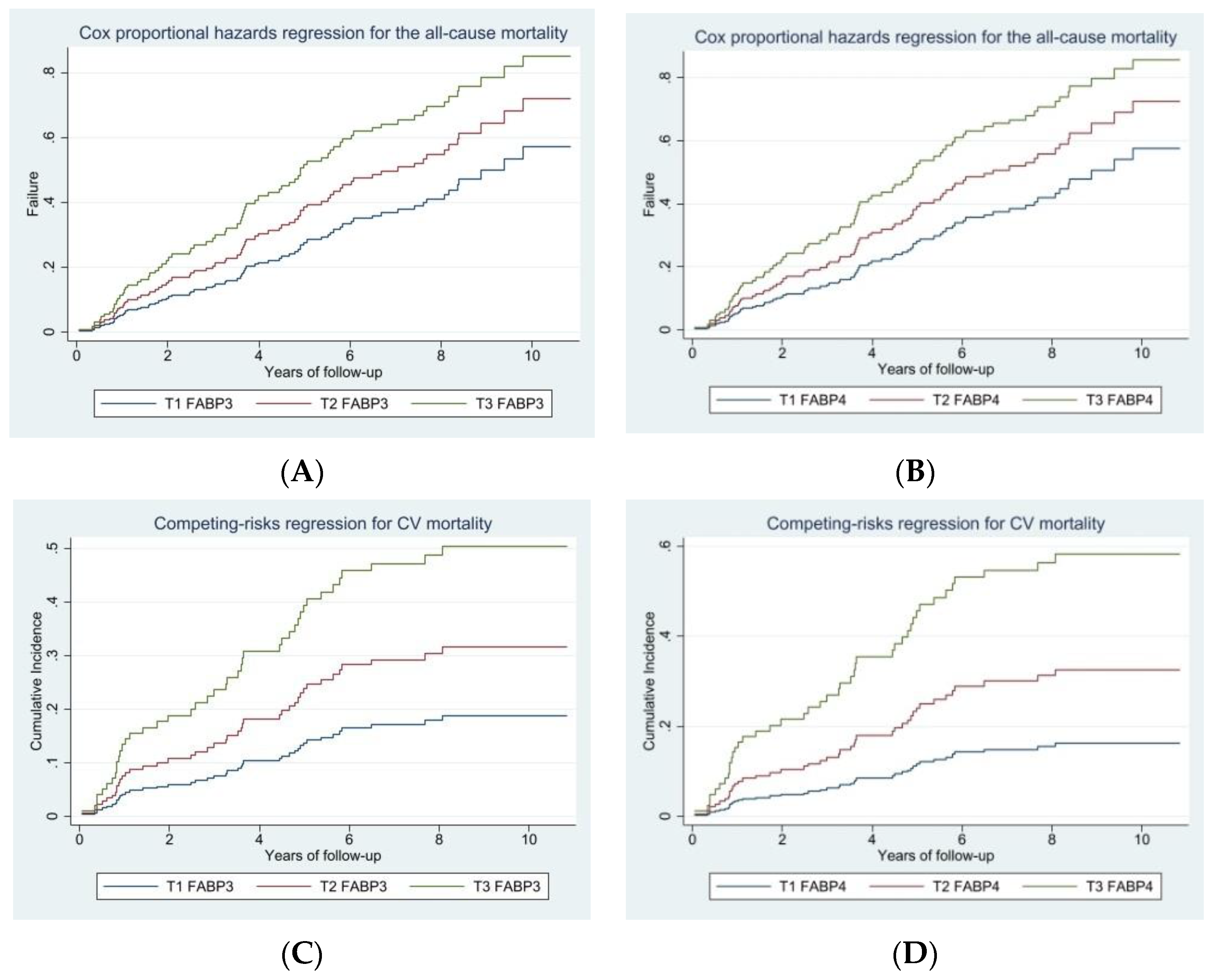
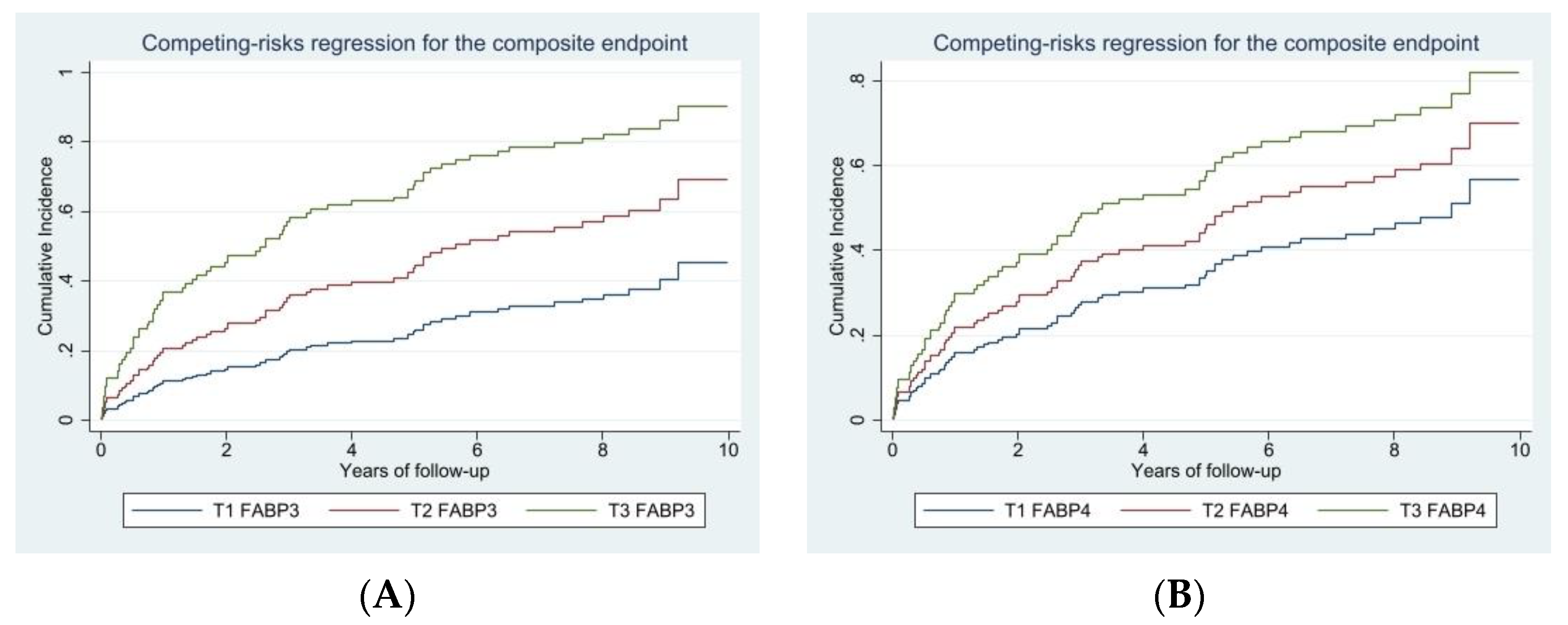
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen-Solal, A.; Beauvais, F.; Logeart, D. Heart failure and diabetes mellitus: Epidemiology and management of an alarming association. J. Card. Fail. 2008, 14, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.D.; Langenberg, C.; Rapsomaniki, E.; Denaxas, S.; Pujades-Rodriguez, M.; Gale, C.P.; Deanfield, J.; Smeeth, L.; Timmis, A.; Hemingway, H. Type 2 diabetes and incidence of cardiovascular diseases: A cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015, 3, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, A.G.; Hundley, W.G.; Massing, M.W.; Bonds, D.E.; Burke, G.L.; Goff, D.C., Jr. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care 2004, 27, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Gravning, J.; Askevold, E.T.; Nymo, S.H.; Ueland, T.; Wikstrand, J.; McMurray, J.J.; Aukrust, P.; Gullestad, L.; Kjekshus, J.; Group, C.S. Prognostic effect of high-sensitive troponin T assessment in elderly patients with chronic heart failure: Results from the CORONA trial. Circ. Heart Fail. 2014, 7, 96–103. [Google Scholar] [CrossRef]
- Bouvy, M.L.; Heerdink, E.R.; Leufkens, H.G.; Hoes, A.W. Predicting mortality in patients with heart failure: A pragmatic approach. Heart 2003, 89, 605–609. [Google Scholar] [CrossRef]
- Levy, W.C.; Mozaffarian, D.; Linker, D.T.; Sutradhar, S.C.; Anker, S.D.; Cropp, A.B.; Anand, I.; Maggioni, A.; Burton, P.; Sullivan, M.D.; et al. The Seattle Heart Failure Model: Prediction of survival in heart failure. Circulation 2006, 113, 1424–1433. [Google Scholar] [CrossRef]
- Pocock, S.J.; Wang, D.; Pfeffer, M.A.; Yusuf, S.; McMurray, J.J.; Swedberg, K.B.; Ostergren, J.; Michelson, E.L.; Pieper, K.S.; Granger, C.B. Predictors of mortality and morbidity in patients with chronic heart failure. Eur. Heart J. 2006, 27, 65–75. [Google Scholar] [CrossRef]
- Roberts, A.W.; Clark, A.L.; Witte, K.K. Review article: Left ventricular dysfunction and heart failure in metabolic syndrome and diabetes without overt coronary artery disease—Do we need to screen our patients? Diabetes Vasc. Dis. Res. 2009, 6, 153–163. [Google Scholar] [CrossRef]
- Palomer, X.; Salvado, L.; Barroso, E.; Vazquez-Carrera, M. An overview of the crosstalk between inflammatory processes and metabolic dysregulation during diabetic cardiomyopathy. Int. J. Cardiol. 2013, 168, 3160–3172. [Google Scholar] [CrossRef]
- Rodriguez-Calvo, R.; Girona, J.; Alegret, J.M.; Bosquet, A.; Ibarretxe, D.; Masana, L. Role of the fatty acid-binding protein 4 in heart failure and cardiovascular disease. J. Endocrinol. 2017, 233, R173–R184. [Google Scholar] [CrossRef]
- Shearer, J.; Fueger, P.T.; Wang, Z.; Bracy, D.P.; Wasserman, D.H.; Rottman, J.N. Metabolic implications of reduced heart-type fatty acid binding protein in insulin resistant cardiac muscle. Biochim. Biophys. Acta 2008, 1782, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.J.; Barcelo-Coblijn, G.; Binas, B.; Glatz, J.F. Heart fatty acid uptake is decreased in heart fatty acid-binding protein gene-ablated mice. J. Biol. Chem. 2004, 279, 34481–34488. [Google Scholar] [CrossRef] [PubMed]
- Lamounier-Zepter, V.; Look, C.; Alvarez, J.; Christ, T.; Ravens, U.; Schunck, W.H.; Ehrhart-Bornstein, M.; Bornstein, S.R.; Morano, I. Adipocyte fatty acid-binding protein suppresses cardiomyocyte contraction: A new link between obesity and heart disease. Circ. Res. 2009, 105, 326–334. [Google Scholar] [CrossRef]
- Iso, T.; Maeda, K.; Hanaoka, H.; Suga, T.; Goto, K.; Syamsunarno, M.R.; Hishiki, T.; Nagahata, Y.; Matsui, H.; Arai, M.; et al. Capillary endothelial fatty acid binding proteins 4 and 5 play a critical role in fatty acid uptake in heart and skeletal muscle. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2549–2557. [Google Scholar] [CrossRef]
- Rodriguez-Calvo, R.; Girona, J.; Rodriguez, M.; Samino, S.; Barroso, E.; de Gonzalo-Calvo, D.; Guaita-Esteruelas, S.; Heras, M.; van der Meer, R.W.; Lamb, H.J.; et al. Fatty acid binding protein 4 (FABP4) as a potential biomarker reflecting myocardial lipid storage in type 2 diabetes. Metabolism 2019, 96, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.D.; He, Y.; Wang, S.; Wong, G.T.; Irwin, M.G.; Xia, Z. Heart-type fatty acid binding protein (H-FABP) as a biomarker for acute myocardial injury and long-term post-ischemic prognosis. Acta Pharmacol. Sin. 2018, 39, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Pyati, A.K.; Devaranavadagi, B.B.; Sajjannar, S.L.; Nikam, S.V.; Shannawaz, M.; Sudharani. Heart-Type Fatty Acid Binding Protein: A Better Cardiac Biomarker than CK-MB and Myoglobin in the Early Diagnosis of Acute Myocardial Infarction. J. Clin. Diagn. Res. 2015, 9, BC08–BC11. [Google Scholar] [CrossRef]
- Collinson, P.; Gaze, D.; Goodacre, S. Comparison of contemporary troponin assays with the novel biomarkers, heart fatty acid binding protein and copeptin, for the early confirmation or exclusion of myocardial infarction in patients presenting to the emergency department with chest pain. Heart 2014, 100, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Basar, O.; Akbal, E.; Koklu, S.; Tuna, Y.; Kocak, E.; Basar, N.; Tok, D.; Erbis, H.; Senes, M. Increased H-FABP concentrations in nonalcoholic fatty liver disease. Possible marker for subclinical myocardial damage and subclinical atherosclerosis. Herz 2013, 38, 417–422. [Google Scholar] [CrossRef]
- Narumi, T.; Shishido, T.; Kiribayashi, N.; Kadowaki, S.; Nishiyama, S.; Takahashi, H.; Arimoto, T.; Miyashita, T.; Miyamoto, T.; Watanabe, T.; et al. Impact of insulin resistance on silent and ongoing myocardial damage in normal subjects: The Takahata study. Exp. Diabetes Res. 2012, 2012, 815098. [Google Scholar] [CrossRef]
- Fuseya, T.; Furuhashi, M.; Yuda, S.; Muranaka, A.; Kawamukai, M.; Mita, T.; Ishimura, S.; Watanabe, Y.; Hoshina, K.; Tanaka, M.; et al. Elevation of circulating fatty acid-binding protein 4 is independently associated with left ventricular diastolic dysfunction in a general population. Cardiovasc. Diabetol. 2014, 13, 126. [Google Scholar] [CrossRef]
- Balci, M.M.; Arslan, U.; Firat, H.; Kocaoglu, I.; Vural, M.G.; Balci, K.G.; Maden, O.; Gurbuz, O.A.; Ardic, S.; Yeter, E. Serum levels of adipocyte fatty acid-binding protein are independently associated with left ventricular mass and myocardial performance index in obstructive sleep apnea syndrome. J. Investig. Med. 2012, 60, 1020–1026. [Google Scholar] [CrossRef]
- Cabre, A.; Valdovinos, P.; Lazaro, I.; Bonet, G.; Bardaji, A.; Masana, L. Parallel evolution of circulating FABP4 and NT-proBNP in heart failure patients. Cardiovasc. Diabetol. 2013, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Djousse, L.; Bartz, T.M.; Ix, J.H.; Kochar, J.; Kizer, J.R.; Gottdiener, J.S.; Tracy, R.P.; Mozaffarian, D.; Siscovick, D.S.; Mukamal, K.J.; et al. Fatty acid-binding protein 4 and incident heart failure: The Cardiovascular Health Study. Eur. J. Heart Fail. 2013, 15, 394–399. [Google Scholar] [CrossRef]
- Baessler, A.; Lamounier-Zepter, V.; Fenk, S.; Strack, C.; Lahmann, C.; Loew, T.; Schmitz, G.; Bluher, M.; Bornstein, S.R.; Fischer, M. Adipocyte fatty acid-binding protein levels are associated with left ventricular diastolic dysfunction in morbidly obese subjects. Nutr. Diabetes 2014, 4, e106. [Google Scholar] [CrossRef] [PubMed]
- Engeli, S.; Utz, W.; Haufe, S.; Lamounier-Zepter, V.; Pofahl, M.; Traber, J.; Janke, J.; Luft, F.C.; Boschmann, M.; Schulz-Menger, J.; et al. Fatty acid binding protein 4 predicts left ventricular mass and longitudinal function in overweight and obese women. Heart 2013, 99, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.L.; Wu, Y.W.; Wu, C.C.; Lin, L.; Wu, Y.C.; Hsu, P.Y.; Jong, Y.S.; Yang, W.S. Association between serum adipocyte fatty-acid binding protein concentrations, left ventricular function and myocardial perfusion abnormalities in patients with coronary artery disease. Cardiovasc. Diabetol. 2013, 12, 105. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, M.; Bao, Y.; Xu, Z.; Li, H.; Zhang, H.; Zhu, W.; Zhang, J.; Xu, A.; Wei, M.; et al. Circulating adipocyte fatty acid-binding protein levels are independently associated with heart failure. Clin. Sci. 2013, 124, 115–122. [Google Scholar] [CrossRef]
- Sharma, S.; Adrogue, J.V.; Golfman, L.; Uray, I.; Lemm, J.; Youker, K.; Noon, G.P.; Frazier, O.H.; Taegtmeyer, H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004, 18, 1692–1700. [Google Scholar] [CrossRef]
- Levelt, E.; Mahmod, M.; Piechnik, S.K.; Ariga, R.; Francis, J.M.; Rodgers, C.T.; Clarke, W.T.; Sabharwal, N.; Schneider, J.E.; Karamitsos, T.D.; et al. Relationship Between Left Ventricular Structural and Metabolic Remodeling in Type 2 Diabetes. Diabetes 2016, 65, 44–52. [Google Scholar] [CrossRef] [PubMed]
- McGavock, J.M.; Lingvay, I.; Zib, I.; Tillery, T.; Salas, N.; Unger, R.; Levine, B.D.; Raskin, P.; Victor, R.G.; Szczepaniak, L.S. Cardiac steatosis in diabetes mellitus: A 1H-magnetic resonance spectroscopy study. Circulation 2007, 116, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.C.; Delgado, V.; Bertini, M.; van der Meer, R.W.; Rijzewijk, L.J.; Hooi Ewe, S.; Siebelink, H.M.; Smit, J.W.; Diamant, M.; Romijn, J.A.; et al. Myocardial steatosis and biventricular strain and strain rate imaging in patients with type 2 diabetes mellitus. Circulation 2010, 122, 2538–2544. [Google Scholar] [CrossRef] [PubMed]
- Rijzewijk, L.J.; van der Meer, R.W.; Smit, J.W.; Diamant, M.; Bax, J.J.; Hammer, S.; Romijn, J.A.; de Roos, A.; Lamb, H.J. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J. Am. Coll. Cardiol. 2008, 52, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Abass, M.A.; Arafa, M.H.; El-Shal, A.S.; Atteia, H.H. Asymmetric dimethylarginine and heart-type fatty acid-binding protein 3 are risk markers of cardiotoxicity in carbon monoxide poisoning cases in Zagazig university hospitals. Hum. Exp. Toxicol. 2017, 36, 247–255. [Google Scholar] [CrossRef]
- Zhou, M.; Bao, Y.; Li, H.; Pan, Y.; Shu, L.; Xia, Z.; Wu, D.; Lam, K.S.; Vanhoutte, P.M.; Xu, A.; et al. Deficiency of adipocyte fatty-acid-binding protein alleviates myocardial ischaemia/reperfusion injury and diabetes-induced cardiac dysfunction. Clin. Sci. 2015, 129, 547–559. [Google Scholar] [CrossRef]
- McCann, C.J.; Glover, B.M.; Menown, I.B.; Moore, M.J.; McEneny, J.; Owens, C.G.; Smith, B.; Sharpe, P.C.; Young, I.S.; Adgey, J.A. Prognostic value of a multimarker approach for patients presenting to hospital with acute chest pain. Am. J. Cardiol. 2009, 103, 22–28. [Google Scholar] [CrossRef]
- Matsumoto, S.; Nakatani, D.; Sakata, Y.; Suna, S.; Shimizu, M.; Usami, M.; Hara, M.; Sumitsuji, S.; Nanto, S.; Sasaki, T.; et al. Elevated serum heart-type fatty acid-binding protein in the convalescent stage predicts long-term outcome in patients surviving acute myocardial infarction. Circ. J. 2013, 77, 1026–1032. [Google Scholar] [CrossRef]
- Shirakabe, A.; Hata, N.; Kobayashi, N.; Okazaki, H.; Shinada, T.; Tomita, K.; Yamamoto, M.; Tsurumi, M.; Matsushita, M.; Yamamoto, Y.; et al. Serum heart-type fatty acid-binding protein level can be used to detect acute kidney injury on admission and predict an adverse outcome in patients with acute heart failure. Circ. J. 2015, 79, 119–128. [Google Scholar] [CrossRef]
- Lu, Y.C.; Lee, T.L.; Hsuan, C.F.; Hung, W.C.; Wu, C.C.; Wang, C.P.; Wei, C.T.; Yu, T.H.; Chung, F.M.; Lee, Y.J.; et al. Elevated plasma fatty acid-binding protein 3 is related to prolonged corrected QT interval and reduced ejection fraction in patients with stable angina. Int. J. Med. Sci. 2021, 18, 2076–2085. [Google Scholar] [CrossRef]
- Liu, G.; Ding, M.; Chiuve, S.E.; Rimm, E.B.; Franks, P.W.; Meigs, J.B.; Hu, F.B.; Sun, Q. Plasma Levels of Fatty Acid-Binding Protein 4, Retinol-Binding Protein 4, High-Molecular-Weight Adiponectin, and Cardiovascular Mortality among Men with Type 2 Diabetes: A 22-Year Prospective Study. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2259–2267. [Google Scholar] [CrossRef]
- Lee, C.H.; Cheung, C.Y.Y.; Woo, Y.C.; Lui, D.T.W.; Yuen, M.M.A.; Fong, C.H.Y.; Chow, W.S.; Xu, A.; Lam, K.S.L. Circulating Adipocyte Fatty Acid-Binding Protein Concentrations Predict Multiple Mortality Outcomes among Men and Women with Diabetes. Clin. Chem. 2018, 64, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Hobaus, C.; Herz, C.T.; Pesau, G.; Wrba, T.; Koppensteiner, R.; Schernthaner, G.H. FABP4 and Cardiovascular Events in Peripheral Arterial Disease. Angiology 2018, 69, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Komamura, K.; Sasaki, T.; Hanatani, A.; Kim, J.; Hashimura, K.; Ishida, Y.; Ohkaru, Y.; Asayama, K.; Tanaka, T.; Ogai, A.; et al. Heart-type fatty acid binding protein is a novel prognostic marker in patients with non-ischaemic dilated cardiomyopathy. Heart 2006, 92, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, K.; Kilcullen, N.; Morrell, C.; Thistlethwaite, S.J.; Sivananthan, M.U.; Hassan, T.B.; Barth, J.H.; Hall, A.S. Heart-type fatty acid-binding protein predicts long-term mortality and re-infarction in consecutive patients with suspected acute coronary syndrome who are troponin-negative. J. Am. Coll. Cardiol. 2010, 55, 2590–2598. [Google Scholar] [CrossRef]
- Kutsuzawa, D.; Arimoto, T.; Watanabe, T.; Shishido, T.; Miyamoto, T.; Miyashita, T.; Takahashi, H.; Niizeki, T.; Takeishi, Y.; Kubota, I. Ongoing myocardial damage in patients with heart failure and preserved ejection fraction. J. Cardiol. 2012, 60, 454–461. [Google Scholar] [CrossRef]
- Liu, M.; Yuan, X.; Qiu, X.; Shan, X.; Lin, D.; Zhu, L. Prognostic role of heart-type fatty acid binding protein in pulmonary embolism: A meta-analysis. Thromb. Res. 2015, 135, 20–25. [Google Scholar] [CrossRef]
- Qian, H.Y.; Huang, J.; Yang, Y.J.; Yang, Y.M.; Li, Z.Z.; Zhang, J.M. Heart-type Fatty Acid Binding Protein in the Assessment of Acute Pulmonary Embolism. Am. J. Med. Sci. 2016, 352, 557–562. [Google Scholar] [CrossRef]
- Zhang, H.W.; Jin, J.L.; Cao, Y.X.; Liu, H.H.; Zhang, Y.; Guo, Y.L.; Wu, N.Q.; Gao, Y.; Xu, R.X.; Hua, Q.; et al. Prognostic utility of heart-type fatty acid-binding protein in patients with stable coronary artery disease and impaired glucose metabolism: A cohort study. Cardiovasc. Diabetol. 2020, 19, 15. [Google Scholar] [CrossRef]
- Tso, A.W.; Lam, T.K.; Xu, A.; Yiu, K.H.; Tse, H.F.; Li, L.S.; Law, L.S.; Cheung, B.M.; Cheung, R.T.; Lam, K.S. Serum adipocyte fatty acid-binding protein associated with ischemic stroke and early death. Neurology 2011, 76, 1968–1975. [Google Scholar] [CrossRef]
- Peeters, W.; de Kleijn, D.P.; Vink, A.; van de Weg, S.; Schoneveld, A.H.; Sze, S.K.; van der Spek, P.J.; de Vries, J.P.; Moll, F.L.; Pasterkamp, G. Adipocyte fatty acid binding protein in atherosclerotic plaques is associated with local vulnerability and is predictive for the occurrence of adverse cardiovascular events. Eur. Heart J. 2011, 32, 1758–1768. [Google Scholar] [CrossRef]
- Furuhashi, M.; Ishimura, S.; Ota, H.; Hayashi, M.; Nishitani, T.; Tanaka, M.; Yoshida, H.; Shimamoto, K.; Hotamisligil, G.S.; Miura, T. Serum fatty acid-binding protein 4 is a predictor of cardiovascular events in end-stage renal disease. PLoS ONE 2011, 6, e27356. [Google Scholar] [CrossRef]
- von Eynatten, M.; Breitling, L.P.; Roos, M.; Baumann, M.; Rothenbacher, D.; Brenner, H. Circulating adipocyte fatty acid-binding protein levels and cardiovascular morbidity and mortality in patients with coronary heart disease: A 10-year prospective study. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2327–2335. [Google Scholar] [CrossRef]
- Djousse, L.; Maziarz, M.; Biggs, M.L.; Ix, J.H.; Zieman, S.J.; Kizer, J.R.; Lemaitre, R.N.; Mozaffarian, D.; Tracy, R.P.; Mukamal, K.J.; et al. Plasma Fatty Acid binding protein 4 and risk of sudden cardiac death in older adults. Cardiol. Res. Pract. 2013, 2013, 181054. [Google Scholar] [CrossRef]
- Takagi, W.; Miyoshi, T.; Doi, M.; Okawa, K.; Nosaka, K.; Nishibe, T.; Matsuo, N.; Hirohata, S.; Ito, H. Circulating adipocyte fatty acid-binding protein is a predictor of cardiovascular events in patients with stable angina undergoing percutaneous coronary intervention. BMC Cardiovasc. Disord. 2017, 17, 258. [Google Scholar] [CrossRef]
- Naka, K.K.; Papathanassiou, K.; Bechlioulis, A.; Pappas, K.; Tigas, S.; Makriyiannis, D.; Antoniou, S.; Kazakos, N.; Margeli, A.; Papassotiriou, I.; et al. Association of vascular indices with novel circulating biomarkers as prognostic factors for cardiovascular complications in patients with type 2 diabetes mellitus. Clin. Biochem. 2018, 53, 31–37. [Google Scholar] [CrossRef]
- Egbuche, O.; Biggs, M.L.; Ix, J.H.; Kizer, J.R.; Lyles, M.F.; Siscovick, D.S.; Djousse, L.; Mukamal, K.J. Fatty Acid Binding Protein-4 and Risk of Cardiovascular Disease: The Cardiovascular Health Study. J. Am. Heart Assoc. 2020, 9, e014070. [Google Scholar] [CrossRef]
- Saito, N.; Furuhashi, M.; Koyama, M.; Higashiura, Y.; Akasaka, H.; Tanaka, M.; Moniwa, N.; Ohnishi, H.; Saitoh, S.; Ura, N.; et al. Elevated circulating FABP4 concentration predicts cardiovascular death in a general population: A 12-year prospective study. Sci. Rep. 2021, 11, 4008. [Google Scholar] [CrossRef] [PubMed]
- Brankovic, M.; Akkerhuis, K.M.; Mouthaan, H.; Brugts, J.J.; Manintveld, O.C.; van Ramshorst, J.; Germans, T.; Umans, V.; Boersma, E.; Kardys, I. Cardiometabolic Biomarkers and Their Temporal Patterns Predict Poor Outcome in Chronic Heart Failure (Bio-SHiFT Study). J. Clin. Endocrinol. Metab. 2018, 103, 3954–3964. [Google Scholar] [CrossRef] [PubMed]
- Alonso, N.; Lupon, J.; Barallat, J.; de Antonio, M.; Domingo, M.; Zamora, E.; Moliner, P.; Galan, A.; Santesmases, J.; Pastor, C.; et al. Impact of diabetes on the predictive value of heart failure biomarkers. Cardiovasc. Diabetol. 2016, 15, 151. [Google Scholar] [CrossRef] [PubMed]
- Teis, A.; Cediel, G.; Amigo, N.; Julve, J.; Aranyo, J.; Andres-Cordon, J.; Puig-Jove, C.; Castelblanco, E.; Gual-Capllonch, F.; Ferrer-Sistach, E.; et al. Particle size and cholesterol content of circulating HDL correlate with cardiovascular death in chronic heart failure. Sci. Rep. 2021, 11, 3141. [Google Scholar] [CrossRef]
- Gottdiener, J.S.; Bednarz, J.; Devereux, R.; Gardin, J.; Klein, A.; Manning, W.J.; Morehead, A.; Kitzman, D.; Oh, J.; Quinones, M.; et al. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J. Am. Soc. Echocardiogr. 2004, 17, 1086–1119. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- World Medical, A. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef] [PubMed]
- Sautin, Y.Y.; Johnson, R.J. Uric acid: The oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Kurajoh, M.; Fukumoto, S.; Yoshida, S.; Akari, S.; Murase, T.; Nakamura, T.; Ishii, H.; Yoshida, H.; Nagata, Y.; Morioka, T.; et al. Uric acid shown to contribute to increased oxidative stress level independent of xanthine oxidoreductase activity in MedCity21 health examination registry. Sci. Rep. 2021, 11, 7378. [Google Scholar] [CrossRef]
- Lorenzo, O.; Picatoste, B.; Ares-Carrasco, S.; Ramirez, E.; Egido, J.; Tunon, J. Potential role of nuclear factor kappaB in diabetic cardiomyopathy. Mediat. Inflamm. 2011, 2011, 652097. [Google Scholar] [CrossRef]
| Characteristics | All N = 240 | T2D N = 114 | Non-T2D N = 126 | * p-Value |
|---|---|---|---|---|
| Age, years | 69.0 [58.5–77.0] | 71.0 [63.0–77.0] | 66.5 [53.0–78.0] | 0.063 |
| Sex, women | 70 (29.17) | 36 (31.58) | 34 (26.98) | 0.434 |
| Ethnicity, Caucasian | 234 (97.50) | 111 (97.37) | 123 (97.62) | 0.388 |
| Smoking | ||||
| Current smoker | 34 (14.70) | 14 (12.28) | 20 (15.87) | 0.425 |
| Former smoker | 104 (43.33) | 48 (42.11) | 56 (44.44) | 0.715 |
| BMI, Kg/m2 | 26.47 [23.59–30.21] | 27.06 [23.95–31.24] | 26.04 [23.31–28.84] | 0.075 |
| Hypertension | 161 (67.08) | 88 (77.19) | 73 (57.94) | 0.002 |
| Hypercholesterolemia | 154 (64.17) | 92 (80.70) | 62 (49.21) | <0.001 |
| Oral antidiabetic drugs | 84 (35.00) | 84 (73.68) | 0 (0) | <0.001 |
| Insulin treatment | 71 (29.58) | 71 (62.28) | 0 (0) | <0.001 |
| Ischemic heart disease | 100 (41.67) | 54 (47.37) | 46 (36.51) | 0.088 |
| NYHA III and IV | 53 (22.08) | 33 (28.95) | 20 (15.87) | 0.015 |
| LVEF | 34.00 [25.00–42.00] | 34.00 [28.00–42.00] | 34.00 [24.00–44.00] | 0.824 |
| HF duration, months | 6.00 [2.00–45.00] | 8.00 [2.00–39.00] | 5.00 [2.00–48.00] | 0.413 |
| Admission for heart failure | 105 (43.75) | 57 (50.00) | 48 (38.10) | 0.063 |
| Ischemic etiology | 117 (48.75) | 66 (57.89) | 51 (40.48) | 0.007 |
| Total cholesterol, mg/dL | 172.85 [141.23–210.88] | 159.66 [133.86–198.66] | 178.87 [149.77–221.40] | 0.002 |
| LDL cholesterol, mg/dL | 89.36 [74.42–106.60] | 87.74 [68.89–116.28] | 104.72 [83.21–131.61] | <0.001 |
| HDL cholesterol, mg/dL | 46.94 [41.66–54.25] | 43.46 [36.86–52.77] | 48.69 [41.13–55.87] | 0.005 |
| Triglycerides, mg/dL | 120.46 [85.91–170.50] | 126.21 [4.14–173.60] | 114.70 [86.79–162.97] | 0.550 |
| Creatinine, mg/dL | 1.20 [1.00–1.71] | 1.40 [1.04–2.00] | 1.10 [0.96–1.60] | 0.004 |
| Urate, mg/dL | 6.50 [6.50–6.50] | 6.50 [6.50–6.50] | 6.50 [6.50–6.50] | 0.099 |
| eGFR, mL/min/1.73 m2 | 54.20 [34.88–78.52] | 48.59 [28.06–69.58] | 67.19 [38.99–85.62] | 0.001 |
| NTproBNP, ng/L | 2142.50 [763.50–5050.00] | 2675.5 [1104.00–5780.00] | 1820.50 [593.00–3885.00] | 0.005 |
| FABP3, pg/mL | 1596.09 [971.60–2894.00] | 1829.33 [1104.92–3440.49] | 1396.05 [820.3–2362.16] | 0.007 |
| FABP4, ng/mL | 39.90 [25.98–66.63] | 45.5 [27.62–79.82] | 34.1 [24.09–55.3] | 0.006 |
| Deaths | 143 (59.58) | 79 (69.30) | 64 (50.79) | 0.004 |
| Death follow-up, years | 6.67 [2.85–8.55] | 5.22 [2.02–8.17] | 7.13 [3.27–8.90] | 0.009 |
| CV mortality | 73 (31.47) | 44 (40.00) | 29 (23.77) | 0.008 |
| CV mortality and/or admission for HF | 134 (57.26) | 77 (69.37) | 57 (46.34) | 0.002 |
| CV mortality and/or admission for HF follow-up, years | 3.76 [0.79–7.81] | 2.57 [0.61–6.81] | 5.72 [0.90–8.31] | 0.006 |
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
|---|---|---|---|---|
| FABP3, ng/mL | 1.25 (1.09–1.44) | 0.002 | - | - |
| FABP4, ng/dL | - | - | 2.21 (1.12–4.36) | 0.023 |
| Age, years | 1.04 (1.01–1.07) | 0.003 | 1.04 (1.01–1.06) | 0.004 |
| Sex, women | 0.74 (0.39–1.39) | 0.345 | 0.61 (0.32–1.19) | 0.149 |
| Ischemic etiology | 2.07 (1.20–3.57) | 0.009 | 2.15 (1.22–3.80) | 0.008 |
| NYHA III and IV, % | 1.71 (0.97–3.01) | 0.062 | 1.83 (1.05–3.21) | 0.034 |
| HF duration, years | 1.00 (1.00–1.01) | 0.124 | 1.00 (1.00–1.01) | 0.149 |
| LVEF | 1.02 (0.99–1.04) | 0.198 | 1.01 (0.99–1.04) | 0.255 |
| NTproBNP, ng/L | 1.00 (1.00–1.00) | 0.899 | 1.00 (1.00–1.00) | 0.381 |
| Obesity, % | 0.89 (0.49–1.59) | 0.683 | 0.80 (0.45–1.43) | 0.447 |
| eGFR, mL/min/1.73 m2 | 1.01 (1.00–1.02) | 0.150 | 1.01 (0.99–1.02) | 0.396 |
| SHR (95% CI) | p-Value | SHR (95% CI) | p-Value | |
|---|---|---|---|---|
| FABP3, ng/mL | 1.28 (1.09–1.50) | 0.002 | - | - |
| FABP4, ng/dL | - | - | 4.19 (2.21–7.95) | <0.001 |
| Age, years | 1.03 (0.99–1.06) | 0.101 | 1.03 (1.00–1.06) | 0.059 |
| Sex, women | 1.33 (0.57–3.11) | 0.516 | 0.94 (0.38–2.33) | 0.886 |
| Ischemic etiology | 2.57 (1.26–5.26) | 0.010 | 3.08 (1.42–6.68) | 0.004 |
| NYHA III and IV, % | 1.98 (0.97–4.04) | 0.060 | 2.42 (1.18–4.96) | 0.016 |
| HF duration, years | 1.00 (1.00–1.01) | 0.867 | 1.00 (1.00–1.00) | 0.937 |
| LVEF | 1.00 (0.96–1.03) | 0.828 | 1.00 (0.96–1.03) | 0.940 |
| NTproBNP, ng/L | 1.00 (1.00–1.00) | 0.134 | 1.00 (1.00–1.00) | 0.238 |
| Obesity, % | 0.54 (0.22–1.31) | 0.171 | 0.45 (0.19–1.05) | 0.065 |
| eGFR, mL/min/1.73 m2 | 1.02 (1.00–1.03) | 0.079 | 1.02 (1.00–1.03) | 0.030 |
| SHR (95% CI) | p-Value | SHR (95% CI) | p-Value | |
|---|---|---|---|---|
| FABP3, ng/mL | 1.14 (0.98–1.32) | 0.083 | - | - |
| FABP4, ng/dL | - | - | 2.07 (1.11–3.87) | 0.022 |
| Age, years | 1.04 (1.01–1.07) | 0.008 | 1.04 (1.01–1.07) | 0.007 |
| Sex, women | 1.20 (0.66–2.16) | 0.552 | 0.96 (0.53–1.76) | 0.902 |
| Ischemic etiology | 2.21 (1.35–3.60) | 0.002 | 2.31 (1.42–3.74) | 0.001 |
| NYHA III and IV, % | 1.27 (0.67–2.39) | 0.465 | 1.41 (0.77–2.58) | 0.270 |
| HF duration, years | 1.00 (1.00–1.00) | 0.813 | 1.00 (1.00–1.00) | 0.737 |
| LVEF | 0.99 (0.97–1.01) | 0.427 | 0.99 (0.97–1.02) | 0.441 |
| NTproBNP, ng/L | 1.00 (1.00–1.00) | 0.656 | 1.00 (1.00–1.00) | 0.724 |
| Obesity, % | 2.14 (1.19–3.89) | 0.013 | 1.88 (1.02–3.46) | 0.044 |
| eGFR, mL/min/1.73 m2 | 1.00 (0.99–1.02) | 0.702 | 1.00 (0.99–1.01) | 0.625 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Calvo, R.; Granado-Casas, M.; Pérez-Montes de Oca, A.; Julian, M.T.; Domingo, M.; Codina, P.; Santiago-Vacas, E.; Cediel, G.; Julve, J.; Rossell, J.; et al. Fatty Acid Binding Proteins 3 and 4 Predict Both All-Cause and Cardiovascular Mortality in Subjects with Chronic Heart Failure and Type 2 Diabetes Mellitus. Antioxidants 2023, 12, 645. https://doi.org/10.3390/antiox12030645
Rodríguez-Calvo R, Granado-Casas M, Pérez-Montes de Oca A, Julian MT, Domingo M, Codina P, Santiago-Vacas E, Cediel G, Julve J, Rossell J, et al. Fatty Acid Binding Proteins 3 and 4 Predict Both All-Cause and Cardiovascular Mortality in Subjects with Chronic Heart Failure and Type 2 Diabetes Mellitus. Antioxidants. 2023; 12(3):645. https://doi.org/10.3390/antiox12030645
Chicago/Turabian StyleRodríguez-Calvo, Ricardo, Minerva Granado-Casas, Alejandra Pérez-Montes de Oca, María Teresa Julian, Mar Domingo, Pau Codina, Evelyn Santiago-Vacas, Germán Cediel, Josep Julve, Joana Rossell, and et al. 2023. "Fatty Acid Binding Proteins 3 and 4 Predict Both All-Cause and Cardiovascular Mortality in Subjects with Chronic Heart Failure and Type 2 Diabetes Mellitus" Antioxidants 12, no. 3: 645. https://doi.org/10.3390/antiox12030645
APA StyleRodríguez-Calvo, R., Granado-Casas, M., Pérez-Montes de Oca, A., Julian, M. T., Domingo, M., Codina, P., Santiago-Vacas, E., Cediel, G., Julve, J., Rossell, J., Masana, L., Mauricio, D., Lupón, J., Bayes-Genis, A., & Alonso, N. (2023). Fatty Acid Binding Proteins 3 and 4 Predict Both All-Cause and Cardiovascular Mortality in Subjects with Chronic Heart Failure and Type 2 Diabetes Mellitus. Antioxidants, 12(3), 645. https://doi.org/10.3390/antiox12030645